Introduction
Chronic obstructive pulmonary disease (COPD),
characterized by a fixed obstruction of the airway caused by
emphysema, chronic bronchitis, or both, is a common, growing public
health problem that is responsible for a huge economic health
burden worldwide (1). Stable COPD
may lead to declines in lung functions, such as airflow
obstruction, airway function decline, and respiratory muscle
fatigue, which impair patient quality of life; although acute
exacerbations of COPD (AECOPD) have various definitions, they are
commonly characterized by worsened dyspnea and increased volumes of
phlegm and phlegm purulence, usually accompanied by hypoxemia and
worsened hypercapnia (2,3). Small airway lesions (due to chronic
bronchiolitis) and destruction of the alveolar walls (emphysema)
are the two major features of AECOPD (4). Based on available data in 2010, COPD
is one of the 6 leading causes of death, and AECOPD is associated
with high morbidity (5). To date,
there are no methods for preventing AECOPD, and current medical
therapies for AECOPD mainly involve bronchial relaxation and the
use of glucocorticoids and antibiotics, which, however, are always
associated with side effects leading to unsatisfactory prognosis
(6).
Smoking, malnutrition, depression and drug addiction
are the risk factors affecting the quality of life for patients
with AECOPD (7). Furthermore,
skeletal muscle dysfunction is the most severe complication of
COPD, and progression is associated with oxidative stress, skeletal
muscle fiber types, systemic inflammation and mitochondrial
dysfunction (1), bringing great
detrimental effects to patients. In fact, skeletal muscle depletion
has been adopted as a predictor of mortality in patients with COPD
(8). In COPD patients, a lack of
antioxidant capacity and glutathione (GSH) indicated that oxidative
stress is associated with skeletal muscle dysfunction (9). Furthermore, bacterial infection is
another major cause of AECOPD (10). The increasing levels of systemic
inflammatory factors, such as tumor necrosis factor α (TNF-α),
interleukin (IL)-6 and IL-8, may inhibit muscle shrinkage and the
protein degradation of skeletal muscle, thus leading to muscle
atrophy (11). Mitochondrial
dysfunction, in terms of sharply increased transmembrane potential
and reduced mitochondrial density, is one of the factors associated
with abnormal skeletal muscle in COPD (9). Thus, further studies are warranted
in order to explore the mechanism underlying the pathogenesis of
AECOPD.
Previous studies have identified a number of
microRNAs (miRNAs or miRs) that may have significant regulatory
functions in the progression of COPD, such as miR-223, miR-1274a
and miR-15b in lung tissue (12),
let-7c and miR-125b (13), as
well as serum miR-20a, miR-28-3p, miR-34c-5p, miR-100 and miR-7
(14). Furthermore, the
expression of miR-1 in quadriceps may be responsible for muscle
dysfunction in COPD (15). To the
best of our knowledge, however, there have been no reports on
miRNAs contributing to skeletal muscle weakness in patients with
AECOPD.
In order to identify the key genes and miRNAs that
may be responsible for the loss of muscle force during an acute
exacerbation in COPD patients, we downloaded a gene expression
profile dataset GSE10828 (16)
from the Gene Expression Omnibus (GEO) database of the National
Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/geo) (17), which were collected from muscle
samples of patients with AECOPD or stable COPD. The authors
submitting this dataset identified the differentially expressed
genes (DEGs) that may be associated with loss of muscle force
during AECOPD, and performed gene ontology (GO) enrichment analysis
(16). In the present study,
which is based on the identified DEGs, we constructed a
protein-protein interaction (PPI) network and conducted subsequent
module analysis in order to identify genes that play critical roles
in the progression of AECOPD; we also built a miR-gene regulatory
network. This study aimed to deepen our understanding of the
molecular mechanisms underlying the loss of muscle force in
AECOPD.
Materials and methods
Affymetrix microarray data
Only one microarray dataset (GSE10828) (16) was found to be associated with
AECOPD in the GEO database of NCBI (17). The annotation platform of this
dataset is the GPL2891 platform (GE Healthcare/Amersham Biosciences
CodeLink™ UniSet Human 20K I Bioarray, Chalfont, UK). This dataset
were collected from vastus lateralis samples from 4 male patients
with acute COPD and 5 male patients with stable COPD. No
significant differences were found in the basic characteristics
between the patients with AECOPD and stable COPD with regard to
age, body mass index (BMI), forced expiratory volume in the first
second (FEV1), FEV1/forced vital capacity
(FVC), arterial oxygen and carbon dioxide tension (PaO2
and PaCO2), and maximal inspiratory mouth pressure
(PImax), apart from C-reactive protein (CRP) levels at
admission and lower quadriceps force (16).
Data preprocessing and identification of
DEGs
Firstly, the microarray data in .CEL format were
converted into expression measures using the GEOquery package, a
package for retrieving gene expression data sets in R/Bioconductor
(Bioconductor version: Release 3.1, http://www.bioconductor.org/packages/release/bioc/html/GEOquery.html)
(18). The Robust Multi-array
Analysis (RMA) method was used to preprocess the downloaded raw
data by background adjustment, quintile normalization and
summarization (19).
Subsequently, the differential expression values of genes between
the acute COPD samples and the stable COPD samples were calculated
by t-test using the Linear Models for Microarray Data (LIMMA)
package (R/Bioconductor version: Release 3.1, http://www.bioconductor.org/packages/release/bioc/html/limma.html)
(20). Multiple testing
correction was conducted by the Bayesian method (21). Only genes with a false discovery
rate (FDR) <0.01 and |log2 Fold-Change (FC)| >1.5
were identified as DEGs. Finally, the pheatmap package of R
(http://cran.r-project.org/web/packages/pheatmap/index.html)
was used for hierarchical clustering (22) of DEGs based on Euclidean distance
(23), and the result was
visualized using heat maps.
GO analysis
GO enrichment analysis was performed using the
Database for Annotation, Visualization and Integrated Discovery
(DAVID) online tool (version 6.7, http://david.abcc.ncifcrf.gov/) (24) with a p-value <0.05 as
threshold.
Construction of PPI network and
functional analysis of significant modules
The Search Tool for the Retrieval of Interacting
Genes (STRING) (version 10, http://string-db.org/) database was used to predict
the interactions between the encoded proteins of these up- and
downregulated DEGs, respectively (25). Only PPI pairs with a combination
score >0.4 were included. Cytoscape software (version 3.2.1,
http://cytoscape.org/), an open source software
platform for integrating bimolecular interaction networks with
high-throughput expression data and other molecular states into a
unified conceptual framework (26), was used to visualize the resulting
PPIs. The top five genes with the highest connection degrees were
considered to be most closely associated with AECOPD. In addition,
Cluster ONE plugin (27) was used
to select the significant modules of the up- and downregulated
genes in the PPI network, respectively. The top three modules
according to p-values were selected for further analysis.
In addition, the biological processes of DEGs in the
resulting networks were functionally analyzed using DAVID (version
6.7, http://david.abcc.ncifcrf.gov/)
(24) with a p-value
<0.05.
Enrichment analysis of key miRNAs and
regulatory network construction of key miRNAs
We used the gene set enrichment analysis (GSEA)
software (version 2.0.14, http://www.broadinstitute.org/gsea/index.jsp), based
on the whole genome expression profile (28), to predict the miRNAs associated
with AECOPD based on the microarray data. A p-value <0.01 was
selected as threshold.
In addition, we used Cytoscape software to construct
a miRNA-gene regulatory network with the miRNAs and their target
DEGs. A miR-DEG regulatory network is a complicated biological
system in which an miRNA functions in a variety of intracellular
biological processes, such as gene regulation, cell signaling
transduction, protein-protein interactions and metabolism, by
regulating molecules at a transcriptional level. This provides a
deeper understanding of the biological mechanisms involved
(29).
Results
Screening of DEGs and hierarchical
clustering analysis
The expression profiling data were preprocessed
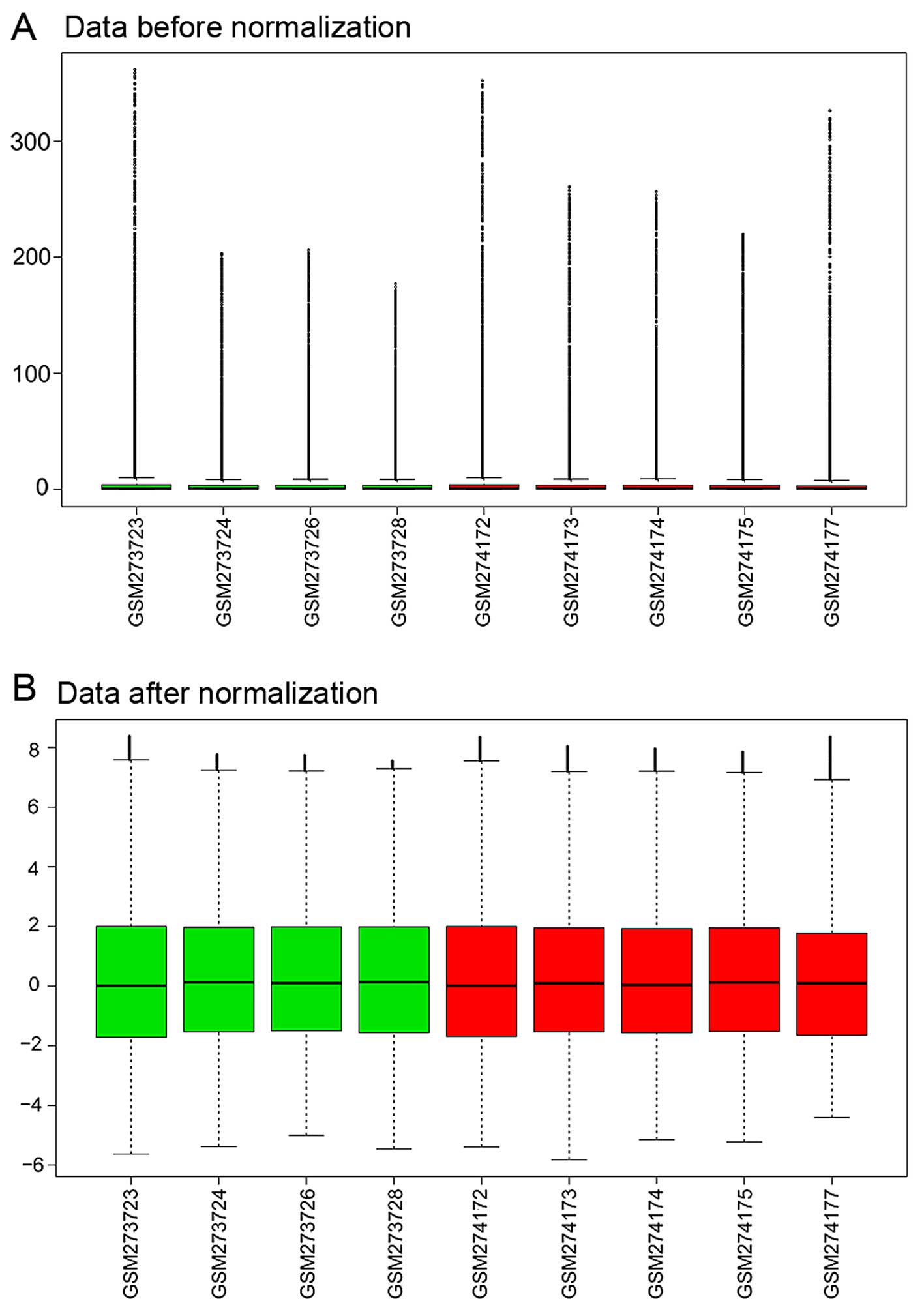
using the R GEOquery package and were normalized by the RMA method
in R (Fig. 1).
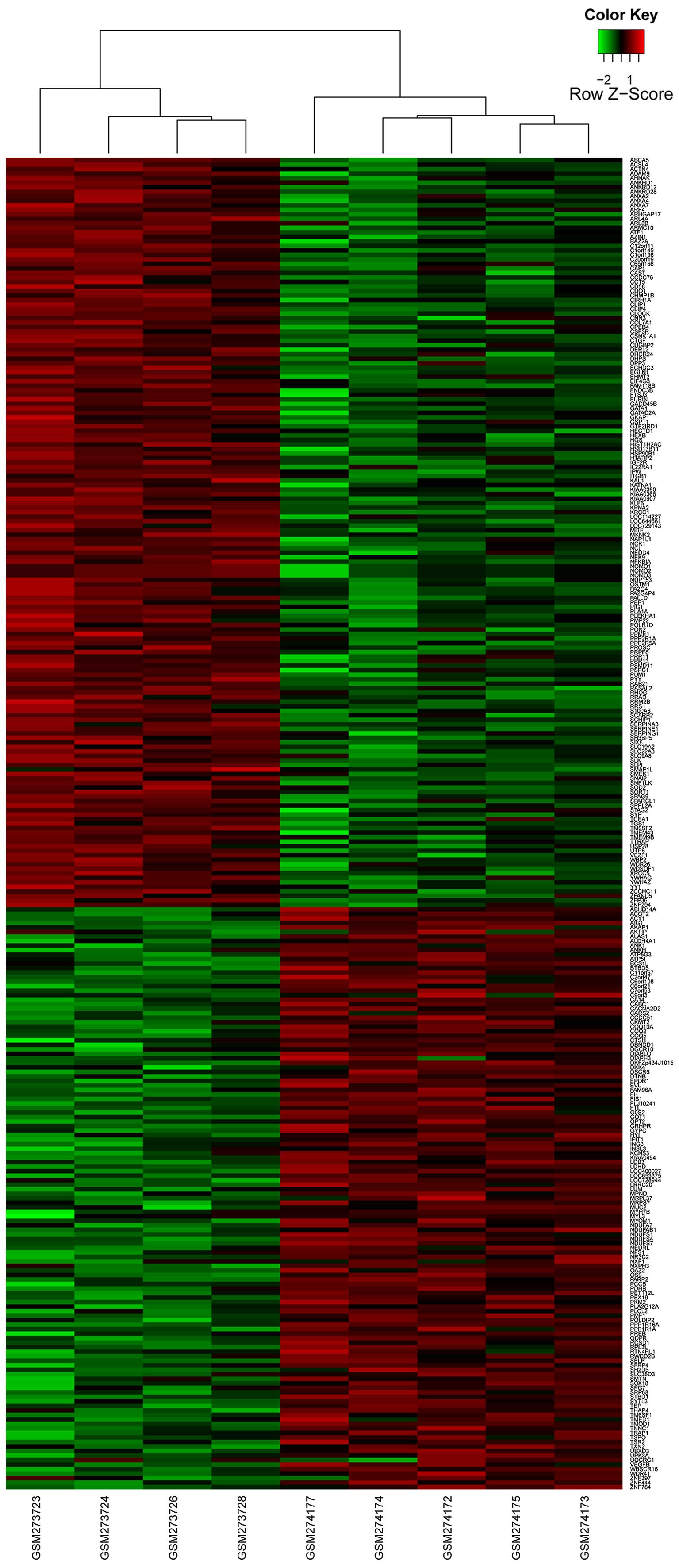
With the cut-offs of FDR <0.01 and
|log2 FC| >1.5, a total of 295 DEGs were identified
between the human AECOPD and stable COPD controls, including 166
upregulated genes and 129 downregulated genes (Fig. 2).
Functional annotation analysis of
DEGs
According to the GO functional annotation, the top
10 enriched biological process terms included cellular respiration,
generation of precursor metabolites and energy, and respiratory
electron transport chain; the top 10 molecular function terms
included cytoskeletal protein binding, serine-type endopeptidase
inhibitor activity, protein complex binding and NADH dehydrogenase
activity. Translocator protein (TSPO), superoxide dismutase
2 (SOD2) and proligeration associated protein (PA2G4)
were the genes enriched in the significant functions (Table I).
 | Table IThe top 10 enriched GO terms in BP
and MF categories. |
Table I
The top 10 enriched GO terms in BP
and MF categories.
| Name | Term | Count | p-value | Genes |
|---|
| BP | GO:0015980 - energy
derivation by oxidation of organic compounds | 11 | 7.94E-05 | NDUFS7, NDUFS4,
GOT1, UQCRC1, PPP1R1A, NDUFA7, NDUFAB1, NDUFS1, PDHB, FH,
SOD2 |
| BP | GO:0045333 -
cellular respiration | 9 | 1.26E-04 | NDUFS7, NDUFS4,
UQCRC1, NDUFA7, NDUFAB1, NDUFS1, PDHB, FH, SOD2 |
| BP | GO:0006091 -
generation of precursor metabolites and energy | 15 | 3.67E-04 | UQCRC1, TXN2,
NDUFA7, NDUFAB1, ATP5G3, PDHB, SOD2, NDUFS7, GOT1, NDUFS4, PKM2,
PPP1R1A, ATP5I, NDUFS1, FH |
| BP | GO:0022904 -
respiratory electron transport chain | 7 | 4.50E-04 | NDUFS7, NDUFS4,
UQCRC1, NDUFA7, NDUFAB1, NDUFS1, SOD2 |
| BP | GO:0006119 -
oxidative phosphorylation | 8 | 8.01E-04 | NDUFS7, NDUFS4,
UQCRC1, NDUFA7, NDUFAB1, ATP5I, ATP5G3, NDUFS1 |
| BP | GO:0007005 -
mitochondrion organization | 9 | 0.001355 | NDUFS7, FIS1,
SPG7, TSPO, YWHAZ, NDUFS4, BCS1L, RRM2B, SOD2 |
| BP | GO:0042773 - ATP
synthesis coupled electron transport | 6 | 0.001691 | NDUFS7, NDUFS4,
UQCRC1, NDUFA7, NDUFAB1, NDUFS1 |
| BP | GO:0042775 -
mitochondrial ATP synthesis coupled electron transport | 6 | 0.001691 | NDUFS7, NDUFS4,
UQCRC1, NDUFA7, NDUFAB1, NDUFS1 |
| BP | GO:0022900 -
electron transport chain | 8 | 0.001937 | NDUFS7, NDUFS4,
UQCRC1, TXN2, NDUFA7, NDUFAB1, NDUFS1, SOD2 |
| BP | GO:0006120 -
mitochondrial electron transport, NADH to ubiquinone | 5 | 0.003914 | NDUFS7, NDUFS4,
NDUFA7, NDUFAB1, NDUFS1 |
| MF | GO:0004857 - enzyme
inhibitor activity | 16 | 1.89E-05 | CAST, PPME1,
SERPING1, AZIN1, FURIN, ANXA4, ANXA2, OAZ2, SH3BP5, YWHAG, COL7A1,
PPP1R1A, KAL1, SERPINE1, SERPINA3, SLPI |
| MF | GO:0008092 -
cytoskeletal protein binding | 19 | 7.97E-04 | ACTN4, CNN3,
TNNC1, MYL3, DIAPH3, EVL, PALLD, ANXA2, LOC729143, SPAG9,
ANK1, SMTN,KATNA1, NCK1, CLIP1, ARL8B, CAP1, MYH7B, TMOD1 |
| MF | GO:0004867 -
serine-type endopeptidase inhibitor activity | 7 | 0.002949 | COL7A1, KAL1,
SERPINE1, SERPINA3, SLPI, SERPING1, FURIN |
| MF | GO:0019899 - enzyme
binding | 18 | 0.003008 | PPME1, DIAPH3,
NFKBIA, FURIN, ANXA2, SPAG9, YWHAG, PA2G4, ANK1, NEDD4, IGF2R,
SERPINE1, SORT1, SYTL3, AKAP1, KPNA2, PA2G4P4, DHCR24,
ADAM9 |
| MF | GO:0032403 -
protein complex binding | 10 | 0.003358 | SYP, INSL3,
YWHAZ, UQCRC1, ACTN4, TXN2, CTGF, NFKBIA, ITGB1, ADAM9 |
| MF | GO:0050136 - NADH
dehydrogenase (quinone) activity | 5 | 0.004217 | NDUFS7, NDUFS4,
NDUFA7, NDUFAB1, NDUFS1 |
| MF | GO:0008137 - NADH
dehydrogenase (ubiquinone) activity | 5 | 0.004217 | NDUFS7, NDUFS4,
NDUFA7, NDUFAB1, NDUFS1 |
| MF | GO:0003954 - NADH
dehydrogenase activity | 5 | 0.004217 | NDUFS7, NDUFS4,
NDUFA7, NDUFAB1, NDUFS1 |
| MF | GO:0016655 -
oxidoreductase activity, acting on NADH or NADPH, quinone or
similar compound as acceptor | 5 | 0.006741 | NDUFS7, NDUFS4,
NDUFA7, NDUFAB1, NDUFS1 |
| MF | GO:0004866 -
endopeptidase inhibitor activity | 8 | 0.007169 | CAST, COL7A1,
KAL1, SERPINE1, SERPINA3, SLPI, SERPING1, FURIN |
PPI network
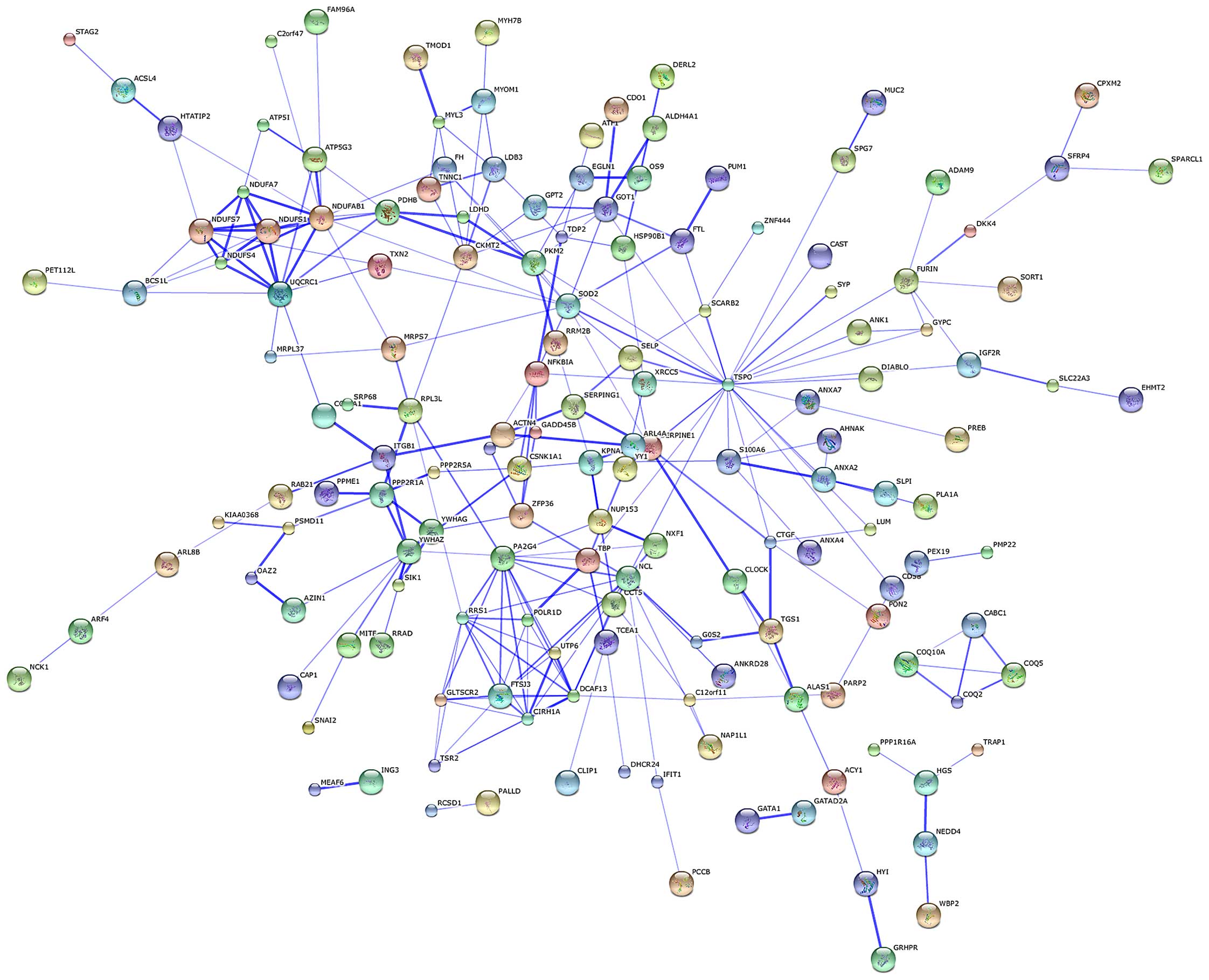
The protein-protein network of the identified DEGs
was mapped using the software STRING in order to predict the
protein interactions. By integrating these correlations,
interaction networks between the target genes and their interactive
genes were constructed (Fig. 3).
In the core of the PPI network, genes belonged to more than one
module. TSPO, nucleolin (NCL), NADH dehydrogenase
(ubiquinone) 1, α/β subcomplex (NDUFAB1), PA2G4 and
SOD2 were the top 5 protein nodes with the highest
connection degrees (Table II and
Fig. 3).
 | Table IIGenes with the top 5 node degrees in
PPI network. |
Table II
Genes with the top 5 node degrees in
PPI network.
| Gene | Degree | Log2FC | p-value |
|---|
| TSPO | 25 | −2.73239 | 0.007907 |
| NCL | 14 | 2.021786 | 0.001702 |
| NDUFAB1 | 13 | −27.2233 | 0.006066 |
| PA2G4 | 12 | 3.607543 | 0.006317 |
| SOD2 | 11 | 21.28352 | 0.005172 |
Module analysis and functional analysis
of DEGs in PPI network
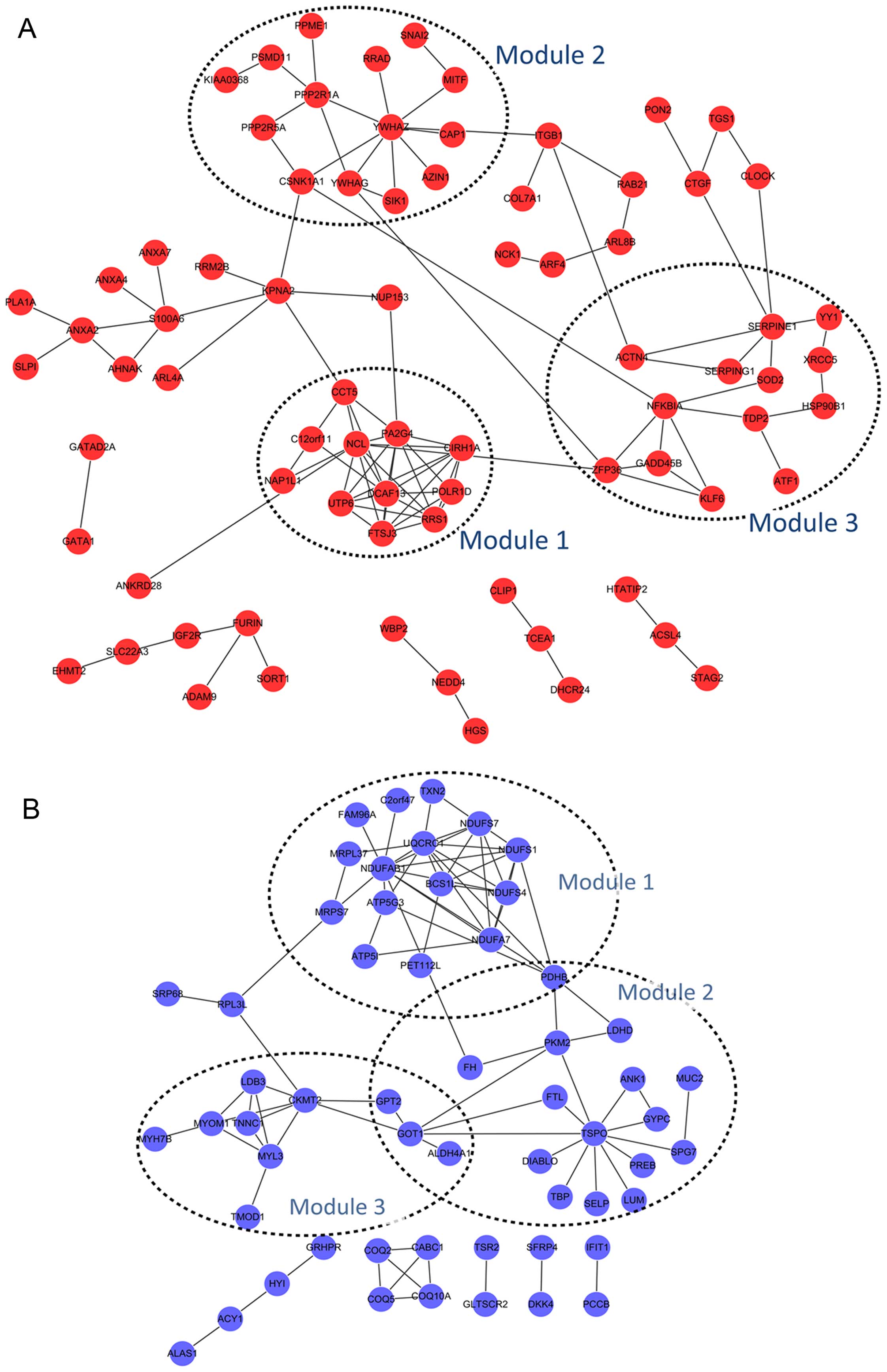
For the upregulated DEGs, three significant modules
were obtained: module 1 consisting of 11 nodes (including NCL) and
33 edges (p-value=3.017E-5), module 2 of 14 nodes and 16 edges
(p-value=3.115E-5), and module 3 of 13 nodes and 17 edges
(p-value=3.194E-5) (Fig. 4A).
Moreover, 3 significant modules were also obtained for the
downregulated DEGs: module 1 consisting of 16 nodes and 35 edges
(p-value=1.418E-6), module 2 of 18 nodes and 21 edges
(p-value=4.592E-6), and module 3 of 10 nodes and 15 edges
(p-value=3.642E-4). Pyruvate dehydrogenase (lipoamide) beta
(PDHB) was commonly detected in module 1 and 2; glutamic
pyruvate transaminase (alanine aminotransferase) 2 (GPT2),
glutamic-oxaloacetic transaminase 1 (GOT1) and aldehyde
dehydrogenase 4 family member A1 (ALDH4A1) were common in
module 2 and 3 (Fig. 4B).
In addition, GO terms of the DEGs in the significant
modules are presented in Table
III. Functions of upregulated DEGs in module 1 were in the
ribosome biogenesis. No GO term of the upregulated DEGs in module 2
was detected. Upregulated genes in module 3, such as X-ray repair
complementing defective repair in Chinese hamster cells 5
(XRCC5) and Kruppel-like factor 6 (KLF6), were
enriched in GO terms including hemopoietic or lymphoid organ
development and immune system development (Table III-A). GO functions of
downregulated DEGs in module 1 were in generation of precursor
metabolites and energy, whereas that in module 2 was in cell death,
and that in module 3 were in muscle contraction and muscle system
process (Table III-B).
 | Table IIIGO terms of the DEGs in significant
modules. |
Table III
GO terms of the DEGs in significant
modules.
| Module | Term | Count | p-value | Genes |
|---|
| A, GO terms of the
upregulated DEGs in the significant modules |
|
| Module 1 | Cluster 1
enrichment score: 4.308352643161865 | | | |
| GO:0042254 -
ribosome biogenesis | 5 | 7.66E-07 | DCAF13,
PA2G4, UTP6, RRS1, FTSJ3 |
| GO:0022613 -
ribonucleoprotein complex biogenesis | 5 | 3.63E-06 | DCAF13,
PA2G4, UTP6, RRS1, FTSJ3 |
| GO:0006364 - rRNA
processing | 4 | 2.48E-05 | DCAF13,
PA2G4, UTP6, FTSJ3 |
| GO:0016072 - rRNA
metabolic process | 4 | 2.82E-05 | DCAF13,
PA2G4, UTP6, FTSJ3 |
| GO:0034470 - ncRNA
processing | 4 | 2.05E-04 | DCAF13,
PA2G4, UTP6, FTSJ3 |
| GO:0034660 - ncRNA
metabolic process | 4 | 3.78E-04 | DCAF13,
PA2G4, UTP6, FTSJ3 |
| GO:0006396 - RNA
processing | 4 | 4.60E-03 | DCAF13,
PA2G4, UTP6, FTSJ3 |
| Module 2 | None | | | |
| Module 3 | Cluster 1
enrichment score: 2.0090957996722376 | | | |
| GO:0043066 -
negative regulation of apoptosis | 4 | 2.51E-03 | XRCC5,
HSP90B1, NFKBIA, SOD2 |
| GO:0043069 -
negative regulation of programmed cell death | 4 | 2.61E-03 | XRCC5,
HSP90B1, NFKBIA, SOD2 |
| GO:0060548 -
negative regulation of cell death | 4 | 2.63E-03 | XRCC5,
HSP90B1, NFKBIA, SOD2 |
| GO:0042981 -
regulation of apoptosis | 5 | 2.92E-03 | XRCC5,
HSP90B1, ACTN4, NFKBIA, SOD2 |
| GO:0043067 -
regulation of programmed cell death | 5 | 3.03E-03 | XRCC5,
HSP90B1, ACTN4, NFKBIA, SOD2 |
| GO:0010941 -
regulation of cell death | 5 | 3.07E-03 | XRCC5,
HSP90B1, ACTN4, NFKBIA, SOD2 |
| GO:0001666 -
response to hypoxia | 3 | 5.05E-03 | HSP90B1,
ACTN4, SOD2 |
| GO:0070482 -
response to oxygen levels | 3 | 5.58E-03 | HSP90B1,
ACTN4, SOD2 |
| GO:0006916 -
anti-apoptosis | 3 | 1.16E-02 | HSP90B1,
NFKBIA, SOD2 |
| GO:0042592 -
homeostatic process | 4 | 2.01E-02 | XRCC5,
HSP90B1, SERPINE1, SOD2 |
| Cluster 2
enrichment score: 1.7537673375612617 | | | |
| GO:0030097 -
hemopoiesis | 3 | 1.50E-02 | XRCC5,
KLF6, SOD2 |
| GO:0048534 -
hemopoietic or lymphoid organ development | 3 | 1.81E-02 | XRCC5,
KLF6, SOD2 |
| GO:0002520 - immune
system development | 3 | 2.02E-02 | XRCC5,
KLF6, SOD2 |
| Cluster 3
enrichment score: 1.1482146347041753 | | | |
| GO:0051252 -
regulation of RNA metabolic process | 6 | 9.85E-03 | ZFP36,
KLF6, YY1, NFKBIA, ATF1,
SOD2 |
| GO:0006357 -
regulation of transcription from RNA polymerase II promoter | 4 | 1.84E-02 | KLF6,
YY1, NFKBIA, SOD2 |
| GO:0006355 -
regulation of transcription, DNA-dependent | 5 | 4.53E-02 | KLF6,
YY1, NFKBIA, ATF1, SOD2 |
|
| B, GO terms of the
downregulated DEGs in the significant modules |
|
| Module 1 | Cluster 1
enrichment score: 8.207305207832107 | | | |
| GO:0006091 -
generation of precursor metabolites and energy | 10 | 1.12E-12 | NDUFS7,
NDUFS4, UQCRC1, TXN2, NDUFA7,
NDUFAB1, ATP5I, ATP5G3, NDUFS1,
PDHB |
| GO:0006119 -
oxidative phosphorylation | 8 | 1.39E-12 | NDUFS7,
NDUFS4, UQCRC1, NDUFA7, NDUFAB1,
ATP5I, ATP5G3, NDUFS1 |
| GO:0045333 -
cellular respiration | 7 | 1.92E-10 | NDUFS7,
NDUFS4, UQCRC1, NDUFA7, NDUFAB1,
NDUFS1, PDHB |
| GO:0022900 -
electron transport chain | 7 | 5.13E-10 | NDUFS7,
NDUFS4, UQCRC1, TXN2, NDUFA7,
NDUFAB1, NDUFS1 |
| GO:0042773 - ATP
synthesis coupled electron transport | 6 | 1.27E-09 | NDUFS7,
NDUFS4, UQCRC1, NDUFA7, NDUFAB1,
NDUFS1 |
| GO:0042775 -
mitochondrial ATP synthesis coupled electron transport | 6 | 1.27E-09 | NDUFS7,
NDUFS4, UQCRC1, NDUFA7, NDUFAB1,
NDUFS1 |
| GO:0015980 - energy
derivation by oxidation of organic compounds | 7 | 2.11E-09 | NDUFS7,
NDUFS4, UQCRC1, NDUFA7, NDUFAB1,
NDUFS1, PDHB |
| GO:0022904 -
respiratory electron transport chain | 6 | 2.53E-09 | NDUFS7,
NDUFS4, UQCRC1, NDUFA7, NDUFAB1,
NDUFS1 |
| GO:0006120 -
mitochondrial electron transport, NADH to ubiquinone | 5 | 5.62E-08 | NDUFS7,
NDUFS4, NDUFA7, NDUFAB1, NDUFS1 |
| GO:0055114 -
oxidation reduction | 8 | 6.79E-07 | NDUFS7,
NDUFS4, UQCRC1, TXN2, NDUFA7,
NDUFAB1, NDUFS1, PDHB |
| GO:0016310 -
phosphorylation | 8 | 3.09E-06 | NDUFS7,
NDUFS4, UQCRC1, NDUFA7, NDUFAB1,
ATP5I, ATP5G3, NDUFS1 |
| GO:0006793 -
phosphorus metabolic process | 8 | 1.14E-05 | NDUFS7,
NDUFS4, UQCRC1, NDUFA7, NDUFAB1,
ATP5I, ATP5G3, NDUFS1 |
| GO:0006796 -
phosphate metabolic process | 8 | 1.14E-05 | NDUFS7,
NDUFS4, UQCRC1, NDUFA7, NDUFAB1,
ATP5I, ATP5G3, NDUFS1 |
| Cluster 2
enrichment score: 2.1705698016603754 | | | |
| GO:0010257 - NADH
dehydrogenase complex assembly | 3 | 3.82E-05 | NDUFS7,
NDUFS4, BCS1L |
| GO:0032981 -
mitochondrial respiratory chain complex I assembly | 3 | 3.82E-05 | NDUFS7,
NDUFS4, BCS1L |
| GO:0033108 -
mitochondrial respiratory chain complex assembly | 3 | 5.60E-05 | NDUFS7,
NDUFS4, BCS1L |
| GO:0007005 -
mitochondrion organization | 3 | 7.49E-03 | NDUFS7,
NDUFS4, BCS1L |
| GO:0043623 -
cellular protein complex assembly | 3 | 1.02E-02 | NDUFS7,
NDUFS4, BCS1L |
| GO:0034622 -
cellular macromolecular complex assembly | 3 | 3.62E-02 | NDUFS7,
NDUFS4, BCS1L |
| GO:0034621 -
cellular macromolecular complex subunit organization | 3 | 4.47E-02 | NDUFS7,
NDUFS4, BCS1L |
| Cluster 3
enrichment score: 2.16913552816325 | | | |
| GO:0046034 - ATP
metabolic process | 3 | 4.40E-03 | ATP5I,
ATP5G3, NDUFS1 |
| GO:0009205 - purine
ribonucleoside triphosphate metabolic process | 3 | 5.44E-03 | ATP5I,
ATP5G3, NDUFS1 |
| GO:0009199 -
ribonucleoside triphosphate metabolic process | 3 | 5.53E-03 | ATP5I,
ATP5G3, NDUFS1 |
| GO:0009144 - purine
nucleoside triphosphate metabolic process | 3 | 5.90E-03 | ATP5I,
ATP5G3, NDUFS1 |
| GO:0009141 -
nucleoside triphosphate metabolic process | 3 | 6.77E-03 | ATP5I,
ATP5G3, NDUFS1 |
| GO:0009150 - purine
ribonucleotide metabolic process | 3 | 7.49E-03 | ATP5I,
ATP5G3, NDUFS1 |
| GO:0009259 -
ribonucleotide metabolic process | 3 | 8.46E-03 | ATP5I,
ATP5G3, NDUFS1 |
| GO:0006163 - purine
nucleotide metabolic process | 3 | 1.33E-02 | ATP5I,
ATP5G3, NDUFS1 |
| Module 2 | Cluster 1
enrichment score: 2.0431078400255807 | | | |
| GO:0008219 - cell
death | 6 | 1.12E-03 | MUC2,
SPG7, TSPO, PKM2, DIABLO,
TBP |
| GO:0016265 -
death | 6 | 1.15E-03 | MUC2,
SPG7, TSPO, PKM2, DIABLO,
TBP |
| GO:0012501 -
programmed cell death | 4 | 3.31E-02 | MUC2,
TSPO, PKM2, DIABLO |
| GO:0006915 -
apoptosis | 3 | 1.58E-01 | MUC2,
TSPO, DIABLO |
| Module 3 | Cluster 1
enrichment score: 4.256164391089315 | | | |
| GO:0006936 - muscle
contraction | 4 | 2.77E-05 | MYL3,
TNNC1, CKMT2, MYOM1 |
| GO:0003012 - muscle
system process | 4 | 3.66E-05 | MYL3,
TNNC1, CKMT2, MYOM1 |
| GO:0006941 -
striated muscle contraction | 3 | 1.68E-04 | MYL3,
TNNC1, MYOM1 |
Enrichment analysis of key miRNAs
In total, we identified 39 AECOPD-associated miRNAs
using GSEA software at a p-value <0.01 (Table IV).
 | Table IVEnrichment analysis of key
miRNAs. |
Table IV
Enrichment analysis of key
miRNAs.
| Name | Basic groups | Size | ES | NES | NOM | FDR |
|---|
| miR-23a,
miR-23b | AATGTGA | 343 | 0.278676 | 1.438706 | 0 | 0.089588 |
| miR-103,
miR-107 | ATGCTGC | 177 | 0.335453 | 1.486206 | 0 | 0.088895 |
| miR-221,
miR-222 | ATGTAGC | 108 | 0.336992 | 1.396569 | 0 | 0.094994 |
| miR-320 | CAGCTTT | 213 | 0.343332 | 1.552349 | 0 | 0.078454 |
| miR-520f | AAGCACT | 194 | 0.344786 | 1.56579 | 0 | 0.091075 |
| miR-183 | GTGCCAT | 152 | 0.355742 | 1.541346 | 0 | 0.073964 |
| miR-524 | CTTTGTA | 365 | 0.35657 | 1.562035 | 0 | 0.084759 |
| miR-493 | ATGTACA | 266 | 0.384502 | 1.549554 | 0 | 0.074808 |
| miR-494 | ATGTTTC | 128 | 0.386803 | 1.631935 | 0 | 0.135062 |
| miR-498 | GCTTGAA | 92 | 0.397854 | 1.605433 | 0 | 0.115428 |
| miR-1, miR-206 | ACATTCC | 253 | 0.398441 | 1.603156 | 0 | 0.108254 |
| miR-323 | TAATGTG | 131 | 0.400318 | 1.56248 | 0 | 0.087979 |
| miR-373 | TTTTGAG | 194 | 0.406584 | 1.664687 | 0 | 0.125706 |
| miR-485-3p | TGTATGA | 128 | 0.41496 | 1.611675 | 0 | 0.123895 |
| miR-9 | TAGCTTT | 190 | 0.419432 | 1.627284 | 0 | 0.120835 |
| miR-17-3p | ACTGCAG | 87 | 0.421458 | 1.635642 | 0 | 0.150641 |
| miR-409-3p | AACATTC | 120 | 0.421607 | 1.671052 | 0 | 0.156583 |
| miR-422b,
miR-422a | AAGTCCA | 56 | 0.428607 | 1.58016 | 0 | 0.109384 |
| miR-200a | GTAAGAT | 44 | 0.440217 | 1.63578 | 0 | 0.179569 |
| miR-518a-2 | TTTGCAG | 169 | 0.441104 | 1.684444 | 0 | 0.204788 |
| miR-202 | ATAGGAA | 84 | 0.445265 | 1.574246 | 0 | 0.100272 |
| miR-410 | GTTATAT | 76 | 0.466728 | 1.711476 | 0 | 0.295102 |
| miR-217 | ATGCAGT | 95 | 0.477771 | 1.610814 | 0.009328 | 0.113216 |
| miR-15a, miR-16,
miR-15a, miR-195 | | | | | | |
| miR-424,
miR-497 | TGCTGCT | 499 | 0.293906 | 1.427648 | 0.009452 | 0.09335 |
| miR-126 | TAATAAT | 179 | 0.379129 | 1.572559 | 0.009524 | 0.09167 |
| miR-186 | ATTCTTT | 234 | 0.390894 | 1.593438 | 0.009785 | 0.099291 |
| miR-182 | TTGCCAA | 274 | 0.355565 | 1.520188 | 0.00994 | 0.085925 |
| miR-519e | GGCACTT | 105 | 0.382867 | 1.579575 | 0.00994 | 0.104196 |
| miR-527 | CTTTGCA | 192 | 0.316954 | 1.487434 | 0.00996 | 0.089759 |
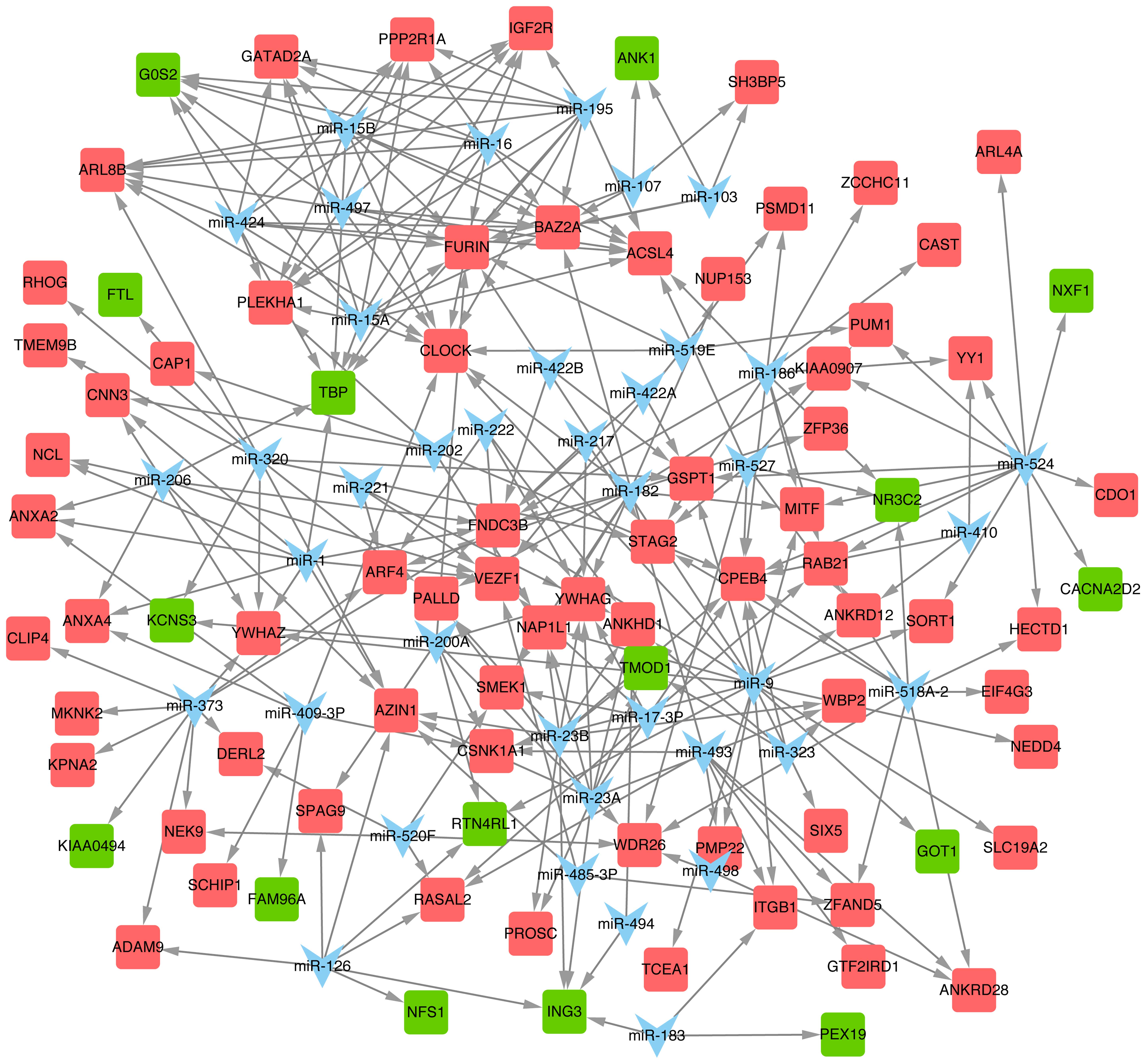
miRNA regulatory network
Thirty-nine predicted miRNAs and their target DEGS
were constructed in an miR-DEG regulatory network (Fig. 5). miR-9, miR-524, miR-23a, miR-15a
and miR-16 were the top 5 miRNAs with the most target DEGs
(Table V), and their target DEGs
included tropomodulin 1 (TMOD1), GOT1, NR3C2
and CPEB4 among others.
 | Table VmiRNAs with the top 5 degrees in
regulatory network. |
Table V
miRNAs with the top 5 degrees in
regulatory network.
| miRNA | Degree | miRNA | Degree |
|---|
| miR-9 | 20 | miR-15A | 11 |
| miR-524 | 13 | miR-15B | 11 |
| miR-23a | 10 | miR-16 | 11 |
| miR-23b | 10 | miR-182 | 11 |
| miR-320 | 10 | miR-186 | 11 |
| miR-373 | 10 | miR-195 | 11 |
| miR-1 | 9 | miR-424 | 11 |
| miR-206 | 9 | miR-497 | 11 |
| miR-518a-2 | 9 | | |
Discussion
Since AECOPD are one of the leading causes of death,
there is an urgent need to investigate the mechanism underlying
AECOPD and to develop an effective preventative strategy.
Microarray-based studies have been performed to analyze the
pathogenesis of AECOPD and to identify the AECOPD-associated genes
in peripheral blood mononuclear cells (30) and skeletal muscle (16). However, no research investigating
AECOPD-associated miRNAs has been reported, to the best of our
knowledge. Hence, the present study was performed in order to
predict AECOPD-associated mRNAs and miRNAs that may be responsible
for the loss of muscle force, and to discuss the molecular
mechanisms underlying the loss of muscle force during AECOPD.
NCL was observed in the Module 1 of the
upregulated genes. It encodes a eukaryotic nucleolar phosphoprotein
that is involved in the synthesis and maturation of ribosomes,
which is mainly located in dense, fibrillar regions of the
nucleolus. Nucleolin is one of the three components consisting of a
D4Z4 repeat (31), in which the
number variation is frequently detected in facioscapulohumeral
muscular dystrophy (32). Thus,
it can be inferred that NCL may also play a role in the loss
of muscle force in AECOPD since it is associated with muscular
function. Furthermore, NCL was also predicted to be
regulated by miR-1 in the present study. miR-1 and miR-206, another
miRNA also observed to regulate the differential gene expression
herein, promote myotube formation (33). Another study reported the reduced
expression of miR-1 in the quadriceps of patients with COPD,
suggesting that miR-1 downregulation may contribute to
COPD-associated skeletal muscle dysfunction (34), and they further observed an
inverse correlation between miR-1 and Akt phosphorylation levels or
HDAC4 protein levels in patients. Thus, it is likely that NCL may
be downregulated during the AECOPD due to the downregulation of
miR-1. SOD2 was another upregulated gene observed in module
3. An imbalance of the oxidation-antioxidant system in the body
represents the principal cause of AECOPD (35). SOD2 (Mn-SOD) is a key enzyme that
prevents cells from damage by eliminating the endogenous free
radicals in the body (36), and
increased expression was found in patients with AECOPD in the
present study. Considering that samples were taken from patients
with an exacerbation on day 4 of hospitalization, the antioxidant
system may be activated by upregulating SOD2 in patients with
AECOPD. However, this hypothesis requires further careful
consideration. Additionally, Togliatto et al have reported
that unacylated ghrelin (UnAG) induced skeletal muscle regeneration
following hindlimb ischemia and was mediated by SOD2 (37). SOD2 may also play similar roles in
muscle dysfunction during AECOPD, which suggests that SOD2 may be
used as a therapeutic target in AECOPD.
In addition, XRCC5 and KLF6 were also
found in module 3 of the upregulated DEGs. Both genes are involved
in immune system development according to GO analysis, suggesting
that the two genes may be important in AECOPD. KLF6 is a member of
the Kruppel-like family of transcription factors that functions as
a tumor suppressor (38).
Mgbemena et al have proven that KLF6 regulated the apoptosis
of lung cells through iNOS expression during respiratory syncytial
virus infection (39). In
addition, KLF6 may also be involved in cell atrophy during an acute
exacerbation. On the other hand, XRCC5 is an ATP-dependent DNA
helicase II or DNA repair protein (40). The role of XRCC5 in COPD has not
been fully elucidated. However, previous findings have revealed
that DNA damage or lack of DNA repair regulated the immune response
to the tissue destruction in COPD (41). Therefore, XRCC5 may be a novel
target for protecting against AECOPD.
TSPO displayed downregulated expression in
patients with AECOPD, and this was observed in module 2. This gene
encodes a protein transformation-related 18-kDa protein that
assists in the recognition of the mitochondrial proteins prior to
intracellular transportation (42). Otherwise, dysfunction of
mitochondria caused by permeability transition would lead to the
apoptosis of muscle cells, which plays a principal role in the
progression of COPD (43). Thus,
the downregulation of TSPO expression observed suggests that
it may have an important role in the loss of muscle force occurring
in AECOPD.
TMOD1 was another key downregulated gene,
observed in module 3. Tropomodulin is a binding protein of
tropomyosin, existing in the muscle cells, and is extracted from
erythrocytes. It is necessary for many key biological functions
including cell migration, differentiation and muscle contraction
(44). The most important cause
of the progression of AECOPD is oxygen deficiency resulting from
several factors, such as the transformation of pulmonary blood
vessel structures, manifested by hyperplasia and hypertrophy of
pulmonary arterial muscle cells, leading to the incrassation of
membranes and fibroblast proliferation (45). In the present study, TMOD1
was predicted to be regulated by miR-23a. It has been reported that
the upregulation of miR-23a inhibits the development of B cells
(46).
GOT1, displaying downregulated expression in
AECOPD, was found in both modules 2 and 3 of the downregulated
genes. GOT1 encodes glutamic-oxaloacetic transaminase 1,
which is a cytoplasmic form of glutamic-oxaloacetic transaminase
that is involved in amino acid metabolism. De Palma et al
have also reported the decreased expression of GOT1 in patients
with Ullrich congenital muscular dystrophy compared to controls
(47). However, there have been
no reports regarding the role of GOT1 in AECOPD, to the best of our
knowledge. However, it has been reported as a putative target gene
of miR-9 by Thulin et al (48), which is consistent with our
predictions. miR-9 is known to play a key role in the activation of
monocytes and/or macrophages during inflammatory responses
(49). More importantly, it is
also reported to play a role in Huntington's disease, a type of
motor neuron disease (50).
Furthermore, the abnormal expression of miR-9 alters motor neuron
subtype differentiation as well as columnar development of spinal
cords in chick embryos (51).
Although the involvement of miR-9 in AECOPD has not been reported
to date, to the best of our knowledge, it is possible that this
miRNA may play a role in the loss of muscle force in AECOPD, and
GOT1 may also be involved this process.
Finally, another two miRNAs, miR-15a and miR-16,
were also found overexpressed in AECOPD patients in the present
study. The pathway of Wnt signaling is known to be a promising
target for mediating the development of COPD (52). A knockout of Wnt2 gene would
induce lung hypoplasia and pulmonary hemorrhage caused by the
abnormal muscle cells (53).
Notably, miR-15a and miR-16-1 are reported to inhibit Wnt signaling
(54). Thus, we hypothesized that
both miR-15a and miR-16 play key roles in preventing the
progression of AECOPD.
In conclusion, the present study identified some
DEGs, such as NCL, GOT1, SOD2, KLF6,
XRCC5, TSPO and TMOD1, and several miRNAs
(e.g., miR-1, miR-9 and miR-23a) which may be associated with the
pathomechanism of AECOPD. Among them, SOD2, KLF6 and
XRCC5 may be involved in AECOPD due to infection via immune
system development. The present study provides in-depth knowledge
of the pathogenesis underlying the loss of muscle force during an
acute exacerbation of COPD, despite using non-experimental methods.
Since the public microarray data used in this study comes from a
small sample size - 4 male patients with acute COPD and 5 male
patients with stable COPD - it is necessary to validate our
findings using experimental methods in a Chinese population with a
larger sample size.
References
|
1
|
Vestbo J, Hurd SS, Agustí AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar
|
|
2
|
Uzun S, Djamin R, Hoogsteden H, Aerts J
and van der Eerden M: Acute exacerbations of chronic obstructive
pulmonary disease. Oncogenesis, Inflammatory and Parasitic Tropical
Diseases of the Lung. Kayembe JM: InTech. Chapter 4. pp. 77–98.
2013, http://dx.doi.org/10.5772/54867.
|
|
3
|
Carrillo A, Ferrer M, Gonzalez-Diaz G,
Lopez-Martinez A, Llamas N, Alcazar M, Capilla L and Torres A:
Noninvasive ventilation in acute hypercapnic respiratory failure
caused by obesity hypoventilation syndrome and chronic obstructive
pulmonary disease. Am J Respir Crit Care Med. 186:1279–1285. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim V, Rogers TJ and Criner GJ: New
concepts in the pathobiology of chronic obstructive pulmonary
disease. Proc Am Thorac Soc. 5:478–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woodhead M, Blasi F, Ewig S, Garau J,
Huchon G, Ieven M, Ortqvist A, Schaberg T, Torres A, van der
Heijden G, et al Joint Taskforce of the European Respiratory
Society and European Society for Clinical Microbiology and
Infectious Diseases: Guidelines for the management of adult lower
respiratory tract infections - full version. Clin Microbiol Infect.
17(Suppl 6): E1–E59. 2011. View Article : Google Scholar
|
|
7
|
Coventry PA, Gemmell I and Todd CJ:
Psychosocial risk factors for hospital readmission in COPD patients
on early discharge services: a cohort study. BMC Pulm Med.
11:492011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Swallow EB, Reyes D, Hopkinson NS, Man WD,
Porcher R, Cetti EJ, Moore AJ, Moxham J and Polkey MI: Quadriceps
strength predicts mortality in patients with moderate to severe
chronic obstructive pulmonary disease. Thorax. 62:115–120. 2007.
View Article : Google Scholar
|
|
9
|
Rabinovich RA, Bastos R, Ardite E, Llinàs
L, Orozco-Levi M, Gea J, Vilaró J, Barberà JA, Rodríguez-Roisin R,
Fernández-Checa JC and Roca J: Mitochondrial dysfunction in COPD
patients with low body mass index. Eur Respir J. 29:643–650. 2007.
View Article : Google Scholar
|
|
10
|
Perotin JM, Dury S, Renois F, Deslee G,
Wolak A, Duval V, De Champs C, Lebargy F and Andreoletti L:
Detection of multiple viral and bacterial infections in acute
exacerbation of chronic obstructive pulmonary disease: a pilot
prospective study. J Med Virol. 85:866–873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Remels AH, Gosker HR, van der Velden J,
Langen RC and Schols AM: Systemic inflammation and skeletal muscle
dysfunction in chronic obstructive pulmonary disease: state of the
art and novel insights in regulation of muscle plasticity. Clin
Chest Med. 28:537–552. vi2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ezzie ME, Crawford M, Cho JH, Orellana R,
Zhang S, Gelinas R, Batte K, Yu L, Nuovo G, Galas D, et al: Gene
expression networks in COPD: microRNA and mRNA regulation. Thorax.
67:122–131. 2012. View Article : Google Scholar
|
|
13
|
Van Pottelberge GR, Mestdagh P, Bracke KR,
Thas O, van Durme YM, Joos GF, Vandesompele J and Brusselle GG:
MicroRNA expression in induced sputum of smokers and patients with
chronic obstructive pulmonary disease. Am J Respir Crit Care Med.
183:898–906. 2011. View Article : Google Scholar
|
|
14
|
Akbas F, Coskunpinar E, Aynaci E, Oltulu
YM and Yildiz P: Analysis of serum micro-RNAs as potential
biomarker in chronic obstructive pulmonary disease. Exp Lung Res.
38:286–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lewis A, Riddoch-Contreras J, Natanek SA,
Donaldson A, Man WD, Moxham J, Hopkinson NS, Polkey MI and Kemp PR:
Downregulation of the serum response factor/miR-1 axis in the
quadriceps of patients with COPD. Thorax. 67:26–34. 2012.
View Article : Google Scholar
|
|
16
|
Crul T, Testelmans D, Spruit MA, Troosters
T, Gosselink R, Geeraerts I, Decramer M and Gayan-Ramirez G: Gene
expression profiling in vastus lateralis muscle during an acute
exacerbation of COPD. Cell Physiol Biochem. 25:491–500. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barrett T, Troup DB, Wilhite SE, Ledoux P,
Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M and
Edgar R: NCBI GEO: Mining tens of millions of expression profiles -
database and tools update. Nucleic Acids Res. 35:D760–D765. 2007.
View Article : Google Scholar
|
|
18
|
Davis S and Meltzer PS: GEOquery: a bridge
between the Gene Expression Omnibus (GEO) and BioConductor.
Bioinformatics. 14:1846–1847. 2013.
|
|
19
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: affy - analysis of Affymetrix GeneChip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gentleman RC, Carey VJ, Bates DM, Bolstad
B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al:
Bioconductor: Open software development for computational biology
and bioinformatics. Genome Biol. 5:R802004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Šmídl V and Quinn A: The variational Bayes
method in signal processing. Signals and Communication Technology.
Springer Berlin; Heidelberg: 2006
|
|
22
|
Szekely GJ and Rizzo ML: Hierarchical
clustering via joint between-within distances: extending Ward's
minimum variance method. J Classif. 22:151–183. 2005. View Article : Google Scholar
|
|
23
|
Mukherjee S, Chen Z and Gangopadhyay A: A
privacy-preserving technique for Euclidean distance-based mining
algorithms using Fourier-related transforms. VLDB J. 15:293–315.
2006. View Article : Google Scholar
|
|
24
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol. 4:32003.
View Article : Google Scholar
|
|
25
|
Jensen LJ, Kuhn M, Stark M, Chaffron S,
Creevey C, Muller J, Doerks T, Julien P, Roth A, Simonovic M, et
al: STRING 8 - a global view on proteins and their functional
interactions in 630 organisms. Nucleic Acids Res. 37:D412–D416.
2009. View Article : Google Scholar
|
|
26
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: a
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujita Y, Hayashida K, Nagai M, Inoue S,
Matsumoto H, Okabe N, Reiprich TH, Sarazin CL and Takizawa M:
Suzaku observation of the Ophiuchus galaxy cluster: one of the
hottest cool core clusters. Publ Astron Soc Jpn Nihon Tenmon
Gakkai. 60:1133–1142. 2008. View Article : Google Scholar
|
|
28
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: a
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Plaisier CL, Pan M and Baliga NS: A
miRNA-regulatory network explains how dysregulated miRNAs perturb
oncogenic processes across diverse cancers. Genome Res.
22:2302–2314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu X, Sun X, Chen C, Bai C and Wang X:
Dynamic gene expressions of peripheral blood mononuclear cells in
patients with acute exacerbation of chronic obstructive pulmonary
disease: a preliminary study. Crit Care. 18:5082014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gabellini D, Green MR and Tupler R:
Inappropriate gene activation in FSHD: a repressor complex binds a
chromosomal repeat deleted in dystrophic muscle. Cell. 110:339–348.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Deutekom JC, Wijmenga C, van Tienhoven
EA, Gruter AM, Hewitt JE, Padberg GW, van Ommen GJ, Hofker MH and
Frants RR: FSHD associated DNA rearrangements are due to deletions
of integral copies of a 3.2 kb tandemly repeated unit. Hum Mol
Genet. 2:2037–2042. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rao PK, Kumar RM, Farkhondeh M,
Baskerville S and Lodish HF: Myogenic factors that regulate
expression of muscle-specific microRNAs. Proc Natl Acad Sci USA.
23:8721–8726. 2006. View Article : Google Scholar
|
|
34
|
Puig-Vilanova E, Aguiló R,
Rodríguez-Fuster A, Martínez-Llorens J, Gea J and Barreiro E:
Epigenetic mechanisms in respiratory muscle dysfunction of patients
with chronic obstructive pulmonary disease. PLoS One.
9:e1115142014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barreiro E, de la Puente B, Minguella J,
Corominas JM, Serrano S, Hussain SN and Gea J: Oxidative stress and
respiratory muscle dysfunction in severe chronic obstructive
pulmonary disease. Am J Respir Crit Care Med. 171:1116–1124. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zelko IN, Mariani TJ and Folz RJ:
Superoxide dismutase multigene family: A comparison of the CuZn-SOD
(SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures,
evolution, and expression. Free Radic Biol Med. 33:337–349. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Togliatto G, Trombetta A, Dentelli P,
Cotogni P, Rosso A, Tschöp MH, Granata R, Ghigo E and Brizzi MF:
Unacylated ghrelin promotes skeletal muscle regeneration following
hindlimb ischemia via SOD-2-mediated miR-221/222 expression. J Am
Heart Assoc. 2:e0003762013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wilson SR, Joshi AD and Elferink CJ: The
tumor suppressor Kruppel-like factor 6 is a novel aryl hydrocarbon
receptor DNA binding partner. J Pharmacol Exp Ther. 345:419–429.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mgbemena V, Segovia J, Chang TH and Bose
S: KLF6 and iNOS regulates apoptosis during respiratory syncytial
virus infection. Cell Immunol. 283:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taccioli GE, Gottlieb TM, Blunt T,
Priestley A, Demengeot J, Mizuta R, Lehmann AR, Alt FW, Jackson SP
and Jeggo PA: Ku80: product of the XRCC5 gene and its role in DNA
repair and V(D)J recombination. Science. 265:1442–1445. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brody JS and Spira A: State of the art.
Chronic obstructive pulmonary disease, inflammation, and lung
cancer. Proc Am Thorac Soc. 3:535–537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Becker T, Vögtle FN, Stojanovski D and
Meisinger C: Sorting and assembly of mitochondrial outer membrane
proteins. Biochim Biophys Acta. 1777:557–563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Baraldo S, Turato G, Badin C, Bazzan E,
Beghé B, Zuin R, Calabrese F, Casoni G, Maestrelli P, Papi A, et
al: Neutrophilic infiltration within the airway smooth muscle in
patients with COPD. Thorax. 59:308–312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fowler VM, Greenfield NJ and Moyer J:
Tropomodulin contains two actin filament pointed end-capping
domains. J Biol Chem. 278:40000–40009. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peinado VI, Pizarro S and Barberà JA:
Pulmonary vascular involvement in COPD. Chest. 134:808–814. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kong KY1, Owens KS, Rogers JH, Mullenix J,
Velu CS, Grimes HL and Dahl R: miR-23A microRNA cluster inhibits
B-cell development. Exp Hematol. 38:629–640. e6212010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
De Palma S, Capitanio D, Vasso M,
Braghetta P, Scotton C, Bonaldo P, Lochmüller H, Muntoni F, Ferlini
A and Gelfi C: Muscle proteomics reveals novel insights into the
pathophysiological mechanisms of collagen VI myopathies. J Proteome
Res. 13:5022–5030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Thulin P, Wei T, Werngren O, Cheung L,
Fisher RM, Grandér D, Corcoran M and Ehrenborg E: MicroRNA-9
regulates the expression of peroxisome proliferator-activated
receptor δ in human monocytes during the inflammatory response. Int
J Mol Med. 31:1003–1010. 2013.PubMed/NCBI
|
|
49
|
Bazzoni F, Rossato M, Fabbri M, Gaudiosi
D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA and
Locati M: Induction and regulatory function of miR-9 in human
monocytes and neutrophils exposed to proinflammatory signals. Proc
Natl Acad Sci USA. 106:5282–5287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Packer AN, Xing Y, Harper SQ, Jones L and
Davidson BL: The bifunctional microRNA miR-9/miR-9*
regulates REST and CoREST and is downregulated in Huntington's
disease. J Neurosci. 28:14341–14346. 2008. View Article : Google Scholar
|
|
51
|
Otaegi G, Pollock A, Hong J and Sun T:
MicroRNA miR-9 modifies motor neuron columns by a tuning regulation
of FoxP1 levels in developing spinal cords. J Neurosci. 31:809–818.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Reuter S, Beckert H and Taube C: Take the
Wnt out of the inflammatory sails: modulatory effects of Wnt in
airway diseases. Lab Invest. 2:177–185. 2015.
|
|
53
|
Goss AM, Tian Y, Cheng L, Yang J, Zhou D,
Cohen ED and Morrisey EE: Wnt2 signaling is necessary and
sufficient to activate the airway smooth muscle program in the lung
by regulating myocardin/Mrtf-B and Fgf10 expression. Dev Biol.
356:541–552. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen CH, Dixon RA, Ke LY and Willerson JT:
Vascular progenitor cells in diabetes mellitus: roles of Wnt
signaling and negatively charged low-density lipoprotein. Circ Res.
9:1038–1040. 2009. View Article : Google Scholar
|