Introduction
Oxidative stress reflects an imbalance between the
production of reactive oxygen species (ROS) and the biological
system's ability to remove them (1,2).
Although some ROS act as cellular messengers in redox signaling,
excessive ROS can arbitrarily react with all components of the
cell, including proteins, lipids and DNA, thereby causing oxidative
stress and damage in these macromolecules (3,4).
This damage is a crucial etiological factor that is implicated in
various human diseases, including aging and cancer, as well as
neurodegenerative and chronic diseases (5,6).
Therefore, the development of safe and effective antioxidants
continues to be an important research target (7,8).
The antioxidant activity of natural compounds
isolated from plants has been reported to counteract free radicals
(9,10). Among such compounds, flavonoids, a
family of well-known polyphenols, are bioactive compounds, which
are widespread in plants and have long received attention in the
development of antioxidants (8,11,12). Morin
(2′,3,4′,5,7-pentahydroxyflavone), a member of the flavonoid
family, is a yellow-colored compound that can be isolated from
members of the Moraceae family, which are used as herbal medicines
(13–15). Morin exhibits certain biological
activities, which include antioxidant (16–18), cytoprotection (19,20), antidiabetic (21,22), anti-inflammatory (23–25) and anticancer properties (26–29). Moreover, morin has been reported
to possess cellular protective effects against oxidative
stress-induced damage through activation of the nuclear
factor-erythroid 2-related factor 2 (Nrf2) signaling pathway
(30–32). However, the mechanism underlying
the action of morin against oxidative stress has not been fully
studied to date.
In the present study, the antioxidant activities of
morin were examined through a series of in vitro tests that
were conducted to examine its superoxide dismutase (SOD)-like and
2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)
(ABTS•+) free radical scavenging activities. We also
investigated the ameliorative effects of morin on cell damage
induced by hydrogen peroxide (H2O2) in V79-4
Chinese hamster lung fibroblasts and the possible mechanism
underlying this cytoprotective effect.
Materials and methods
Reagents and antibodies
Dulbecco's modified Eagle's medium (DMEM), fetal
calf serum (FCS), streptomycin, and penicillin were purchased from
WelGENE Inc. (Daegu, Republic of Korea). Morin,
3-(4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT),
4′,6-diamidino-2-phenylindole (DAPI), N-acetyl-L-cysteine
(NAC), 6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine
iodide (JC-1) and zinc protoporphyrin IX (ZnPP), a specific
inhibitor of heme oxygenase-1 (HO-1), were obtained from
Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). A SOD assay kit
was obtained from Dojindo Molecular Technologies (Tokyo, Japan).
2′,7′-Dichlorodihydrofluorescein diacetate (DCF-DA) and a
fluorescein-conjugated Annexin V (Annexin V-FITC) staining assay
kit were purchased from Molecular Probes (Eugene, OR, USA) and BD
Biosciences (San Jose, CA, USA), respectively. An enhanced
chemiluminescence (ECL) detection system was purchased from
Amersham Co. (Arlington Heights, IL, USA). Primary antibodies
(Table I) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), Cell Signaling
Technology, Inc. (Danvers, MA, USA) and Abcam, Inc. (Cambridge, MA,
USA). Horseradish (HRP)-conjugated secondary antibodies were
obtained from Amersham Co. Morin was dissolved in dimethyl
sulphoxide (DMSO; Sigma-Aldrich Chemical Co.) and then diluted with
medium to the desired concentration prior to use. The final DMSO
concentration was <0.1% in all experiments. All other chemicals
were purchased from Sigma-Aldrich Chemical Co.
 | Table IAntibodies used in the present
study. |
Table I
Antibodies used in the present
study.
| Antibody | Origin | Company | Cat. no. |
|---|
| Actin | Mouse
monoclonal | Santa Cruz
Biotechnology, Inc. | SC-47778 |
| Caspase-3 | Mouse
monoclonal | Santa Cruz
Biotechnology, Inc. | SC-7272 |
| PARP | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. | SC-7150 |
| p-γH2AX | Rabbit
monoclonal | Cell Signaling
Technology, Inc. | 9718 |
| γH2AX | Rabbit
monoclonal | Cell Signaling
Technology, Inc. | 7631 |
| p-Nrf2 | Rabbit
monoclonal | Abcam, Inc. | ab76026 |
| Nrf2 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. | SC-13032 |
| Keap1 | Goat
polyclonal | Santa Cruz
Biotechnology, Inc. | SC-15246 |
| HO-1 | Rabbit
polyclonal | Santa Cruz
Biotechnology, Inc. | SC-10789 |
| NQO1 | Goat
polyclonal | Santa Cruz
Biotechnology, Inc. | SC-16464 |
Measurement of SOD-like activity
The SOD-like activity of morin was measured using a
SOD assay kit according to the manufacturer's instructions.
Briefly, 20 μl of morin stock solution was added to 200
μl of the working solution in the SOD assay kit. The mixture
was incubated at 37°C for 20 min after gentle shaking and then 20
μl of the kit enzyme working solution was added. The
absorbance of the mixtures was measured at 450 nm using an
enzyme-linked immunosorbent assay (ELISA) reader (Molecular
Devices, Sunnyvale, CA, USA), and the SOD activity was calculated
according to the manufacturer's instructions (33). Vitamin C was used as a positive
control.
Measurement of ABTS radical scavenging
activity
The ABTS assay was based on a previously described
method (34) with slight
modifications. ABTS radical cation (ABTS•+) was produced
by the reaction of a 7 mM ABTS solution with potassium persulphate
(2.45 mM). The ABTS•+ solution was diluted with ethanol
to an absorbance of 0.70±0.05 at 734 nm. The mixture was stored in
the dark at room temperature for 12 h before use. After the
addition of 25 μl of morin solution or vitamin C as a
positive control to 2 ml of diluted ABTS•+ solution,
absorbance was measured at 734 nm after exactly 6 min. Inhibition
of the ABTS radical by morin was calculated using the formula: ABTS
scavenging activity (%) = [1 - (absorbance of the sample/absorbance
of the control)] × 100.
Cell culture
The Chinese hamster lung fibroblast V79-4 cell line
was obtained from the American Type Culture Collection (ATCC;
Manassas, MD, USA) and cultured in DMEM containing 10%
heat-inactivated FCS, streptomycin (100 μg/ml) and
penicillin (100 Units/ml). The cells were maintained at 37°C in an
incubator with a humidified atmosphere of 5% CO2.
MTT assay
For the cell viability assay, V79-4 cells were
seeded at 1×105 cells/ml in a 96-well plate and cultured
for 24 h before being treated with the indicated concentrations of
morin for 24 h in the presence or absence of 1 mM
H2O2 with or without 1 h pretreatment of
morin or NAC. After the treatments, the MTT solution (0.5 mg/ml)
was added, followed by a 2-h incubation at 37°C in the dark, after
which the medium was removed. The formazan precipitate was
dissolved in DMSO. The absorbance of the formazan product was
measured at 540 nm using an ELISA reader (35).
Nuclear staining with DAPI
The cells were harvested, washed with PBS, and fixed
with 3.7% paraformaldehyde for 30 min at room temperature. After
washing twice with PBS, the cells were attached on glass slides
using cytospin (Shandon, Pittsburgh, PA, USA) and stained with 2.5
μg/ml DAPI solution for 20 min at room temperature. The
stained cells were washed twice with PBS and then analyzed using a
fluorescence microscope (Carl Zeiss, Deisenhofen, Germany).
Determination of apoptotic cells by flow
cytometry
To assess the induced cell apoptosis rate
quantitatively, the cells were washed with PBS and stained with 5
μl of Annexin V-FITC and 5 μl of propidium iodide
(PI) in each sample according to the manufacturer's protocols.
After a 15-min incubation at room temperature in the dark, the
degree of apoptosis was quantified as a percentage of the Annexin
V-positive and PI-negative (Annexin
V+/PI−cells) cells using a flow cytometer (BD
Biosciences).
Measurement of intracellular ROS
The oxidation-sensitive dye DCF-DA was used to
determine the formation of intracellular ROS. Briefly, the cells
from each treatment were harvested, washed twice with PBS, and then
re-suspended in 10 μM DCF-DA for 30 min at 37°C in the dark.
The production of ROS in the cells was monitored immediately using
a flow cytometer (36).
Measurement of mitochondrial membrane
potential (MMP, Δψm)
The MMP values were determined using the
dual-emission potential-sensitive probe JC-1. Briefly, the cells
were collected and incubated with 10 μM of JC-1 for 20 min
at 37°C in the dark. After the JC-1 was removed, the cells were
washed with PBS to remove the unbound dye, and the amount of JC-1
retained by 10,000 cells per sample was measured by using a flow
cytometer (37).
Determination of DNA damage by comet
assay
After each treatment, the cells were washed with
PBS, and the cell suspension was mixed with 0.5% low melting
agarose (LMA) at 37°C before adding it to the slides precoated with
1.0% normal melting agarose. After the agarose was solidified, the
slides were covered with another 0.5% LMA and then immersed in
lysis buffer [2.5 M NaCl, 500 mM Na-ethylenediaminetetraacetic acid
(EDTA), 1 M Tris buffer, 1% sodium lauroyl sarcosinate and 1%
Triton X-100] for 1 h at 4°C. The slides were later transferred
into an unwinding buffer for another 20 min for DNA unwinding. The
slides were then placed in an electrophoresis tank containing 300
mM NaOH and 1 mM Na-EDTA (pH 13.0). An electrical field was then
applied (300 mA, 25 V) for 20 min at 25°C to draw the negatively
charged DNA toward the anode. The slides were washed three times
for 5 min at 25°C in a neutral-izing buffer (0.4 M Tris, pH 7.5),
and then stained with 20 μg/ml PI. The slides were examined
under a fluorescence microscope.
Protein extraction and western blot
analysis
All cell lysates were lysed in an extraction buffer
[25 mM Tris-Cl (pH 7.5), 250 mM NaCl, 5 mM EDTA, 1% Nonidet P-40,
0.1 mM sodium orthovanadate, 2 μg/ml leupeptin, and 100
μg/ml phenylmethylsulfonyl fluoride]. The protein
concentration in the cell lysate was determined using a
detergent-compatible protein assay from Bio-Rad (Hercules, CA,
USA). In the Western blot analysis, equal amounts of protein (30–50
μg) were separated by 8–10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then were
electro-transferred to polyvinylidene fluoride (PVDF) membranes
(Schleicher & Schuell, Keene, NH, USA). The membranes were
blocked with 5% skim milk for 1 h and then subjected to immunoblot
analysis with the appropriate antibodies. Using an ECL detection
system, immunoreactive bands were detected and exposed to X-ray
film.
HO-1 activity assay
HO-1 enzyme activity was measured as previously
described (38). In brief,
lysates of the cells were prepared, and homogenates containing
biliverdin reductase were obtained from rat liver. After
quantifying the protein concentration, the cell lysates and
homogenates were incubated with nicotinamide adenine dinucleotide
phosphate (NADPH) and hemin for 1 h, whereas the blank samples were
incubated with hemin only. The concentration of bilirubin, which
was the product of degradation by HO-1, was determined as the
difference in absorbance at 464 and 530 nm using an ELISA plate
reader. HO-1 activity was expressed as picomoles of bilirubin per
milligram of protein.
Statistical analysis
All experiments were conducted in triplicate (n=3),
and a one-way ANOVA (SPSS 11.5 statistical software; SPSS, Inc.,
Chicago, IL, USA) was used to compare the mean values of each
treatment. Significant differences were determined using Duncan's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
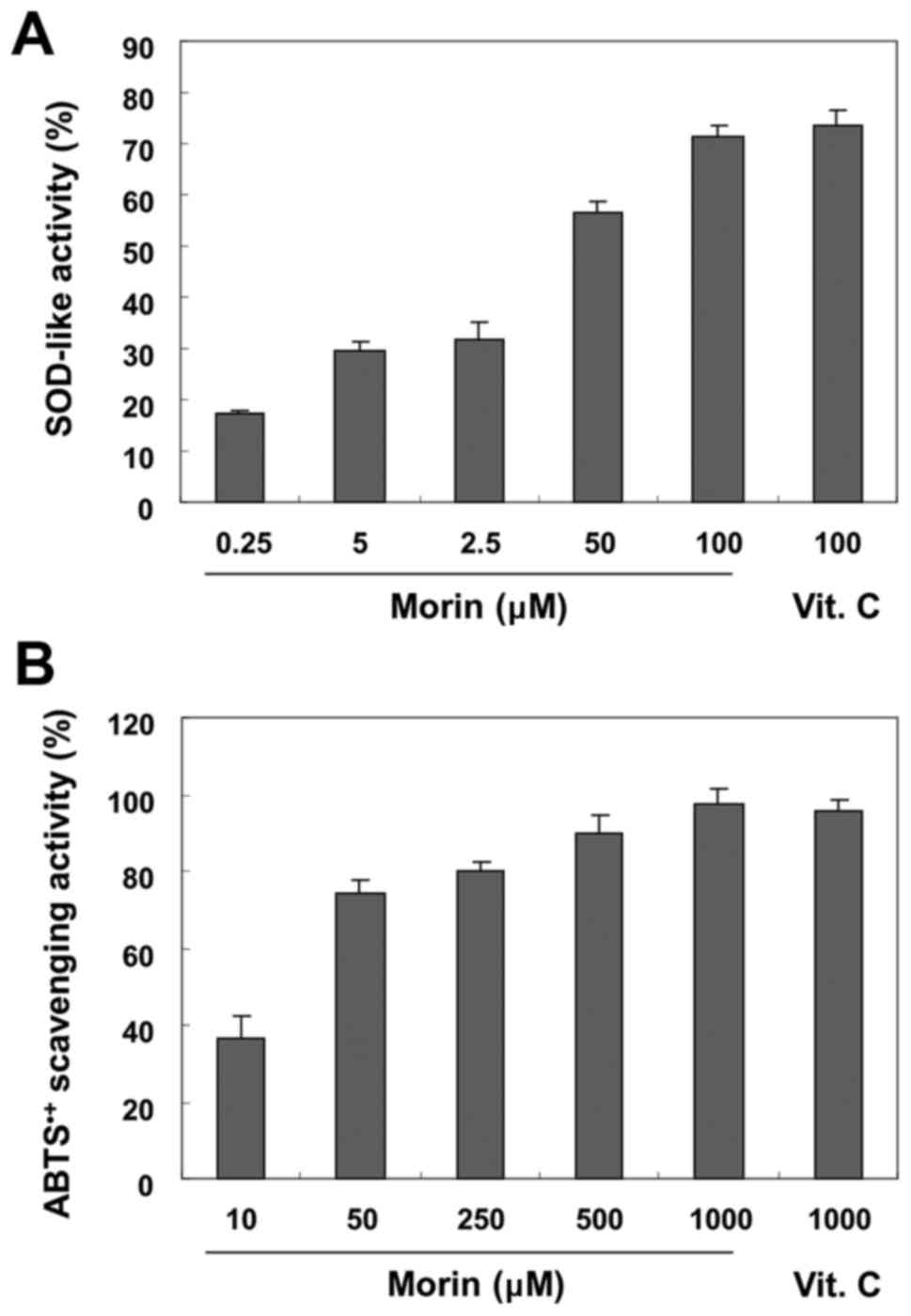
Antioxidant activity of morin
To determine the antioxidant activity of morin, the
SOD-like enzyme and ABTS radical scavenging activities were
evaluated. As shown in Fig. 1A,
the SOD-like enzyme activity of morin was highly increased in a
concentration-dependent manner to the given concentration. For
example, the percentage of SOD-like enzyme activity was ~72% at a
concentration of 100 μM morin, which was very similar after
treatment with the same concentration of vitamin C used as a
positive control. Similar to SOD-like enzyme activity, at different
concentrations (10–1,000 μM), morin was also found to
effectively scavenge ABTS•+ (Fig. 1B).
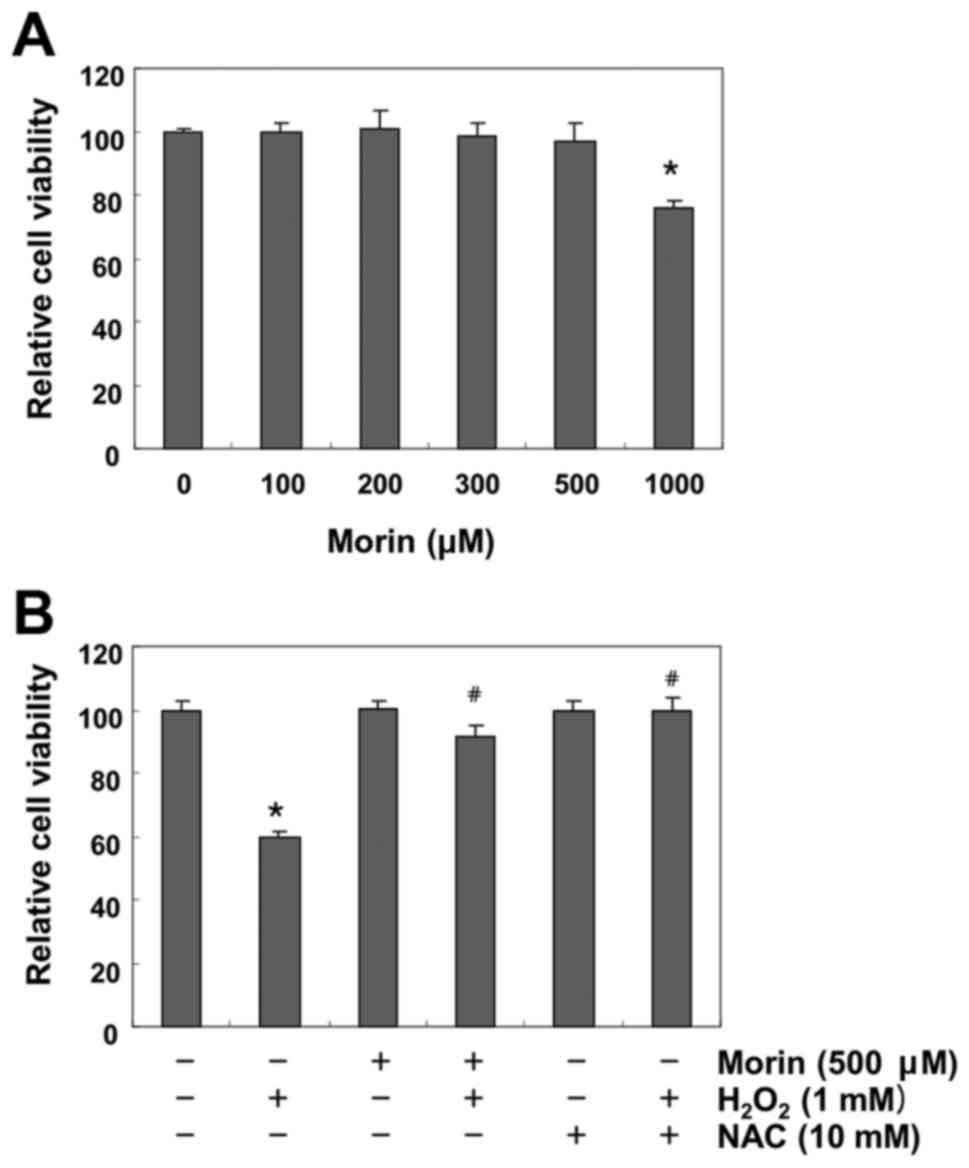
Morin protects against
H2O2-induced inhibition of cell viability in
V79-4 fibroblasts
To exclude the cytotoxicity caused by morin
treatment in V79-4 fibroblasts, the cells were treated with
different concentrations (100–1,000 μM) of morin for 24 h.
The MTT assay indicated that the treatments did not result in any
cytotoxic effect at the concentration of 500 μM, and cell
viability was significantly decreased at concentrations higher than
1,000 μM (Fig. 2A).
Therefore, 500 μM morin was selected for use in the
subsequent examination of the protective effect of morin on
H2O2-induced cytotoxicity. A further MTT
assay revealed that treatment with 1 mM H2O2
significantly reduced cell viability. However, the
H2O2-induced reduction in cell viability was
effectively protected by pretreatment with both 500 μM morin
and 10 mM NAC (Fig. 2B).
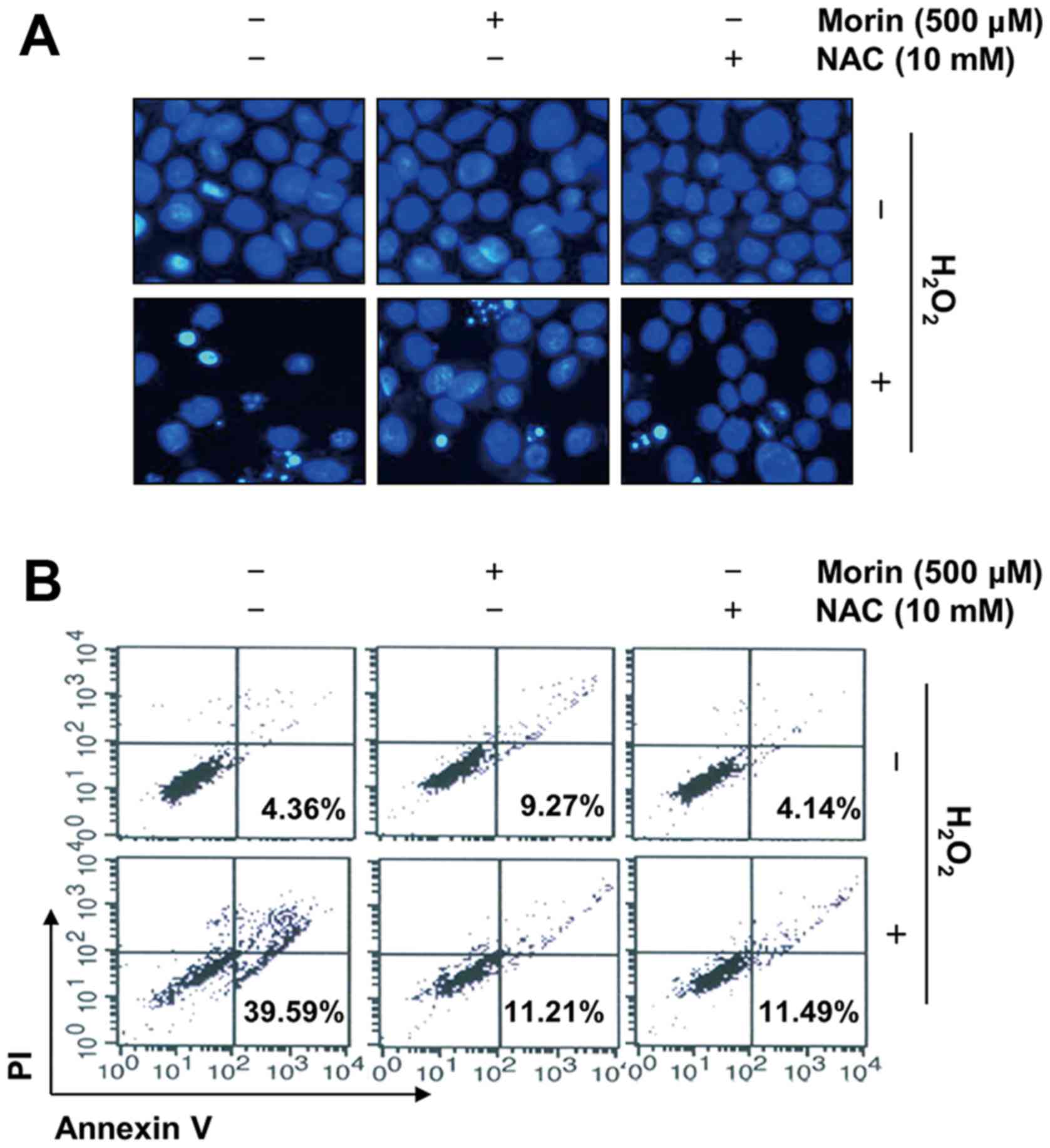
Morin attenuates
H2O2-induced apoptosis in V79-4
fibroblasts
To elucidate the cytoprotective effect of morin
against the H2O2-induced reduction in V79-4
cell viability, we investigated the effects of morin on
H2O2-mediated apoptosis. The results of the
DAPI staining showed that treatment with H2O2
alone significantly increased the number of cells with condensed or
blebbing nuclei. In contrast, when these cells were pretreated with
morin or NAC, these phenomena were markedly reduced (Fig. 3A). The results of the flow
cytometry consistently indicated that the
H2O2 treatment enhanced the population of
Annexin V+/PI− apoptotic cells. However, the
pretreatment of cells with morin or NAC prior to exposure to
H2O2 effectively protected the V79-4 cells
against apoptosis (Fig. 3B).
Morin suppresses
H2O2-induced ROS generation in V79-4
fibroblasts
In a further experiment to study the mechanisms
underlying the protective effect of morin, the intracellular ROS
levels were determined. The results of the flow cytometric analysis
using DCF-DA as a fluorescence probe demonstrated that the
intensity of the DCF-liberated fluorescent signal from the
H2O2-treated cells was markedly increased.
However, the signal was effectively attenuated in the presence of
morin as well as NAC (Fig.
4A).
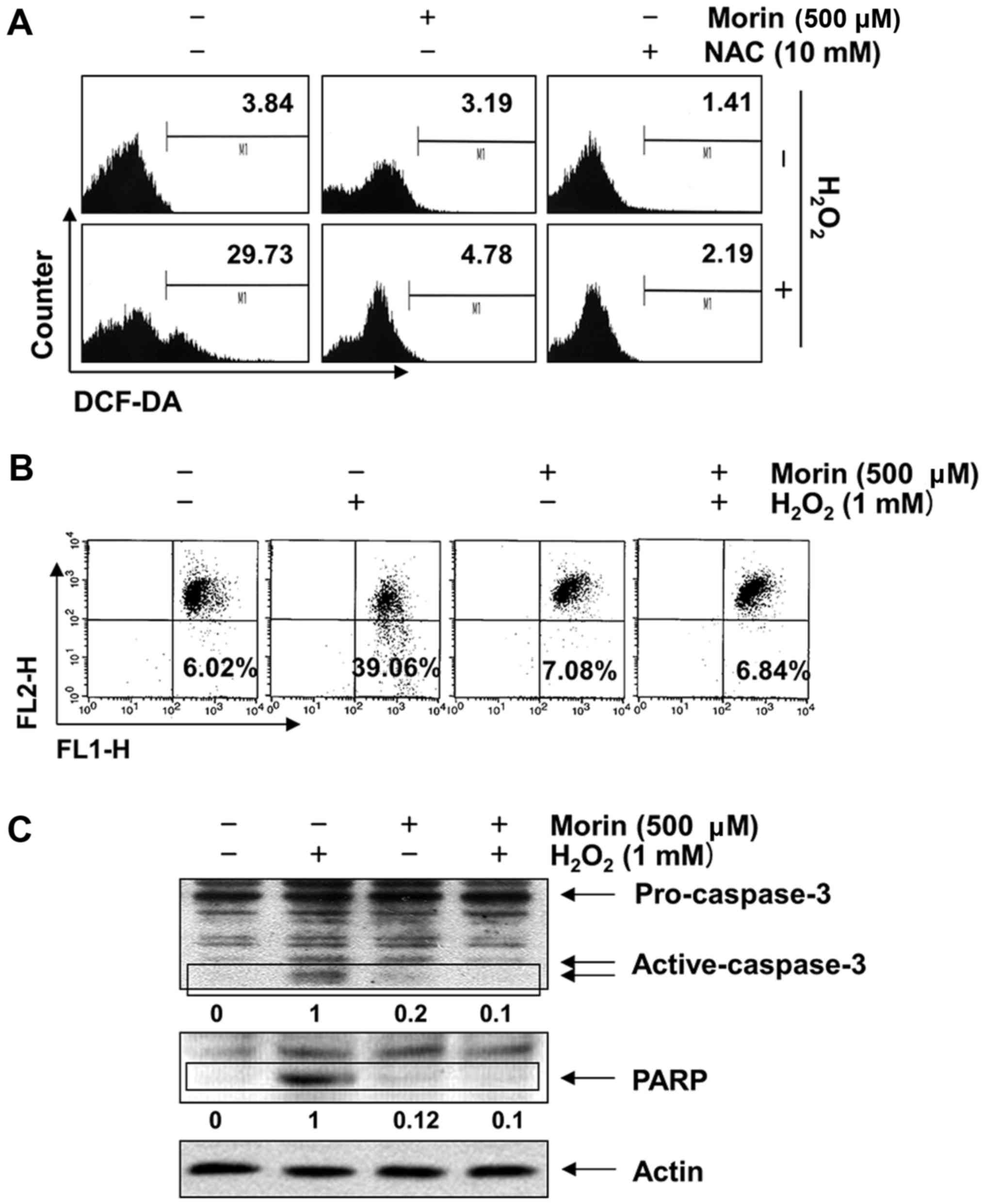 | Figure 4Inhibition of
H2O2-induced generation of ROS, loss of MMP
and activation of caspase-3 by morin in V79-4 lung fibroblasts. The
cells were pretreated with 500 μM morin for 1 h and then
incubated with or without 1 mM H2O2 for (A) 1
h or (B and C) 24 h. (A) To monitor ROS production, the cells were
incubated at 37°C in the dark for 20 min with a new culture medium
containing 10 μM DCF-DA. ROS accumulation was measured using
a flow cytometer. (B) To assess the effects of morin on the
H2O2-induced loss of MMP, the cells were
collected and incubated with 10 μM JC-1 for 20 min at 37°C
in the dark. The cells were then washed once with PBS and analyzed
using a flow cytometer. The data shown represent the mean values
from two different experiments. (C) To assess caspase-3 and PARP
levels, the cells were lysed, and then equal amounts of cell
lysates were separated using sodium dodecyl sulphate-polyacrylamide
gel electrophoresis and transferred to PVDF membranes. The
membranes were probed with anti-caspase-3 and anti-PARP antibodies,
and the proteins were visualized using an ECL detection system. The
relative ratios of expression as determined by the western blotting
are presented at the bottom of each lane as relative values of
actin expression. MMP, mitochondrial membrane potential; NAC,
N-acetyl-L-cysteine; PVDF; polyvinylidene fluoride; PARP,
poly(ADP-ribose) polymerase; ECL, enhanced chemiluminescence. |
Morin inhibits
H2O2-induced mitochondrial dysfunction and
activation of caspase-3 in V79-4 fibroblasts
The mitochondrial-mediated intrinsic apoptosis
pathway is initiated by the loss of mitochondrial membrane
potential (MMP, Δψm) and the subsequent release of pro-apoptotic
proteins, which leads to the activation of caspase-9 and -3
(39,40). We therefore investigated the
effects of morin on the H2O2-induced loss of
MMP and the activation of caspase-3. The results indicated that the
loss of MMP in H2O2-treated V79-4 cells
increased 6.4-fold relative to the untreated control; however, the
reduction in MMP was significantly inhibited by pretreatment with
morin (Fig. 4B). The
immunoblotting results indicated a marked increase in the level of
activated caspase-3 expression in the
H2O2-treated cells compared with the control.
A subsequent increase in cleaved poly(ADP-ribose) polymerase
(PARP), a well-known substrate of caspase-3 (41), was also observed. However, the
results clearly showed that H2O2-induced
caspase-3 activation and PARP degradation were completely abrogated
by pretreatment with morin (Fig.
4C).
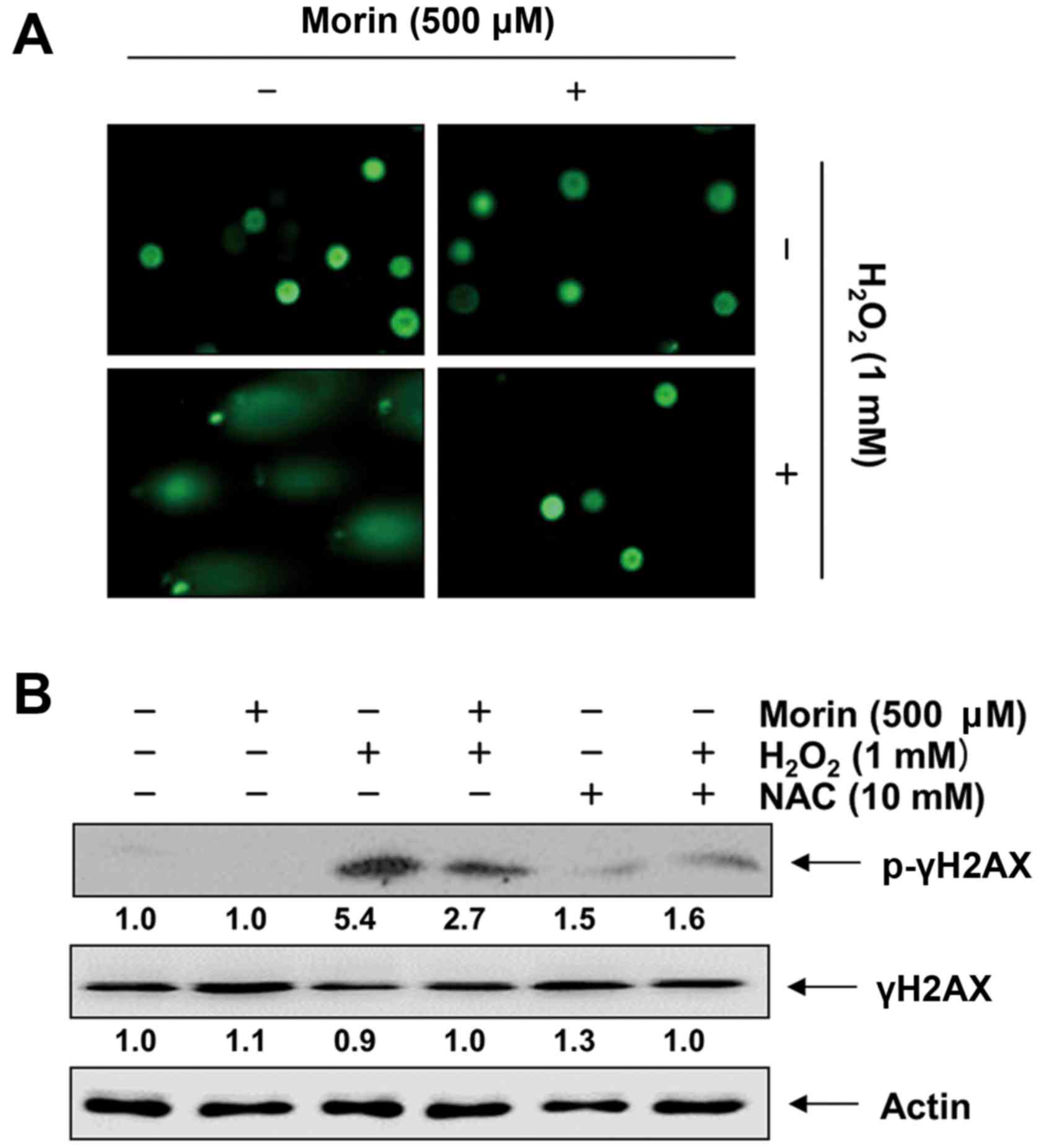
Morin protects against
H2O2-induced DNA damage in V79-4
fibroblasts
We then assessed the protective effects of morin
against H2O2-induced DNA damage by applying
the alkaline comet assay and Western blot analysis. The comet assay
measures single- and double-strand breaks that are caused either
directly by the DNA-damaging system or indirectly by the repair
mechanism (42). As shown in
Fig. 5A, the exposure of cells to
H2O2 increased DNA breaks, resulting in an
increase in fluorescence intensity in the tails of the comet-like
structures. However, pretreatment with morin led to a marked
decrease in damage to DNA (Fig.
5A). Furthermore, as expected, H2O2
enhanced the phosphorylation of histone λH2AX on serine 139, which
was rapidly increased after the induction of DNA double-strand
breaks (43), whereas
pretreatment with morin prevented the increase in the
phosphorylation of histone λH2AX (Fig. 5B).
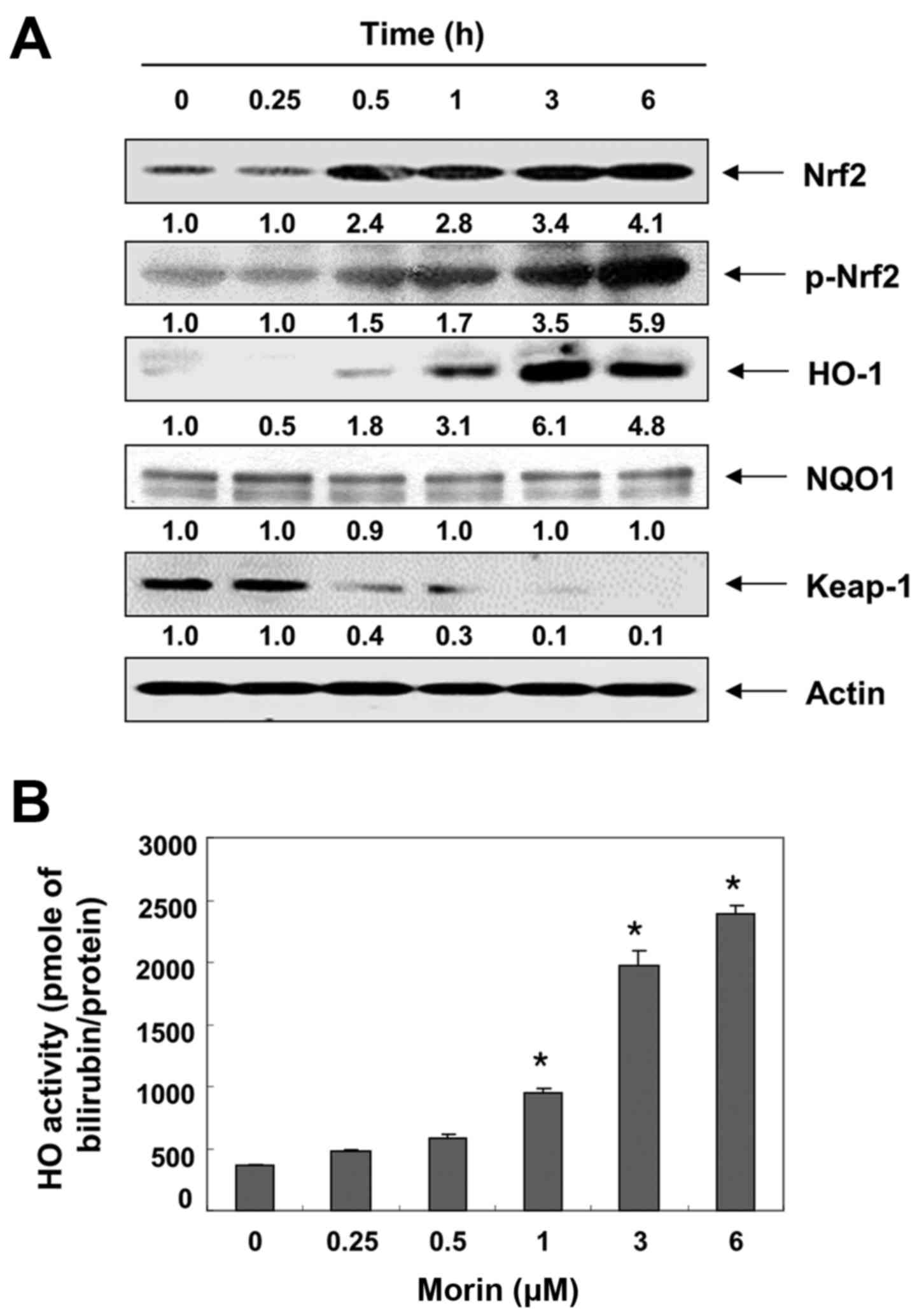
Morin enhances the expression Nrf2 and
HO-1 in V79-4 fibro- blasts
The transcription factor Nrf2 regulates the
expression of antioxidant responsive element (ARE)-driven
antioxidant and cytoprotective genes, including HO-1 and quinone
oxidoreductase-1 (NQO-1), to control the cellular defense against
oxidative stress (44,45). Thus, we examined the effects of
morin on the levels of Nrf2, HO-1 and NQO-1 expression, and found
that treatment with morin gradually increased Nrf2 expression and
its phosphorylation, but not NQO-1 expression, in a time-dependent
manner. It concomitantly decreased the level of Kelch-like
ECH-associated protein 1 (Keap1), which is a negative regulator of
Nrf2 (Fig. 6A). Morin also
enhanced HO-1 expression as well as HO-1 activity in a
time-dependent manner (Fig.
6B).
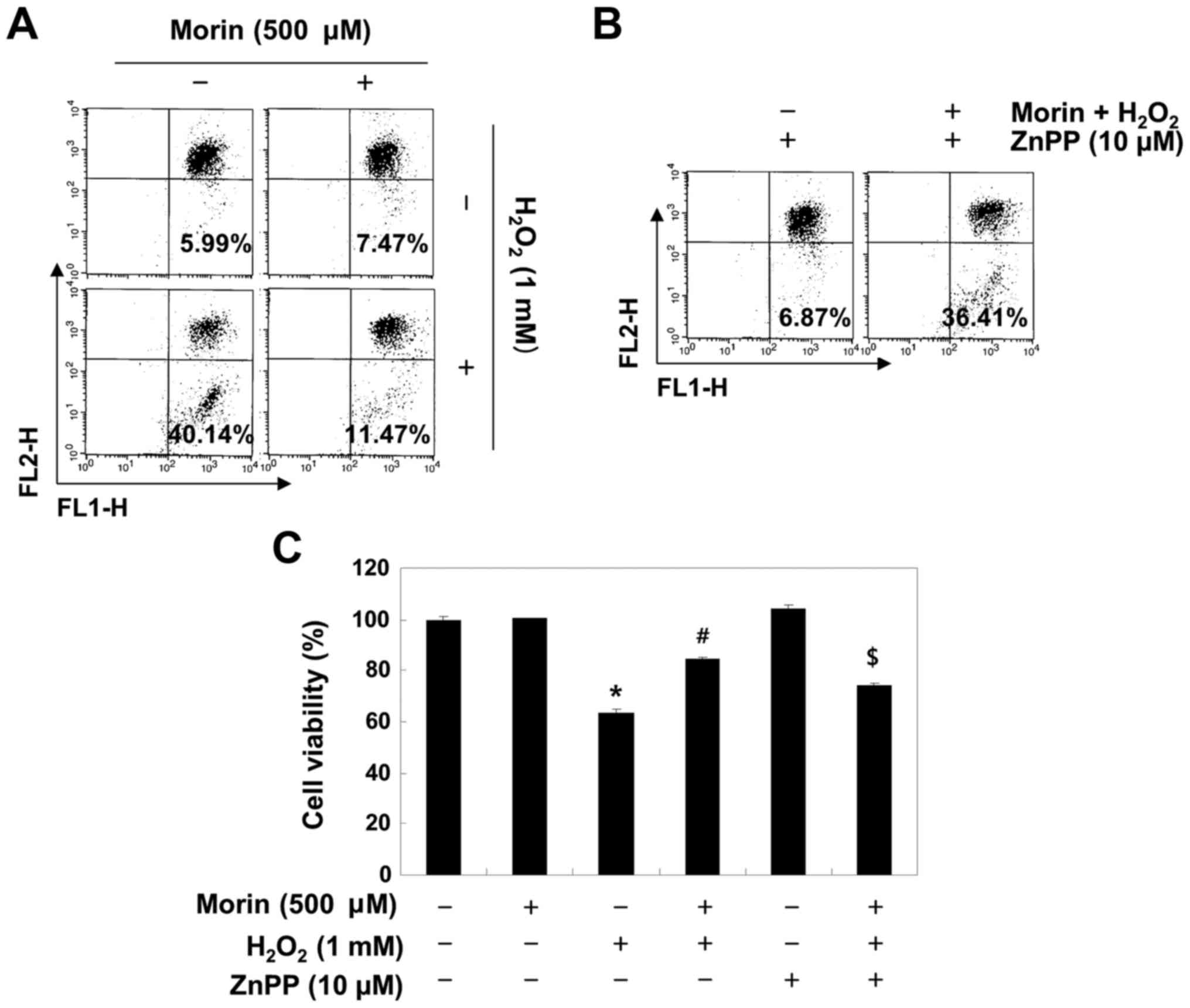
The Nrf2/HO-1 pathway is involved in the
protective effects of morin in H2O2-treated
V79-4 fibroblasts
To determine whether the morin-induced antioxidant
and cytoprotective activities against oxidative stress were
mediated through the activation of the Nrf2/HO-1 pathway, V79-4
cells were pretreated with or without a specific inhibitor of HO-1,
ZnPP, and the levels of ROS and cell viability were assessed. As
shown in Fig. 7, ZnPP abrogated
the protective effect of morin on the
H2O2-induced production of ROS and the
reduction in cell viability.
Discussion
Oxidative stress, represented as the increase in
ROS, is an abnormal phenomenon when the production of free radicals
exceeds the antioxidant capacity. Extreme increases in ROS can
destroy the cytoprotective defense mechanism by the emasculation of
antioxidant systems, leading to the development of several human
diseases (5,6). The accumulation of ROS directly
causes base modification and DNA strand breaks, resulting in DNA
damage (3,4). Accumulation of ROS also induces
mitochondrial dysfunction, resulting in a decrease in MMP, and
activation of caspase-9 and -3 through the release of mitochondrial
apoptotic factors into the cytoplasm. These factors are known to be
strong stimulators of the mitochondrial-mediated intrinsic
apoptosis pathway (46,47). In the present study, the results
showed that morin had a strong antioxidant capacity as determined
by SOD-like activity and ABTS scavenging ability assays, which are
widely used to analyze the antioxidant potential of candidate
materials. Additional data from the MTT assay and flow cytometry
demonstrated that morin significantly rescued cell viability and
reduced the apoptosis caused by H2O2-induced
oxidative stress in V79-4 lung fibroblasts. We also found that the
protective effects were associated with the suppression of ROS
accumulation and DNA damage, which indicates that morin may enhance
the antioxidant and DNA repair systems. In addition, the results of
the JC-1 staining and immunoblotting showed that morin
significantly restored the H2O2-induced loss
of MMP at the basal level, the activation of caspase-3, and the
cleavage of PARP, which is a substrate protein of activated
caspase-3 (41) in V79-4 cells.
These results indicate that the ability of morin to attenuate
oxidative stress was partly dependent on inhibition of
mitochondrial-related apoptosis.
Previous evidence strongly suggests that the
Nrf2-mediated signaling pathway is essential in protecting cells
against oxidative stress. Nrf2, a basic leucine zipper
transcription factor, plays an important role in the
transcriptional regulation of phase II enzymes by binding to AREs
(44,45). Under basal conditions,
Nrf2-dependent transcription is suppressed by Keap1, a negative
regulator of Nrf2, which facilitates the degradation of Nrf2
through ubiquitin-mediated proteasomal degradation (45,48). Upon the modification of specific
thiols by insult, Keap1 triggers the dissociation of Nrf2 from the
Nrf2-Keap1 complex in the cytoplasm and allows Nrf2 to translocate
into the nucleus, where it subsequently activates the AREs present
in the promoter regions of an array of genes (44,48). Moreover, several previous studies
have found that the phosphorylation of Nrf2 (Try568) led to the
nuclear export of Nrf2 (49,50). Therefore, we investigated the Nrf2
pathway to determine whether it contributes to the protective
effects of morin against H2O2-induced
oxidative stress. In the present study, our results strongly
support the ability of morin to stimulate the Nrf2 pathway; morin
treatment increased Nrf2 accumulation and phosphorylation and
decreased Keap1 expression in a time-dependent manner. Following
the treatment of V79-4 cells with morin, we also observed
significant increases in HO-1 expression and activity, while NQO1
was unaffected by the morin treatment. HO-1 is an Nrf2 downstream
target and a powerful indirect antioxidant enzyme, which is a
rate-limiting enzyme in heme catabolism. This enzyme converts heme
to beneficial byproducts such as carbon monoxide and bilirubin,
which can directly scavenge free radicals and repair DNA damage
caused by oxidative stress (48).
However, pretreatment with ZnPP, an inhibitor of HO-1, markedly
abrogated the protective effects of morin against
H2O2-induced MMP loss and inhibition of the
growth of the V79-4 cells (Fig.
7). Therefore, our results support the supposition that the
cytoprotective effect of morin against oxidative stress in V79-4
cells is mediated through activation of the Nrf2/HO-1 signaling
pathway.
In conclusion, the present study provides evidence
that morin protects lung fibroblast V79-4 cells from oxidative
stress-induced DNA damage and cell death via the suppression of ROS
generation and mitochondrial dysfunction. This process is also
associated with the involvement of Nrf2 activation and the
upregulation of the expression of its downstream antioxidant gene
HO-1 in order to protect cells from oxidative stress. Therefore,
morin may be of therapeutic value in the prevention and treatment
of various human diseases associated with oxidative stress.
Acknowledgments
This study was supported by the Functional Districts
of the Science Belt Support Program, Ministry of Science, ICT and
Future Planning and Basic Science Research Program through a grant
from the National Research Foundation of Korea (NRF) funded by the
Korea government (no. 2015R1A2A2A01004633).
References
|
1
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lyakhovich A and Graifer D:
Mitochondria-mediated oxidative stress: Old Target for New drugs.
Curr Med Chem. 22:3040–3053. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ermakov AV, Konkova MS, Kostyuk SV,
Izevskaya VL, Baranova A and Veiko NN: Oxidized extracellular DNA
as a stress signal in human cells. Oxid Med Cell Longev.
2013:6497472013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang HY and Lee TH: Antioxidant enzymes as
redox-based biomarkers: A brief review. BMB Rep. 48:200–208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alfadda AA and Sallam RM: Reactive oxygen
species in health and disease. J Biomed Biotechnol.
2012:9364862012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cha MY, Kim DK and Mook-Jung I: The role
of mitochondrial DNA mutation on neurodegenerative diseases. Exp
Mol Med. 47:e1502015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ginter E, Simko V and Panakova V:
Antioxidants in health and disease. Bratisl Lek Listy. 115:603–606.
2014.
|
|
8
|
Cirillo G, Curcio M, Vittorio O, Iemma F,
Restuccia D, Spizzirri UG, Puoci F and Picci N: Polyphenol
conjugates and human health: A perspective review. Crit Rev Food
Sci Nutr. 56:326–337. 2016. View Article : Google Scholar
|
|
9
|
Landete JM: Dietary intake of natural
antioxidants: Vitamins and polyphenols. Crit Rev Food Sci Nutr.
53:706–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kehrer JP and Klotz LO: Free radicals and
related reactive species as mediators of tissue injury and disease:
Implications for Health. Crit Rev Toxicol. 45:765–798. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kancheva VD and Kasaikina OT:
Bio-antioxidants - a chemical base of their antioxidant activity
and beneficial effect on human health. Curr Med Chem. 20:4784–4805.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bondonno CP, Croft KD, Ward N, Considine
MJ and Hodgson JM: Dietary flavonoids and nitrate: Effects on
nitric oxide and vascular function. Nutr Rev. 73:216–235. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stockert JC, Colman OD and Cañete M:
Fluorescence reaction of leukocyte granules by morin. Acta
Histochem Suppl. 31:243–252. 1985.PubMed/NCBI
|
|
14
|
Srinivas NR: Recent trends in preclinical
drug-drug interaction studies of flavonoids - Review of case
studies, issues and perspectives. Phytother Res. 29:1679–1691.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caselli A, Cirri P, Santi A and Paoli P:
Morin: A Promising natural drug. Curr Med Chem. 23:774–791. 2016.
View Article : Google Scholar
|
|
16
|
Kim JM, Lee EK, Park G, Kim MK, Yokozawa
T, Yu BP and Chung HY: Morin modulates the oxidative stress-induced
NF-kappaB pathway through its anti-oxidant activity. Free Radic
Res. 44:454–461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Komirishetty P, Areti A, Sistla R and
Kumar A: Morin mitigates chronic constriction injury (CCI)-induced
peripheral neuropathy by inhibiting oxidative stress induced PARP
over-activation and neuroinflammation. Neurochem Res. 41:2029–2042.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ola MS, Aleisa AM, Al-Rejaie SS,
Abuohashish HM, Parmar MY, Alhomida AS and Ahmed MM: Flavonoid,
morin inhibits oxidative stress, inflammation and enhances
neurotrophic support in the brain of streptozotocin-induced
diabetic rats. Neurol Sci. 35:1003–1008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
MadanKumar P, NaveenKumar P, Manikandan S,
Devaraj H and NiranjaliDevaraj S: Morin ameliorates chemically
induced liver fibrosis in vivo and inhibits stellate cell
proliferation in vitro by suppressing Wnt/β-catenin signaling.
Toxicol Appl Pharmacol. 277:210–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ganguli A, Das A, Nag D, Bhattacharya S
and Chakrabarti G: Potential role of autophagy in smokeless tobacco
extract-induced cytotoxicity and in morin-induced protection in
oral epithelial cells. Food Chem Toxicol. 90:160–170. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paoli P, Cirri P, Caselli A, Ranaldi F,
Bruschi G, Santi A and Camici G: The insulin-mimetic effect of
Morin: A promising molecule in diabetes treatment. Biochim Biophys
Acta. 1830:3102–3111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sendrayaperumal V, Iyyam Pillai S and
Subramanian S: Design, synthesis and characterization of
zinc-morin, a metal flavonol complex and evaluation of its
antidiabetic potential in HFD-STZ induced type 2 diabetes in rats.
Chem Biol Interact. 219:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qureshi AA, Guan XQ, Reis JC, Papasian CJ,
Jabre S, Morrison DC and Qureshi N: Inhibition of nitric oxide and
inflammatory cytokines in LPS-stimulated murine macrophages by
resveratrol, a potent proteasome inhibitor. Lipids Health Dis.
11:762012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dhanasekar C, Kalaiselvan S and Rasool M:
Morin, a bioflavonoid suppresses monosodium urate crystal-induced
inflammatory immune response in RAW 264.7 macrophages through the
inhibition of inflammatory mediators, intracellular ROS levels and
NF-κB activation. PLoS One. 10:e01450932015. View Article : Google Scholar
|
|
25
|
Dilshara MG, Jayasooriya RG, Lee S, Choi
YH and Kim GY: Morin downregulates nitric oxide and prostaglandin
E2 production in LPS-stimulated BV2 microglial cells by
suppressing NF-κB activity and activating HO-1 induction. Environ
Toxicol Pharmacol. 44:62–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gupta SC, Phromnoi K and Aggarwal BB:
Morin inhibits STAT3 tyrosine 705 phosphorylation in tumor cells
through activation of protein tyrosine phosphatase SHP1. Biochem
Pharmacol. 85:898–912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manna SK, Aggarwal RS, Sethi G, Aggarwal
BB and Ramesh GT: Morin (3,5,7,2′,4′-pentahydroxyflavone) abolishes
nuclear factor-kappaB activation induced by various carcinogens and
inflammatory stimuli, leading to suppression of nuclear
factor-kappaB-regulated gene expression and up-regulation of
apoptosis. Clin Cancer Res. 13:2290–2297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park C, Lee WS, Go SI, Nagappan A, Han MH,
Hong SH, Kim GS, Kim GY, Kwon TK, Ryu CH, et al: Morin, a flavonoid
from Moraceae, induces apoptosis by induction of BAD protein in
human leukemic cells. Int J Mol Sci. 16:645–659. 2014. View Article : Google Scholar
|
|
29
|
Hyun HB, Lee WS, Go SI, Nagappan A, Park
C, Han MH, Hong SH, Kim G, Kim GY, Cheong J, et al: The flavonoid
morin from Moraceae induces apoptosis by modulation of Bcl-2 family
members and Fas receptor in HCT 116 cells. Int J Oncol.
46:2670–2678. 2015.PubMed/NCBI
|
|
30
|
Park JY, Kang KA, Kim KC, Cha JW, Kim EH
and Hyun JW: Morin induces heme oxygenase-1 via ERK-Nrf2 signaling
pathway. J Cancer Prev. 18:249–256. 2013. View Article : Google Scholar
|
|
31
|
Rizvi F, Mathur A and Kakkar P: Morin
mitigates acetaminophen-induced liver injury by potentiating Nrf2
regulated survival mechanism through molecular intervention in
PHLPP2-Akt-Gsk3β axis. Apoptosis. 20:1296–1306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mathur A, Rizvi F and Kakkar P: PHLPP2
down regulation influences nuclear Nrf2 stability via
Akt-1/Gsk3β/Fyn kinase axis in acetaminophen induced oxidative
renal toxicity: Protection accorded by morin. Food Chem Toxicol.
89:19–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee YJ, Koh EK, Kim JE, Go J, Song SH,
Seong JE, Son HJ, Kang BC and Hwang DY: Beneficial effects of
ethanol extracts of Red Liriope platyphylla on vascular dysfunction
in the aorta of spontaneously hypertensive rats. Lab Anim Res.
31:13–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sreejayan N and Rao MN: Nitric oxide
scavenging by curcuminoids. J Pharm Pharmacol. 49:105–107. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim SH, Kang SH and Kang BS: Therapeutic
effects of dihydroartemisinin and transferrin against glioblastoma.
Nutr Res Pract. 10:393–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eom SA, Kim DW, Shin MJ, Ahn EH, Chung SY,
Sohn EJ, Jo HS, Jeon SJ, Kim DS, Kwon HY, et al: Protective effects
of PEP-1-Catalase on stress-induced cellular toxicity and
MPTP-induced Parkinson's disease. BMB Rep. 48:395–400. 2015.
View Article : Google Scholar :
|
|
37
|
Chun SK, Go K, Yang MJ, Zendejas I, Behrns
KE and Kim JS: Autophagy in ischemic livers: A critical role of
Sirtuin 1/Mitofusin 2 axis in autophagy induction. Toxicol Res.
32:35–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alaoui-Jamali MA, Bismar TA, Gupta A,
Szarek WA, Su J, Song W, Xu Y, Xu B, Liu G, Vlahakis JZ, et al: A
novel experimental heme oxygenase-1-targeted therapy for
hormone-refractory prostate cancer. Cancer Res. 69:8017–8024. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hensley P, Mishra M and Kyprianou N:
Targeting caspases in cancer therapeutics. Biol Chem. 394:831–843.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
MacKenzie SH and Clark AC: Targeting cell
death in tumors by activating caspases. Curr Cancer Drug Targets.
8:98–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Azqueta A, Slyskova J, Langie SA, O'Neill
Gaivão I and Collins A: Comet assay to measure DNA repair: Approach
and applications. Front Genet. 5:2882014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphory-lation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dinkova-Kostova AT, Holtzclaw WD, Cole RN,
Itoh K, Wakabayashi N, Katoh Y, Yamamoto M and Talalay P: Direct
evidence that sulfhydryl groups of Keap1 are the sensors regulating
induction of phase 2 enzymes that protect against carcinogens and
oxidants. Proc Natl Acad Sci USA. 99:11908–11913. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gan L and Johnson JA: Oxidative damage and
the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys
Acta. 1842:1208–1218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Yang J and Yi J: Redox sensing by
proteins: Oxidative modifications on cysteines and the consequent
events. Antioxid Redox Signal. 16:649–657. 2012. View Article : Google Scholar
|
|
47
|
Clerkin JS, Naughton R, Quiney C and
Cotter TG: Mechanisms of ROS modulated cell survival during
carcinogenesis. Cancer Lett. 266:30–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jaramillo MC and Zhang DD: The emerging
role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev.
27:2179–2191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kaspar JW and Jaiswal AK: Tyrosine
phosphorylation controls nuclear export of Fyn, allowing Nrf2
activation of cytoprotective gene expression. FASEB J.
25:1076–1087. 2011. View Article : Google Scholar :
|
|
50
|
Jain AK and Jaiswal AK: Phosphorylation of
tyrosine 568 controls nuclear export of Nrf2. J Biol Chem.
281:12132–12142. 2006. View Article : Google Scholar : PubMed/NCBI
|