Introduction
Pulmonary arteriolar smooth muscle cells (PASMCs)
play an important role in pulmonary arterial remodeling and hypoxic
pulmonary vasoconstriction during sustained hypoxia or ischemia and
reperfusion. A limited understanding of the basic cellular
mechanisms governing this disease process has led to few
therapeutic options and continued morbidity and mortality (1,2).
In the pulmonary vasculature, mitochondria
contribute to physiological intracellular signaling pathways
through production of reactive oxygen species and play the role of
oxygen sensors that coordinate hypoxic pulmonary vasoconstriction
(3,4). Mitochondrial alterations have been
observed in pulmonary arteries in pulmonary hypertension and
mitochondrial dysfunction plays a role in the pathogenesis of
pulmonary hypertension (4).
Several intra- and extra-mitochondrial causes of mitochondrial
dysfunction in pulmonary hypertension have been described in
pulmonary arteries, whereas ischemia and decreased angiogenesis
appear to be the main triggers of mitochondrial dysfunction
(5). Mitochondrial-targeted
therapies are currently being investigated and may open novel
therapeutic perspectives in hypoxic pulmonary vasoconstriction and
pulmonary hypertension. Decreased oxidative capacity and energy
production are due to decreased mitochondrial biogenesis. It is
unknown what mechanism of mitochondrial biogenesis reprogramming in
PASMCs occurs during hypoxia/reoxygenation (H/R).
Recently, histone deacetylase sirtuin 1 (SIRT1) has
emerged as a crucial regulator of mitochondrial function in
vascular smooth muscle, liver, kidney and heart (6–9).
SIRT1 and SIRT3 also play roles in pulmonary hypertension (10,11). The effects of SIRT1 on
mitochondrial biogenesis are mediated by PGC-1α (12). Knockdown of Sirt3 blocks
PGC-1α-dependent gene expression of subunits I and II
(mitochondrial encoded) as well as subunit VIIa (nuclear encoded)
from cytochrome c oxidase, respectively (13). SIRT3 is required for
PGC-1α-induced mitochondrial-related gene expression and
mitochondrial biogenesis. SIRT1 and SIRT3 have been reported to
play an important role in mitochondrial morphology and function in
aorterial smooth muscle cells (6). Cyclophilin D (CyPD), a
peptidylprolyl isomerase F (PPIase), modulates mitochondrial
membrane permeability (14).
SIRT1/SIRT3 mediates deacetylation of CyPD to regulate
mitochondrial membrane permeability and mitochondrial function
during hemorrhagic shock (6).
However, both SIRT1 and SIRT3, in regard to mitochondrial
biogenesis, are still lacking in PASMCs during H/R.
Mitochondrial transcription factor A (TFAM) is a
multi-functional DNA binding protein that is essential for
transcriptional activation and mitochondrial DNA (mtDNA)
organization. TFAM is an important mitochondrial biogenesis marker
(15). In a clinical trial,
caloric restriction improved skeletal muscle mitochondrial function
and mitochondrial biogenesis through regulation of SIRT1, PGC-1α
and TFAM gene expression (16).
TFAM can also act as one of the mitochondrial damage-associated
molecular patterns (DAMPs), which may be associated with vascular
damage (17).
Polydatin is a glucoside of resveratrol, a natural
polyphenolic compound. Polydatin protects multiple organs from
ischemia/reperfusion injury (18–20). A previous study from our
laboratory showed that polydatin protects against ASMC
mitochondrial injury in hemorrhagic shock (21). Recently, we found that polydatin
protects mitochondria through activation of SIRT1 in the liver and
small intestine during hemorrhagic shock (20,22). Yet, the role of SIRT1 in
mitochondrial biogenesis during hypoxia and reperfusion remains
unclear.
We hypothesized that SIRT1 may play an important
role in protection against mitochondrial biogenesis reprogramming
and function in PASMCs during H/R. Polydatin protects mitochondrial
biogenesis through SIRT1. The purpose of this study was to
determine: i) the expression and activity of SIRT1 in PASMCs during
H/R, ii) the expression of TFAM in mitochondria and the release of
TFAM during H/R, iii) the role of PGC-1α/SIRT3/CyPD in the effect
of SIRT1 on mitochondrial biogenesis during H/R, and iv) the effect
of polydatin on mitochondrial biogenesis.
Materials and methods
Materials
Antibodies against human SIRT1 (#2310), SIRT3
(#2627), PGC-1α (#2178) and acetylated-lysine (#9441) were all
purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
The antibody against human CyPD (#ab110324) and mtTFA Human
SimpleStep ELISA™ kit were purchased from Abcam, Inc. (Cambridge,
MA, USA). The SIRT3 Deacetylase Fluorometric assay kit was purch
ased from CycLex Co., Ltd. (Nagano, Japan). SIRT1 open reading
frame (ORF) and control vector were both obtained from the
Functional Genomics Facility of the University of Colorado.
Lipofectamine 2000 was purchased from Life Technologies, Inc.
(Grand Island, NY, USA). TransDux™ transduction reagent was
purchased from System Biosciences, Inc. (Mountain View, CA, USA).
Human Mitochondrial to Nuclear DNA Ratio kit was purchased from
Clontech Laboratories, Inc. (Mountain View, CA, USA). CellTiter-Glo
Luciferase-based assay was purchased from Promega (Madison, WI,
USA). Specific siRNA for human SIRT3 and PGC-1α and scrambled siRNA
were purchased from Integrated DNA Technologies, Inc. (Coralville,
IA, USA). HiPerFect® transfection reagent and other
transfection-related reagents were purchased from Qiagen (Valencia,
CA, USA). Dulbecco's modified Eagle's medium (DMEM) was purchased
from Life Technologies. All other chemicals and reagents were from
Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Cell preparation and culture
Human PASMCs were cultured by explant outgrowth from
the branches between an artery and a bronchiole harvested for lung
transplantation. Cells were grown in DMEM supplemented with 10%
(vol/vol) fetal bovine serum (FBS), 100 U/ml penicillin, 100
μg/ml streptomycin and 1.25 μg/ml amphotericin B. The
cells exhibited the typical 'hill and valley' growth morphology of
SMCs. PASMC isolates lacked endothelial cells and fibroblasts as
verified by α-SMA, fibroblast-specific protein 1 (FSP1) and von
Willebrand factor (vWF) staining. Cells from passages 3–6 were used
for the experiments.
Mitochondrial DNA content
Genomic DNA (including mtDNA) was extracted from
human PASMCs. Mitochondrial DNA content was measured using the
human mitochondrial to Nuclear DNA Ratio kit according to the
manufacturer's instructions. The copy number of mtDNA was
calculated as the average of the values and calculated based on the
combinations of the primers. Data analysis was performed and the
mtDNA copy number was determined by relative quantification.
Mitochondrial TFAM levels and TFAM
release
Mitochondrial TFAM levels and TFAM release were
measured with the enzyme-linked immunosorbent assay TFAM
SimpleStep. To measure mitochondrial TFAM levels, the mitochondria
in the PASMCs were isolated. To measure the release of TFAM, cell
culture supernatants were collected. Particulates were removed by
centrifugation for 15 min at 10,000 × g at 2–8°C. Then
mitochondrial TFAM levels and TFAM release were assessed according
to the assay procedure of the TFAM SimpleStep ELISA kit.
Measurements were carried out in quadruplicates.
Measurement of intracellular ATP
PASMC ATP levels were measured by a luciferase-based
assay (CellTiter-Glo; Promega) according to the manufacturer's
protocols. The luminescence was recorded using an automatic
microplate reader (SpectraMax M5; Molecular Devices, Sunnyvale, CA,
USA).
Western blot analysis
Total protein was isolated from the PASMCs by
homogenization in cold RIPA lysis buffer containing protease
inhibitors and phosphatase inhibitors (Roche, Indianapolis, IN,
USA). Equal amounts of proteins were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to a polyvinylidene difluoride membrane. After blocking
in 5% bovine serum albumin (BSA) in Tris-buffered saline/Tween-20
(50 mM Tris, pH 7.5, 500 mM sodium chloride, and 0.05% Tween-20)
for 1 h at room temperature, the membranes were incubated overnight
at 4°C with primary antibodies against SIRT1, SIRT3, PGC-1α, CyPD
and β-actin. After incubation with a secondary antibody conjugated
to horseradish peroxidase for 1 h at room temperature,
immunoreactive signals were detected by chemiluminescence using ECL
detection reagent, visualized using a chemiluminescence detection
system (Image Station 4000R; Kodak, Rochester, NY, USA), and
analyzed using Quantity One software (version 4.52).
SIRT3 deacetylase activity assay
For this assay the SIRT3 Deacetylase Fluorometric
assay kit was used. Briefly, PASMCs were homogenized in 500
μl of immunoprecipitation buffer (T-PER® Tissue
Protein Extraction Reagent 78510; Pierce, Rockford, IL, USA). After
immunoprecipitation of SIRT3, the final reaction mixtures (50
μl) contained 50 mM Tris-HCL (pH 8.8), 4 mM
MgCl2, 0.5 mM DTT, 0.25 mA/ml lysyl endopeptidase, 1
μM Trichostatin A, 200 μM NAD+ and 5
μl of extract. The fluorescence intensity (Ex 340 nm, Em 460
nm) was measured using an automatic microplate reader (SpectraMax
M5; Molecular Devices, Sunnyvale, CA, USA).
H/R
Human PASMCs were cultured in a hypoxic incubator
(3% oxygen, 5% carbon and 92% dioxide) for 24–48 h and then
cultured in a normal incubator (95% air and 5% CO2) for
24–48 h.
Gene knockdown and overexpression
To knockdown SIRT3 and PGC-1α, human PASMCs (60–70%
confluent) in 24-well plates were incubated with a mixture of siRNA
(60 nM) (Life Technologies) and transfection reagent (Qiagen) for
48 h (35). Control cells were
treated with scrambled siRNA and transfection reagent.
SIRT1 expression plasmids, control plasmid and
lentiviral packaging plasmids (pVSVG, pRSV-Rev and pMDL) (GE
Dharmacon, Lafayette, CO, USA) were purified using the HiSpeed
Plasmid Midi kit (Qiagen). Lentiviruses that overexpress SIRT1 and
scrambled control were generated by co-transfection of 293T cells
using Lipofectamine 2000. After 48 h, lentiviral supernatants were
collected and concentrated. Human PASMCs were infected with a
lentivirus overexpressing SIRT1 or scrambled control using TransDux
transduction reagent. The expression of GFP was examined under a
fluorescence microscope.
Co-immunoprecipitation
Human PASMCs (80–90% confluent) in 6-well plates
were gently rinsed 3 times with phosphate-buffered saline (PBS).
Cells were lysed in Pierce cell lysis buffer (25 mM Tris-HCl, 150
mM NaCl, 1 mM EDTA, 1% NP-40 and 5% glycerol, pH 7.4), and the
lysates were centrifuged at 13,000 × g for 10 min at 4°C. The
cleared lysates were incubated with anti-acetylated-lysine antibody
(Cell Signaling Technology, Boston, MA, USA) overnight at 4°C with
rocking. Next, protein A/G-agarose beads (20 μl of 50% bead
slurry) were added and the mixtures were incubated with gentle
rocking for 3 h at 4°C. The immunoprecipitates were washed 3 times
with 0.5 ml of lysis buffer and resuspended in 100 μl of 2X
SDS sample buffer. CyPD protein level in the immunoprecipates was
analyzed by immunoblotting.
Statistical analysis
Statistical analyses were performed using SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). All values were
presented as mean ± standard error (SE). One-way or two-way
analysis of variance (ANOVA) was used to compare the differences
between two groups or among multiple groups, respectively, followed
by the post hoc Bonferroni/Dunn test. P-values <0.05 were
considered statistically significant.
Results
Expression and activation of SIRT1 are
reduced by H/R in human PASMCs
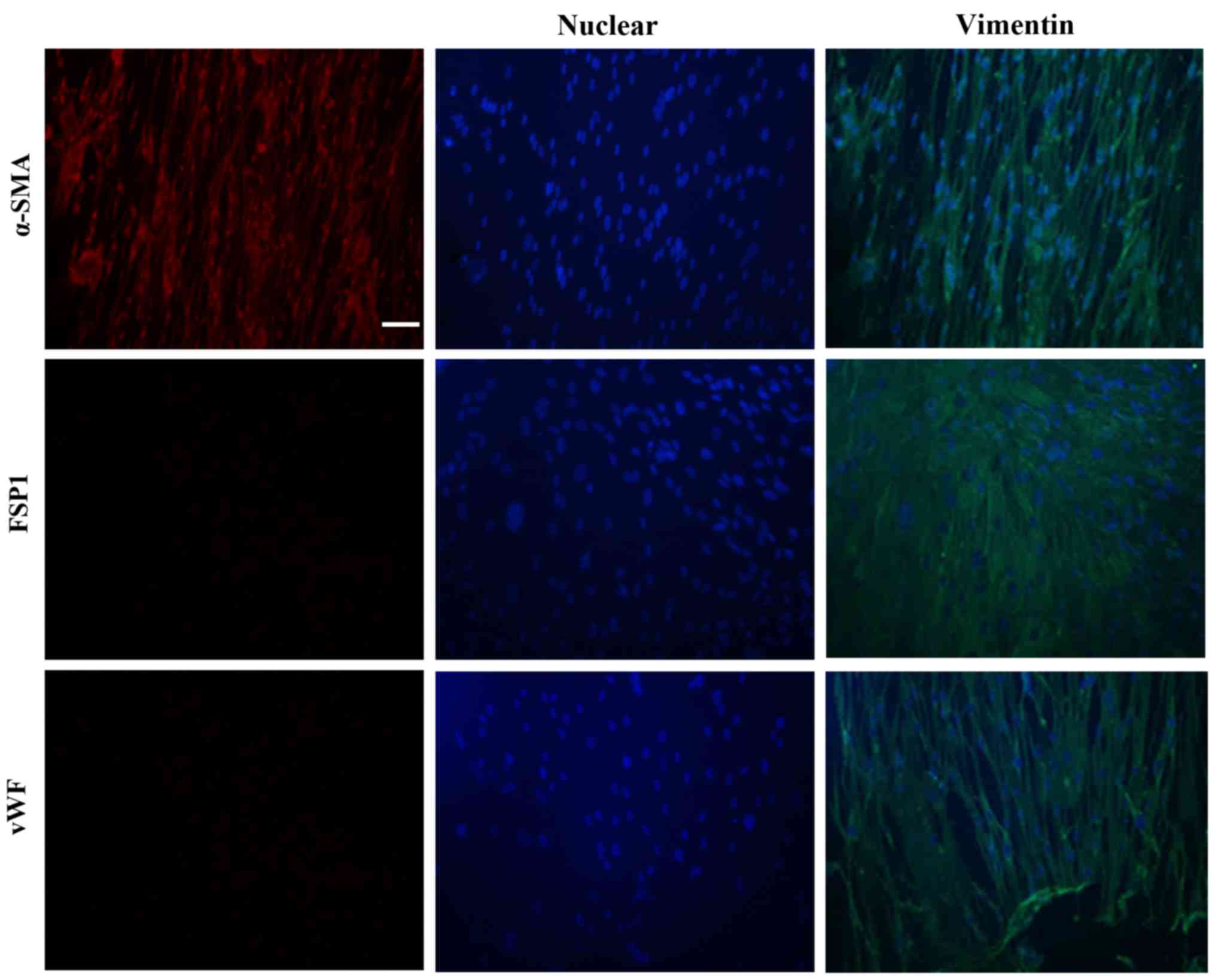
We applied antibodies against α-SMA, FSP1, and vWF
to determine whether the cultured human PASMCs were contaminated by
fibroblasts and endothelial cells. No signals for FSP1 and vWF were
detected (Fig. 1), indicating
that fibroblasts and endothelial cells were not detected in the
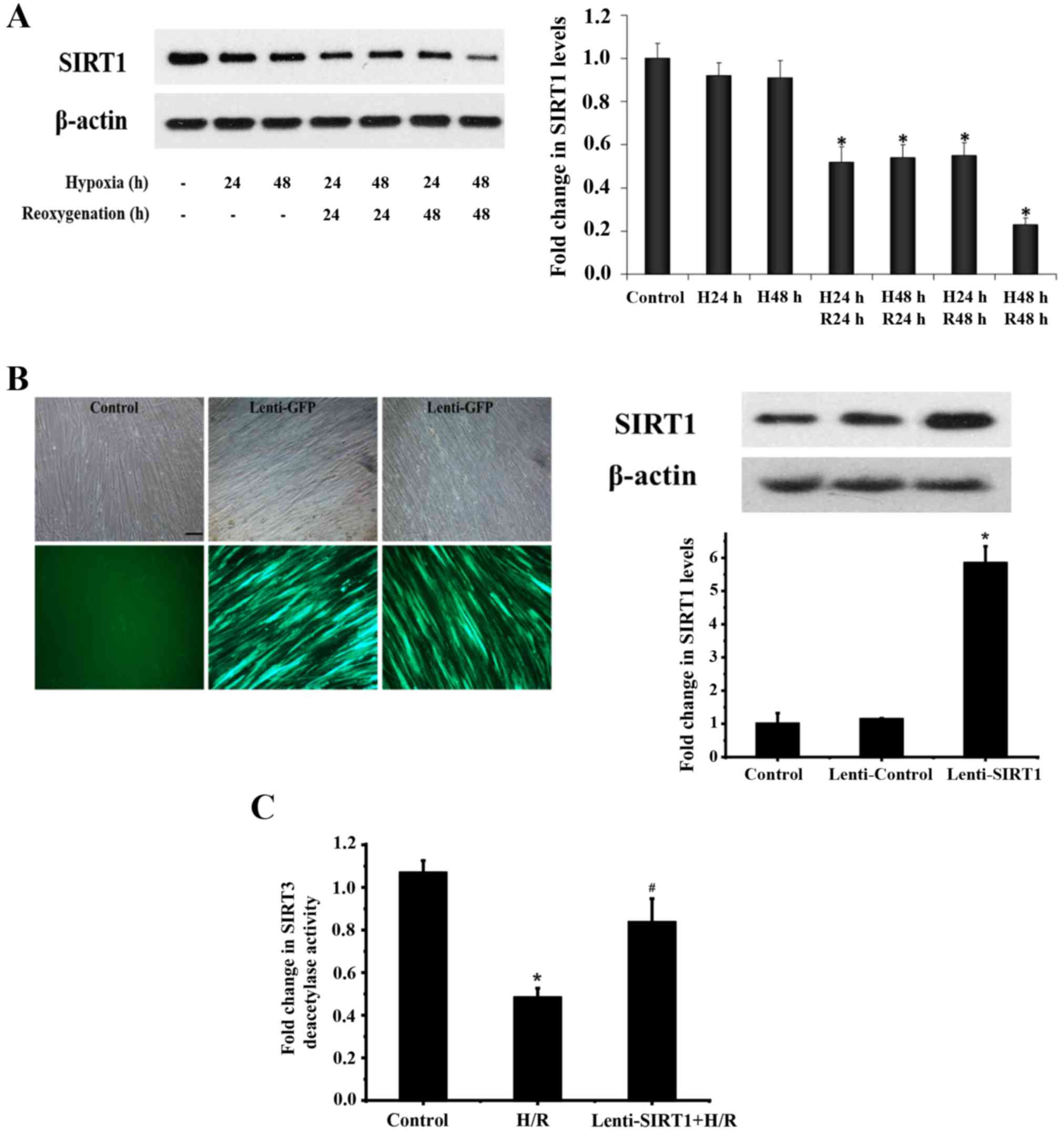
isolates. PASMCs were exposed to H/R. As shown in Fig. 2A, the expression of SIRT1 was
reduced in the PASMCs during hypoxia for 24–48 h and reoxygenation
for 24–48 h following hypoxia.
To determine the role of SIRT1 in SIRT3 deacetylase
activity during H/R, human PASMCs were infected with a lentivirus
for the overexpression of SIRT1 (Fig.
2B). While SIRT3 deacetylase activity was reduced in cells
during 24 h of hypoxia and 24 h of reoxygenation, overexpression of
SIRT1 restored SIRT3 deacetylase activity reduced by H/R (Fig. 2C).
Expression and release of mitochondrial
TFAM are altered by H/R in human PASMCs
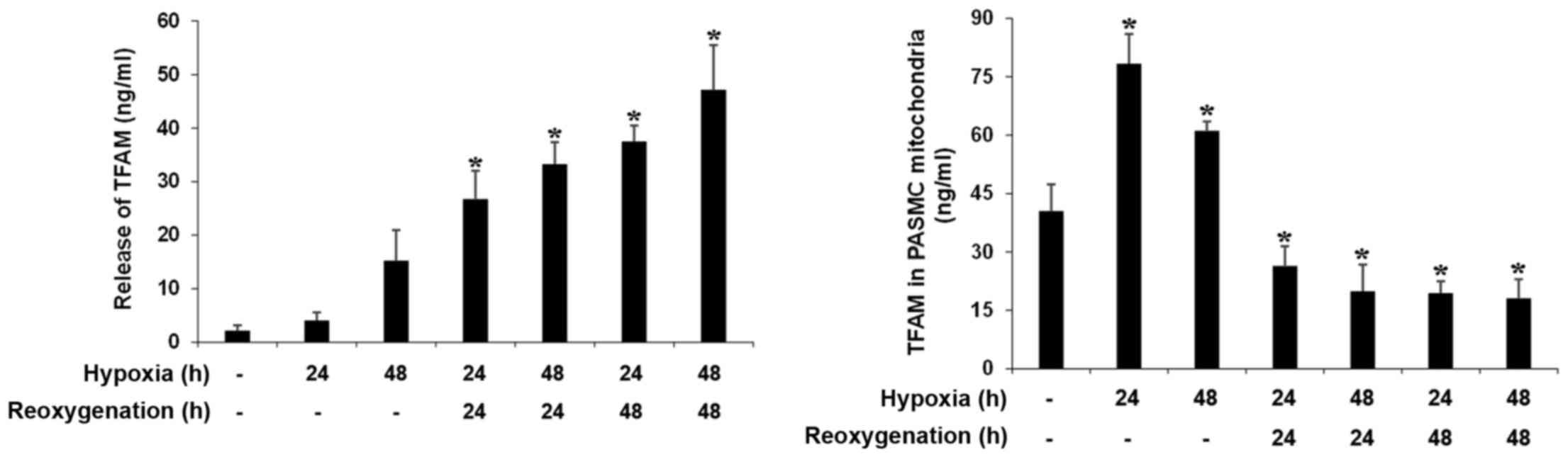
To determine PASMC mitochondrial biogenesis
reprogramming, we detected expression of TFAM in human PASMCs
exposed to H/R. As shown in Fig.
3, TFAM levels in the mitochondria were increased at 24 and 48
h of hypoxia, while TFAM levels in the mitochondria were decreased
after reoxygenation. Interestingly, we found the release of TFAM by
PASMCs was markedly increased after reoxygenation (Fig. 3).
PGC-1α/SIRT3/CyPD mediates the effect of
SIRT1 on TFAM in PASMCs during H/R
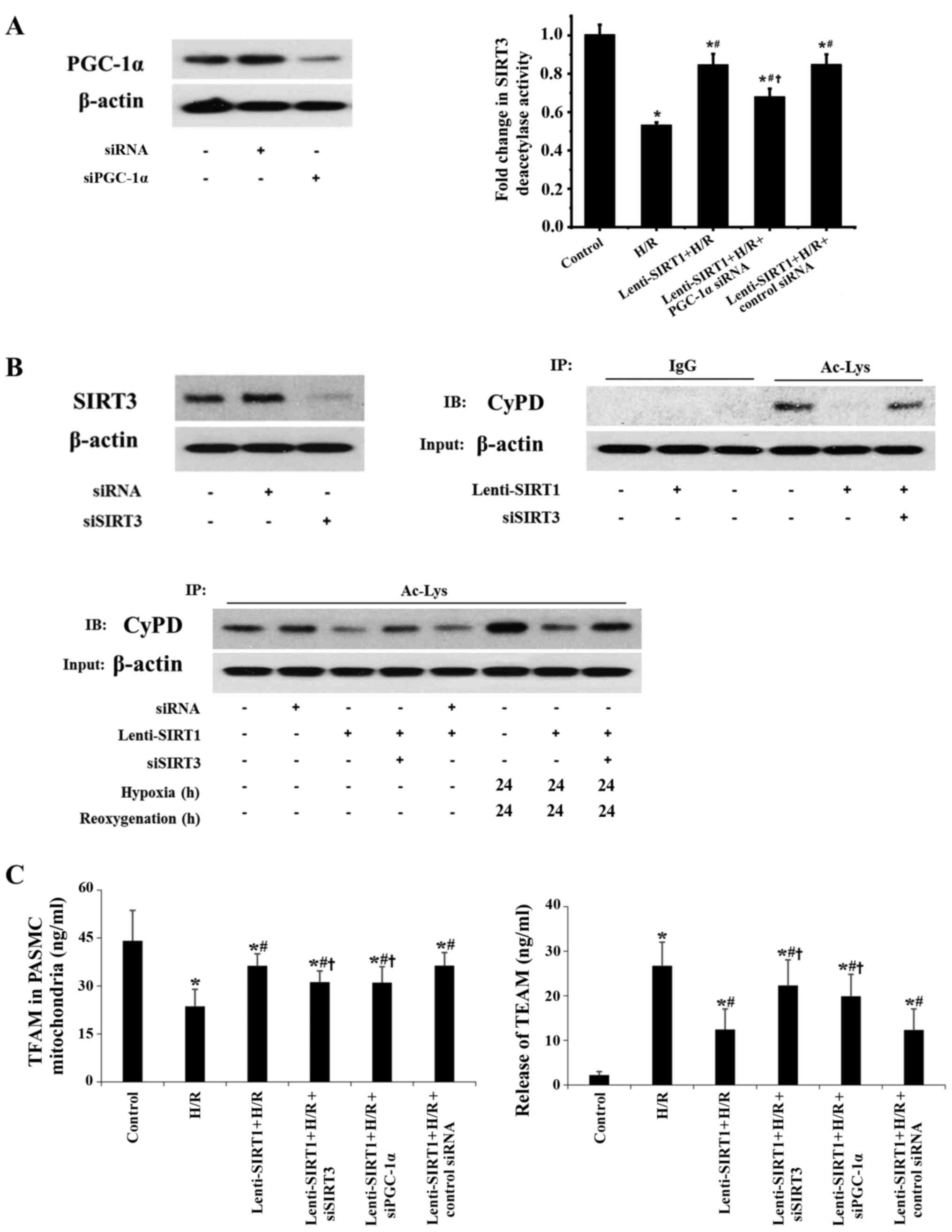
To determine how SIRT1 exerts an effect on SIRT3
activity, we knocked down PGC-1α, a downstream target of SIRT1.
Knockdown of PGC-1α abrogated restoration of H/R-induced
deacetylase activity of SIRT3 by SIRT1 (Fig. 4A). Overexpression of SIRT1 reduced
the acetylation of mitochondrial CyPD, an important mitochondrial
member that is involved in regulating the mitochondrial
permeability transition, during H/R (Fig. 4B). It has been reported that SIRT3
regulates CyPD deacetylation in cardiac muscle (23). Therefore, we transfected PASMCs
with SIRT3 siRNA and found that knockdown of SIRT3 inhibited the
effect of SIRT1 on the acetylation of mitochondrial CyPD during H/R
(Fig. 4B). These data suggest
that SIRT3 deacetylase activity is necessary for SIRT1-regulated
acetylation of CyPD during H/R. Importantly, overexpression of
SIRT1 increased TFAM levels in mitochondria during H/R (Fig. 4C). Knockdown of SIRT3 or PGC-1α
suppressed the protective effect of SIRT1 on TFAM levels in the
mitochondria (Fig. 4C). For
release of TFAM by PASMCs, overexpression of SIRT1 decreased the
levels of TFAM during H/R. Knockdown of SIRT3 or PGC-1α suppressed
the effect of SIRT1 on the release of TFAM (Fig. 4C).
Stimulation of mitochondrial biogenesis
in PASMCs requires functional SIRT1
To determine whether SIRT1 can stimulate
mitochondrial biogenesis, we examined the mitochondrial DNA (mtDNA)
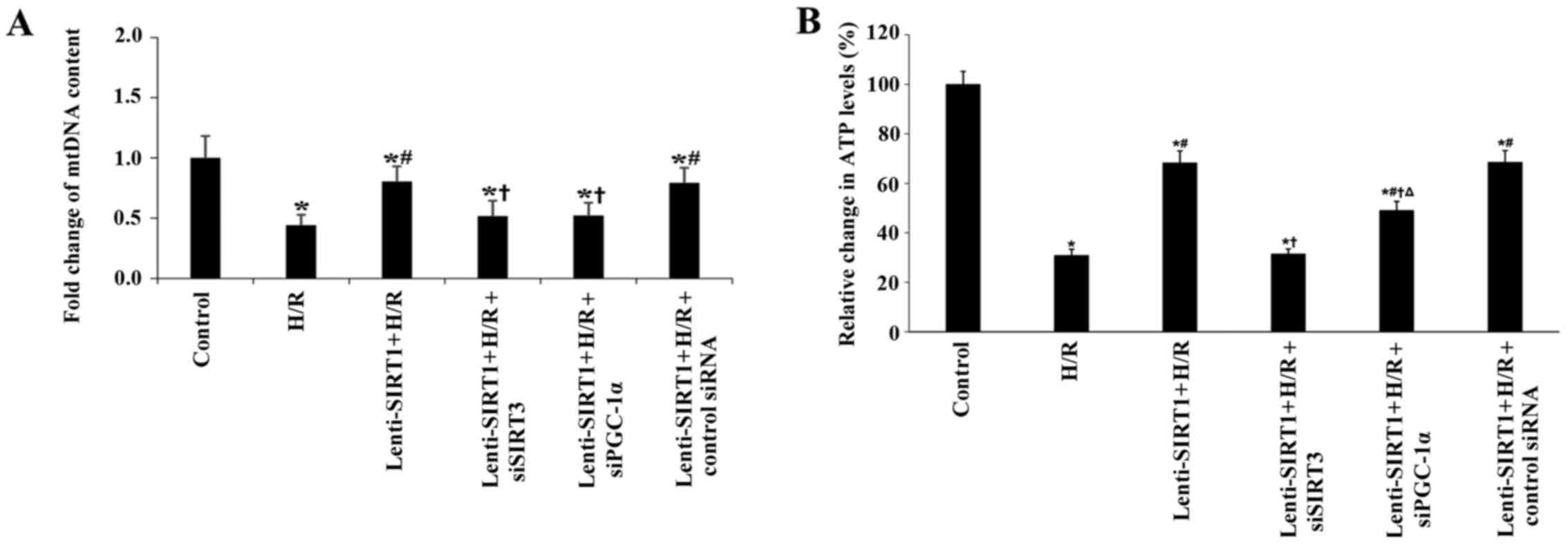
content in PASMCs. As shown in Fig.
5, mtDNA content was decreased 2.5-fold by H/R. Overexpression
of SIRT1 increased mtDNA content. Knockdown of SIRT3 or PGC-1α
suppressed the effect of SIRT1 on mtDNA content. Furthermore,
overexpression of SIRT1 increased cellular ATP levels during H/R.
Knockdown of SIRT3 or PGC-1α suppressed the effect of SIRT1 on ATP
levels (Fig. 5).
Polydatin improves mitochondrial
biogenetic reprogramming and mitochondrial function in PASMCs, but
has no effect on SIRT1-knockdown PASMCs
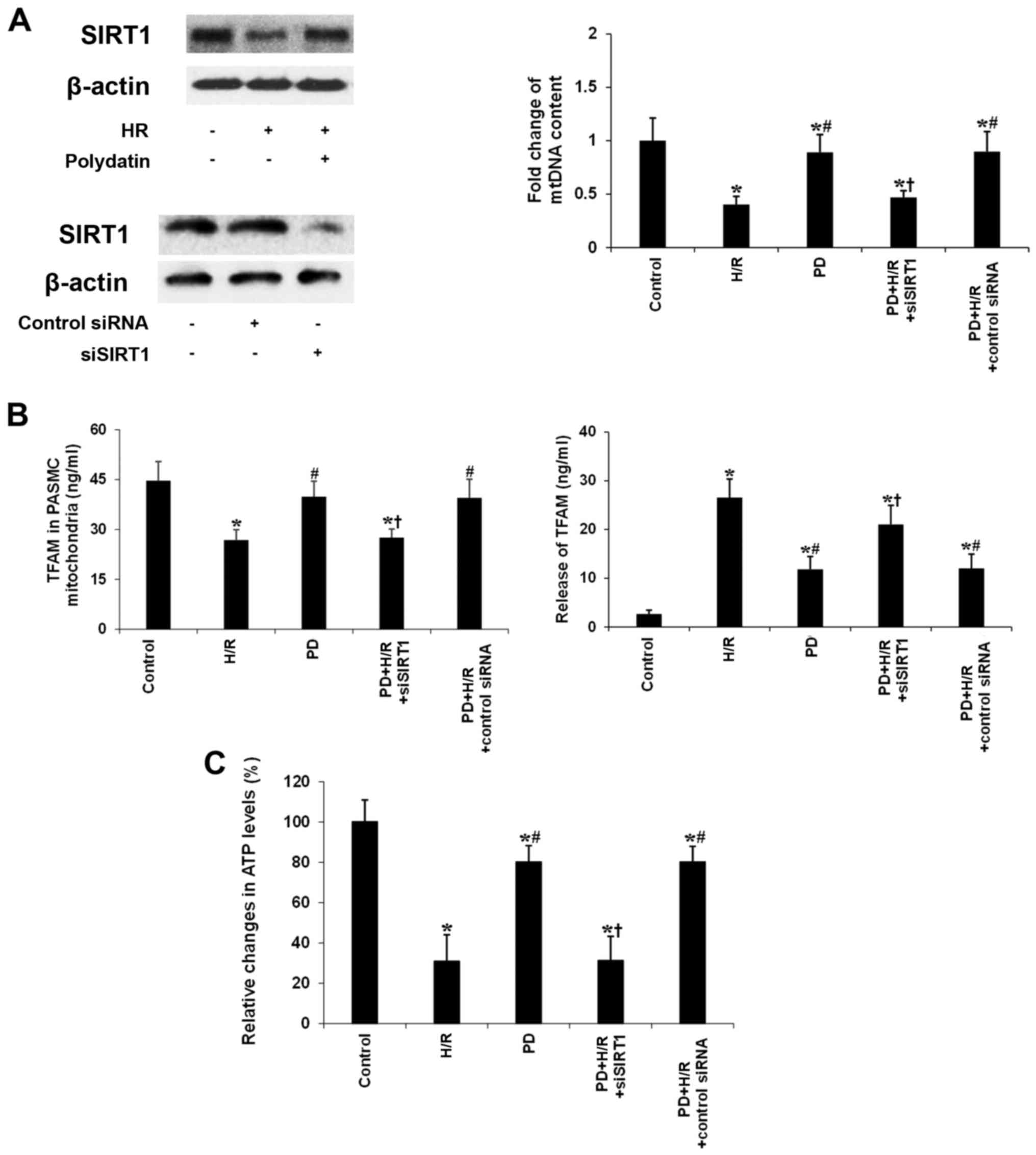
To determine the role of polydatin in mitochondrial
biogenesis reprogramming, we treated SIRT1-knockdown PASMCs with
polydatin during H/R. As shown in Fig. 6A, polydatin (50 nM) restored SIRT1
levels in human PASMCs exposed to 24 h reoxygenation after 24 h
hypoxia. Polydatin (50 nM) increased mtDNA content in the human
PASMCs exposed to 24 h reoxygenation after 24 h hypoxia. Knockdown
of SIRT1 suppressed the effect of polydatin on mtDNA content. We
also found that polydatin (50 nM) increased the expression of TFAM
in mitochondria during H/R (Fig.
6B). Knockdown of SIRT1 suppressed the effect of polydatin (50
nM) on the expression of TFAM in mitochondria (Fig. 6B). Conversely, polydatin (50 nM)
decreased the release of TFAM in the mitochondria during H/R
(Fig. 6B). Knockdown of SIRT1
suppressed the effect of polydatin (50 nM) on the release of TFAM
(Fig. 6B). Importantly, polydatin
(50 nM) increased ATP levels in the PASMCs during H/R (Fig. 6C). Knockdown of SIRT1 suppressed
the effect of polydatin (50 nM) on ATP levels (Fig. 6C).
Discussion
Several in vivo and in vitro studies
have demonstrated that histone deacetylase SIRT1 activity is
decreased in human lung epithelial cells, macrophages, and lungs of
mice exposed to cigarette smoke and in the lungs of patients with
chronic obstructive pulmonary disease (COPD) (24,25). Clinical evidence suggests that
SIRT1 also affects the clock-dependent metabolome and mitochondrial
functions/biogenesis, which are altered during the pathogenesis of
airway diseases such as COPD (26). Acute regulation of SIRT1 in
response to stressful conditions, such as ischemia and reperfusion
are likely to differ according to the type of injury; SIRT1 was
found to be downregulated in mice exposed to 20 min ischemia
followed by 24 h reperfusion (27), but upregulated in hearts subjected
to short periods of ischemia and reperfusion (28). We demonstrated that human
pulmonary arteriolar smooth muscle cells (PASMCs) exposed to H/R
(mimicking ischemia and reperfusion) have decreased SIRT1
expression compared to control untreated cells. Our previous study
showed that SIRT1 positively modulates SIRT3 activity, which plays
an important role in protecting mitochondrial function in ASMCs
during hemorrhagic shock (6). To
investigate the effect of H/R on SIRT1 activity and function, we
examined the effect of the overexpression of SIRT1 on SIRT3
deacetylase activity in PASMCs exposed to H/R. Our data showed that
H/R decreased SIRT3 activity, and overexpression of SIRT1 increased
SIRT3 activity in the PASMCs. These results suggest that increased
expression of SIRT1 during H/R may protect the mitochondrial
biogenesis and function in ASMCs affected by human pulmonary
diseases.
Ischemic injury-induced mitochondrial biogenesis
reprogramming includes alterations in mtDNA content and TFAM
protein expression in the brain and heart (29,30). It was reported that acute ischemic
injury significantly increased TFAM protein expression in the
retina at 12–72 h (31). TFAM is
rapidly increased in the early neurodegenerative events of neonatal
hypoxic-ischemic brain injury (32). Mice lack ing TFAM were found to
have impaired mtDNA transcription and loss of mtDNA, which led to
bioenergetic dysfunction and embryonic lethality (33). In contrast, overexpression of TFAM
mediated delayed neuronal death following transient forebrain
ischemia in mice (34). Our data
showed that TFAM levels were rapidly increased in PASMCs at 24–48 h
hypoxia, suggesting these responses may support endogenous repair
mechanisms for mtDNA damage following hypoxic-ischemic lung injury.
TFAM levels were decreased at 24–48 h reoxygenation, suggesting
that mitochondrial function and ultrastructure were damaged.
Interestingly, we found that the release of TFAM was increased by
PASMCs exposed to H/R. Damage-associated molecular patterns are
derived from many sources within the cell, including the plasma
membrane, nucleus, cytosol, endoplasmic reticulum and mitochondria.
TFAM is a transcription factor for mitochondrial DNA. It is
composed of two HMG boxes and a basic carboxyl terminal region
(C-tail). Like many other HMG family proteins, TFAM has the ability
to bind to DNA in a sequence-independent manner. A growing body of
evidence suggests that TFAM may play a crucial role in maintaining
mitochondrial DNA as a main component of the nucleoid. Although
TFAM is structurally related to HMGB1, it remains unknown whether
it can be identified as a DAMP. TFAM can be released into the
circulation after hemorrhage to initiate inflammatory responses.
Further study is needed to demonstrate the role of TFAM in PASMCs
during H/R.
The mechanisms involved in acute regulation of SIRT1
in response to stressful conditions, such as ischemia and
reperfusion, are poorly understood. SIRT3 is also emerging as a
regulatory protein in the modulation of additional mitochondrial
programs. SIRT1 and SIRT3 have been reported to play an important
role in mitochondrial morphology and function in ASMCs. However,
knowledge of both SIRT1 and SIRT3 in regard to mitochondrial
biogenesis is still lacking in PASMCs during H/R. PGC-1α stimulates
mitochondrial biogenesis and promotes the remodeling of muscle
tissue to a fiber-type composition that is metabolically more
oxidative and less glycolytic in nature (35). SIRT1-promoting mitochondrial
biogenesis via PGC-1α occurs in heart and myotubes (36,37). We found that SIRT1 regulated SIRT3
activity through PGC-1α in ASMCs exposed to H/R. Inhibition of
CyPD-dependent mitochondrial permeability transition pore opening
preserved TFAM protein expression and ameliorated mitochondrial
dysfunction, leading to protection against hypoxic/ischemic retinal
injury (38). We found that
knockdown of SIRT3 inhibited the effect of SIRT1 on the acetylation
of mitochondrial CyPD under normoxia and during H/R. Knockdown of
SIRT3 or PGC-1α suppressed the inhibitory effects of SIRT1 on the
decrease in mitochondrial TFAM expression and increase in the
release of TFAM from PASMCs during H/R. In keeping with its role as
a protective mediator in mitochondrial energy production,
overexpression of SIRT1 preserved mtDNA content and ATP production
in human PASMCs exposed to H/R. Knockdown of SIRT3 or PGC-1α
suppressed the protective effect of SIRT1. Some PGC-1α-independent
pathways, such as HIF-α, were also found to be involved in
SIRT1-mediated mitochondrial regulation in myoblasts and cancer
cells (39,40). Further study will be performed to
determine the PGC-1α-independent pathways involved in
SIRT1-mediated mitochondrial biogenesis and function in PASMCs.
Thus, modulation of SIRT1 and its downstream signaling pathways has
the potential to protect mitochondrial biogenesis and function in
PASMCs during H/R.
Data concerning the effects of natural compounds on
mitochondrial biogenesis are quite controversial. Several studies
indicate that resveratrol and quercetin have some beneficial
effects on mitochondrial biogenesis and activity (41). Conversely, other authors have
shown that muscle mitochondrial biogenesis should be attributed
exclusively to exercise and that quercetin supplementation in the
diet had a negligible effect on mitochondria in mice fed with a
high-fat diet (42). Oral
resveratrol was found to have no effect on mitochondrial biogenesis
in skeletal muscle (43). In this
study, polydatin, a natural precursor of resveratrol, restored
mtDNA and TFAM in mitochondria by enhancing expression of SIRT1 in
human PASMCs exposed to H/R. Interestingly, we found that polydatin
decreased the release of TFAM through SIRT1 in PASMCs exposed to
H/R. Our data showed that poly datin had a protective effect on
mitochondrial biogenesis and function by enhancing SIRT1
expression.
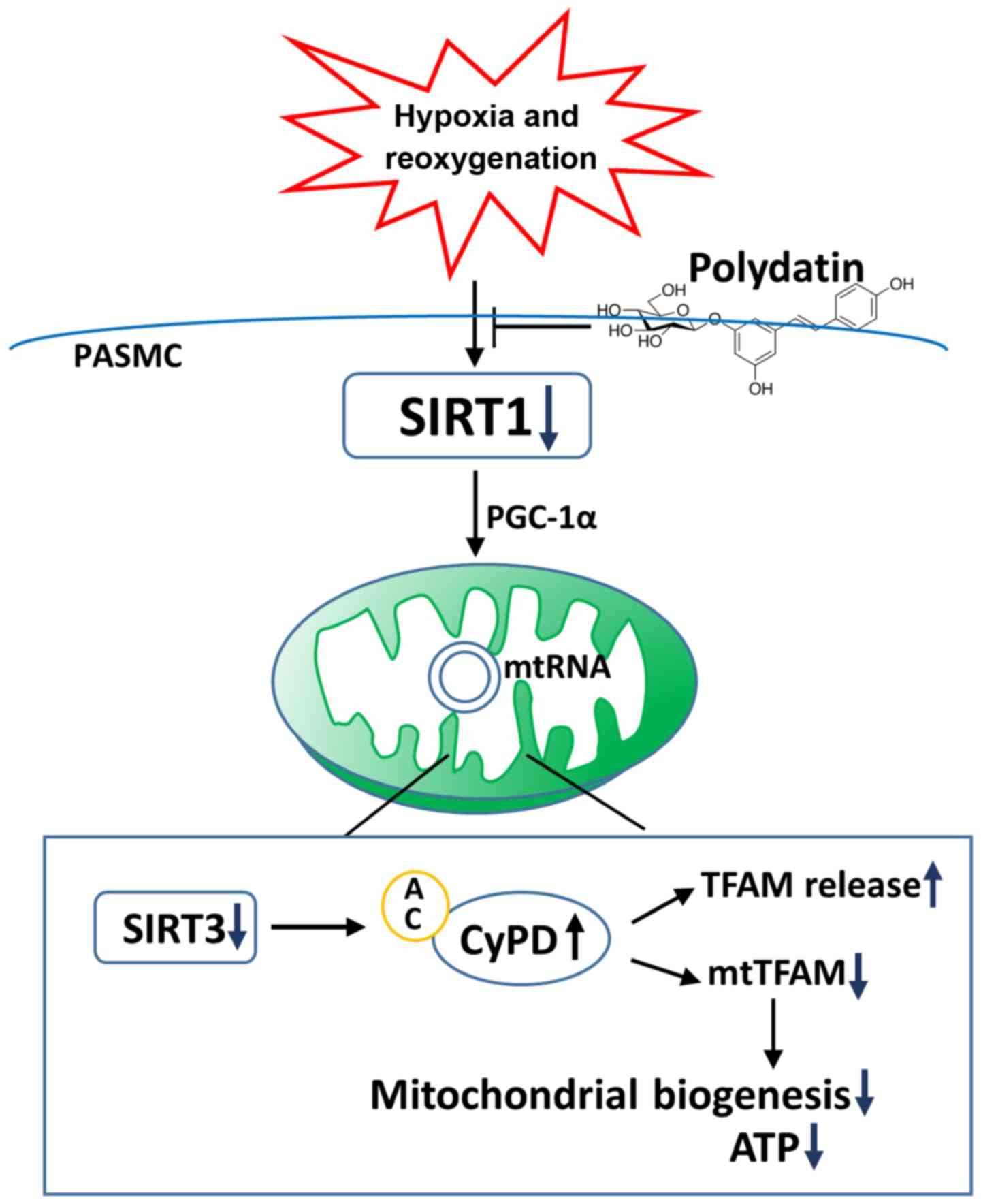
In conclusion, our results showed that the
expression and activity of SIRT1 are decreased in PASMCs exposed to
H/R (Fig. 7). PGC-1α/SIRT3/CyPD
mediates the effect of SIRT1 on TFAM expression in mitochondria and
the release of TFAM, and mitochondrial biogenesis and function
(Fig. 7). Polydatin protects
mitochondrial biogenesis and function by enhancing expression of
SIRT1 in human PASMCs (Fig. 7).
Furthermore, these data indicate the possibility that mitochondrial
biogenesis reprogramming may be manipulated as a strategy by which
to enhance tolerance against ischemic pulmonary injury.
Acknowledgments
This study was supported by the Natural Science
Foundation of Guangdong Province (no. 2015A030313297) and the
National Science Foundation of China (nos. 81200234 and
81600381).
References
|
1
|
Rabe KF, Hurd S, Anzueto A, Barnes PJ,
Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R,
van Weel C, et al Global Initiative for Chronic Obstructive Lung
Disease: Global strategy for the diagnosis, management, and
prevention of chronic obstructive pulmonary disease: GOLD executive
summary. Am J Respir Crit Care Med. 176:532–555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gibson PG and Simpson JL: The overlap
syndrome of asthma and COPD: What are its features and how
important is it? Thorax. 64:728–735. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Evans AM, Hardie DG, Peers C and Mahmoud
A: Hypoxic pulmonary vasoconstriction: Mechanisms of
oxygen-sensing. Curr Opin Anaesthesiol. 24:13–20. 2011. View Article : Google Scholar :
|
|
4
|
Freund-Michel V, Khoyrattee N, Savineau
JP, Muller B and Guibert C: Mitochondria: Roles in pulmonary
hypertension. Int J Biochem Cell Biol. 55:93–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Cell biology of ischemia/reperfusion injury. Int Rev
Cell Mol Biol. 298:229–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li P, Meng X, Bian H, Burns N, Zhao KS and
Song R: Activation of sirtuin 1/3 improves vascular hyporeactivity
in severe hemorrhagic shock by alleviation of mitochondrial damage.
Oncotarget. 6:36998–37011. 2015.PubMed/NCBI
|
|
7
|
Powell RD, Swet JH, Kennedy KL, Huynh TT,
McKillop IH and Evans SL: Resveratrol attenuates hypoxic injury in
a primary hepatocyte model of hemorrhagic shock and resuscitation.
J Trauma Acute Care Surg. 76:409–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jian B, Yang S, Chaudry IH and Raju R:
Resveratrol restores sirtuin 1 (SIRT1) activity and pyruvate
dehydrogenase kinase 1 (PDK1) expression after hemorrhagic injury
in a rat model. Mol Med. 20:10–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jian B, Yang S, Chaudry IH and Raju R:
Resveratrol improves cardiac contractility following
trauma-hemorrhage by modulating Sirt1. Mol Med. 18:209–214. 2012.
View Article : Google Scholar :
|
|
10
|
Waypa GB, Osborne SW, Marks JD,
Berkelhamer SK, Kondapalli J and Schumacker PT: Sirtuin 3
deficiency does not augment hypoxia-induced pulmonary hypertension.
Am J Respir Cell Mol Biol. 49:885–891. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paulin R, Dromparis P, Sutendra G, Gurtu
V, Zervopoulos S, Bowers L, Haromy A, Webster L, Provencher S,
Bonnet S, et al: Sirtuin 3 deficiency is associated with inhibited
mitochondrial function and pulmonary arterial hypertension in
rodents and humans. Cell Metab. 20:827–839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Menzies KJ and Hood DA: The role of SirT1
in muscle mitochondrial turnover. Mitochondrion. 12:5–13. 2012.
View Article : Google Scholar
|
|
13
|
Kong X, Wang R, Xue Y, Liu X, Zhang H,
Chen Y, Fang F and Chang Y: Sirtuin 3, a new target of PGC-1alpha,
plays an important role in the suppression of ROS and mitochondrial
biogenesis. PLoS One. 5:e117072010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baines CP, Kaiser RA, Purcell NH, Blair
NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA,
Dorn GW, et al: Loss of cyclophilin D reveals a critical role for
mitochondrial permeability transition in cell death. Nature.
434:658–662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marzetti E, Calvani R, Cesari M, Buford
TW, Lorenzi M, Behnke BJ and Leeuwenburgh C: Mitochondrial
dysfunction and sarcopenia of aging: From signaling pathways to
clinical trials. Int J Biochem Cell Biol. 45:2288–2301. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Civitarese AE, Carling S, Heilbronn LK,
Hulver MH, Ukropcova B, Deutsch WA, Smith SR and Ravussin E;
CALERIE Pennington Team: Calorie restriction increases muscle
mitochondrial biogenesis in healthy humans. PLoS Med. 4:e762007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chaung WW, Wu R, Ji Y, Dong W and Wang P:
Mitochondrial transcription factor A is a proinflammatory mediator
in hemorrhagic shock. Int J Mol Med. 30:199–203. 2012.PubMed/NCBI
|
|
18
|
Liu HB, Meng QH, Huang C, Wang JB and Liu
XW: Nephroprotective effects of polydatin against
ischemia/reperfusion injury: A role for the PI3K/Akt signal
pathway. Oxid Med Cell Longev. 2015:3621582015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li XH, Gong X, Zhang L, Jiang R, Li HZ, Wu
MJ and Wan JY: Protective effects of polydatin on septic lung
injury in mice via upregulation of HO-1. Mediators Inflamm.
2013:3540872013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng Z, Yang Y, Dai X, Xu S, Li T, Zhang
Q, Zhao KS and Chen Z: Polydatin ameliorates injury to the small
intestine induced by hemorrhagic shock via SIRT3
activation-mediated mitochondrial protection. Expert Opin Ther
Targets. 20:645–652. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Song R, Chen Y, Zhao M and Zhao
KS: Polydatin - a new mitochondria protector for acute severe
hemorrhagic shock treatment. Expert Opin Investig Drugs.
22:169–179. 2013. View Article : Google Scholar
|
|
22
|
Li P, Wang X, Zhao M, Song R and Zhao KS:
Polydatin protects hepatocytes against mitochondrial injury in
acute severe hemorrhagic shock via SIRT1-SOD2 pathway. Expert Opin
Ther Targets. 19:997–1010. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hafner AV, Dai J, Gomes AP, Xiao CY,
Palmeira CM, Rosenzweig A and Sinclair DA: Regulation of the mPTP
by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses
age-related cardiac hypertrophy. Aging (Albany NY). 2:914–923.
2010. View Article : Google Scholar
|
|
24
|
Becker RH, Sha S, Frick AD and Fountaine
RJ: The effect of smoking cessation and subsequent resumption on
absorption of inhaled insulin. Diabetes Care. 29:277–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rajendrasozhan S, Yang SR, Kinnula VL and
Rahman I: SIRT1, an antiinflammatory and antiaging protein, is
decreased in lungs of patients with chronic obstructive pulmonary
disease. Am J Respir Crit Care Med. 177:861–870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao H, Sundar IK, Huang Y, Gerloff J,
Sellix MT, Sime PJ and Rahman I: Disruption of sirtuin 1-mediated
control of circadian molecular clock and inflammation in chronic
obstructive pulmonary disease. Am J Respir Cell Mol Biol.
53:782–792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsu CP, Zhai P, Yamamoto T, Maejima Y,
Matsushima S, Hariharan N, Shao D, Takagi H, Oka S and Sadoshima J:
Silent information regulator 1 protects the heart from
ischemia/reperfusion. Circulation. 122:2170–2182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cattelan A, Ceolotto G, Bova S, Albiero M,
Kuppusamy M, De Martin S, Semplicini A, Fadini GP, de Kreutzenberg
SV and Avogaro A: NAD(+)-dependent SIRT1 deactivation has a key
role on ischemia-reperfusion-induced apoptosis. Vascul Pharmacol.
70:35–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uittenbogaard M and Chiaramello A:
Mitochondrial biogenesis: a therapeutic target for
neurodevelopmental disorders and neurodegenerative diseases. Curr
Pharm Des. 20:5574–5593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pisano A, Cerbelli B, Perli E, Pelullo M,
Bargelli V, Preziuso C, Mancini M, He L, Bates MG, Lucena JR, et
al: Impaired mitochondrial biogenesis is a common feature to
myocardial hypertrophy and end-stage ischemic heart failure.
Cardiovasc Pathol. 25:103–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee D, Kim KY, Noh YH, Chai S, Lindsey JD,
Ellisman MH, Weinreb RN and Ju WK: Brimonidine blocks glutamate
excitotoxicity-induced oxidative stress and preserves mitochondrial
transcription factor a in ischemic retinal injury. PLoS One.
7:e470982012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yin W, Signore AP, Iwai M, Cao G, Gao Y
and Chen J: Rapidly increased neuronal mitochondrial biogenesis
after hypoxic-ischemic brain injury. Stroke. 39:3057–3063. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Larsson NG, Wang J, Wilhelmsson H, Oldfors
A, Rustin P, Lewandoski M, Barsh GS and Clayton DA: Mitochondrial
transcription factor A is necessary for mtDNA maintenance and
embryogenesis in mice. Nat Genet. 18:231–236. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hokari M, Kuroda S, Kinugawa S, Ide T,
Tsutsui H and Iwasaki Y: Overexpression of mitochondrial
transcription factor A (TFAM) ameliorates delayed neuronal death
due to transient forebrain ischemia in mice. Neuropathology.
30:401–407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang H and Ward WF: PGC-1alpha: A key
regulator of energy metabolism. Adv Physiol Educ. 30:145–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lehman JJ, Barger PM, Kovacs A, Saffitz
JE, Medeiros DM and Kelly DP: Peroxisome proliferator-activated
receptor gamma coactivator-1 promotes cardiac mitochondrial
biogenesis. J Clin Invest. 106:847–856. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu Z, Puigserver P, Andersson U, Zhang C,
Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et
al: Mechanisms controlling mitochondrial biogenesis and respiration
through the thermogenic coactivator PGC-1. Cell. 98:115–124. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim SY, Shim MS, Kim KY, Weinreb RN,
Wheeler LA and Ju WK: Inhibition of cyclophilin D by cyclosporin A
promotes retinal ganglion cell survival by preventing mitochondrial
alteration in ischemic injury. Cell Death Dis. 5:e11052014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gomes AP, Price L, Ling AJ, Moslehi JJ,
Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard
BP, et al: Declining NAD(+) induces a pseudohypoxic state
disrupting nuclear-mitochondrial communication during aging. Cell.
155:1624–1638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Joo HY, Yun M, Jeong J, Park ER, Shin HJ,
Woo SR, Jung JK, Kim YM, Park JJ, Kim J, et al: SIRT1 deacetylates
and stabilizes hypoxia-inducible factor-1α (HIF-1α) via direct
interactions during hypoxia. Biochem Biophys Res Commun.
462:294–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lagouge M, Argmann C, Gerhart-Hines Z,
Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P,
Elliott P, et al: Resveratrol improves mitochondrial function and
protects against metabolic disease by activating SIRT1 and
PGC-1alpha. Cell. 127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kwon SM, Park HG, Jun JK and Lee WL:
Exercise, but not quercetin, ameliorates inflammation,
mitochondrial biogenesis, and lipid metabolism in skeletal muscle
after strenuous exercise by high-fat diet mice. J Exerc Nutrition
Biochem. 18:51–60. 2014. View Article : Google Scholar
|
|
43
|
Higashida K, Kim SH, Jung SR, Asaka M,
Holloszy JO and Han DH: Effects of resveratrol and SIRT1 on PGC-1α
activity and mitochondrial biogenesis: A reevaluation. PLoS Biol.
11:e10016032013. View Article : Google Scholar
|