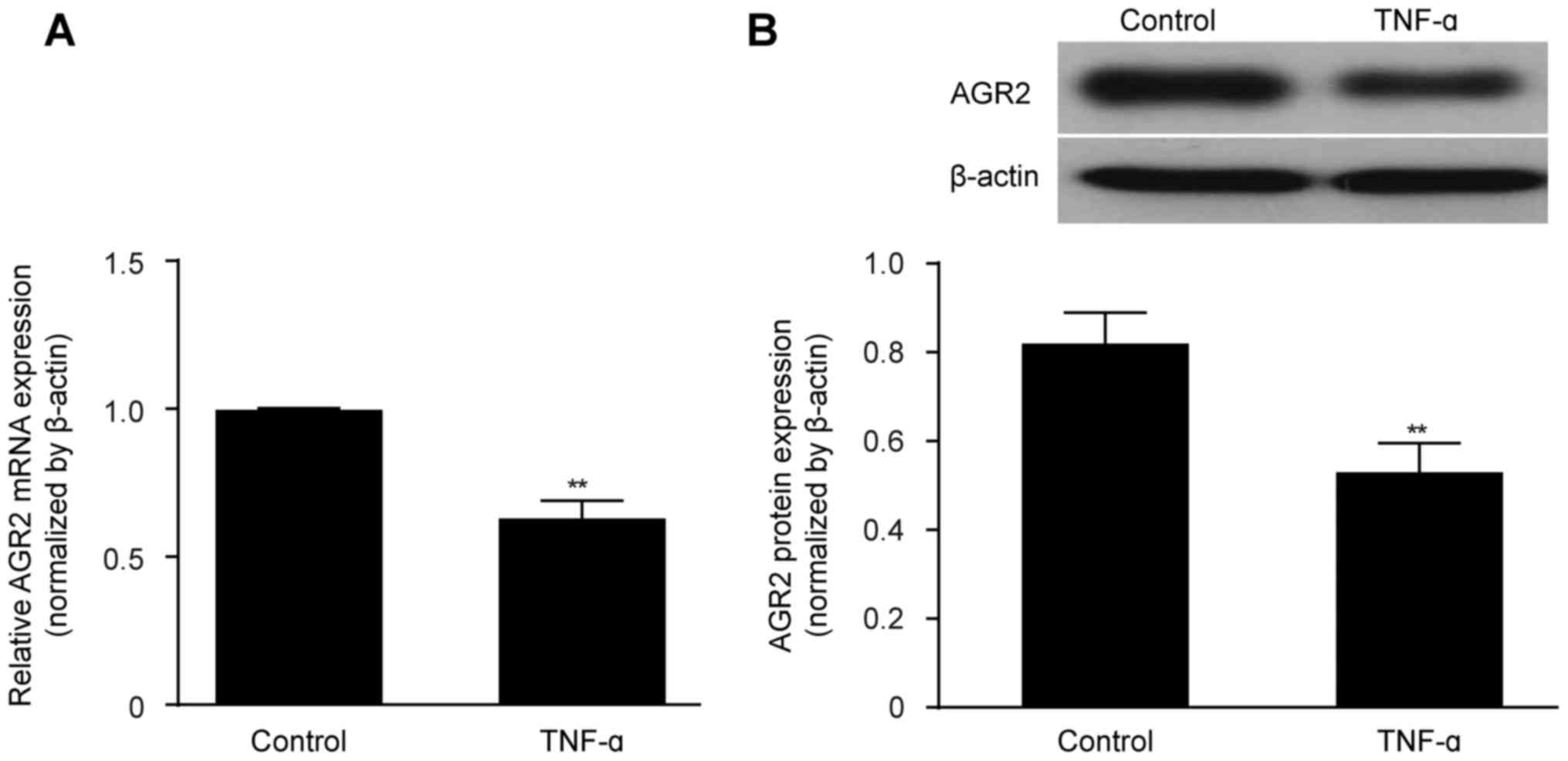
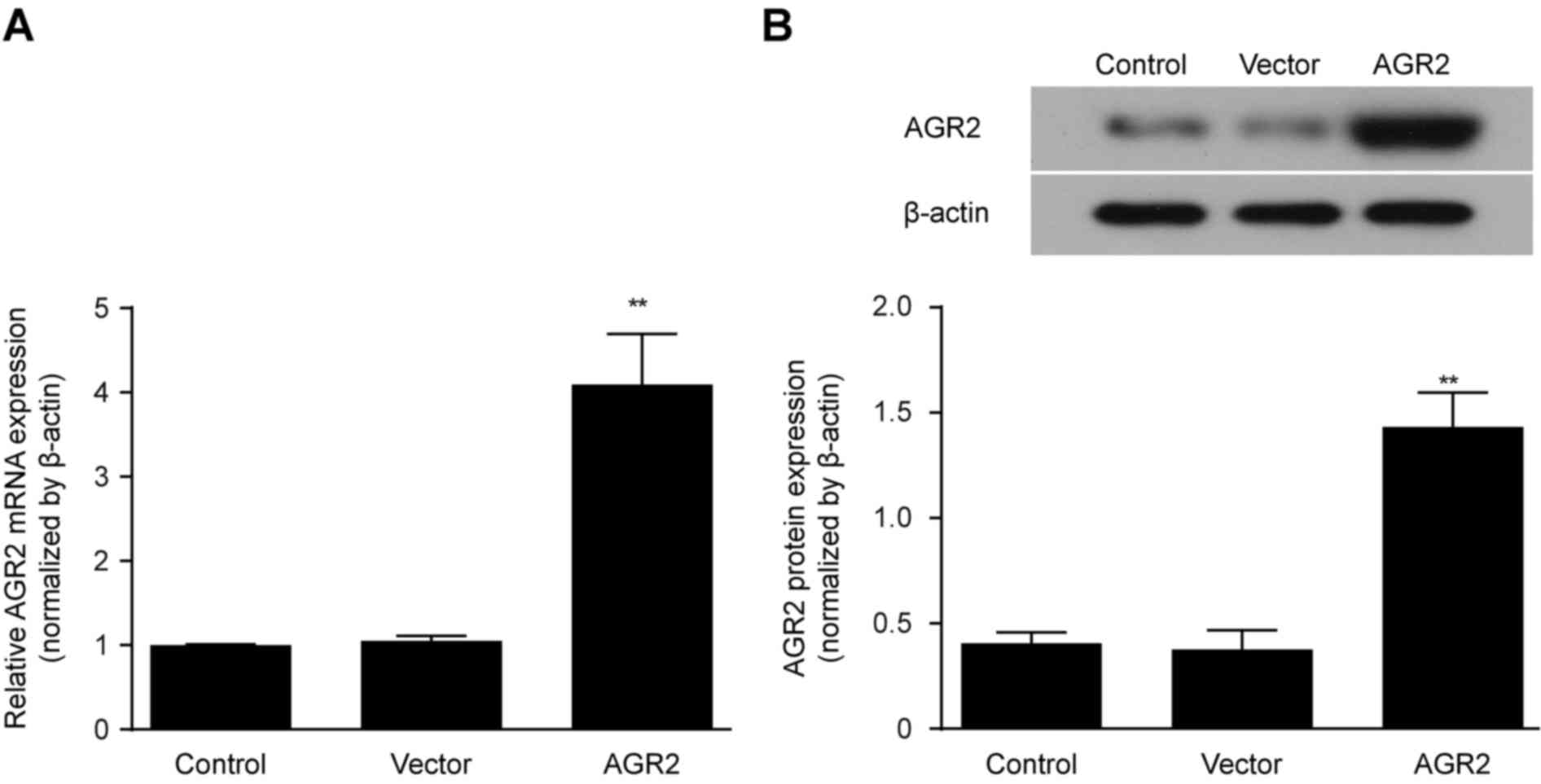
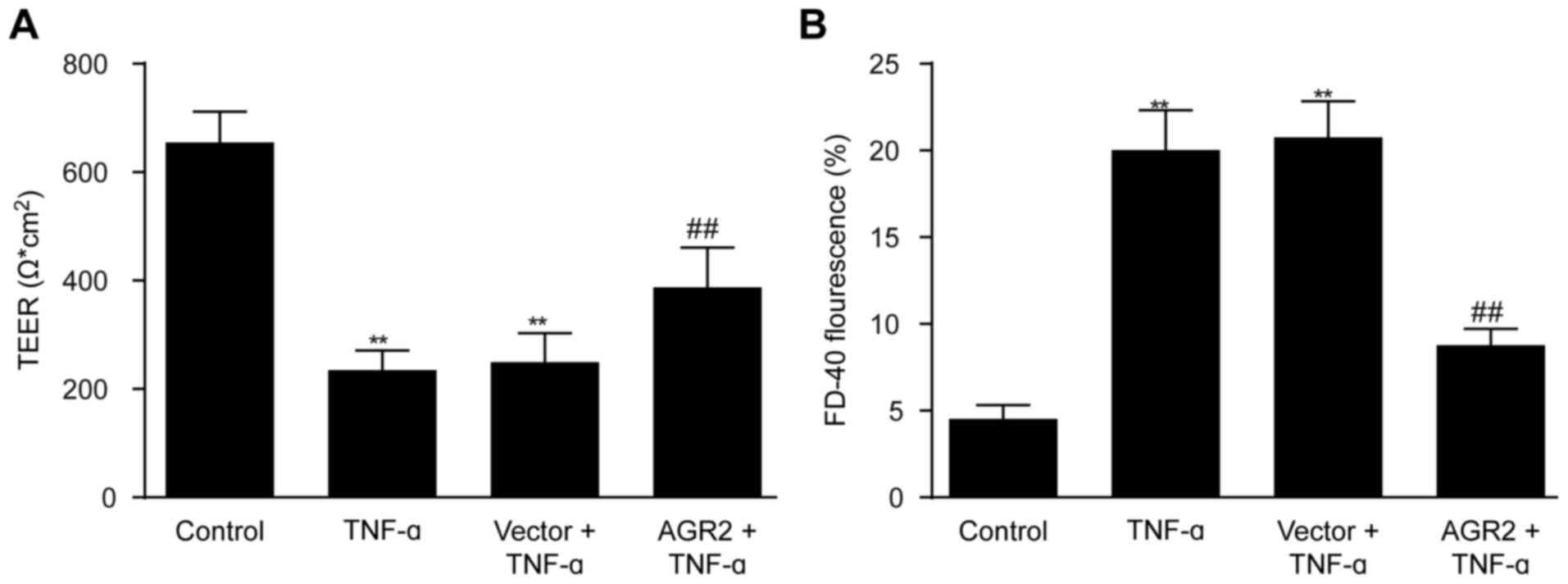
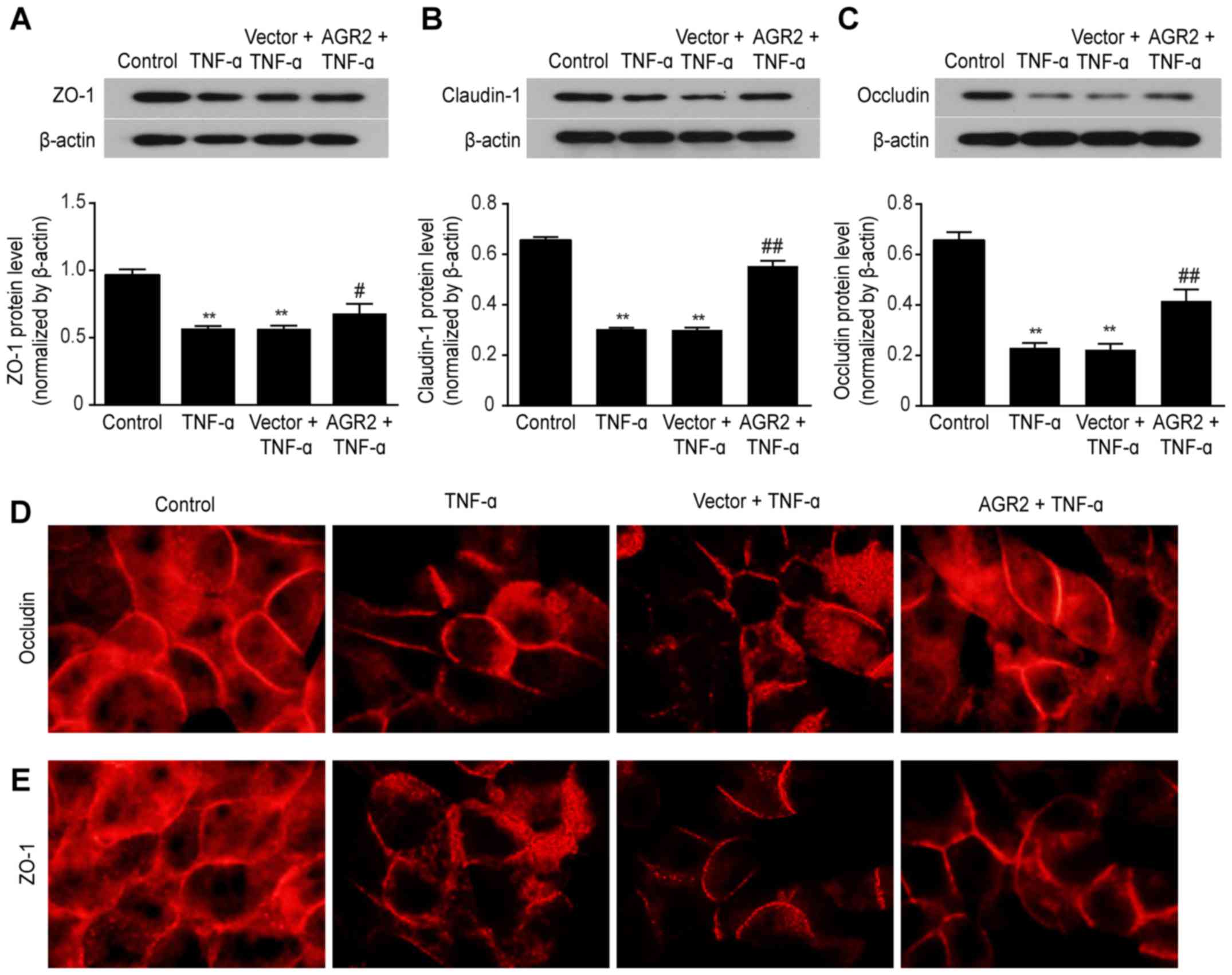
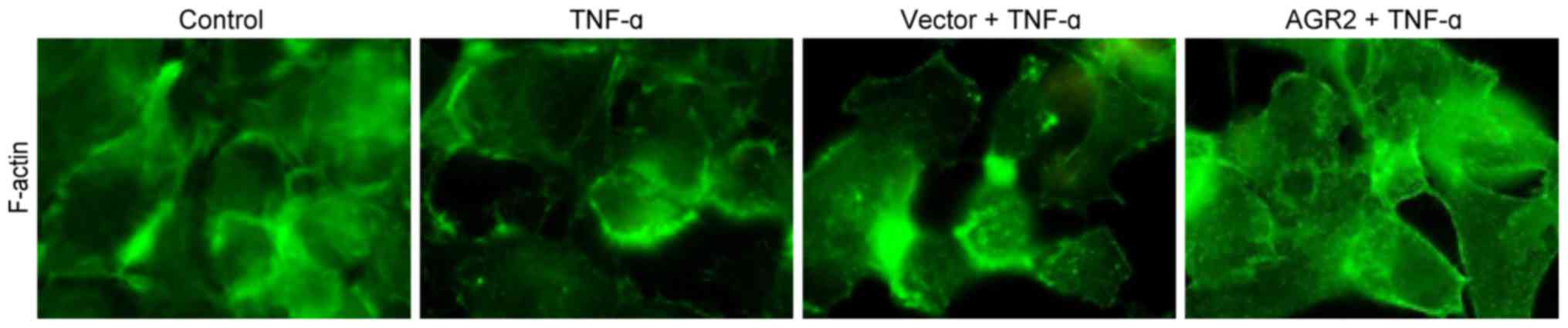
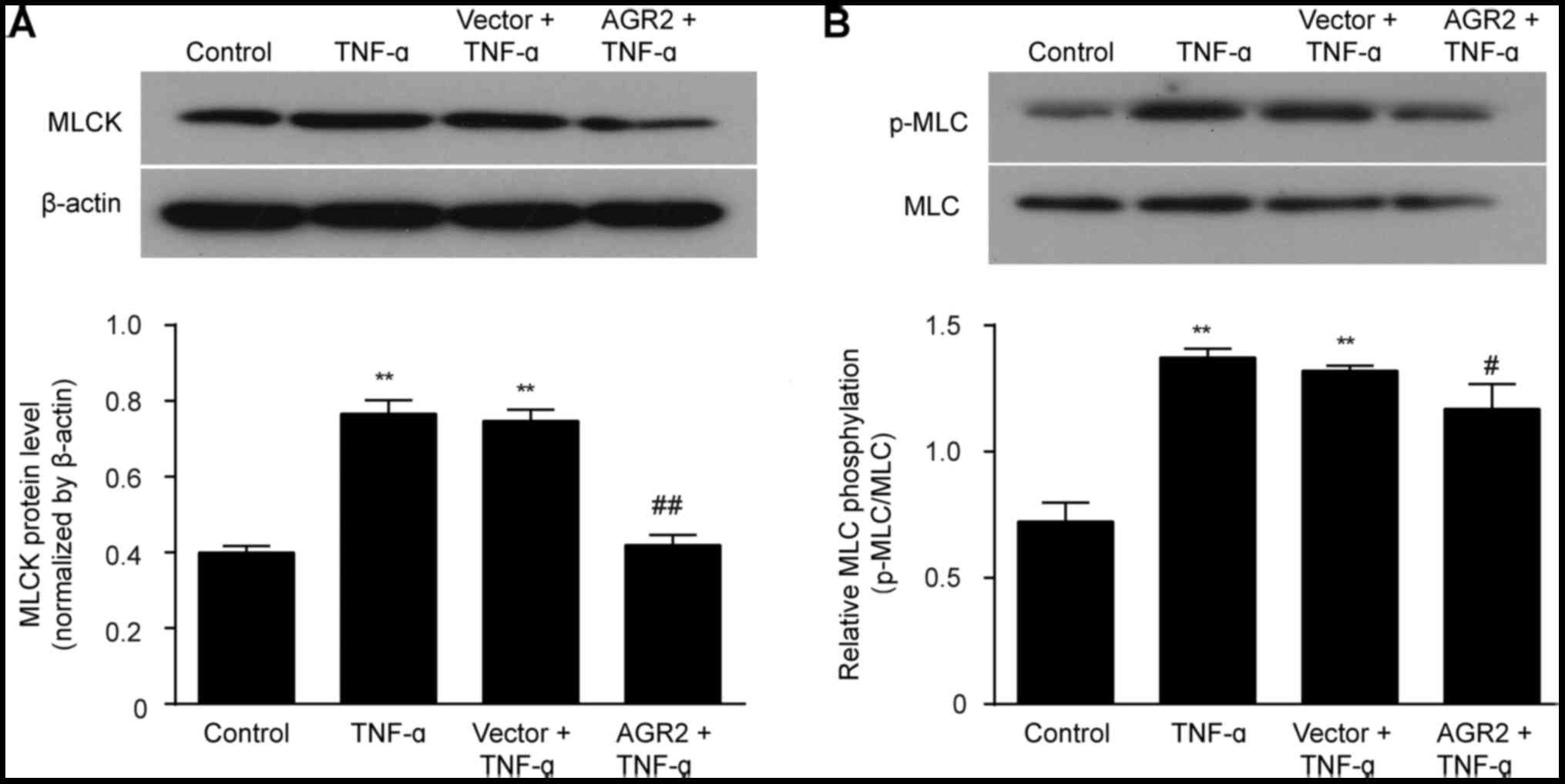
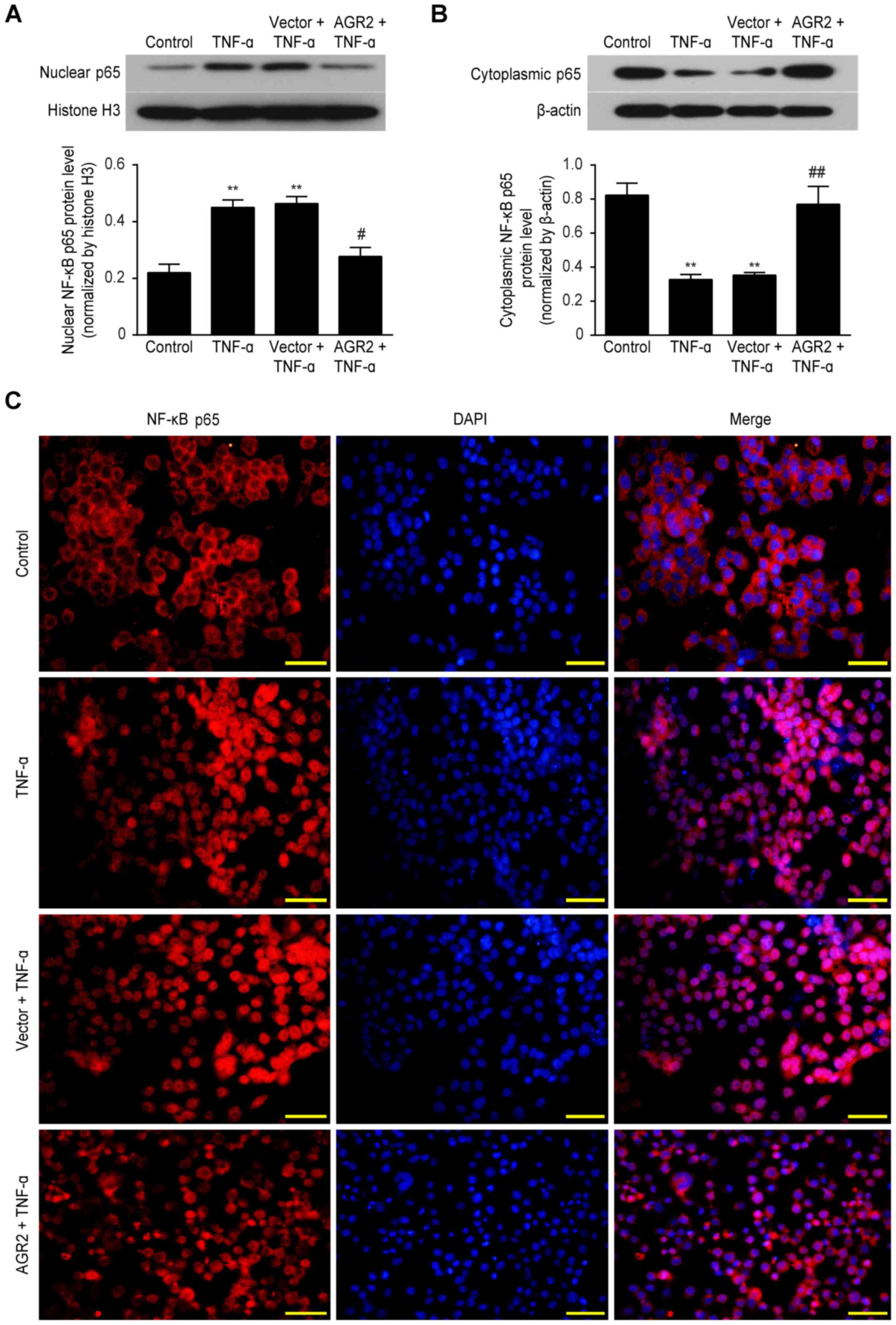
|
1
|
Salim SY and Söderholm JD: Importance of
disrupted intestinal barrier in inflammatory bowel diseases.
Inflamm Bowel Dis. 17:362–381. 2011. View Article : Google Scholar
|
|
2
|
Petersson J, Schreiber O, Hansson GC,
Gendler SJ, Velcich A, Lundberg JO, Roos S, Holm L and Phillipson
M: Importance and regulation of the colonic mucus barrier in a
mouse model of colitis. Am J Physiol Gastrointest Liver Physiol.
300:G327–G333. 2011. View Article : Google Scholar
|
|
3
|
Fernández-Blanco JA, Estévez J,
Shea-Donohue T, Martínez V and Vergara P: Changes in epithelial
barrier function in response to parasitic infection: implications
for IBD pathogenesis. J Crohns Colitis. 9:463–476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edelblum KL and Turner JR: The tight
junction in inflammatory disease: communication breakdown. Curr
Opin Pharmacol. 9:715–720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alipour M, Zaidi D, Valcheva R, Jovel J,
Martínez I, Sergi C, Walter J, Mason AL, Wong GK, Dieleman LA, et
al: Mucosal barrier depletion and loss of bacterial diversity are
primary abnormalities in paediatric ulcerative colitis. J Crohns
Colitis. 10:462–471. 2016. View Article : Google Scholar :
|
|
6
|
Vetrano S, Rescigno M, Cera MR, Correale
C, Rumio C, Doni A, Fantini M, Sturm A, Borroni E, Repici A, et al:
Unique role of junctional adhesion molecule-A in maintaining
mucosal homeostasis in inflammatory bowel disease.
Gastroenterology. 135:173–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neurath MF and Travis SP: Mucosal healing
in inflammatory bowel diseases: a systematic review. Gut.
61:1619–1635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki T, Yoshinaga N and Tanabe S:
Interleukin-6 (IL-6) regulates claudin-2 expression and tight
junction permeability in intestinal epithelium. J Biol Chem.
286:31263–31271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amasheh M, Grotjohann I, Amasheh S, Fromm
A, Söderholm JD, Zeitz M, Fromm M and Schulzke JD: Regulation of
mucosal structure and barrier function in rat colon exposed to
tumor necrosis factor alpha and interferon gamma in vitro: a novel
model for studying the pathomechanisms of inflammatory bowel
disease cytokines. Scand J Gastroenterol. 44:1226–1235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Wang P, Cao M, Li M and Wang F:
Protective role of oligomycin against intestinal epithelial barrier
dysfunction caused by IFN-γ and TNF-α. Cell Physiol Biochem.
29:799–808. 2012. View Article : Google Scholar
|
|
11
|
Michielan A and D'Incà R: Intestinal
permeability in inflammatory bowel disease: pathogenesis, clinical
evaluation, and therapy of leaky gut. Mediators Inflamm.
2015:6281572015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki T: Regulation of intestinal
epithelial permeability by tight junctions. Cell Mol Life Sci.
70:631–659. 2013. View Article : Google Scholar
|
|
13
|
Shen L, Weber CR, Raleigh DR, Yu D and
Turner JR: Tight junction pore and leak pathways: a dynamic duo.
Annu Rev Physiol. 73:283–309. 2011. View Article : Google Scholar
|
|
14
|
Chen Y, Li D, Dai Z, Piao X, Wu Z, Wang B,
Zhu Y and Zeng Z: L-methionine supplementation maintains the
integrity and barrier function of the small-intestinal mucosa in
post-weaning piglets. Amino Acids. 46:1131–1142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turner JR: Intestinal mucosal barrier
function in health and disease. Nat Rev Immunol. 9:799–809. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marchiando AM, Shen L, Graham WV, Edelblum
KL, Duckworth CA, Guan Y, Montrose MH, Turner JR and Watson AJ: The
epithelial barrier is maintained by in vivo tight junction
expansion during pathologic intestinal epithelial shedding.
Gastroenterology. 140:1208–1218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Assasi N, Blackhouse G, Xie F, Marshall
JK, Irvine EJ, Gaebel K, Robertson D, Campbell K, Hopkins R and
Goeree R: Patient outcomes after anti TNF-alpha drugs for Crohn's
disease. Expert Rev Pharmacoecon Outcomes Res. 10:163–175. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang QY, Sun AM, Song J, Chen Y, Wang JD
and Li CG: Cytokine tumor necrosis factor alpha induces intestinal
epithelial barrier dysfunction. Cytokine. 58:226–230. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huys L, Van Hauwermeiren F, Dejager L,
Dejonckheere E, Lienenklaus S, Weiss S, Leclercq G and Libert C:
Type I interferon drives tumor necrosis factor-induced lethal
shock. J Exp Med. 206:1873–1882. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi N, Vanlaere I, de Rycke R,
Cauwels A, Joosten LA, Lubberts E, van den Berg WB and Libert C:
IL-17 produced by Paneth cells drives TNF-induced shock. J Exp Med.
205:1755–1761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki M, Nagaishi T, Yamazaki M, Onizawa
M, Watabe T, Sakamaki Y, Ichinose S, Totsuka M, Oshima S, Okamoto
R, et al: Myosin light chain kinase expression induced via tumor
necrosis factor receptor 2 signaling in the epithelial cells
regulates the development of colitis-associated carcinogenesis.
PLoS One. 9:e883692014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng Y and Teitelbaum DH: Tumour necrosis
factor-induced loss of intestinal barrier function requires TNFR1
and TNFR2 signalling in a mouse model of total parenteral
nutrition. J Physiol. 591:3709–3723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du J, Chen Y, Shi Y, Liu T, Cao Y, Tang Y,
Ge X, Nie H, Zheng C and Li YC: 1,25-Dihydroxyvitamin D protects
intestinal epithelial barrier by regulating the myosin light chain
kinase signaling pathway. Inflamm Bowel Dis. 21:2495–2506. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Xu J, Mei Q, Han L and Huang J:
Myosin light chain kinase inhibitor inhibits dextran sulfate
sodium-induced colitis in mice. Dig Dis Sci. 58:107–114. 2013.
View Article : Google Scholar
|
|
25
|
Park SW, Zhen G, Verhaeghe C, Nakagami Y,
Nguyenvu LT, Barczak AJ, Killeen N and Erle DJ: The protein
disulfide isomerase AGR2 is essential for production of intestinal
mucus. Proc Natl Acad Sci USA. 106:6950–6955. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng W, Rosenstiel P, Huse K, Sina C,
Valentonyte R, Mah N, Zeitlmann L, Grosse J, Ruf N, Nürnberg P, et
al: Evaluation of AGR2 and AGR3 as candidate genes for inflammatory
bowel disease. Genes Immun. 7:11–18. 2006. View Article : Google Scholar
|
|
27
|
Lai YR, Lu YF, Lien HW, Huang CJ and Hwang
SP: Foxa2 and Hif1ab regulate maturation of intestinal goblet cells
by modulating agr2 expression in zebrafish embryos. Biochem J.
473:2205–2218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao F, Edwards R, Dizon D, Afrasiabi K,
Mastroianni JR, Geyfman M, Ouellette AJ, Andersen B and Lipkin SM:
Disruption of Paneth and goblet cell homeostasis and increased
endoplasmic reticulum stress in Agr2−/− mice. Dev Biol.
338:270–279. 2010. View Article : Google Scholar
|
|
29
|
Su L, Shen L, Clayburgh DR, Nalle SC,
Sullivan EA, Meddings JB, Abraham C and Turner JR: Targeted
epithelial tight junction dysfunction causes immune activation and
contributes to development of experimental colitis.
Gastroenterology. 136:551–563. 2009. View Article : Google Scholar :
|
|
30
|
Liu Z, Li N and Neu J: Tight junctions,
leaky intestines, and pediatric diseases. Acta Paediatr.
94:386–393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin N, Xu LF and Sun M: The protective
effect of trefoil factor 3 on the intestinal tight junction barrier
is mediated by toll-like receptor 2 via a I3K/Akt dependent
mechanism. Biochem Biophys Res Commun. 440:143–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu LF, Teng X, Guo J and Sun M: Protective
effect of intestinal trefoil factor on injury of intestinal
epithelial tight junction induced by platelet activating factor.
Inflammation. 35:308–315. 2012. View Article : Google Scholar
|
|
33
|
Blander JM: Death in the intestinal
epithelium-basic biology and implications for inflammatory bowel
disease. FEBS J. 283:2720–2730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao YT, Yan WH, Cao Y, Yan JK and Cai W:
Neutralization of IL-6 and TNF-α ameliorates intestinal
permeability in DSS-induced colitis. Cytokine. 83:189–192. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen S, Zhu J, Chen G, Zuo S, Zhang J,
Chen Z, Wang X, Li J, Liu Y and Wang P: 1,25-Dihydroxyvitamin D3
preserves intestinal epithelial barrier function from TNF-alpha
induced injury via suppression of NF-kB 65 mediated MLCK-P-MLC
signaling pathway. Biochem Biophys Res Commun. 460:873–878. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He F, Peng J, Deng XL, Yang LF, Camara AD,
Omran A, Wang GL, Wu LW, Zhang CL and Yin F: Mechanisms of tumor
necrosis factor-alpha-induced leaks in intestine epithelial
barrier. Cytokine. 59:264–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao M, Wang P, Sun C, He W and Wang F:
Amelioration of IFN-γ and TNF-α-induced intestinal epithelial
barrier dysfunction by berberine via suppression of MLCK-MLC
phosphorylation signaling pathway. PLoS One. 8:e619442013.
View Article : Google Scholar
|
|
38
|
Zhang J, Lu Y, Wei J, Li L and Han L:
Protective effect of carboxytmethylpachymaran on TNF-alpha-induced
damage in Caco-2 cell monolayers. Int J Biol Macromol. 93(Pt A):
506–511. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuhlmann CR, Tamaki R, Gamerdinger M,
Lessmann V, Behl C, Kempski OS and Luhmann HJ: Inhibition of the
myosin light chain kinase prevents hypoxia-induced blood-brain
barrier disruption. J Neurochem. 102:501–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boivin MA, Ye D, Kennedy JC, Al-Sadi R,
Shepela C and Ma TY: Mechanism of glucocorticoid regulation of the
intestinal tight junction barrier. Am J Physiol Gastrointest Liver
Physiol. 292:G590–G598. 2007. View Article : Google Scholar
|
|
41
|
Cunningham KE and Turner JR: Myosin light
chain kinase: pulling the strings of epithelial tight junction
function. Ann NY Acad Sci. 1258:34–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ye D and Ma TY: Cellular and molecular
mechanisms that mediate basal and tumour necrosis
factor-alpha-induced regulation of myosin light chain kinase gene
activity. J Cell Mol Med. 12:1331–1346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Al-Sadi R, Guo S, Ye D, Rawat M and Ma TY:
TNF-alpha modulation of intestinal tight junction permeability is
mediated by NIK/IKK-alpha axis activation of the canonical NF-κB
pathway. Am J Pathol. 186:1151–1165. 2016. View Article : Google Scholar : PubMed/NCBI
|