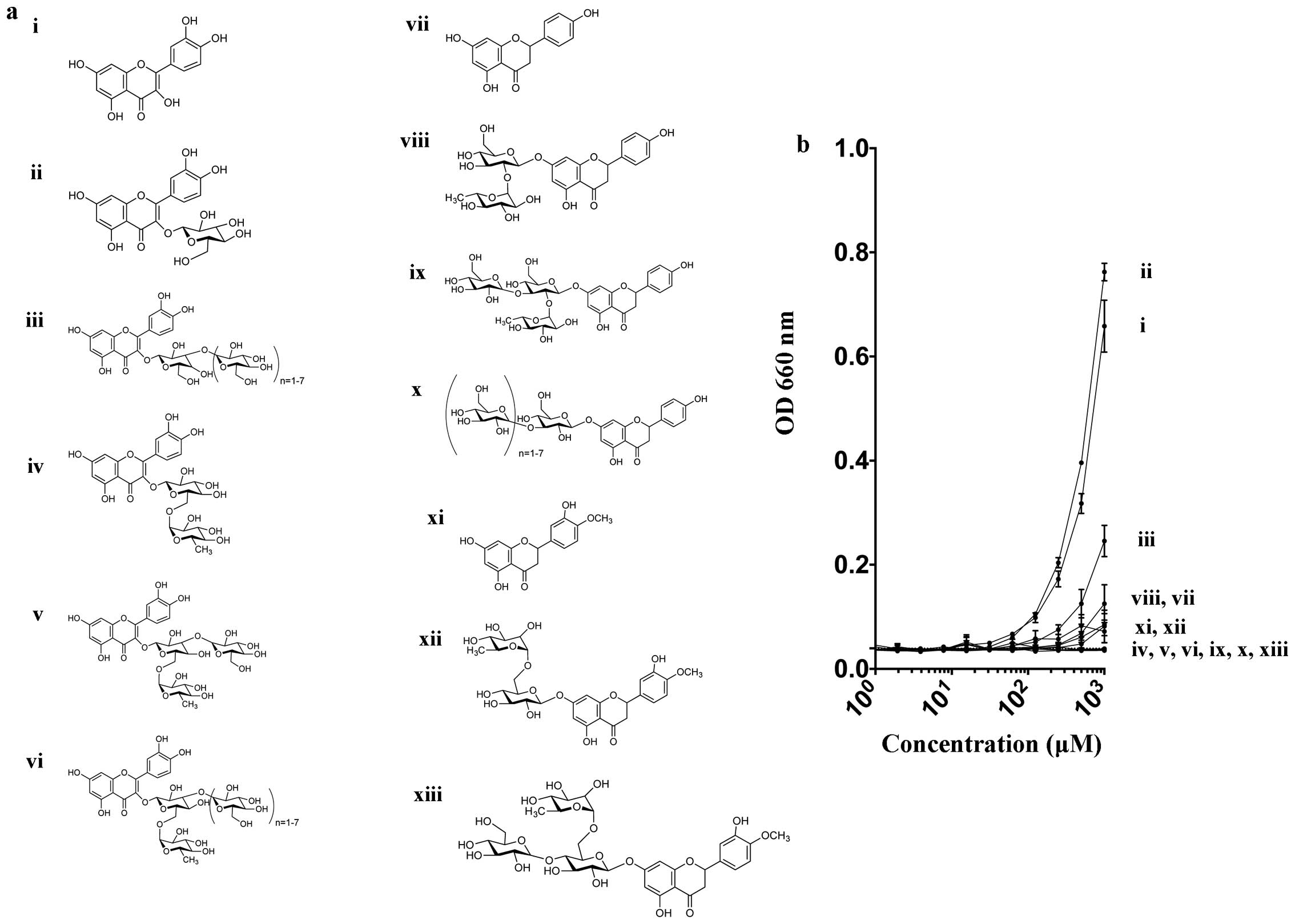
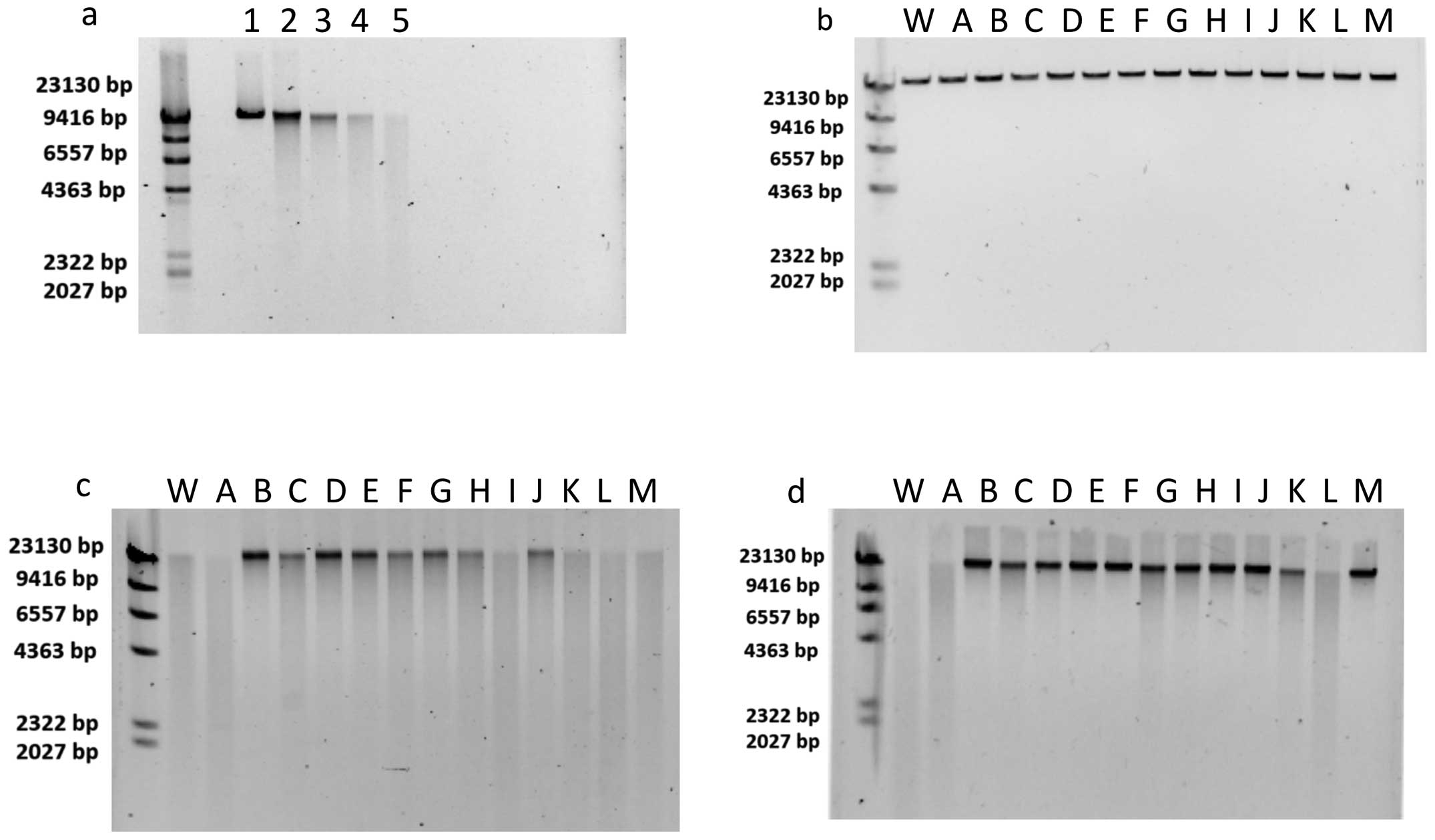
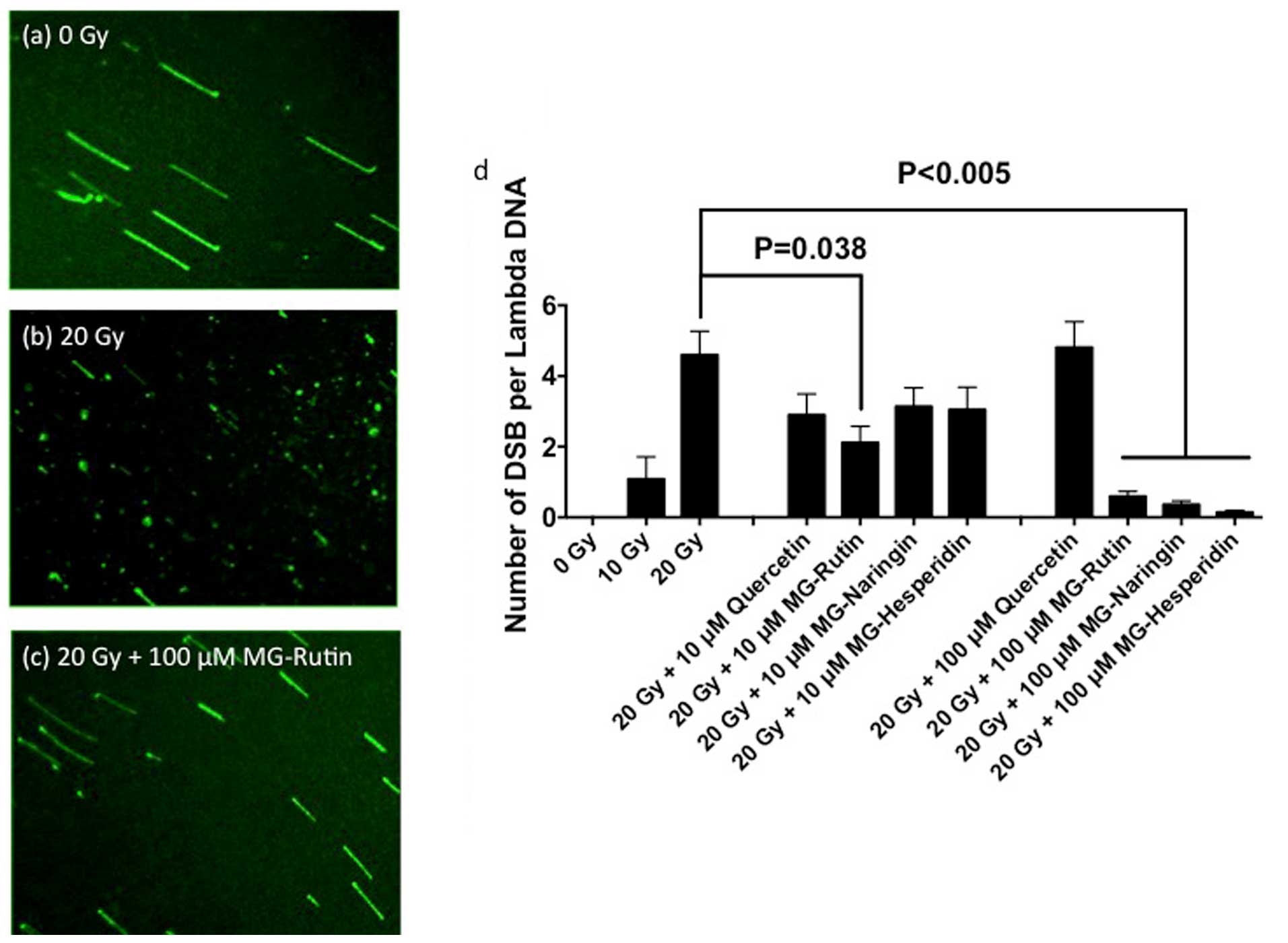
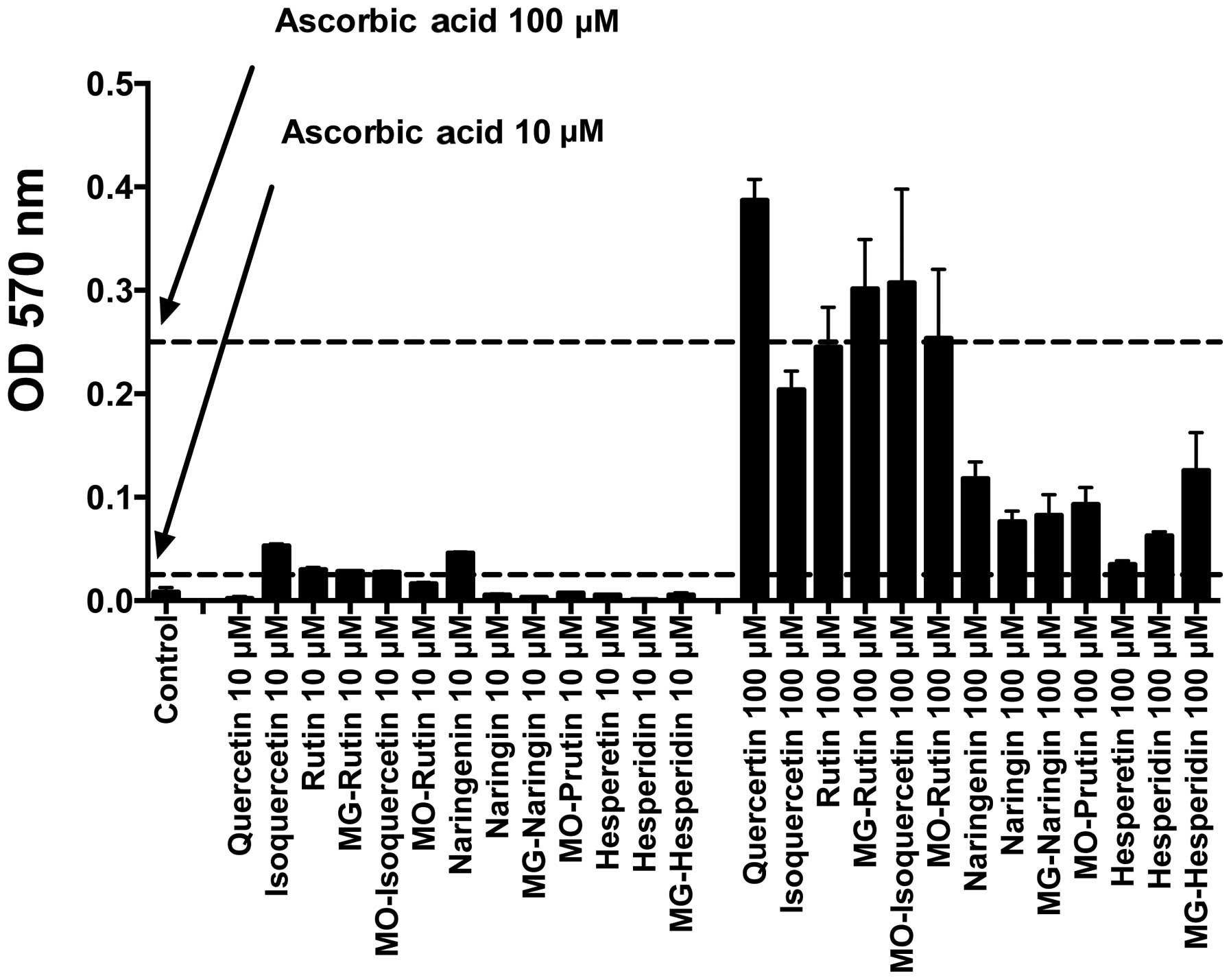
|
1
|
Harman D: Aging: a theory based on free
radical and radiation chemistry. J Gerontol. 11:298–300. 1956.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ward JF: DNA damage produced by ionizing
radiation in mammalian cells: identities, mechanisms of formation,
and reparability. Prog Nucleic Acid Res Mol Biol. 35:95–125. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lobo V, Patil A, Phatak A and Chandra N:
Free radicals, antioxidants and functional foods: impact on human
health. Pharmacogn Rev. 4:118–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Best BP: Nuclear DNA damage as a direct
cause of aging. Rejuvenation Res. 12:199–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andersson DI, Slechta ES and Roth JR:
Evidence that gene amplification underlies adaptive mutability of
the bacterial lac operon. Science. 282:1133–1135. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baumann P and West SC: Role of the human
RAD51 protein in homologous recombination and double-stranded-break
repair. Trends Biochem Sci. 23:247–251. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar S and Pandey AK: Chemistry and
biological activities of flavonoids: an overview. Scientific World
Journal. 2013:1627502013. View Article : Google Scholar
|
|
8
|
Harborne JB and Paxman GJ: Genetics of
anthocyanin product in the radish. Hered Edinb. 19:505–506. 1964.
View Article : Google Scholar
|
|
9
|
Thompson M, Williams CR and Elliot GE:
Stability of flavonoid complexes of copper(II) and flavonoid
antioxidant activity. Anal Chim Acta. 85:375–381. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Svobodová A, Psotová J and Walterová D:
Natural phenolics in the prevention of UV-induced skin damage. A
review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
147:137–145. 2003. View Article : Google Scholar
|
|
11
|
Rahman K: Studies on free radicals,
antioxidants, and co-factors. Clin Interv Aging. 2:219–236.
2007.PubMed/NCBI
|
|
12
|
Plumb GW, De Pascual-Teresa S,
Santos-Buelga C, Cheynier V and Williamson G: Antioxidant
properties of catechins and proanthocyanidins: effect of
polymerisation, galloylation and glycosylation. Free Radic Res.
29:351–358. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimoi K, Yoshizumi K, Kido T, Usui Y and
Yumoto T: Absorption and urinary excretion of Quercetin, Rutin, and
alphaG-Rutin, a water soluble flavonoid, in rats. J Agric Food
Chem. 51:2785–2789. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Engen A, Maeda J, Wozniak DE, Brents CA,
Bell JJ, Uesaka M, Aizawa Y and Kato TA: Induction of cytotoxic and
genotoxic responses by natural and novel Quercetin glycosides.
Mutat Res Genet Toxicol Environ Mutagen. 784–785:15–22. 2015.
View Article : Google Scholar
|
|
15
|
Maeda J, Roybal EJ, Brents CA, Uesaka M,
Aizawa Y and Kato TA: Natural and glucosyl flavonoids inhibit
poly(ADP-ribose) polymerase activity and induce synthetic lethality
in BRCA mutant cells. Oncol Rep. 31:551–556. 2014.
|
|
16
|
Sunada S, Fujisawa H, Cartwright IM, Maeda
J, Brents CA, Mizuno K, Aizawa Y, Kato TA and Uesaka M:
Monoglucosyl-Rutin as a potential radioprotector in mammalian
cells. Mol Med Rep. 10:10–14. 2014.PubMed/NCBI
|
|
17
|
Chandrasekharan DK, Kagiya TV and Nair
CKK: Radiation protection by 6-palmitoyl ascorbic acid-2-glucoside:
studies on DNA damage in vitro, ex vivo, in vivo and oxidative
stress in vivo. J Radiat Res (Tokyo). 50:203–212. 2009. View Article : Google Scholar
|
|
18
|
Kobashigawa S, Kashino G, Suzuki K,
Yamashita S and Mori H: Ionizing radiation-induced cell death is
partly caused by increase of mitochondrial reactive oxygen species
in normal human fibroblast cells. Radiat Res. 183:455–464. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kemp LM, Sedgwick SG and Jeggo PA: X-ray
sensitive mutants of Chinese hamster ovary cells defective in
double-strand break rejoining. Mutat Res. 132:189–196.
1984.PubMed/NCBI
|
|
20
|
Wlodek D and Olive PL: Physical basis for
detection of DNA double-strand breaks using neutral filter elution.
Radiat Res. 124:326–333. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olive PL, Wlodek D and Banáth JP: DNA
double-strand breaks measured in individual cells subjected to gel
electrophoresis. Cancer Res. 51:4671–4676. 1991.PubMed/NCBI
|
|
22
|
Kato TA, Okayasu R, Jeggo PA and Fujimori
A: ASPM influences DNA double-strand break repair and represents a
potential target for radiotherapy. Int J Radiat Biol. 87:1189–1195.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujii Y, Genet MD, Roybal EJ, Kubota N,
Okayasu R, Miyagawa K, Fujimori A and Kato TA: Comparison of the
bromodeoxyuridine-mediated sensitization effects between low-LET
and high-LET ionizing radiation on DNA double-strand breaks. Oncol
Rep. 29:2133–2139. 2013.PubMed/NCBI
|
|
24
|
Oshige M, Yamaguchi K, Matsuura S, Kurita
H, Mizuno A and Katsura S: A new DNA combing method for biochemical
analysis. Anal Biochem. 400:145–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karioti A, Hadjipavlou-Litina D, Mensah
ML, Fleischer TC and Skaltsa H: Composition and antioxidant
activity of the essential oils of Xylopia aethiopica (Dun) A. Rich.
(Annonaceae) leaves, stem bark, root bark, and fresh and dried
fruits, growing in Ghana. J Agric Food Chem. 52:8094–8098. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bansal V, Sharma A, Ghanshyam C and Singla
ML: Coupling of chromatographic analyses with pretreatment for the
determination of bioactive compounds in Emblica officinalis juice.
Anal Methods. 6:410–418. 2014. View Article : Google Scholar
|
|
27
|
Solimani R: The flavonols Quercetin, Rutin
and morin in DNA solution: UV-vis dichroic (and mid-infrared)
analysis explain the possible association between the biopolymer
and a nucleophilic vegetable-dye. Biochim Biophys Acta.
1336:281–294. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujimori M, Kadota K, Shimono K, Shirakawa
Y, Sato H and Tozuka Y: Enhanced solubility of Quercetin by forming
composite particles with transglycosylated materials. J Food Eng.
149:248–254. 2015. View Article : Google Scholar
|
|
29
|
Khanna KK and Jackson SP: DNA
double-strand breaks: signaling, repair and the cancer connection.
Nat Genet. 27:247–254. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoo HB, Lim HM, Yang I, Kim SK and Park
SR: Flow cytometric investigation on degradation of macro-DNA by
common laboratory manipulations. J Biophys Chem. 2:102–111. 2011.
View Article : Google Scholar
|