Introduction
Sepsis is defined as a systemic inflammatory
response to infections caused by bacteria, fungi, viruses or
parasites. It can be divided into three severities with increasing
severity, namely, sepsis, severe sepsis and septic shock (1). A recent report indicated that the
incidence of sepsis is more frequent than previously reported and
that over 700 in 100,000 patients admitted to a medical emergency
department are diagnosed with sepsis of any severity (2). Sepsis is assoicated with many other
disorders, thus making its diagnosis difficult. Many efforts have
been made to improve the diagnostic methods and increase the
survival rates, such as measuring the lactate level immediately and
obtaining blood cultures prior to the administration of antibodies
(3). Existing management methods
include anti-biotics (4), blood
cleansing (5), vasopressor agents
(3), and so forth. Although the
pathophysiology of sepsis has been a research hotspot, further
detailed studies are still urgently required.
An increased percentage of regulatory T cells (Treg
cells) is one of the main characteristics of sepsis (6). Treg cells are specialized for immune
suppression and they are indispensable to the proper control of the
adaptive immune response (7).
Ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1; also
known as CD39) and 5′-ectonucleotidase (NT5E; also known as CD73)
are primarily expressed in Treg cells as surface markers (8,9),
both catalyzing adenosine generation (10). Particularly, CD39 converts
adenosine triphosphate (ATP) and adenosine diphosphate (ADP) to
adenosine monophosphate (AMP), and CD73 dephosphorylates AMP to
adenosine (8). Since studies have
demonstrated the pro-inflammatory functions of ATP (11), and the anti-inflammatory functions
of adenosine and adenosine A2A receptor (12,13), CD39 and CD73 can play synergistic
roles in inhibiting inflammation. Besides, both CD39 and CD73
reduce the mortality assoicated with sepsis (14,15), implying their close association
with sepsis. However, the factors leading to the high expression of
CD39 and CD73 on the Treg cell surface during sepsis remain
unclear.
This study aimed to elucidate the molecular
mechanisms responsible for the high expression of CD39 and CD73 on
the Treg cell surface, and to investigate the regulatory factors of
the CD39/CD73/adenosine pathway. For this purpose, a mouse model of
sepsis was constructed using the cecal ligation and puncture (CLP)
method. Treg cells were isolated and cultured for drug treatment or
siRNA transfection. We analyzed the regulatory effects of adenosine
and the specific adenosine A2A receptor agonist,
2-p-(2-carboxyethyl)-phenylethylamino-5′-N-ethylcarboxamidoadenosine
(CGS21680). The functions of E2F transcription factor 1 (E2F-1) and
cyclic adenosine monophosphate (cAMP) responsive element-binding
protein (CREB) as regards the regulation of CD39 or CD73 were
verified. Subsequently, we detected changes in the adenosine cycle
caused by these factors. Our results shed insight as to why CD39
and CD73 are enriched on the Treg cell surface, and enhance our
understanding of the CD39/CD73/adenosine pathway.
Materials and methods
CLP and cell culture
All the experiments were performed based on the
guidelines of our institute and the Regulation for the
Administration of Affairs Concerning Experimental Animals (approved
by the State Council of China). Clean grade BALB/c mice weighing 20
to 35 g (aged 30 to 40 days) were purchased from HFK Bioscience
(Beijing, China) and raised for 2 days for acclimatization. Before
the CLP operation, the mice were starved for 12 h. A total of 10
mice was randomly selected for the operation and anesthetized with
xylazine (10 mg per kg body weight). CLP was performed as
previously described (16). At
day 7 post-operation, the death rate was approximately 50%. Treg
cells (CD4+ and CD25+) were isolated from the
peritoneal lavage and splenocytes of the 5 living mice using a flow
cytometer and the Mouse Regulatory T cell Staining kit
(eBioscience, San Diego, CA, USA), and maintained in Roswell Park
Memorial Institute (RPMI)-1640 medium (Invitrogen, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin, 200 µg/ml streptomycin and 0.25 µg/ml
amphotericin (Invitrogen). The cells were cultured in a humidified
atmosphere with 5% CO2 at 37°C, and the medium was
changed every 2 days.
High performance liquid chromatography
(HPLC) analysis
At 48 h post-transfection, the cells were treated
with ATP (substrate of CD39) or AMP (substrate of CD73;
Sigma-Aldrich, Shanghai, China) at a concentration of 1 mM for 1 h.
The supernatant was then collected for the detection of the ATP,
AMP and adenosine concentrations using the HPLC SpectraSYSTEM
(Thermo Fisher Scientific, Waltham, MA, USA). Tests were conducted
in the octadecyl silane-C18 column at room temperature. The
injection volume of each test was 20 µl. Potassium phosphate
buffer (50 mM, pH 6.5) was used as the mobile phase, with a flow
velocity of 1 ml/min. The ultraviolet radiation wavelength for
detection was 254 nm.
Flow cytometry
Peritoneal lavage and splenocytes were collected
from the mice based on the methods of a previous study (17). Briefly, the peritoneal lavage and
the spleen tissue of the 5 living mice were collected after the
mice were anesthetized and sacrificed. For the collection of the
peritoneal lavage, 5 ml phosphate-buffered saline was injected
intraperitoneally and samples were obtained after 30 min of gently
pressing the abdomen. The cells were then isolated and cultured
according to the abovementioned method. Treg cells were isolated
using the CD4+CD25+ Regulatory T Cell
Isolation kit (Miltenyi Biotec, Teterow, Germany). αCD4 (RM4-5)
fluorochrome-labeled antibody (BD Biosciences, Heidelberg,
Germany), CD25 (PC61.5) antibody and Armenian and Syrian hamster
IgG (BioLegend, San Diego, CA, USA) were used to label the Treg
cells. The purity of the Treg cells was detected using a flow
cytometer (BD FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA).
Drug treatment and siRNA
transfection
The Treg cells were treated with CGS21680 (100 nM,
Biochempartner, Shanghai, China), or adenosine (10 µM, MCE,
Shanghai, China) at 37°C. Transcription factors in the promoter
sequences of CD39 and CD73 were predicted using UCSC genome browser
(http://genome.ucsc.edu) and VISTA Enhancer Brower
(http://genome.lbl.gov/vista/index.shtml). The E2F-1-
and CREB-specific siRNA and negative control siRNA were purchased
from Cell Signaling Technology (CST, Boston, MA, USA). The Treg
cells were plated on 60-mm dishes and transfected with the
corresponding siRNA treated with Lipofectamine® RNAiMAX
Transfection Reagent (Invitrogen) after 24 h of incubation at the
concentration of 1 pmol per 0.3 µl reagent.
Luciferase assay
The cells were transfected with Cignal E2F Reporter
(luc) kit or CREB-luc (Qiagen, Shanghai, China) using Lipofectamine
2000 transfection reagent (Invitrogen). The transfected cells were
lysed and the luciferase activities were analyzed using the
Dual-Luciferase® reporter assay system (Promega,
Madison, WI, USA) and measured as relative light units using a
luminometer (Turner Designs, Sunnyvale, CA, USA).
Western blot analysis
The protein samples were extracted from the cells
using lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl,
1% Nonidet P 40 (NP-40), 0.1% sodium dodecyl sulfate (SDS), and
0.5% sodium deoxycholate. The proteins of the same amounts were
then separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE)
and transferred onto nitrocellulose membranes. The blots were
blocked with 5% skim milk at 4°C overnight and incubated with the
primary antibodies [GAPDH (sc-365062), CD39 (sc-33558), CD73
(sc-14684), E2F-1 (sc-251), signal transducer and activator of
transcription (STAT)5 (sc-836), p-STAT5 (sc-12893), CREB
(sc-377154) or p-CREB-specific (sc-7978), Santa Cruz Biotechnology,
Dallas, TX, USA] at room temperature for 2 h, followed by
incubation with peroxidase-conjugated secondary antibody
(anti-β-actin antibody, Sigma-Aldrich) at room temperature for 1 h.
GAPDH was used as the internal reference. Positive bands were
detected using enhanced chemiluminescence reagents and analyzed
using the Kodak Digital Imaging System (Rochester, NY, USA) in
triplicate.
Statistical analysis
All experiments were repeated 3 times and the
results are presented as the means ± standard deviation (SD).
Statistical analyses were performed using Statistical Product and
Service Solutions (SPSS) 19.0 software and one-way analysis of
variance (ANOVA). Differences were considered statistically
significant at P<0.05.
Results
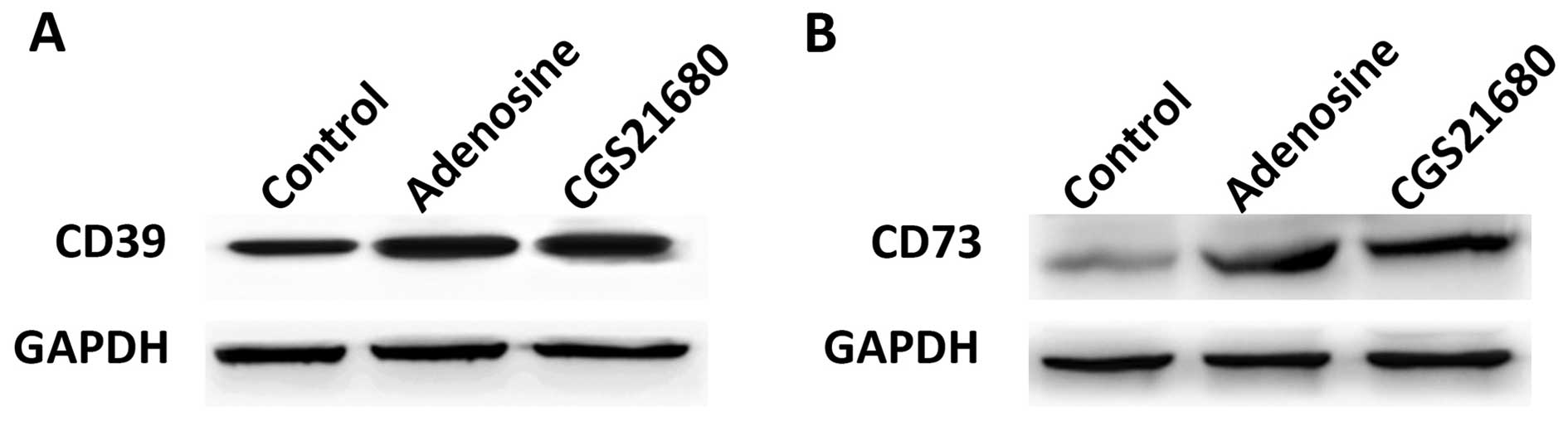
Adenosine and CGS21680 upregulate CD39
and CD73
Western blot analysis was used to examine the
effects of adenosine and CGS21680 on the expression of CD39 and
CD73. At 24 h post-treatment with adenosine or CGS21680, both the
CD39 and CD73 expression levels were upregulated compared to the
control group (Fig. 1A and B),
indicating that CD39 and CD73 are upregulated by adenosine and
CGS21680. We then performed further experiments to determine the
direct regulatory factors of CD39 and CD63.
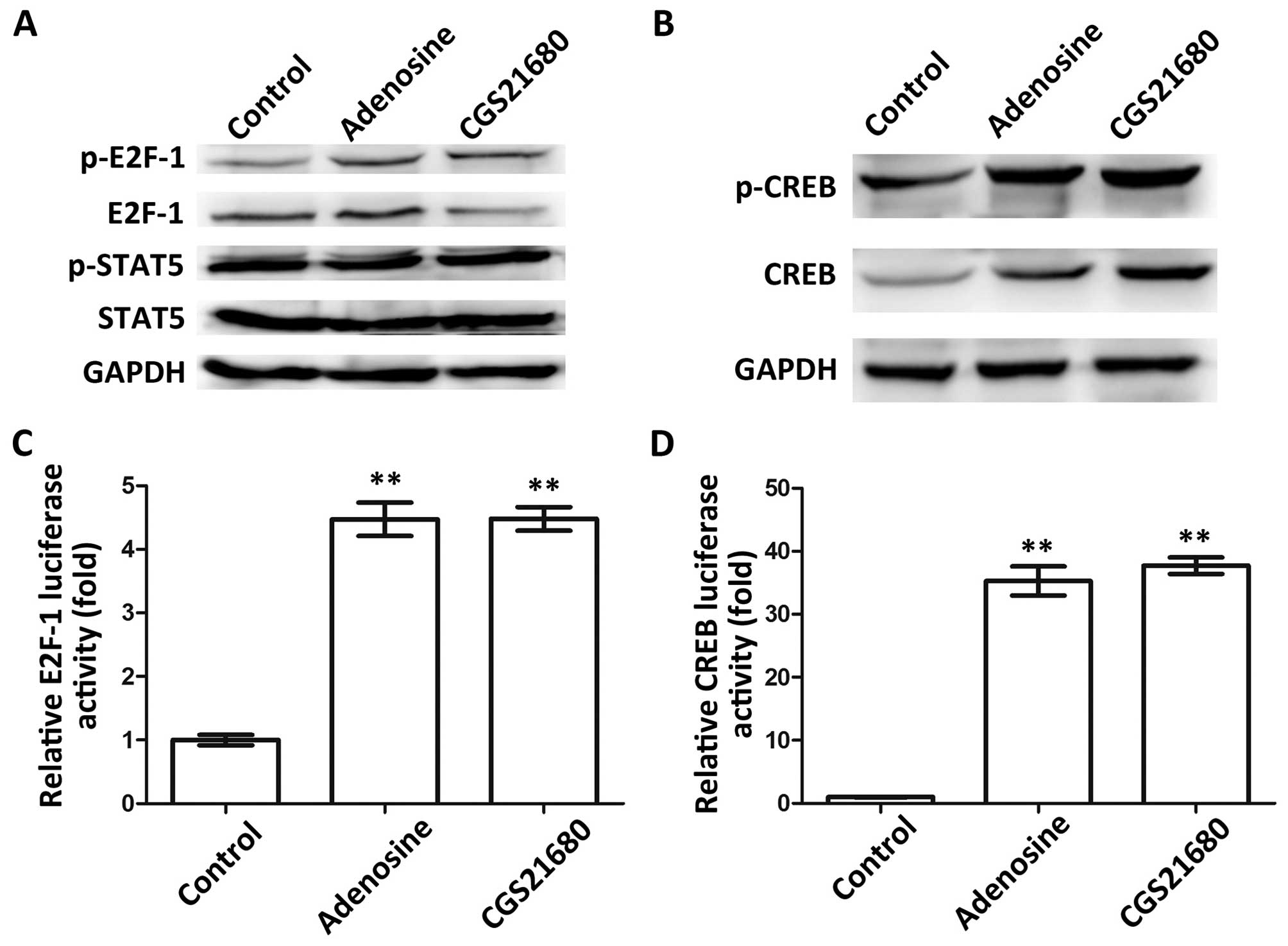
Adenosine and CGS21680 upregulate E2F-1
and CREB
The predicted transcription factors, E2F-1 and STAT5
in the CD39 promoter, and CREB in the CD73 promoter (data not
shown), were selected for this study. We conjectured that these
factors may be regulated by adenosine or CGS21680, and may thus
regulate CD39 and CD73. We thus performed a serious of experiments
for verification. First, the expression levels of E2F-1 and STAT5
were measured by western blot analysis to verify whether they are
regulated by adenosine or CGS21680 (Fig. 2A). The results revealed that
adenosine upregulated the expression of p-E2F-1 and E2F-1. CGS21680
upregulated p-E2F-1; however, it did not affect E2F-1 expression.
On the contrary, the expression patterns of p-STAT5 and STAT5 were
not affected by either adenosine or CGS21680, indicating that STAT5
is not regulated by adenosine or CGS21680. Similarly, the
expression levels of CREB and p-CREB were both upregulated by
adenosine and CGS21680 (Fig. 2B).
Thus, we only referred to E2F-1 and CREB in the following
experiments. Dual-luciferase reporter assay indicated that E2F-1
was expressed at significantly higher levels in the Treg cells
treated with adenosine or CGS21680 (P<0.01, Fig. 2C), which further confirmed tht
E2F-1 is regulated by adenosine and CGS21680. Furthermore, the
expression of CREB was significantly increased by adenosine and
CGS21680 (P<0.01, Fig. 2D),
inferring that adenosine and CGS21680 upregulate CREB. Taken
together, these data indicate that the two transcription regulatory
factors, E2F-1 and CREB, may be regulated by adenosine and
CGS21680.
Adenosine and CGS21680 upregulate CD39
and CD73 via E2F-1 and CREB
We then verified the effects of E2F-1 and CREB on
CD39 and CD73 using their specific siRNA. The knockdown of E2F-1
led to the downregulation of both p-E2F-1 and E2F-1 (Fig. 3A), and the knockdown of CREB
inhibited the expression of p-CREB and CREB protein as expected
(Fig. 3B). We then examined the
effect of E2F-1 or CREB knockdown on CD39 and CD73, and found that
the inhibition of E2F-1 resulted in the downregulation of CD39
(Fig. 3C). There was no change in
CD39 expression when the transfected cells were treated with
adenosine (si-E2F-1 + adenosine vs. si-E2F-1), while CGS21680
compensated to some extent for the inhibition of E2F-1. These
results indicate that CGS21680 and E2F-1 are both positive
regulators of CD39. As for CREB, its knockdown also downregulated
the expression of CD73 (Fig. 3D),
indicating that CREB regulates CD73. Adenosine and CGS21680
slightly increased the expression of CD73 in the transfected cells
(si-CREB + adenosine or si-CREB + CGS21680 vs. si-CREB), implying
that adenosine and CGS21680 upregulated CD73 by promoting CREB.
Taken together, our data indicate that adenosine and CGS21680
promote CD39 and CD73 by upregulating E2F-1 or CREB, constituting
the CD39/CD73/adenosine pathway. Therefore, the regulatory
functions of adenosine, CGS21680, E2F-1 and CREB are the possible
reasons for the high expression of CD39 and CD73 on the Treg cell
surface.
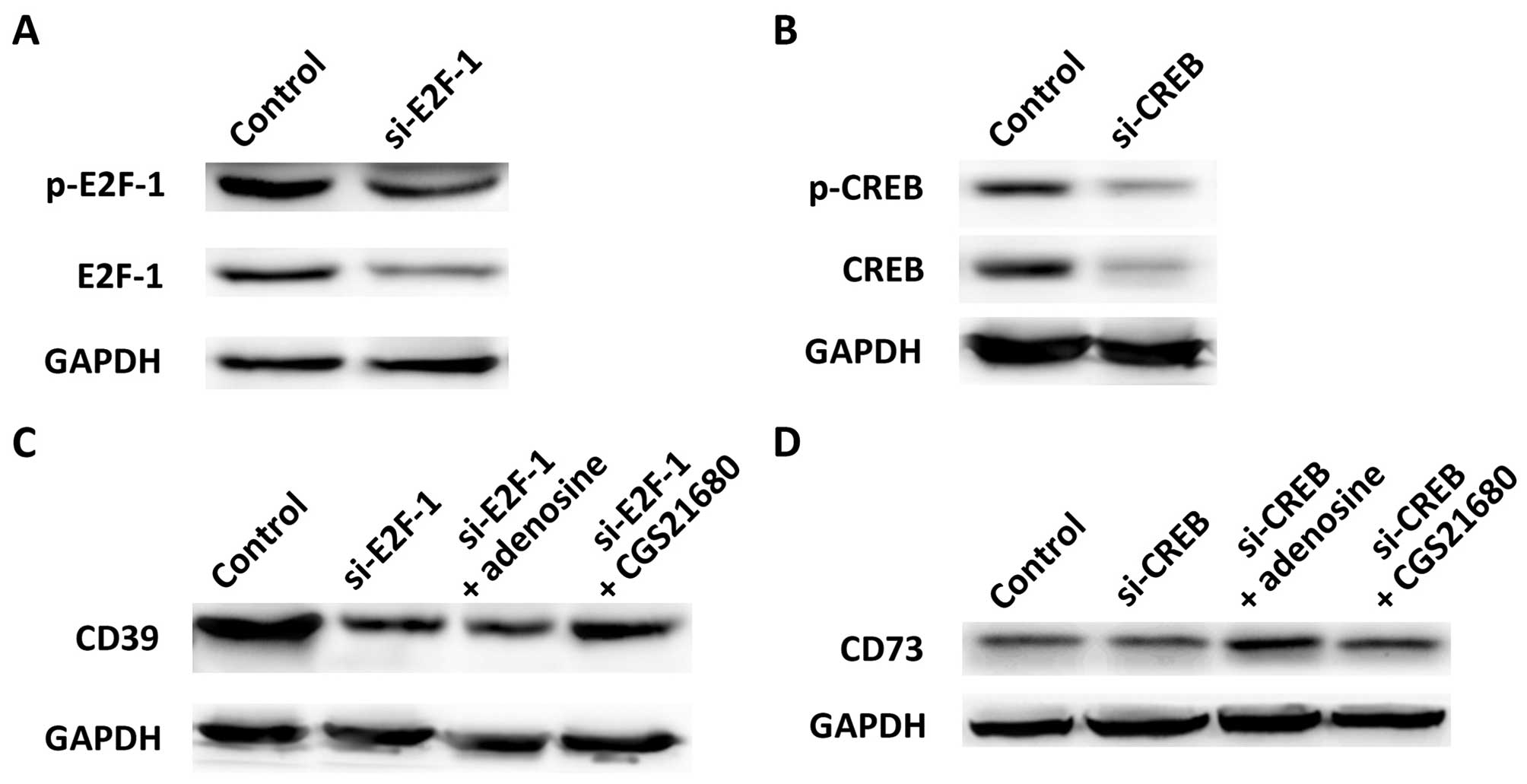 | Figure 3Regulation of CD39 and CD73 by E2F-1
and CREB. (A) E2F-1-specific siRNA inhibited the expression of
p-E2F-1 and E2F-1. (B) CREB-specific siRNA inhibited the expression
of p-CREB and CREB. (C) E2F-1, adenosine and CGS21680 regulated
CD39. (D) CREB, adenosine and CGS21680 regulated CD73. GAPDH was
the internal reference in western blot analysis. E2F-1, E2F
transcription factor 1; p-E2F-1, phosphorylated E2F-1; CREB, cAMP
responsive element-binding protein; p-CREB, phosphorylated CREB;
si-E2F-1, E2F-1-specific siRNA; si-CREB, CREB-specific siRNA;
CGS21680, specific adenosine A2A receptor agonist; CD39,
ectonucleoside triphosphate diphosphohydrolase 1; CD73,
5′-ectonucleotidase. |
Adenosine and CGS21680 promote adenosine
generation
After the cells were treated with ATP, the
production of ATP and AMP in the supernatant was detected and
compared, as shown in Fig. 4A.
The amount of ATP decreased sharply in both the CGS21680-treated
and untreated cells when detected at 5 min after ATP treatment. In
particular, the changes in the amount of ATP in the
CGS21680-treated cells were more significant than those in the
untreated cells (P<0.05), lasting until the final detection at
80 min post-ATP treatment. Furthermore, the amount of AMP, the
hydrolysate of ATP, kept increasing until 40 min post-ATP treatment
and the CGS21680-treated cells exhibited significant differences
before this time point when compared with the untreated cells
(P<0.05). Similarly, after adding AMP to the cells, AMP
decreased gradually and adenosine, the hydrolysate of ATP,
increased accordingly (Fig. 4B).
Significant differences were observed between the CGS21680-treated
and untreated cells in the changes in the amount of both AMP and
adenosine (P<0.05). These results indicated that the adenosine
cycle was accelerated in the presence of CGS21680.
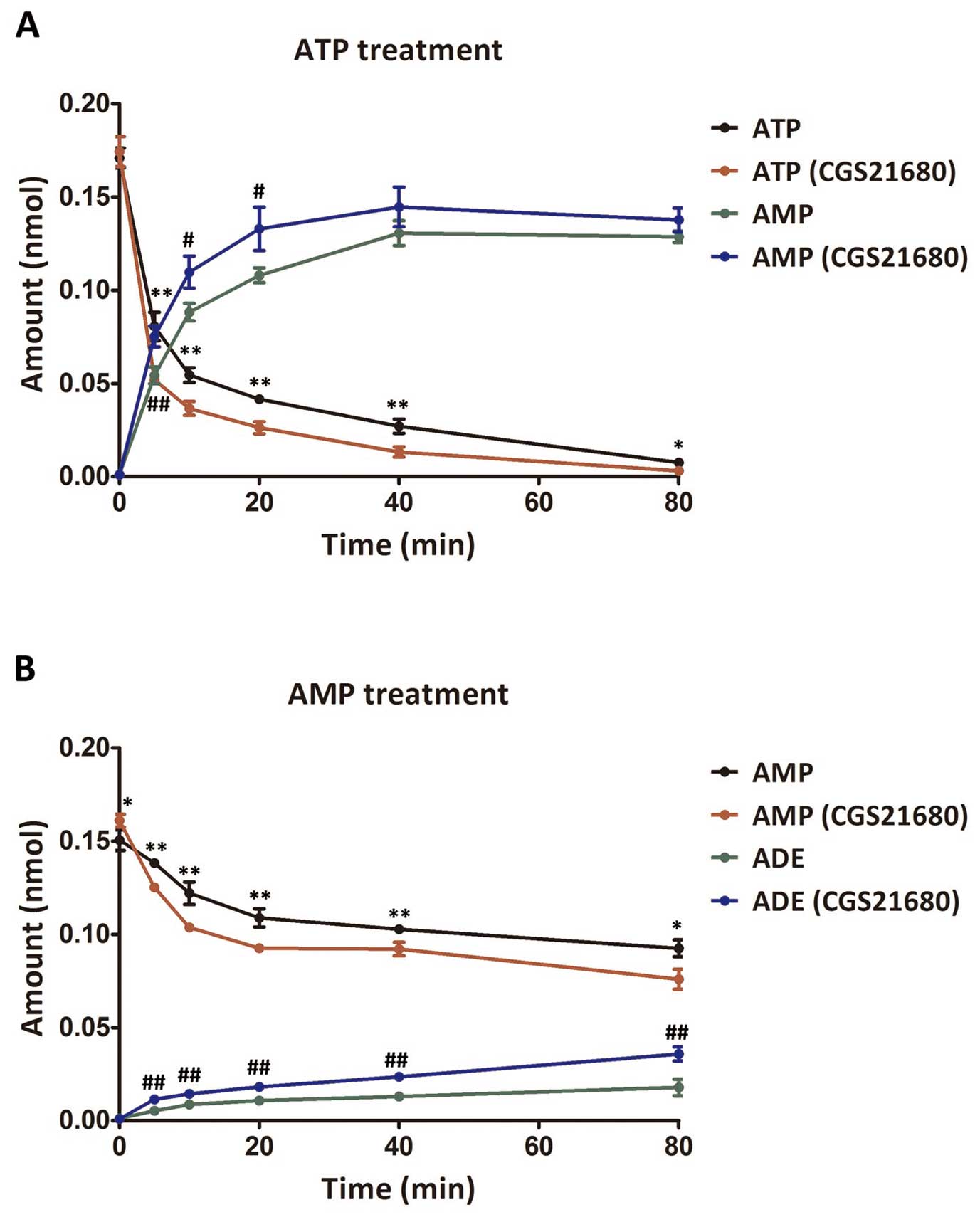 | Figure 4Amount of ATP, AMP and ADE in the
supernatant after the addition of ATP or AMP to the
CGS21680-treated cells. (A) Changes in the amount of ATP and AMP
after the addition of ATP. * and ** indicate extremely significant
differences (P<0.01) and significant differences (P<0.05) in
the amount of ATP between the control group and the
CGS21680-treated group. # and ## indicate extremely significant
differences (P<0.01) and significant differences (P<0.05) in
the amount of AMP between the control group and the
CGS21680-treated group, respectively. (B) Changes in the amount of
AMP and adenosine after AMP treatment. * and ** indicate extremely
significant differences (P<0.01) and significant differences
(P<0.05) in the amount of AMP between the control group and the
CGS21680-treated group, respectively. ## indicates extremely
significant differences (P<0.01) in the amount of adenosine
between the control group and the CGS21680-treated group. Amounts
were detected at 0, 5, 10, 20, 40, 60 and 80 min post-ATP or -AMP
treatment. ATP, adenosine triphosphate; AMP, adenosine
monophosphate; ADE, adenosine; CGS21680, specific adenosine
A2A receptor agonist. |
Based on the above-mentioned results that E2F-1 may
regulate CD39, and that CREB may regulate CD73, we conducted
further analysis by adding ATP and AMP, the corresponding
substrates of CD39 and CD73. First, the changes in the adenosine
cycle were analyzed after ATP treatment in the 3 groups of cells,
namely the cells with no pre-treatment, the cells transfected with
si-E2F-1 and the transfected cells treated with CGS21680 (Fig. 5A). After the addition of ATP, the
amount of ATP in the supernatant decreased, while the amount of AMP
increased. Significant differences were observed between the
untreated cells and the si-E2F-1-transfected cells (P<0.05).
Slight differences were observed between the transfected cells and
the transfected cells treated with CGS21680, with no significance
(P>0.05). A similar detection was also performed after AMP
treatment among the cells with no pre-treatment, the
si-CREB-transfected cells and the transfected cells treated with
CGS21680 (Fig. 5B). The trends in
the changes in AMP or adenosine content were consistent among the 3
groups, with significant differences only observed in the amount of
adenosine between the untreated cells and the si-CREB-transfected
cells at 5, 10 and 40 min post-AMP treatment (P<0.05).
Therefore, the promoting effects of CREB on the adenosine cycle
were ambiguous. Overall, these results suggest that E2F-1, CREB may
promote the adenosine cycle and the effects of their inhibition may
be compensated by CGS21680 to a certain extent.
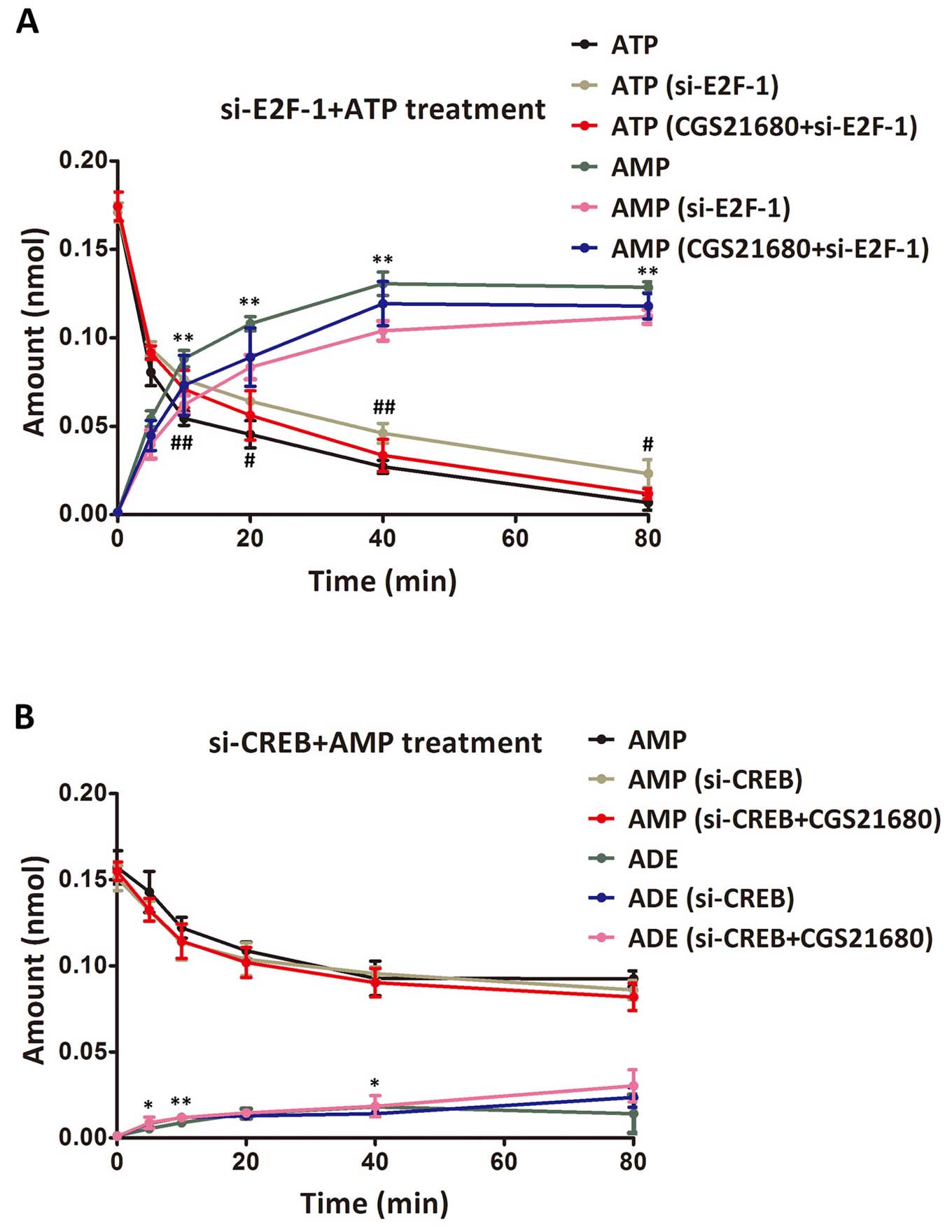 | Figure 5Amount of ATP, AMP and ADE in the
supernatant after the addition of ATP or AMP to the transfected
cells. (A) Changes in the amount of ATP and AMP after the addition
of ATP. # and ## indicate extremely significant differences
(P<0.01) and significant differences (P<0.05) in the amount
of ATP between the control group and the si-E2F-1-transfected
group, respectively. ** indicates extremely significant differences
(P<0.01) in the amount of AMP between the control group and the
si-E2F-1-transfected group. No significant difference was observed
between the si-E2F-1-transfected group and the transfected group
treated with CGS21680 (P>0.05). (B) Changes in the amount of AMP
and adenosine after AMP treatment. ** and * indicate extremely
significant differences (P<0.01) and significant differences
(P<0.05) in the amount of AMP between the control group and the
si-CREB-transfected group, respectively. No significant difference
was observed in the other comparisons (P>0.05). Amounts are
detected at 0, 5, 10, 20, 40, 60 and 80 min post-ATP or -AMP
treatment. ATP, adenosine triphosphate; AMP, adenosine
monophosphate; ADE, adenosine; CGS21680, specific adenosine
A2A receptor agonist; E2F-1, phosphorylated E2F-1;
si-E2F-1, E2F-1-specific siRNA; CREB, cAMP responsive
element-binding protein; si-CREB, CREB-specific siRNA. |
Discussion
This study discusses the molecular mechanisms
resulting in the high expression of CD39 and CD73 on the Treg cell
surface during sepsis to analyze the regulatory factors of the
CD39/CD73/adenosine pathway. We proved that the expression of CD39
and CD73 is upregulated by adenosine and the specific adenosine
A2A receptor agonist, CGS21680. Adenosine and CGS21680
upregulated E2F-1 and CREB, the predicted transcription regulatory
factors of CD39 and CD73. E2F-1 and CREB were further verified to
promote the expression of CD39 and CD73. Besides, the results
indicated that adenosine production was accelerated in the presence
of CGS21680, as well as E2F-1 and CREB. Thus, adenosine and the
adenosine A2A receptor play roles as signal transducer
factors, upregulating E2F-1 and CREB to increase the expression of
CD39 and CD73, and promote adenosine generation in Treg cells
during sepsis.
CD39 and CD73 are two vital proteins catalyzing the
conversion of ATP/ADP to AMP and AMP to adenosine, respectively.
Previous studies have found that CD39 and CD73 can be regulated by
various factors. For example, CD39 is upregulated by specificity
protein 1 (Sp1) (18) and STAT3,
and is suppressed by growth factor independent 1 transcription
repressor (GFI-1) (19). CD73 can
be induced by interferon-β-1a (20) and inhibited by GFI-1 (19). The two proteins are both expressed
broadly in various cell types and tissues (21,22). Nevertheless, the reason for their
high expression levels on the Treg cell surface during sepsis is
the topic of this study. We selected three predicted transcription
regulatory factors in the promoters of CD39 and CD73, among which
E2F-1 and CREB were likely to be regulated by adenosine and
CGS21680, and could promote CD39 and CD73, respectively. Therefore,
E2F-1 and CREB were two direct regulatory factors leading to the
high expression of CD39 and CD73 on the Treg cell surface during
sepsis. In addition, adenosine and CGS21680 seemed to be two
indirect activators of CD39 and CD73 by upregulating E2F-1 and
CREB. As the specific adenosine A2A receptor agonist,
CGS21680 divides adenosine receptor A2A from
A2B based on different affinities (23), representing the promoted activity
of adenosine A2A receptor in this study. Thus, it could
be inferred that adenosine and adenosine A2A receptor
are two regulatory factors for the indirect promotion of CD39 and
CD73.
CD39 and CD73 are viewed as immunological switches,
shifting the immune cell activities from an ATP-driven
pro-inflammatory state to an adenosine-mediated anti-inflammatory
state (24). The overexpression
of CD39 increases adenosine production to inhibit activated
T-lymphocytes in mesenchymal stromal cells (25). The low expression of CD73 reduces
adenosine generation, resulting in the enhanced severity of
juvenile idiopathic arthritis (26), and suppresses pro-inflammatory
responses in endothelial cells (27) and gastritis cells (28). The roles of adenosine and its
receptors in inflammation have been discussed in existing studies.
Patients with septic shock possess high adenosine plasma
concentrations (29). The
activation of adenosine A2A receptor by agonists can
inhibit inflammation caused by Helicobacter (30), and has been shown to increase
survival in murine models of sepsis (31,32). For this study, adenosine,
CGS21680, E2F-1 and CREB in the CD39/CD73/adenosine pathway all
promoted CD39 and CD73 indirectly or directly, accelerating ATP
hydrolysis and adenosine generation. Therefore, these factors may
facilitate the anti-inflammatory activities of Treg cells during
sepsis.
In summary, this study discusses the molecular
mechanisms responsible for the high expression of CD39 and CD73 on
the Treg cell surface, and the possible regulatory factors of the
CD39/CD73/adenosine pathway during sepsis. Together with adenosine
and adenosine A2A receptor, E2F-1 and CREB upregulate
CD39 and CD73, respectively, and promote adenosine generation to
participate in the anti-inflammatory activities of Treg cells
during sepsis. Our study offers more detailed information of the
regulatory relationship of the CD39/CD73/adenosine pathway,
facilitating further research on the treatment of sepsis using
adenosine, adenosine receptor agonists and other methods concerning
the regulation of adenosine metabolism.
Acknowledgments
This study was supported by grant nos. 81471895 and
81401575 from the National Natural Science Foundation of China to
T.Y. and R.B. (Beijing, China).
References
|
1
|
Jawad I, Lukšić I and Rafnsson SB:
Assessing available information on the burden of sepsis: Global
estimates of incidence, prevalence and mortality. J Glob Health.
2:0104042012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Henriksen DP, Laursen CB, Jensen TG,
Hallas J, Pedersen C and Lassen AT: Incidence rate of
community-acquired sepsis among hospitalized acute medical
patients-a population-based survey. Crit Care Med. 43:13–21. 2015.
View Article : Google Scholar
|
|
3
|
Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opa SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R,
et al Surviving Sepsis Campaign Guidelines Committee including The
Pediatric Subgroup: Surviving Sepsis Campaign: International
guidelines for management of severe sepsis and septic shock, 2012.
Intensive Care Med. 39:165–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marik PE: Early management of severe
sepsis: concepts and controversies. Chest. 145:1407–1418. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang JH, Super M, Yung CW, Cooper RM,
Domansky K, Graveline AR, Mammoto T, Berthet JB, Tobin H,
Cartwright MJ, et al: An extracorporeal blood-cleansing device for
sepsis therapy. Nat Med. 20:1211–1216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saito K, Wagatsuma T, Toyama H, Ejima Y,
Hoshi K, Shibusawa M, Kato M and Kurosawa S: Sepsis is
characterized by the increases in percentages of circulating
CD4+CD25+ regulatory T cells and plasma
levels of soluble CD25. Tohoku J Exp Med. 216:61–68. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borsellino G, Kleinewietfeld M, Di Mitri
D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D,
Bernardi G, Dell'Acqua ML, et al: Expression of ectonucleotidase
CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular
ATP and immune suppression. Blood. 110:1225–1232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobie JJ, Shah PR, Yang L, Rebhahn JA,
Fowell DJ and Mosmann TR: T regulatory and primed uncommitted CD4 T
cells express CD73, which suppresses effector CD4 T cells by
converting 5′-adenosine monophosphate to adenosine. J Immunol.
177:6780–6786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deaglio S, Dwyer KM, Gao W, Friedman D,
Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al:
Adenosine generation catalyzed by CD39 and CD73 expressed on
regulatory T cells mediates immune suppression. J Exp Med.
204:1257–1265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Z and Jin T: Extracellular high dosages
of adenosine triphosphate induce inflammatory response and insulin
resistance in rat adipocytes. Biochem Biophys Res Commun.
402:455–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cronstein BN: Adenosine, an endogenous
anti-inflammatory agent. J Appl Physiol (1985). 76:5–13. 1994.
|
|
13
|
Sullivan GW: Adenosine A2A receptor
agonists as anti-inflammatory agents. Curr Opin Investig Drugs.
4:1313–1319. 2003.
|
|
14
|
Csóka B, Németh ZH, Törő G, Koscsó B,
Kókai E, Robson SC, Enjyoji K, Rolandelli RH, Erdélyi K, Pacher P,
et al: CD39 improves survival in microbial sepsis by attenuating
systemic inflammation. FASEB J. 29:25–36. 2015. View Article : Google Scholar :
|
|
15
|
Haskó G, Csóka B, Koscsó B, Chandra R,
Pacher P, Thompson LF, Deitch EA, Spolarics Z, Virág L, Gergely P,
et al: Ecto-5′-nucleotidase (CD73) decreases mortality and organ
injury in sepsis. J Immunol. 187:4256–4267. 2011. View Article : Google Scholar
|
|
16
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immuno-design of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar
|
|
17
|
Busse M, Traeger T, Pötschke C, Billing A,
Dummer A, Friebe E, Kiank C, Grunwald U, Jack RS, Schütt C, et al:
Detrimental role for CD4+ T lymphocytes in murine
diffuse peritonitis due to inhibition of local bacterial
elimination. Gut. 57:188–195. 2008. View Article : Google Scholar
|
|
18
|
Eltzschig HK, Köhler D, Eckle T, Kong T,
Robson SC and Colgan SP: Central role of Sp1-regulated CD39 in
hypoxia/ischemia protection. Blood. 113:224–232. 2009. View Article : Google Scholar :
|
|
19
|
Chalmin F, Mignot G, Bruchard M, Chevriaux
A, Végran F, Hichami A, Ladoire S, Derangère V, Vincent J, Masson
D, et al: Stat3 and Gfi-1 transcription factors control Th17 cell
immuno-suppressive activity via the regulation of ectonucleotidase
expression. Immunity. 36:362–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bellingan G, Maksimow M, Howell DC, Stotz
M, Beale R, Beatty M, Walsh T, Binning A, Davidson A, Kuper M, et
al: The effect of intravenous interferon-beta-1a (FP-1201) on lung
CD73 expression and on acute respiratory distress syndrome
mortality: An open-label study. Lancet Respir Med. 2:98–107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dwyer KM, Deaglio S, Gao W, Friedman D,
Strom TB and Robson SC: CD39 and control of cellular immune
responses. Purinergic Signal. 3:171–180. 2007. View Article : Google Scholar
|
|
22
|
Thompson LF, Eltzschig HK, Ibla JC, Van De
Wiele CJ, Resta R, Morote-Garcia JC and Colgan SP: Crucial role for
ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J
Exp Med. 200:1395–1405. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wan W, Sutherland GR and Geiger JD:
Binding of the adenosine A2 receptor ligand [3H]CGS 21680 to human
and rat brain: Evidence for multiple affinity sites. J Neurochem.
55:1763–1771. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Antonioli L, Pacher P, Vizi ES and Haskó
G: CD39 and CD73 in immunity and inflammation. Trends Mol Med.
19:355–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saldanha-Araujo F, Ferreira FI, Palma PV,
Araujo AG, Queiroz RH, Covas DT, Zago MA and Panepucci RA:
Mesenchymal stromal cells up-regulate CD39 and increase adenosine
production to suppress activated T-lymphocytes. Stem Cell Res
(Amst). 7:66–74. 2011. View Article : Google Scholar
|
|
26
|
Botta Gordon-Smith S, Ursu S, Eaton S,
Moncrieffe H and Wedderburn LR: Correlation of low CD73 expression
on synovial lymphocytes with reduced adenosine generation and
higher disease severity in juvenile idiopathic arthritis. Arthritis
Rheumatol. 67:545–554. 2015. View Article : Google Scholar
|
|
27
|
Grünewald JK and Ridley AJ: CD73 represses
pro-inflammatory responses in human endothelial cells. J Inflamm
(Lond). 7:102010. View Article : Google Scholar
|
|
28
|
Alam MS, Kurtz CC, Rowlett RM, Reuter BK,
Wiznerowicz E, Das S, Linden J, Crowe SE and Ernst PB: CD73 is
expressed by human regulatory T helper cells and suppresses
proinflammatory cytokine production and Helicobacter felis-induced
gastritis in mice. J Infect Dis. 199:494–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin C, Leone M, Viviand X, Ayem ML and
Guieu R: High adenosine plasma concentration as a prognostic index
for outcome in patients with septic shock. Crit Care Med.
28:3198–3202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alam MS, Kurtz CC, Wilson JM, Burnette BR,
Wiznerowicz EB, Ross WG, Rieger JM, Figler RA, Linden J, Crowe SE
and Ernst PB: A2A adenosine receptor (AR) activation inhibits
pro-inflammatory cytokine production by human CD4+
helper T cells and regulates Helicobacter-induced gastritis and
bacterial persistence. Mucosal Immunol. 2:232–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moore CC, Martin EN, Lee GH, Obrig T,
Linden J and Scheld WM: An A2A adenosine receptor agonist, ATL313,
reduces inflammation and improves survival in murine sepsis models.
BMC Infect Dis. 8:1412008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sullivan GW, Fang G, Linden J and Scheld
WM: A2A adenosine receptor activation improves survival in mouse
models of endotoxemia and sepsis. J Infect Dis. 189:1897–1904.
2004. View
Article : Google Scholar : PubMed/NCBI
|