Introduction
Inflammation is an innate immune response mediated
by macrophages and a panel of pro-inflammatory mediators, such as
tumor necrosis factor α (TNF-α), interleukin (IL)-1β, IL-6, nitric
oxide (NO) and macrophage chemoattractant protein (MCP)-1.
Macrophages promote inflammation and play a crucial role in
cytokine secretion (1).
Inflammatory diseases, such as atherosclerosis (2), acute lung injury (3) and pulmonary fibrosis (4) are characterized by the
overexpression of these cytokines and pro-inflammatory mediators.
Thus, blocking the release of cytokines from activated macrophages
may provide a mechanism for the treatment of inflammatory
disorders.
Bacteria, viruses and alcohol promote the release of
inflammatory cytokines from macrophages. Lipopolysaccharides (LPS)
in the outer wall of Gram-negative bacteria are bound by Toll-like
receptor 4 (TLR4) on the macrophage surface, thereby activating
macrophages (5,6) and triggering the activation of
several intracellular signaling pathways, such as nuclear factor κB
(NF-κB), Janus kinase-signal transducers and activators of
transcription (JAK-STATs) and mitogen-activated protein kinases
(MAPKs). These signaling cascades regulate the expression of target
genes involved in inflammatory cytokine production (7,8).
Small endogenous RNA molecules known as micro-RNAs
(miRNAs or miRs) have been identified as regulators of the
inflammatory response, which act by specifically binding the 3′UTR
of target miRNAs, marking them for degradation or suppressing
translation (9). Several miRNAs
have been implicated in the control of inflammatory processes,
including miR-155, which plays a pro-inflammatory role in the
LPS-stimulated immune response. The miRNA targets of miR155 include
pro-apoptotic and anti-inflammatory proteins, such as the
suppressor of cytokine signaling 1 (SOCS1) (10). SOCS1 inhibits JAK and STAT. It
thereby creates a negative feedback loop in LPS-induced signaling
pathways (10,11).
Resveratrol is a polyphenolic compound found in
grapes and traditional Chinese medicinal plants, such as
Polygonum cuspidatum. It influences a variety of molecular
targets, and many of them are associated with inflammation and
immunity (12,13). In this study, we examined the
specific effects of resveratrol on the production of
pro-inflammatory cytokines by LPS-stimulated RAW264.7 murine
macrophages. Our findings suggest that resveratrol inhibits STAT
activation and enhances SOCS1 expression by attenuating the
production of miR-155.
Materials and methods
Materials and reagents
Resveratrol (>99%, HPLC; molecular weight,
228.24, Trans-; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in
DMSO to produce an 80 mM stock solution and stored at −20°C. The
stock solution was diluted with medium to the desired concentration
immediately prior to use. The SB203580 (Cat. no. S8307) and AG490
(Cat. no. T3434) were purchased from Sigma-Aldrich. Enzyme-linked
immunosorbent assay (ELISA) kits for murine IL-6 and TNF-α, sICAM1
and CXCL10 were obtained from R&D Systems (Minneapolis, MN,
USA). The BCA™ protein assay kit and MTT reagent were purchased
from Beyotime (Shanghai, China). Escherichia coli LPS
(O55:B5) was obtained from Sigma-Aldrich. Antibodies directed
against phosphorylated (p-)p38 MAPK (Thr180/Tyr182; Cat. no. 9211),
p44/42 MAPK [extracellular signal-regulated kinase1/2 (ERK1/2);
Cat. no. 4695], p-p44/42 MAPK (ERK1/2; Cat. no. 4376), STAT1 (42H3;
Cat. no. 9175S), p-Tyr701 STAT1 (p-STAT1; Cat. no. 7649S), STAT3
(79D7; Cat. no. 4904), p-Tyr705 STAT3 (pSTAT3; Cat. no. 9145),
SOCS1 (Cat. no. 3950) and β-actin (Cat. no. 4970), and
HRP-conjugated anti-rabbit IgG (Cat. no. 7074) were obtained from
Cell Signaling Technology (Beverly, MA, USA). Antibodies against
c-Jun NH2-terminal kinase (JNK)1 (Cat. no. 3496-1), JNK1
(pY185)/JNK2(pY185)/JNK3(Py223) (Cat. no. 2155-1) and Crk/p38 (Cat.
no. 5359-1) were purchased from Epitomics (Burlingame, CA, USA).
The mimic and inhibitor of miR-155 (micrON™ mmu-miR-155-5p mimic,
micrOFF™ mmu-miR-155-5p inhibitor, micrON™ mimic and micrOFF™
inhibitor negative control) and the Bulge-loop™ miRNA RT-qPCR
primers for miR-155 and U6 (internal control for normalization)
were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum
(FBS), and TRIzol reagent were purchased from Gibco/BRL (Grand
Island, NY, USA).
Cell culture
The RAW264.7 murine macrophages were purchased from
the American Type Culture Collection (ATCC, Rockville, MD, USA).
The cells were cultivated in DMEM supplemented with 10%
heat-inactivated FBS and antibiotics (100 U/ml penicillin and 100
µg/ml streptomycin) at 37°C in an atmosphere containing 5%
CO2.
Cell viability assay
For cell viability assays, the cells
(1×104 cells/well) were seeded in 96-well plates,
pre-treated with 1, 5, 10, 20, or 40 µM resveratrol for 1 h,
and then stimulated with 1 µg/ml LPS for 24 h. Cell
viability was assessed by MTT assay according to the manufacturer's
instructions. The results are expressed as fold changes relative to
the control. Three replicates were performed for each
treatment.
Determination of cytokine secretion
The cells were seeded in 24-well culture plates at a
density of 1×105 cells/well and serum-starved overnight
prior to treatment. Following adhesion, the cells were
pre-incubated with resveratrol (1, 5, 10, and 20 µM) for 1 h
and stimulated with 1 µg/ml LPS for 24 h. The culture media
were collected and centrifuged at 1,000 × g to remove debris. IL-6,
TNF-α, soluble intercellular adhesion molecule 1 (sICAM1) and C-X-C
motif chemokine 10 (CXCL10) in the media were quantified by ELISA
according to manufacturer's instructions. The absorbance was read
at 450 nm using a microplate reader (Bio-Rad Laboratories Inc.,
Hercules, CA, USA), and cytokine levels were calculated from
standard curves. Three replicates were performed for each
treatment.
Determination of cytokine expression
The cells were seeded in 6-wells culture plates at a
density of 1×106 cells/well and serum-starved overnight.
The cells were then pre-incubated with resveratrol (1, 5, 10, and
20 µM) for 1 h and stimulated with 1 µg/ml LPS for 4
h. Total RNA was isolated using TRIzol reagent according to
manufacturer's instructions, and as previously described (14) and single-strand cDNA was
synthesized from 2 µg total RNA using the PrimeScript™ II
1st-strand cDNA Synthesis kit (Takara Biotechnology, Co., Ltd.,
Dalian, China). qPCR was performed on a C1000 Thermal Cycler
(Bio-Rad) with SYBR-Green (Invitrogen, Carlsbad, CA, USA). Each 25
µl reaction contained 12.5 µl SYBR Premix, 0.5
µl each primer (10 µM), 1 µl cDNA and 10.5
µl RNase-free dH2O. The cycling conditions were
as follows: step 1, 94°C for 3 min; step 2, 35 cycles at 94°C for
20 s, 57°C for 20 sec, 72°C for 30 sec; step 3, dissociation. The
data were collected and analyzed using on-instrument software.
Relative gene expression was determined by the 2−ΔΔCt
method, as previously described (15). The primer sequences were as
follows: TNF-α sense, 5′-GCAGAGAGGTTGACTTTC-3′ and antisense,
5′-CTACTCCCAGGTTCTCTTCAA-3′; IL-6 sense,
5′-AGTTGTGCAATGGCAATTCTGA-3′ and antisense,
5′-AGGACTCTGGCTTTGTCTTTCT-3′; sICAM sense,
5′-AGAAGGACTGCTTGGGGAA-3′ and antisense, 5′-CCT
CTGGCGGTAATAGGTG-3′; CXCL10 sense, 5′-GGATCCC TCTCGCAAGGA-3′ and
antisense, 5′-ATCGTGGCAATGATCTCAACA-3′; SOCS1 sense,
5′-CACTTCTGGCTGGAGACC-3′ and antisense, 5′-TGGAGAGGTAGGAGTGGAA-3′;
and β-actin sense, 5′-TGCTGTCCCTGTATGCCTCT-3′ and antisense,
5′-TTTGATGTCACGCACGATTT-3′. Each assay was normalized to
β-actin.
Analysis of miR-155 expression by
qPCR
Total cellular RNA was obtained as described above.
The reverse transcription (RT) of 1 µg total RNA was
performed using the PrimeScript™ RT reagent kit (Takara
Biotechnology, Co., Ltd.). Stem-loop RT-PCR was performed with
SYBR-Green (Invitrogen). The cycling conditions were as follows:
step 1, 94°C for 3 min; step 2, 35 cycles at 94°C for 15 sec, 57°C
for 15 sec, 72°C for 25 sec; step 3, dissociation. Each assay was
performed in triplicate and normalized to U6 expression. The primer
sequences for miR155 and U6 are the property of Guangzhou RiboBio
Co., Ltd.
Protein extraction and western blot
analysis
The cells were seeded in 6-well culture plates at a
density of 1×106 cells/well and serum-starved overnight.
The cells were pre-incubated with resveratrol (1, 5, 10, and 20
µM) for 1 h and then stimulated with 1 µg/ml LPS for
30 min prior to assaying for p-p38 MAPK, p-AKT, p-p44/42 MAPK and
p-JNK; and for 2 h prior to assaying for p-STAT1 and p-STAT3; LPS
stimulation was performed for 24 h for SOCS1 analysis. We used
SB203580 (20 µM) and AG490 (20 µM) to specifically
block p38 MAPK and JAK, respectively. The RAW264.7 cells were
pre-treated with resveratrol, SB203580 or AG490 for 1 h, followed
by stimulation with LPS. Cell lysates were obtained at 30 min and 2
h following the LPS challenge and the levels of p-p38 and
p-STAT1/STAT3 were assessed by western blot analysis.
Following incubation, the cells were harvested and
washed 3 times with ice-cold PBS. Total cellular protein was
extracted with cell lysis buffer (Cell Signaling Technology,
Beverly, MA, USA) and quantified using a bicinchoninic acid protein
assay kit (Beyotime). Equal amounts of lysate (30–50 µg
protein) were separated by SDS-PAGE and transferred onto
nitrocellulose membranes (Millipore, Billerica, MA, USA). The
membranes were blocked with 5% BSA-Tris-buffered saline with
Tween-20 for 1 h and incubated overnight at 4°C with primary
monoclonal antibodies. The membranes were incubated with
HRP-conjugated anti-rabbit IgG for 2 h at room temperature after
washing 3 times in TBST, and the bands were visualized with a
chemiluminescent substrate (ECL-Plus; Millipore) for 2–5 min using
Quantity One (v. 4.62) software (Bio-Rad Laboratories Inc.).
Small interfering RNA (siRNA) and miRNA
transfection
siRNA sequences targeting SOCS1 were designed by
Guangzhou RiboBio Co., Ltd.. The siRNA was transfected into the
RAW264.7 cells according to the manufacturer's instructions using
Lipofectamine® RNAiMAX (Invitrogen). The cells were
incubated with 10 nM SOCS1 siRNA for 6 h. Following transfection,
the supernatant was replaced with fresh medium and the cells were
pre-treated with resveratrol, followed by stimulation with LPS.
The mmu-miR-155-5p inhibitor and negative control
(10 nM) were transfected into the cells for 6 h; the cells were
washed and pre-treated with resveratrol, followed by stimulation
with LPS.
Statistical analysis
All data are expressed as the means ± SEM. For
statistical comparisons, the data were analyzed by ANOVA and
Scheffe's post-hoc test or the Kruskal-Wallis and Mann-Whitney
test. A P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Effect of resveratrol on cellular
cytotoxicity
The cytotoxicity of resveratrol to RAW264.7 murine
macrophages was determined by MTT assay. Resveratrol had no effect
on cell viability following treatment for 24 h at concentrations of
0–20 µM (data not shown), indicating no cytotoxic effects at
the dosages and time points used in this study. For all subsequent
experiments, non-toxic concentrations of resveratrol (0–20
µM) were used.
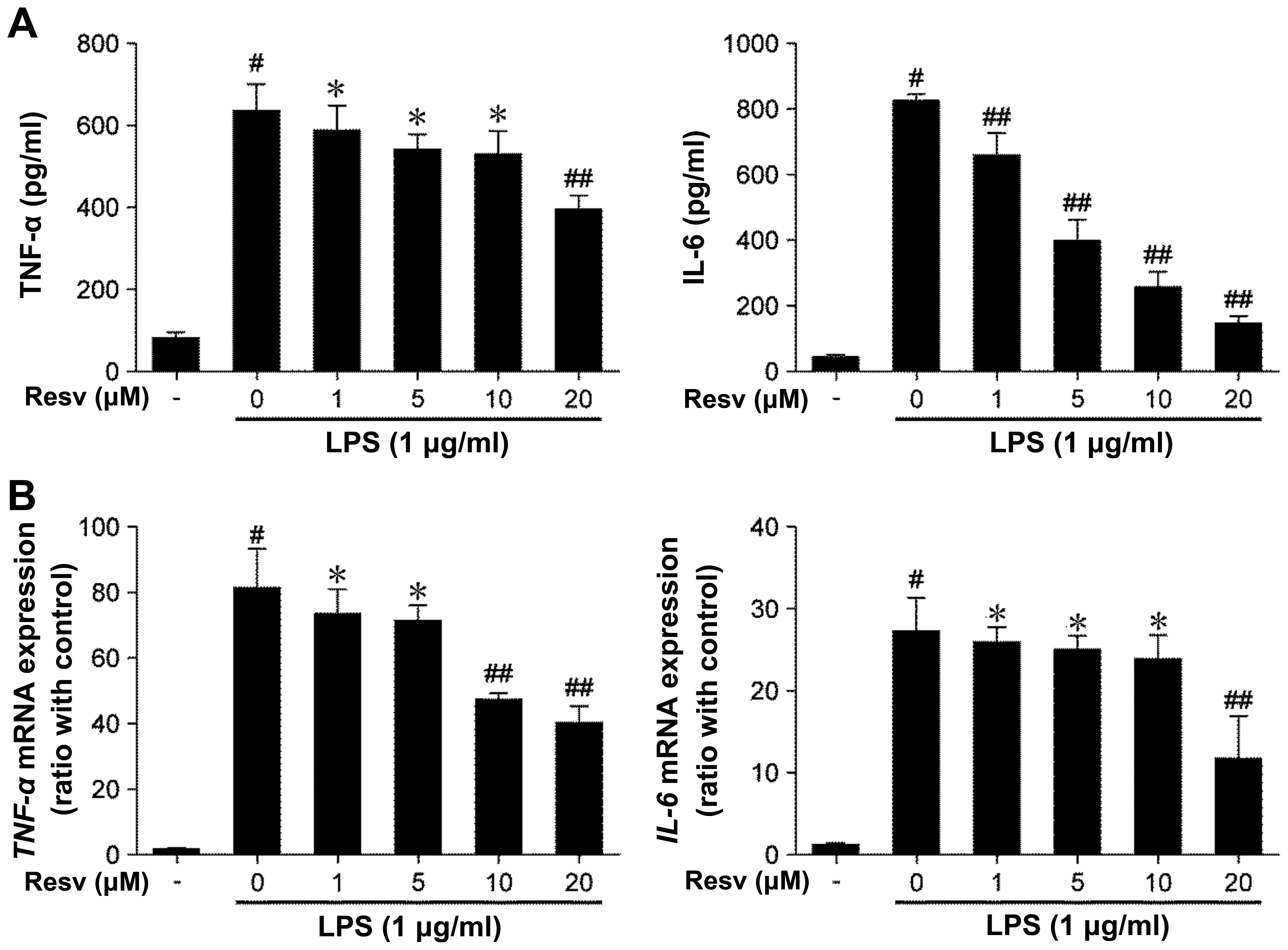
Resveratrol suppresses the production of
cytokines in LPS-stimulated RAW264.7 cells
LPS induces the release of cytokines from
macrophages (16). Thus, in this
study, we investigated whether resveratrol inhibits the production
of cytokines in LPS-stimulated RAW264.7 cells. Stimulation with LPS
alone induced the production of TNF-α and IL-6, whereas treatmetn
with resveratrol inhibited the release of these cytokines in a
dose-dependent manner (Fig.
1A).
We then investigated whether resveratrol influences
the miRNA expression of TNF-α and IL-6. Indeed, resveratrol
downregulated the miRNA expression of these cytokines (Fig. 1B). These results suggest that
resveratrol attenuates the transcript and protein expression of
inflammatory cytokines in LPS-stimulated RAW264.7 macrophages.
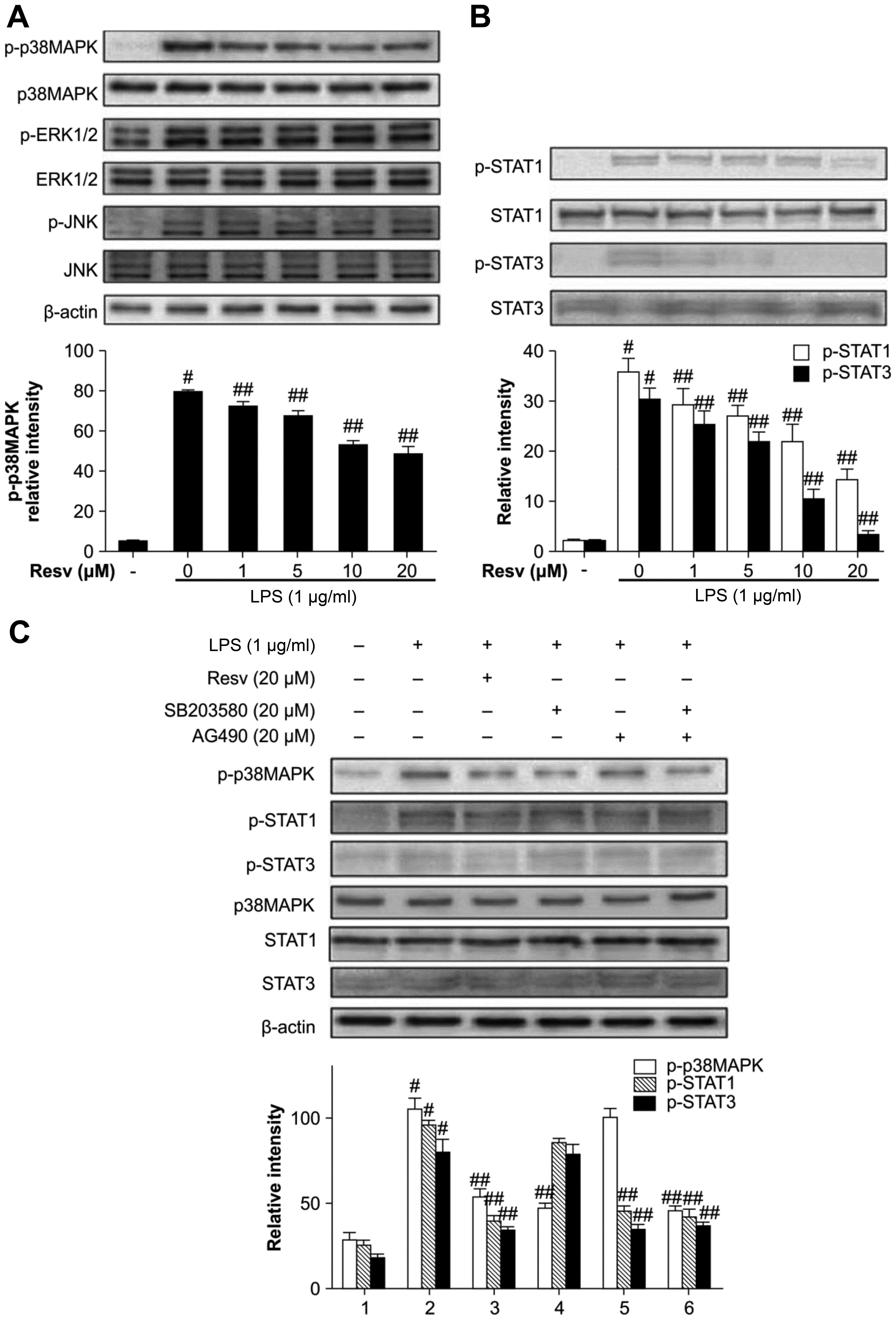
Resveratrol inhibits the LPS-induced
activation of p38 MAPK and JAK/STATs
MAPKs are the mediators of important signaling
events that control the synthesis and release of inflammatory
cytokines by activated macrophages (17). Thus, in this study, to determine
whether resveratrol influences the LPS-mediated activation of MAPK
signaling, we measured the active form of MAPK signaling components
using specific antibodies to p-ERK1/2, p-JNK1/2 and p-p38 MAPK. LPS
rapidly activated the phosphorylation of p38, ERK1/2, JNK1/2 (all
P<0.01). Resveratrol inhibited the phosphorylation of p38 in a
dose-dependent manner, but had no effect on ERK1/2 and JNK1/2
phosphorylation (Fig. 2A).
STAT1 and STAT3 are key transcription factors in
immunity and play roles in the inflammatory signaling cascades
triggered by LPS (18). In this
study, we examined the hypothesis that resveratrol blocks the
LPS-induced phosphorylation of STAT1 and STAT3. Indeed, resveratrol
inhibited LPS-induced STAT1 and STAT3 phosphorylation in RAW264.7
cells in a dose-dependent manner (Fig. 2B).
We then compared resveratrol to other signaling
inhibitors from the LPS-induced inflammatory response. We used
SB203580 and AG490 to specifically block p38 MAPK and JAK,
respectively. SB203580 only inhibited p38 phosphorylation and AG490
inhibited STAT1/STAT3 phosphorylation. By contrast, resveratrol
inhibited both factors and this indicates that it has a broader
range of inhibitory activity (Fig.
2C).
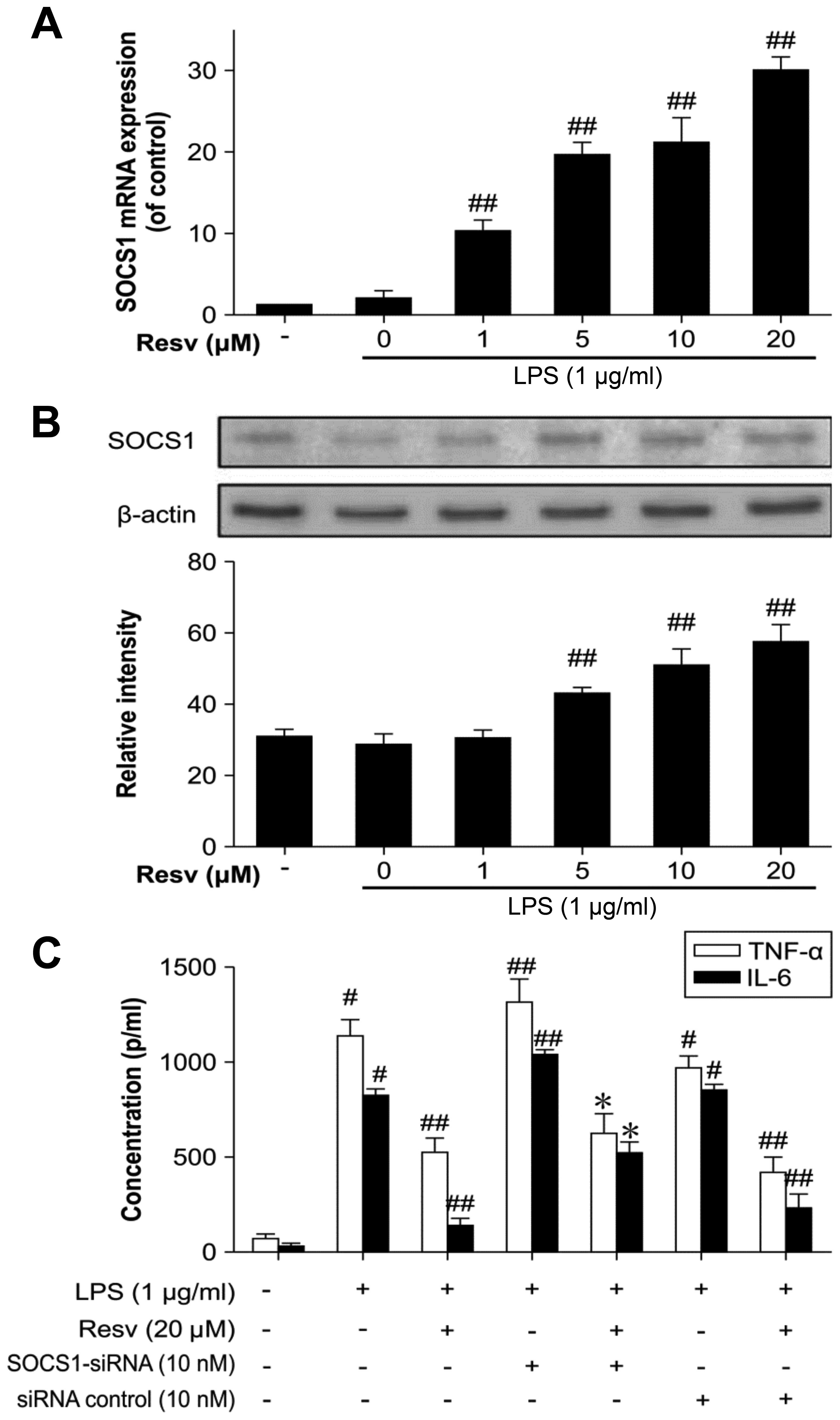
Resveratrol upregulates SOCS1 expression
in LPS-stimulated RAW264.7 cells
SOCS proteins function via the suppression of the
JAK/STAT pathway. SOCS1 acts as a pseudo-substrate, interacting
with and inhibiting JAK tyrosine kinase activity, thereby
suppressing cytokine signal transduction (19). In this study, we examined whether
resveratrol induces SOCS1 expression to suppress JAK/STAT signaling
in LPS-stimulated RAW264.7 cells pre-treated with resveratrol.
Indeed, resveratrol induced SOCS1 expression particularly at a
higher concentration, indicating its function as an
anti-inflammatory agent via the promotion of SOCS1 expression
(Fig. 3A and B).
We then used siRNA targeting SOCS1 to confirm the
status of SOCS1 in this inflammatory response. Following
transfection of the cells with SOCS1 siRNA, TNF-α and IL-6
expression increased in the LPS-stimulated macrophages and the
anti-inflammatory effect of resveratrol was somewhat reduced
(Fig. 3C).
Resveratrol downregulates miR-155 in
LPS-stimulated RAW264.7 cells
miR-155 plays an important role in modulating immune
processes (20). Thus, we
determined whether resveratrol influences miR-155 production by
measuring miR-155 expression with a stem-loop RT-PCR method. LPS
alone induced miR-155 expression, whereas resveratrol inhibited its
expression in a dose-dependent manner (Fig. 4A).
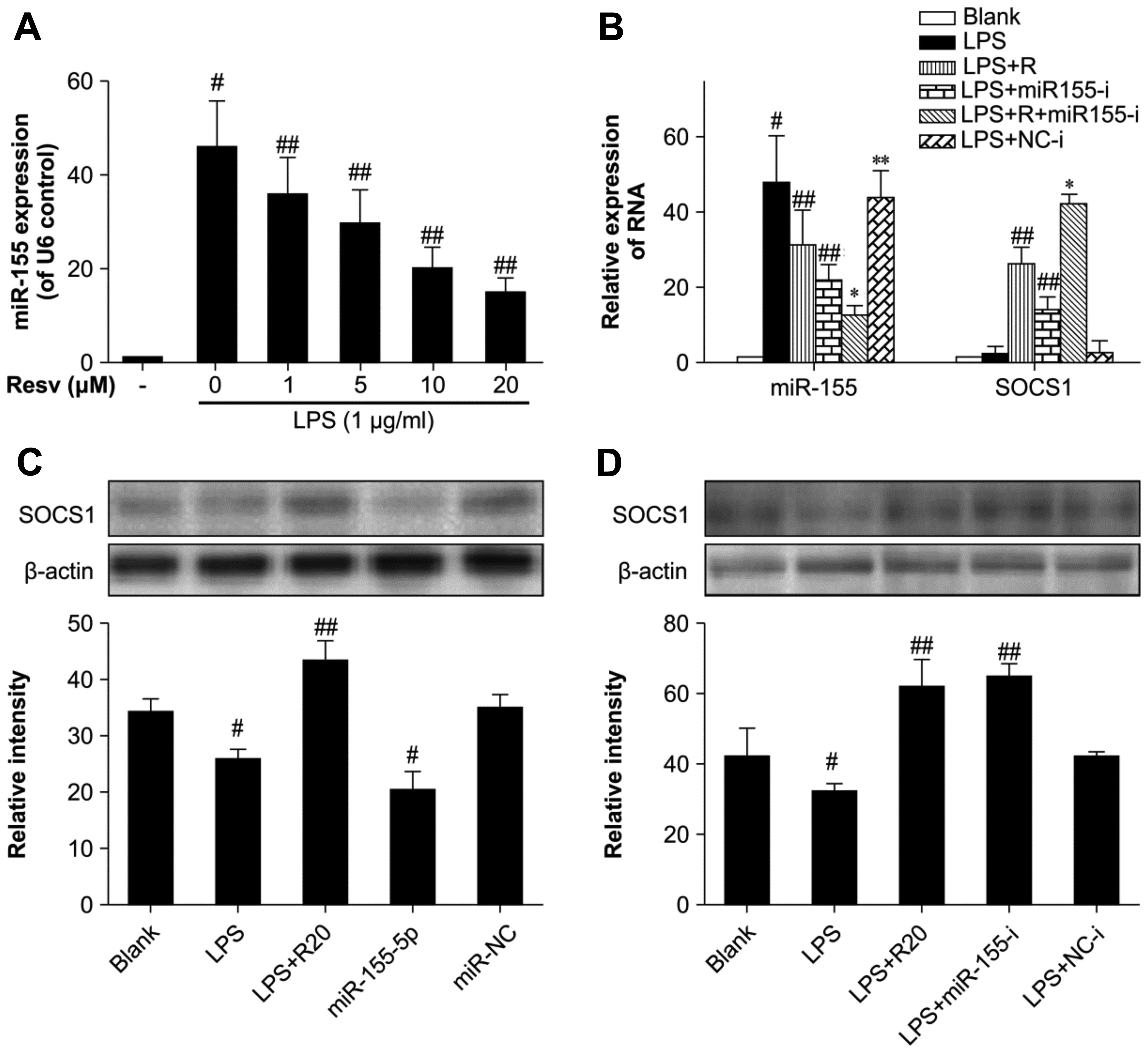 | Figure 4The influence of resveratrol on
microRNA (miR)-155 expression in lipopolysaccharide (LPS)-induced
inflammation. (A) Cells were pre-treated with resveratrol for 1 h
and then stimulated with 1 µg/ml LPS for 4 h. miR-155
expression was assayed by stem-loop RT-PCR with U6 as an endogenous
control. (B) Cells were transfected with miR-155 inhibitor and
miRNA negative control, then treated with 20 µM resveratrol
and 1 µg/ml LPS as above, suppressor of cytokine signaling 1
(SOCS1) expression was normalized against the β-actin control. (C
and D) Cells were transfected with miR-155 mimic and inhibitor,
then treated as above and SOCS1 was assayed by western blot
analysis. Bands were quantified by densitometry. The results are
presented as the means ± SD of 3 independent experiments.
#P<0.01 vs. blank control, ##P<0.01 vs.
LPS group, *P<0.01 vs. LPS + Resv group and LPS +
miR155-i group, **P<0.01 vs. blank control but
P>0.05 vs. LPS group. Blank control, untreated cells; LPS group,
cells stimulated with only LPS; LPS + R20, cells stimulated with
LPS and treated with resveratrol at 20 µM; LPS + miR155-i
group, cells transfected with miR-155 inhibitor then stimulated
with LPS. |
We used micrOFF™ mmu-miR-155-5p inhibitor (10 nM) to
neutralize miR-155 expression and the micrON™ mmu-miR-155-5p mimic
(5 nM) in resveratrol- and LPS-treated cells and observed an
increased expression of SOCS1 in the presence of miR-155-5p
inhibition and a decreased expression with miR-155-5p
overexpression (Fig. 4B–D). These
results demonstrated that resveratrol upregulated SOCS1 by
downregulating miR-155 and has the same effect as an miR-155
inhibitor.
Discussion
Resveratrol is a polyphenolic compound found in
grapes and in the Chinese herb, Polygonum cuspidatum. It
interacts with multiple molecular targets, many of them associated
with inflammation and immunity (13). In this study, we provide strong
evidence that resveratrol suppresses the production of
pro-inflammatory cytokines and inhibits the activation of the p38
MAPK and STAT1/STAT3 signaling pathways by upregulating SOCS1
expression in response to LPS stimulation.
LPS, the major component of the cell wall of
Gram-negative bacteria, interacts with TLR4 on macrophages, which
then produce pro-inflammatory cytokines, such as IL-6, TNF-α,
CXCL10. These pro-inflammatory cytokines mediate cell damage and
tissue destruction (21,22). TNF-α is the earliest and most
important cytokine during the inflammatory reaction, which can
activate macrophages and then promote the release of various
mediators (23,24). Several important common pathways
have been identified, including the MAPK pathways. The MAPKs are
intracellular serine/threonine protein kinases and include ERK1/2,
p38 MAPK and JNK. They are involved in diverse cellular processes,
including cell growth, proliferation, differentiation, cell death
and immune responses (25). In
our study, resveratrol inhibited the expression of IL-6 and TNF-α
at the miRNA and protein level. Resveratrol has been shown to
modulate the LPS-TLR4 pathway and suppress the activation of
nuclear factor (NF)-κB (26,27). Furthermore, in our study,
resveratrol inhibited the LPS-induced phosphorylation of p-38 MAPK,
but not of that ERK1/2 and JNK, indicating that p-38 MAPK is a
molecular target for resveratrol. By using specific kinase
inhibitors for p-38 MAPK, we confirmed this anti-inflammatory
effect.
The JAK-STAT cascade is an essential signaling
pathway in the immune and inflammatory responses (28). LPS receptor binding induces the
phosphorylation of receptor-associated JAK, which in turn leads to
STAT phosphorylation. In addition to LPS, other stimuli such as
cytokines and growth factors can also activate JAK-STAT signaling
systems (29). Phosphorylated
STATs are dissociated from the receptor complex and then form
homodimers or heterodimers, which translocate to the nucleus where
they regulate the transcription of pro-inflammatory target genes.
STAT1 and STAT3 are vital modulators in inflammatory signaling
cascades triggered by LPS (30).
In this study, resveratrol suppressed the phosphorylation of STAT1
and STAT3 2 h after the LPS challenge. We noted STAT1 and STAT3
phosphorylation was decreased much later than the activation of
MAPK, indicating that STAT1/3 may be downstream targets of
resveratrol. Previous studies have suggested that the serine 727 of
STAT1 and STAT3 can be phosphorylated by p38 MAPK (31–34). However, we did not find evidence
indicating that the phosphorylation of STAT1 and STAT3 is induced
by p38 MAPK, as SB203580, a specific inhibitor of p38 MAPK, did not
block the downstream signal of STAT1/3. We suggest that resveratrol
may have extensive anti-inflammatory effects as it interferes with
p38 MAPK and STAT1/STAT3.
SOCS1 plays a vital role in the negative regulation
of cytokines and TLR-mediated signaling pathways (35). SOCS1 blocks signaling by
interacting with phosphotyrosine residues of JAK2 and STATs
(19). SOCS1-deficient
macrophages secrete more pro-inflammatory cytokines such as TNF-α
and IL-6 (36). In our study,
SOCS1 transcript expression was slightly upregulated in
LPS-stimulated macrophages; however, resveratrol increased SOCS1
expression, particularly at higher concentrations. We speculated
that resveratrol acts as an anti-inflammatory agent partly due to
the upregulation of SOCS1 expression, and SOCS1 negatively
regulates inflammation. This effect is lost upon the RNA silencing
of SOCS1 in RAW264.7 macrophages. Thus, the absence of SOCS1 caused
more inflammatory cytokines to be released, such as TNF-α and IL-6
than the presence of SOCS1. We confirmed that resveratrol exerted
an anti-inflammatory effect by enhancing SOCS1 expression.
miRNAs regulated immune responses. miR-155 has been
found in several immune cell types, such as macrophages, monocytes
and dendritic cells (37). Many
of the miR-155 targets encode anti-inflammatory proteins, such as
SOCS1. LPS can induce the expression of miR-155, and the effect of
miR155 is to combine with the 3′UTR miRNA of SOCS1, then
downregulate the protein expression of SOCS1. In this study, a
decrease in SOCS1 expression was observed following transfection
with a miR-155-5p mimic, which caused the overexpression of
miR-155, and induced the expression of pro-inflammatory cytokines.
By contrast, an increase in SOCS1 expression had the opposite
effect following transfection with a miR-155 inhibitor, and had the
same effect as resveratrol. Our study provides compelling evidence
of the inhibition of miR-155 by resveratrol through the
upregulation of SOCS1. The mutual restrictive associatoin between
miR-155 and SOCS1 may be the mechanism responsible for the
anti-inflammatory effect of resveratrol. SOCS1, as a negative
regulator, inhibited the TLR-mediated JAK/STAT inflammatory
cascade. Resveratrol also inhibited the p38 MAPK signaling pathway.
Thus, resveratrol exerts multiple anti-inflammatory effects in
LPS-stimulated inflammatory cells. This study highlights the
potential therapeutic value of resveratrol in the treatment of
inflammatory diseases.
Acknowledgments
This study was funded by National Natural Science
Foundation of China (grant no. 81273678).
References
|
1
|
Brummer E, Capilla J, Bythadka L and
Stevens DA: Production of IL-6, in contrast to other cytokines and
chemokines, in macrophage innate immune responses: effect of serum
and fungal (Blastomyces) challenge. Cytokine. 39:163–170. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goodman RB, Pugin J, Lee JS and Matthay
MA: Cytokine-mediated inflammation in acute lung injury. Cytokine
Growth Factor Rev. 14:523–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Driscoll KE, Maurer JK, Higgins J and
Poynter J: Alveolar macrophage cytokine and growth factor
production in a rat model of crocidolite-induced pulmonary
inflammation and fibrosis. J Toxicol Environ Health. 46:155–169.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berghaus LJ, Moore JN, Hurley DJ,
Vandenplas ML, Fortes BP, Wolfert MA and Boons GJ: Innate immune
responses of primary murine macrophage-lineage cells and RAW 264.7
cells to ligands of toll-like receptors 2, 3, and 4. Comp Immunol
Microbiol Infect Dis. 33:443–454. 2010. View Article : Google Scholar :
|
|
6
|
Froidevaux C, Roger T, Martin C, Glauser
MP and Calandra T: Macrophage migration inhibitory factor and
innate immune responses to bacterial infections. Crit Care Med.
29(Suppl 7): S13–S15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roger T, Chanson AL, Knaup-Reymond M and
Calandra T: Macrophage migration inhibitory factor promotes innate
immune responses by suppressing glucocorticoid-induced expression
of mitogen-activated protein kinase phosphatase-1. Eur J Immunol.
35:3405–3413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baeuerle PA: IkappaB-NF-kappaB structures:
at the interface of inflammation control. Cell. 95:729–731. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Juarez MT, Kui JS, Thomas J, Heller BA and
Timmermans MC: microRNA-mediated repression of rolled leaf1
specifies maize leaf polarity. Nature. 428:84–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cardoso AL, Guedes JR, Pereira de Almeida
L and Pedroso de Lima MC: miR-155 modulates microglia-mediated
immune response by down-regulating SOCS-1 and promoting cytokine
and nitric oxide production. Immunology. 135:73–88. 2012.
View Article : Google Scholar :
|
|
11
|
Kimura A, Naka T, Muta T, Takeuchi O,
Akira S, Kawase I and Kishimoto T: Suppressor of cytokine
signaling-1 selectively inhibits LPS-induced IL-6 production by
regulating JAK-STAT. Proc Natl Acad Sci USA. 102:17089–17094. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gambini J, López-Grueso R, Olaso-González
G, Inglés M, Abdelazid K, El Alami M, Bonet-Costa V, Borrás C and
Viña J: Resveratrol: distribution, properties and perspectives. Rev
Esp Geriatr Gerontol. 48:79–88. 2013.in Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Švajger U and Jeras M: Anti-inflammatory
effects of resveratrol and its potential use in therapy of
immune-mediated diseases. Int Rev Immunol. 31:202–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simões AE, Pereira DM, Amaral JD, Nunes
AF, Gomes SE, Rodrigues PM, Lo AC, D'Hooge R, Steer CJ, Thibodeau
SN, et al: Efficient recovery of proteins from multiple source
samples after TRIzol(®) or TRIzol(®)LS RNA extraction and long-term
storage. BMC Genomics. 14:1812013. View Article : Google Scholar
|
|
15
|
Ihsan A, Wang X, Liu Z, Wang Y, Huang X,
Liu Y, Yu H, Zhang H, Li T, Yang C and Yuan Z: Long-term mequindox
treatment induced endocrine and reproductive toxicity via oxidative
stress in male wistar rats. Toxicol Appl Pharmacol. 252:281–288.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bølling AK, Samuelsen JT, Morisbak E,
Ansteinsson V, Becher R, Dahl JE and Mathisen GH: Dental monomers
inhibit LPS-induced cytokine release from the macrophage cell line
RAW264.7. Toxicol Lett. 216:130–138. 2013. View Article : Google Scholar
|
|
17
|
Huang JL, Zhang YL, Wang CC, Zhou JR, Ma
Q, Wang X, Shen XH and Jiang CL: Enhanced phosphorylation of MAPKs
by NE promotes TNF-α production by macrophage through α adrenergic
receptor. Inflammation. 35:527–534. 2012. View Article : Google Scholar
|
|
18
|
Kaplan MH: STAT signaling in inflammation.
JAK-STAT. 2:e241982013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Croker BA, Kiu H and Nicholson SE: SOCS
regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol.
19:414–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vigorito E, Kohlhaas S, Lu D and Leyland
R: miR-155: an ancient regulator of the immune system. Immunol Rev.
253:146–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garzón E, Holzmuller P, Bras-Gonçalves R,
Vincendeau P, Cuny G, Lemesre JL and Geiger A: The Trypanosoma
brucei gambiense secretome impairs lipopolysaccharide-induced
maturation, cytokine production, and allostimulatory capacity of
dendritic cells. Infect Immun. 81:3300–3308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang FM, Reyna SM, Granados JC, Wei SJ,
Innis-Whitehouse W, Maffi SK, Rodriguez E, Slaga TJ and Short JD:
Inhibition of neddylation represses lipopolysaccharide-induced
proinflammatory cytokine production in macrophage cells. J Biol
Chem. 287:35756–35767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mukhopadhyay S, Hoidal JR and Mukherjee
TK: Role of TNFalpha in pulmonary pathophysiology. Respir Res.
7:1252006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Butt Y, Kurdowska A and Allen TC: Acute
lung injury: A clinical and molecular review. Arch Pathol Lab Med.
140:345–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trempolec N, Dave–Coll N and Nebreda AR:
SnapShot: p38 MAPK signaling. Cell. 152:9242013. View Article : Google Scholar
|
|
26
|
Byun EB, Sung NY, Park JN, Yang MS, Park
SH and Byun EH: Gamma-irradiated resveratrol negatively regulates
LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages.
Int Immunopharmacol. 25:249–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma C, Wang Y, Dong L, Li M and Cai W:
Anti-inflammatory effect of resveratrol through the suppression of
NF-κB and JAK/STAT signaling pathways. Acta Biochim Biophys Sin
(Shanghai). 47:207–213. 2015. View Article : Google Scholar
|
|
28
|
Mertens C and Darnell JE Jr: SnapShot:
JAK-STAT signaling. Cell. 131:6122007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Shea JJ, Gadina M and Schreiber RD:
Cytokine signaling in 2002: new surprises in the Jak/Stat pathway.
Cell. 109(Suppl): S121–S131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murray PJ: The JAK-STAT signaling pathway:
Input and output integration. J Immunol. 178:2623–2629. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kovarik P, Stoiber D, Eyers PA, Menghini
R, Neininger A, Gaestel M, Cohen P and Decker T: Stress-induced
phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated
protein kinase whereas IFN-gamma uses a different signaling
pathway. Proc Natl Acad Sci USA. 96:13956–13961. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shuai K and Liu B: Regulation of JAK-STAT
signalling in the immune system. Nat Rev Immunol. 3:900–911. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galdiero M, Vitiello M, D'Isanto M, Raieta
K and Galdiero E: STAT1 and STAT3 phosphorylation by porins are
independent of JAKs but are dependent on MAPK pathway and plays a
role in U937 cells production of interleukin-6. Cytokine.
36:218–228. 2006. View Article : Google Scholar
|
|
34
|
Sakaguchi M, Oka M, Iwasaki T, Fukami Y
and Nishigori C: Role and regulation of STAT3 phosphorylation at
Ser727 in melanocytes and melanoma cells. J Invest Dermatol.
132:1877–1885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Linossi EM, Babon JJ, Hilton DJ and
Nicholson SE: Suppression of cytokine signaling: the SOCS
perspective. Cytokine Growth Factor Rev. 24:241–248. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guenterberg KD, Lesinski GB, Mundy-Bosse
BL, Karpa VI, Jaime-Ramirez AC, Wei L and Carson WE III: Enhanced
anti-tumor activity of interferon-alpha in SOCS1-deficient mice is
mediated by CD4+ and CD8+ T cells. Cancer
Immunol Immunother. 60:1281–1288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O'Connell RM, Taganov KD, Boldin MP, Cheng
G and Baltimore D: MicroRNA-155 is induced during the macrophage
inflammatory response. Proc Natl Acad Sci USA. 104:1604–1609. 2007.
View Article : Google Scholar : PubMed/NCBI
|