Introduction
Rheumatoid arthritis (RA), a chronic systemic
autoimmune disease primarily affecting the joints, is characterized
by synovitis, synovial tissue proliferation, cartilage and bone
destruction, and ultimately leads to physical disability (1). RA affects approximately 1% of the
adult population worldwide.
Currently, the exact pathogenesis of RA remains
unclear, but accumulating evidence suggests that fibroblast-like
synoviocytes (FLS) play an important role in the development of RA
because of their resistance to apoptosis and extensive
proliferative ability (2). In RA
patients, defective apoptosis of synovial cells is closely related
to synovial tissue hyperplasia (3). RA-FLS exhibit features of tumor-like
damage and erosion, which may lead to further synovial tissue
hyperplasia and destruction of joint cartilage (4). Thus, inhibition of RA-FLS
proliferation and induction of their apoptosis are under
consideration as a therapeutic strategy for the treatment of
RA.
The mitochondrial pathway plays a critical role in
FLS apoptosis in RA, with the Bax, Bcl-2, and caspase-3 proteins,
in particular, being implicated. Bcl-2 and Bax are key regulators
of the mitochondrial apoptosis pathway (5). Bax promotes apoptosis, whereas Bcl-2
exerts a wide range of anti-apoptotic effects. Caspase-3 is a key
executive mediator of apoptosis and the common downstream effector
of many apoptotic signaling pathways.
The Janus kinase/signal transducers and activators
of transcription (JAK/STAT) signaling pathway is important for many
cellular processes, including growth, differentiation,
proliferation and immune regulation (6). Aberrant JAK/STAT signaling has been
related to the occurrence of many human diseases, including RA and
cancer (7). Previous study has
indicated that activating JAK/STAT through interferon-γ confers
apoptotic resistance to cells in synovial tissues of inflammatory
RA, leading to a significantly increased density of synovial cells
(3). The same study showed that
JAK/STAT exerts anti-apoptotic effects via translational regulation
(3).
In recent years, JAKs have generated great interest
as therapeutic targets given their key roles in signal transduction
(8). Activated JAKs trigger a
signaling cascade that leads to the phosphorylation of STATs.
Phosphorylated STATs form as active dimers, translocate to the
nucleus, and bind to specific response elements to activate or
inhibit the expression of the target genes. There are seven STAT
family members, of which STAT1 and STAT3 represent the major
activators (9). STAT1 expression
and activity increases in early synovial tissues of human RA, and
STAT3 promotes synovial fibroblast survival (10,11). Some studies have suggested that
the activation of STAT1 and STAT3 is important to synovial cell
proliferation (12).
The traditional approach for controlling RA relies
on disease-modifying antirheumatic drugs (DMARDs), including
methotrexate (MTX), leflunomide and sulphasalazine. DMARDs have
certain clinical benefits, but coincide with a series of toxic
events (13). As an alternative
treatment for RA, plant extracts are being considered due to lower
toxicity profiles and minimal side effects (14,15). Matrine, a traditional Chinese
medicine, is an alkaloid isolated from plants in the genus
Sophora, comprised of four rings of quinolizidine classes
(C15H24N2O). Matrine exhibits
different biological properties, including anti-inflammatory
(16), antitumor (17), anti-viral (18), anti-fibrosis (19), anti-arrhythmia (20), anti-asthmatic (21) and immunosuppressive activity
(22). However, the effects of
matrine on the proliferation and apoptosis of FLS have yet to be
reported.
Collagen-induced arthritis (CIA) is an experimental
model of human RA that has been widely used to study the
pathogenesis of RA and evaluate the effects of therapeutic
interventions (23,24). The purpose of this study was to
investigate the effect of matrine on FLS proliferation and
apoptosis in a rat model of CIA. We also assessed the mechanisms by
which matrine functions to modulate these cellular behaviors.
Materials and methods
Induction of CIA
Male Sprague-Dawley (SD) rats (7–8 weeks) were
purchased from the Experimental Animal Center of the Third Military
Medical University (Chongqing, China). Animal care and treatment
were completed by the Ethics Committee of the Experimental Animal
Administration of the Third Military Medical University. CIA was
immune-induced by bovine type II collagen (Chondrex, Inc., Redmond,
WA, USA). Type II collagen (2 mg/ml) dissolved in 0.1 M acetic acid
was emulsified with an equal volume of complete Freund's adjuvant
(CFA) or incomplete Freund's adjuvant (IFA; MP Biomedicals, Santa
Ana, CA, USA). The emulsion (300 µl) was injected into the
base of the tail on day 0 for the first immunization and on day 7
for the second immunization. An equal volume of saline was
administered to rats in the control group at the same time and into
the same site.
Culture and drug treatment of FLS
Forty-two days after the initial immunization,
randomly chosen rats from the CIA and control groups were
sacrificed. Synovial tissues from the knee joints were isolated,
minced and cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS; Gibco, Grand Island, NY, USA) at 37°C in a
humidified atmosphere under 5% CO2. After FLS
identification based on cell morphology and immunofluorescent
vimentin labeling (25), cells
were used from passages 3 to 5 in ensuing experiments. Cell
treatments included the JAK2 inhibitor, AG490 (25 µmol/l;
Abmole Bioscience, Houston, TX, USA) or matrine (0.75 mg/ml;
Aladdin Reagents Co., Ltd., Shanghai, China).
Cell proliferation assay
Cell proliferation was analyzed using the Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan).
Cells were seeded in three 96-well plates at a density of
3×104 cells/well and treated with increasing
concentrations (0, 0.25, 0.5, 0.75, 1.0, 1.5 and 2.0 mg/ml) of
matrine for 24, 48 and 72 h. Then, 10 µl of CCK-8 solution
was added to each well, and the cells were incubated for another 2
h at 37°C. The optical density (OD) was measured using a
micro-plate reader (Beijing Purkinje General Instrument Co., Ltd.,
Beijing, China) at a wavelength of 450 nm. The cell growth
inhibition rate (GIR) was measured and the half maximal inhibitory
concentration (IC50) was calculated using GraphPad Prism
5 (GraphPad Software, San Diego, CA, USA).
Cell cycle analysis
FLS were collected in the logarithmic growth phase
and seeded in culture flasks at a density of 2×105
cells/ml. After synchronization in serum-free medium for 24 h, FLS
were treated with matrine, AG490, matrine+AG490, or left untreated
for 24 h. After drug treatment, the cells were collected, suspended
in cold phosphate-buffered saline (PBS), centrifuged, and then
fixed with 75% cold ethanol and stored at 4°C for 24 h. The cells
were stained with propidium iodide (PI) solution (Cell Cycle and
Apoptosis Analysis kit; Beyotime Institute of Biotechnology,
Nanjing, China) for 30 min at 37°C in the dark. DNA content was
analyzed by flow cytometry (Beckman Coulter, Brea, CA, USA).
Apoptosis analysis
FLS apoptosis was assessed with the Annexin
V-FITC/PI double labeling method combined with flow cytometry.
Briefly, the cells were seeded at a density of 2×105
cells/ml in five culture flasks for 24 h and synchronized. After
exposure to drugs for 24 h, the cells were collected, re-suspended
in PBS, counted, centrifuged and resuspended in 195 µl
binding buffer according to the manufacturer's instructions. The
cell suspension was incubated in 5 µl of Annexin V-FITC and
10 µl PI (Annexin V-FITC Apoptosis Detection kit; Beyotime
Institute of Biotechnology) for 20 min at room temperature in the
dark. Apoptotic cells were analyzed by flow cytometry.
Western blot analysis
The cells were collected in radioimmunoprecipitation
assay (RIPA) lysis buffer (Chongqing Golden Wheat Biotechnology,
Chongqing, China). The protein concentration was determined using
the bicinchoninic acid (BCA) protein assay (Beyotime Institute of
Biotechnology). Proteins were separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene difluoride (PVDF) membranes. Then,
the membranes were blocked with 5% skim milk for 2 h at room
temperature. The membranes were incubated overnight at 4°C in
antibodies against β-actin (1:1,000; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), p-JAK2 (1:1000; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China), p-STAT1 and p-STAT3
(1:1,000; both from BBI Life Sciences Corporation, Shanghai,
China). The next day, the membranes were washed three times in
Tris-buffered saline containing 0.05% Tween-20 (TBST) and incubated
in horseradish peroxidase-conjugated anti-rabbit secondary antibody
(1:1,000; Beyotime Institute of Biotechnology) for 2 h at room
temperature. The protein bands were visualized with ECL reagents
(Beyotime Institute of Biotechnology).
For the rat experiments, synovial tissues were
isolated and ground into a powder with liquid nitrogen. Proteins
were collected in RIPA buffer, and the expression levels of
phosphorylated JAK2, STAT1 and STAT3 were detected by western blot
analysis as described above.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extr acted from cultured FLS with
TRIzol reagent according to the manufacturer's instructions (Sangon
Biotech Co., Ltd., Shanghai, China). The RNA was
reverse-transcribed with AMV First Strand cDNA Synthesis kit
(Sangon Biotech Co., Ltd.) in the presence of random primers and
RNase-free ddH2O. The primers were designed by Sangon
Biotech Co., Ltd. and are listed in Table I. β-actin was used as an
endogenous control. The conditions for the amplifications were as
follows: 3 min hold at 95°C, followed by 7 sec denaturation at
95°C, 10 sec annealing at 57°C, and 15 sec extension at 72°C for 40
cycles. The PCR products were analyzed using StepOne Plus™
Real-Time PCR system (Applied Biosystems, Grand Island, NY, USA).
The qRT-PCR data were analyzed using the 2−ΔΔCT
method.
 | Table IPrimers used for qRT-PCR
analysis. |
Table I
Primers used for qRT-PCR
analysis.
| Genes | Primer sequence
(5′-3′) | Accession no. |
|---|
| Bax | F:
GGCGATGAACTGGACAACAA
R: GCAAAGTAGAAAAGGGCAACC | NM 017059.2 |
| Bcl-2 | F:
ACGAGTGGGATACTGGAGATGA
R: CTCAGGCTGGAAGGAGAAGAT | NM 016993.1 |
| Caspase-3 | F:
ATCCACGAGCAGAGTCAAAGG
R: CAAGCCAACCAAGTTCACACA | NM 012922.2 |
| β-actin | F:
CGTAAAGACCTCTATGCCAACA
R: AGCCACCAATCCACACAGAG | NM 031144.3 |
CIA drug treatments
Rats with CIA were divided randomly into a control
group, CIA group, matrine group [100 mg/kg/day by oral gavage
(19)], and MTX group [Shanghai
Sine Pharmaceuticals Co., Ltd., Shanghai, China; 2 mg/kg/week by
oral gavage (26)]. Control and
CIA rats received saline at a dose of 10 ml/kg/day by oral gavage.
Drugs were administered from days 11 to 52 after the first
immunization.
Evaluation of arthritis index (AI)
AI was assessed by the presence of edema and/or
erythema in the paws that started before gavage, occurred once a
week, and lasted for six weeks. Assessment standards have been
previously described (27) as
follows: 0, no redness or swelling; 1, light redness or swelling in
a few toes; 2, redness and swelling in most toe joints and toes; 3,
serious redness and swelling in feet and below ankle joint; and 4,
redness and swelling in feet and ankle joint. The AI was calculated
by cumulation of the scores from all four paws, with a maximum
value of 16.
The pathological alteration of ankle
joints
Rats received matrine or MTX over the course of six
weeks before they were sacrificed by cervical dislocation. The
ankles were removed and fixed in 4% paraformaldehyde (Guangfu Fine
Chemical Research Institute, Tianjin, China) and decalcified for 16
h with 8% nitrate decalcification solution (Chengdu Kelong Chemical
Co., Ltd., Chengdu, China). Then, the joints were washed,
dehydrated, brightened, embedded in paraffin, sectioned and stained
with hematoxylin and eosin (H&E). Pathological alterations were
detected with a light microscope (BX51M; Olympus, Tokyo,
Japan).
Immunohistochemical analysis
The rats were sacrificed and then their synovial
tissues were isolated from their knee joints and embedded in
paraffin. Sections (5-µm thick) were cut on a microtome and
then dewaxed in water. Endogenous peroxidases were inactivated and
antigen retrieval was conducted using a boiling method. Then, the
sections were blocked in goat serum for 15 min at room temperature
and incubated overnight at 4°C with antibodies against Bax (1:100)
and Bcl-2 (1:100) (both from BBI Life Science Corporation), and
caspase-3 (1:100; Nanjing Jiancheng Bioengineering Institute). A
biotin-labeled secondary antibody and SABC solution were added
dropwise, successively and incubated for 20 min at room
temperature. The sections were developed with DAB, re-stained with
hematoxylin, dehydrated, brightened and mounted with neutral gum.
Images were captured on a light microscope (Olympus) and OD values
were calculated with IPP software.
Statistical analysis
Quantitative data are presented as mean ± standard
deviation (SD). All data were analyzed using SPSS 19.0 software
(SPSS, Inc., Chicago, IL, USA). Statistical significance was
assessed by one-way analysis of variance (ANOVA) or the
Games-Howell test according to the homogeneity of variance.
P-values <0.05 were considered to indicate a statistically
significant difference.
Results
Identification of FLS
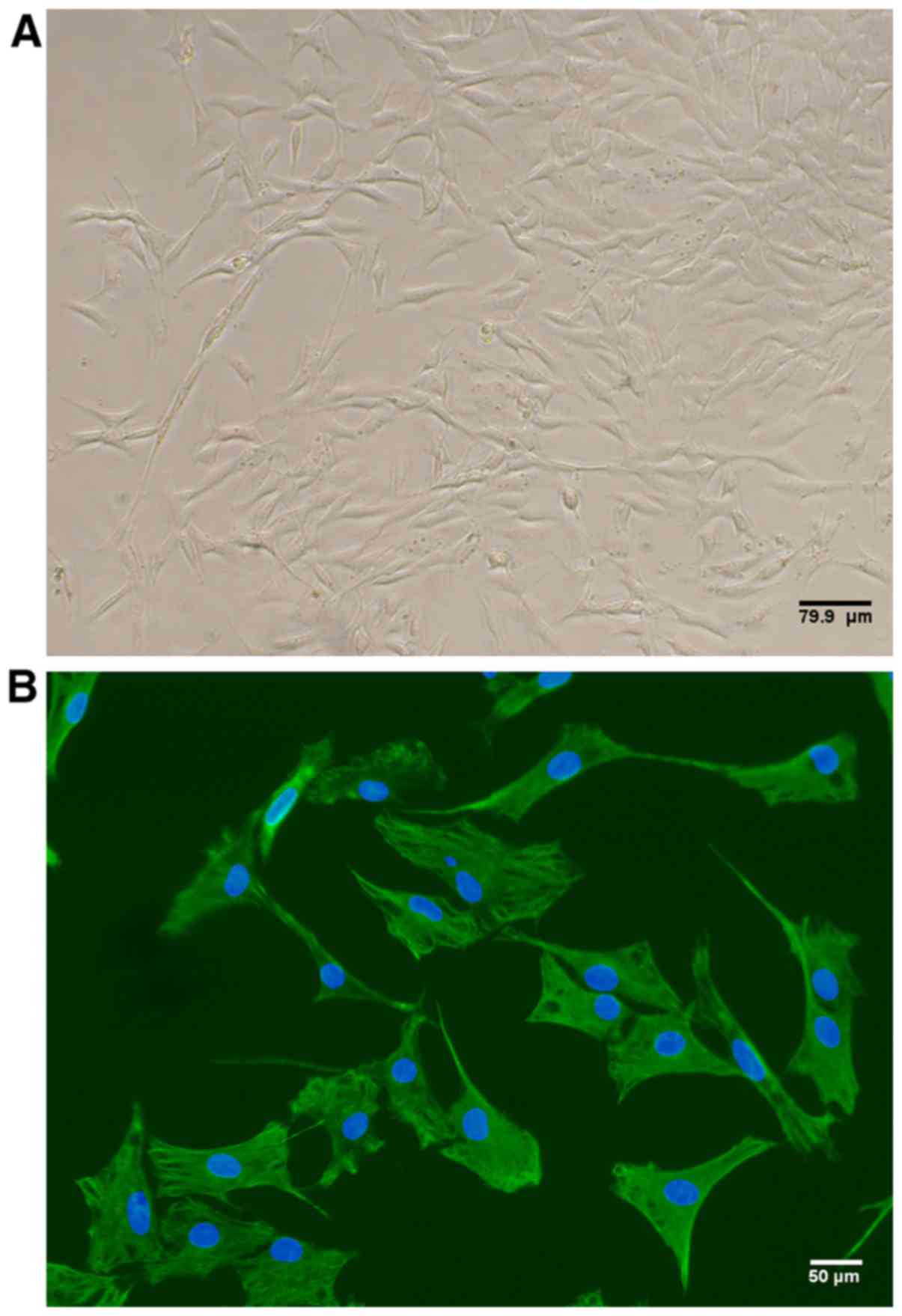
By passage three, the cellular morphology of
cultured rat FLS appeared spindle-shaped, as is characteristic of
fibroblasts (Fig. 1A). In
addition, 100% of the cultured cells expressed vimentin as detected
by immunofluorescence, thus confirming FLS identity (Fig. 1B).
Matrine inhibits the proliferation of
rat-derived FLS in vitro
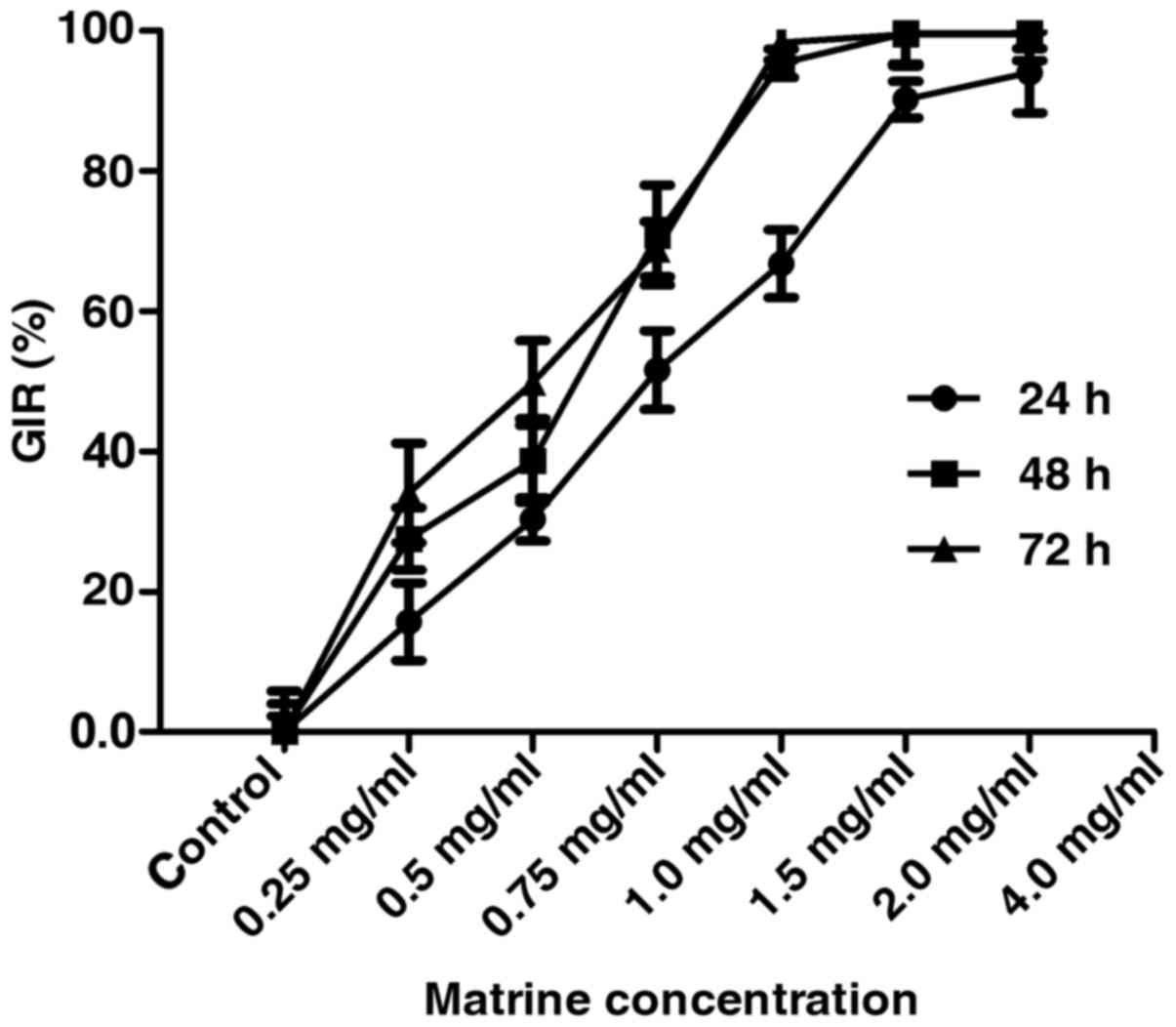
CCK-8 assay assessment of cell viability of the FLS
isolated from the CIA rats treated with varying concentrations (0,
0.25, 0.5, 0.75, 1.0, 1.5 and 2.0 mg/ml) of matrine showed that
matrine inhibited FLS growth in a concentration-dependent manner at
24, 48 and 72 h (P<0.01; Fig.
2). The IC50 of matrine inhibitory activity was
estimated to be 0.75 mg/ml at 24 h, and these parameters were used
in subsequent experiments.
Matrine induces cell cycle arrest of
rat-derived FLS in vitro
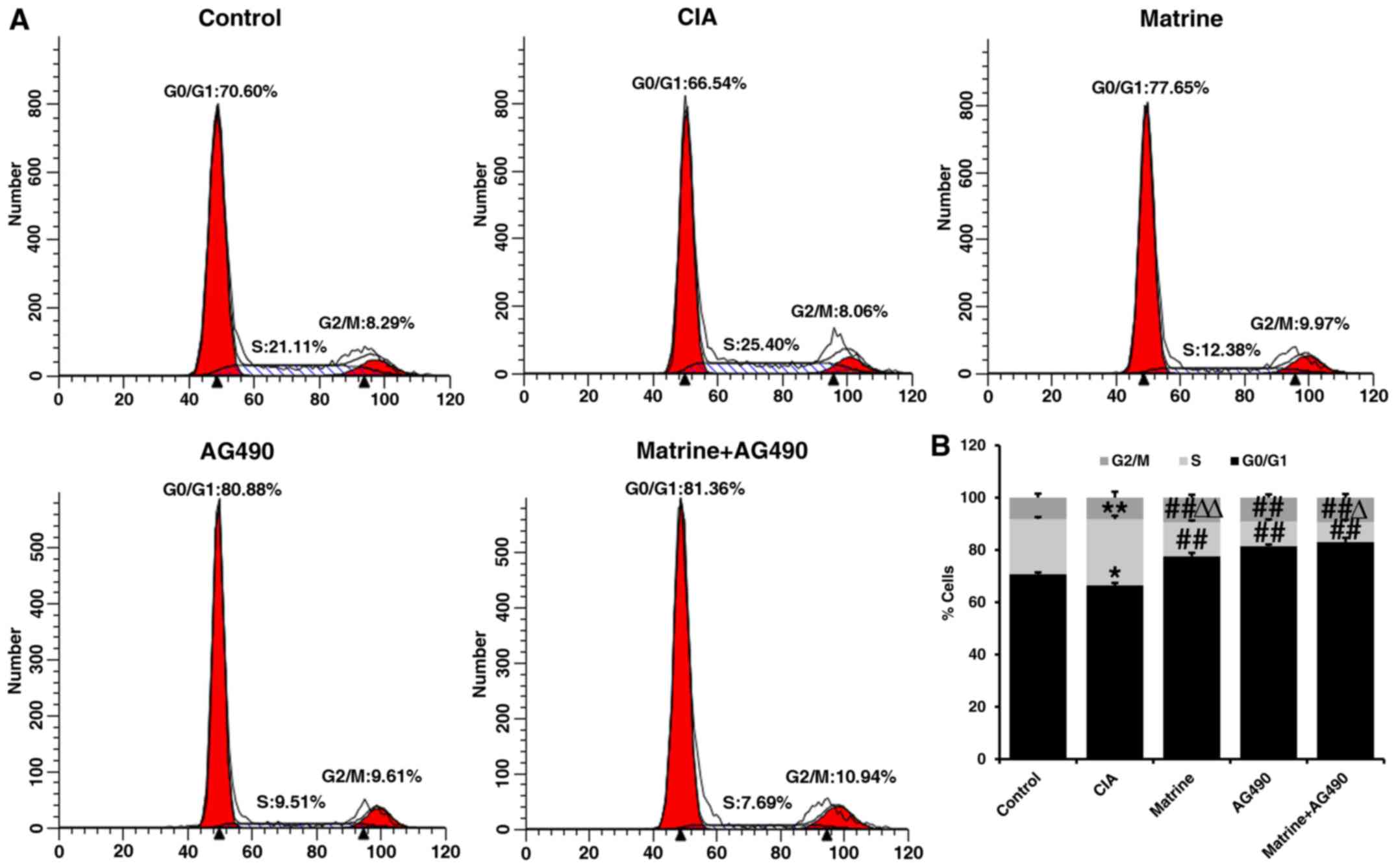
Flow cytometry of FLS revealed that the percentage
of cells in S phase was increased (P<0.01), whereas the
percentage of cells in the G0/G1 phase was decreased (P<0.05) in
the CIA group compared with the control group (Fig. 3). Compared with the CIA group, the
percentages of FLS in the S phase were significantly decreased in
cells treated with matrine, the specific JAK2 inhibitor AG490
(inhibits phosphorylation of STAT1/3 in RA-FLS), or both for 24 h
(P<0.01; Fig. 3).
Additionally, the percentages of cells in the G0/G1 phase were also
increased in the drug-treated groups compared with CIA alone
(P<0.01). The percentage of cells in the S phase in the
AG490-treated group was less than that in the matrine group
(P<0.01), but greater than that in the matrine+AG490 group
(P<0.05) (Fig. 3). These
findings suggest that matrine inhibits cell proliferation by
inducing cell cycle arrest at the G0/G1 phase.
Matrine induces apoptosis of rat-derived
FLS in vitro
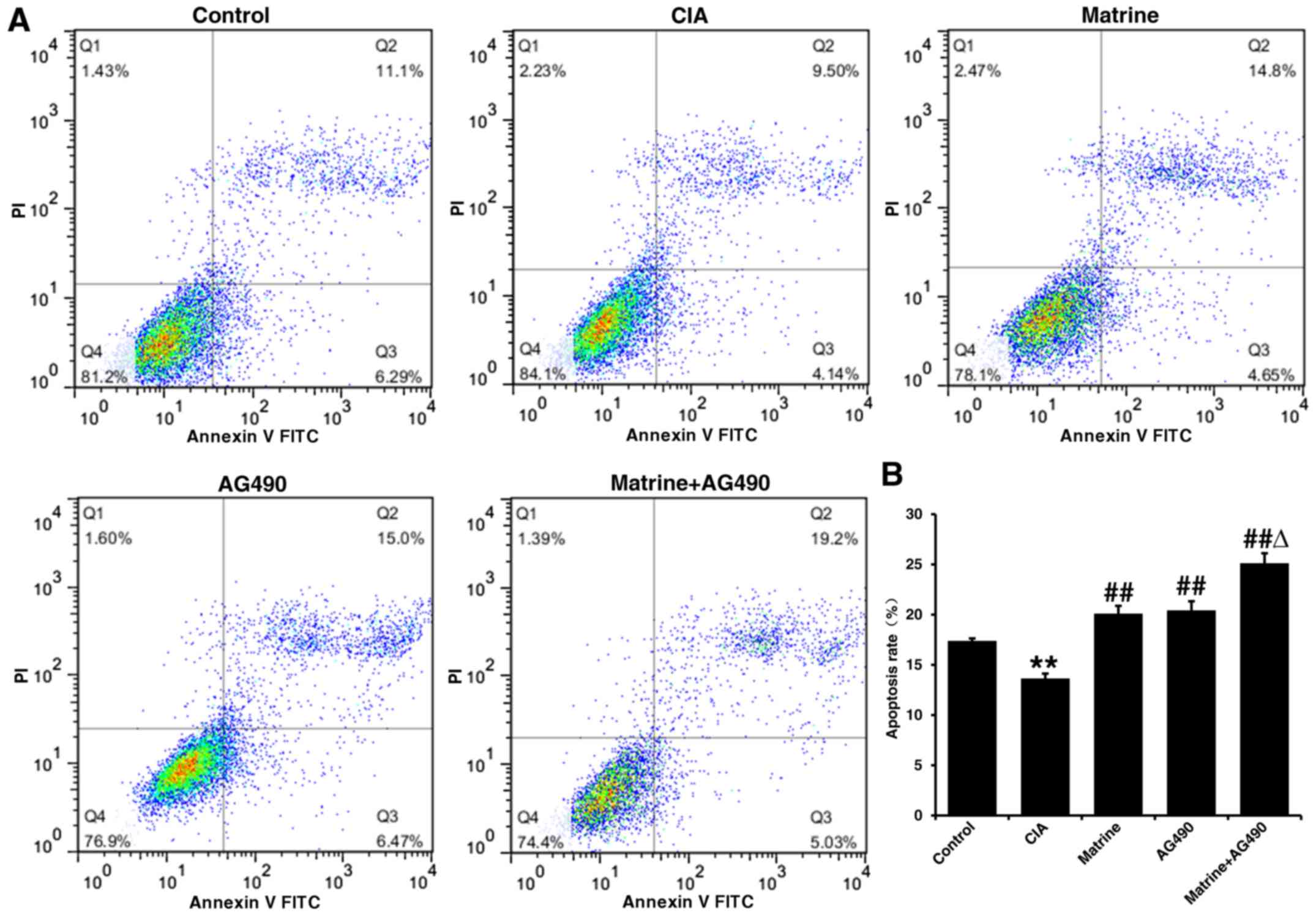
Annexin V-FITC/PI double staining and flow cytometry
indicated that the apoptosis rate of FLS isolated from control rats
was 17.24±0.24% compared with 13.64±0.49% in the CIA group
(P<0.01; Fig. 4). All drug
treatments resulted in significantly higher apoptosis rates than
that observed for the CIA group (P<0.01; matrine, 20.10±0.77%;
AG490, 20.42±0.91%; matrine+AG490, 25.11±1.00%). The apoptosis rate
did not differ between the matrine and AG490 groups. These results
indicate that matrine treatment increased FLS apoptosis.
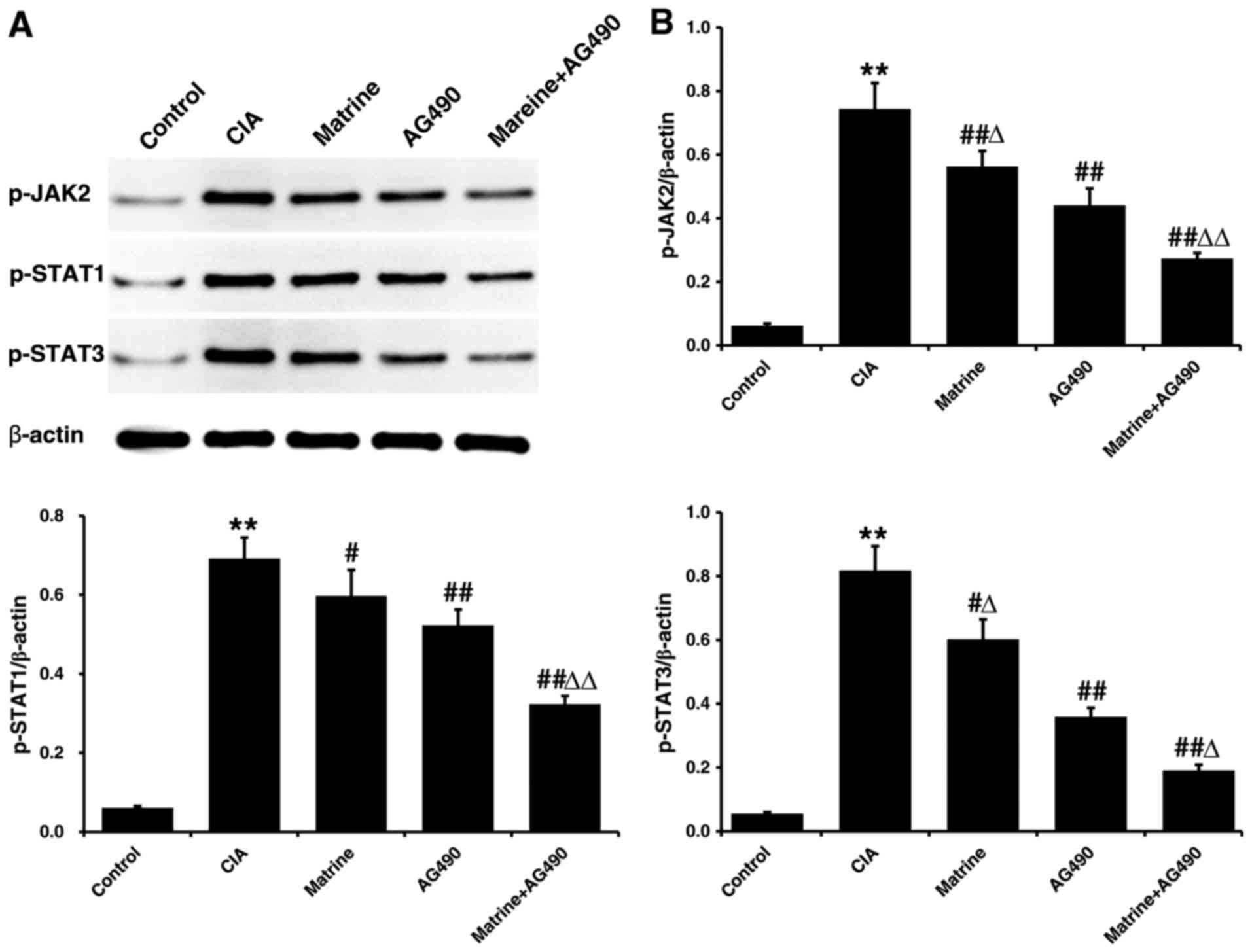
Matrine suppresses JAK/STAT signaling
pathway activation in rat-derived FLS in vitro
Protein expression levels of p-JAK2, p-STAT1 and
p-STAT3 in FLS isolated after 24 h of drug treatment were only
faintly detectable in the control group, and markedly higher in the
CIA group (P<0.01 vs. control; Fig. 5). All phosphorylated protein
levels were decreased relative to CIA alone when FLS from CIA rats
were treated with matrine (p-JAK2, P<0.01; p-STAT1 and p-STAT3,
P<0.05), AG490 (all P<0.01), or matrine+AG490 (all
P<0.01). In addition, p-JAK2 and p-STAT3 were inhibited to a
greater extent by treatment with AG490 than by treatment with
matrine (P<0.05). However, p-STAT1 levels did not differ between
the matrine and AG490 groups (P>0.05).
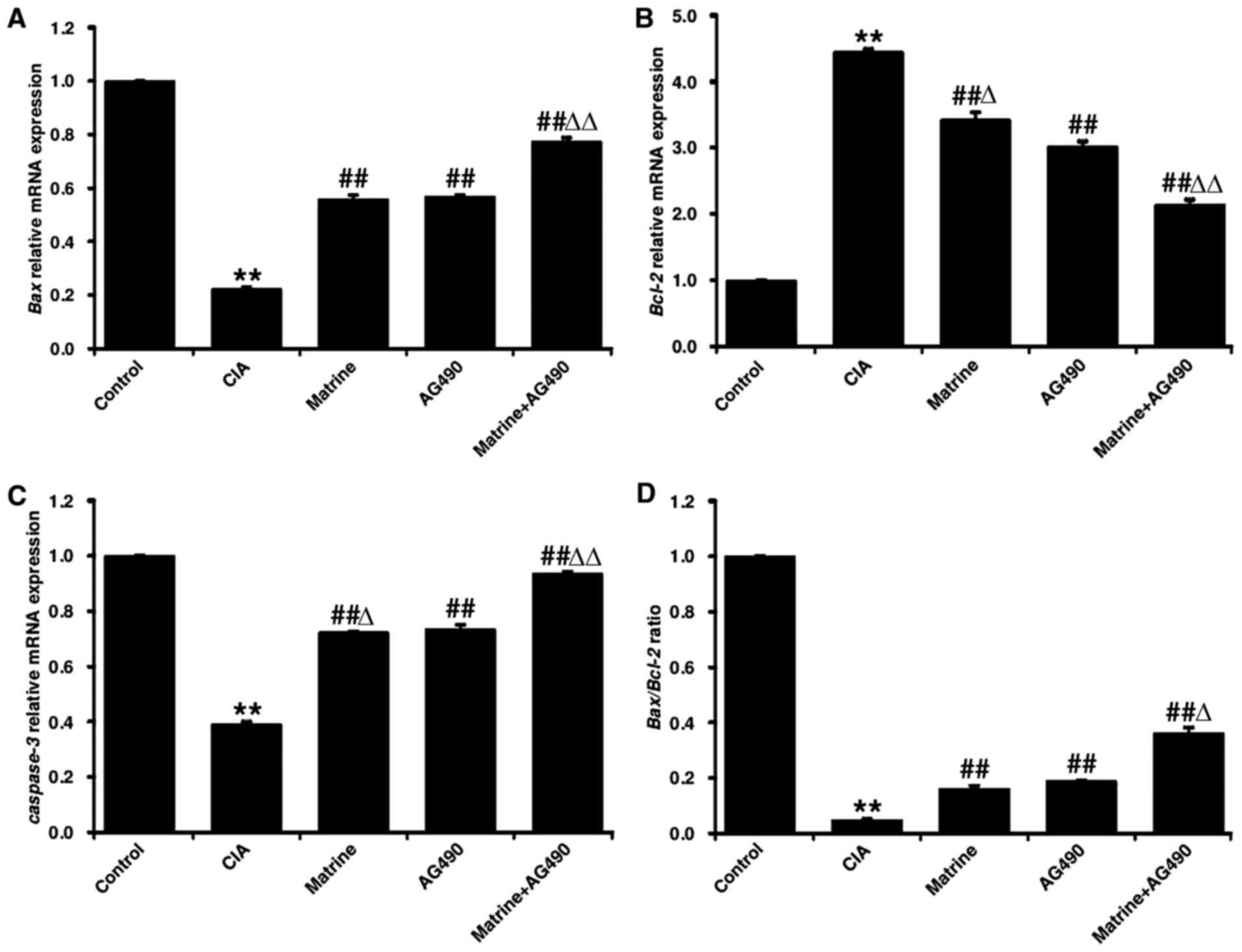
Matrine induces upregulation of
pro-apoptotic markers in rat-derived FLS in vitro
As shown in Fig.
6, qRT-PCR analysis revealed that the expression levels of Bax
and caspase-3 in the CIA group were decreased, whereas Bcl-2
expression was increased, compared to levels observed for the
control group FLS (P<0.01). Treatment with matrine, AG490, or
matrine+AG490 markedly increased the mRNA expression of Bax and
caspase-3, and decreased expression of Bcl-2 (P<0.01 vs. CIA
alone). Matrine treatment resulted in higher levels of Bcl-2
(Fig. 6B) and lower levels of
caspase-3 (Fig. 6C) than did the
AG490 treatment (P<0.05). Bax levels did not differ between the
matrine and AG490 groups (P>0.05; Fig. 6A). Finally, the Bax:Bcl-2 ratio
was increased in all drug-treated groups compared with CIA alone
(P<0.01; Fig. 6D).
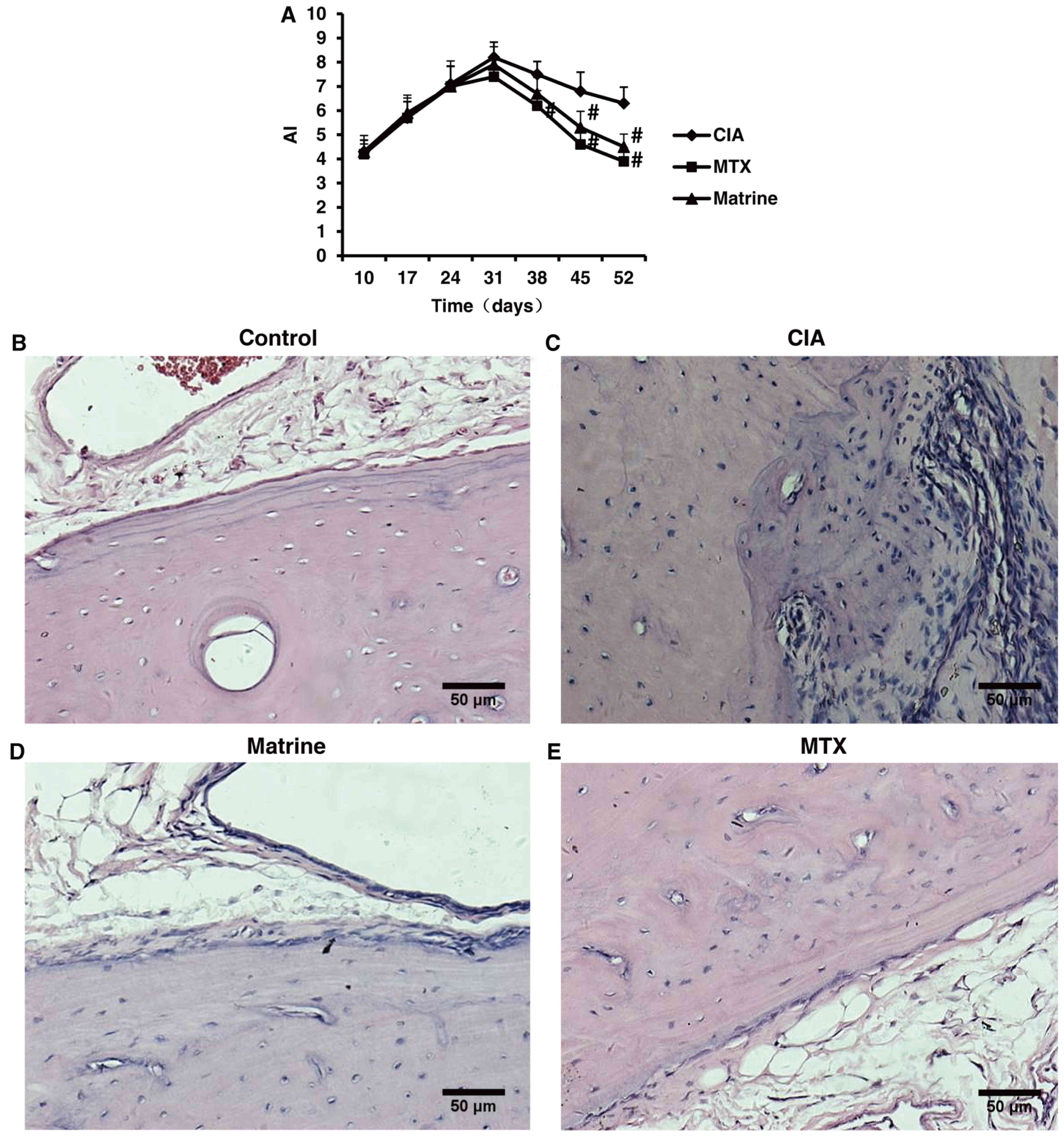
Therapeutic effects of matrine in rats
with CIA
Evaluation of AI and pathological alterations of the
ankle to determine arthritis severity in the CIA model indicated
that onset of secondary arthritis appeared around day 10 after
primary immunization and peaked on day 31. Matrine treatment
decreased AI compared with no treatment (P<0.05; Fig. 7A). There were no statistically
significant differences between the matrine and MTX groups
(P>0.05).
Microscopic assessment of ankle structure revealed
that control rats displayed smooth articular surfaces with no
evidence of inflammatory cell infiltration or cartilage destruction
(Fig. 7B). In contrast, the
ankles of rats in the CIA group exhibited significant synovial
tissue hyperplasia, inflammatory cell infiltration and cartilage
erosion (Fig. 7C). Treatment with
matrine or MTX improved CIA ankle pathologies (Fig. 7B–D).
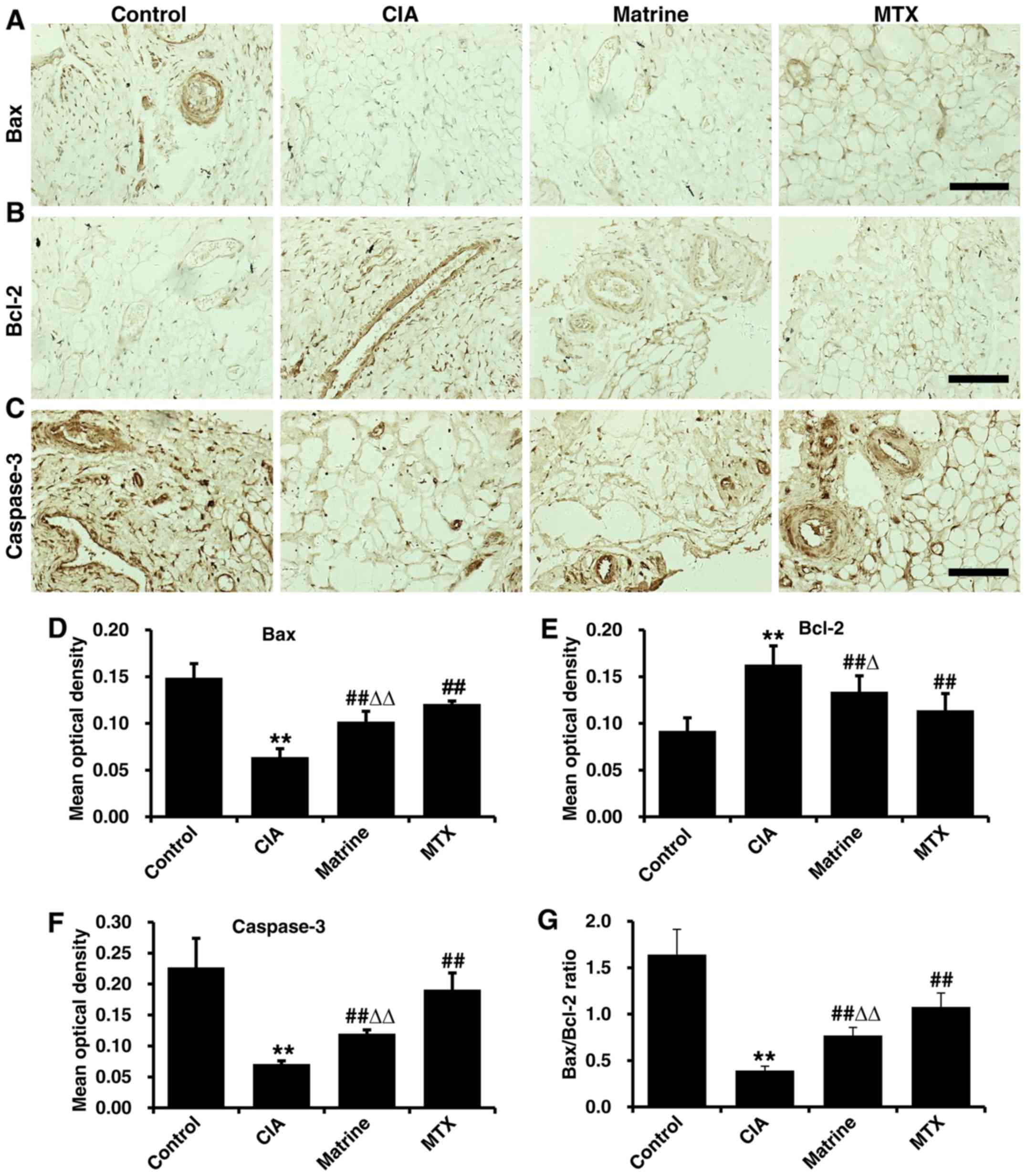
Effects of matrine on apoptotic marker
expression in synovial tissues from CIA rats
Immunohistochemical analysis demonstrated Bax, Bcl-2
and caspase-3 expression in the nucleus and cytoplasm in synovial
tissue cells (positivity indicated with tan granules; Fig. 8A–C). Quantification of
immunohistochemical OD values revealed that the expression of Bax
and caspase-3 were decreased, whereas Bcl-2 expression was
increased in the CIA group compared with the control group
(P<0.01). Treatment with matrine or MTX rescued Bax and
caspase-3 levels and attenuated Bcl-2 expression, but matrine had
greater effects on reduction of Bax and caspase-3 (P<0.01) and
augmentation of Bcl-2 (P<0.05) relative to MTX (Fig. 8D–F). Finally, the Bax:Bcl-2 ratio
was increased in the matrine group compared with CIA alone
(P<0.01; Fig. 8G).
Effects of matrine on JAK/STAT signaling
in synovial tissues from rats with CIA
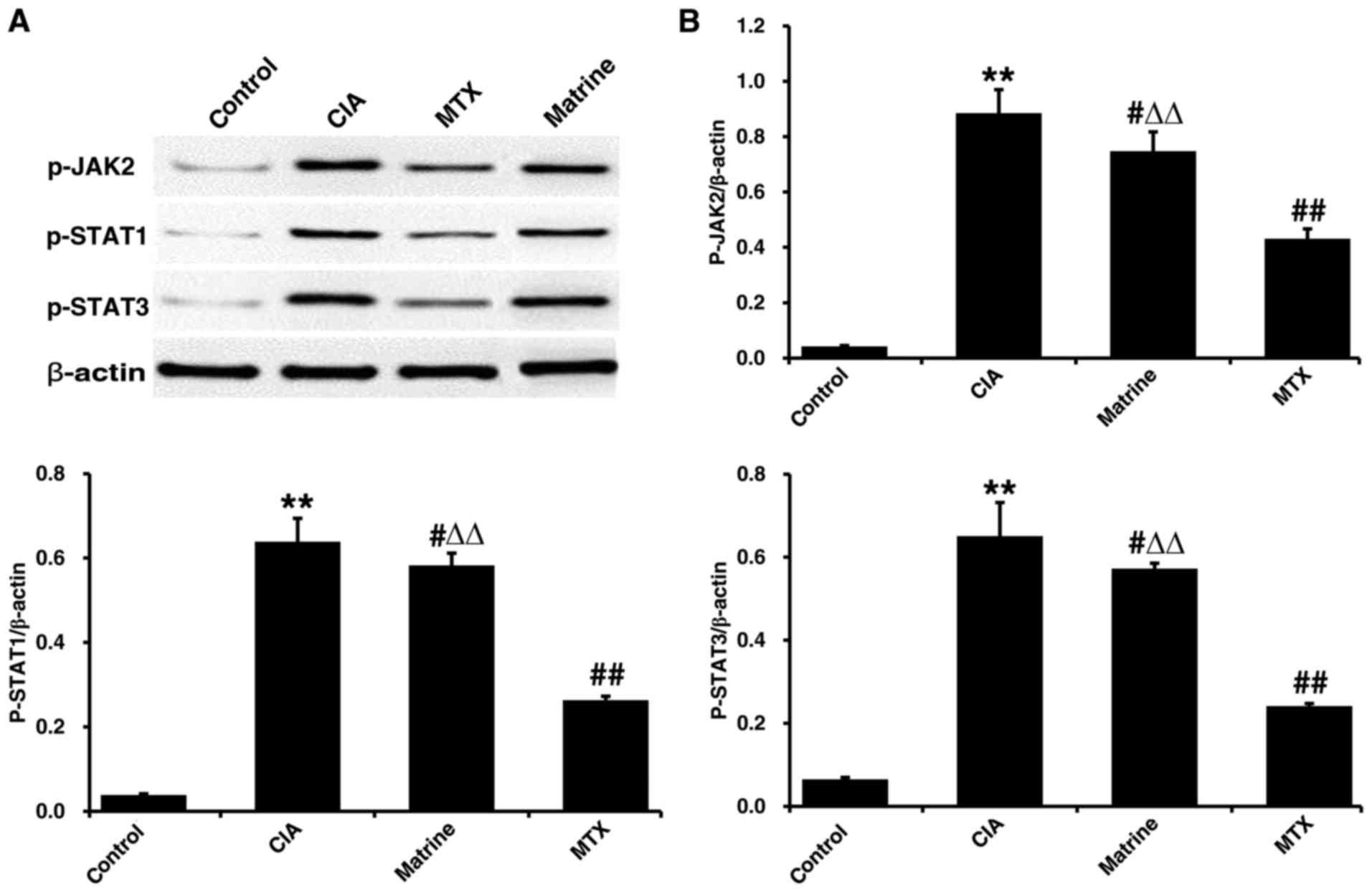
Phosphorylated JAK2, STAT1 and STAT3 levels were
examined in synoviocytes from rats with CIA by western blot
analysis. Protein expression levels of p-JAK2, p-STAT1 and p-STAT3
were detected at low levels in the control rats, whereas CIA
resulted in increased phosphorylation (P<0.01; Fig. 9). Matrine treatment reduced
phosphorylated levels of all three proteins compared with CIA alone
(P<0.01). MTX treatment was more potent than matrine at reducing
phosphorylation (P<0.01).
Discussion
In the present study, we investigated the effects of
matrine treatment on the proliferation, apoptosis and activation of
JAK/STAT signaling in FLS isolated from rats with CIA. We found
that matrine exerted therapeutic effects on CIA, inhibited FLS
proliferation, and induced apoptosis at least partly through
downregulation of JAK/STAT signaling.
RA is a heterogeneous disease characterized by
prominent synoviocyte hyperplasia and a potential imbalance between
FLS overgrowth and apoptosis (28). RA pathogenesis is affected by
multiple etiologies, including genetic, epigenetic and
environmental factors, as well as by immune disturbance and
cytokine factors. DMARDs, non-steroidal anti-inflammatory drugs
(NSAIDs), steroid hormones, and biologics are typically used to
treat RA, but are associated with severe adverse effects, including
gastrointestinal lesions, cardiovascular complications, and
reproductive toxicity (29).
Therefore, the exploitation of plant-derived drugs as anti-RA
agents with potent effects and lower toxicity profiles has drawn
much attention (30). Matrine is
the primary active component of the traditional Chinese medicinal
herb Sophora flavescens Ait. and is associated with cell
growth inhibition and apoptosis induction in cancer cells (17,31). FLS exhibit proliferative,
aggressive, and tumor-like behaviors in patients with RA (28), and may therefore be optimal
targets for matrine activity. While some of the effects of matrine
are related to the etiological factors underlying RA, the mechanism
of action of this compound remains uncharacterized.
The pathological changes to the blood and articular
tissue in the CIA model are similar to those in human RA,
highlighting the value of this system for studying human arthritis
(32). In this study, we assessed
the effects of matrine on arthritis severity in rats using AI and
ankle pathological analysis and found that matrine reduced AI and
improved ankle structure. Our in vitro experiments clearly
indicated that matrine markedly inhibited cell proliferation in a
concentration-dependent manner, blocked G0/G1 cell cycle
progression, and induced apoptosis in FLS derived from rats with
CIA. These findings demonstrated for the first time that matrine
can trigger FLS apoptosis, which may reflect a mechanism of action
related to its anti-RA effects.
Increasing evidence suggests that a reduced level of
apoptosis in vivo is closely associated with FLS
proliferation in RA (33).
Apoptosis is a distinct form of cell death initiated by various
physiological and pathological stimuli (34). The Bcl-2 family consists of key
regulatory proteins involved in mitochondrial apoptotic pathways
(2). A decrease in Bcl-2 levels
or an increase in Bax levels can trigger signals to initiate the
apoptotic cascade (33). Various
studies have examined the relative expression status of Bcl-2
family members in RA (35).
Pro-apoptotic Bax was shown to be decreased, whereas anti-apoptotic
Bcl-2 was increased in FLS from RA patients compared with those
from osteoarthritis patients (36). Furthermore, an increase in the
Bax:Bcl-2 ratio has been demonstrated to promote apoptosis
(28). As demonstrated in the
present study, the expression of Bcl-2 decreased, whereas that of
Bax increased, resulting in an elevated Bax:Bcl-2 ratio, suggesting
that matrine promoted the apoptosis of FLS from rats with CIA.
It is well-known that apoptosis requires the
activation of a series of caspases, including initiator and
effector caspases (33).
Following caspase activation, an increasing number of cellular
substrates, including the DNA repair protein PARP, are degraded or
cleaved, resulting in cell death (28). The activation of caspase-3 plays a
crucial role in the initiation of apoptosis (33). A significant decrease in
procaspase-3 and -9 expression and a significant increase in
caspase-3 and -9 expression were observed in RA-induced FLS
apoptosis (37). Accordingly, we
also found that matrine significantly induced the activation of
caspase-3 in FLS from rats with CIA.
The JAK/STAT signal transduction pathway is
activated by many cytokines and growth factors that regulate gene
expression, cell proliferation and differentiation (11). Suppression of JAK/STAT activity
leads to the induction of an apoptotic response (38). FLS in the intimal lining in RA
have been demonstrated to express high levels of STAT1 (10), and STAT3 expression was found to
be elevated in the synovial lining in RA and in an experimental
arthritis model (39). Persistent
activation of STAT3 contributes to the expression of anti-apoptotic
molecules that restrain the induction of programmed cell death
(40), whereas STAT3 blockade
promotes apoptosis in RA-FLS (9).
Previous studies have provided evidence that the JAK2/STAT1/3
signaling pathway may be the upstream mechanism by which FLS
proliferation is controlled (12). Studies have reported that matrine
suppresses proliferation and induces apoptosis in human
cholangiocarcinoma cells by blocking JAK2/STAT3 signaling (17). The appearance of RA-FLS with
tumor-like aggressive phenotypes (41) may also justify the use of tumor
therapies for RA. We found that phosphorylation of JAK, STAT1 and
STAT3 were suppressed by matrine in FLS from rats with CIA,
suggesting that matrine induced apoptosis via suppression of
JAK2/STAT1/3 signaling.
In conclusion, the results of this study demonstrate
that matrine has potent anti-proliferative and pro-apoptotic
effects on FLS. Notably, matrine treatment was clinically
beneficial in a rat model of arthritis that recapitulates human
pathology. Therefore, matrine may represent a novel therapeutic
agent for RA that counters the activation of JAK2/STAT1/3
signaling.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 80360574). The authors would like
to thank Professor Qiumei Dong and Sangon Biotech Co., Ltd. for the
technical assistance.
References
|
1
|
Zhang W, Zhu J, Du Z, Yu J, Xu Y and Wang
F: Intraarticular gene transfer of SPRY2 suppresses
adjuvant-induced arthritis in rats. Appl Microbiol Biotechnol.
99:6727–6735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu H, Yang Y, Cai X, Gao Y, Du J and Chen
S: The effects of arctigenin on human rheumatoid arthritis
fibroblast-like synoviocytes. Pharm Biol. 53:1118–1123. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamai M, Kawakami A, Tanaka F, Miyashita
T, Nakamura H, Iwanaga N, Izumi Y, Arima K, Aratake K, Huang M, et
al: Significant inhibition of TRAIL-mediated fibroblast-like
synovial cell apoptosis by IFN-gamma through JAK/STAT pathway by
translational regulation. J Lab Clin Med. 147:182–190. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zong M, Lu T, Fan S, Zhang H, Gong R, Sun
L, Fu Z and Fan L: Glucose-6-phosphate isomerase promotes the
proliferation and inhibits the apoptosis in fibroblast-like
synoviocytes in rheumatoid arthritis. Arthritis Res Ther.
17:1002015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang M, Zeng S, Qiu Q, Xiao Y, Shi M, Zou
Y, Yang X, Xu H and Liang L: Niclosamide induces apoptosis in human
rheumatoid arthritis fibroblast-like synoviocytes. Int
Immunopharmacol. 31:45–49. 2016. View Article : Google Scholar
|
|
6
|
Coskun M, Salem M, Pedersen J and Nielsen
OH: Involvement of JAK/STAT signaling in the pathogenesis of
inflammatory bowel disease. Pharmacol Res. 76:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seavey MM and Dobrzanski P: The many faces
of Janus kinase. Biochem Pharmacol. 83:1136–1145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghoreschi K, Laurence A and O'Shea JJ:
Selectivity and therapeutic inhibition of kinases: to be or not to
be? Nat Immunol. 10:356–360. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SK, Park KY, Yoon WC, Park SH, Park
KK, Yoo DH and Choe JY: Melittin enhances apoptosis through
suppression of IL-6/sIL-6R complex-induced NF-κB and STAT3
activation and Bcl-2 expression for human fibroblast-like
synoviocytes in rheumatoid arthritis. Joint Bone Spine. 78:471–477.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasperkovitz PV, Verbeet NL, Smeets TJ,
van Rietschoten JG, Kraan MC, van der Pouw Kraan TC, Tak PP and
Verweij CL: Activation of the STAT1 pathway in rheumatoid
arthritis. Ann Rheum Dis. 63:233–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krause A, Scaletta N, Ji JD and Ivashkiv
LB: Rheumatoid arthritis synoviocyte survival is dependent on
Stat3. J Immunol. 169:6610–6616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Fang Y, Wang Y, Wang Z, Zou Q, Shi
Y, Chen J and Peng D: Inhibitory effect of curcumol on Jak2-STAT
signal pathway molecules of fibroblast-like synoviocytes in
patients with rheumatoid arthritis. Evid Based Complement Alternat
Med. 2012:7464262012.PubMed/NCBI
|
|
13
|
Culshaw S, McInnes IB and Liew FY: What
can the periodontal community learn from the pathophysiology of
rheumatoid arthritis? J Clin Periodontol. 38(Suppl 11): 106–113.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee WS, Lim JH, Sung MS, Lee EG, Oh YJ and
Yoo WH: Ethyl acetate fraction from Angelica sinensis inhibits
IL-1β-induced rheumatoid synovial fibroblast proliferation and
COX-2, PGE2, and MMPs production. Biol Res. 47:412014. View Article : Google Scholar
|
|
15
|
Xin W, Huang C, Zhang X, Xin S, Zhou Y, Ma
X, Zhang D, Li Y, Zhou S, Zhang D, et al: Methyl salicylate
lactoside inhibits inflammatory response of fibroblast-like
synoviocytes and joint destruction in collagen-induced arthritis in
mice. Br J Pharmacol. 171:3526–3538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Wang Y, Zhang Y, Chen LP and Wang
JY: Effect of matrine on NO and ADMA metabolism pathways in serum
and tissues of mice with lipopolysaccharide-induced intestine
tissue inflammation. Zhongguo Zhong Yao Za Zhi. 39:2318–2321.
2014.In Chinese. PubMed/NCBI
|
|
17
|
Yang N, Han F, Cui H, Huang J, Wang T,
Zhou Y and Zhou J: Matrine suppresses proliferation and induces
apoptosis in human cholangiocarcinoma cells through suppression of
JAK2/STAT3 signaling. Pharmacol Rep. 67:388–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun N, Wang ZW, Wu CH, Li E, He JP, Wang
SY, Hu YL, Lei HM and Li HQ: Antiviral activity and underlying
molecular mechanisms of matrine against porcine reproductive and
respiratory syndrome virus in vitro. Res Vet Sci. 96:323–327. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu JL, Li JH, Chengz RG, Ma YM, Wang XJ
and Liu JC: Effect of matrine on transforming growth factor β1 and
hepatocyte growth factor in rat liver fibrosis model. Asian Pac J
Trop Med. 7:390–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Wu Y, Deng L, Chen L, Zhao D, Lv
L, Chen X, Man J, Wang Y, Shan H, et al: The alkaloid matrine of
the root of Sophora flavescens prevents arrhythmogenic effect of
ouabain. Phytomedicine. 21:931–935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu Q, Wang J, Ma Z and Ma S:
Anti-asthmatic effects of matrine in a mouse model of allergic
asthma. Fitoterapia. 94:183–189. 2014. View Article : Google Scholar
|
|
22
|
Liu N, Kan QC, Zhang XJ, Xv YM, Zhang S,
Zhang GX and Zhu L: Upregulation of immunomodulatory molecules by
matrine treatment in experimental autoimmune encephalomyelitis. Exp
Mol Pathol. 97:470–476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan X, Garrett-Sinha LA, Sarkar D and
Yang S: Deletion of IFT20 in early stage T lymphocyte
differentiation inhibits the development of collagen-induced
arthritis. Bone Res. 2:140382014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caplazi P, Baca M, Barck K, Carano RA,
DeVoss J, Lee WP, Bolon B and Diehl L: Mouse models of rheumatoid
arthritis. Vet Pathol. 52:819–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ota F, Maeshima A, Yamashita S, Ikeuchi H,
Kaneko Y, Kuroiwa T, Hiromura K, Ueki K, Kojima I and Nojima Y:
Activin A induces cell proliferation of fibroblast-like
synoviocytes in rheumatoid arthritis. Arthritis Rheum.
48:2442–2449. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang WD, Wang PM, Han RX and Zhang TS:
Efficacy and safety evaluation of fire needling for rats with
rheumatoid arthritis. Zhongguo Zhen Jiu. 33:334–338. 2013.In
Chinese. PubMed/NCBI
|
|
27
|
Baharav E, Mor F, Halpern M and Weinberger
A: Lactobacillus GG bacteria ameliorate arthritis in Lewis rats. J
Nutr. 134:1964–1969. 2004.PubMed/NCBI
|
|
28
|
Yan C, Kong D, Ge D, Zhang Y, Zhang X, Su
C and Cao X: Mitomycin C induces apoptosis in rheumatoid arthritis
fibroblast-like synoviocytes via a mitochondrial-mediated pathway.
Cell Physiol Biochem. 35:1125–1136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng CJ, Zhao XX, Ai HW, Lin B, Han T,
Jiang YP, Xing X and Qin LP: Therapeutic effects of standardized
Vitex negundo seeds extract on complete Freund's adjuvant induced
arthritis in rats. Phytomedicine. 21:838–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang M, Li K, Nie Y, Wei Y and Li X:
Antirheumatoid arthritis activities and chemical compositions of
phenolic compounds-rich fraction from Urtica atrichocaulis, an
endemic plant to China. Evid Based Complement Alternat Med.
2012:8182302012.
|
|
31
|
Shao Q, Zhao X and Yao L: Matrine inhibits
the growth of retinoblastoma cells (SO-Rb50) by decreasing
proliferation and inducing apoptosis in a mitochondrial pathway.
Mol Biol Rep. 41:3475–3480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi M, Cui F, Liu AJ, Ma HJ, Cheng M, Song
SX, Yuan F, Li DP and Zhang Y: The protective effects of chronic
intermittent hypobaric hypoxia pretreatment against
collagen-induced arthritis in rats. J Inflamm (Lond). 12:232015.
View Article : Google Scholar
|
|
33
|
Luo Y, Wei Z, Chou G, Wang Z, Xia Y and
Dai Y: Noriso-boldine induces apoptosis of fibroblast-like
synoviocytes from adjuvant-induced arthritis rats. Int
Immunopharmacol. 20:110–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Correia C, Lee SH, Meng XW, Vincelette ND,
Knorr KL, Ding H, Nowakowski GS, Dai H and Kaufmann SH: Emerging
understanding of Bcl-2 biology: implications for neoplastic
progression and treatment. Biochim Biophys Acta. 1853:1658–1671.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee SY, Kwok SK, Son HJ, Ryu JG, Kim EK,
Oh HJ, Cho ML, Ju JH, Park SH and Kim HY: IL-17-mediated Bcl-2
expression regulates survival of fibroblast-like synoviocytes in
rheumatoid arthritis through STAT3 activation. Arthritis Res Ther.
15:R312013. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jie L, Du H, Huang Q, Wei S, Huang R and
Sun W: Tanshinone IIA induces apoptosis in fibroblast-like
synoviocytes in rheumatoid arthritis via blockade of the cell cycle
in the G2/M phase and a mitochondrial pathway. Biol Pharm Bull.
37:1366–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yeh CT, Huang WC, Rao YK, Ye M, Lee WH,
Wang LS, Tzeng DT, Wu CH, Shieh YS, Huang CY, et al: A
sesquiterpene lactone antrocin from Antrodia camphorata negatively
modulates JAK2/STAT3 signaling via microRNA let-7c and induces
apoptosis in lung cancer cells. Carcinogenesis. 34:2918–2928. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shouda T, Yoshida T, Hanada T, Wakioka T,
Oishi M, Miyoshi K, Komiya S, Kosai K, Hanakawa Y, Hashimoto K, et
al: Induction of the cytokine signal regulator SOCS3/CIS3 as a
therapeutic strategy for treating inflammatory arthritis. J Clin
Invest. 108:1781–1788. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu H and Pope RM: The role of apoptosis
in rheumatoid arthritis. Curr Opin Pharmacol. 3:317–322. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li GF, Qin YH and Du PQ: Andrographolide
inhibits the migration, invasion and matrix metalloproteinase
expression of rheumatoid arthritis fibroblast-like synoviocytes via
inhibition of HIF-1α signaling. Life Sci. 136:67–72. 2015.
View Article : Google Scholar : PubMed/NCBI
|