Introduction
Urotensin II (UII), the most potent vasoconstrictor
known, was first recognized for its constrictive and natriuretic
properties in fish almost 40 years ago. The UII peptide was
subsequently isolated from frog brain and, later, the prepro-UII
cDNA was characterized in various mammals, including humans. In the
central nervous system (CNS) of tetrapods, UII is expressed
primarily in the motor neurons of the brainstem and spinal cord.
The biological functions of UII are mediated through a G
protein-coupled receptor, termed UT, that exhibits high sequence
similarity with the somatostatin receptors. The UT gene is widely
expressed in the CNS and in peripheral organs. Consistent with
their broad distribution, UT and UII exert a broad range of
behavioral effects and regulate endocrine, cardiovascular, kidney
and immune functions (1,2).
Diabetic nephropathy (DN) is a severe microvascular
complication commonly observed in patients with diabetes, and is
also the leading cause of end-stage renal disease (ESRD).
Tubulointerstitial nephropathy is closely associated with the
impairment of renal function in the pathogenesis of diabetic
nephropathy. Advanced glycation end products (AGEs) are formed by
the Maillard process, a non-enzymatic reaction between ketones or
aldehydes and the amino groups of proteins, lipids and nucleic
acids, and are known to contribute to the aging of macromolecules.
Under conditions of hyperglycemia and/or oxidative stress, this
process begins with the conversion of reversible Schiff base
adducts to more stable, covalently-bound Amadori rearrangement
products. AGEs play important roles in the pathogenesis of diabetic
nephropathy by upregulating the expression of cytokines in tubular
epithelial cells and stimulating the accumulation of extracellular
matrix (ECM) (3,4). UII is a potent vasoconstrictor
peptide, which promotes proliferation and ECM synthesis, in both an
autocrine and paracrine manner in renal epithelial cells (5,6).
Elevated UII levels have been detected in the plasma of patients
with diabetes (7). In addition,
the UII and UT genes have been shown to be upregulated in both the
aorta and kidneys of non-obese diabetic rats (8). Peaks in the renal expression levels
of UII and UT have been observed in epithelial cells of the distal
tubule, the proximal tubule and collecting tubule epithelial cells
(9), suggesting that UII may be
involved in the etiology of DN.
Transforming growth factor β1 (TGF-β1) is expressed
primarily in the kidneys and has been shown to promote renal
fibrosis (10,11). Dai et al (12) found that TGF-β1 modulated the
pro-fibrogenic effects of UII in neonatal cardiac fibroblasts via
UT. However, the specific mechanisms through which UII promotes the
synthesis of ECM by tubular epithelial cells remain unknown. This
study aimed to determine the fibrotic effects of UII in cultured
rat proximal renal tubular epithelial cells (NRK-52E cells) in the
presence of AGEs, and to identify the signaling pathways involved
in these effects.
Materials and methods
Preparation of AGE-bovine serum albumin
(BSA)
Briefly, BSA and glucose were dissolved in
phosphate-buffered saline (PBS) at final concentrations of 0.5
mol/l of glucose and 10 mg/ml of BSA. The solution was sterilized
by ultrafiltration, incubated at 37°C for 90 days, and finally
dialyzed against PBS to remove free glucose. As a control, BSA was
incubated in parallel without D-glucose. No endotoxin was
detectable in these preparations. The levels of AGE-BSA and BSA
were 15.1 and 4.3 arbitrary units detected by spectrofluorimetry
[using a fluorescence photometer (INFINITE 200 PRO; Tecan Austria
GmbH, Grödig, Austria)] with an excitation wavelength of 370 nm, an
emission wavelength of 440 nm, and a split of 3 nm.
Cell culture and experimental design
Rat proximal tubular epithelial cells (NRK-52E
cells) were purchased from the Stem Cell Bank, Chinese Academy of
Sciences, Shanghai, China. The cells were resuspended in Dulbecco's
modified Eagle's medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 20% FBS and 100 IU/ml antibiotics. A suspension
of NRK-52E cells was plated into tissue culture flasks and
incubated at 37°C in 5% CO2.
Effects of UII on the proliferation of
NRK-52E cells
The cells were cultured for 48 h with various
concentrations (10−10, 10−9, 10−8
and 10−7 mol/l) of UII (Sigma-Aldrich). Nimodipine
(Bayer, Leverkusen, Germany)-pre-treated cells were incubated with
10−5 mol/l nimodipine for 5 min and then cultured with
10−8 mol/l UII for 48 h. The EDTA-treated cells were
pre-treated with EDTA for 30 min and then cultured with
10−8 mol/l UII for 48 h. For the final 2 h of the
culture period, 10−4 mol/l 5-bromodeoxyuridine (BrdU;
Sigma-Aldrich) were added before the cells and the supernatant were
collected. The cells in the control group were cultured for 48 h
without being subjected to any treatment.
Effects of AGEs on the protein expression
of UII in NRK-52E cells
The cells were cultured with AGE-BSA (100 mg/l) or
BSA (control) and serum-free DMEM (blank control) for 48 h and then
collected for western blot analysis.
Effects of AGEs on the UII mRNA
expression and protein secretion of fibronectin (FN) and collagen
(Col)IV in NRK-52E cells
To examine the effects of AGE-BSA at various
concentrations, the cells were cultured with 0, 25, 50, 100 and 200
mg/l AGE-BSA or BSA (control). The cells and supernatants were
collected at 48 h post-treatment. In a separate time-course
experiment, the cells were cultured with AGE-BSA at 100 mg/l or BSA
(control). The cells and supernatant were collected at 0, 2, 8, 16,
24 or 48 h post-treatment.
Effects of UII on the expression of
TGF-β1, FN, and ColIV in NRK-52E cells
The cells were cultured with 10−8 mol/l
UII for 48 h. The urantide-pre-treated cells were pre-treated with
urantide (10−6 mol/l; Shanghai Huada Tianyuan Biology
Co., Ltd., Shanghai, China) for 30 min and then cultured with
10−8 mol/l UII for 48 h. The anti-TGF-β1
antibody-pre-treated cells were pre-treated with anti-TGF-β1
antibody (10 µg/ml; monoclonal nouse; Cat no. MAB240,
R&D Systems Inc., Minneapolis, MN, USA) for 30 min and then
cultured with 10−8 mol/l UII for 48 h. The cells and
culture supernatants were collected. The cells in the control group
were cultured for 48 h without any special treatment.
Flow cytometry
The cells were released with 0.25% trypsin, washed
with PBS and fixed in 70% ethanol at 4°C overnight. The cells were
collected by centrifugation (1000 × g) and washed with PBS. The
cells were resuspended in PBS. The cell concentration was adjusted
to 1.0×106 and RNaseA was added, at 37°C in a water bath
for 30 min and then stained with propidium iodide at 4°C for 30 min
in the dark. The distribution of the cells was analyzed using
Modfit LT 3.0 software (BD FACSCanto II Flow Cytometer, BD
Biosciences, San Jose, CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
The medium was collected from the cultured NRK-52E
cells with or without the treatments described above and
centrifuged (1000 × g, 4°C) immediately. The supernatant was then
assayed using a BrdU ELISA kit (Sigma-Aldrich) or ELISA kits for FN
and ColIV (R&D Systems Inc.) according to the manufacturer's
instructions. The absorbance of the colored products was determined
using a microplate reader (VERSA max, sn: BNR05706; Molecular
Devices, Sunnyvale, CA, USA) set to 490 nm.
RT-PCR
Total RNA was extracted from the cultured NRK-52E
cells using TRIzol reagent (Gibco Life Technologies, Carlsbad, CA,
USA). Primers for UII, TGF-β1, FN, ColIV and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed and
synthesized by Shanghai Biological Engineering (Shanghai, China).
The sequences of these primers are presented in Table I.
 | Table IUpstream and downstream primers for
UII, TGF-β1, FN, Col IV and GAPDH. |
Table I
Upstream and downstream primers for
UII, TGF-β1, FN, Col IV and GAPDH.
| Primer | Sequence | Length (bp) |
|---|
| UII sense |
5′-TGCCTGCTCTTCGTAGGACT-3′ | 242 |
| UII antisense |
5′-AGAGCCTTCCTCAAGCTT-3′ | |
| TGF-β1 sense |
5′-CCAAGGAGACGGAATACAGG-3′ | 412 |
| TGF-β1
antisense |
5′-GTGTTGGTTGTAGAGGGCAAG-3′ | |
| FN sense |
5′-CCTTTCTGAGCAGCAACC-3′ | 353 |
| FN antisense |
5′-AAGGACCACAGGAGCAGT-3′ | |
| Col IV sense |
5′-CTTCGCCTCCAGGAACGA-3′ | 401 |
| Col IV
antisense |
5′-TGGGCTTCTTGAACATCTCG-3′ | |
| GAPDH sense |
5′-ACCACAGTCCATGCCATCAC-3′ | 450 |
| GAPDH
antisense |
5′-TCCACCACCCTGTTGCTGTA-3′ | |
Total RNA (0.5 µg) was amplified using the
Titan™ One Tube RT-PCR kit (Boehringer-Mannheim, Shanghai, China).
Twenty-five cycles of replication were used. The products were
separated by agarose gel electrophoresis and visualized by ethidium
bromide staining. Bands were digitized using a Tanon-1000 gel image
system (Shanghai, China). The ratios of UII, TGF-β1, FN and ColIV
band density to GAPDH band density in the various groups are
presented.
Western blot analysis
The protein level of UII was analyzed by western
blot analysis. Equal amounts of lysates were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto Bio-Rad Trans-Blot nitrocellulose membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were
incubated in blocking buffer (Tris-buffered saline containing 0.1%
polysorbate 20 and 5% non-fat dried milk) for 1 h at room
temperature followed by incubation with the appropriate primary
antibody (anti-UII; 1:1,000 dilution; polyclonal goat, Cat. no.
sc-21096; Santa Cruz Biotechnology, Inc.; Santa Cruz, CA, USA)
overnight at 4°C with gentle shaking. The membranes were washed 3
times (15 min each) with washing buffer (Tris-buffered saline
containing 0.1% polysorbate 20) and incubated with goat anti-mouse
secondary antibody (1:2,000 dilution; rabbit-anti-goat, Cat. no.
sc-2031; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. After washing as described above, the protein of
interest was detected using enhanced chemiluminescence reagents
from Amresco (Solon, OH, USA). Protein expression levels are
expressed as a ratio to GAPDH (polyclonal rabbit, Cat. no. 2118;
Cell Signaling Technology, Danvers, MA, USA) levels. Protein bands
were detected and analyzed with a gel imaging system.
Statistical analysis
All data were analyzed using the statistical
software package SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and are
expressed as the means ± standard deviation. Comparisons were
performed using one-way and two-way ANOVA. A P-value of <0.05
was considered to indicate a statistically significant
difference.
Results
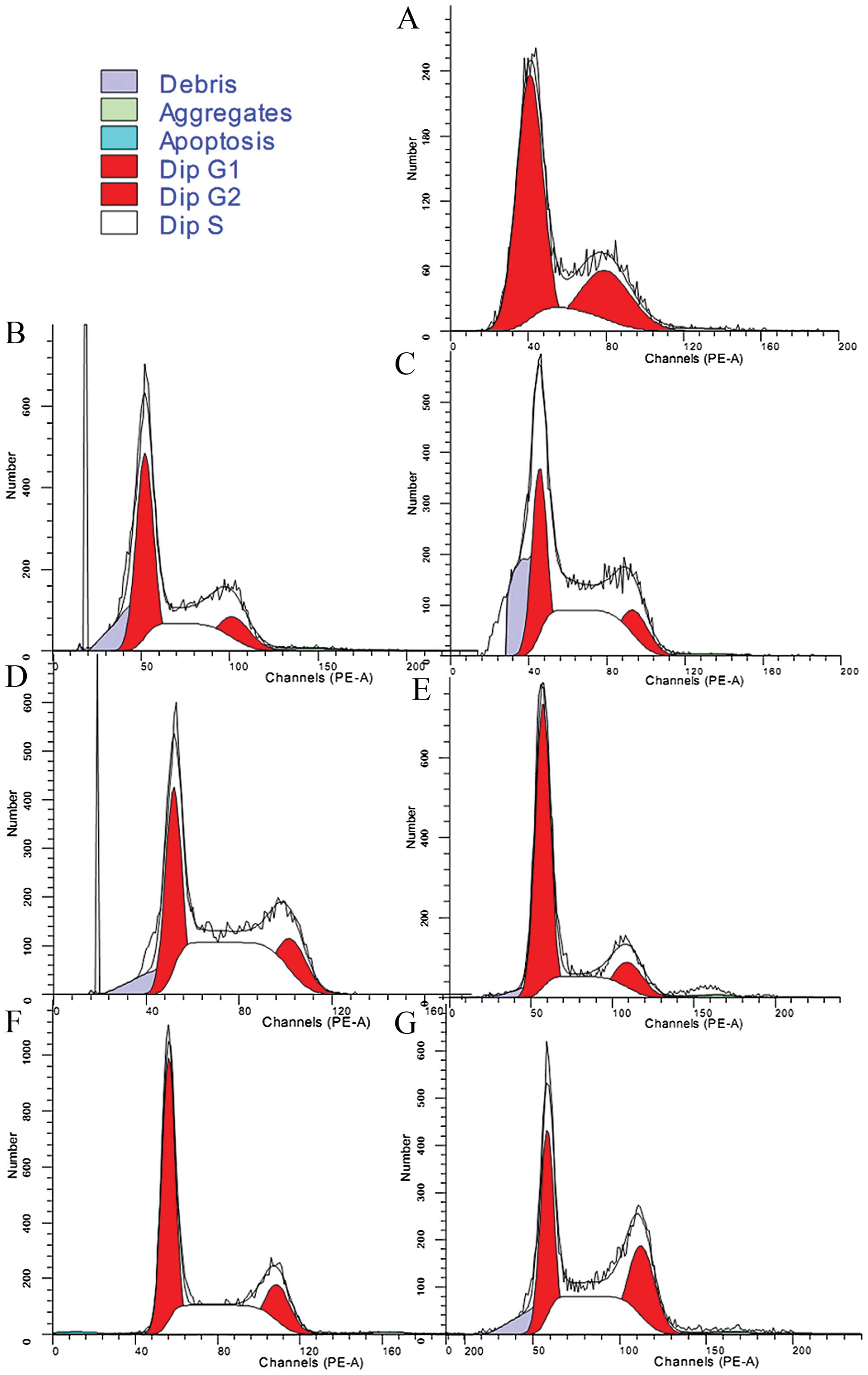
UII stimulates the proliferation of
NRK-52E cells
The cell proliferation cycle includes 4 phases, the
G1, S, G2 and M phases; the S phase is the DNA synthesis phase and
increased cell proliferation was indicated by an increase in the
number of cells in the S phase. UII promoted the proliferation of
the NRK-52E cells in a concentration-dependent manner following
treatment at 10−10 to 10−8 mol/l.
Unexpectedly, treatment with UII at 10−7 mol/l failed to
significantly increase cell proliferation relative to the controls
(Table II and Fig. 1). The promoting effect of UII on
cell proliferation was partially blocked by treatment with
nimodipine and EDTA (Fig. 1 and
Table III).
 | Table IIEffects of UII at various
concentrations on the proliferation of NRK-52E cells. |
Table II
Effects of UII at various
concentrations on the proliferation of NRK-52E cells.
| Group | n | Value of A | G1 Phase (%) | S Phase (%) | G2 Phase (%) |
|---|
| Control | 6 | 0.556±0.039 | 56.46±2.88 | 20.46±4.44 | 23.56±3.75 |
| 10−10
mol/l | 6 | 0.491±0.038a | 49.63±3.06 | 26.96±3.35a | 22.75±3.36 |
| 10−9
mol/l | 6 | 0.281±0.037a | 37.48±2.06 | 44.26±3.28a | 18.12±1.82 |
| 10−8
mol/l | 6 | 0.291±0.023a | 34.03±1.92 | 48.12±2.22a | 18.18±1.81 |
| 10−7
mol/l | 6 | 0.524±0.035 | 63.33±2.46 | 20.85±2.21 | 16.81±2.46 |
 | Table IIIEffects of nimodipine and EDTA on the
proliferation of NRK-52E cells induced by UII. |
Table III
Effects of nimodipine and EDTA on the
proliferation of NRK-52E cells induced by UII.
| Group | n | Value of A | G1 Phase (%) | S Phase (%) | G2 Phase (%) |
|---|
| Control | 6 | 0.556±0.039 | 56.46±2.88 | 20.46±4.44 | 23.56±3.75 |
| 10−8
mol/l UII | 6 | 0.291±0.023a | 34.03±1.92 | 48.12±2.22a | 18.18±1.81 |
| 10−8 UII
+ Nim | 6 | 0.466±0.037a,b | 46.91±1.67 | 34.45±2.83a,b | 17.91±1.44 |
| 10−8 UII
+ EDTA | 6 | 0.451±0.035a,b | 47.51±1.22 | 32.90±2.52a,b | 19.40±2.15 |
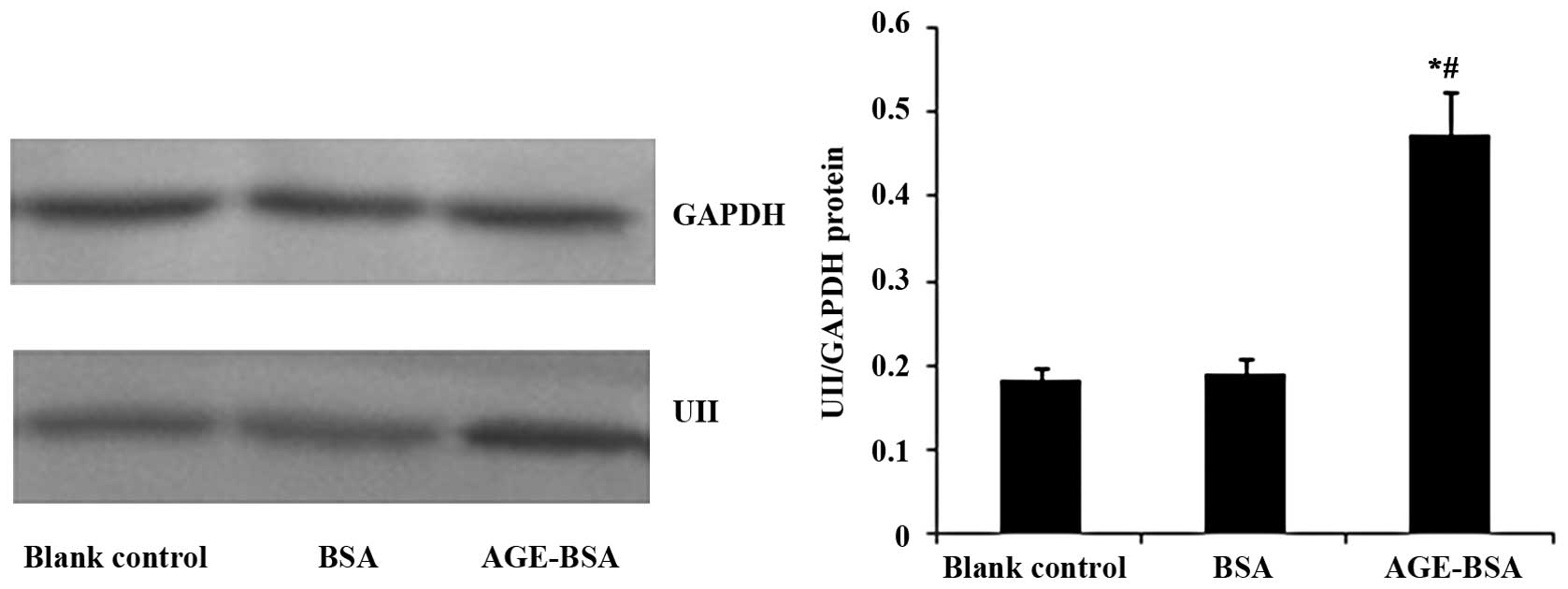
AGE-BSA upregulates the protein
expression of UII in NRK-52E cells
The AGE-BSA-stimulated UII protein expression in
NRK-52E cells was examined by western blot analysis. AGE-BSA
upregulated UII protein expression in the NRK-52E cells compared
with the blank control and BSA groups (Fig. 2).
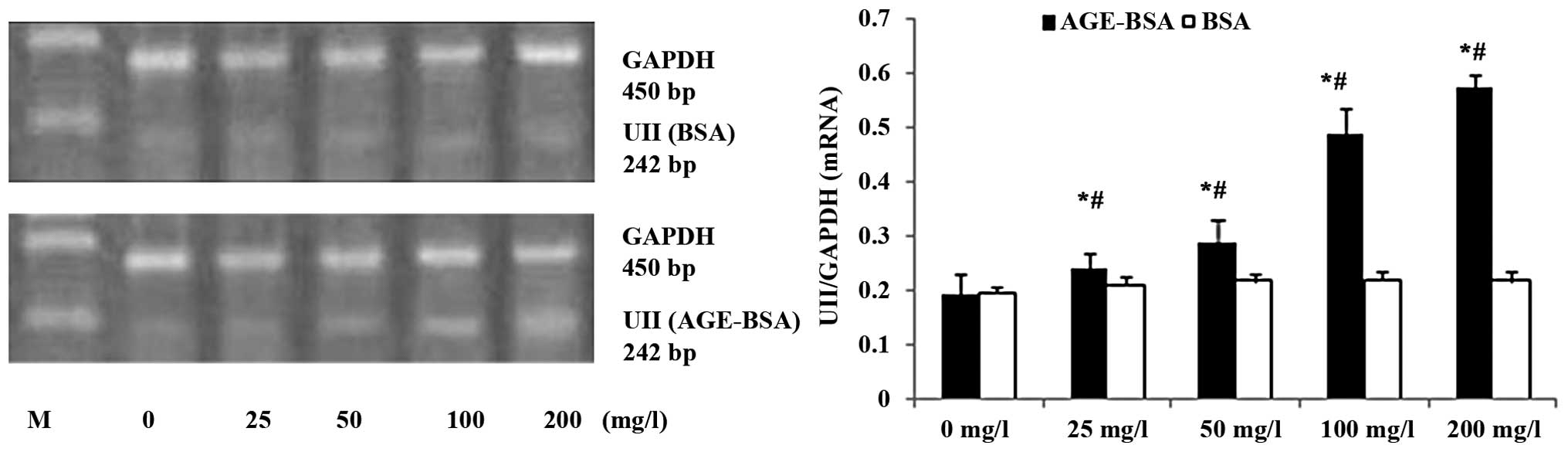
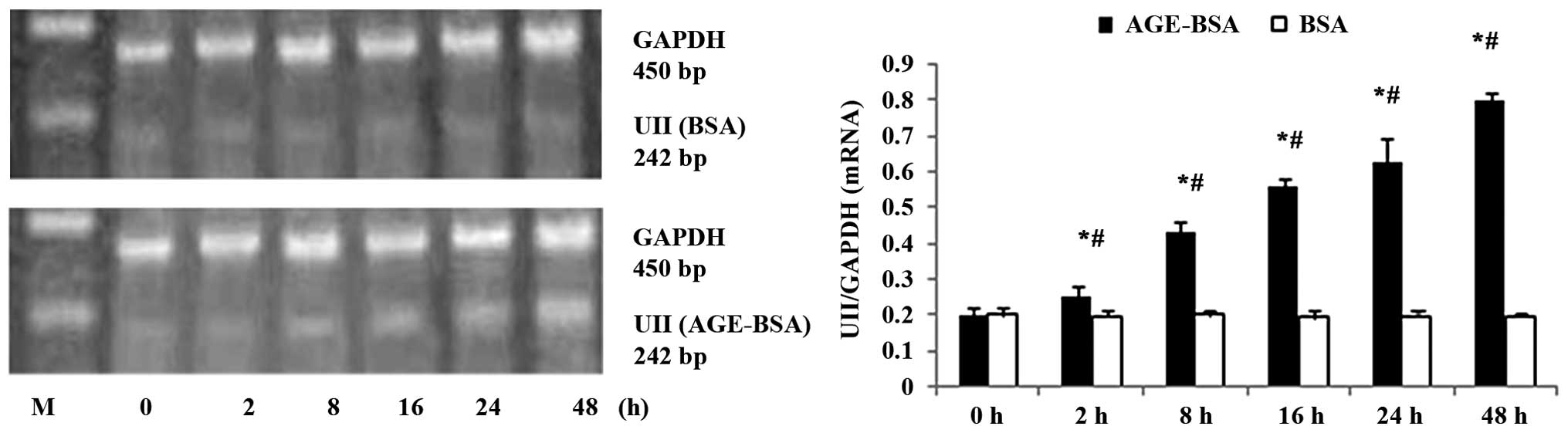
AGE-BSA upregulates mRNA expression of
UII in NRK-52E cells
AGE-BSA upregulated UII mRNA expression in the
NRK-52E cells in a concentration-dependent manner, and the NRK-52E
cells treated with 100 mg/l AGE-BSA exhibited a time-dependent
increase in UII mRNA expression between 2 and 48 h of treatment
(Figs. 3 and 4).
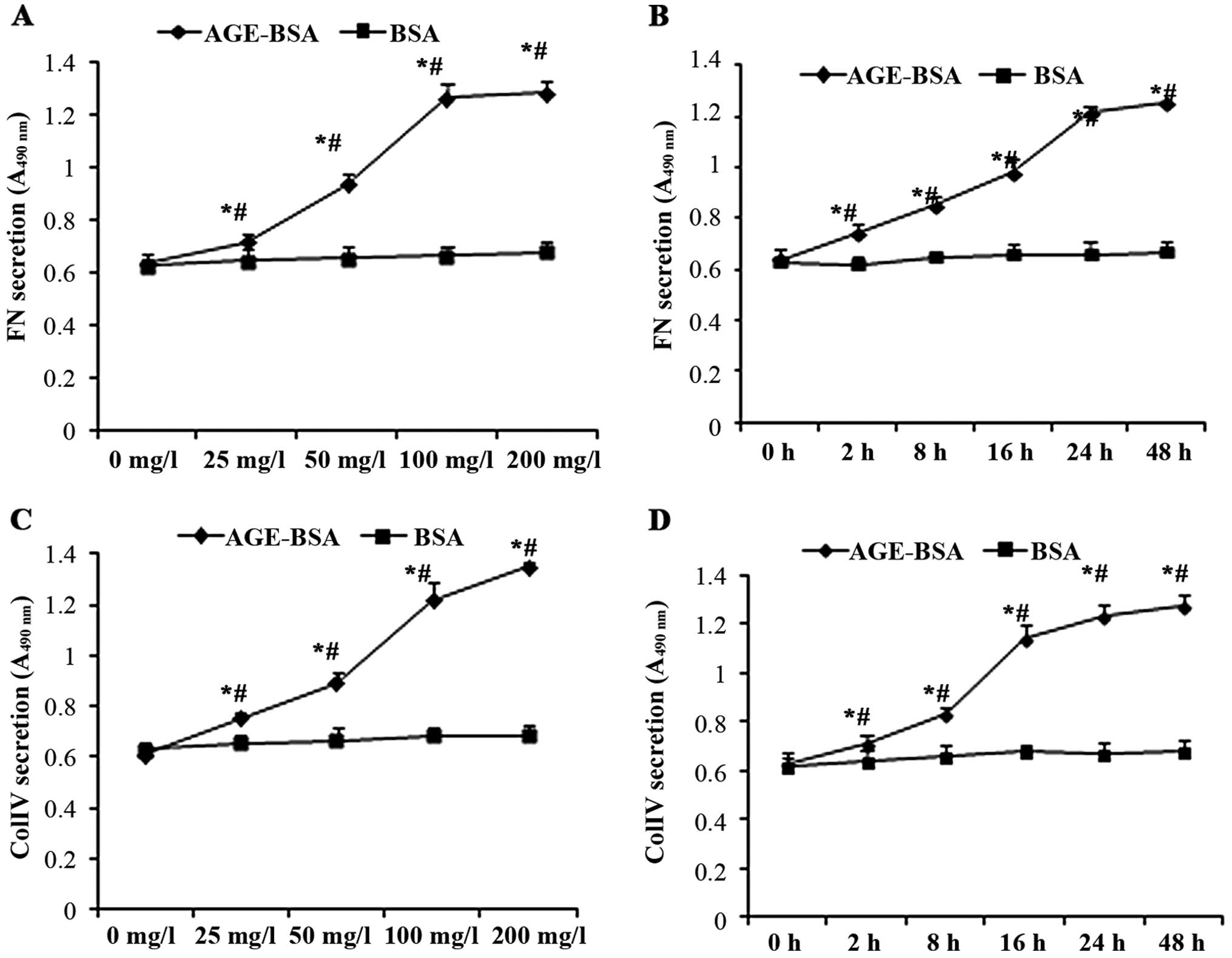
AGE-BSA stimulates the secretion of FN
and ColIV by NRK-52E cells
The concentrations of FN and ColIV in the
supernatant of NRK-52E cells were increased following treatment
with AGE-BSA in a time- and concentration-dependent manner compared
with the control (BSA-treated cells) (Fig. 5).
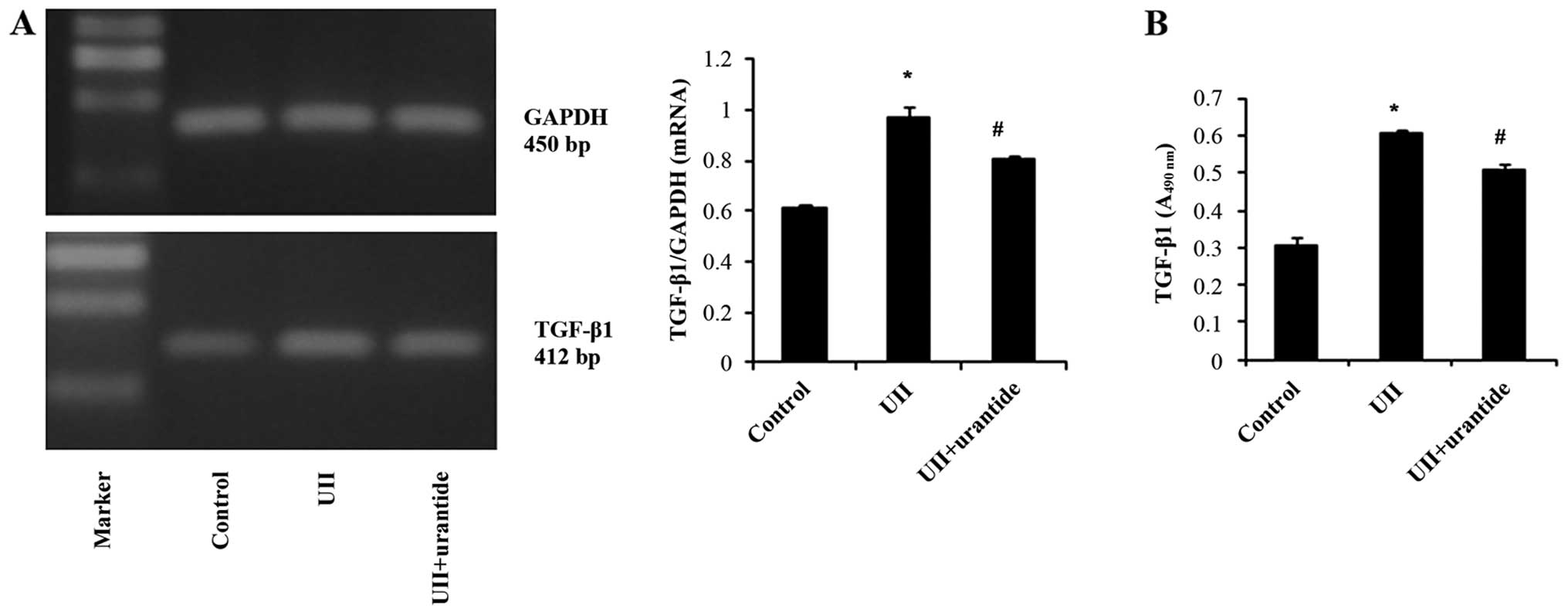
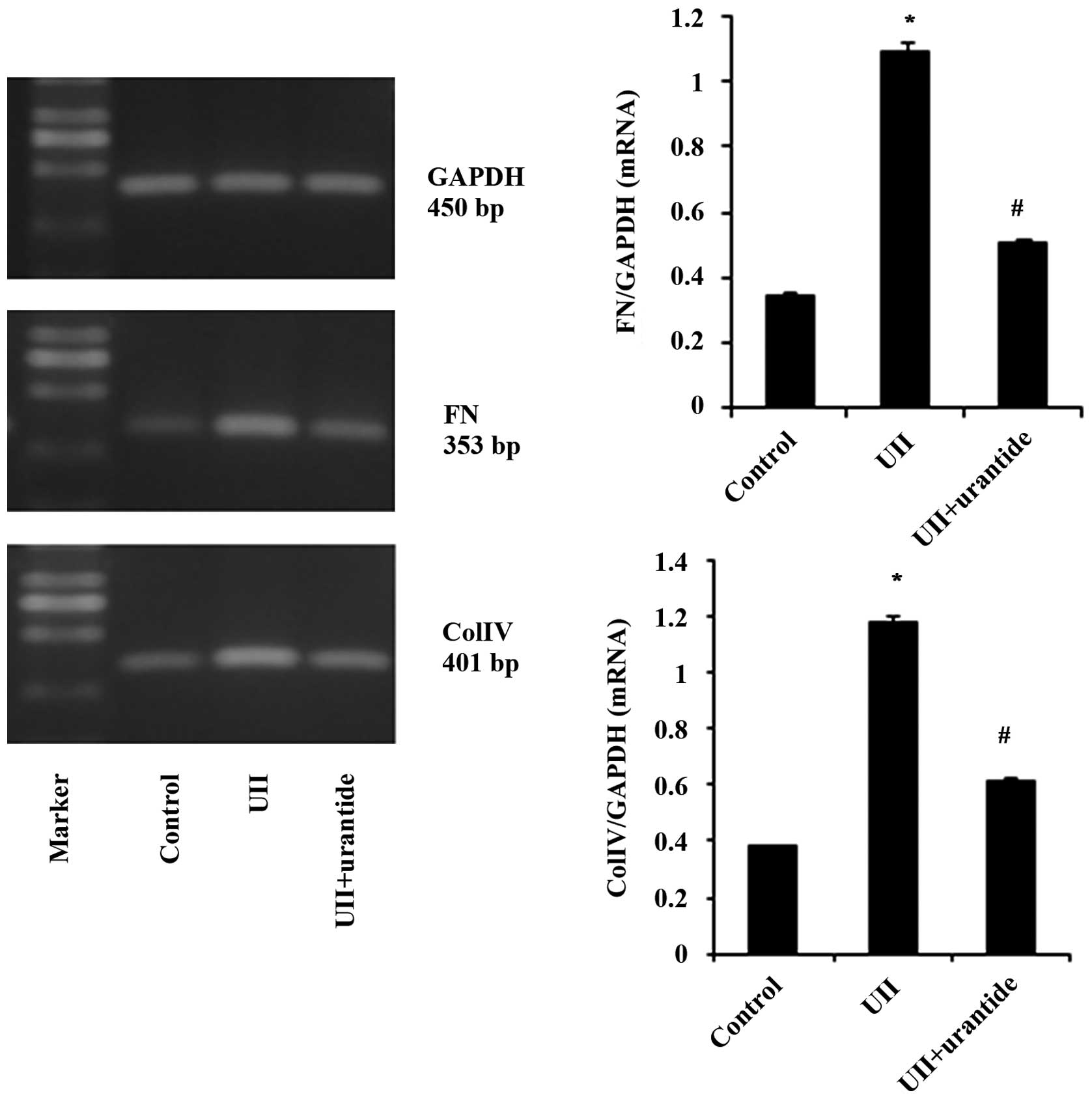
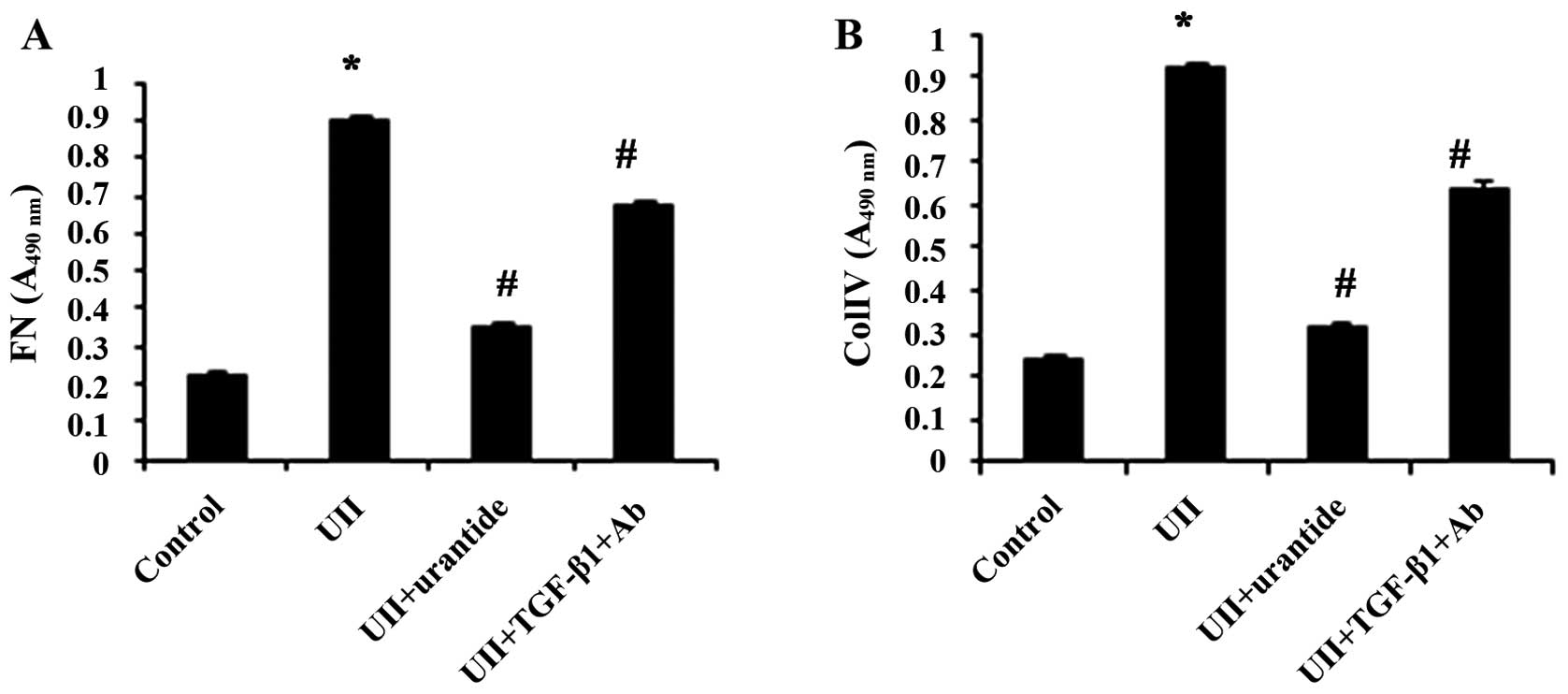
UII upregulates the mRNA expression and
protein secretion of TGF-β1, FN and ColIV in NRK-52E cells
The NRK-52E cells treated with UII exhibited an
increased protein and mRNA expression of TGF-β1, as well as a
concomitant upregulation of mRNA expression and protein secretion
of FN and ColIV. Urantide attenuated these effects, while
anti-TGF-β1 antibody also inhibited the UII-stimulated secretion of
FN and ColIV (Figs. 6Figure 7–8).
Discussion
UII, a somatostatin-like vasoconstrictor peptide
initially isolated from fish urophysis, has been identified in
mammals, and in particular, in the nervous system, cardiovascular
tissues and kidneys (13,14). Studies showing that UII promotes
cell proliferation and ECM accumulation (15–18) have been confirmed in airway and
vascular smooth muscle cells, renal epithelial cells and renal
carcinoma cell lines (19–24).
Early in vitro studies suggested that UII was
an autocrine and paracrine growth factor for renal epithelial
cells, acting via a mechanism encompassing the activation of both
the protein kinase C (PKC) and extracellular signal-regulated
kinase (ERK)1/2 pathways, as well as Ca2+ influx via
voltage-dependent Ca2+ channels (25). The present study demonstrated that
UII increased the percentage of cells in the S phase in cultures of
NRK-52E cells at concentrations between
10−10–10−8 mol/l. This promoting effect on
proliferation was not observed with UII at a concentration of
10−7 mol/l, possibly reflecting the saturation of UT.
The attenuation of the promoting effect of UII on proliferation by
nimodipine and EDTA, which reduce the influx of extracellular
calcium, confirmed that this action is mediated by the influx of
extracellular calcium ions.
It has been shown that the proximal renal tubule
reabsorbs large quantities of AGEs when blood AGE concentrations
are elevated. AGEs can potentially alter the structure and function
of the kidneys, leading to increased glomerular hyperfiltration,
basement membrane thickness, glomerulosclerosis and/or
tubulointerstitial fibrosis in diabetes. Moreover, several in
vivo and in vitro studies have implicated TGF-β in
AGE-induced renal damage in diabetes (26–28). EMC proteins, such as ColI, III, V
and VII, and FN are normally distributed in the renal interstitium,
while others such as laminin and ColIV are normally expressed in
the basal membrane of the tubules. The expression of FN has been
shown to be regulated by TGF-β, which is regarded as an early
biomarker of fibrosis (29).
This study investigated the roles of UII in the
AGE-induced overproduction of EMC in NRK-52E cells. UII has been
shown to induce collagen synthesis and secretion through a
mechanism involving the upregulation of TGF-β1 expression and
secretion in rat aortic vascular smooth muscle cells (30), implying that TGF-β1/Smad2/3
signaling may mediate the effects of UII in vascular fibrosis.
Previous data from our group demonstrated that the upregulation of
TGF-β1 expression by UII and GPR14-targeted RNA interference
decreased the UII-induced upregulation of TGF-β1 (31). The present study found that in
NRK-52E cells, the inhibition of UII function attenuated the
increases in TGF-β1, FN, and ColIV mRNA expression, and that an
anti-TGF-β1 antibody attenuated the UII-induced increase in FN and
ColIV protein secretion. At the same time, AGEs increased the
protein and mRNA expression of UII, and increased the protein
secretion of FN and ColIV.
The findings of this study suggested that the
AGE-induced upregulation of UII plays an important role in
TGF-β1-mediated EMC synthesis, most likely through autocrine and/or
paracrine mechanisms, and implicated the UII–TGF-β1 signaling
pathway in renal fibrosis. It can be reasonably speculated that
such a mechanism contributes to tubulointerstitial nephropathy in
diabetes patients. That said, it remains unclear as to whether
renal fibrosis is caused by the hyperglycemia-induced
overproduction of EMC components, or whether TGF-β1 is involved in
the UII-induced phenotypic differentiation of renal tubular
epithelial cells into myofibroblasts. Further studies are warranted
in order to elucidate the underlying mechanisms.
Acknowledgments
This study was supported by the Scientific Research
of Heilongjiang Province Health Department (grant no. 2013008 to
Lin Tian) and the Postdoctoral Scientific Research Developmental
Fund of Heilongjiang Province (grant no. LBH-Q14121 to Lin
Tian).
References
|
1
|
You Z, Al Kindi H, Abdul-Karim A, Barrette
PO and Schwertani A: Blocking the urotensin II receptor pathway
ameliorates the metabolic syndrome and improves cardiac function in
obese mice. FASEB J. 28:1210–1220. 2014. View Article : Google Scholar
|
|
2
|
Vaudry H, Leprince J, Chatenet D, Fournier
A, Lambert DG, Le Mével JC, Ohlstein EH, Schwertani A, Tostivint H
and Vaudry D: International Union of Basic and Clinical
Pharmacology. XCII Urotensin II, urotensin II-related peptide, and
their receptor: from structure to function. Pharmacol Rev.
67:214–258. 2015. View Article : Google Scholar
|
|
3
|
Forbes JM, Cooper ME, Oldfield MD and
Thomas MC: Role of advanced glycation end products in diabetic
nephropathy. J Am Soc Nephrol. 14(Suppl 3): S254–S258. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamagishi S: Role of advanced glycation
end products (AGEs) and receptor for AGEs (RAGE) in vascular damage
in diabetes. Exp Gerontol. 46:217–224. 2011. View Article : Google Scholar
|
|
5
|
Shenouda A, Douglas SA, Ohlstein EH and
Giaid A: Localization of urotensin-II immunoreactivity in normal
human kidneys and renal carcinoma. J Histochem Cytochem.
50:885–889. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsu YH, Chen TH, Chen YC, Cheng CY, Sue
YM, Chen JR and Chen CH: Urotensin II exerts antiapoptotic effect
on NRK-52E cells through prostacyclin-mediated peroxisome
proliferator-activated receptor alpha and Akt activation. Mol Cell
Endocrinol. 381:168–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Totsune K, Takahashi K, Arihara Z, Sone M,
Ito S and Murakami O: Increased plasma urotensin II levels in
patients with diabetes mellitus. Clin Sci (Lond). 104:1–5. 2003.
View Article : Google Scholar
|
|
8
|
Xie N and Liu L: Elevated expression of
urotensin II and its receptor in great artery of type 2 diabetes
and its significance. Biomed Pharmacother. 63:734–741. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Langham RG, Kelly DJ, Gow RM, Zhang Y,
Dowling JK, Thomson NM and Gilbert RE: Increased expression of
urotensin II and urotensin II receptor in human diabetic
nephropathy. Am J Kidney Dis. 44:826–831. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lam S, van der Geest RN, Verhagen NA, Daha
MR and van Kooten C: Secretion of collagen type IV by human renal
fibroblasts is increased by high glucose via a TGF-beta-independent
pathway. Nephrol Dial Transplant. 19:1694–1701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JT, Kato M, Lanting L, Castro N, Nam
BY, Wang M, Kang SW and Natarajan R: Repression of let-7 by
transforming growth factor-β1-induced Lin28 upregulates collagen
expression in glomerular mesangial cells under diabetic conditions.
Am J Physiol Renal Physiol. 307:F1390–F1403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai HY, Kang WQ, Wang X, Yu XJ, Li ZH,
Tang MX, Xu DL, Li CW, Zhang Y and Ge ZM: The involvement of
transforming growth factor-beta1 secretion in urotensin II-induced
collagen synthesis in neonatal cardiac fibroblasts. Regul Pept.
140:88–93. 2007. View Article : Google Scholar
|
|
13
|
Coulouarn Y, Lihrmann I, Jegou S, Anouar
Y, Tostivint H, Beauvillain JC, Conlon JM, Bern HA and Vaudry H:
Cloning of the cDNA encoding the urotensin II precursor in frog and
human reveals intense expression of the urotensin II gene in
motoneurons of the spinal cord. Proc Natl Acad Sci USA.
95:15803–15808. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ames RS, Sarau HM, Chambers JK, Willette
RN, Aiyar NV, Romanic AM, Louden CS, Foley JJ, Sauermelch CF,
Coatney RW, et al: Human urotensin-II is a potent vasoconstrictor
and agonist for the orphan receptor GPR14. Nature. 401:282–286.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsushita M, Shichiri M, Imai T, Iwashina
M, Tanaka H, Takasu N and Hirata Y: Co-expression of urotensin II
and its receptor (GPR14) in human cardiovascular and renal tissues.
J Hypertens. 19:2185–2190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang YG, Li YG, Liu BG, Wei RH, Wang DM,
Tan XR, Bu DF, Pang YZ and Tang CS: Urotensin II accelerates
cardiac fibrosis and hypertrophy of rats induced by isoproterenol.
Acta Pharmacol Sin. 28:36–43. 2007. View Article : Google Scholar
|
|
17
|
Guidolin D, Albertin G, Oselladore B,
Sorato E, Rebuffat P, Mascarin A and Ribatti D: The pro-angiogenic
activity of urotensin-II on human vascular endothelial cells
involves ERK1/2 and PI3K signaling pathways. Regul Pept. 162:26–32.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Albertin G, Guidolin D, Sorato E,
Oselladore B, Tortorella C and Ribatti D: Urotensin-II-stimulated
expression of pro-angiogenic factors in human vascular endothelial
cells. Regul Pept. 172:16–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sauzeau V, Le Mellionnec E, Bertoglio J,
Scalbert E, Pacaud P and Loirand G: Human urotensin II-induced
contraction and arterial smooth muscle cell proliferation are
mediated by RhoA and Rho-kinase. Circ Res. 88:1102–1104. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe T, Pakala R, Katagiri T and
Benedict CR: Synergistic effect of urotensin II with serotonin on
vascular smooth muscle cell proliferation. J Hypertens.
19:2191–2196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsushita M, Shichiri M, Fukai N, Ozawa
N, Yoshimoto T, Takasu N and Hirata Y: Urotensin II is an
autocrine/paracrine growth factor for the porcine renal epithelial
cell line, LLCPK1. Endocrinology. 144:1825–1831. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi K, Totsune K, Murakami O,
Arihara Z, Noshiro T, Hayashi Y and Shibahara S: Expression of
urotensin II and its receptor in adrenal tumors and stimulation of
proliferation of cultured tumor cells by urotensin II. Peptides.
24:301–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai HY, He T, Li XL, Xu WL and Ge ZM:
Urotensin-2 promotes collagen synthesis via ERK1/2-dependent and
ERK1/2-independent TGF-β1 in neonatal cardiac fibroblasts. Cell
Biol Int. 35:93–98. 2011. View Article : Google Scholar
|
|
24
|
Xu S, Wen H and Jiang H: Urotensin II
promotes the proliferation of endothelial progenitor cells through
p38 and p44/42 MAPK activation. Mol Med Rep. 6:197–200.
2012.PubMed/NCBI
|
|
25
|
Adebiyi A: Rgs2 regulates urotensin
II-induced intracellular Ca2+ elevation and contraction
in glomerular mesangial cells. J Cell Physiol. 229:502–511. 2014.
View Article : Google Scholar
|
|
26
|
Fukami K, Ueda S, Yamagishi S, Kato S,
Inagaki Y, Takeuchi M, Motomiya Y, Bucala R, Iida S, Tamaki K, et
al: AGEs activate mesangial TGF-beta-Smad signaling via an
angiotensin II type I receptor interaction. Kidney Int.
66:2137–2147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamagishi S, Fukami K, Ueda S and Okuda S:
Molecular mechanisms of diabetic nephropathy and its therapeutic
intervention. Curr Drug Targets. 8:952–959. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishibashi Y, Matsui T, Takeuchi M and
Yamagishi S: Metformin inhibits advanced glycation end products
(AGEs)-induced renal tubular cell injury by suppressing reactive
oxygen species generation via reducing receptor for AGEs (RAGE)
expression. Horm Metab Res. 44:891–895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eismann U, Sommer M, Kosmehl H, Appenroth
D, Fleck C and Stein G: Fibronectin splice variants-prognostic
markers for the stage of renal interstitial fibrosis in the rat.
Nephron. 92:379–388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao J, Ding W, Song N, Dong X, Di B, Peng
F and Tang C: Urotensin II-induced collagen synthesis in cultured
smooth muscle cells from rat aortic media and a possible
involvement of transforming growth factor-β1/Smad2/3 signaling
pathway. Regul Pept. 182:53–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tian L, Li C, Qi J, Fu P, Yu X, Li X and
Cai L: Diabetes-induced upregulation of urotensin II and its
receptor plays an important role in TGF-beta1-mediated renal
fibrosis and dysfunction. Am J Physiol Endocrinol Metab.
295:E1234–E1242. 2008. View Article : Google Scholar : PubMed/NCBI
|