Introduction
Non-alcoholic fatty liver disease (NAFLD) is a
hepatic manifestation of metabolic syndrome. The prevalence of
NAFLD is estimated to range from 25 to 37% of the general
population (1–4), and it constitutes a common
etiological factor of chronic liver disease worldwide.
Non-alcoholic steatohepatitis (NASH) is a progressive form of
NAFLD. Patients with NASH have a high risk of life-threatening
complications, including cardiovascular disease, liver cirrhosis,
and hepatocellular carcinoma (5,6).
In addition, NASH is predicted to become the leading cause of liver
transplantation in the USA by the year 2020 (2). Thus, NAFLD/NASH is becoming a major
social issue worldwide.
The first-line therapy for NASH is life-style
intervention. Although diet and exercise alleviate the severity of
NASH, long-term adherence to life-style interventions is poor
(7,8). Pioglitazone, an insulin-sensitizing
anti-diabetic agent, leads to metabolic and histologic improvement
in patients with NASH (9).
However, the indication of pioglitazone is restricted to NASH
patients with diabetes mellitus (10). Furthermore, severe liver disease
is a contraindication of pioglitazone because of adverse events,
including severe hepatitis. Vitamin E is also reported to attenuate
histological changes of the liver in patients with NASH (11). However, its efficacy is
controversial (12). Furthermore,
long-term administration of vitamin E is reported to increase
all-cause mortality (13).
Accordingly, the development of new, safe, easily obtainable, and
low-cost therapeutic agents for NASH is required.
Whole-wheat consumption is known to reduce the risk
of obesity, type 2 diabetes, and cardiovascular disease (14,15). Supplementation with bran, the
outer layers of wheat grains, improves glycemic and lipid
metabolism in patients with impaired glucose tolerance (16,17). Recently, we developed a simple
peptide extraction method and identified two bioactive peptides
[leucine-arginine-proline (LRP) and leucine-glutamine-proline
(LQP)] from wheat bran by autolysis reactions (18). These peptides inhibit angiotensin
I-converting enzyme (ACE), which causes oxidative stress (19,20). In addition, these peptides contain
leucine, a branched-chain amino acid, which regulates lipid
metabolism via activation of the AMP-activated protein kinase
(AMPK) signaling pathway (21).
Since increased oxidative stress and downregulation of AMPK are
major pathogenic factors of NASH, these wheat-bran autolytic
peptides may be potent therapeutic supplements for the treatment of
NASH.
The aim of the present study was to investigate the
therapeutic efficacy of LRP and LQP for NASH in a mouse model. In
addition, we investigated the effects of these peptides on
oxidative stress and the AMPK signaling pathway.
Materials and methods
Materials
All the reagents were purchased from Wako Pure
Chemical Industries (Osaka, Japan) unless otherwise indicated.
Animals
NASH was induced in 7-week-old male C57Bl/6
wild-type mice by feeding a high-fat diet containing 71% total
calories as fat (CELA Japan, Tokyo, Japan) (22). The mice were housed individually
in an air-conditioned room at 22±3°C and 55±10% humidity, and with
a 12-h light/dark cycle. All mice were permitted ad libitum
consumption of water throughout the experimental period.
All animal experiments were conducted in accordance
with the National Institutes of Health Guidelines for the Care and
Use of Laboratory Animals, and were approved by the University of
Kurume Institutional Animal Care and Use Committee.
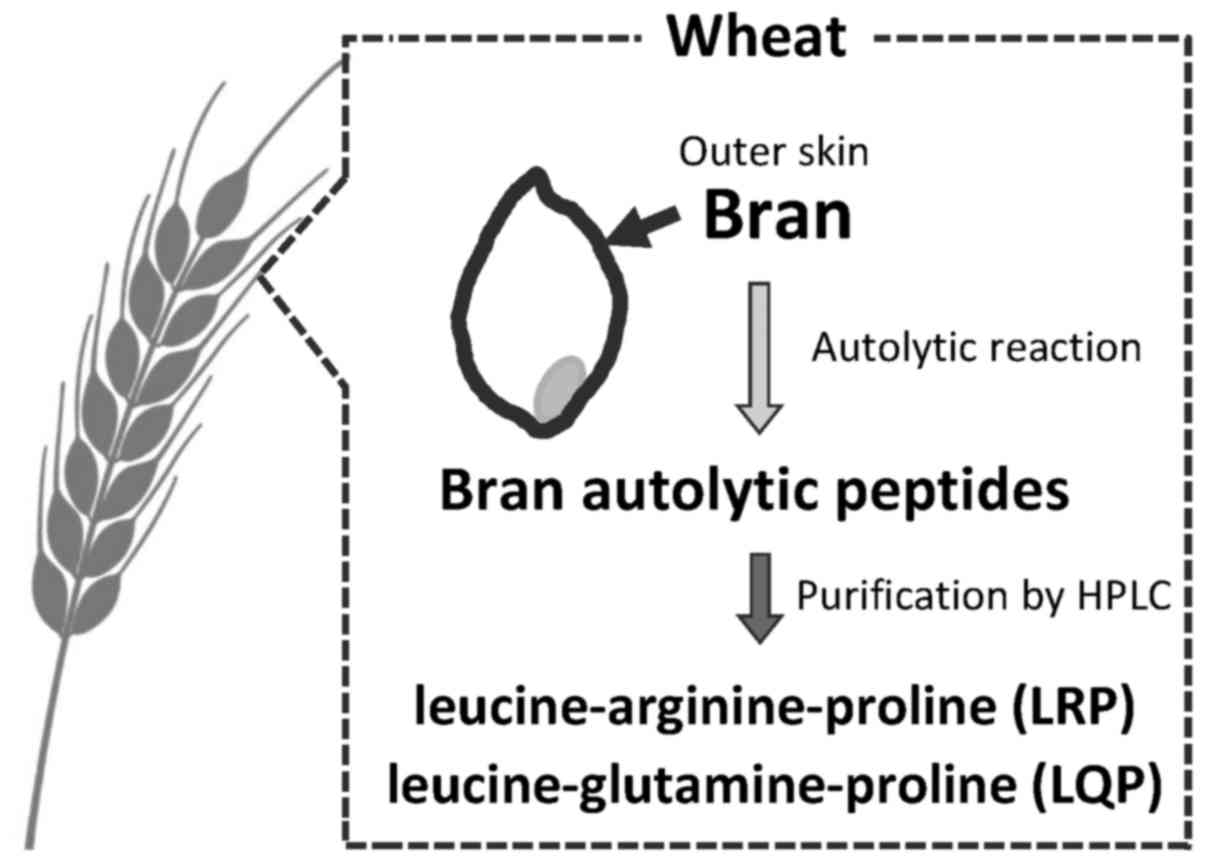
Purification of two bioactive peptides,
LRP and LQP, from wheat bran
Two bioactive peptides, LRP and LQP, were purified
from wheat bran using HPLC as previously described (18). Briefly, wheat samples were milled
in a test mill to obtain bran (Fig.
1). Bran samples were suspended in 50 mM citrate-phosphate
buffer (pH 3.2) and autolyzed at 40°C for 12 h, followed by high
performance liquid chromatography purification (Shimadzu LC-10AD
system; Shimadzu, Kyoto, Japan) (Fig.
1). The amino acid sequence was analyzed using a Shimadzu
PPSQ-21 protein sequencer (18).
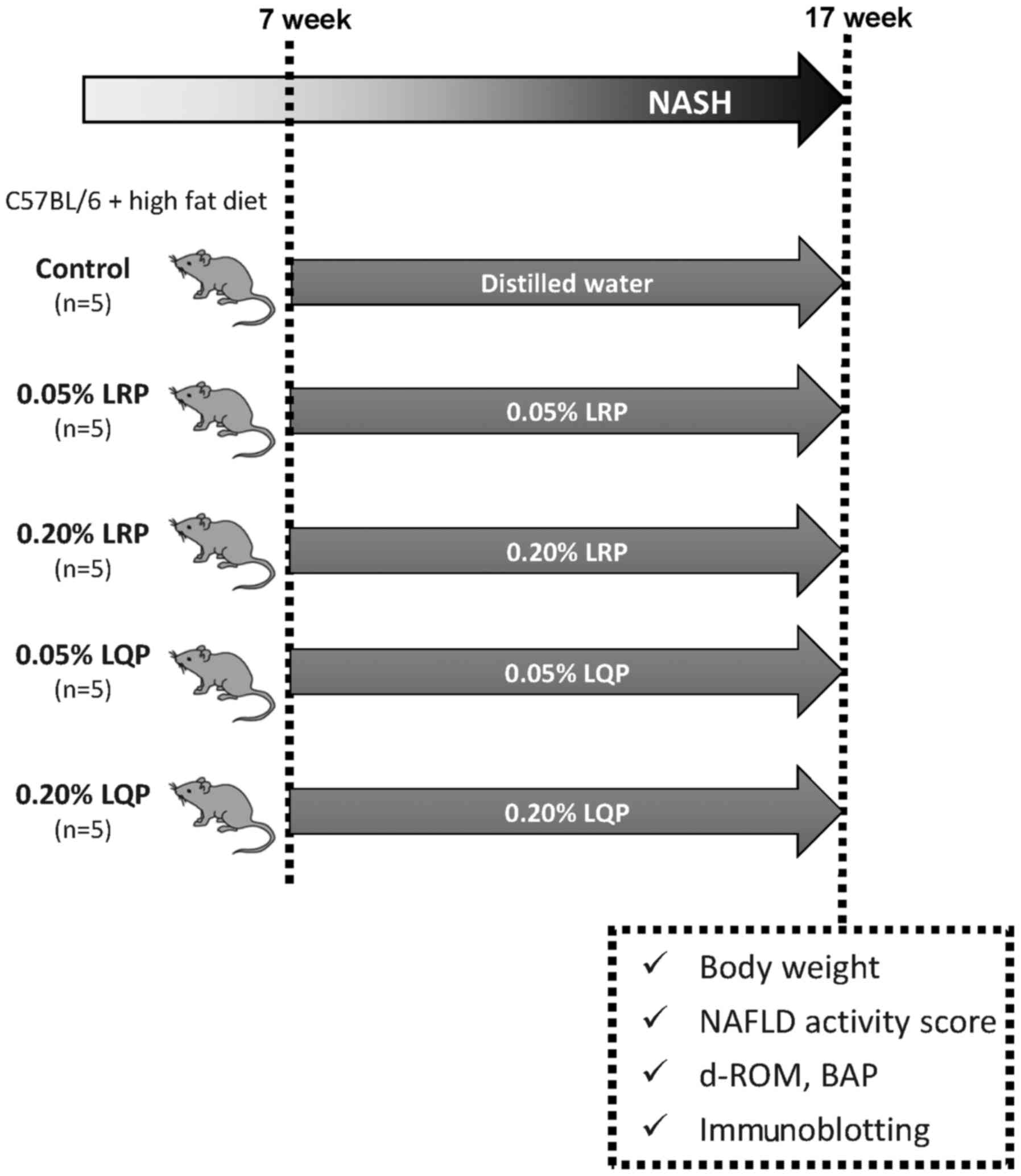
Treatment protocol
Seven-week-old male C57BL/6 mice were fed with a
high-fat diet for 10 weeks to induce NASH, and were administered
0.05% LRP-supplemented water (0.05% LRP; n=5), 0.20%
LRP-supplemented water (0.20% LRP; n=5), 0.05% LQP-supplemented
water (0.05% LQP; n=5), 0.20% LQP-supplemented water (0.20% LQP;
n=5), or distilled water (control; n=5) ad libitum (Fig. 2). At week 17, body weight was
measured. Then, the mice were sacrificed and the serum and livers
were collected under anesthesia (Fig.
2).
Liver histology
Random histological sampling was performed
throughout this study as previously described (23,24). Liver samples were fixed overnight
in 10% buffered formalin and embedded in paraffin. All the sections
were cut at 5 µm and stained with hematoxylin and eosin (25).
NAFLD activity score
Activity of NAFLD was evaluated by NAFLD activity
score, in which the following findings were evaluated
semi-quantitatively: steatosis (0–3 points), lobular inflammation
(0–2 points), hepatocellular ballooning (0–2 points), and fibrosis
(0–4 points) (26).
Measurement of reactive oxygen
metabolites and antioxidant capacity
To analyze reactive oxygen metabolites and
antioxidant capacity in serum, diacron reactive oxygen metabolite
(d-ROM) and biological antioxidant potential (BAP) were measured by
a free radical analyzer system (FREE Carpe Diem; Wismerll Co.,
Ltd., Tokyo, Japan) as previously described (27,28). The d-ROM measurements were
determined based on the ability of transition metals to catalyze
the formation of colored free radicals, and the results were
expressed in arbitrary units (U.Carr), where 1 U.Carr was 0.8 mg/l
of H2O2. In BAP measurements, 10 µl of serum
was added to a solution containing FeCl3. Reduction of
Fe3+ to Fe2+ caused a chromatic change, which
was measured at 505 nm using a photometer. The results of BAP
measurements were expressed in µmol/l of the reduced ferric
ions.
Immunoblotting
Immunoblotting was performed as previously described
(29,30) using the following antibodies:
anti-phospho-AMPKα (Thr172; Cat. no. 07-681), anti-AMPKα-pan (Cat.
no. 07-181) (both from Merck Millipore Corp., Darmstadt, Germany),
anti-phospho-acetyl-CoA carboxylase (ACC) (Ser79; Cat. no. 3661)
and anti-ACC (Cat. no. 3662) (both from Cell Signaling Technology
Inc., Danvers, MA, USA). Equal amounts of protein (20 µg) from
liver homogenates were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. The resolved proteins
were transferred electrophoretically onto polyvinylidene difluoride
membranes. The membranes were incubated with the primary antibodies
mentioned above at 1:1,000 dilution, and were subsequently
incubated with the secondary antibodies at 1:10,000 dilution (Cat.
no. NA9340-1ML; GE Healthcare Japan K.K., Tokyo, Japan). The
membranes were then incubated with a chemiluminescence reagent (ECL
kit) and analyzed by a luminescent image analyzer (LAS-4000) (both
from GE Healthcare Japan K.K.).
Changes in immunoblotting intensity for
phospho-AMPK, AMPK, phospho-ACC and ACC were quantitated by
measuring pixel intensities using an image processing program,
ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA)
(http://rsb.info.nih.gov/ij/) (31). Changes in immunoblotting intensity
for phospho-AMPK, AMPK, phospho-ACC and ACC were quantitated by
measuring pixel intensities using an image processing program,
ImageJ.
Statistical analysis
Data are expressed as mean ± SD. Statistical
comparisons among multiple groups were performed by ANOVA followed
by Scheffe's post-hoc test (JMP Pro version 12.01; SAS Institute
Inc., Cary, NC, USA). P<0.05 was considered significant.
Results
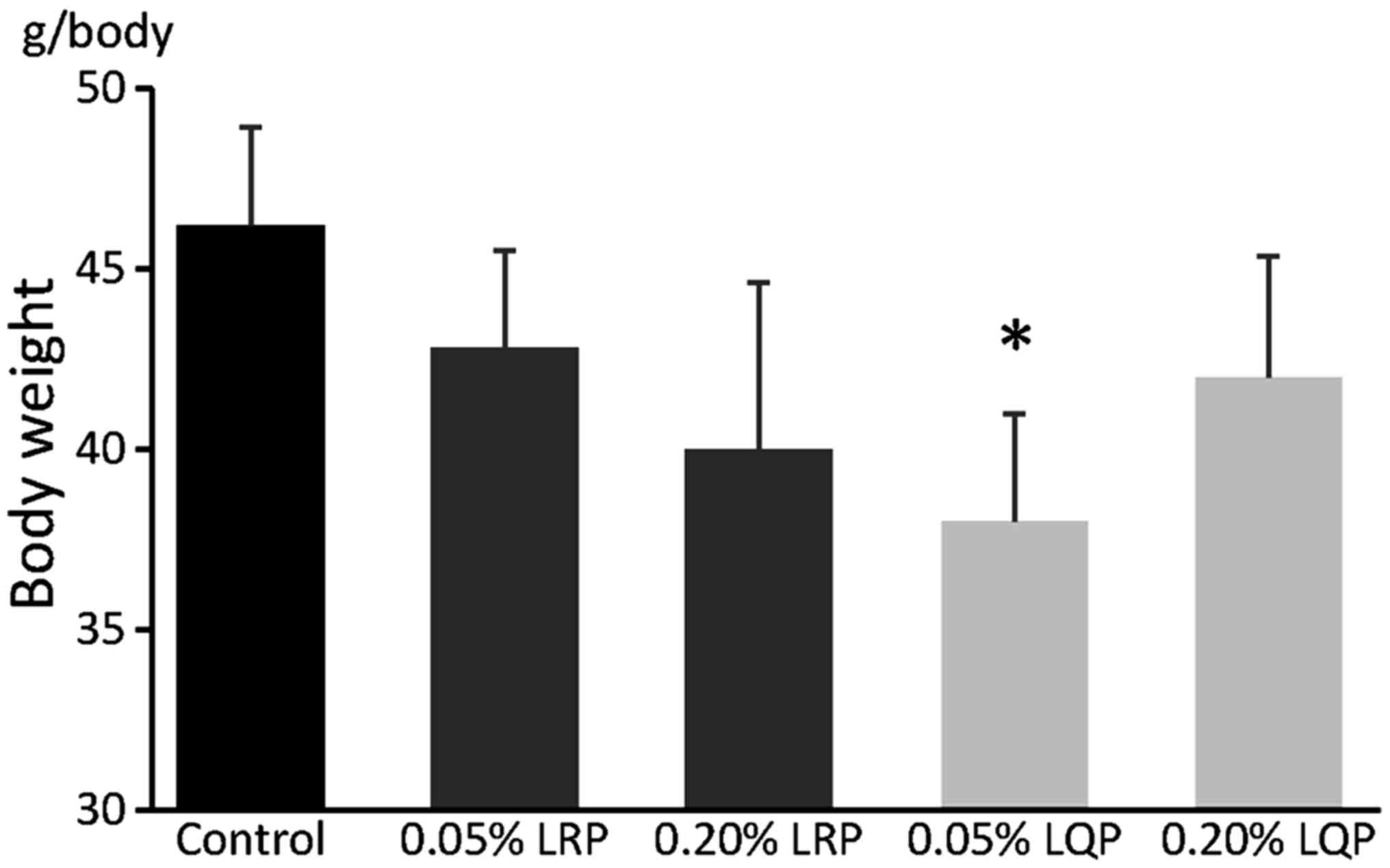
Effects of LRP and LQP on body
weight
Body weight in the 0.05% LQP group was significantly
lower compared to that in the control group (46.0±3.0 vs. 38.0±3.3
g/body, P=0.02) (Fig. 3). No
significant differences were seen in body weight between the
control group and the 0.05% LRP, 0.20% LRP or 0.20% LQP groups
(Fig. 3).
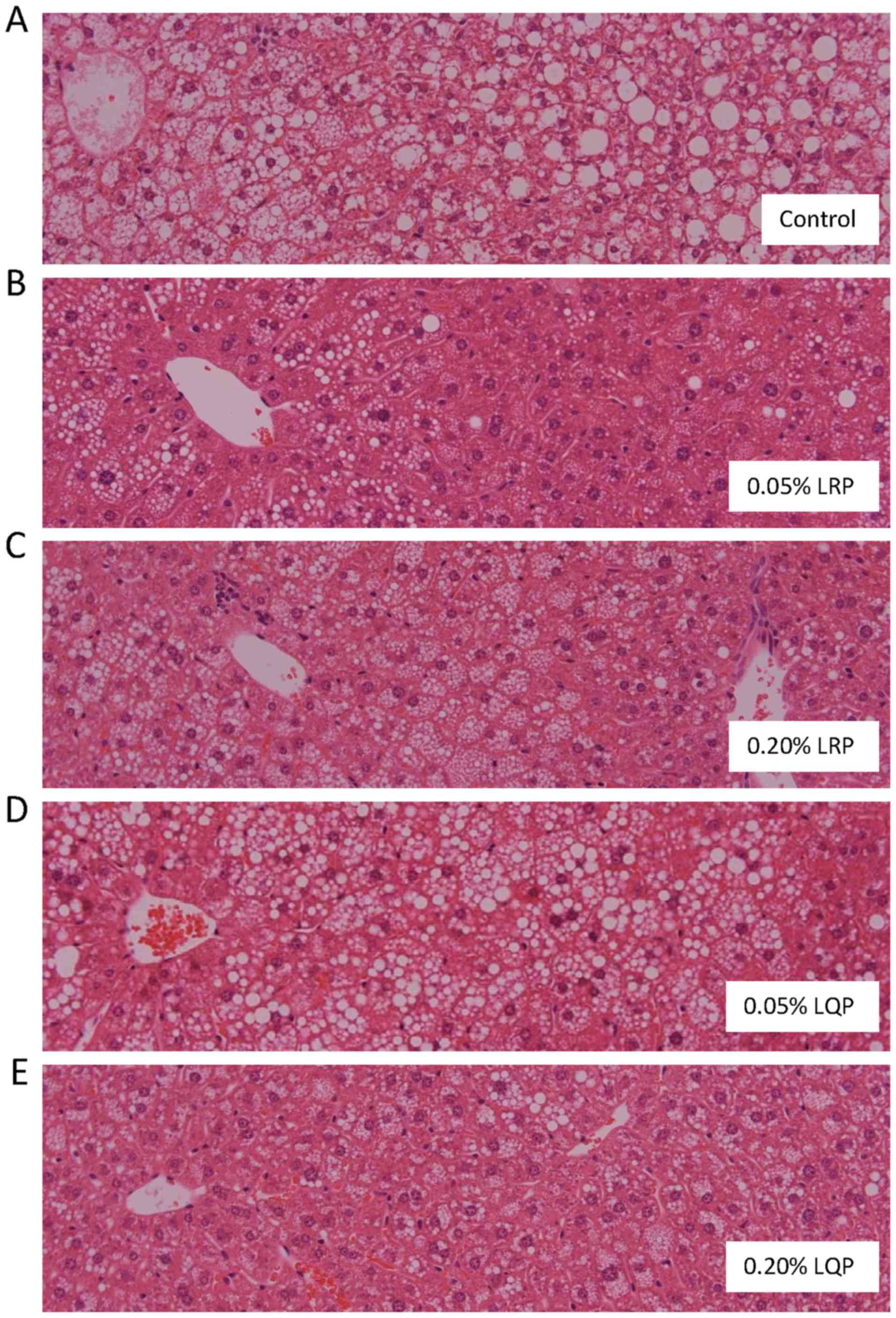
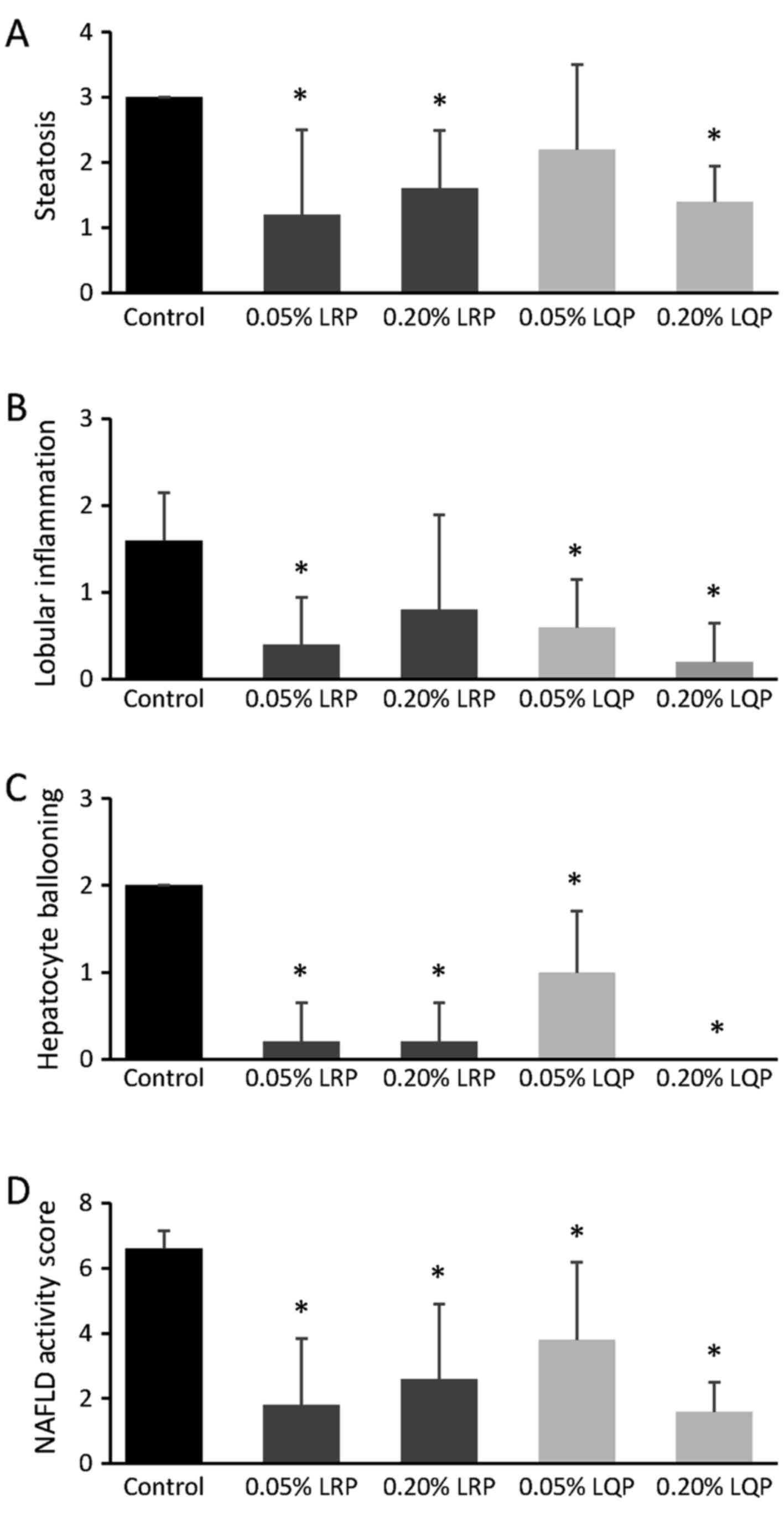
Effects of LRP and LQP on liver histology
and NAFLD activity score
Severe hepatic steatosis was identified in the
Control and 0.05% LQP groups (Fig. 4A
and D). However, amelioration of hepatic steatosis was observed
in the 0.05% LRP, 0.20% LRP and 0.20% LQP groups (Fig. 4B, C and E). Hepatic fibrosis was
rarely seen in any group, including the control group.
The grade of steatosis was significantly decreased
in the 0.05% LRP, 0.20% LRP, and 0.20% LQP groups compared to that
in the control group (Fig. 5A).
The grade of lobular inflammation was significantly decreased in
the 0.05% LRP, 0.05% LQP and 0.20% LQP groups compared to that in
the control group (Fig. 5B). The
grade of hepatocyte ballooning and NAFLD activity score were
significantly decreased in all the treated groups compared to that
in the control group (Fig. 5C and
D).
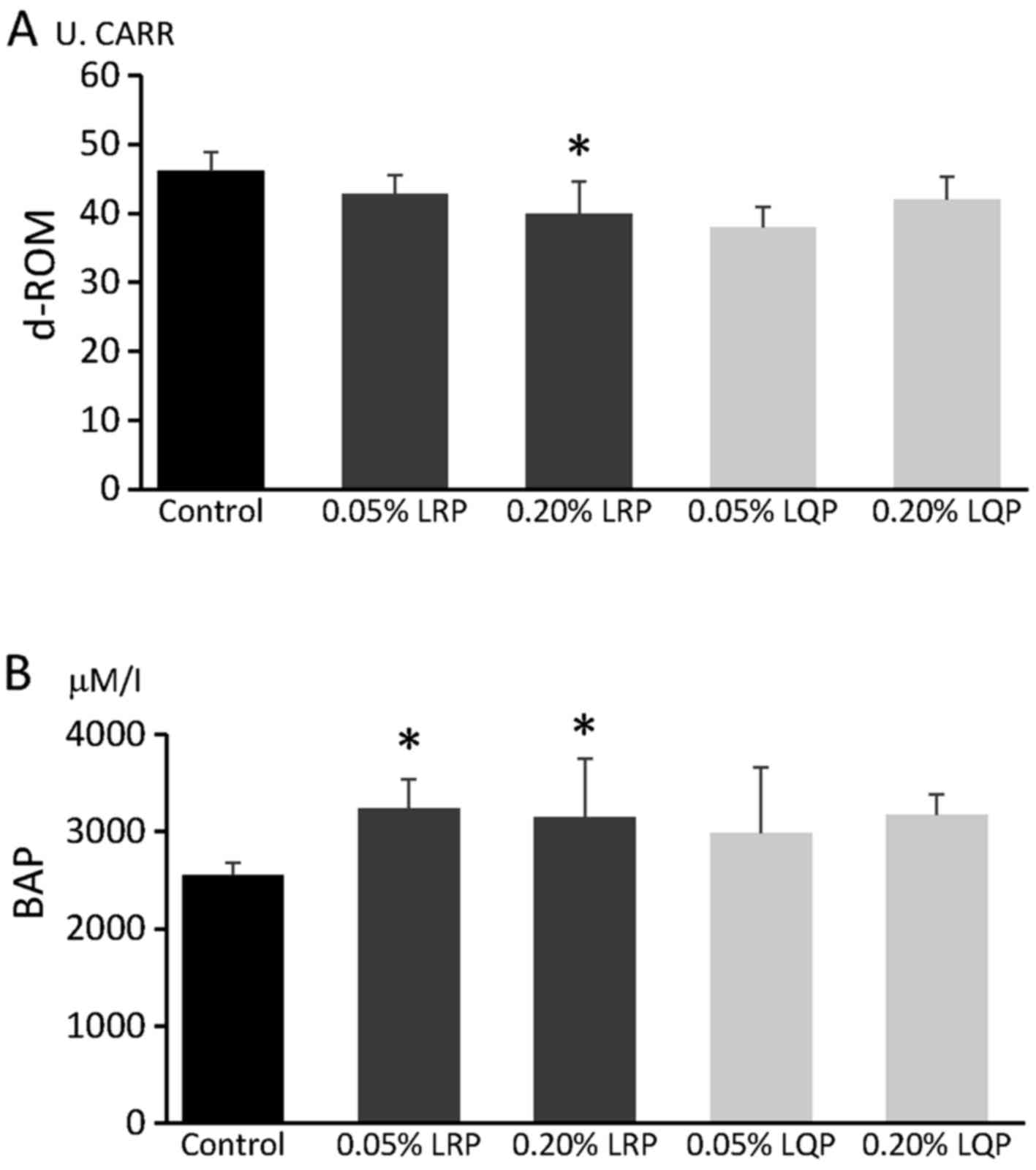
Effects of LRP and LQP on d-ROM and
BAP
Serum d-ROM levels were significantly decreased in
the 0.20% LRP group, but not in the 0.05% LRP, 0.05% LQP, and 0.20%
LQP groups, compared to that in the control group (Fig. 6A). Serum BAP levels were
significantly higher in the 0.05% LRP and 0.20% LRP groups, but not
in the 0.05% LQP and 0.20% LQP groups, compared to that in the
control group (Fig. 6B).
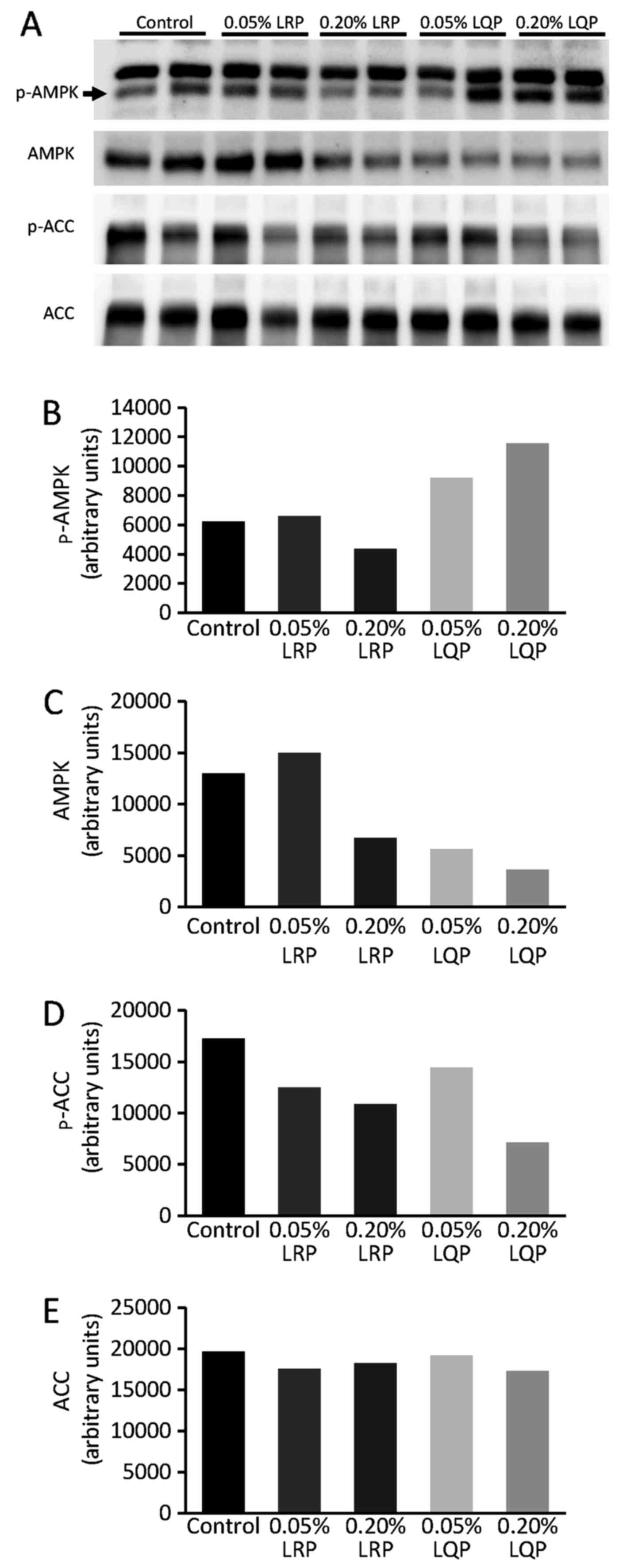
Effects of LRP and LQP on the AMPK
signaling pathway
Immunoblotting analysis showed that the hepatic
expression of phospho-AMPK was increased in the 0.20% LQP group
compared to that in the control group (Fig. 7A and B). The hepatic expression of
phospho-ACC, a downstream molecule of AMPK, was suppressed in the
0.20% LQP group compared to that in the control group (Fig. 7D).
Discussion
We demonstrated that LRP and LQP alleviated the
severity of NASH in high-fat diet-fed mice. In addition, we
revealed that LRP suppressed oxidative stress, while LQP
upregulated the AMPK/ACC signaling pathway. Thus, LRP and LQP may
attenuate NASH through different mechanisms. LRP and LQP are easily
obtainable at a low cost; therefore, these wheat-bran autolytic
peptides may be clinically applicable therapeutic agents in
patients with NASH.
In general, 7–10% weight loss is required to
attenuate hepatic steatosis in patients with NASH (32). In this study, although significant
weight loss was observed in the 0.05% LQP group, no significant
attenuation of hepatic steatosis was observed. On the other hand, a
significant attenuation of hepatic steatosis without weight loss
was identified in the 0.05% LRP, 0.20% LRP and 0.20% LQP groups.
These findings indicate that the mechanisms underlying LRP- and
LQP-induced attenuation of hepatic steatosis are independent of
weight loss. Increase in oxidative stress is a major NASH
pathogenic factor (33).
Recently, Ni et al reported that silymarin, derived from the
milk thistle plant, upregulates NADPH oxidase components and
antioxidant enzymes, and attenuates hepatic steatosis without
significant weight loss in a mouse model of NAFLD (34). This led us to investigate the
effects of LRP and LQP on oxidative stress.
There are several markers for evaluating oxidative
stress, including 8-hydroxyguanosine, 4-hydroxynonenal protein, and
activity of glutathione and superoxide dismutase (35,36). Additionally, the levels of d-ROM
and BAP have been used to evaluate oxidative status. d-ROM levels
are the total levels of peroxidized metabolites, whereas BAP
reflects serum antioxidant capacity. These markers are known to
correlate with the development of metabolic syndrome,
cardiovascular disease, and hepatocellular carcinoma (37–39). In this study, a significant
decrease in d-ROM was observed in the 0.20% LRP group, and a
significant increase in BAP was observed in the 0.05% LRP and 0.20%
LRP groups. These data indicate that LRP upregulates antioxidant
capacity, leading to an attenuation of NASH. Although the
mechanisms underlying LRP-induced upregulation of antioxidant
capacity remain unclear, we previously reported that LRP inhibits
the activity of angiotensin I-converting enzyme (ACE) (18). Fiordaliso et al reported
that an ACE inhibitor reduces oxidative stress in the heart and
aorta in a rat model of diabetes mellitus (19). In addition, Privratsky et
al reported that ACE inhibition reduces the activation of NADPH
oxidase and suppresses the production of oxygen radicals in a rat
model of hypertension (20).
Thus, LRP may attenuate NASH through the downregulation of
oxidative stress in high-fat diet-fed mice.
LRP and LQP contain leucine, a branched-chain amino
acid, which regulates lipid metabolism via the AMPK signaling
pathway (21). Therefore, we
evaluated the effects of LRP and LQP on phosphorylation of AMPK and
ACC, a downstream lipogenic molecule of AMPK. In the 0.20% LQP
group, the expression of phospho-AMPK and phospho-ACC was increased
and decreased, respectively. These data indicate that 0.20% LQP
activates the AMPK/ACC pathway, leading to the suppression of de
novo lipogenesis. Recently, leucine, along with metformin or a
phosphodiesterase 5 inhibitor, was reported to attenuate hepatic
steatosis, insulin sensitivity, and glycemic control via the
upregulation of AMPK in obese mice (40,41). These findings support our
hypothesis that LQP attenuates NASH through activation of the
AMPK/ACC signaling pathway.
There are several limitations in this study. Our
results showed that liver function and glucose metabolisms were
improved. However, we did not perform any biochemical examination
for liver function and glucose metabolisms such as glucose
tolerance test and insulin tolerance test. In addition, although
LRP and LQP may attenuate NASH through upregulation of the
transporter of fatty acid, we did not evaluate the expression and
function of the transporter of fatty acid such as CD36 and CPT1.
These are uncertainty areas in this field, and should be clarified
in further research.
In conclusion, we demonstrated that LRP and LQP
attenuate NASH in mice fed a high-fat diet. LRP and LQP may
attenuate NASH through different mechanisms. LRP suppressed
oxidative stress, while LQP upregulated the AMPK/ACC signaling
pathway. Since the worldwide prevalence of NASH is high, treatment
availability and cost are important issues. LRP and LQP are easily
obtainable at a low cost; therefore, these wheat-bran autolytic
peptides may represent clinically applicable therapeutic agents in
patients with NASH.
Abbreviations:
|
LRP
|
leucine-arginine-proline
|
|
LQP
|
leucine-glutamine-proline
|
|
NASH
|
non-alcoholic steatohepatitis
|
|
AMPK
|
AMP-activated protein kinase
|
|
d-ROM
|
diacron reactive oxygen metabolite
|
|
BAP
|
biological antioxidant potential
|
|
ACC
|
acetyl-CoA carboxylase
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
ACE
|
angiotensin I-converting enzyme
|
Acknowledgments
This study is supported by the Research Program on
Hepatitis from Japan Agency for Medical Research and Development,
AMED.
References
|
1
|
Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N,
Fujimoto K, Chayama K and Saibara T; JSG-NAFLD: Prevalence and
associated metabolic factors of nonalcoholic fatty liver disease in
the general population from 2009 to 2010 in Japan: A multicenter
large retrospective study. J Gastroenterol. 47:586–595. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wree A, Broderick L, Canbay A, Hoffman HM
and Feldstein AE: From NAFLD to NASH to cirrhosis - new insights
into disease mechanisms. Nat Rev Gastroenterol Hepatol. 10:627–636.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Younossi ZM, Koenig AB, Abdelatif D, Fazel
Y, Henry L and Wymer M: Global epidemiology of non-alcoholic fatty
liver disease-meta-analytic assessment of prevalence, incidence and
outcomes. Hepatology. 64:73–84. 2016. View Article : Google Scholar
|
|
4
|
Hashimoto E and Tokushige K: Prevalence,
gender, ethnic variations, and prognosis of NASH. J Gastroenterol.
46(Suppl 1): 63–69. 2011. View Article : Google Scholar
|
|
5
|
Tokushige K, Hashimoto E and Kodama K:
Hepatocarcinogenesis in non-alcoholic fatty liver disease in Japan.
J Gastroenterol Hepatol. 28(Suppl 4): 88–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baffy G, Brunt EM and Caldwell SH:
Hepatocellular carcinoma in non-alcoholic fatty liver disease: An
emerging menace. J Hepatol. 56:1384–1391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nobili V, Manco M, Devito R, Di Ciommo V,
Comparcola D, Sartorelli MR, Piemonte F, Marcellini M and Angulo P:
Lifestyle intervention and antioxidant therapy in children with
nonalcoholic fatty liver disease: A randomized, controlled trial.
Hepatology. 48:119–128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oza N, Eguchi Y, Mizuta T, Ishibashi E,
Kitajima Y, Horie H, Ushirogawa M, Tsuzura T, Nakashita S,
Takahashi H, et al: A pilot trial of body weight reduction for
nonalcoholic fatty liver disease with a home-based lifestyle
modification intervention delivered in collaboration with
interdisciplinary medical staff. J Gastroenterol. 44:1203–1208.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Belfort R, Harrison SA, Brown K, Darland
C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, et
al: A placebo-controlled trial of pioglitazone in subjects with
nonalcoholic steatohepatitis. N Engl J Med. 355:2297–2307. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sumie S, Kawaguchi T, Kawaguchi A,
Kuromatsu R, Nakano M, Satani M, Yamada S, Okamura S, Yonezawa Y,
Kakuma T, et al: Effect of pioglitazone on outcome following
curative treatment for hepatocellular carcinoma in patients with
hepatitis C virus infection: A prospective study. Mol Clin Oncol.
3:115–120. 2015.
|
|
11
|
Sanyal AJ, Chalasani N, Kowdley KV,
McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE,
Tonascia J, Unalp A, et al NASH CRN: Pioglitazone, vitamin E, or
placebo for nonalcoholic steatohepatitis. N Engl J Med.
362:1675–1685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lavine JE, Schwimmer JB, Van Natta ML,
Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO,
Sanyal AJ, Chalasani N, et al Nonalcoholic Steatohepatitis Clinical
Research Network: Effect of vitamin E or metformin for treatment of
nonalcoholic fatty liver disease in children and adolescents: The
TONIC randomized controlled trial. JAMA. 305:1659–1668. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miller ER III, Pastor-Barriuso R, Dalal D,
Riemersma RA, Appel LJ and Guallar E: Meta-analysis: High-dosage
vitamin E supplementation may increase all-cause mortality. Ann
Intern Med. 142:37–46. 2005. View Article : Google Scholar
|
|
14
|
Ye EQ, Chacko SA, Chou EL, Kugizaki M and
Liu S: Greater whole-grain intake is associated with lower risk of
type 2 diabetes, cardiovascular disease, and weight gain. J Nutr.
142:1304–1313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vetrani C, Costabile G, Luongo D, Naviglio
D, Rivellese AA, Riccardi G and Giacco R: Effects of whole-grain
cereal foods on plasma short chain fatty acid concentrations in
individuals with the metabolic syndrome. Nutrition. 32:217–221.
2016. View Article : Google Scholar
|
|
16
|
Bosello O, Ostuzzi R, Armellini F,
Micciolo R and Scuro LA: Glucose tolerance and blood lipids in
bran-fed patients with impaired glucose tolerance. Diabetes Care.
3:46–49. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brodribb AJ and Humphreys DM: Diverticular
disease: Three studies. Part III - Metabolic effect of bran in
patients with diverticular disease. Br Med J. 1:428–430. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nogata Y, Nagamine T, Yanaka M and Ohta H:
Angiotensin I converting enzyme inhibitory peptides produced by
autolysis reactions from wheat bran. J Agric Food Chem.
57:6618–6622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fiordaliso F, Cuccovillo I, Bianchi R, Bai
A, Doni M, Salio M, De Angelis N, Ghezzi P, Latini R and Masson S:
Cardiovascular oxidative stress is reduced by an ACE inhibitor in a
rat model of streptozotocin-induced diabetes. Life Sci. 79:121–129.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Privratsky JR, Wold LE, Sowers JR, Quinn
MT and Ren J: AT1 blockade prevents glucose-induced cardiac
dysfunction in ventricular myocytes: Role of the AT1 receptor and
NADPH oxidase. Hypertension. 42:206–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zarfeshani A, Ngo S and Sheppard AM:
Leucine alters hepatic glucose/lipid homeostasis via the
myostatin-AMP-activated protein kinase pathway - potential
implications for nonalcoholic fatty liver disease. Clin
Epigenetics. 6:272014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mantena SK, Vaughn DP Jr, Andringa KK,
Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW,
Darley-Usmar VM and Bailey SM: High fat diet induces dysregulation
of hepatic oxygen gradients and mitochondrial function in vivo.
Biochem J. 417:183–193. 2009. View Article : Google Scholar :
|
|
23
|
Kawaguchi T, Sakisaka S, Sata M, Mori M
and Tanikawa K: Different lobular distributions of altered
hepatocyte tight junctions in rat models of intrahepatic and
extrahepatic cholestasis. Hepatology. 29:205–216. 1999. View Article : Google Scholar
|
|
24
|
Kawaguchi T, Sakisaka S, Mitsuyama K,
Harada M, Koga H, Taniguchi E, Sasatomi K, Kimura R, Ueno T, Sawada
N, et al: Cholestasis with altered structure and function of
hepatocyte tight junction and decreased expression of canalicular
multispecific organic anion transporter in a rat model of colitis.
Hepatology. 31:1285–1295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawaguchi T, Itou M, Taniguchi E and Sata
M: Exendin 4, a glucagon like peptide 1 receptor agonist, modulates
hepatic fatty acid composition and Δ5 desaturase index in a murine
model of non alcoholic steatohepatitis. Int J Mol Med. 34:782–787.
2014.PubMed/NCBI
|
|
26
|
Kleiner DE, Brunt EM, Van Natta M, Behling
C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS,
Unalp-Arida A, et al Nonalcoholic Steatohepatitis Clinical Research
Network: Design and validation of a histological scoring system for
nonalcoholic fatty liver disease. Hepatology. 41:1313–1321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Korenaga M, Nishina S, Korenaga K,
Tomiyama Y, Yoshioka N, Hara Y, Sasaki Y, Shimonaka Y and Hino K:
Branched-chain amino acids reduce hepatic iron accumulation and
oxidative stress in hepatitis C virus polyprotein-expressing mice.
Liver Int. 35:1303–1314. 2015. View Article : Google Scholar :
|
|
28
|
Tanito M, Kaidzu S, Takai Y and Ohira A:
Status of systemic oxidative stresses in patients with primary
open-angle glaucoma and pseudoexfoliation syndrome. PLoS One.
7:e496802012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawaguchi T, Yoshida T, Harada M, Hisamoto
T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, et
al: Hepatitis C virus downregulates insulin receptor substrates 1
and 2 through upregulation of suppressor of cytokine signaling 3.
Am J Pathol. 165:1499–1508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawaguchi T, Ide T, Taniguchi E, Hirano E,
Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H, et al:
Clearance of HCV improves insulin resistance, beta-cell function,
and hepatic expression of insulin receptor substrate 1 and 2. Am J
Gastroenterol. 102:570–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marchesini G, Petta S and Dalle Grave R:
Diet, weight loss, and liver health in nonalcoholic fatty liver
disease: Pathophysiology, evidence, and practice. Hepatology.
63:2032–2043. 2016. View Article : Google Scholar
|
|
33
|
Albano E, Mottaran E, Occhino G, Reale E
and Vidali M: Review article: Role of oxidative stress in the
progression of non-alcoholic steatosis. Aliment Pharmacol Ther.
22(Suppl 2): 71–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ni X and Wang H: Silymarin attenuated
hepatic steatosis through regulation of lipid metabolism and
oxidative stress in a mouse model of nonalcoholic fatty liver
disease (NAFLD). Am J Transl Res. 8:1073–1081. 2016.PubMed/NCBI
|
|
35
|
Maeda K, Koda M, Matono T, Sugihara T,
Yamamoto S, Ueki M, Murawaki Y, Yamashita N and Nishiyama S:
Preventive effects of ME3738 on hepatic fibrosis induced by bile
duct ligation in rats. Hepatol Res. 38:727–735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tomiyama Y, Nishina S, Hara Y, Kawase T
and Hino K: Hepatic oxidative stress in ovariectomized transgenic
mice expressing the hepatitis C virus polyprotein is augmented
through suppression of adenosine monophosphate-activated protein
kinase/proliferator-activated receptor gamma co-activator 1 alpha
signaling. Hepatol Res. 44:E229–E239. 2014. View Article : Google Scholar
|
|
37
|
Suzuki Y, Imai K, Takai K, Hanai T,
Hayashi H, Naiki T, Nishigaki Y, Tomita E, Shimizu M and Moriwaki
H: Hepatocellular carcinoma patients with increased oxidative
stress levels are prone to recurrence after curative treatment: A
prospective case series study using the d-ROM test. J Cancer Res
Clin Oncol. 139:845–852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vassalle C, Bianchi S, Battaglia D, Landi
P, Bianchi F and Carpeggiani C: Elevated levels of oxidative stress
as a prognostic predictor of major adverse cardiovascular events in
patients with coronary artery disease. J Atheroscler Thromb.
19:712–717. 2012.PubMed/NCBI
|
|
39
|
Kim JH, Baik HW, Yoon YS, Joung HJ, Park
JS, Park SJ, Jang EJ, Park SW, Kim SJ, Kim MJ, et al: Measurement
of antioxidant capacity using the biological antioxidant potential
test and its role as a predictive marker of metabolic syndrome.
Korean J Intern Med. 29:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu L, Bruckbauer A, Li F, Cao Q, Cui X, Wu
R, Shi H, Zemel MB and Xue B: Interaction between metformin and
leucine in reducing hyperlipidemia and hepatic lipid accumulation
in diet-induced obese mice. Metabolism. 64:1426–1434. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fu L, Li F, Bruckbauer A, Cao Q, Cui X, Wu
R, Shi H, Xue B and Zemel MB: Interaction between leucine and
phosphodiesterase 5 inhibition in modulating insulin sensitivity
and lipid metabolism. Diabetes Metab Syndr Obes. 8:227–239.
2015.PubMed/NCBI
|