Introduction
Diabetes poses a significant global health concern.
It is estimated that 382 million individuals are suffering from
diabetes, and this number is expected to increase to 592 million by
the year 2035 (1). Although
pancreatic islet transplantation has been proposed as an effective
therapy in diabetes, it is largely limited by the shortage of islet
donors, poor islet survival and the requirement for lifelong
immunosuppression (2). In recent
years, biomaterials have been used as an immunoisolation technique
in islet transplantation. This technique aims at producing
biological barriers which prevent immune cell migration and
maintain the long-term function of transplanted islets (3,4).
Biomaterials putatively offer several potential benefits, such as
delivering proteins and growth factors, protecting the islets from
immune rejection without the use of an immunosuppressor, and
increasing the safety and clinical effect of the procedure
(5–7). A number of natural and synthetic
materials have been investigated in islet transplantation,
including alginate, polyvinyl alcohol and silk hydrogel (8–10).
However, there are still significant challenges that need to be
resolved, such as methods of providing oxygen and nutrients to
coated islets, reducing the damage due to the inflammatory
response, and selecting suitable sites for transplantation
(11).
The porcine small intestinal submucosa (SIS) is a
new bioactive material composed of collagen I and fibronectin. SIS
includes proteoglycan, glycosaminoglycan, glycoprotein and growth
factors (12). Compared with
other synthetic materials, SIS is easy to handle and elicits no
immune response in the recipient organism (13). As a safe material, SIS has been
successfully applied in clinical practice, including general
pediatric surgery, urology and neurosurgery (14). SIS has a 3-dimensional
microarchitecture that contains many cytokines, and can serve as a
scaffold for cell growth and proliferation (15,16). Several research groups, including
ours, have demonstrated that SIS can improve the islet survival
rate, boost insulin secretion and reduce cell apoptosis in
vitro (17–19); however, the precise mechanisms and
the effects in vivo remain unclear.
The mesenchymal stromal cell (MSC) is an adult stem
cell (20) that is considered a
suitable candidate for regenerative medicine and cell-based
therapy, due to its ease of isolation, self-renewal potential,
multipotency and immunomodulatory function (21,22). MSCs can promote angiogenesis by
producing a large number of cytokines. In addition, MSCs have
anti-apoptotic, anti-inflammatory and mitogenic effects. It has
been reported that MSCs can maintain islet organization and
morphology, improve graft revascularization, suppress inflammatory
damage and mediate immune responses, promoting prolonged graft
survival and enhanced islet function (23–27).
It is unknown whether a scaffold containing both SIS
and MSCs may improve islet function and islet survival. Thus, in
the present study, in an aim to clarify this issue, we investigated
the effects of a SIS-MSC scaffold on islet function and survival
in vitro and in vivo.
Materials and methods
Rats
The Animal Care and Use Committee of Xi'an Jiaotong
University approved all the animal protocols. Sprague-Dawley rats
were purchased from the Laboratory Animal Center, Xi'an Jiaotong
University, Xi'an, China. Bamei pigs were purchased from Xi'an
Zhuque market, Xi'an, China. Rats (Laboratory Animal Center, Xi'an
Jiaotong University, Xi'an, China) were raised in a
specific-pathogen-free laboratory (temperature 18–26°C, relative
humidity 40–70%) and were provided with free access to food and
water.
Rat MSC isolation and identification
MSCs were acquired from Sprague-Dawley rats (n=8;
male, 3 weeks of age, 60–80 g). The rats were sacrificed by the
spinal dislocation method before obtaining the bones. Briefly, the
femurs and tibiae were removed in a sterile environment and the
bone cavity was lightly flushed with phosphate-buffered saline
(PBS) using a 21-gauge needle. The flushed samples were centrifuged
at 1,000 rpm for 5 min, and the supernatant was removed. The cells
were resuspended in complete medium (Cyagen Biosciences, Guangzhou,
China), and incubated by the adherence culture method, as
previously descrbied (28) to
isolate the MSCs. MSCs were identified by surface molecular markers
[CD90 (anti-mouse/rat CD90.1(Thy-1.1) PE; 12-0900-81, eBioscience,
San Diego, CA USA) and CD34 (anti-mouse CD34 FITC; 11-0341-82,
eBioscience)] with the use of a flow cytometer, and their ability
to differentiate into osteoblast-like and adipocyte-like cells was
assessed by Alizarin Red S and Oil Red O staining (both from Cyagen
Biosciences). All experiments were carried out using MSCs at
passage 3–6.
Isolation of SIS from Bamei pigs and
SIS-MSC scaffold preparation
SIS isolation and preparation was performed as
previously described (29).
Briefly, Bamei pigs (male, 6 months of age, 100–120 kg) were
sacrificed by a lethal injection of sodium pentobarbital and the
SIS was prepared within 4 h. The jejunum was washed with water, and
fat, tunica serosa, and tunica muscularis were removed. The jejunum
was dipped in 1 g/l peroxyacetic acid (Shaanxi Three Bridge
Chemical Co., Ltd., Shaanxi, China) to sterilize and maintained in
PBS until use. SIS was identified by hematoxylin and eosin
(H&E) staining and scanning electron microscopy (Hitachi,
Tokyo, Japan).
To generate the SIS-MSC scaffold, the SIS was cut
into 22×22 mm sections and each section was placed on a Nunclon
35-mm petri dish (Thermo Fisher Scientific, Waltham, MA, USA).
Approximately 1×106 MSCs were seeded in the dish and
cultured for 48 h to form a confluent monolayer.
Rat islet isolation and
identification
Islets were isolated from Sprague-Dawley rats (n=10;
male, 8 weeks of age, 280–300 g) as previously described (30). The rats were anesthetized by an
intraperitoneal injection of pentobarbital before obtaining the
pancreas for the islets. The pancreas was digested with 1 mg/ml
collagenase P (Roche, Mannheim, Germany) and purified by
Histopaque-1077 (Sigma, St. Louis, MO, USA). After washing with
RPMI-1640 medium (Gibco, Carlsbad, CA, USA), the islets were
distributed into groups of 200 for culture. Islets were identified
by dithizone and their viability was estimated by acridine
orange/propidium iodide (AO/PI), described below (both from
Gibco).
Experimental design
The islets were divided into 3 groups as follows:
group A, islets; group B, SIS-islets; and group C, SIS-MSC-islets.
In the controls (group A), 200 fresh islets were cultured alone in
non-treated 35-mm Petri dishes. For group B, 200 fresh islets were
seeded on SIS. For group C, 200 fresh islets were seeded on the
SIS-MSC scaffold.
RPMI-1640 containing 10% fetal calf serum was used
for all co-culture configurations. All islets were cultured in a
carbon dioxide incubator (37°C, 5% CO2).
In vitro islet function tests
The islets from the 3 groups were collected on days
7 and 14. The viability rate of the islets was estimated by AO/PI
as follows: viability rate, % =
numbersgreen/numbersgreen + red ×100.
Islet function was measured by a glucose-stimulated
insulin secretion test. Briefly, the islets were incubated in
RPMI-1640 containing 1.67 mmol/l glucose for 2 h, and then
incubated in RPMI-1640 containing 16.7 mmol/l glucose for a further
2 h. Insulin secretion was assessed with an enzyme-linked
immunosorbent assay (ELISA) kit (Mercodia, Uppsala, Sweden). The
insulin release stimulation index (SI) was calculated as follows:
SI = insulin concentration (16.7 mmol/l)/insulin concentration
(1.67 mmol/l).
Immunohistological analysis and cytokine
detection
In each group, the islets and cultured supernatants
were obtained at day 7. The islets were fixed in 10%
paraformaldehyde (Beyotime, Shanghai, China) overnight and embedded
in paraffin. The paraffin sections were stained with a rabbit
insulin monoclonal antibody (1:100; Cell Signaling Technology,
Danvers, MA, USA), followed by 3,3′-diaminobenzidine (DAB)
immunostaining. Images were acquired using a light microscope
(Olympus, Tokyo, Japan). Cytokines [vascular endothelial growth
factor A (VEGFA), ciliary neurotrophic factor (CNTF), epidermal
growth factor (EGF), hepatocyte growth factor (HGF) and tumor
necrosis factor (TNF)] in cultured supernatants were detected using
an ELISA kit (Mercodia) in accordance with the manufacturer's
instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA was extracted from the islets on day 14 using an
RNeasy kit (Qiagen, Valencia, CA, USA). Reverse transcription was
performed using a FastQuant RT kit with gDNase (Tiangen, Beijing,
China) from 2 mg total RNA. Quantitative PCR (qPCR) was performed
using a maxima SYBR-Green qPCR master mix (Thermo Fisher
Scientific) protocol on the instructions of CFX96 (Bio-Rad,
Hercules, CA, USA). The PCR products were amplified using the
following primer sets: for glyceraldehyde 3-phosphate dehydrogenase
(Gapdh), 5′-TACCCACGGCAAGTTCAACG-3′ and
5′-CACCAGCATCACCCCATTTG-3′; for pancreatic and duodenal homeobox 1
(Pdx1), 5′-GGAACGCTGGAACAGGGAAG-3′ and
5′-CAGTCTCGGTTCCATTCGGG-3′; for insulin 1 (Ins1),
5′-GACCATTGATTCCGTGACAT-3′ and 5′-CACCAGAGCATAGGAGCGAC-3′; and for
vascular endothelial growth factor A (Vegfa),
5′-AGGAGTACCCCGATGAGATA-3′ and 5′-ATCTCTCCTATGTGCTGGCT-3′.
Every sample was tested in triplicate and the
results were quantified using the Livak 2−ΔΔCT method,
where CT is the cycle threshold. Relative gene expression was
determined by the ΔΔCT method relative to the GAPDH gene
expression. All data are presented as the means ± SEM. A value of
P<0.05 was considered to indicate a statistically significant
difference compared to the control group.
Immunofluorescence staining
The cultured islets were collected on day 14 and
treated using a cell smear centrifuge. The smears were fixed in 4%
paraformaldehyde and permeabilized with Triton X-100 (Beyotime).
After blocking with 10% donkey serum, the smears were incubated
serially with a rabbit insulin monoclonal antibody (#3014) or a
mouse cluster of differentiation (CD)31 monoclonal antibody (#3528)
(1:100; both from Cell Signaling Technology). The smears were then
incubated with secondary antibody (donkey anti-rabbit Alexa 488,
711-545-152, 1:500 or donkey anti-mouse Cy3, 715-165-150, 1:500;
Jackson Immunoresearch, West Grove, PA, USA). Finally, the smears
ware counterstained with 4′,6-diamidino-2-phenylindole (DAPI;
Beyotime). Images were obtained using a confocal laser scanning
microscope (Leica). Graphical representations are expressed as the
average of mean fluorescence intensity (MFI) using arbitrary units
(AU) ± SD. A value of P<0.05 was considered to indicate a
statistically significant difference compared with the control
group, as determined by the Tukey-Kramer post test.
Islet transplantation
Sprague-Dawley rats (n=30; male, 6–8 weeks of age,
250–300 g) were rendered diabetic by a single intraperitoneal
injection of streptozotocin (STZ, 50 mg/kg). The rats were
anesthetized by an intraperitoneal injection of pentobarbital
before transplantation. The rats were randomly apportioned into 3
groups as follows: group A, islets (control); group B, SIS-islets;
and group C, SIS-MSC-islets. In the controls (group A), the rats
received 1,000-islet transplantation under the skin. In group B,
1,000 SIS-coated islets were folded to a size of 1×1 cm and fixed
on the back under the skin of the rats. In group C,
5×106 MSCs were seeded onto SIS to generate an SIS-MSC
scaffold within 48 h, and 1,000 islets were coated and folded as in
group B for transplantation. No immunosuppressive protocols were
applied in the recipient rats.
Graft survival and function
To assess islet graft survival and function, blood
glucose and insulin concentrations were monitored. Blood was
obtained from the tail vein using a syringe. Successful islet
function was defined as blood glucose levels <11.1 mmol/l on 2
consecutive days. Graft rejection was defined as blood glucose
levels >11.1 mmol/l on 2 consecutive days. The mean survival
time of the grafts was recorded.
Statistical analysis
The statistical significance of the differences was
determined using one-way analysis of variance. Statistical analyses
were performed using SPSS17.0 software. A P-value <0.05 was
considered to indicate a statistically significant difference.
Differences among the groups with regard to blood glucose and
insulin concentration were analyzed by multivariate analysis.
Results
Characterization of MSCs, islets and
SIS
To characterize the MSCs, bone marrow cells isolated
from rats were stained for surface molecular marks (CD90 and CD34)
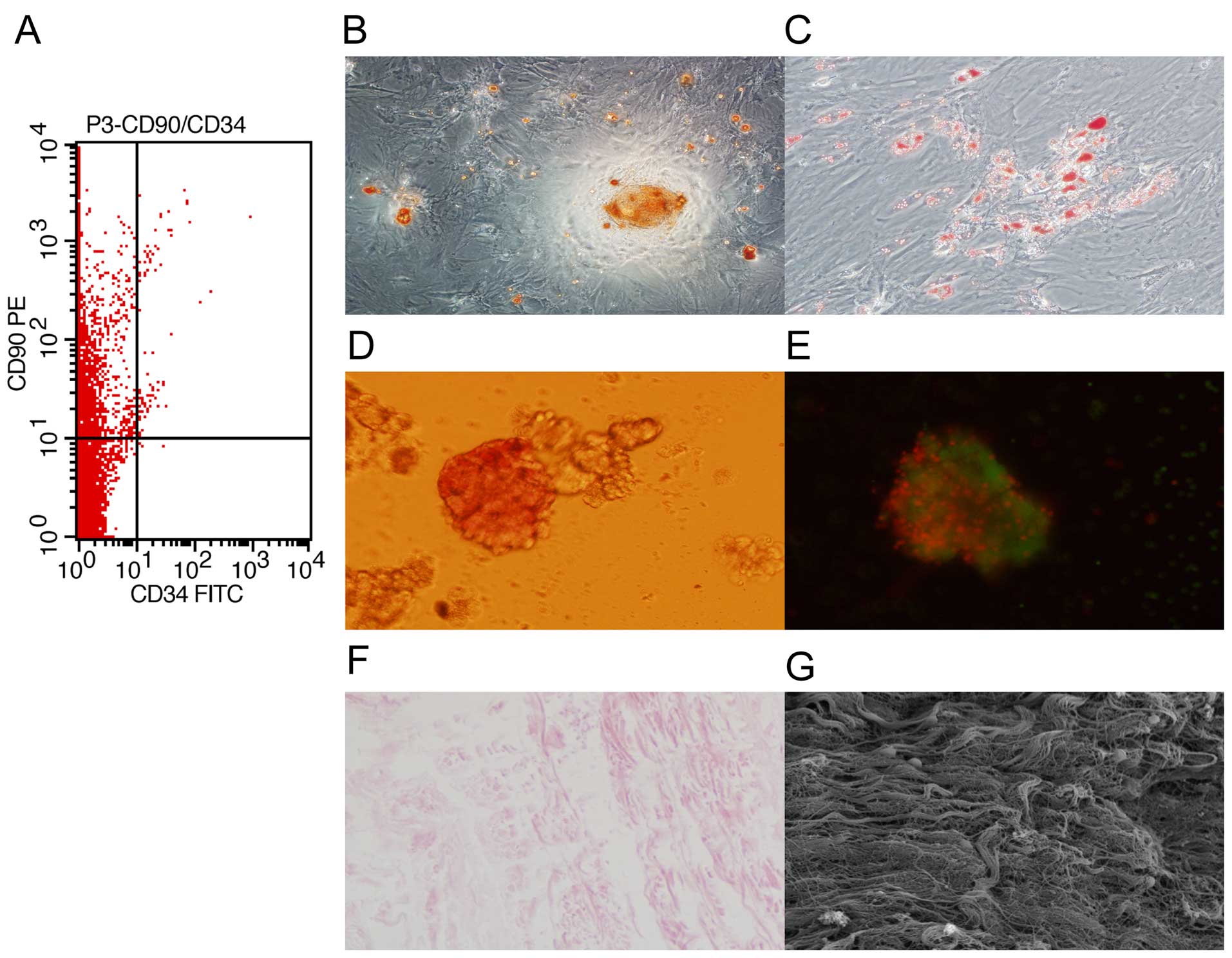
and analyzed by flow cytometry. We found that the cells were
positive for CD90 and negative for CD34 (Fig. 1A). In addition, the cells were
able to differentiate into osteoblast-like and adipocyte-like cells
(Fig. 1B and C). These results
confirmed that the isolated cells were MSCs.
To characterize the islets, islets isolated from
rats were identified with dithizone and AO/PI staining. We found
that the islets were stained with dithizone and AO/PI (Fig. 1D and E). These results indicated
that islet isolation was successful.
To characterize SIS, the SIS from Bamei pigs was
observed respectively under a light microscope and scanning
electron microscope. We found that the SIS was composed of collagen
fiber with no cells (Fig. 1F and
G), indicating that the isolation was successful.
SIS-MSC scaffold enhances islet viability
and function in vitro
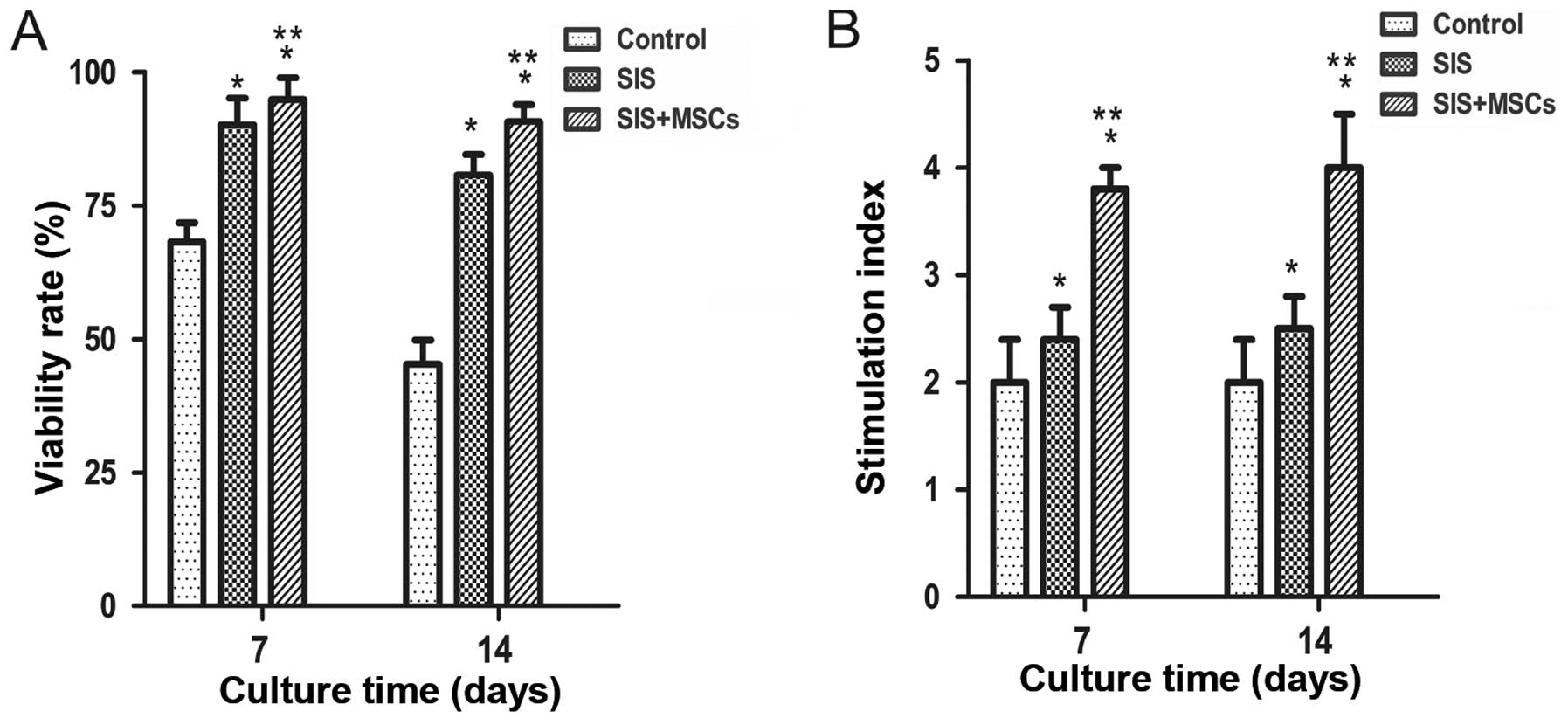
To examine the effects of the SIS and SIS-MSC
scaffold on islets, their viability and function were examined
in vitro. We found that the viability was significantly
higher in both the SIS group and SIS-MSC group than in the control
group (Fig. 2A). The cell
viability in the SIS-MSC group appeared to be superior to that of
the SIS group. These results suggest that the SIS and SIS-MSC
scaffold increase islet viability.
Islet function was determined with a
glucose-stimulated insulin secretion test on days 7 and 14. We
found that the SI was significantly higher in both the SIS and
SIS-MSC groups relative to the control group (Fig. 2B; P<0.05). The SI was
significantly higher in the SIS-MSC group compared with the SIS
group (P<0.05). These findings suggest that the SIS and SIS-MSC
scaffolds enhanced islet function, and that the SIS-MSC scaffold
was superior to the SIS scaffold.
SIS-MSC scaffold increases insulin
expression in islets in vitro
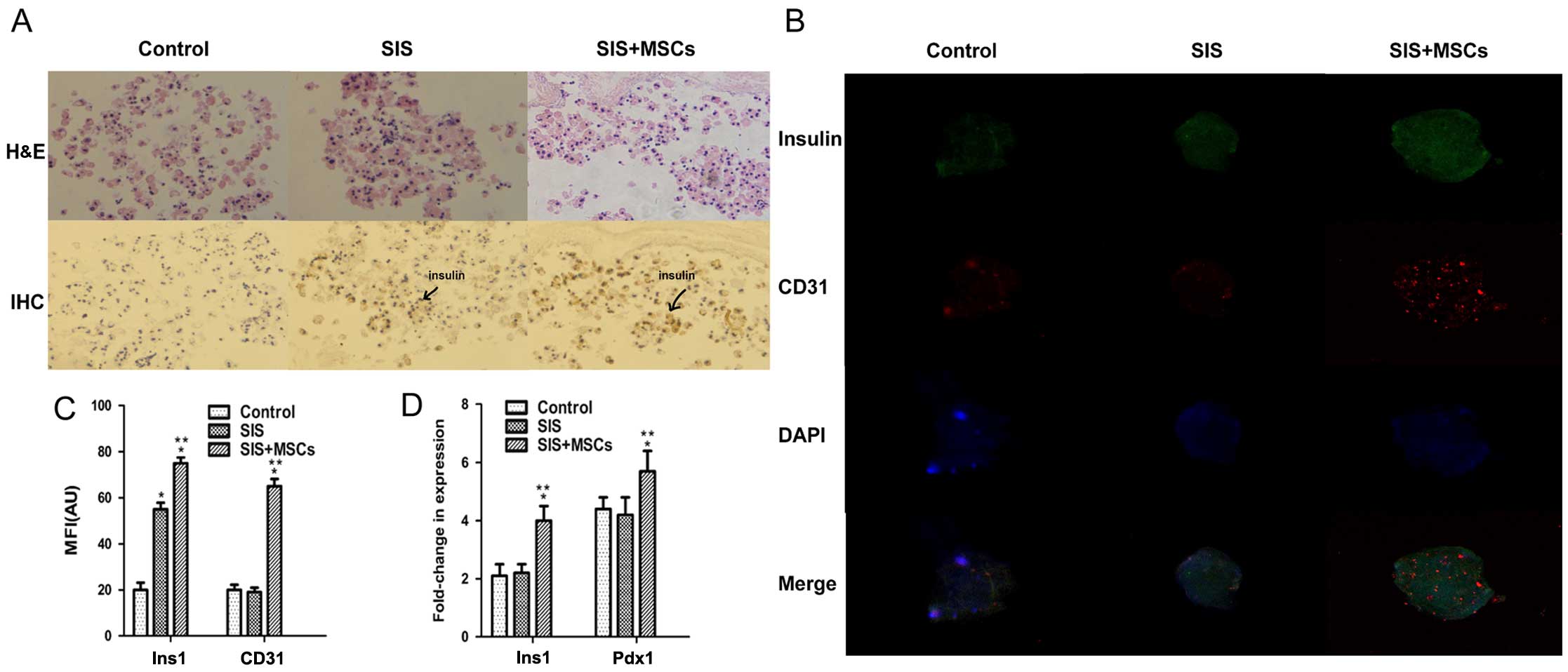
To investigate whether SIS-MSC increases insulin
secretion, we analyzed the intensity of insulin staining in the
islets by immunohistochemistry and immunofluorescence staining. The
results of immunohistochemistry revealed that the intensity of
insulin was significantly higher in the SIS-MSC group than in
either the SIS group or the control group (Fig. 3A). The insulin signal was
undetectable and islet morphology became loose in the control
group, whereas the insulin signal was detected and islet morphology
was compact in the SIS and SIS-MSC groups. Consistently, the
results of immunofluorescence staining indicated that the MFI of
insulin was markedly higher in the SIS-MSC group than in the SIS or
the control groups (Fig. 3B and
C). These results revealed that the SIS-MSC scaffold was
associated with an increase in insulin levels and may prevent islet
destruction.
Subsequently, we examined the gene expression levels
of Ins1 and Pdx1 by RT-qPCR. We found that the levels
of Ins1 and Pdx1 were significantly higher in the
SIS-MSC group than in the SIS and the control groups, and that
there was no significant difference in mRNA levels of Ins1
or Pdx1 between the control and SIS groups (Fig. 3D). These results suggest that the
SIS-MSC scaffold rather than the SIS scaffold upregulates the gene
expression of Pdx1 and Ins1.
SIS-MSC scaffold increases CD31
expression in islets in vitro
CD31 is a marker of the vascular endothelium
(31). To investigate whether the
SIS-MSC scaffold improves the microcirculation of islets, we
performed an immunofluorescence analysis for CD31. Although the
islets were positive for CD31 in the 3 groups, the MFI of CD31 was
significantly higher in the SIS-MSC group than in the SIS and the
control group (Fig. 3B–C). These
results suggest that SIS-MSC scaffold boosts islet
microcirculation.
SIS-MSC scaffold increases growth factor
secretion and decreases TNF secretion in vitro
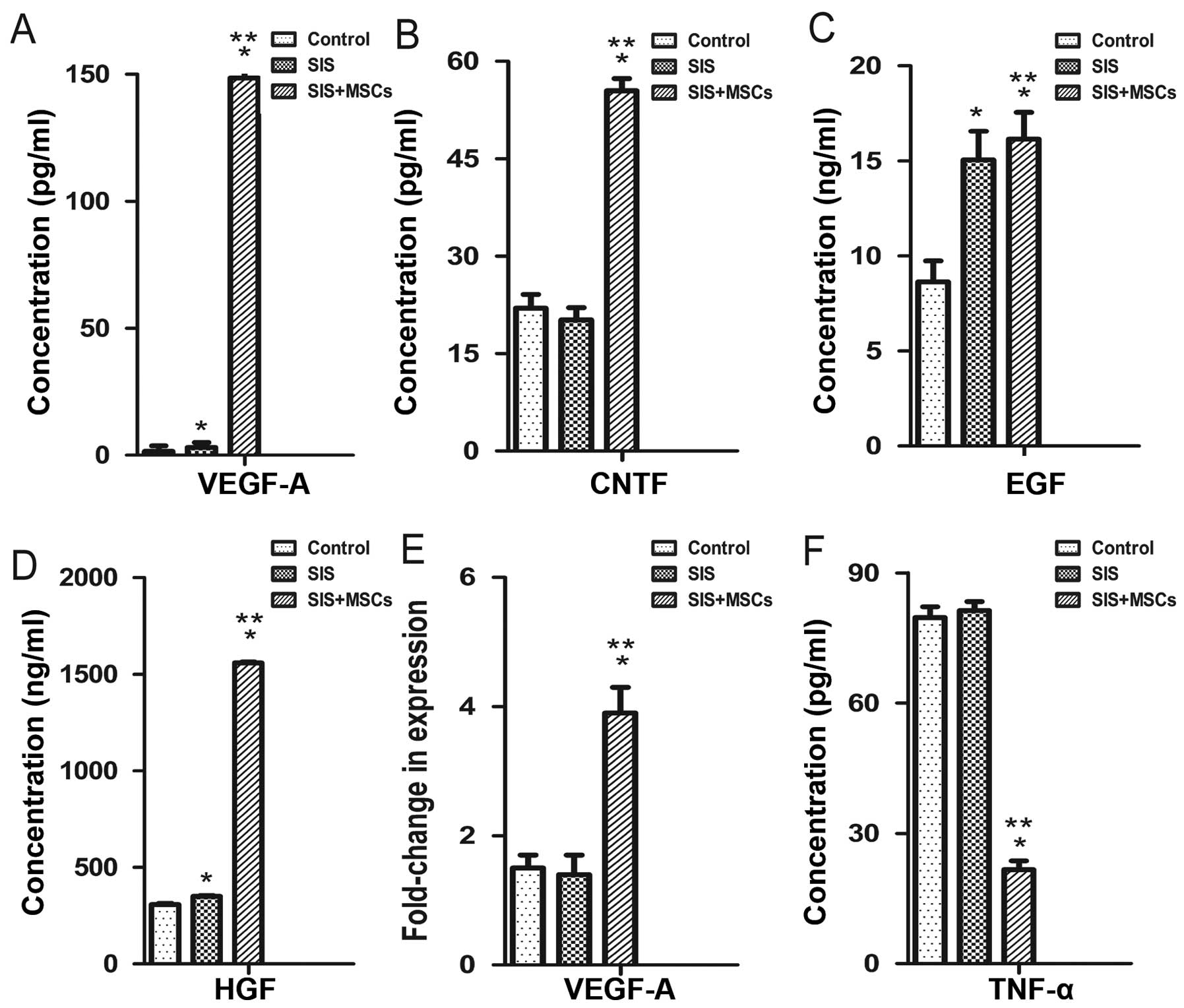
We examined the effects of the SIS-MSC scaffold on
cytokine secretion using ELISA. The concentrations of VEGFA, CNTF,
EGF and HGF in culture media were significantly higher in the
SIS-MSC group than in the SIS group or the control group (Fig. 4A–D). Consistently, the results of
RT-qPCR revealed that the mRNA levels of Vegfa were
significantly higher in the SIS-MSC group compared with the SIS or
the control groups (Fig. 4E). By
contrast, the concentrations of TNF in the culture media were
significantly lower in the SIS-MSC group than in the SIS or the
control groups (Fig. 4F). These
results suggest that MSCs can secrete growth factors and may
decrease inflammation.
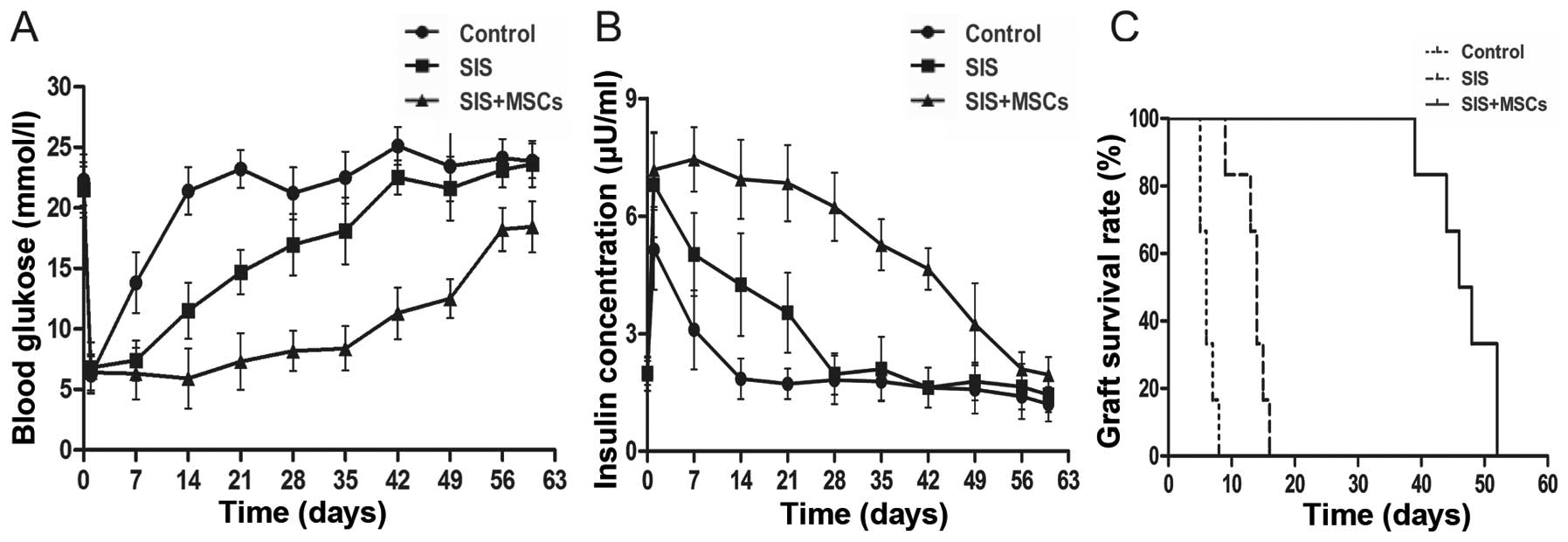
SIS-MSC scaffold improves islet function
and graft survival in vivo
To examine the effects of the SIS-MSC scaffold on
graft survival and function, we performed islet transplantation in
rats and monitored the blood levels of glucose and insulin. While
the blood glucose levels were significantly lower in both the SIS
and the SIS-MSC groups than in the control group, these levels were
markedly lower in the SIS-MSC group than in the SIS group (Fig. 5A). Consistently, the blood insulin
levels and graft survival time were significantly higher in the
SIS-MSC group relative to the SIS or the control groups (Fig. 5B and C). These findings suggest
that the SIS-MSC scaffold improves islet function and prolongs
graft survival.
Discussion
In this study, we investigated the effects of the
SIS-MSC scaffold on islet function and survival. We found that the
SIS-MSC scaffold significantly improved islet function and survival
in vitro and in vivo.
MSCs have become a promising source for cell-based
therapies (32,33). It has been reported that the
co-culture of islets with MSCs has beneficial effects, including
maintaining morphological changes, conserving islet function and
preventing an early inflammatory reaction (34,35). Recently, SIS has been used
clinically as a safe material to repair vascular, urogenital and
musculoskeletal tissues. SIS is a superior biomaterial due to its
biodegradability, biocompatibility, and low rate of peritoneal
adhesions (36). In this study,
we generated a new scaffold containing both MSCs and SIS and
investigated its effect on islets.
In the pancreas, extracellular matrix (ECM)
encircles the islets to provide support, mediate adhesion and
activate signaling pathways (10). Upon isolation and purification,
the loss of ECM and cell-cell interactions leads to rapid islet
death (37). Our findings
demonstrated that SIS and SIS-MSC scaffolds increased the viability
and function of islets. These results suggest that SIS, which has a
3-dimensional structure, may protect the ECM and cell-cell
interactions, thus decreasing the loss of islets.
Our study demonstrated that the expression of
insulin and Pdx1 was upregulated in islets coated by
SIS-MSC. Pdx1 is an important transcription factor that plays an
essential role in the development of the pancreas, islet
differentiation and the maintenance of β-cell function (38). It may also regulate islet cell
proliferation and apoptosis (39). Previous studies have indicated
that MSCs are associated with an increase in the expression of some
islet-related genes, particularly Pdx1 and insulin (39,40).
Our results revealed that the SIS-MSC scaffold may
conserve islet microcirculation and maintain islet morphology. A
dense vascular network in islets is essential for efficient insulin
secretion and oxygen transfer (41). In islet transplantation, islets
are isolated from the remainder of the pancreas. This process
destroys the vasculature within the islets (18). Our results revealed that the
SIS-MSC scaffold increased CD31 expression, a marker of vascular
endothelium.
Our in vivo results revealed that both the
SIS and SIS-MSC scaffolds prolonged the survival of grafts
following islet transplantation. SIS, as a physical immunobarrier,
can protect islets from contact with blood and avoid an instant
blood-mediated inflammatory reaction. However, we found that islet
function and graft survival were markedly improved in diabetic rats
receiving islets coating the SIS-MSC scaffold, compared with rats
receiving islets coating the SIS, suggesting that MSCs contribute
to the prolongation of islet survival. It has been shown that MSCs
secrete cytokines as a nutrition source for islets. VEGFA can
promote vascular development, HGF may enhance endogenous β-cell
regeneration (40) and EGF can
promote metaplastic-ductal formation (42). We found that the concentrations of
VEGFA, CNTF, EGF and HGF in the culture media were significantly
higher in the SIS-MSC group than in the SIS group. Of note, the
concentrations of TNF, which play important pro-inflammatory and
pro-apoptotic roles in islet transplantation (43), were lower in the SIS-MSC group,
suggesting that MSCs may decrease inflammation.
In conclusion, the findings of our study
demonstrated that the SIS-MSC scaffold significantly improved islet
function and islet survival in vitro and in vivo.
This improvement may be associated with the upregulation of insulin
expression, the improvement of islet microcirculation and the
secretion of cytokines.
Acknowledgments
This study was supported by the National Nature
Science Foundation of China (grant no. 81270548).
References
|
1
|
Shi Y and Hu FB: The global implications
of diabetes and cancer. Lancet. 383:1947–1948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inverardi L, Kenyon NS and Ricordi C:
Islet transplantation: Immunological perspectives. Curr Opin
Immunol. 15:507–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olabisi RM: Cell microencapsulation with
synthetic polymers. J Biomed Mater Res A. 103:846–859. 2015.
View Article : Google Scholar :
|
|
4
|
Shim JB, Ankeny RF, Kim H, Nerem RM and
Khang G: A study of a three-dimensional PLGA sponge containing
natural polymers co-cultured with endothelial and mesenchymal stem
cells as a tissue engineering scaffold. Biomed Mater. 9:0450152014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Sullivan ES, Vegas A, Anderson DG and
Weir GC: Islets transplanted in immunoisolation devices: A review
of the progress and the challenges that remain. Endocr Rev.
32:827–844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su J, Hu BH, Lowe WL Jr, Kaufman DB and
Messersmith PB: Anti-inflammatory peptide-functionalized hydrogels
for insulin-secreting cell encapsulation. Biomaterials. 31:308–314.
2010. View Article : Google Scholar
|
|
7
|
Calafiore R, Basta G, Luca G, Boselli C,
Bufalari A, Giustozzi GM, Moggi L and Brunetti P:
Alginate/polyaminoacidic coherent microcapsules for pancreatic
islet graft immunoisolation in diabetic recipients. Ann NY Acad
Sci. 831:313–322. 1997. View Article : Google Scholar
|
|
8
|
Qi M, Gu Y, Sakata N, Kim D, Shirouzu Y,
Yamamoto C, Hiura A, Sumi S and Inoue K: PVA hydrogel sheet
macroencapsulation for the bioartificial pancreas. Biomaterials.
25:5885–5892. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lamb M, Storrs R, Li S, Liang O, Laugenour
K, Dorian R, Chapman D, Ichii H, Imagawa D, Foster C III, et al:
Function and viability of human islets encapsulated in alginate
sheets: In vitro and in vivo culture. Transplant Proc.
43:3265–3266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davis NE, Beenken-Rothkopf LN, Mirsoian A,
Kojic N, Kaplan DL, Barron AE and Fontaine MJ: Enhanced function of
pancreatic islets co-encapsulated with ECM proteins and mesenchymal
stromal cells in a silk hydrogel. Biomaterials. 33:6691–6697. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qureshi KM, Lee J, Paget MB, Bailey CJ,
Curnow SJ, Murray HE and Downing R: Low gravity rotational culture
and the integration of immunomodulatory stem cells reduce human
islet allo-reactivity. Clin Transplant. 29:90–98. 2015. View Article : Google Scholar
|
|
12
|
McPherson TB and Badylak SF:
Characterization of fibronectin derived from porcine small
intestinal submucosa. Tissue Eng. 4:75–83. 1998. View Article : Google Scholar
|
|
13
|
Prevel CD, Eppley BL, Summerlin DJ, Sidner
R, Jackson JR, McCarty M and Badylak SF: Small intestinal
submucosa: Utilization as a wound dressing in full-thickness rodent
wounds. Ann Plast Surg. 35:381–388. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
D'Eredità R: Porcine small intestinal
submucosa (SIS) myringoplasty in children: A randomized controlled
study. Int J Pediatr Otorhinolaryngol. 79:1085–1089. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrand BK, Kokini K, Badylak SF, Geddes
LA, Hiles MC and Morff RJ: Directional porosity of porcine
small-intestinal submucosa. J Biomed Mater Res. 27:1235–1241. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Badylak S, Liang A, Record R, Tullius R
and Hodde J: Endothelial cell adherence to small intestinal
submucosa: An acellular bioscaffold. Biomaterials. 20:2257–2263.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woods EJ, Walsh CM, Sidner RA, Zieger MA,
Mullin S, Lakey JR, Ricordi C and Critser JK: Enhanced recovery of
cryopreserved islets using SIS. Transplant Proc. 36:1139–1142.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiaohui T, Wujun X, Xiaoming D, Xinlu P,
Yan T, Puxun T and Xinshun F: Small intestinal submucosa improves
islet survival and function in vitro culture. Transplant Proc.
38:1552–1558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lakey JRT, Troendle J, Zieger MAJ, Geary
WA, Voytek S and Critser JK: Improved islet survival and in vitro
function using small intestinal submucosa. Transplant Proc. 30:383.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zaher W, Harkness L, Jafari A and Kassem
M: An update of human mesenchymal stem cell biology and their
clinical uses. Arch Toxicol. 88:1069–1082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Secunda R, Vennila R, Mohanashankar AM,
Rajasundari M, Jeswanth S and Surendran R: Isolation, expansion and
characterisation of mesenchymal stem cells from human bone marrow,
adipose tissue, umbilical cord blood and matrix: A comparative
study. Cytotechnology. 67:793–807. 2015. View Article : Google Scholar :
|
|
22
|
Nagamura-Inoue T and Mukai T: Umbilical
cord is a rich source of mesenchymal stromal cells for cell
therapy. Curr Stem Cell Res Ther. Oct 26–2015.Epub ahead of print.
PubMed/NCBI
|
|
23
|
Ding Y, Xu D, Feng G, Bushell A, Muschel
RJ and Wood KJ: Mesenchymal stem cells prevent the rejection of
fully allogenic islet grafts by the immunosuppressive activity of
matrix metal-loproteinase-2 and -9. Diabetes. 58:1797–1806. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Itakura S, Asari S, Rawson J, Ito T,
Todorov I, Liu CP, Sasaki N, Kandeel F and Mullen Y: Mesenchymal
stem cells facilitate the induction of mixed hematopoietic
chimerism and islet allograft tolerance without GVHD in the rat. Am
J Transplant. 7:336–346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin JY, Jeong JH, Han J, Bhang SH, Jeong
GJ, Haque MR, Al-Hilal TA, Noh M, Byun Y and Kim BS:
Transplantation of heterospheroids of islet cells and mesenchymal
stem cells for effective angiogenesis and antiapoptosis. Tissue Eng
Part A. 21:1024–1035. 2015. View Article : Google Scholar :
|
|
26
|
Yoshimatsu G, Sakata N, Tsuchiya H, Minowa
T, Takemura T, Morita H, Hata T, Fukase M, Aoki T, Ishida M, et al:
The co-transplantation of bone marrow derived mesenchymal stem
cells reduced inflammation in intramuscular islet transplantation.
PLoS One. 10:e01175612015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borg DJ, Weigelt M, Wilhelm C, Gerlach M,
Bickle M, Speier S, Bonifacio E and Hommel A: Mesenchymal stromal
cells improve transplanted islet survival and islet function in a
syngeneic mouse model. Diabetologia. 57:522–531. 2014. View Article : Google Scholar
|
|
28
|
Gao X, Song L, Shen K, Wang H, Qian M, Niu
W and Qin X: Bone marrow mesenchymal stem cells promote the repair
of islets from diabetic mice through paracrine actions. Mol Cell
Endocrinol. 388:41–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Badylak SF, Lantz GC, Coffey A and Geddes
LA: Small intestinal submucosa as a large diameter vascular graft
in the dog. J Surg Res. 47:74–80. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rackham CL, Chagastelles PC, Nardi NB,
Hauge-Evans AC, Jones PM and King AJ: Co-transplantation of
mesenchymal stem cells maintains islet organisation and morphology
in mice. Diabetologia. 54:1127–1135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W, Zhao R, Liu J, Tian M, Lu Y, He T,
Cheng M, Liang K, Li X, Wang X, et al: Small islets transplantation
superiority to large ones: Implications from islet microcirculation
and revascularization. J Diabetes Res. 2014:1920932014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carlsson PO, Schwarcz E, Korsgren O and Le
Blanc K: Preserved β-cell function in type 1 diabetes by
mesenchymal stromal cells. Diabetes. 64:587–592. 2015. View Article : Google Scholar
|
|
33
|
Franchi F, Peterson KM, Xu R, Miller B,
Psaltis PJ, Harris PC, Lerman LO and Rodriguez-Porcel M:
Mesenchymal Stromal Cells Improve Renovascular Function in
Polycystic Kidney Disease. Cell Transplant. 24:1687–1698. 2015.
View Article : Google Scholar :
|
|
34
|
Sumi S and Yanai G: Fusion of mesenchymal
stem cells and islet cells for cell therapy. Methods Mol Biol.
1313:107–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rackham CL, Vargas AE, Hawkes RG, Amisten
S, Persaud SJ, Austin ALF, King AJF and Jones PM: Annexin A1 is a
key modulator of Mesenchymal Stromal Cell mediated improvements in
islet function. Diabetes. 65:129–139. 2016.
|
|
36
|
Costa RG, Lontra MB, Scalco P, Cavazzola
LT and Gurski RR: Polylactic acid film versus acellular porcine
small intestinal submucosa mesh in peritoneal adhesion formation in
rats. Acta Cir Bras. 24:128–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schaschkow A, Mura C, Bietiger W, Peronet
C, Langlois A, Bodin F, Dissaux C, Bruant-Rodier C, Pinget M,
Jeandidier N, et al: Impact of an autologous oxygenating matrix
culture system on rat islet transplantation outcome. Biomaterials.
52:180–188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin P, Li W, Yao Z, Sun Y, Wang L, Li S
and Chen L: Oral administration of PDX1 confers protection against
insulitis in the non-obese diabetic (NOD) mice. Biochem Biophys Res
Commun. 466:656–663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yuan H, Liu H, Tian R, Li J and Zhao Z:
Regulation of mesenchymal stem cell differentiation and insulin
secretion by differential expression of Pdx-1. Mol Biol Rep.
39:7777–7783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boumaza I, Srinivasan S, Witt WT,
Feghali-Bostwick C, Dai Y, Garcia-Ocana A and Feili-Hariri M:
Autologous bone marrow-derived rat mesenchymal stem cells promote
PDX-1 and insulin expression in the islets, alter T cell cytokine
pattern and preserve regulatory T cells in the periphery and induce
sustained normoglycemia. J Autoimmun. 32:33–42. 2009. View Article : Google Scholar
|
|
41
|
Zanone MM, Favaro E and Camussi G: From
endothelial to beta cells: Insights into pancreatic islet
microendothelium. Curr Diabetes Rev. 4:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lai M, Cai K, Hu Y, Zhang Y, Li L, Luo Z,
Hou Y, Li J, Ding X and Chen X: Construction of microenvironment
onto titanium substrates to regulate the osteoblastic
differentiation of bone marrow stromal cells in vitro and
osteogenesis in vivo. J Biomed Mater Res A. 101:653–666. 2013.
View Article : Google Scholar
|
|
43
|
SoRelle JA, Itoh T, Peng H, Kanak MA,
Sugimoto K, Matsumoto S, Levy MF, Lawrence MC and Naziruddin B:
Withaferin A inhibits pro-inflammatory cytokine-induced damage to
islets in culture and following transplantation. Diabetologia.
56:814–824. 2013. View Article : Google Scholar : PubMed/NCBI
|