Introduction
Clinically, acute ischemic cerebrovascular disease
is very common with high morbidity, mortality and disability
(1). Cerebral ischemia can
trigger nerve cell damage (1).
The fundamental measures for preventing ischemic injury are to
recover the blood supply as soon as possible (2). With the recovery of blood perfusion
to the ischemic area after continuing cerebral ischemia for a
certain period of time, the original cells at the ischemic area
cannot recover (2). Thus, this
aggravates ischemic injury. This phenomenon is known as cerebral
ischemia/reperfusion injury (CIRI) (3). The pathogenesis of CIRI is more
complex than that of pure hypoxic-ischemic brain damage and is
associated with nerve cell apoptosis, the breakdown of high energy
phosphate compounds, lipid peroxidation, inflammatory reactions,
the toxic effects of excitatory amino acids, calcium overload, the
functions of nitric oxide (NO) and endothelial cell dysfunction
(4–6). However, the specific mechanisms
responsible for the pathogenesis of CIRI have not yet been fully
elucidated.
Sirtuin 1 (SIRT1) is a type of histone deacetylase
dependent on NAD+, which is the highest factor in
homologous genes of mammals in silent information regulator SIR2
(7). Studies have confirmed that
SIRT1 is extensively expressed in tissues and organs and is
associated with metabolic syndrome, tumors and neurodegenerative
diseases (8–10). A previous study demonstrated that
icariin exerted neuroprotective effects through the induction of
SIRT1, and that these effects were reversed by the use of a SIRT1
inhibitor (11). Moreover, SIRT1
can regulate many transcription factors and receptors, such as
hypermethylated in cancer 1 (HIC1), C-terminal-binding protein
(CtBP), p53, forkhead box O3 (FOXO3A) and E2F transcription factor
1 (E2F1) (9,11,12). The hippocampus is a key structure
of learning and memory in the brain. The expression of SIRT1 in the
brain is the material basis for its participation in regulating
cognitive functions (13). SIRT1
plays a role in neuronal differentiation (13). It has also been shown that SIRT1
participates in the dendronization of neuronal dentritic cells
(11). The overexpression of
SIRT1 in nurtured hippocampal neurons enhances the dendronization
of dendrites (14).
During the later decades of an individual's life,
the morbidity and death rates triggered by cerebral ischemia are
relatively high. Owing to delayed neuronal death (DND), despite
survival after an attack, nervous system sequelae, such as motor
dysfunction, and language and cognitive deficits may occur
(15). It has been proven that
apoptosis is closely associated with DND. Some apoptosis-related
genes have also been found. The tumor suppressor gene, p53, has
been found to participate in the DND of brain neurons following
ischemia. It has dual functions in cerebral ischemia (16). When DNA is damaged, p53 causes
cell cycle arrest, which helps the repair of DNA. When DNA cannot
be restored, it can induce cell apoptosis (17).
During the process of ischemia/reperfusion, many
genes undergo alterations, resulting in the inhibition of some
proteins, while the expression of other harmful proteins is
significantly upregulated (18).
As important components in genetic expression, the levels and
functions of mRNAs are regulated by several factors. Studies have
found that microRNAs participate in various biological process,
such as embryogenesis, proliferation, differentiation and cell
death (19,20). Thus, it has been predicted that
microRNAs regulate genec expression in over a third of animals. In
diseases of the nervous system, the abnormal expression of
microRNAs is associated with the dysfunction of synaptogenesis and
synaptic reorganization, degenerative diseases, tumors and epilepsy
(18,20). A recent study indicated that
microRNAs (microRNA-107) participate in the development of CIRI
(21). In the present study,
therefore, we wished to determine whether SIRT1 exerts
neuroprotective effects by attenuating CIRI and explored the
mechanisms involved.
Materials and methods
Animals, model of CIRI and drug
treatment
Sprague-Dawley male rats (n=56, weighing, 230–260 g)
were purchased from Founder Animal's Pharmaceutcal Co., Ltd.
(Cangzhou, China) and kept (22±1°C, 55±5% humidity) on a 12 h
light/dark cycle with ad libitum access to food and water.
All experimental procedures were approved by the Committee of
Cangzhou Central Hospital in accordance with the NIH Guide for the
Care and Use of Laboratory Animals. The rats were anesthetized with
chloral hydrate [300 mg/kg, intraperitoneal (i.p.) injection]. LPS
(1 mg/ml; Sigma, St. Louis, MO USA) was injected into the corpus
callosum in order to induce CIRI. After 1 day, focal cerebral
ischemia was induced by right-sided endovascular middle cerebral
artery occlusion. The suture was pulled back and the rats were
allowed to recover after 2 h of ischemia. Firstly, 32 rats were
randomly assigned into 4 groups as follows: i) the sham-operated
(control, n=8) group; ii) the vehicle group (CIRI model, n=8); iii)
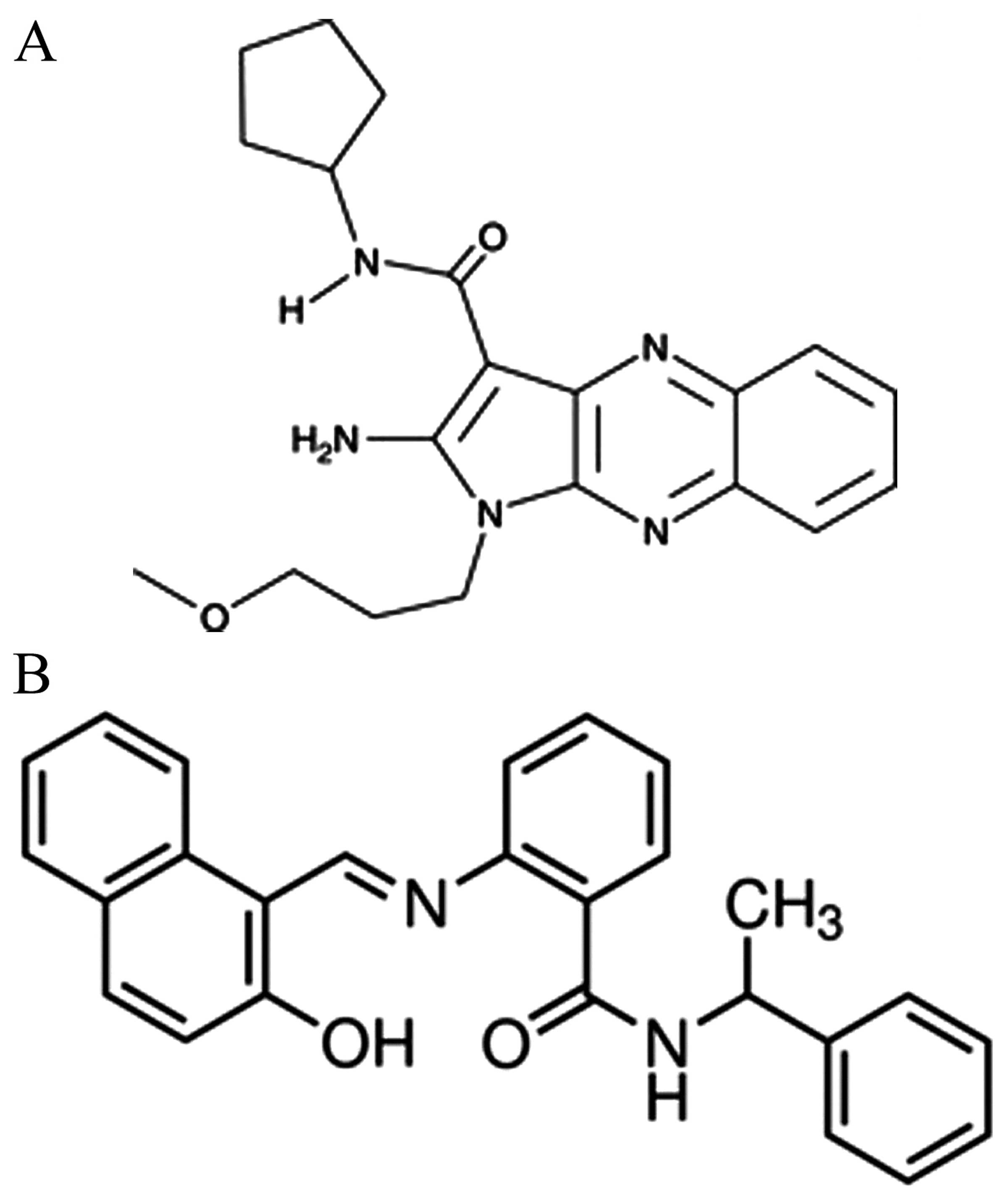
the group treated with 5 mg/kg of sc-222315 (SIRT1 activator 3,
n=8; chemical structure shown in Fig.
1A); and iv) the group treated with 10 mg/kg group of sc-222315
(n=8). A total of 16 rats were treated with SIRT1 activator 3 for
48 h by intraperitoneal injection. Subsequently, 24 rats were
randomly assigned into 3 groups as follows: i) the sham-operated
(control, n=8) group; ii) the vehicle group (CIRI model, n=8); and
the group treated with 10 mg/kg of sirtinol (S7942; SIRT1
inhibitor, n=8; chemical structure shown in Fig. 1B). A total of 8 rats were treated
with sirtinol for 48 h by intraperitoneal injection. The rats were
then anesthetized with chloral hydrate (300 mg/kg, by
intraperitoneal injection) and sacrificed by decollation. The
brains were immediately removed for determining the infarct volume,
and the brain tissue samples were washed with PBS and stored at -80
°C until use.
Measurement of infarct volume and
neurological deficit score
The brain tissue samples were cut into coronal
slices of 2 mm for determining the infarct volume using 2%
triphenyltetrazolium chloride (TTC; Sigma). The integrating 6
selected sections were used to calculate the infarct volume, and
the corrected infarct volumes were calculated to compensate for
brain edema. The neurological deficit score was analyzed based on
the following criteria and five-point scoring system: no
neurological injury symptoms was given a score of 0; inability to
entirely extend the front jaw on the heterolateral side was given a
score of 1; rotation while crawling and falling to the
heterolateral side was given a score of 2; inability to walk
without assistance was given a score of 3; unconsciousness was
given a score of 4.
Measurement of inflammation and caspase-3
activity
Brain samples were homogenized in 50 mM Tris buffer
and were then subjected to centrifugation at 12,000 × g for 10 min
at 4°C for determining the activity of tumor necrosis factor-α
(TNF-α), interleukin (IL)-6, IL-10, and caspase-3 in the rat brains
using ELISA kits in accordance with the manufacturer's instructions
(Elabscience Biology Co., Ltd., Wuhan, China).
Western blot analysis
The brain samples were homogenized in 50 mM Tris
buffer, and the protein concentration in the supernatant was
determined using the Micro BCA protein assay kit with bovine serum
albumin as the standard (Pierce, Rockford, IL, USA) following
centrifugation at 12,000 × g at 4°C for 10 min. Total protein (50
μg) was subjected to 10–12% sodium dodecyl sulfate
(SDS)-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA).
The membranes were incubated with 5% non-fat dry milk in Tween-20
(0.1%)-containing TBS for 1 h, followed by incubation with primary
antibodies against cyclooxygenase (COX)-2 (sc-7951), inducible NO
synthase (iNOS; sc-650), p53 (sc-47698) and β-actin (sc-47778) (all
from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C
overnight. The membranes were washed with TBST and then incubated
with anti-mouse or anti-rabbit secondary antibody (sc-2354 or
sc-2768; Santa Cruz Biotechnology, Inc.) and examined using the ECL
kit (Amersham, Piscataway, NJ, USA).
Real-time PCR
Total RNA was extracted from the brain samples using
TRIzol reagent according to the manufacturer's instructions
(Sigma). The gene expression of microRNA-22 was detected by TaqMan
MicroRNA assays using looped-primer reverse transcription (RT)-PCR.
The conditions for PCR were as follows: at 95°C for 10 min; at 95°C
for 30 sec and 60°C for 30 sec followed by 40 cycles.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an
internal control. The sequences of the primers used were as
follows: GAPDH sense, 5′-GAAGGTGAAGGTCGGAGTC-3′ and antisense,
5′-GAAGATGGTGATGGGATTTC-3′; and microRNA-22 sense,
5′-AACTCGAGCGAGTCCTCTCCTAGGACTA-3′ and antisense,
5′-TTGCGGCCGCACACAAAGCTTAAATATG-3′.
Statistical analysis
Data are expressed as the means ± SD and analyzed
for statistical significance using one-way ANOVA, followed by
Scheffe's test for multiple comparisons. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
SIRT1 decreases the infarct volume and
improves the neurological deficit score in a rat model of CIRI
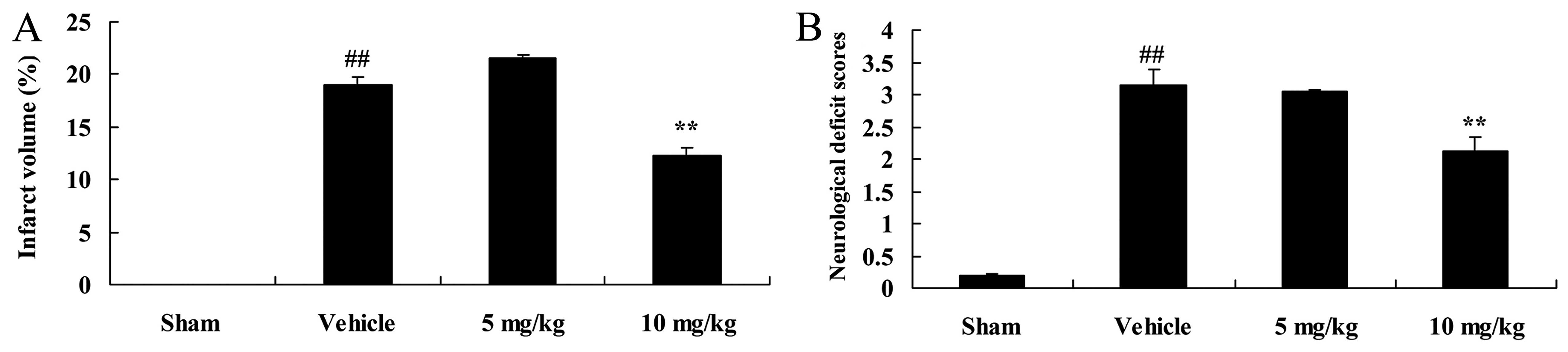
Initially, we examined the effects of SIRT1 on the
infarct volume and neurological deficit score in rats with CIRI. As
shown in Fig. 2, the rats with
CIRI exhibited a significant increase in infarct volume and the
neurological deficit score, compared with the rats in the
sham-operated group. However, treatment with 10 mg/kg of SIRT1
activator 3 significantly attenuated the CIRI-induced increase int
he infarct volume and neurological deficit score in the rats
(Fig. 2).
SIRT1 attenuates inflammation in a rat
model of CIRI
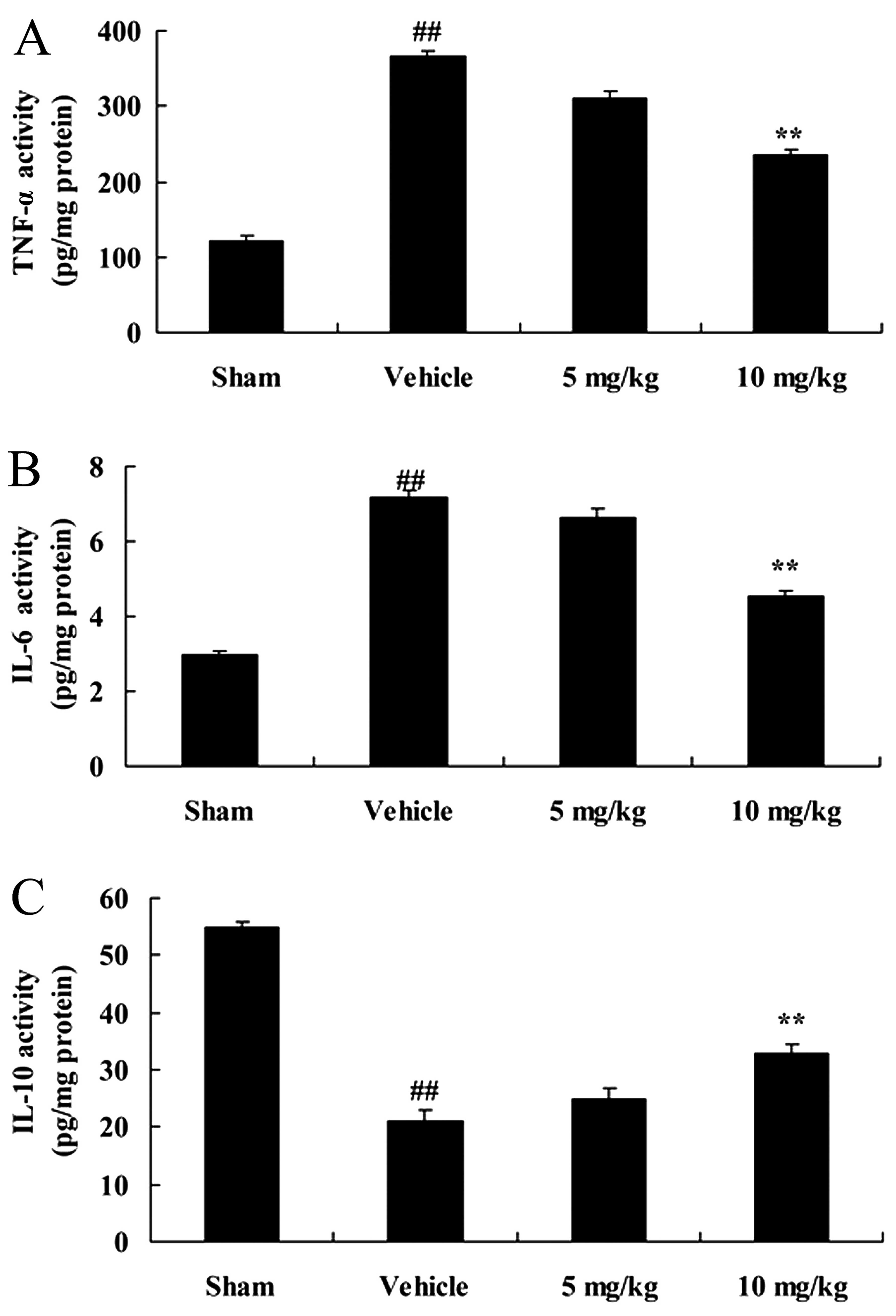
We then examined the neuroprotective effects of
SIRT1 on inflammatory reactions in our rat model of CIRI. The
activity of TNF-α and II-6 was significantly enhanced, and that of
IL-10 was significantly inhibited following CIRI, compared with the
sham-operated group (Fig. 3).
However, treatment with 10 mg/kg of SIRT1 activator 3 significantly
suppressed the activity of TNF-α and II-6, and increased that of
IL-10 in the rats with CIRI (Fig.
3).
SIRT1 decreases COX-2 protein expression
in a rat model of CIRI
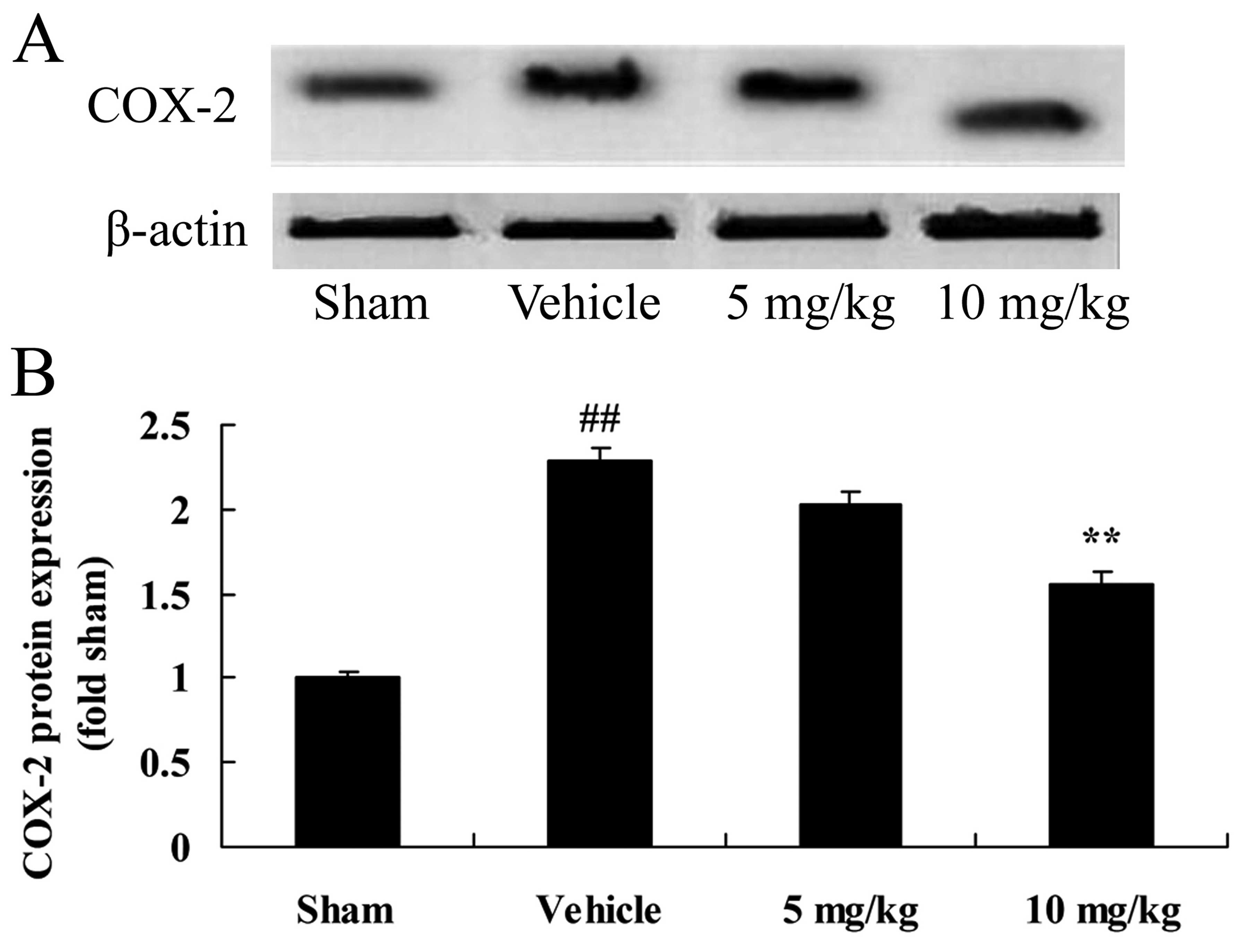
Furthermore, to clarify the regulatory mechanisms of
SIRT1 in rats with CIRI, we first examined COX-2 protein expression
in our rat model of CIRI. As shown in Fig. 4, CIRI significantly increased the
protein expression of COX-2 in the rats with CIRI, compared with
those in the sham-operated group. The induction of COX-2 protein
expression following CIRI was significantly attenuated by treatment
with 10 mg/kg of SIRT1 activator 3 in the rats with CIRI (Fig. 4).
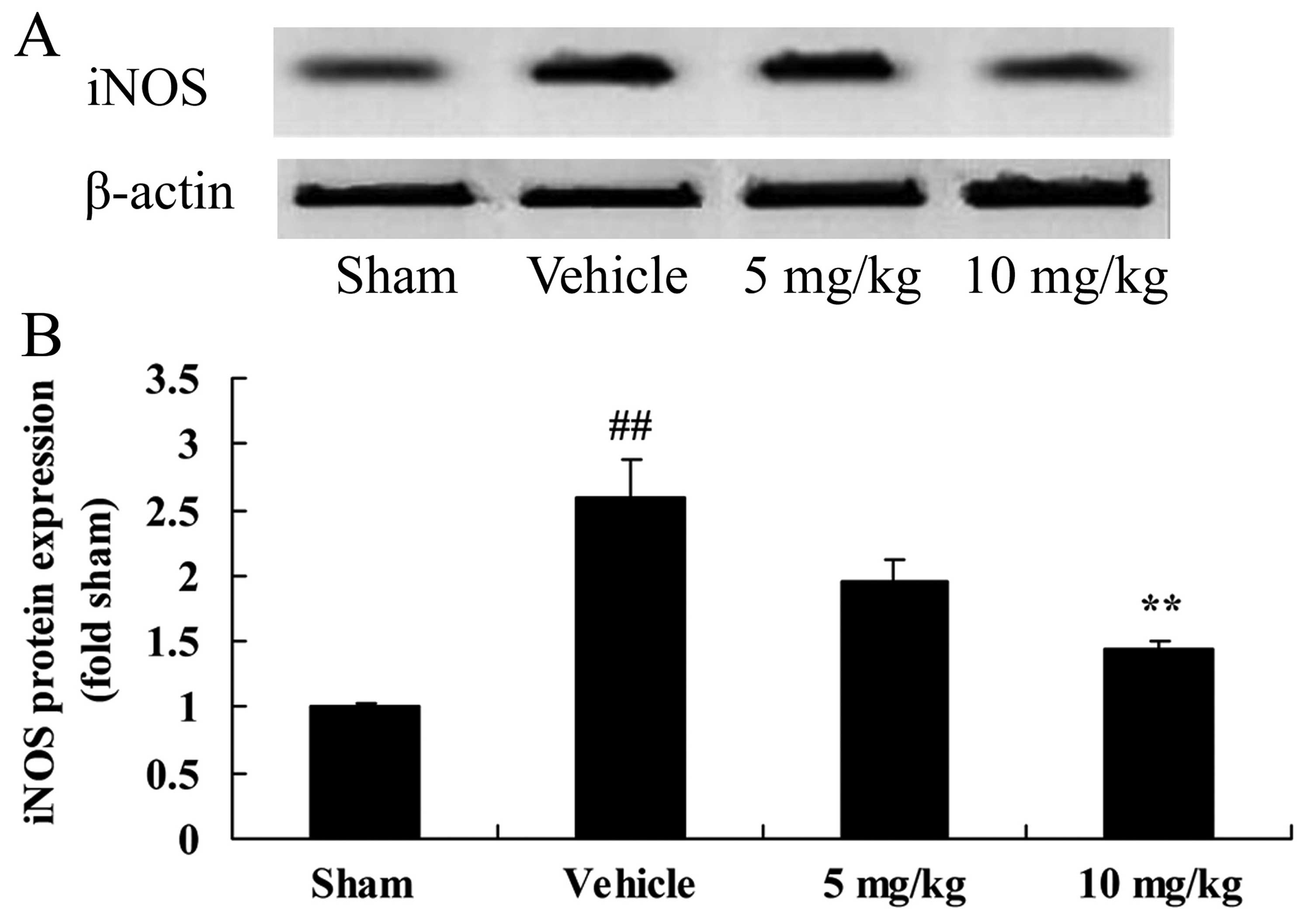
SIRT1 decreases iNOS protein expression
in a rat model of CIRI
The neuroprotective effects of SIRT1 against CIRI
may be modulated by various mechanisms, such as the iNOS pathway.
Compared with the sham-operated group, CIRI significantly increased
iNOS protein expression in the rats with CIRI (Fig. 5). By contrast, treatment with 10
mg/kg of SIRT1 activator 3 significantly decreased iNOS protein
expression in the rats with CIRI (Fig. 5).
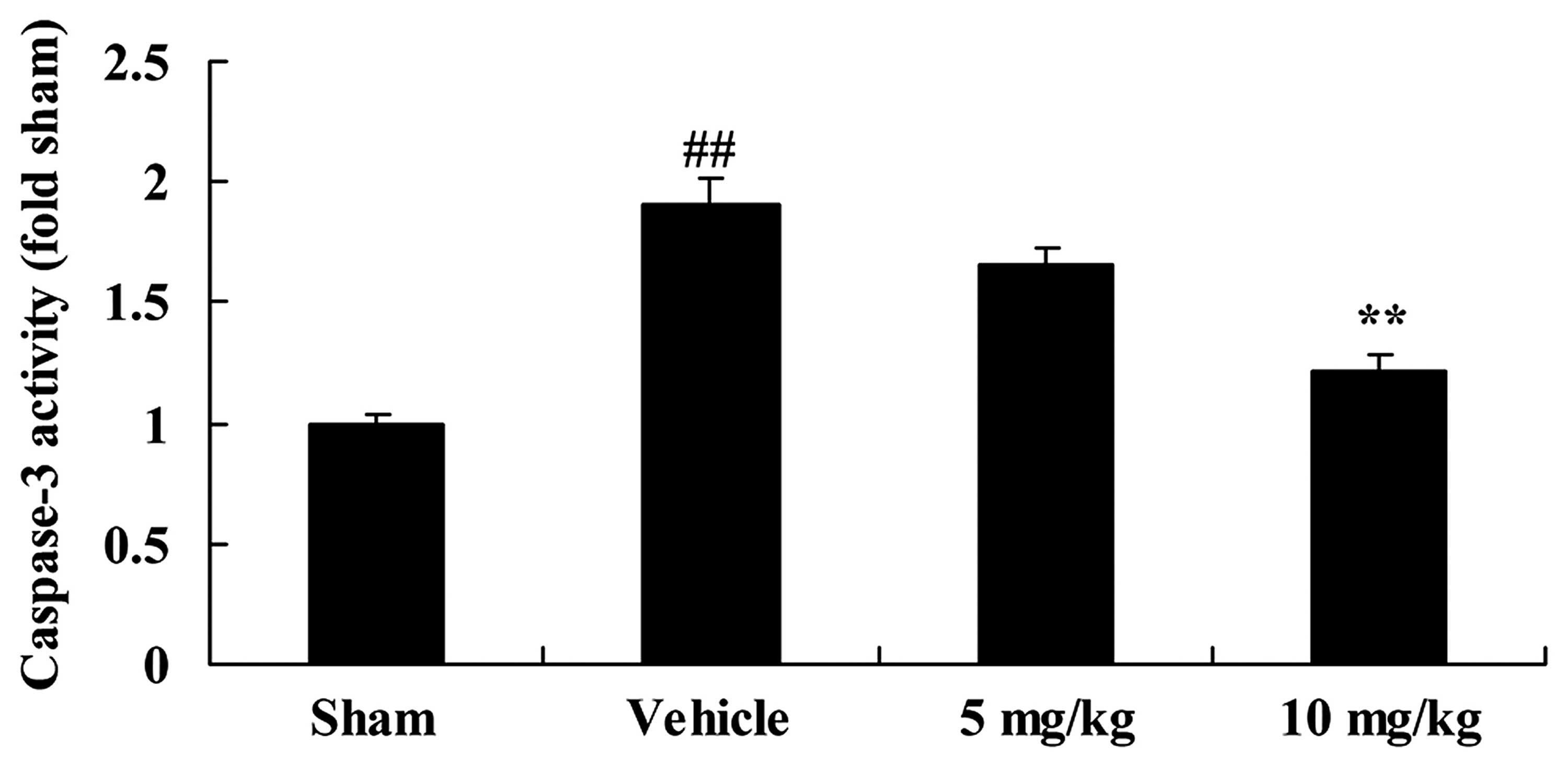
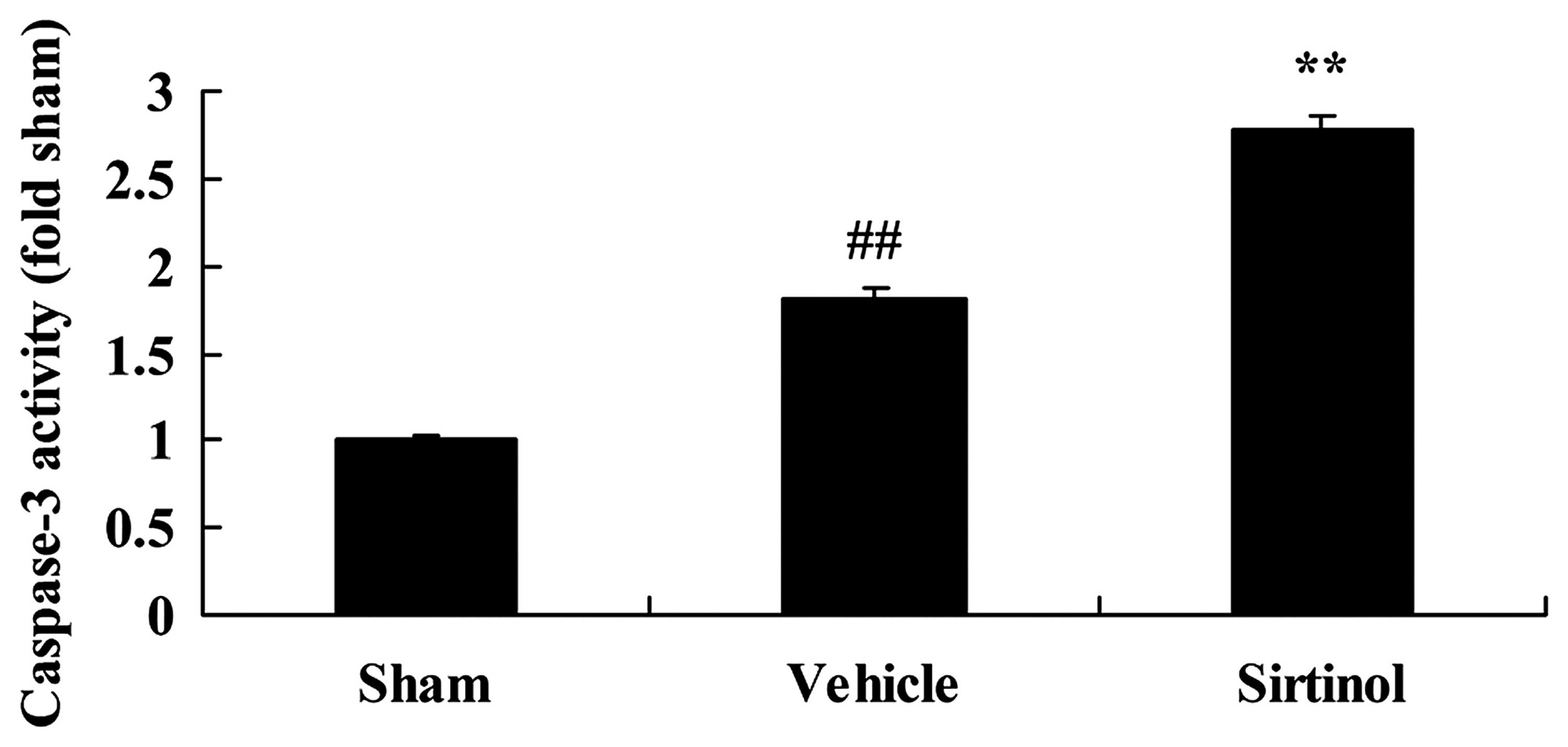
SIRT1 decreases caspase-3 activity in a
rat model of CIRI
To examine the role of caspase-3 activity in the
effects of SIRT1 on CIRI, caspase-3 activity was measured using an
ELISA kit. Indeed, there was a significant increase in caspase-3
activity in the rats with CIRI, compared with those in the
sham-operated group (Fig. 6). By
contrast, treatment with 10 mg/kg of SIRT1 activator 3
significantly decreased caspase-3 activity in the rts with CIRI
(Fig. 6).
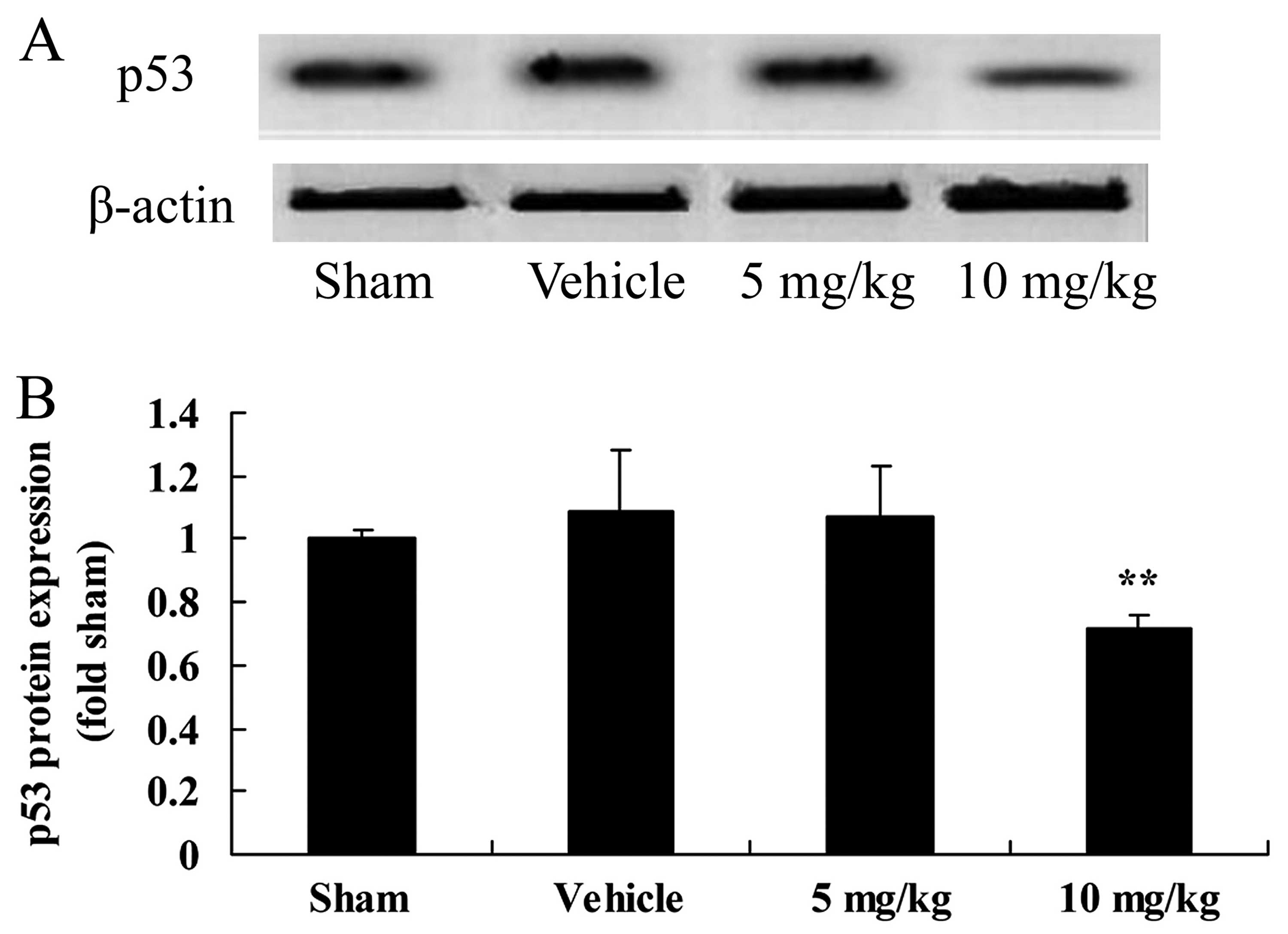
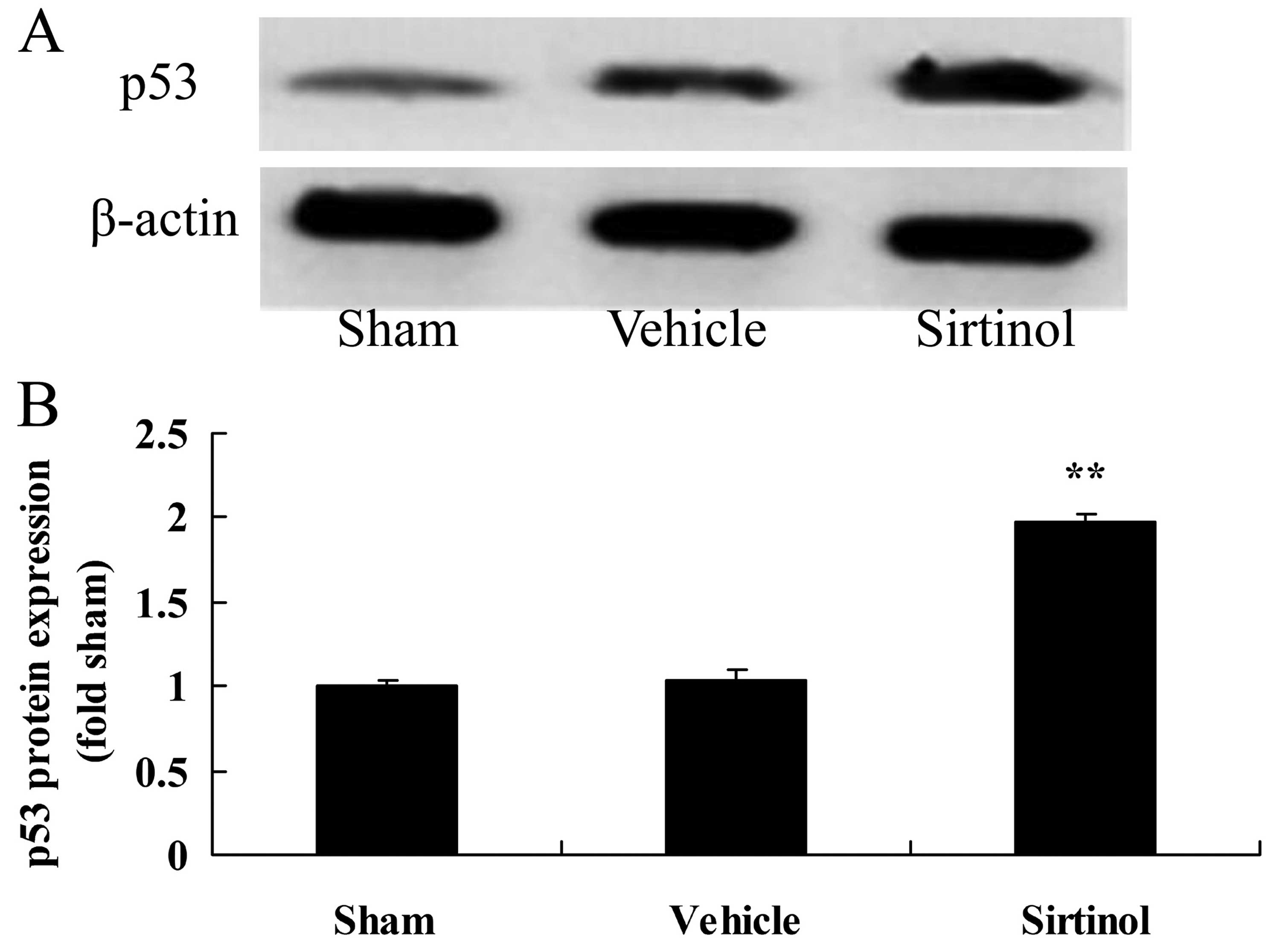
SIRT1 decreases p53 protein expression in
a rat model of CIRI
To examine the expression profile of p53 protein in
the brain tissue samples of rats with CIRI, we performed western
blot analysis. The results revealed that the protein expression of
p53 was upregulated following CIRI, although this did not reach
statistical significance (Fig.
7). Furthermore, treatment with 10 mg/kg of SIRT1 activator 3
significantly decreased p53 expression in the rats with CIRI
(Fig. 7).
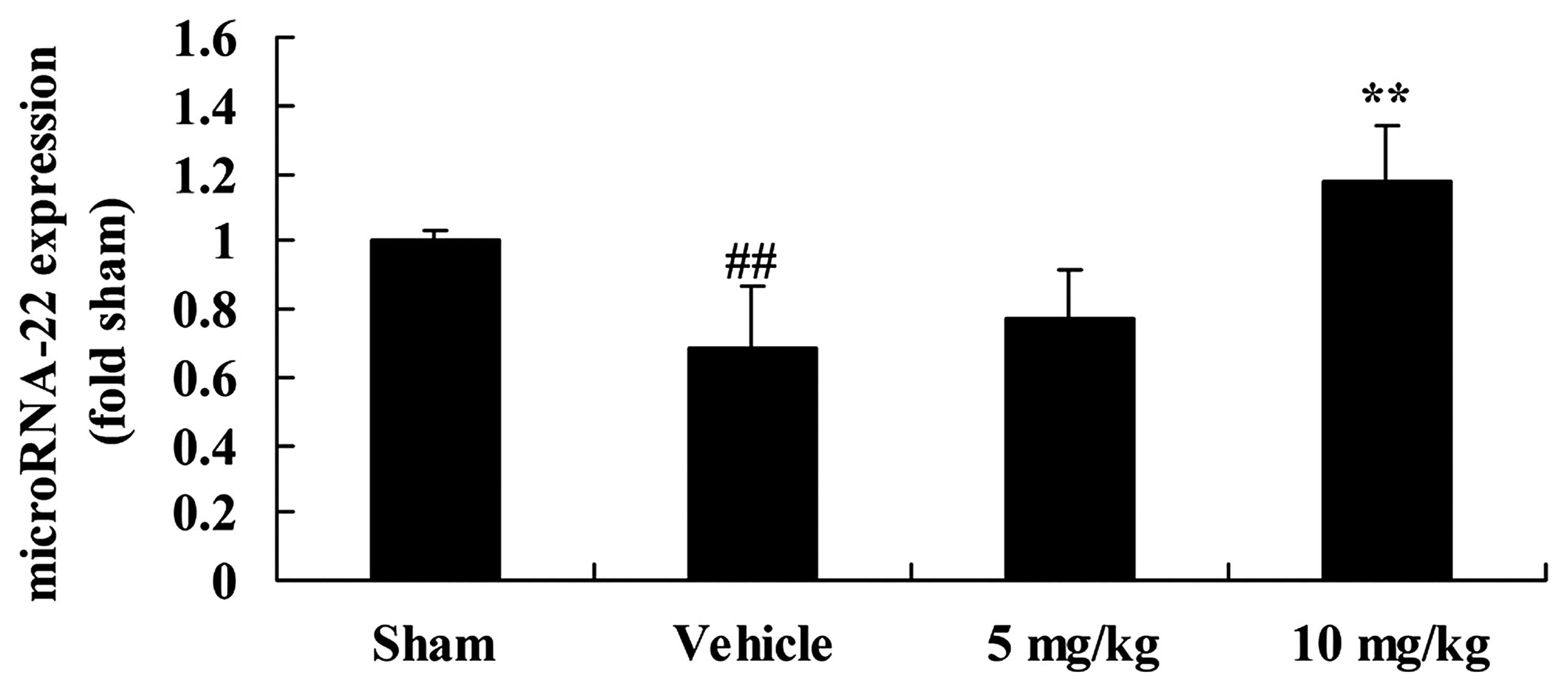
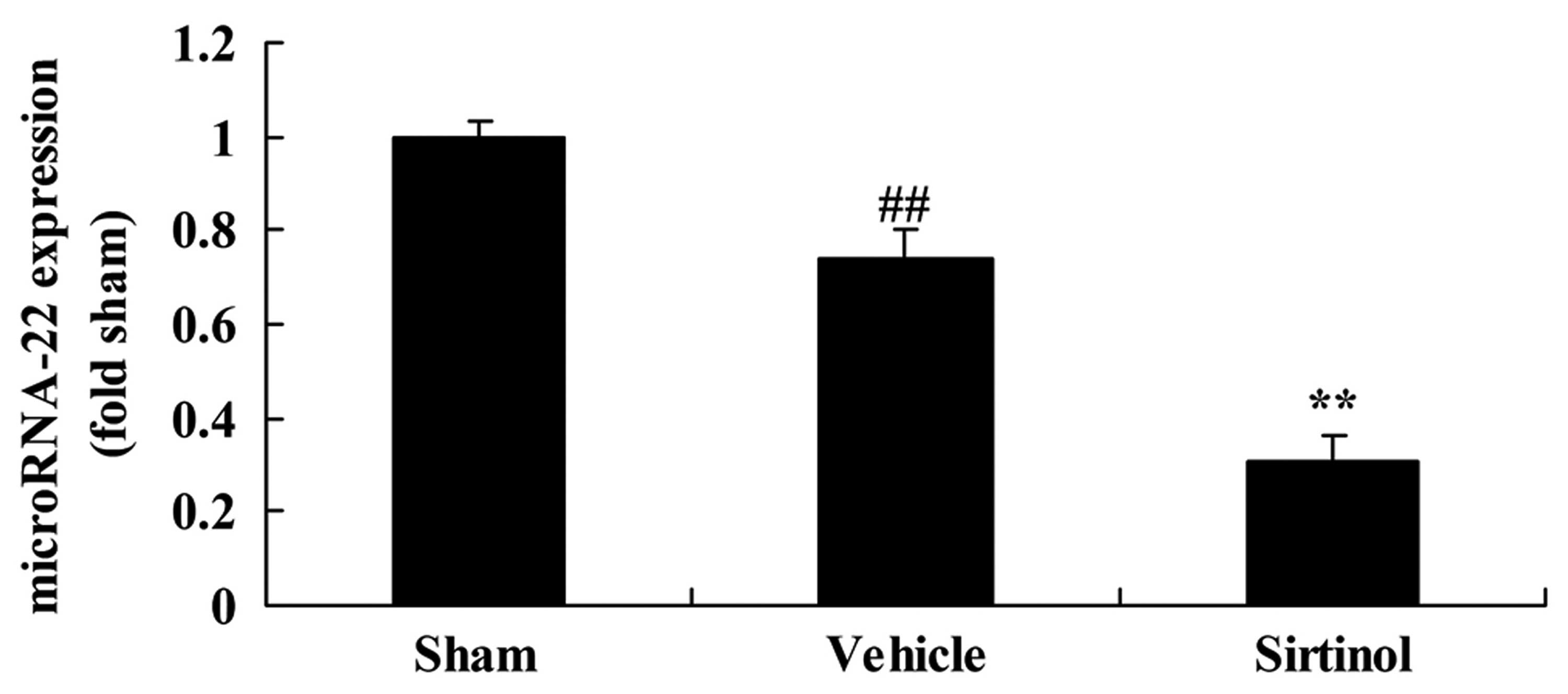
SIRT1 increases microRNA-22 expression in
a rat model of CIRI
We examined the correlation between the effects of
SIRT1 and microRNA-22 expression in our rat model of CIRI. The
expression of microRNA-22 in the rats with CIRI was significantly
inhibited, compared with that in the sham-operated group (Fig. 8). Moreover, the decrease in
microRNA-22 expression in the rats with CIRI was reversed by
treatment with 10 mg/kg of SIRT1 activator 3 (Fig. 8).
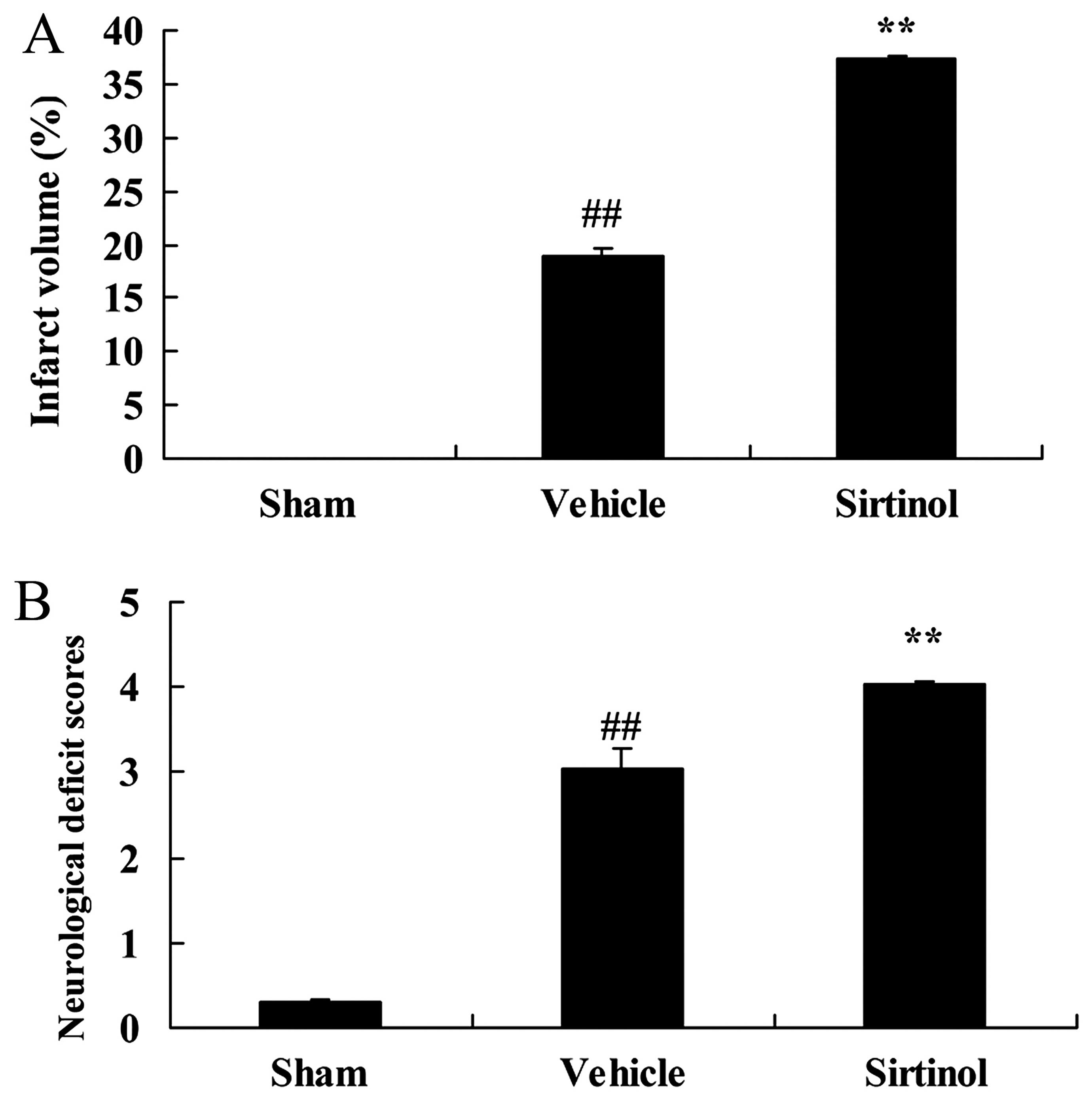
Downregulation of SIRT1 increases the
infarct volume and neurological deficit score in a rat model of
CIRI
We then investigated the effects of the
downregulation of SIRT1 on infarct volume and neurological deficit
score in our rat model of CIRI. As shown by our results, the
downregulation of SIRT1 (by treatment with 10 mg/kg of sirtinol)
significantly increased the infarct volume and neurological deficit
score in the rats with CIRI, compared with the CIRI model group
(Fig. 9).
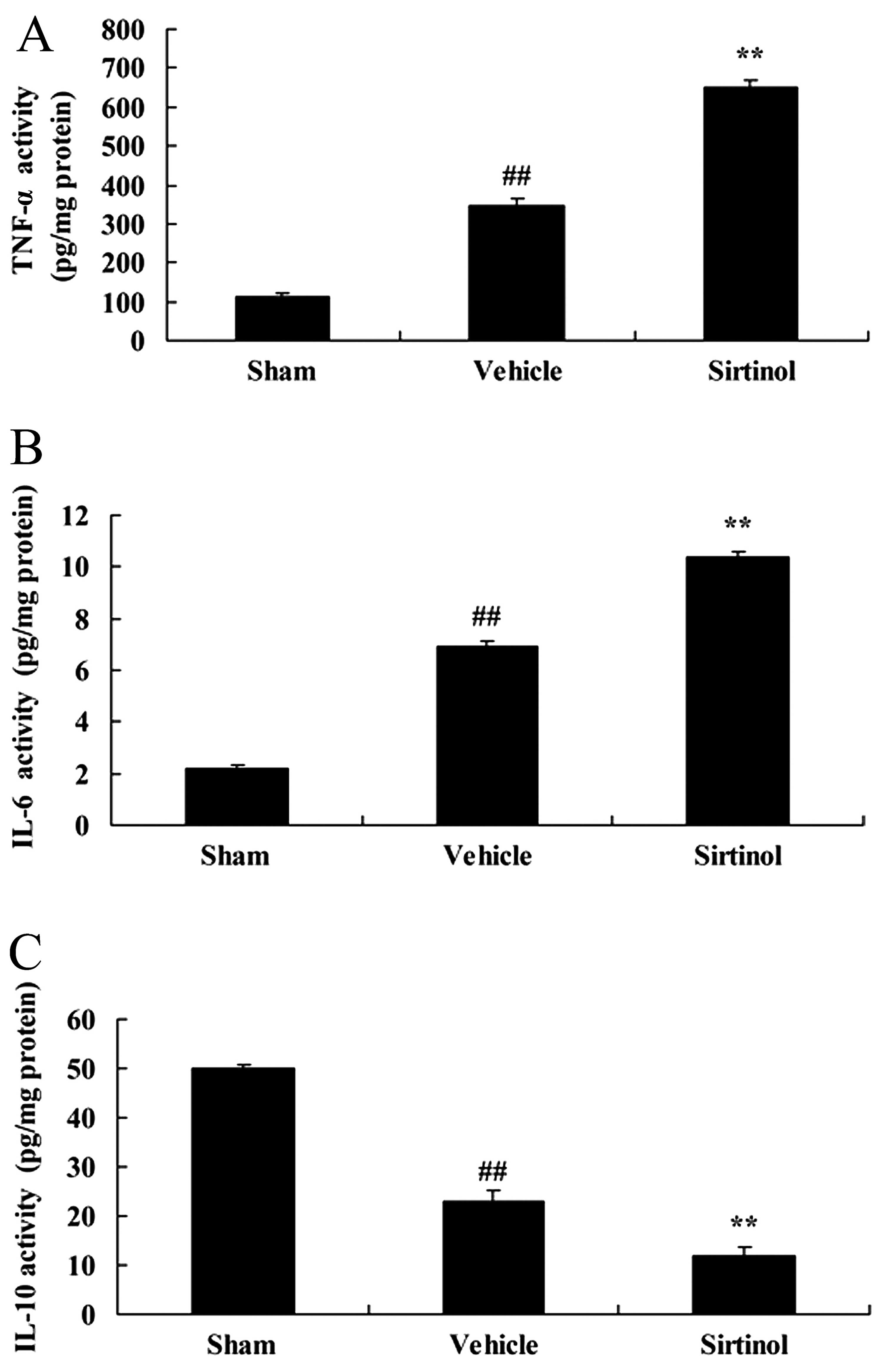
Downregulation of SIRT1 enhances
inflammation in a rat mdoel of CIRI
Subsequently, to further examine the effects of
SIRT1 in CIRI brain tissue, we examined the expression of
inflammation-related markers in rats with CIRI. The downregulation
of SIRT1 (10 mg/kg of sirtinol) significantly increased the
activity of TNF-α and II-6, and inhibited IL-10 activity in the
rats with CIRI, compared with the CIRI model group (Fig. 10).
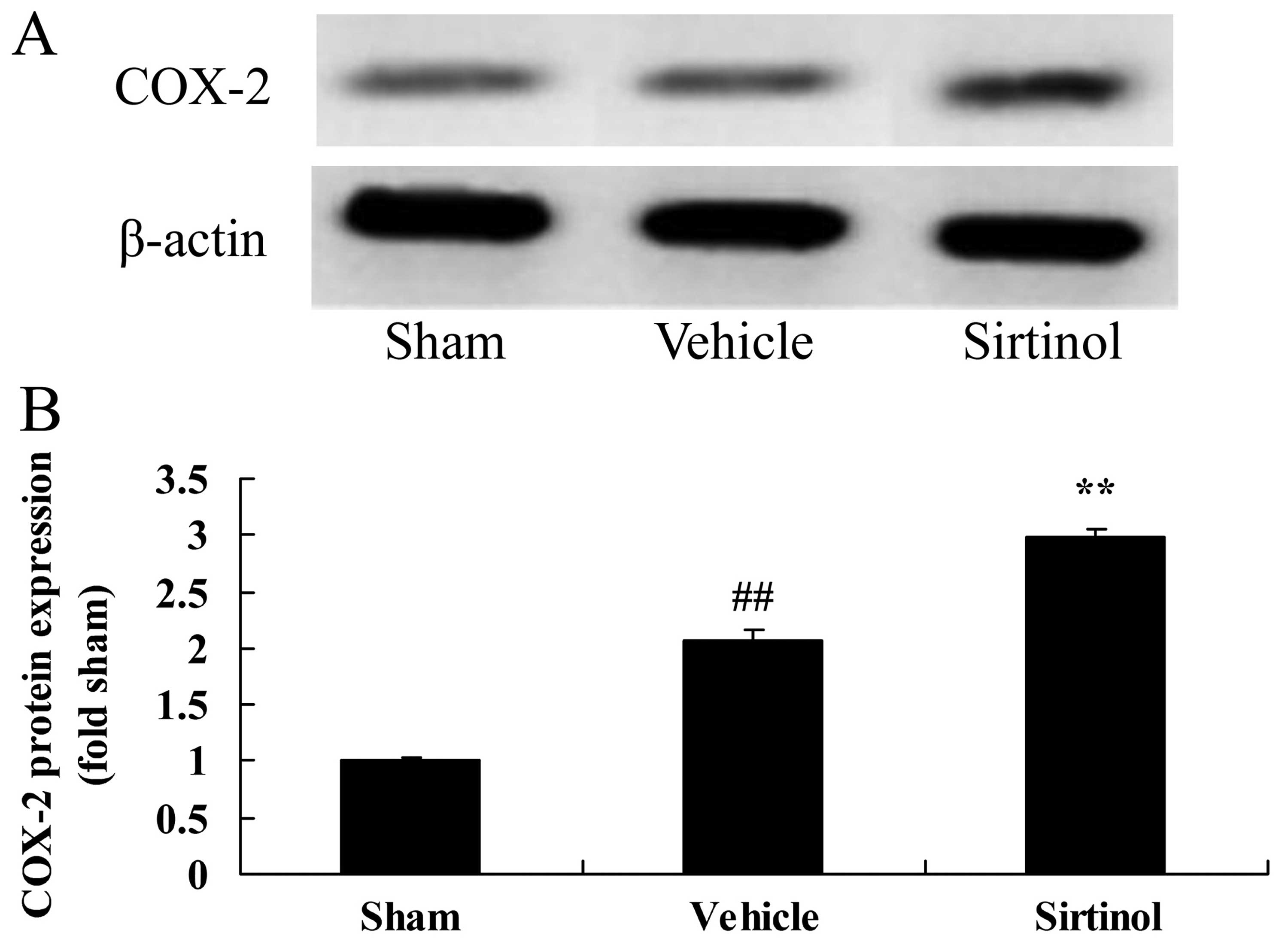
Downregulation of SIRT1 increases COX-2
protein expression in a rat model of CIRI
We then examined COX-2 protein expression following
the downregulation of SIRT1 by western blot analysis. The
downregulation of SIRT1 (by treatment with 10 mg/kg of sirtinol)
significantly increased the protein expression of COX-2 in the rats
with CIRI, compared with the CIRI model group (Fig. 11).
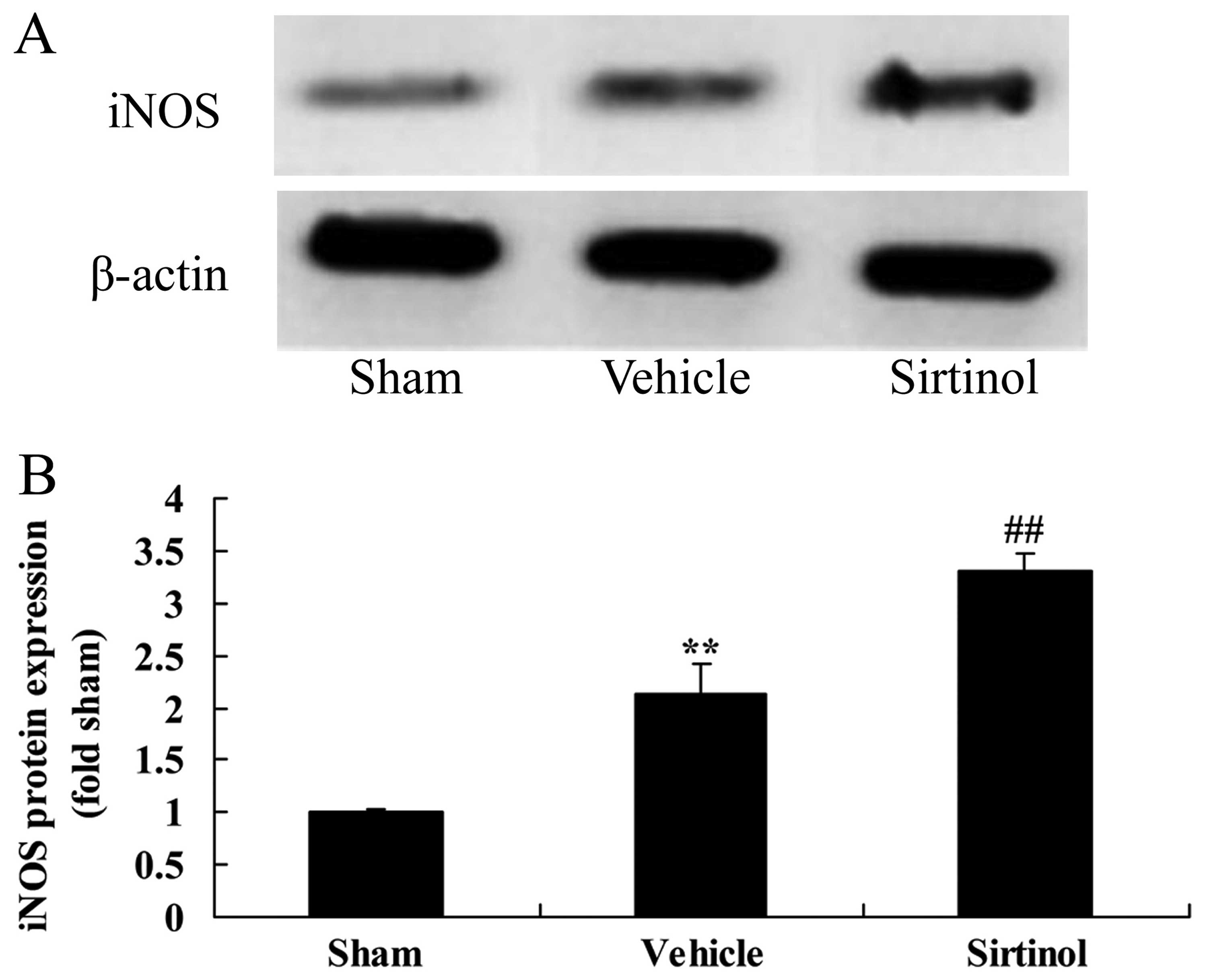
Downregulation of SIRT1 increases iNOS
protein expression in a rat model of CIRI
We then further examined the effects of the
downregulation of SIRT1 on iNOS protein expression in the rats with
CIRI. As shown in Fig. 12, the
downregulation of SIRT1 (by treatment with 10 mg/kg of sirtinol)
significantly enhanced the protein expression of iNOS in the rats
with CIRI, compared with the CIRI model group.
Downregulation of SIRT1 increases
caspase-3 activity in a rat model of CIRI
We then sought to determine whether SIRT1 regulates
caspase-3 activity using a SIRT1 inhibitor. According to the
results of ELISA, the downregulation of SIRT1 (by treatment with 10
mg/kg of sirtinol) significantly increased caspase-3 activity in
the rats with CIRI, compared with the CIRI model group (Fig. 13).
Downregulation of SIRT1 increases p53
protein expression in a rat model of CIRI
As p53 signaling is activated with the upregulation
of SIRT1, we also examined p53 protein expression in the rats with
CIRI following treatment with 10 mg/kg of sirtinol. As shown in
Fig. 14, treatment with 10 mg/kg
of sirtinol significantly increased p53 protein expression in the
rats with CIRI, compared with the CIRI model group.
Downregulation of SIRT1 decreased
microRNA-22 expression in a rat model of CIRI
We also performed real-time PCR to examine the
effects of the downregulation of SIRT1 on microRNA-22 expression in
our rat model of CIRI. Indeed, treatment with 10 mg/kg of sirtinol
significantly inhibited microRNA-22 expression in the rats with
CIRI, compared with the CIRI model group (Fig. 15).
Discussion
During the process of embryonic development, SIRT1
is highly expressed in the brain, spinal cord and dorsal root
ganglion (11). In adult brains,
SIRT1 is highly expressed in the cortex, hippocampus and
epencephalon, while it has a lower expression in aged neurons
(14). At present, there is
evidence to indicate that SIRT1 plays versatile roles in the
central nervous system, such as neurogenesis and neuroprotection.
The increase in the gene expression of SIRT1 can reduce
β-amyloidotic enzyme deposition in rats models of ischemic
stroke-induced brain injury and facilitate neuronal survival
(13). In addition, SIRT1 plays
an important role in the prevention of axonal degenerative disease.
SIRT1 can decide the fate of neuronal precursor cells (22). In our study, we found that SIRT1
exerted neuroprotective effects by decreasing the infarct volume
and neurological deficit score in a rat model of CIRI.
Collectively, our results suggested that SIRT1 may prove to be an
important therapeutic target for the treatment of CIRI. Ding et
al also suggested that SIRT1 protected against myocardial
ischemia-reperfusion injury in diabetic rats (23).
p53 genes are the key regulating point of cellular
signaling pathways under a number of pathological conditions and
complete adaptive responses by interacting with target genes
(24). p53 and its downstream
genes play an essential role in cerebral ischemic cell apoptosis
(25). Downstream genes of p53
have two functions, the regulation of the cell cycle and the
regulation of cell apoptosis, such as the Bcl-2 family (16). In this study, SIRT1 exerted
neuroprotective effects by attenuating p53 protein expression in
rats with CIRI. These data suggest that the decreased expression of
SIRT1 may contribute to the increased expression of p53 protein in
rats with CIRI. Sin et al suggested that the effects of
resveratrol on muscle injury were mediated through SIRT1, p53 and
caspase-3 in skeletal muscle (26).
With neurotransmitter functions, NO is an important
signaling and effector molecule, which extensively participates in
physiological and pathological events (27). In the process of CIRI, according
to different periods of ischemia and reperfusion, cell types and
procedures of NO production, NO can exert dual functions of injury
or protection (28). It has been
suggested that during CIRI, NOS is the key determinant of whether
NO has dual functions (29). At
an advanced stage of CIRI, NO generated by iNOS can intensify the
toxicity of glutamic acid and result in delayed neuronal damage
(27). Particularly, the close
integration of iNOS and calmodulin further aggravates DND (30,31). In this study, we found that SIRT1
exerted neuroprotective effects by suppressing iNOS protein
expression in rats with CIRI.
COX-2 is a key enzyme in the metabolic pathway of
arachidonic acid (AA) (32). Also
known as prostaglandin G/H synthetase, COX is the catalyzing enzyme
of the metabolic transformation of prostaglandins (PGs) and
hromboxane (TX) from AA (24).
COX exists in tissues in the forms of two subtypes. COX-1 is
structural and has certain activities in normal conditions with the
basic functions of maintaining physiological processes and the
stability of the inner environment (33). COX-2 is inducible and is rarely
expressed in normal tissues, while it is highly expressed in
tissues during inflammation (34). Many inflammation simulating
factors, such as LPS, IL-1, TNF, serum, epidermal growth factor-α
and platelet-activating factor can induce the genetic expression of
COX-2 and trigger an increase in the levels of PGE2, PGI2 and PGE1
at inflammatory sites (33). COX
and its metabolites can aggravate CIRI at the vasculature,
blood-brain barrier and neurons (33). The present study clearly
demonstrated that SIRT1 exerted neuroprotective effects by
suppressing COX-2 protein expression in rats with CIRI. Gano et
al reported that the SIRT1 activator, SRT1720, reversed
vascular endothelial dysfunction through COX-2 and inflammation in
aged mice (35).
Neuro-inflammation during CIRI is key factor giving
rise to breakdown, encephaledema and nerve injury. Various measures
to relieve the inflammatory response can significantly protect the
blood-brain barrier and prevent encephaledema and nerve injury. It
has been found that as the dominant ingredient of astragalus,
formononetin can markedly reduce the expression of iNOS, IL-1β and
TNF-α, and decrease the activity of matrix metalloproteinases
(MMPs) and the permeability of the blood-brain barrier, as well as
improve nerve function deficit and exert marked neuroprotective
effects (36). Our results
demonstrated that SIRT1 exerted neuroprotective effect by
suppressing the expression of inflammation-related markers in rats
with CIRI.
A fair amount of evidence has indicated that
apoptosis is strongly linked with nerve cell damage caused by
cerebral hypoxia-ischemia (37).
Following CIRI, DND may occur at the ischemia-sensitive area and is
closely related with molecular mechanisms in the apoptotic process
(38). Following focal brain
ischemia reperfusion, apoptotic pathways are activated at the
ischemia penumbra, resulting in neuronal damage (39). There are three major pathways of
cell apoptosis, namely the extrinsic, intrinsic and endoplasmic
reticulum (40). As a type of
proteolytic enzymes playing key roles, the family of caspases runs
through the beginning to the end of the three pathways. As an
important family member in the caspase family, caspase-3 plays an
essential role in launching, and executing cell apoptosis and cell
death (40,41). Our present in vivo results
demonstrated that SIRT1 exerted neuroprotective effect by
inhibiting caspase-3 activity in rats with CIRI. Sin et al
suggested that the effects of resveratrol on muscle injury were
mediated through SIRT1, p53 and caspase-3 in skeletal muscle
(26).
MicroRNAs are widely involved in the regulation of
nervous system growth and development, as well as in the
maintenance of various biological functions (42). MicroRNAs have been found to be
expressed in cerebral tissues, and to play a role in the growth and
differentiation of cerebral tissues (42). As a complex organ with various
cell types, cerebral tissues have different cellular components and
synaptic connections at different sites (43). Neurons differentiate from
progenitor cells. Studies have confirmed that microRNAs promote the
differentiation of progenitor cells into certain nerve cells by
regulating the expression of target genes and promote
differentiated nerve cells maintain innate features (20). In cerebral tissues, cells of
different types have individual expression profiles (44). In the present study, we also
demonstrated that SIRT1 exerted neuroprotective effects by
increasing microRNA-22 expression in rats with CIRI. Zhang et
al demonstrated that microRNA-22 functions as a tumor
suppressor in renal cell carcinoma through SIRT1 (45).
In conclusion, in the present study, we demonstrated
that SIRT1 exerted neuroprotective effects by decreasing the
infarct volume and neurological deficit score, suppressing
inflammation, decreasing COX-2 and iNOS expression, and inhibiting
caspase-3 activity in a rat model of CIRI through the
p53/microRNA-22 pathway. Our results open a new line of
investigation aimed to determine the neuroprotective effects of
SIRT1, which may prove to be an important therapeutic target in
CIRI.
References
|
1
|
Bots ML, Ford I, Lloyd SM, Laurent S,
Touboul PJ and Hennerici MG; Prevention of Cerebrovascular and
Cardiovascular Events of Ischemic Origin With Terutroban in
Patients With a History of Ischemic Stroke or Transient Ischemic
Attack Vascular Ultrasound Study Investigators: Thromboxane
prostaglandin receptor antagonist and carotid atherosclerosis
progression in patients with cerebrovascular disease of ischemic
origin: A randomized controlled trial. Stroke. 45:2348–2353. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ibaraki M, Ohmura T, Matsubara K and
Kinoshita T: Reliability of CT perfusion-derived CBF in relation to
hemodynamic compromise in patients with cerebrovascular
steno-occlusive disease: A comparative study with 15O PET. J Cereb
Blood Flow Metab. 35:1280–1288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rozanski M, Richter TB, Grittner U, Endres
M, Fiebach JB and Jungehulsing GJ: Elevated levels of hemoglobin
A1c are associated with cerebral white matter disease in patients
with stroke. Stroke. 45:1007–1011. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Li J, Yang Y, Wang X, Zhang Z and
Zhang L: Neuronal apoptosis in cerebral ischemia/reperfusion area
following electrical stimulation of fastigial nucleus. Neural Regen
Res. 9:727–734. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dornbos D III and Ding Y: Mechanisms of
neuronal damage and neuroprotection underlying ischemia/reperfusion
injury after physical exercise. Curr Drug Targets. 13:247–262.
2012. View Article : Google Scholar
|
|
6
|
Szabó C: Physiological and
pathophysiological roles of nitric oxide in the central nervous
system. Brain Res Bull. 41:131–141. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ran M, Li Z, Yang L, Tong L, Zhang L and
Dong H: Calorie restriction attenuates cerebral ischemic injury via
increasing SIRT1 synthesis in the rat. Brain Res. 1610:61–68. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang
YJ, Wu WN, Dong LD and Chen JG: Protection by tetrahydroxystilbene
glucoside against cerebral ischemia: Involvement of JNK, SIRT1, and
NF-kappaB pathways and inhibition of intracellular ROS/RNS
generation. Free Radic Biol Med. 47:229–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Q, Wang SY, Fleuriel C, Dominique L,
Rocheleau JV, Piston DW and Goodman RHL: Metabolic regulation of
SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc Natl
Acad Sci USA. 112:E8192015. View Article : Google Scholar
|
|
10
|
Martin A, Tegla CA, Cudrici CD, Kruszewski
AM, Azimzadeh P, Boodhoo D, Mekala AP, Rus V and Rus H: Role of
SIRT1 in autoimmune demyelination and neurodegeneration. Immunol
Res. 61:187–197. 2015. View Article : Google Scholar
|
|
11
|
Wang L, Zhang L, Chen ZB, Wu JY, Zhang X
and Xu Y: Icariin enhances neuronal survival after oxygen and
glucose deprivation by increasing SIRT1. Eur J Pharmacol.
609:40–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santo EE and Paik J: FOXO3a &
haematopoietic stem cells: Goodbye PI3K, hello SIRT1? Cell Cycle.
15:879–880. 2016. View Article : Google Scholar
|
|
13
|
Miao Y, Zhao S, Gao Y, Wang R, Wu Q, Wu H
and Luo T: Curcumin pretreatment attenuates inflammation and
mitochondrial dysfunction in experimental stroke: The possible role
of Sirt1 signaling. Brain Res Bull. 121:9–15. 2016. View Article : Google Scholar
|
|
14
|
Meng Z, Li J, Zhao H, Liu H, Zhang G, Wang
L, Hu HE, Li DI, Liu M, Bi F, et al: Resveratrol relieves
ischemia-induced oxidative stress in the hippocampus by activating
SIRT1. Exp Ther Med. 10:525–530. 2015.PubMed/NCBI
|
|
15
|
Araki T and Kogure K: Prevention of
delayed neuronal death in gerbil hippocampus by a novel vinca
alkaloid derivative (vinconate). Mol Chem Neuropathol. 11:33–43.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo YB, Bao XJ, Xu SB, Zhang XD and Liu
HY: Honokiol induces cell cycle arrest and apoptosis via p53
activation in H4 human neuroglioma cells. Int J Clin Exp Med.
8:7168–7175. 2015.PubMed/NCBI
|
|
17
|
Leak RK, Zhang L, Luo Y, Li P, Zhao H, Liu
X, Ling F, Jia J, Chen J and Ji X: Peroxiredoxin 2 battles
poly(ADP-ribose) polymerase 1- and p53-dependent prodeath pathways
after ischemic injury. Stroke. 44:1124–1134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y, Deng H, Xu S and Zhang J: MicroRNAs
regulate mitochondrial function in cerebral ischemia-reperfusion
injury. Int J Mol Sci. 16:24895–24917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ni J, Wang X, Chen S, Liu H, Wang Y, Xu X,
Cheng J, Jia J and Zhen X: MicroRNA let-7c-5p protects against
cerebral ischemia injury via mechanisms involving the inhibition of
microglia activation. Brain Behav Immun. 49:75–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Pan Y, Cheng B, Chen J and Bai B:
Identification of conserved and novel microRNAs in cerebral
ischemia-reperfusion injury of rat using deep sequencing. J Mol
Neurosci. 54:671–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang ZB, Zhang Z, Li TB, Lou Z, Li SY,
Yang H, Yang J, Luo XJ and Peng J: Upregulation of brain-enriched
miR-107 promotes excitatory neurotoxicity through downregulation of
glutamate transporter-1 expression following ischaemic stroke. Clin
Sci (Lond). 127:679–689. 2014. View Article : Google Scholar
|
|
22
|
Kalaivani P, Ganesh M, Sathiya S, Ranju V,
Gayathiri V and Saravana Babu C: Alteration in bioenergetic
regulators, SirT1 and Parp1 expression precedes oxidative stress in
rats subjected to transient cerebral focal ischemia: molecular and
histopathologic evidences. J Stroke Cerebrovasc Dis. 23:2753–2766.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding M, Lei J, Han H, Li W, Qu Y, Fu E, Fu
F and Wang X: SIRT1 protects against myocardial
ischemia-reperfusion injury via activating eNOS in diabetic rats.
Cardiovasc Diabetol. 14:1432015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chai YS, Hu J, Lei F, Wang YG, Yuan ZY, Lu
X, Wang XP, Du F, Zhang D, Xing DM, et al: Effect of berberine on
cell cycle arrest and cell survival during cerebral ischemia and
reperfusion and correlations with p53/cyclin D1 and PI3K/Akt. Eur J
Pharmacol. 708:44–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tu YF, Lu PJ, Huang CC, Ho CJ and Chou YP:
Moderate dietary restriction reduces p53-mediated neurovascular
damage and microglia activation after hypoxic ischemia in neonatal
brain. Stroke. 43:491–498. 2012. View Article : Google Scholar
|
|
26
|
Sin TK, Yung BY, Yip SP, Chan LW, Wong CS,
Tam EW and Siu PM: SIRT1-dependent myoprotective effects of
resveratrol on muscle injury induced by compression. Front Physiol.
6:2932015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen J, Ma S, Chan P, Lee W, Fung PC,
Cheung RT, Tong Y and Liu KJ: Nitric oxide downregulates caveolin-1
expression in rat brains during focal cerebral ischemia and
reperfusion injury. J Neurochem. 96:1078–1089. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kidd GA, Hong H, Majid A, Kaufman DI and
Chen AF: Inhibition of brain GTP cyclohydrolase I and
tetrahydrobiopterin attenuates cerebral infarction via reducing
inducible NO synthase and peroxynitrite in ischemic stroke. Stroke.
36:2705–2711. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heeba GH and El-Hanafy AA: Nebivolol
regulates eNOS and iNOS expressions and alleviates oxidative stress
in cerebral ischemia/reperfusion injury in rats. Life Sci.
90:388–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen YF, Wang YW, Huang WS, Lee MM, Wood
WG, Leung YM and Tsai HY: Trans-cinnamaldehyde, an essential oil in
cinnamon powder, ameliorates cerebral ischemia-induced brain injury
via inhibition of neuroinflammation through attenuation of iNOS,
COX-2 expression and NFκ-B signaling pathway. Neuromolecular Med.
18:322–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren B, Zhang YX, Zhou HX, Sun FW, Zhang
ZF, Wei Z, Zhang CY and Si DW: Tanshinone IIA prevents the loss of
nigrostriatal dopaminergic neurons by inhibiting NADPH oxidase and
iNOS in the MPTP model of Parkinson's disease. J Neurol Sci.
348:142–152. 2015. View Article : Google Scholar
|
|
32
|
Han X, Li H, Su L, Zhu W, Xu W, Li K, Zhao
Q, Yang H and Liu H: Effect of celecoxib plus standard chemotherapy
on serum levels of vascular endothelial growth factor and
cyclooxygenase-2 in patients with gastric cancer. Biomed Rep.
2:183–187. 2014.PubMed/NCBI
|
|
33
|
Vaibhav K, Shrivastava P, Javed H, Khan A,
Ahmed ME, Tabassum R, Khan MM, Khuwaja G, Islam F, Siddiqui MS, et
al: Piperine suppresses cerebral ischemia-reperfusion-induced
inflammation through the repression of COX-2, NOS-2, and NF-κB in
middle cerebral artery occlusion rat model. Mol Cell Biochem.
367:73–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gaur V and Kumar A: Effect of nonselective
and selective COX-2 inhibitors on memory dysfunction, glutathione
system, and tumor necrosis factor alpha level against cerebral
ischemia reperfusion injury. Drug Chem Toxicol. 35:218–224. 2012.
View Article : Google Scholar
|
|
35
|
Gano LB, Donato AJ, Pasha HM, Hearon CM
Jr, Sindler AL and Seals DR: The SIRT1 activator SRT1720 reverses
vascular endothelial dysfunction, excessive superoxide production,
and inflammation with aging in mice. Am J Physiol Heart Circ
Physiol. 307:H1754–H1763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun K, Fan J and Han J: Ameliorating
effects of traditional Chinese medicine preparation, Chinese
materia medica and active compounds on ischemia/reperfusion-induced
cerebral microcirculatory disturbances and neuron damage. Acta
Pharm Sin B. 5:8–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu H, Liu P, Tang H, Jing J, Lv X, Chen L,
Jiang L, Xu J and Li J: Oleuropein, a natural extract from plants,
offers neuroprotection in focal cerebral ischemia/reperfusion
injury in mice. Eur J Pharmacol. 775:113–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Wang P, Li S, Wang S, Li Y, Liang
N and Wang M: Mdivi-1 prevents apoptosis induced by
ischemia-reperfusion injury in primary hippocampal cells via
inhibition of reactive oxygen species-activated mitochondrial
pathway. J Stroke Cerebrovasc Dis. 23:1491–1499. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang JF, Shi LL, Zhang L, et al:
MicroRNA-25 negatively regulates cerebral ischemia/reperfusion
injury-induced cell apoptosis through Fas/FasL pathway. J Mol
Neurosci. 58:507–516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yaidikar L and Thakur S: Punicalagin
attenuated cerebral ischemia-reperfusion insult via inhibition of
proinflammatory cytokines, up-regulation of Bcl-2, down-regulation
of Bax, and caspase-3. Mol Cell Biochem. 402:141–148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tao T, Li CL, Yang WC, Zeng XZ, Song CY,
Yue ZY, Dong H and Qian H: Protective effects of propofol against
whole cerebral ischemia/reperfusion injury in rats through the
inhibition of the apoptosis-inducing factor pathway. Brain Res.
1644:9–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stary CM, Xu L, Sun X, Ouyang YB, White
RE, Leong J, Li J, Xiong X and Giffard RG: MicroRNA-200c
contributes to injury from transient focal cerebral ischemia by
targeting Reelin. Stroke. 46:551–556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu P, Zhao H, Wang R, Wang P, Tao Z, Gao
L, Yan F, Liu X, Yu S, Ji X, et al: MicroRNA-424 protects against
focal cerebral ischemia and reperfusion injury in mice by
suppressing oxidative stress. Stroke. 46:513–519. 2015. View Article : Google Scholar
|
|
44
|
Brandenburger T, Grievink H, Heinen N,
Barthel F, Huhn R, Stachuletz F, Kohns M, Pannen B and Bauer I:
Effects of remote ischemic preconditioning and myocardial ischemia
on microRNA-1 expression in the rat heart in vivo. Shock.
42:234–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang S, Zhang D, Yi C, Wang Y, Wang H and
Wang J: MicroRNA-22 functions as a tumor suppressor by targeting
SIRT1 in renal cell carcinoma. Oncol Rep. 35:559–567. 2016.
|