Introduction
Gastric cancer is one of the most common
malignancies worldwide, and is the second leading cause of
cancer-related mortality; it causes >730,000 cancer-related
deaths annually (1,2). More than 70% of new cases and deaths
occur in developing countries, particularly in Eastern Asia,
Eastern Europe and South America (1); gastric cancer rates have decreased
substantially in most parts of the world (3). Most cases are caused by
Helicobacter pylori (H. pylori) infection, but
tobacco smoking and dietary factors, such as intake of smoked
foods, salted fish and meat, and pickled vegetables, have also been
associated with a risk for gastric cancer development (1). To date, surgery is the most common
treatment option for gastric cancer, followed by chemotherapy and
radiation therapy, or a combination of both. However, surgical
interventions are curative in <40% of cases as gastric cancer
patients are typically diagnosed at advanced stages. Thus, in order
to improve gastric cancer survival and reduce its incidence,
methods for early diagnosis and a better understanding of the
molecular mechanisms responsible for gastric cancer development and
progression are required.
The development of gastric cancer, as with other
types of cancer, is caused by risk factor-induced genetic
alterations, such as the activation of oncogenes and the silencing
of tumor suppressor genes (4–6).
For example, H. pylori infection plays two major roles in
gastric cancer development: it induces inflammation of the gastric
mucosa and alters gene expression via the induction of mutations
and DNA methylation (7). Indeed,
H. pylori infection promotes the methylation and silencing
of trefoil factor family 2 (TFF2), leading to gastric cancer
development in humans (8). The
TFF family includes secreted proteins characterized by a triple
loop structure and the trefoil domain, and they are expressed in
the gut (8–10). TFF1 is secreted by surface mucous
and the pit epithelium in the fundus and antrum, whereas TFF2 is
restricted to the fundic mucous neck cells, antrum and Brunner's
glands, and TFF3 is found in intestinal cells (5). A previous study demonstrated that
TFF1 is a stomach-specific tumor suppressor gene; however, the role
of TFF2 in gastric cancer progression is less well understood
(5). Recently, TFF2 was shown to
play a protective role in the digestive tract (11); however, other studies have
indicated that it is related to gastric diseases. For example, TFF2
expression has been shown to rapidly increase in gastrointestinal
ulcerative diseases, particularly in the regenerating epithelium
(12) or following non-steroidal
anti-inflammatory drug treatment (13). Another study demonstrated that the
level of TFF2 was markedly lower in the serum and tumor tissues of
gastric cancer patients than in normal tissues, and this may be due
to methylation of the TFF2 promoter (14,15). However, it is not clear that TFF2
functions as a tumor suppressor in gastric cancer development. In a
preliminary yeast two-hybrid screen, we previously found that the
transcription factor, Sp3, is a candidate protein that binds to and
potentially mediates the effects of TFF2 in gastric cancer cells
(16). Thus, in this study, we
characterized the interaction between TFF2 and Sp3 in the
regulation of gastric cancer cell viability, apoptosis and invasion
capacity.
Materials and methods
Cell lines and culture conditions
The normal human gastric mucosal cell line, GES-1,
the gastric cancer cell line, BGC-823, and 293 cells were obtained
from the Life Science College of Xiamen University (Xiamen, China)
and maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco,
Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS)
and 100 U/ml penicillin-streptomycin (both from Invitrogen,
Carlsbad, CA, USA) at 37°C in a humidified incubator containing 5%
CO2.
Immunofluorescent detection of protein
distribution
The gastric cancer cells were seeded onto coverslips
in 6-well plates and grown for 24 h. The cells were then fixed with
freshly prepared 4% paraformaldehyde solution for 30 min on ice.
After washing with phosphate-buffered saline (PBS) containing 0.1%
Triton X-100, the cells were incubated overnight with anti-TFF2
(orb214658; Biorbyt LLC, Berkeley, CA, USA) and/or anti-Sp3 (D20;
Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies at a
dilution of 1:200. The following day, the cells were washed 3 times
with PBS and then further incubated with fluorescein isothiocyanate
(FITC)-conjugated goat anti-rabbit antibody (sc-3839; Santa Cruz
Biotechnology) and/or Texas Red-conjugated goat anti-mouse antibody
(1:600; T-862; Jackson ImmunoResearch Laboratories, West Grove, PA,
USA). The cell nuclei were counterstained with
4′,6-diamidino-2-phenylindole (DAPI; Gibco). The stained cells were
viewed and scored under a BMX-60 microscope (Olympus, Tokyo, Japan)
equipped with a cooled charge-coupled device and sensored camera
(Cooke, Auburn Hills, MI, USA) and SlideBook software (Intelligent
Imaging Innovations, Denver, CO, USA). At least 500 cells in each
condition were reviewed and scored to calculate TFF2 and Sp3
positivity, and each experiment was repeated 3 times.
Construction of TFF2-containing plasmids
and gene transfection
The GES-1 normal human gastric mucosal cells were
grown, and total cellular RNA was isolated using TRIzol®
reagent (Invitrogen) and reverse-transcribed into cDNA according to
the manufacturer's instructions. To amplify TFF2, the primers used
were 5′-AGAGAATTCGGATCCATGGGACGGCGAGACG-3′ (forward) and
5′-TGGCTCGAGCCCGGGGTAATGGCAGTCTTCCACAGAC-3′ (reverse). PCR
amplification was performed using primer enzyme (Fermentas,
Vilnius, Lithuania) for 30 cycles of 94°C for 30 sec, 58°C for 1
min, and 72°C for 30 sec. The PCR products were then separated on
1.2% agarose gels, and TFF2 cDNA was recovered using a DNA Gel
Extraction kit (Tiangene, Beijing, China). The recovered TFF2 cDNA
was then cloned into the pcDNA6.0/HA-tag vector (Invitrogen) at the
BamHI and XhoI sites. Following amplification, the
plasmid was DNA-sequenced for correct vector construction by
Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
The vector-only and pcDNA6.0/HA-tag/TFF2 plasmids were transfected
into gastric cancer cells using Lipofectamine 2000 (Invitrogen)
according to the manufacturer's instructions. We also expressed
exogenous TFF2 in the 293 cells by pcDNA6.0/HA-tag/TFF2
transfection.
Construction of the Sp3 small hairpin RNA
(shRNA) vector and gene transfection
To knock down Sp3 expression, a pU6 expression
vector carrying shRNA targeting Sp3 (Sp3 shRNA) was constructed
according to the methods described in previous studies (17,18). The shRNA sense and antisense
sequences targeting Sp3 cDNA were designed and synthesized by
Invitrogen and inserted into the pU6 vector. Two different sets of
Sp3 shRNA sequences were selected according to a previous study
(19), i.e., Sp3 shRNA 1
(forward,
5′-CACCGGTGGGGCTTTCATTTCAAACGTGTGCTGTCCGTTTGAAGTGAAGGCTCCACCTTTTT-3′
and reverse,
5′-GCATAAAAAGGTGGAGCCTTCACTTCAAACGGACAGCACACGTTTGAAATGAAAGCCCCACC-3′)
and Sp3 shRNA 2 (forward,
5′-CACCGGTGGGAGAGGTATCGATTACGTGTGCTGTCCGTAATTGGTACCTCTTCCACCTTTTT-3′
and reverse,
5′-GCATAAAAAGGTGGAAGAGGTACCAATTACGGACAGCACACGTAATCGATACCTCTCCCACC-3′).
Following amplification, these plasmids were sequenced to confirm
the shRNA construction. Following amplification, these plasmids
were sequenced to confirm shRNA construction. shRNA1 was found to
be more effective (data not shown). In the following paragraphs,
Sp3 shRNA refers to Sp3 shRNA1 These vectors and the negative
control pU6 vector were then transfected into the gastric cancer
cells using Lipofectamine 2000 (Invitrogen) according to the
manufacturer's instructions.
Protein extraction, immunoprecipitation
and western blot analysis
Total cell lysates were harvested from
5×106 cells using 300 μl of lysis buffer
containing complete protease inhibitor cocktail (Roche, Basel,
Switzerland) by rotation at 4°C for 30 min and then centrifuged at
12,000 × g for 20 min. The supernatant was collected, and the
protein concentration was measured using the protein assay kit
(Bio-Rad Laboratories, Hercules, CA, USA). For western blot
analysis, an equal volume of cell lysate was denatured in sodium
dodecyl sulfate (SDS) sample buffer, separated on a 10–12%
SDS-polyacrylamide gel with electrophoresis solution, and then
transferred onto a polyvinylidene difluoride membrane (Bio-Rad
Laboratories). The membranes were blocked in PBS-T (PBS containing
0.1% Tween-20) and 5% bovine serum albumin (Difco,
Becton-Dickinson, Franklin Lakes, NJ, USA) at room temperature for
1 h and incubated with specific primary antibodies [anti-TFF2
(orb214658; Biorbyt LLC) and anti-Sp3 (D20; Santa Cruz
Biotechnology)] in PBS-T at 4°C for 2 h. Following 3 washes with
PBS-T, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (62-6520)
or horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (81-6120) (both from Zymed, San Diego, CA, USA) in PBS-T
at room temperature for 1 h. The membranes were then washed 3 times
with PBS-T, and the protein level was detected using the ECL
detection system (Sigma Biosciences, Santa Clara, CA, USA).
For immunoprecipitation and western blot analysis,
lysates of GES-1 cells expressing both TFF2 and Sp3 proteins were
incubated with glutathione-sepharose beads and immunoprecipitated
with an anti-Sp3 antibody. The precipitates were subjected to
western blot analysis using anti-TFF2 and anti-Sp3 antibodies. The
cell lysate incubated with antibody-free A/G-agarose served as a
negative control.
Bromodeoxyuridine (BrdU) incorporation
assay
To detect cell proliferation, we utilized the BrdU
cell proliferation kit (Roche Diagnostics, Indianapolis, IN, USA).
Briefly, the BGC-823 human gastric cancer cells were grown in
96-well plates and transfected with TFF2, siSp3, or control vectors
for 24–48 h. Subsequently, 10 μl/ml BrdU was added to the
cell cultures, and followed by further incubation at 37°C for 12 or
24 h. The medium was then removed, and the cells were fixed with
fresh 4% paraformaldehyde solution for 30 min at room temperature.
To detect BrdU levels in the cells, an anti-BrdU antibody was added
to each well followed by incubation for 90 min at room temperature.
Following 3 washes with PBS-T, the cells were further incubated
with the substrate solution for 30 min to develop color. After the
addition of H2SO4, the absorbance of the
cells at 450 nm was measured using a spectrophotometer [Eppendorf
BioPhotometer D30, Eppendorf Biotechnology International Trade
(Shanghai) Co., Ltd.].
Flow cytometric analysis
To analyze changes in cell apoptosis, the BGC-823
cells were grown in 96-well plates and transfected with TFF2,
siSp3, or control vectors for 24–48 h with complete medium
containing 7.5 mg/ml cisplatin. The cells were pelleted and
resuspended in 0.3 ml of PBS containing 3% calf serum. The cell
pellets were then fixed by the drop-wise addition of 0.7 ml of
methanol during gentle vortexing and stored overnight at −20°C. The
following day, the fixed cells were pelleted, washed twice in PBS
containing 3% calf serum, and stained with Annexin V (Dojindo,
Kumamoto, Japan) containing 100 μg/ml ribonuclease A
according to the instructions provided with the kit and then with
propidium iodide (PI, 50 μg/ml in PBS and 0.1% Triton X-100;
Dojindo) and protected from light until analysis. The cells were
analyzed using a FACSCalibur flow cytometer (Becton-Dickinson).
Apoptosis antibody array
To determine the role of TFF2 and Sp3 binding in the
regulation of gastric cancer cell apoptosis, the Proteome Profiler™
Human Apoptosis array kit (ARY009; R&D Systems, Minneapolis,
MN, USA) was used to detect the relative expression levels of 35
apoptosis-related proteins according to the manufacturer's
instructions in BGC-823 cells showing differential expression of
TFF2 compared to that in the control cells.
Tumor cell invasion assay
The BGC-823 cells were grown in 96-well plates and
transfected with TFF2, siSp3, or control vectors for 24–48 h, and
then 1–5×104 cells without FBS were re-seeded in the top
chamber of each insert (BD Biosciences, Franklin Lakes, NJ, USA)
and the bottom chamber was filled with 20% FBS and incubated for 22
h. At the end of the experiments, cells that migrated or invaded
the surface of the filter were fixed with methanol for 30 min at
4°C and stained with 0.1% crystal violet containing 20% ethanol for
10 min at room temperature, and the total numbers of cells were
enumerated under an IX71 inverted microscope (Olympus).
Statistical analysis
All statistical analyses were performed using
Student's t-tests or one-way analysis of variance (ANOVA)
implemented in GraphPad Prism 5.01 of GraphPad Software, Inc. (La
Jolla, CA, USA) to evaluate differences between groups. A value of
P<0.05 was considered to indicate a statistially significant
difference.
Results
Co-localization of TFF2 and Sp3 in GES-1
cells
Our previous study involving a yeast two-hybrid
analysis revealed that TFF2 binds to Sp3, leading to antitumor
activity in gastric cancer cells (16); thus, in this study, we first
examined the co-localization of TFF2 and Sp3 in the normal gastric
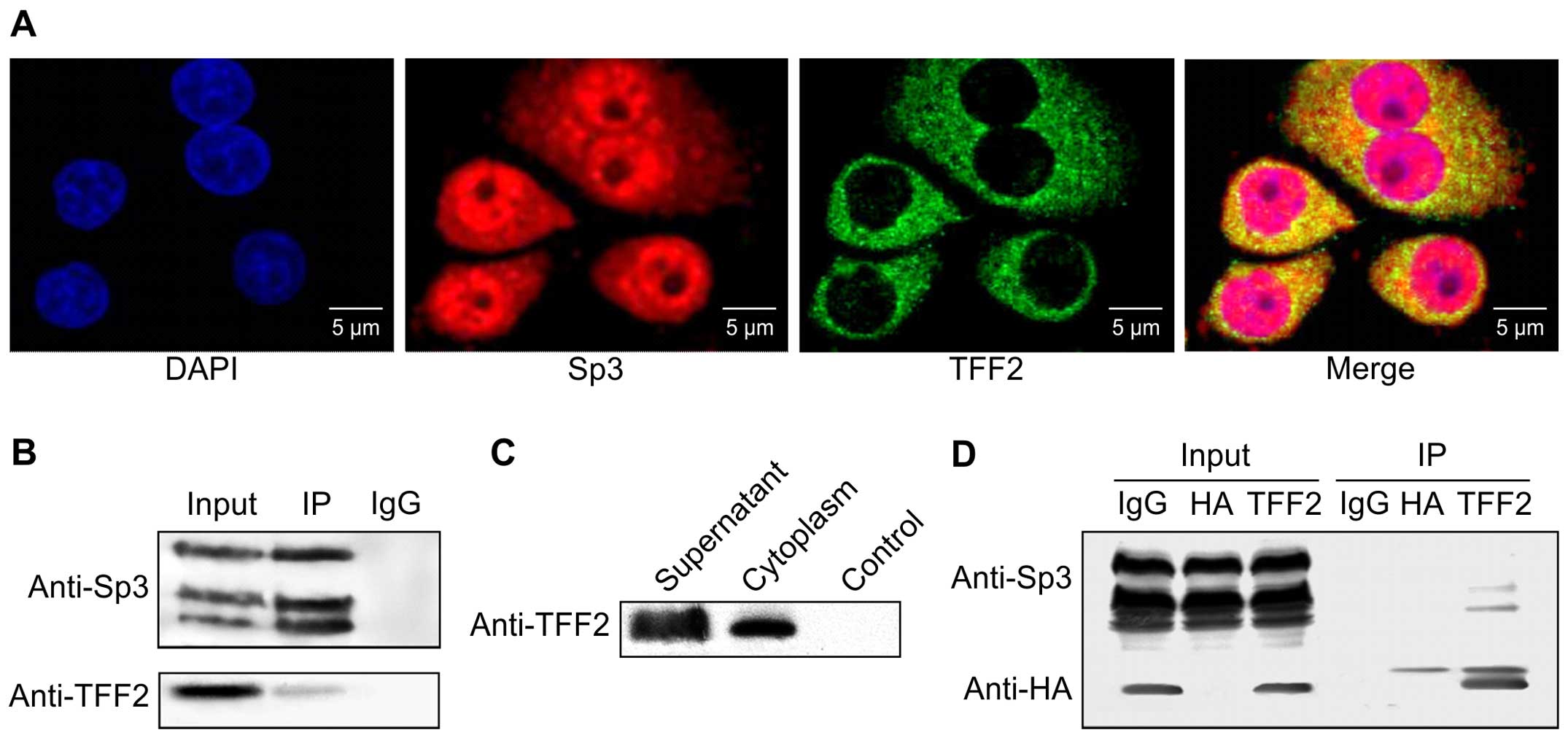
mucosal cell line, GES-1, using immunofluorescence. Our results
revealed that the TFF2 protein was concentrated in the cytoplasm of
GES-1 cells, whereas Sp3 was distributed in both the nuclei and
cytoplasm (Fig. 1A). The proteins
overlapped in the cytoplasm of the GES-1 cells.
Subsequently, using immunoprecipitation and western
blot analysis, we confirmed that endogenous TFF2 binds to Sp3
protein in GES-1 cells (Fig. 1B).
We also expressed exogenous TFF2 in the 293 cells by
pcDNA6.0/HA-tag/TFF2 transfection (Fig. 1C). As shown by the results of
western blot analysis, exogenous TFF2 was able to bind to Sp3
protein (Fig. 1D).
Effects of TFF2 expression on BGC-823
cell proliferation and apoptosis and the effect of Sp3
knockdown
We assessed the effects of TFF2 expression on the
regulation of BGC-823 cell proliferation and apoptosis, and
examined whether Sp3 knockdown alters the effects of TFF2 on
gastric cancer cells. We constructed 4 strains of stably
transfected BGC-823 cells: control cells, TFF2-overexpressing
cells, cells in which Sp3 was knocked down, and TFF2-overexpressing
cells in which Sp3 was also knocked down (namely, pcDNA6.0 + pU6,
TFF2 + pU6, pcDNA6.0 + siSp3 and TFF2 + siSp3 cells, respectively).
The levels of TFF2 and Sp3 expression in the gastric cancer cells
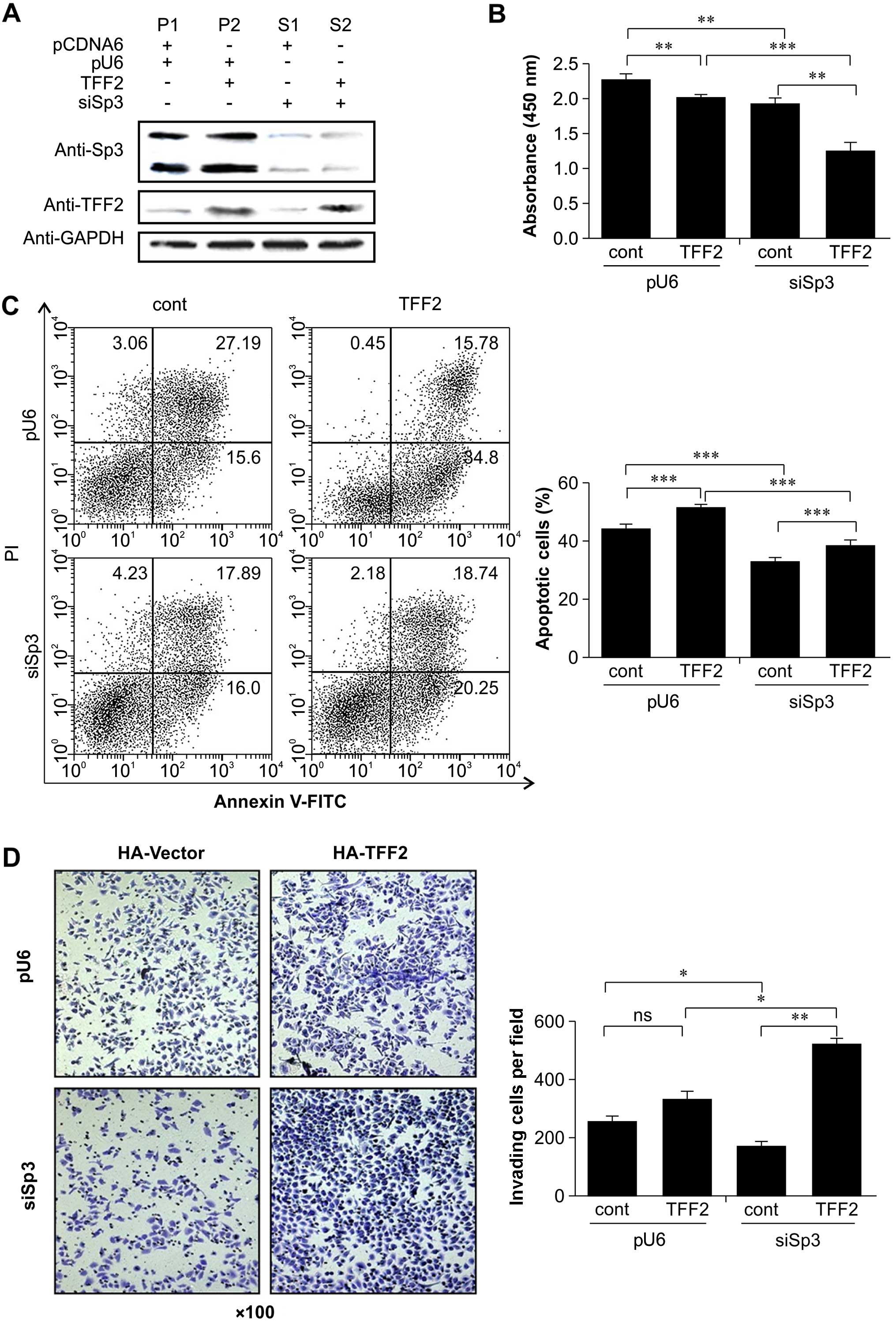
were examined by western blot analysis (Fig. 2A). We then assessed the changes in
cell proliferation and apoptosis. The results of BrdU incorporation
assay revealed that Sp3 promoted the proliferation of BGC-823 cells
(pcDNA6.0 + pU6 vs. pcDNA6.0 + siSp3, Student's t-test, P<0.01),
whereas TFF2 decreased cell proliferation (pcDNA6.0 + siSp3 vs.
TFF2 + siSp3, Student's t-test, P<0.05) (Fig. 2B). However, there was no
significant effect on cell proliferation when both TFF2 and Sp3
(TFF2 + pU6) were overexpressed (Fig.
2B).
 | Figure 2Effects of trefoil factor family 2
(TFF2) expression on gastric cancer cell proliferation and
apoptosis and the effect of stable Sp3 knockdown in BGC-823 gastric
cancer cells. (A) Western blot analysis of TFF2 and Sp3 expression
in gastric cancer cells. BGC-823 cells were grown and stably
transfected with HA-TFF2, pU6-siSp3, or control vectors, and cell
lysates were subjected to western blot analysis for TFF2 and siSp3
expression. GAPDH was used as a loading control. (B)
Bromodeoxyuridine (BrdU) incorporation assay. The 4 types of
BGC-823 cell sublines were grown and incubated with BrdU for up to
22 h and then subjected to spectrophotometer analysis of optical
absorbance levels at 450 nm. Data are expressed as the means (nm) ±
SD of 3 separate experiments and were analyzed by Student's
t-tests. The BrdU incorporation assay showed that Sp3 promoted the
proliferation of BGC-823 cells (pcDNA6.0 + pU6 vs. pcDNA6.0 +
siSp3, P<0.01), whereas TFF2 reduced cell proliferation
(pcDNA6.0 + siSp3 vs. TFF2 + siSp3, P<0.05). (C) Flow cytometric
analysis of BCG-823 cells following incubation with cisplatin for
36 h and subsequent staining with Annexin V and propidium iodide
(PI). Dots in Annexin V−/PI−, Annexin
V+/PI−, and Annexin
V+/PI+ indicate intact cells, cells
undergoing early apoptosis and dead cells, respectively. Data are
shown as the means (%) ± SD of 3 separate experiments and were
analyzed by Student's t-tests. We compared the frequency of
apoptotic cells for pcDNA + pU6 (45.85%), pU6 + TFF2 (51.03%),
siSp3 + pcDNA (38.12%), and siSp3 + TFF2 (41.17%) cells. In cells
with a high expression of Sp3 and overexpression of TFF2, gastric
cancer cell apoptosis was significantly higher than that in the
control cells (P<0.05). In Sp3 knockdown gastric cancer cells,
apoptosis decreased significantly (P<0.05). In cells with Sp3
knockdown and overexpression of TFF2, the apoptosis level did not
decrease obviously. These results indicated that TFF2 and Sp3 alone
significantly promoted the apoptosis of gastric cancer cells. TFF2
and Sp3 in combination further enhanced the levels of gastric
cancer cell apoptosis. Accordingly, TFF2 and Sp3 had a synergistic
effect on gastric cancer cell apoptosis. (D) Tumor cell invasion
assay for the four BGC-823 cell sublines. Data are shown as the
means ± SD of 3 separate experiments and were analyzed by Student's
t-tests. Compared with the empty vector group, gastric cancer cells
with a high expression of Sp3 and overexpression of TFF2 did not
differ significantly with respect to invasive ability (P>0.05).
In cells with Sp3 knockdown, the invasive ability decreased
significantly (P<0.05). In gastric cancer cells with Sp3
knockdown and TFF2 overexpression, the invasive ability markedly
increased. These results indicated that TFF2 and Sp3 alone
significantly promoted the invasive ability of gastric cancer
cells. The combination of TFF2 and Sp3 did not further enhance the
invasive ability of gastric cancer cells. This suggested that TFF2
and Sp3 had an antagonistic effect on gastric cancer cell invasion.
ns, not significant; ***P<0.01,
**P<0.05, and *P<0.1 compared to the
control. Groups in the bar charts are as follows: pU6 cont
(control), pcDNA6.0 + pU6; pU6 TFF2, TFF2 + pU6; siSp3 cont,
pcDNA6.0 + siSp3; and siSp3 TFF2, TFF2 + siSp3. |
Finally, the data from the flow cytometric apoptosis
assay indicated that TFF2 overexpression or Sp3 knockdown induced
apoptosis (Fig. 2C). The BCG-823
cells were incubated with cisplatin for 36 h, stained with Annexin
V and PI, and analyzed by flow cytometry. We then compared the
proportion of apoptotic cells between the pcDNA + pU6 (45.85%), pU6
+ TFF2 (51.03%), siSp3 + pcDNA (38.12%) and siSp3 + TFF2 (41.17%)
groups. The number of apoptotic cells was significantly higher in
the gastric cancer cells with a high expression of Sp3 and with
overexpression of TFF2 than in the cells with a high expression of
Sp3 and a normal expression of TFF2 (P<0.05). After Sp3 was
knocked down, gastric cancer cell apoptosis decreased significantly
(P<0.05). With the combination of Sp3 knockdown and the
overexpression of TFF2, the level of gastric cancer cell apoptosis
did not decrease to a level significantly lower than that in the
control cells. These results indicated that TFF2 and Sp3 alone
significantly promoted the apoptosis of gastric cancer cells. TFF2
and Sp3 in combination further enhanced the apoptosis of gastric
cancer cells. Accordingly, TFF2 and Sp3 had a synergistic effect on
gastric cancer cell apoptosis.
Effects of TFF2 expression on BGC-823
cell invasion capacity and the effect of Sp3 knockdown
We assessed the effects of TFF2 expression on the
regulation of BGC-823 cell invasion capacity and examined whether
Sp3 knockdown alters the effects of TFF2 in gastric cancer cells. A
tumor cell invasion assay was performed for the 4 BGC-823 cell
sublines. Compared with the empty vector group, the gastric cancer
cells with a high expression of Sp3 and overexpression of TFF2 did
not exhibit a significant difference with respect to invasive
ability (Student's t-test, P>0.05). In the gastric cancer cells
in which Sp3 was knocked down, the invasive ability was
significantly lower than that of the control cells (pcDNA6.0 + pU6;
Student's t-test, P<0.05). In the gastric cancer cells in which
Sp3 was knocked down and with TFF2 overexpression, the invasive
ability was markedly greater (Fig.
2D). These results indicated that TFF2 and Sp3 alone
significantly promoted the invasive ability of the gastric cancer
cells. TFF2 and Sp3 together, did not further enhance the invasive
ability of the gastric cancer cells. This suggests that TFF2 and
Sp3 have an antagonistic effect on gastric cancer cell
invasion.
Potential signaling pathway in
TFF2-induced tumor cell apoptosis and interaction with Sp3
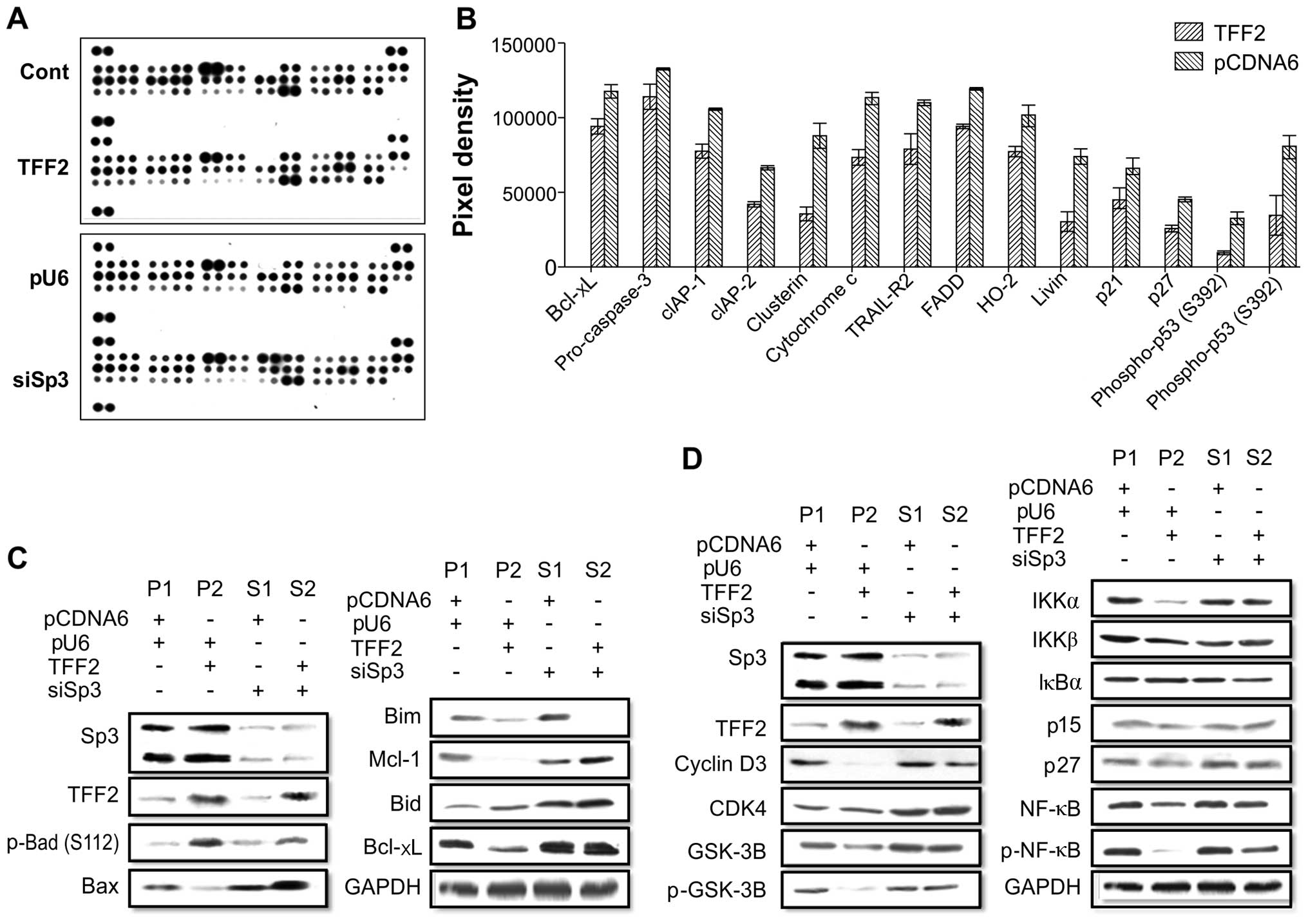
To confirm the TFF2-induced apoptotic effects and
the interaction between TFF2 and Sp3 in gastric cancer cells, we
used the Proteome Profiler™ Human Apoptosis array kit. We detected
14 apoptosis-related proteins that were differentially expressed in
the TFF2-overexpressing gastric cancer cells compared to the
pcDNA6.0 control cells (Fig. 3A and
B). Using 4 different stable gastric cancer sublines, we found
that the pro-apoptotic protein, Bid, was upregulated, but the
pro-apoptotic protein, Bax, was downregulated; however, the
anti-apoptotic proteins, Bcl-xL and Mcl-1, were downregulated in
the TFF2-overexpressing cells (Fig.
3C). TFF2 expression also downregulated the protein expression
of NF-κB (Fig. 3D). However,
following Sp3 knockdown, the effects of TFF2 on the expression of
these proteins were reduced (Fig. 3C
and D). These results demonstrated that TFF2 and Sp3 induced
apoptotic effects via related signaling proteins. There was an
antagonistic interaction between TFF2 and Sp3 with respect to
cancer cell invasion. The high expression of TFF2 and Sp3 alone
regulated the cell cycle in gastric cancer by blocking of the
effect of GSK-3, p-NF-κB, IKKα/IKKβ, and other signaling pathway
proteins. Thus, TFF2 and Sp3 have a greater effect in combination
with respect to gastric cancer cell apoptosis.
Discussion
Increasing evidence has indicated that TFFs play
critical roles in protecting the gastrointestinal tract from
inflammation or tumorigenesis; they are involved in the repair of
the gastrointestinal epithelium and the suppression of tumor
formation in the stomach. By contrast, TFF2 deficiency promotes
inflammation in gastric mucosae and is associated with gastric
malignancy (15). However, few
studies have examined its role at the cellular and molecular level,
and the underlying molecular mechanisms that mediate its effects
are unknown (20). Our current
study demonstrated that the transcription factor, Sp3, acts as a
novel TFF2 binding partner in the GES-1 gastric mucosal cell line.
Our data further suggested that TFF2 binds to the short isoform of
the Sp3 protein, but not to the long isoform. Additionally, we
showed that TFF2 binds to the cytoplasmic form of the Sp3 protein.
However, the biological significance of this phenomenon remains to
be determined. Indeed, the Sp3 protein is ubiquitously expressed in
all mammalian cells (21). It
belongs to the specificity protein/Krüppel-like factor (SP/KLF)
transcription factor family containing a specific DNA-binding
domain composed of a combination of three conserved
Cys2His2 zinc fingers (22). Sp3 regulates the expression of a
number of genes; consistent with this, our data demonstrated that
TFF2 binds to Sp3 in the cytoplasm, resulting in the increased
expression of genes involved in the suppression of cell
proliferation and the induction of apoptosis (23). The Sp3 protein exists in multiple
isoforms: two long fragments and two short ones (24–26). Previous research on Sp3 has
focused on its DNA-binding sites and gene regulation (27). Thus, multiple promoters have known
binding sites for Sp3, which is clearly important for gene
expression regulation (28).
However, the interaction between TFF2 and Sp3 has not been
documented. In this study, to the best of our knowledge, we
demonstrated for the first time that TFF2 interacts with Sp3 to
mediate the biological functions of TFF2 in gastric cancer
cells.
Furthermore, we assessed the biological function of
the TFF2 protein in gastric cancer cells and clarified the role of
Sp3 by establishing 4 stable cell lines with different expression
levels for each locus. Using BrdU incorporation and flow cytometric
assays, we found that TFF2 expression inhibited cell proliferation,
but that it promoted the apoptosis of BGC-823 cells. Sp3 knockdown
antagonized the effects of TFF2 on gastric cancer cells, indicating
that the interaction between TFF2 and Sp3 is essential for TFF2
function in gastric cancer cells. Further studies are required to
confirm this preliminary observation.
TFF2 and Sp3 both promote invasion in gastric cancer
cells. However, the effect was less notable in cells overexpressing
TFF2 and Sp3. A previous study demonstrated that despite its role
as a tumor suppressor, TFF2 can induce gastric cancer cell invasion
(29), although the mechanisms
underlying this effect are unknown. We extended these observations
by exploring the molecular mechanisms through which TFF2 and Sp3
interact to regulate tumor cell apoptosis. We identified 14
differentially expressed proteins in response to TFF2 expression in
gastric cells. Our data were consistent with those of previous
studies showing that the Bcl-2 family (30) and NF-κB signaling proteins
(31,32) are important in gastrointestinal
tumorigenesis. TFF2 expression downregulated the expression of
Bcl-xL and NF-κB family signal proteins in the 4 stable cell lines
expressing different levels of TFF2 and Sp3. We also demonstrated
that the expression of pro-apoptotic proteins (e.g., Bid) may be
induced in response to TFF2 expression in gastric cancer cells.
However, following Sp3 knockdown in these cells, the effects of
TFF2 protein were significantly reduced, further indicating that
Sp3 protein mediates TFF2 antitumor activity.
In conclusion, in the present study, we demonstrated
that endogenous TFF2 binds to Sp3 in GES-1 cells and that the
effects of TFF2 antitumor activity (e.g., the inhibition of tumor
proliferation and the induction of apoptosis) are mediated by this
interaction. However, further studies are required to confirm the
precise mechanisms through which TFF2 interacts with the Sp3
protein to mediate the effects of TFF2 in gastric cancer cells.
Acknowledgments
This study was supported in part by grant
3502Z20126015 from the Science and Technology Innovation Foundation
of Xiamen (Fujian, China). The authors are grateful to members of
the Department of Gastroenterology, Zhongshan Hospital Xiamen
University. We would also like to thank Ms. Diana Monteiro for
assisting with the manuscript revision.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: A global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ganapathy S, Sengupta S, Wawrzyniak PK,
Huber V, Buda F, Baumeister U, Würthner F and de Groot HJ: Zinc
chlorins for artificial light-harvesting self-assemble into
antiparallel stacks forming a microcrystalline solid-state
material. Proc Natl Acad Sci USA. 106:11472–11477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang P, Yu G and Zhang Y, Xiang Y, Zhu Z,
Feng W, Lee W and Zhang Y: Promoter hypermethylation and
downregulation of trefoil factor 2 in human gastric cancer. Oncol
Lett. 7:1525–1531. 2014.PubMed/NCBI
|
|
6
|
Yu G, Jiang P, Xiang Y and Zhang Y, Zhu Z,
Zhang C, Lee S, Lee W and Zhang Y: Increased expression of
protease-activated receptor 4 and trefoil factor 2 in human
colorectal cancer. PLoS One. 10:e01226782015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiba T, Marusawa H, Seno H and Watanabe
N: Mechanism for gastric cancer development by Helicobacter pylori
infection. J Gastroenterol Hepatol. 23:1175–1181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Q, Chen MY, He CY, Sun LP and Yuan Y:
Promoter polymorphisms in trefoil factor 2 and trefoil factor 3
genes and susceptibility to gastric cancer and atrophic gastritis
among Chinese population. Gene. 529:104–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsai YC, Hsiao WH, Lin SH, Yang HB, Cheng
HC, Chang WL, Lu CC and Sheu BS: Genomic single nucleotide
polymorphisms in the offspring of gastric cancer patients
predispose to spasmolytic polypeptide-expressing metaplasia after
H. pylori infection. J Biomed Sci. 22:162015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Belovari T, Bijelić N, Tolušić Levak M and
Baus Lončar M: Trefoil factor family peptides TFF1 and TFF3 in the
nervous tissues of developing mouse embryo. Bosn J Basic Med Sci.
15:33–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanisch FG, Bonar D, Schloerer N and
Schroten H: Human trefoil factor 2 is a lectin that binds
α-GlcNAc-capped mucin glycans with antibiotic activity against
Helicobacter pylori. J Biol Chem. 289:27363–27375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujimoto K, Fujii G, Taguchi K, Yasuda K,
Matsuo Y, Hashiyama A, Mutoh M, Tanaka H and Wada M: Involvement of
trefoil factor family 2 in the enlargement of intestinal tumors in
Apc(Min/+) mice. Biochem Biophys Res Commun. 463:859–863. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ortiz-Masiá D, Hernández C, Quintana E,
Velázquez M, Cebrián S, Riaño A, Calatayud S, Esplugues JV and
Barrachina MD: iNOS-derived nitric oxide mediates the increase in
TFF2 expression associated with gastric damage: Role of HIF-1.
FASEB J. 24:136–145. 2010. View Article : Google Scholar
|
|
14
|
Aikou S, Ohmoto Y, Gunji T, Matsuhashi N,
Ohtsu H, Miura H, Kubota K, Yamagata Y, Seto Y, Nakajima A, et al:
Tests for serum levels of trefoil factor family proteins can
improve gastric cancer screening. Gastroenterology. 141:837–845.e1.
72011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peterson AJ, Menheniott TR, O'Connor L,
Walduck AK, Fox JG, Kawakami K, Minamoto T, Ong EK, Wang TC, Judd
LM, et al: Helicobacter pylori infection promotes methylation and
silencing of trefoil factor 2, leading to gastric tumor development
in mice and humans. Gastroenterology. 139:2005–2017. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhan XJ, Ren JL, Xu HZ, Dong J, Zhou F,
Zhou F, Pan JS and Xiao HM: Identification of genes encoding human
trefoil factor 2-interacting proteins by screening a cDNA library
of gastric cancer cells. World Chin J Dig Chin. 17:2767–2772.
2009.
|
|
17
|
Miyagishi M and Taira K: U6
promoter-driven siRNAs with four uridine 3′ overhangs efficiently
suppress targeted gene expression in mammalian cells. Nat
Biotechnol. 20:497–500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guleng B, Tateishi K, Ohta M, Kanai F,
Jazag A, Ijichi H, Tanaka Y, Washida M, Morikane K, Fukushima Y, et
al: Blockade of the stromal cell-derived factor-1/CXCR4 axis
attenuates in vivo tumor growth by inhibiting angiogenesis in a
vascular endothelial growth factor-independent manner. Cancer Res.
65:5864–5871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jazag A, Ijichi H, Kanai F, Imamura T,
Guleng B, Ohta M, Imamura J, Tanaka Y, Tateishi K, Ikenoue T, et
al: Smad4 silencing in pancreatic cancer cell lines using stable
RNA interference and gene expression profiles induced by
transforming growth factor-beta. Oncogene. 24:662–671. 2005.
View Article : Google Scholar
|
|
20
|
Kim HJ, Kim JC, Min JS, Kim MJ, Kim JA,
Kor MH, Yoo HS and Ahn JK: Aqueous extract of Tribulus terrestris
Linn induces cell growth arrest and apoptosis by down-regulating
NF-κB signaling in liver cancer cells. J Ethnopharmacol.
136:197–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meinders M, Kulu DI, van de Werken HJ,
Hoogenboezem M, Janssen H, Brouwer RW, van Ijcken WF, Rijkers EJ,
Demmers JA, Krüger I, et al: Sp1/Sp3 transcription factors regulate
hallmarks of megakaryocyte maturation and platelet formation and
function. Blood. 125:1957–1967. 2015. View Article : Google Scholar
|
|
22
|
Hertel J, Hirche C, Wissmann C, Ebert MP
and Höcker M: Transcription of the vascular endothelial growth
factor receptor-3 (VEGFR3) gene is regulated by the zinc finger
proteins Sp1 and Sp3 and is under epigenetic control: Transcription
of vascular endothelial growth factor receptor 3. Cell Oncol
(Dordr). 37:131–145. 2014. View Article : Google Scholar
|
|
23
|
Davie JR, He S, Li L, Sekhavat A, Espino
P, Drobic B, Dunn KL, Sun JM, Chen HY, Yu J, et al: Nuclear
organization and chromatin dynamics - Sp1, Sp3 and histone
deacetylases. Adv Enzyme Regul. 48:189–208. 2008. View Article : Google Scholar
|
|
24
|
Katsuyama M, Hirai H, Iwata K, Ibi M,
Matsuno K, Matsumoto M and Yabe-Nishimura C: Sp3 transcription
factor is crucial for transcriptional activation of the human NOX4
gene. FEBS J. 278:964–972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoffmann C, Zimmermann A, Hinney A,
Volckmar AL, Jarrett HW, Fromme T and Klingenspor M: A novel
SP1/Sp3 dependent intronic enhancer governing transcription of the
UCP3 gene in brown adipocytes. PLoS One. 8:e834262013. View Article : Google Scholar
|
|
26
|
Lian S, Potula HH, Pillai MR, Van Stry M,
Koyanagi M, Chung L, Watanabe M and Bix M: Transcriptional
activation of Mina by Sp1/3 factors. PLoS One. 8:e806382013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zelko IN, Mueller MR and Folz RJ:
Transcription factors sp1 and sp3 regulate expression of human
extracellular superoxide dismutase in lung fibroblasts. Am J Respir
Cell Mol Biol. 39:243–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wilson AJ, Chueh AC, Tögel L, Corner GA,
Ahmed N, Goel S, Byun DS, Nasser S, Houston MA, Jhawer M, et al:
Apoptotic sensitivity of colon cancer cells to histone deacetylase
inhibitors is mediated by an Sp1/Sp3-activated transcriptional
program involving immediate-early gene induction. Cancer Res.
70:609–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tu SP, Chi AL, Ai W, Takaishi S,
Dubeykovskaya Z, Quante M, Fox JG and Wang TC: p53 inhibition of
AP1-dependent TFF2 expression induces apoptosis and inhibits cell
migration in gastric cancer cells. Am J Physiol Gastrointest Liver
Physiol. 297:G385–G396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hiraki M, Kitajima Y, Sato S, Nakamura J,
Hashiguchi K, Noshiro H and Miyazaki K: Aberrant gene methylation
in the peritoneal fluid is a risk factor predicting peritoneal
recurrence in gastric cancer. World J Gastroenterol. 16:330–338.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Auyeung KK, Law PC and Ko JK: Astragalus
saponins induce apoptosis via an ERK-independent NF-κB signaling
pathway in the human hepatocellular HepG2 cell line. Int J Mol Med.
23:189–196. 2009.PubMed/NCBI
|
|
32
|
Jani TS, DeVecchio J, Mazumdar T, Agyeman
A and Houghton JA: Inhibition of NF-kappaB signaling by quinacrine
is cytotoxic to human colon carcinoma cell lines and is synergistic
in combination with tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) or oxaliplatin. J Biol Chem.
285:19162–19172. 2010. View Article : Google Scholar : PubMed/NCBI
|