Introduction
Fluorosis, a disease caused by excessive fluoride
intake through water and food, has been documented repeatedly in
many field investigations and community surveys in a number of
countries worldwide, such as Africa, China, Germany, India, United
Republic of Tanzania and the US (1). In China, fluorosis includes 3 types:
water-type fluorosis, coal-burning-type fluorosis and brick
tea-type fluorosis. Excessive exposure to fluoride can lead to
disruptions in bone metabolism and enamel development, causing
skeletal fluorosis and dental fluorosis, respectively. Dental
fluorosis is a less serious disease that causes a chalk-like
discoloration of teeth with unsightly mottled spots or lines on
tooth enamel. However, skeletal fluorosis is a crippling disease
that causes pain and damage to bones and joints (2). Moreover, there is no established
strategy to date for the treatment of patients with skeletal
fluorosis. Thus, it is crucial to to obtain a better understanding
of the mechanisms regulating the pathogenesis of fluorosis for the
prevention of the potential health hazards of fluoride.
The characteristic pathological change associated
with fluorosis is the acceleration of bone turnover, in which
osteoclasts (OCs) play a critical role (3). An OC is a type of bone cell that
disassembles and digests the composite of hydrated protein and
mineral by secreting acid and collagenase, a process known as bone
resorption. OCs play a critical role in the maintenance, repair and
remodeling of bones. Monocytes/macrophages are recognized as OC
precursors. Monocyte/macrophage precursors undergo 3 stages during
their differentiation into functionally mature OCs. First,
monocyte/macrophage precursors become pre-osteoclasts; second,
mononuclear pre-osteoclastss fuse together to become non-functional
multi-nucleated OCs expressing tartrate-resistant acid phosphatase
(TRAP) and lacking ruffled membranes (RM); finally, non-functional
multi-nucleated OCs are fully developed into functional OCs which
secrete H+ and enzymes (4–8).
This differentiation process is regulated by various factors.
Nuclear factor of activated T-cells 1 (NFATc1), a master regulator
of osteoclastogenesis, regulates a number of OC-specific genes,
such as TRAP, cathepsin K and ATPase H+ transporting V0
subunit D2 (ATP6v0d2), which are known to be involved in bone
resorption. It has been demonstrated that NFATc1 binds directly to
the promoter regions and induces the expression of fusion-mediating
molecules, such as ATP6v0d2, thereby regulating OC multi-nucleation
(9). In mature OCs, ATP6v0d2, as
a component of V-type H+-ATPase, mediates the
acidification of the resorption lacuna, which is a prerequisite for
the secretion of enzymes (10,11). The efficient acidification of the
resorption lacuna requires the neutralizing current through
chloride channel-7 (ClC-7). ClC-7 is a Cl−/H+
antiporter, that resides mainly in late endosomes, lysosomes and RM
of multi-nucleated OCs (12–14). The stability of ClC-7 depends on
its association with osteopetrosis-associated transmembrane protein
1 (Ostm1), and Ostm1 serves as a β-subunit to support the function
of ClC-7 (15,16). It has been demonstrated that the
loss of function of ClC-7 or of its association with Ostm1 in
humans and mice result in osteopetrosis (12). Thus, ClC-7 is crucial for OC bone
resorption. Our previous study reported that fluoride decreased OC
bone resorption via the inhibition of NFATc1 (45); however, to date, it has not been
determined whether ClC-7 in OCs is involved in the pathogenesis of
fluorosis.
The brick tea-type fluorosis caused by the
consumption of fluoride from drinking tea, particularly brick tea
compressed by green tea or black tea. It is a public health concern
in the Northwest China, where minorities drink brick tea as
drinking water. It is estimated that 31 million individuals in that
region are affected (17). In
addition, it is considered that the dental fluorosis detection rate
in children and the skeletal fluorosis detection rate in areas
affected by the brick tea-type fluorosis are higher than the rates
in areas affected by water type-fluorosis (18); however, a lower number of patients
with severe dental fluorosis and disabilities were found in areas
affected by the brick tea-type fluorosis. Thus, it is worthy to
explore whether some ingredients in brick tea can antagonize the
adverse effects of fluoride. It is proposed that oxidative stress
plays a critical role in the pathogenesis of fluorosis (19,20). Tea polyphenols (TPs), the most
bio-active ingredient in brick tea (21), have received considerable
attention due to ther antioxidant effects and they have been shown
to exert beneficial effects on health, including maintaining bone
mass (22,23). Nevertheless, the potential effect
of TPs on fluorosis and the underlying mechanisms have not yet been
elucidated. The role of OCs in skeletal fluorosis has attracted
increased attention (24,25,45). TPs have been demonstrated to be
potent bone-supporting agents. Therefore, the present study aimed
to investigate the potential protective effects of TPs against
fluorosis using a mouse model and to explore the underlying
mechanisms with particular focus on ClC-7.
Materials and methods
Animals and treatments
A total of 40 healthy, 3-week-old male C57BL/6 mice
(Vital River Laboratories Animals Technology Co., Ltd., Beijing,
China) were allowed to acclimatize to an environmentally controlled
room with a 12-h light/dark cycle in an animal care facility for 7
days before beginning the experiment. Subsequently, these mice were
randomly divided into 4 groups (n=10/group) by weight as follows:
the distilled water (control group), 100 mg/l fluoridated water (F
group), water containing 10 g/l TP (TP group) and water containing
100 mg/l fluoride and 10 g/l TP (F + TP group). The animals were
allowed to drink the experimental water for 15 weeks. Sodium
fluoride (guaranteed reagent) was purchased from Tianjin Guangfu
Fine Chemical Research Institute (Tianjin, China). TPs with a
purity >98% were purchased from Zhengzhou Chengda Chemical
Products Co., Ltd. (Zhengzhou, China), and the catechins account
for approximately 70% of the extractable solids. Distilled water
mixed with TPs and/or fluoride and was prepared fresh daily. Body
weights were monitored weekly throughout the treatment period. At
the end of the experiment, all mice were euthanized by ether
asphyxiation. The 4 legs were aseptically removed and dissected
free of adherent soft tissue without disarticulation at the joint.
All animal procedures were approved by the Animal Care and Use
Committee of Harbin Medical University, Harbin, China.
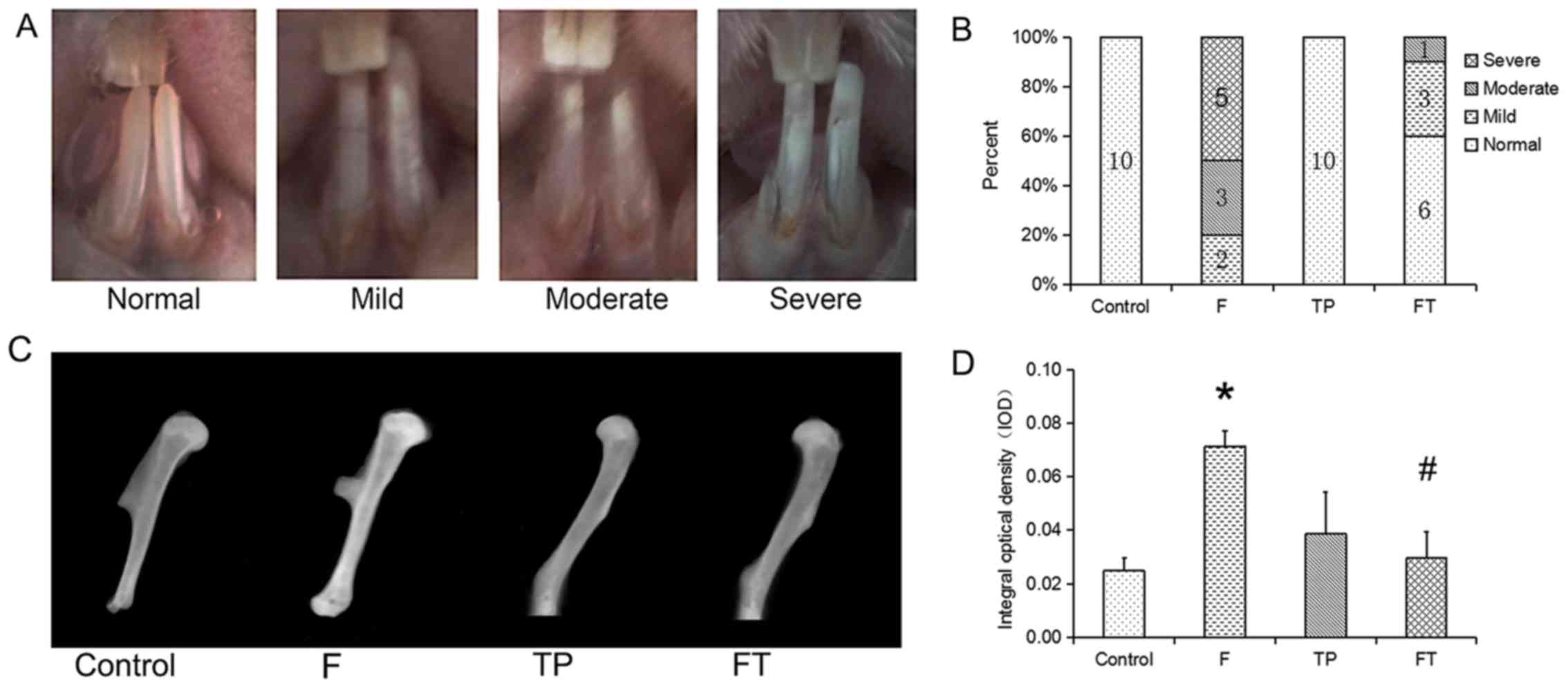
Dental fluorosis phenotyping
The dental fluorosis status of each mouse was
evaluated and scored by 2 independent examiners based on the
following modified Dean Index (26,27) (Fig.
1A).
Assessment of skeletal fluorosis
To measure bone mineral density, the humerus bones
of the mice were excised and cleaned of soft tissue, leaving the
growth plates intact. The humerus bones were then examined by X-ray
film using a Digital X-Ray Specimen System 4000 Pro (Kodak,
Rochester, NY, USA). The integral optical density (IOD) of each
X-ray film was analyzed using Image-Pro Plus software to reflect
bone mineral density, as previousy described (28).
Determination of bone fluoride
content
Femur bone samples were collected and cleaned by
removing the soft tissues, splitting open and removing all marrow
with cold PBS. The specimens were dried for 4 h at 105°C and were
then weighed. Prior to ashing, the bones were heated in a
smoke-free environment in a fume hood. The bones were then ashed
for 6 h at 550°C and weighed again, dissolved in 1N HCl, and
neutralized by the addition of 1N NaOH. A standard method with the
ion-specific F electrode (Shanghai Weiye Instrument Plant,
Shanghai, China) was used to determine the F−
concentration of each dissolvent in the Institute for Endemic
Fluorosis Control, Center for Endemic Disease Control, Chinese
Center for Disease Control and Prevention (Harbin Medical
University).
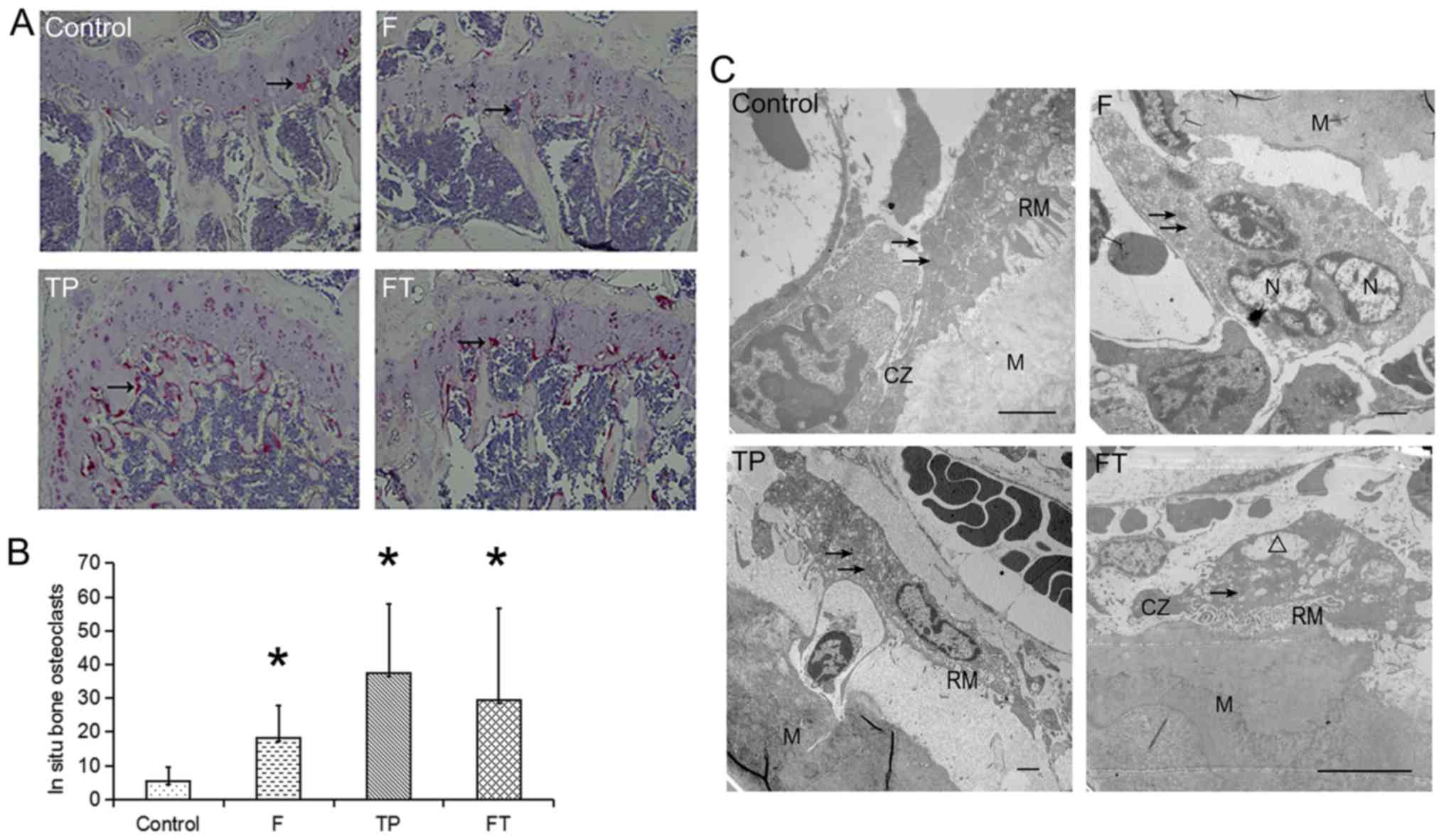
In situ bone OC numbers
The long bones were transected at a distance from
the knee joints to avoid damaging the joint. Following the removal
of the soft tissues, the joints were fixed in 10% formaldehyde for
7 days. The formalin-fixed tissues were then decalcified in 5% EDTA
for 1 month prior to paraffin-embedding. The paraffin-embedded
specimens were then sectioned (6 µm). The slides were
stained using the TRAP staining kit (Shanghai Sunbio Co., Shanghai,
China) and counterstained with hematoxylin. The number of
TRAP+ cells was counted across 4 fields of view just
under the growth plate of each bone section by light microscopy at
×20 magnification in an Olympus BX-51 microscope (Olympus Corp.,
Tokyo, Japan) and digital images were captured. Data were reported
as the mean number of TRAP+ cells within the 4 fields
per bone section as the formation of OCs.
Assessment of OC ultrastructure in the
transmission electron microscope
The metaphyses were fixed in 2.5% glutaraldehyde for
4 weeks at 4°C and the fixed tissues were decalcified in 10% EDTA
for 4 weeks. The specimens were post-fixed for 2 h in 2% aqueous
OsO4, dehydrated in acetone, and embedded in Epon
(Electron Microscopy Sciences, Hatfield, PA, USA). Ultrathin
sections were cut from blocks and then stained with uranyl acetate
and lead citrate. The specimens were observed under an H-7650
transmission electron microscope (Hitachi, Ltd., Tokyo, Japan), as
previously described (29,30).
The cell surface structures, facing matrix, were defined as RM, an
area with multiple folding of the cell membrane and clear zone
(CZ), attachment of the cell to the bone surface.
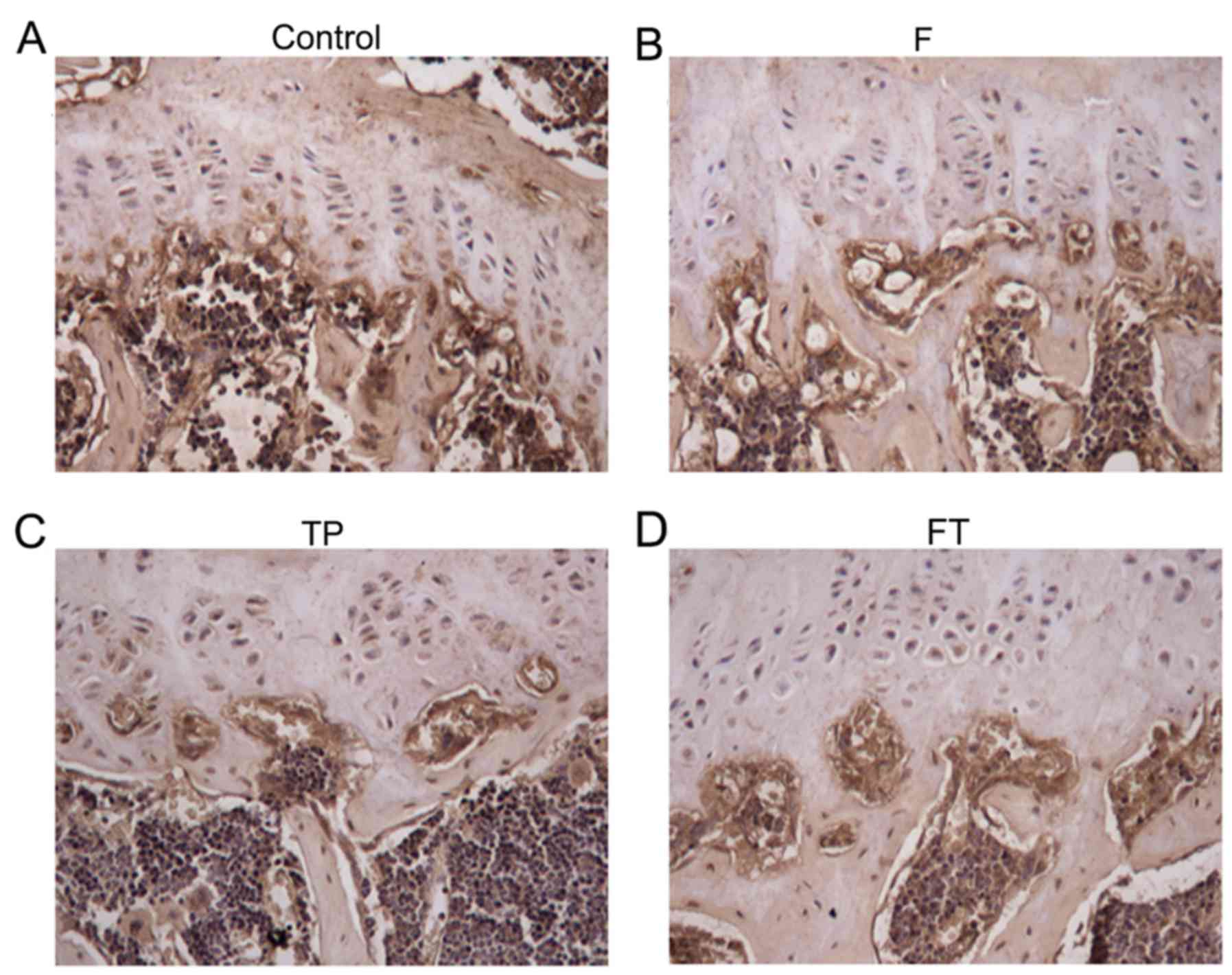
Immunohistochemistry
The sections were treated with 0.3%
H2O2 in ethanol to inactivate endogenous
peroxidase. The sections were digested with 0.2% Triton (Amresco,
LLC, Solon, OH, USA), blocked with 5% BSA (SABC kit, Wuhan Boster
Biological Technology, Ltd., Wuhan, China), incubated for 2 h with
a 1:100 dilution of anti-rabbit ClC-7 antibody (ab86196; Abcam,
Cambridge, UK) at 37°C, treated with a biotinylated anti-rat
antibody, and DAB chromogenic liquid (SABC kit). The sections were
counterstained with hematoxylin.
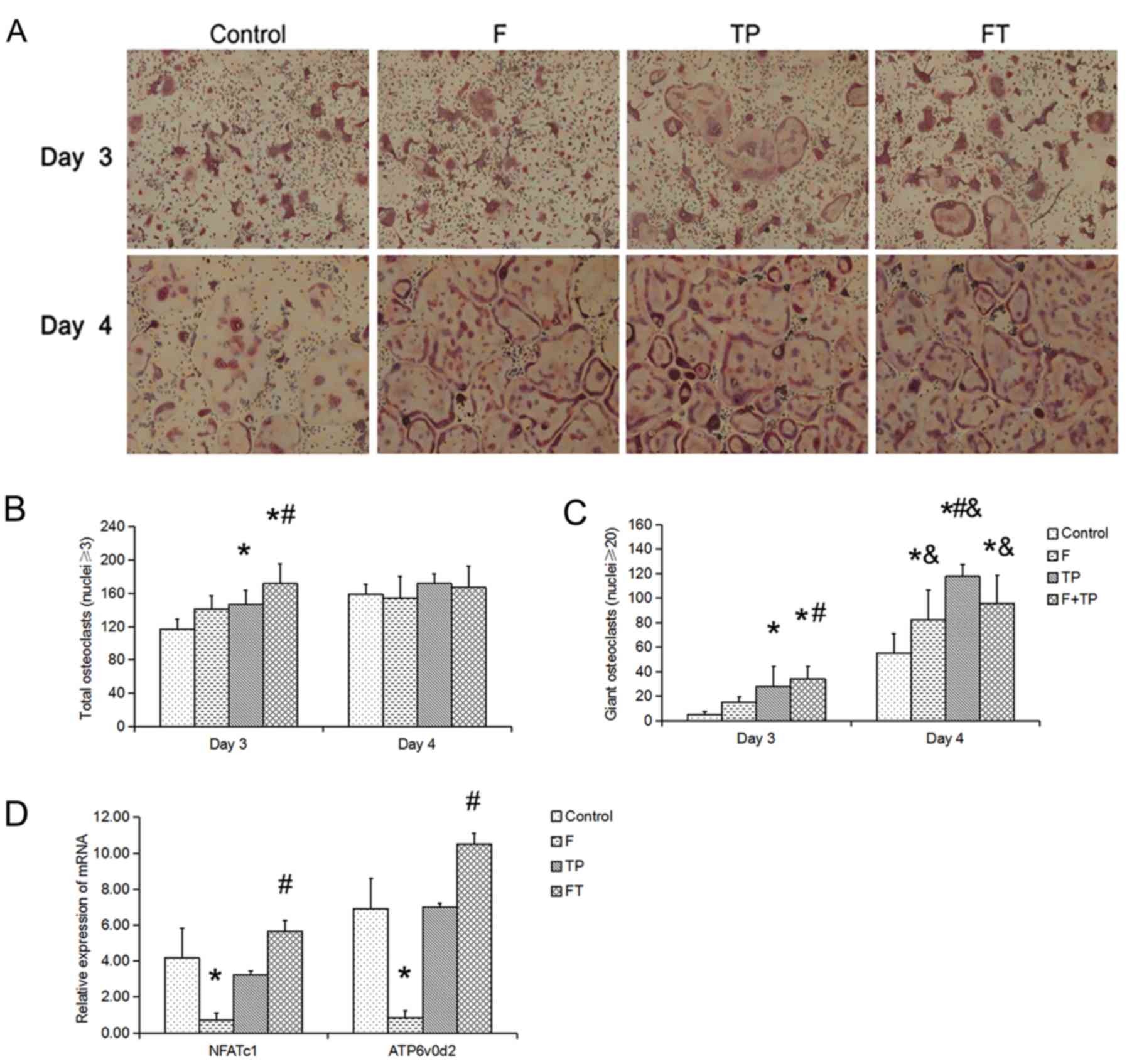
Osteoclastogenesis
Bone marrow-derived macrophages (BMMs) were prepared
as described previously (4,31)
and osteoclastogenesis was induced by receptor activator of NF-κB
ligand (RANKL) and macrophage colony-stimulating factor (M-CSF).
The femora and tibiae were aseptically removed and dissected free
of adherent soft tissue. The bone ends were removed and the marrow
cavity was flushed out into a Petri dish by slowly injecting α-MEM
at one end of the bone using a sterile 5 ml syringe. The bone
marrow suspension was passed repeatedly through a glass pipette to
obtain a single cell suspension. The bone marrow cells were then
resuspended in α-MEM, incubated for 24 h, and plated in 6-well
plates with thye addition of 5 ng/ml M-CSF (Peprotech, Inc., Rocky
Hill, NJ, USA) to deplete the stromal cells of cell preparations.
Non-adherent cells were replated in 12-well plates at a density of
2×106 cells/well for extracting DNA and protein or in
96-well plates at a density of 1×105 cells/well in α-MEM
for OC identification, containing 30 ng/ml M-CSF for 3 days to
obtain the BMMs. Each group included 5 parallel wells. The BMMs
were incubated with 30 ng/ml M-CSF and 100 ng/ml RANKL (Peprotech,
Inc.) for another 4 days which were named day 1 to 4 accordingly.
The medium was replaced every 2 days.
OCs were identified and stained using the TRAP
staining kit (Shanghai Sunbio Co.) on days 3 and 4.
TRAP+ multinucleated cells were counted as total OCs (≥3
nuclei) or giant OCs (≥20 nuclei). Data are presented as the means
± SD.
Determination of NFATc1 and ATP6v0d2 mRNA
expression in OCs in vitro
Gene expression in the cells was assessed by
quantitative PCR (qPCR). The induced cells on day 4 were dissolved
in TRIzol reagent and total RNA was extracted using the total
isolation kit (Takara Bio, Dalian, China). First-strand cDNA was
synthesized using 1 µg total RNA with the PrimeScript RT
reagent kit with gDNA Eraser according to the manufacturer's
instructions (Takara Bio). qPCR was performed on the Real-Time
Fluorescence PCR Instrument (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) using SYBR-Green chemistry. The following commercial
available primer were used for PCR: GAPDH (forward,
5′-CTGACGTGCCGCCTGGAGAAAC-3′, and reverse,
5′-CCCGGCATCGAAGGTGGAAGAGT-3′), NFATc1 (forward,
5′-CTGCAACGGGAAACGGAAGAGAAG-3′, and reverse,
5′-TATACACCCCCAGACCGCATAGC-3′), ATP6v0d2 (forward,
5′-ACAGACGCGCTTTAATCATCACTC-3′, and reverse,
5′-ATCCCCCTTCCTTTCCTCAATAAC-3′) and Ostm1 (forward,
5′-GGGGAGAAACAAGGACAAACACAA-3′; reverse,
5′-ATACCCAAACACTCCTGCCTTCCT-3′). The relative mRNA expression
levels were calculated based on threshold cycle number (Ct) and
normalized to GAPDH using the comparative Ct method.
Western blot analysis
The cells were lysed in RIPA buffer (Beyotime
Institute of Biotechnology, Shanghai, China) and protein
concentrations of cellular extract were measured using an enhanced
BCA Protein Assay kit (Beyotime Institute of Biotechnology). An
automated capillary-based simple western system (Simon;
ProteinSimple, Santa Clara, CA, USA) was then used to quantify the
protein levels of ClC-7. Compared to the traditional western blot
analysis, automation allows for the more accurate and reproducible
assessment of protein levels, as previously demonstrated (32). In brief, 16 ng of protein samples
were used for analysis. The proteins were immunoprobed using
anti-ClC-7 antibody (ab86196; Abcam) or anti-GAPDH antibody (G9545;
Sigma, St. Louis, MO, USA). Other reagents were using Simon-Rabbit
(15–150 kDa) Master kit provided by ProteinSimple (San Jose, CA,
USA). For quantitative analysis, the ClC-7 signals were normalized
against GAPDH.
Chloride permeation
Cl− permeation was measured using the
fluorescent Cl− indicator,
N-ethoxycarbonylmethyl-6-methoxy quinolinium bromide (MQAE),
as previously described (33).
The mechanism of detection is diffusion-limited collisional
quenching of MQAE fluorescence. The degree of fluorescence
quenching in MQAE indicates an increase in the Cl−
concentration. OCs were loaded with 6 mmol·l−1 MQAE in a
chloride-free buffer containing 140 mmol/l NaNO3, 5
mmol/l KNO3, 5 mmol/l
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1
mmol/l Mg(NO3)2 and 5 mmol/l glucose for 60
min at 37°C. Just before detection, the culture buffer was changed
to a chloride buffer of similar composition with Cl− as
the substituting anion. At the very moment, OCs were exposed to
excitation light for 20 times at an interval of 5 sec on the stage
of an inverted microscope (Nikon Diaphot; Nikon, Tokyo, Japan) and
the digitized image was recorded. The original fluorescence
intensity was measured by changing from a chloride buffer to
chloride-free buffer at time zero. The mean relative intensity was
calculated by dividing the fluorescence intensity by the original
fluorescence, as previously described (34).
Statistical analysis
All experiments were repeated 5 times, and
representative results are presented. Data are presented as the
means ± SD and analyzed using SPSS 16.0 software. A value of
P<0.05 was considered to indicate a statistically significant
difference. The normality of distribution and homogeneity of
variance were tested. Dental fluorosis among the 4 groups was
analyzed using the Chi-square test. The IOD of the humerus bone,
TRAP+ cells in vivo and in vitro, the
relative mRNA expression of 4 genes, and ClC-7 protein levels in
vitro were analyzed by two-way ANOVA followed by Fisher's LSD
post hoc test to evaluate the effects of fluoride, TPs or their
combination. Linear by linear association was used to evaluate the
association between the fluorescence intensity and detection
time.
Results
Body weight and bone fluoride
Neither fluoride nor TP significantly affected the
body weights of the mice throughout the study period (data not
shown). The fluoride content in long bone was detected to reflect
the difference of fluoride accumulation among the groups. The data
indicated that the bone fluoride content in the F group
(4098.64±58.84 mg/kg) and F + TP group (3855.70±140.77 mg/kg) was
significantly higher than that in the control group (876.26±14.46
mg/kg) (P<0.001 and P=0.002, respectively); however, there was
no difference between the F group and F + TP group. The fluoride
content in the TP group (970.27±17.05 mg/kg) was similar to that of
the control group (876.26±14.46 mg/kg) (data not shown).
Dental fluorosis
Compared with the mice receiving distilled water or
TPs, the mice receiving fluoride (F and F + TP groups) had a higher
prevalence of dental fluorosis. From the 6th week, the teeth of the
mice in the F group were characterized by a change in color and
thin white horizontal lines. At the 15th week, half of the mice in
the F group had severely fluorosed enamel with discrete or
confluent pitting. It is worth noting that the prevalence of dental
fluorosis in the F + TP group was lower than that in the F group
(P<0.05; Fig. 1B).
Skeletal fluorosis
A radiographic examination of the excised humerus
bones confirmed the status of long bone growth (Fig. 1C). Marrow space, growth plate and
cortial margins were apparent in the control specimens. However,
the specimens in the F group exhibited a thickened cortial margin
and a brighter performance. However, there was no apparent change
in intensity in the TP group and F + TP group by the naked eye. The
IOD of the humerus (Fig. 1D) in
the F group was significantly higher than that in the control group
(P=0.024). Of note, the IOD of the humerus in the F + TP group was
significantly lower than that in the F group P=0.033); there was
thus an interaction between fluoride and TPs (P=0.015).
In situ bone OC numbers
TRAP+ cells were detected as a measure of
OC formation in situ and the majority of these cells
appeared in the zone of cartilage calcification (Fig. 2A). The number of OCs was
consistently elevated in all the treatment groups (Fig. 2B). Specifically, compared with the
control group, the number of OCs increased 7-fold in the TP group,
5-fold in the F + TP group and 3-fold in the F group. Furthermore,
the OCs in the TP group were more intense than those in the other
groups by the naked eye.
OC ultrastructure
Ultrastructural analyses of the proximal metaphyses
of femurs were performed using a transmission electron microscope
(Fig. 2C). In the sections from
the control group and TP group, the OCs with well-developed. RM and
CZ were frequently attached to the bone and these features were
considered to reflect capacity of bone resorption. Many vesicles
containing electron-dense material (arrows) were also observed in
the OCs. By contrast, a large proportion of OCs in the F group
lacked the morphological signs of proper polarization, e.g., RM and
CZ. Instead, the OCs exhibited a matrix-facing area with no
characteristic cell surface structures, which can be considered as
an immature developed cell. Moreover, the OCs in F + TP group
exhibited vacuolation degeneration.
Immunohistochemistry of ClC-7 protein in
long bone
Immunohistochemical staining of ClC-7 was performed
on proximal metaphyses of femurs (Fig. 3). In the control group, ntense
immunostaining was observed in the connecting zone of cartilage
calcification and ossification. The location of ClC-7 protein was
similar among the 4 experimental groups.
OC potential
To examine the effects of fluoride and/or TPs on OC
formation, TRAP+ multi-nucleated OCs were observed in
all groups (Fig. 4). The
morphology of the OCs was similar among the 4 groups (Fig. 4A). On day 3, the number of OCs
(Fig. 4B) in the TP and F + TP
groups was significantly higher than the control group (P=0.031 and
P<0.001, respectively) and the number in the F + TP group was
significantly higher than that in the F group (P=0.023). On day 3,
the number of giant OCs (Fig. 4C)
in the TP and F + TP groups was significantly higher than the
control group (P=0.014 and P=0.002, respectively) and the number in
the F + TP group was also significantly higher than that in the F
group (P=0.030). These differences were evident until day 4. On day
4, the number of giant OCs in the 4 groups increased, compared with
that at day 3. Of note, the number of giant OCs in the control
group was significantly lower than the other 3 groups.
mRNA expression of NFATc1, ATP6v0d2 and
Ostm1 in OCs in vitro
We examined the effect fluoride and/or TP
administration on the mRNA expression of NFATc1 and ATP6v0d2
(Fig. 4D). The expression of
NFATc1 and ATP6v0d2 in the F group was significantly lower than
that in the control group (P=0.043 and P=0.039, respectively). The
mRNA of both genes in the F + TP group was significantly higher
than that in the F group (NFATc1, P=0.009; ATP6v0d2, P= 0.002).
mRNA expression of Ostm1 in OCs in
vitro
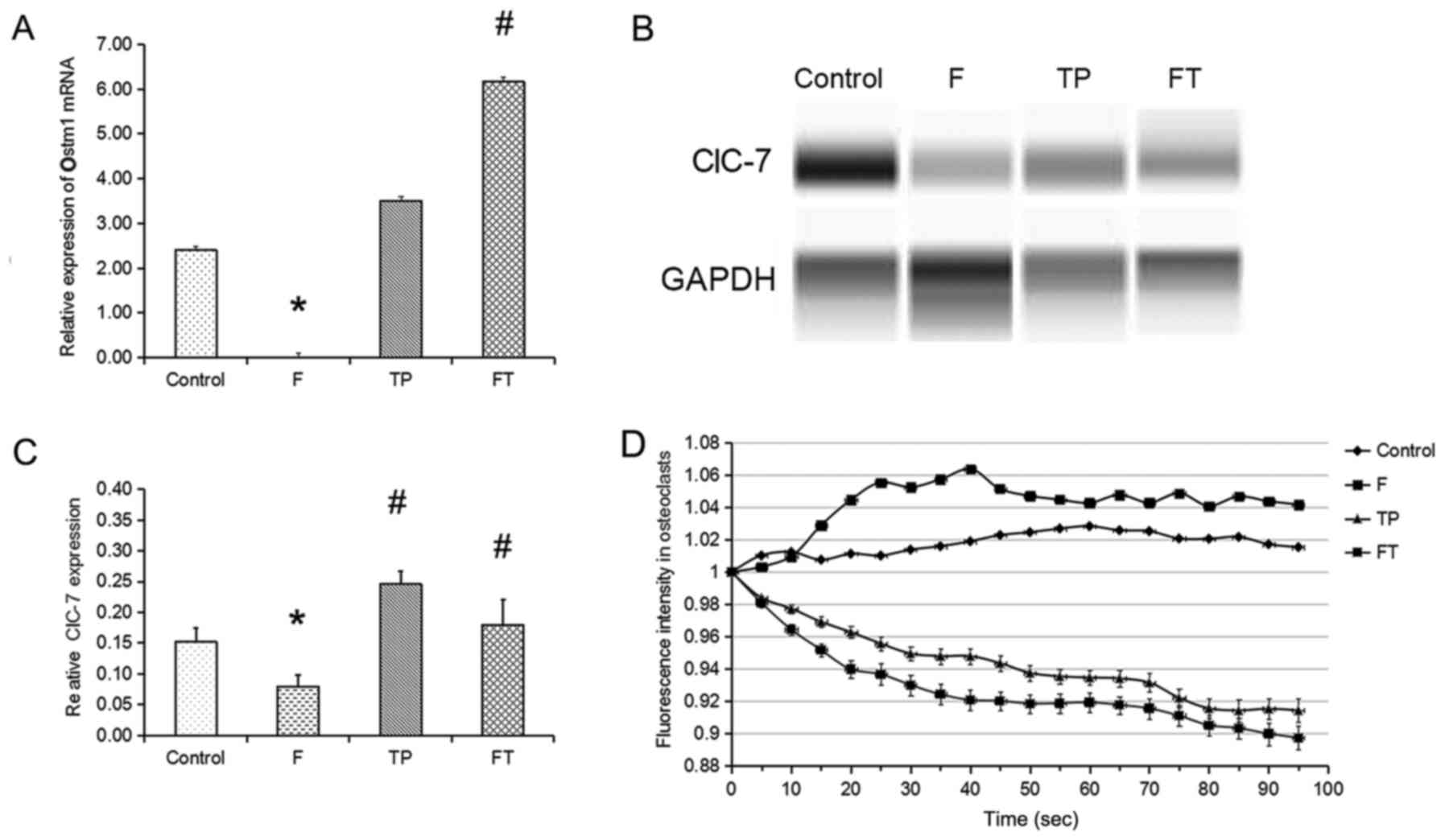
Ostm1 mRNA expression in the F group decreased
almost 600-fold compared to the control group (Fig. 5A). The mRNA expression of Ostm1 in
the F + TP group was significantly higher than that in the F group
(Ostm1, P= 0.033).
ClC-7 expression in OCs in vitro
Giant OCs were regarded as having absorbing
capacity, and thereafter the protein level of ClC-7 on day 4 was
detected (Fig. 5B and C). The
protein level of ClC-7 in the F group (0.08±0.02) was significantly
lower than that in the control group (0.15±0.02, P=0.006). ClC-7
protein expression levels in the TP group (0.24±0.02) and F + TP
group (0.16±0.05) were both significantly higher than the level in
the F group (P=0.012 and P= 0.03, respectively). There was thus an
interaction between fluoride and TPs (P=0.029).
Fluorescence intensity in OCs in
vitro
The ability of ClC in OCs to transport
Cl− in all groups was detected on day 4 (Fig. 5D). Within 90 sec, the fluorescence
intensity was continuously declining in the TP and F + TP groups
(P<0.001 and P=0.024, respectively), suggesting that
Cl− could be accumulated in OCs in these groups;
however, the fluorescence intensity in the F group exhibited an
increasing trend (P<0.001), suggesting that OCs in the F group
did not retain Cl−.
Discussion
The skeletal fluorosis phenotype of the murine model
and human disease is characterized by a combination of
osteosclerosis, osteomalacia and osteoporosis in varying degrees.
The low bone mass in C57BL/6 mice appears to be sensitive to the
effects of fluoride on bone cells (35–39). Therefore, in the present study,
C57BL/6 mice were utilized to investigate the potential protective
effects of TPs against chronic fluorosis. In the F group, the mice
had a higher prevalence of dental fluorosis and severe fluorosed
enamel with discrete or confluent pitting. A radiographic
examination of excised humerus bones revealed a thickened cortial
margin and significantly higher IOD values. Such results were in
agreement with those of another study using female C57BL/6 mice
(35), indicating that fluorosis
model was successfully constructed. The bone fluoride content
remained consistent between the F group and F + TP group,
suggesting that TP supplementation did not affect the accumulation
of fluoride in the bone. However, compared to the F group, the F +
TP group exhibited a significant mitigation of fluorosis, as shown
by the prevalence of dental fluorosis and IOD value of the humerus,
suggesting that TPs exerted a beneficial effect on bone
homeostasis, protecting against the toxic effects of fluoride.
In our mouse model, the consumption of 100 mg/l
fluoride stimulated bone formation. Bone remodeling is a complex,
yet coordinated process that is responsible by two main types of
cells, osteoblasts and OCs. Fluoride, as an anabolic agent, is
capable of promoting osteoblast proliferation, whereas the
influence of fluoride on OCs remains controversial. In our study,
100 mg/l fluoride increased the number of TRAP+ cells in
the region below the growth plate, suggesting that fluoride
stimulated the formation of OCs, which is consistent with a
previous report using theC3H/HeJ (C3H) inbred mouse strain with 50
mg/l fluoride for 3 weeks (37).
However, ultrastructural analysis revealed that the OCs in the F
group exhibited an underveloped RM and CZ, and failed to attach to
the bone matrix, which is consistent with previous animal and cell
studies (40,41). It is known that OCs resorb bone by
establishing a circle between themselves and the bone surface, and
that the formation and development of specific membrane structures,
such as RM and CZ, are important for bone resorption (42). Moreover, patients with defective
acidification have increased numbers of OCs with decreased bone
resorption (43,44). Therefore, our results suggested
that fluoride increased the number of OCs; however, these OCs
exhibited defective acidification and could not resorb bone matrix.
Compared to the the F group, mice in F + TP group had a greater
number of TRAP+ cells in the region below the growth
plate, and most OCs exhibited a well-developed RM and CZ,
suggesting that TP alleviated defective acidification induced by
fluoride. OCs are terminally differentiated multi-nucleated cells
derived from mononuclear hematopoietic cells of the
monocyte/macrophage lineage in bone marrow. RANKL and M-CSF are
essential and sufficient for osteoclastogenesis from BMMs. Thus,
primary BMMs isolated from mouse long bones are utilized as OC
precursors to generate mature OCs ex vivo. In this study, we
isolated primary BMMs from differently treated mice to observe the
effects of fluoride and/or TPs on OC formation and for ex
vivo osteoclastogenesis assays. We noted that fluoride had
little effect on osteoclatogenesis; this result was similar to that
of our previous study (45); the
number of OCs (>3 nuclei) or the number of giant OCs (>20
nuclei) in mice in the F + TP group was greater than that in the F
group on day 3, suggesting that TPs promoted osteoclastogenesis in
association with exposure to high levels of fluoride.
Altough the molecular mechanisms by which fluoride
regulates bone resorption have not yet been illustrated, a previous
in vitro study by our group indicated that fluoride
inhibited the resorption activity of OCs by inhibiting NFATc1 and
its downstream genes, such as Atp6v0d2 (45). NFATc1 plays an important role in
osteoclastogenesis by regulating the fusion of pre-osteoclastss and
bone resorption (8,46,47). Atp6v0d2 is an essential component
of the OC-specific proton pump that mediates extracellular
acidification in bone resorption (6). In our mouse model, we further
examined the effect of fluoride and/or TPs on the expression of
NFATc1 and Atp6v0d2 in new OCs at the mRNA level. Our results
revealed that the expression of NFATc1 and ATP6v0d2 in the F group
was significantly lower than that in the control group, which is
consistent with our previous study, whereas the mRNA expression of
both genes in the F + TP group was significantly higher than that
in the F group, suggesting that TP attenuated the inhibitory effect
on these two genes induced by fluoride.
ClC-7, as one member of the ClC family, may support
bone resorption by electrically shunting the H+-ATPase
that acidifies the resorption lacuna of OCs, or by facilitating the
insertion of proton-pump containing vesicles into the RM. It is
known to be associated with osteopetrosis, that is characterized by
OC dysfunction, impaired bone resorption and poor bone remodeling
(48). In osteopetrosis, OCs are
developed without an RM and fail to generate an acidic bone
resorption compartment or resorb bone. In our model, OCs in the F
group exhibited an underveloped RM and CZ, and failed to attach to
the bone matrix; thus, we investigated the effect of fluoride
and/or TPs on ClC-7. In the present study, the ClC-7 level in the F
group was significantly lower than that in the control group;
however, ClC-7 expression in the F + TP group was significantly
higher than that in the F group, suggesting that TPs attenuated the
inhibitory effect on ClC-7 protein induced by fluoride.
ClC-7, as a Cl−/H+ antiporter,
is important for lysosomal acidification. It is the primary
Cl− permeation pathway in lysosomes (15). Thus, we further detected
Cl− permeation using fluorescent the Cl−
indicator, MQAE (33). The degree
of fluorescence quenching in MQAE indicates an increase in
Cl− in cells. In the F group, new OCs did not retain
Cl−; however, new OCs in the F + TP group retained
Cl−. These results were consistent with the change in
the protein expression of ClC-7. ClC-7 can reach the lysosome and
can be degraded in the lysosome due to the lack of N-linked
glycosylation sites. Ostm1, a type I membrane protein with heavily
glycosylated amino-terminal portion and a short cytoplasmic tail,
shields ClC-7 from degradation by lysosomal proteases (15,16,49). Thus, we investigated the influence
of fluoride and/or TPs on Ostm1 expression at the mRNA level. Our
results indicated that in new OCs in the F group, Ostm1 mRNA
expression was significantly lower than that in the control group;
however, Ostm1 mRNA expression in the FT group was significantly
higher than that in the F group, suggesting that fluoride decreased
ClC-7 expression by inhibiting Ostm1; TPs, however, attenuated this
effect. This may be one of the reasons as to why the severity of
fluorosis in areas affected by brick tea-type fluorosis is milder
than that in areas affected by water-type fluorosis. Thus, perhaps
the use of TPs could be considered for the prevention of fluorosis
in areas affected by water-type fluorosis.
In conclusion, in the present study, we demonstrated
that TPs exerted beneficial effects against fluorosis by regulating
OC resorption ability. We reported that fluoride inhibited OC
resorption by decreased ClC-7 and Ostm1 expression, whereas TPs
attenuated these inhibitory effects of fluoride.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81072252).
References
|
1
|
WHO: http://www.who.int/water_sanitation_health/publications/fluoride-in-drinking-water/en/.
Accessed Nov 10, 2011.
|
|
2
|
Wang C, Gao Y, Wang W, Zhao L, Zhang W,
Han H, Shi Y, Yu G and Sun D: A national cross-sectional study on
effects of fluoride-safe water supply on the prevalence of
fluorosis in China. BMJ Open. 2:e0015642012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun D and Gao Y: Molecular mechanism of
pathogenesis of osteofluorosis: a discussion in the view of bone
turnover. Chin J Endemiol. 27:239–241. 2008.
|
|
4
|
Suda T, Takahashi N, Udagawa N, Jimi E,
Gillespie MT and Martin TJ: Modulation of osteoclast
differentiation and function by the new members of the tumor
necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Väänänen HK, Zhao H, Mulari M and Halleen
JM: The cell biology of osteoclast function. J Cell Sci.
113:377–381. 2000.PubMed/NCBI
|
|
7
|
Väänänen HK, Karhukorpi EK, Sundquist K,
Wallmark B, Roininen I, Hentunen T, Tuukkanen J and Lakkakorpi P:
Evidence for the presence of a proton pump of the vacuolar
H(+)-ATPase type in the ruffled borders of osteoclasts. J Cell
Biol. 111:1305–1311. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim K, Lee SH, Ha Kim J, Choi Y and Kim N:
NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and
the dendritic cell-specific transmembrane protein (DC-STAMP). Mol
Endocrinol. 22:176–185. 2008. View Article : Google Scholar
|
|
10
|
Baron R, Neff L, Louvard D and Courtoy PJ:
Cell-mediated extracellular acidification and bone resorption:
Evidence for a low pH in resorbing lacunae and localization of a
100-kD lysosomal membrane protein at the osteoclast ruffled border.
J Cell Biol. 101:2210–2222. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blair HC, Teitelbaum SL, Ghiselli R and
Gluck S: Osteoclastic bone resorption by a polarized vacuolar
proton pump. Science. 245:855–857. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kornak U, Kasper D, Bösl MR, Kaiser E,
Schweizer M, Schulz A, Friedrich W, Delling G and Jentsch TJ: Loss
of the ClC-7 chloride channel leads to osteopetrosis in mice and
man. Cell. 104:205–215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jentsch TJ, Stein V, Weinreich F and
Zdebik AA: Molecular structure and physiological function of
chloride channels. Physiol Rev. 82:503–568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Q, Wei Q, He A, Jia R and Xiao Y:
CLC-7: A potential therapeutic target for the treatment of
osteoporosis and neurodegeneration. Biochem Biophys Res Commun.
384:277–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leisle L, Ludwig CF, Wagner FA, Jentsch TJ
and Stauber T: ClC-7 is a slowly voltage-gated
2Cl(−)/1H(+)-exchanger and requires Ostm1 for transport activity.
EMBO J. 30:2140–2152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lange PF, Wartosch L, Jentsch TJ and
Fuhrmann JC: ClC-7 requires Ostm1 as a beta-subunit to support bone
resorption and lysosomal function. Nature. 440:220–223. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun D, Gao Y, Zhao L, Yu G, Wu L and Li Q:
A cross-sectional survey on drinking brick-tea type fluorosis in
China. Chin J Endemiol. 27:513–517. 2008.
|
|
18
|
Sun D, Gao Y, Yu G, Liu Y, Zhao X, Wu L,
Li Q and Sun Y: Prevalence characteristics of drink-tea type
fluorosis. Chin J Endemiol. 27:121–123. 2008.
|
|
19
|
Varol E, Icli A, Aksoy F, Bas HA, Sutcu R,
Ersoy IH, Varol S and Ozaydin M: Evaluation of total oxidative
status and total antioxidant capacity in patients with endemic
fluorosis. Toxicol Ind Health. 29:175–180. 2013. View Article : Google Scholar
|
|
20
|
Wang Q, Cui KP, Xu YY, Gao YL, Zhao J, Li
DS, Li XL and Huang HJ: Coal-burning endemic fluorosis is
associated with reduced activity in antioxidative enzymes and
Cu/Zn-SOD gene expression. Environ Geochem Health. 36:107–115.
2014. View Article : Google Scholar
|
|
21
|
Cabrera C, Artacho R and Giménez R:
Beneficial effects of green tea - a review. J Am Coll Nutr.
25:79–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen CL, Yeh JK, Cao JJ and Wang JS: Green
tea and bone metabolism. Nutr Res. 29:437–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen CL, Yeh JK, Stoecker BJ, Chyu MC and
Wang JS: Green tea polyphenols mitigate deterioration of bone
microarchitecture in middle-aged female rats. Bone. 44:684–690.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Junrui P, Bingyun L, Yanhui G, Xu J, Darko
GM and Dianjun S: Relationship between fluoride exposure and
osteoclast markers during RANKL-induced osteoclast differentiation.
Environ Toxicol Pharmacol. 46:241–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu XL, Song J, Liu KJ, Wang WP, Xu C,
Zhang YZ and Liu Y: Role of inhibition of osteogenesis function by
Sema4D/Plexin-B1 signaling pathway in skeletal fluorosis in vitro.
J Huazhong Univ Sci Technolog Med Sci. 35:712–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dean HT: Fluorine in the control of dental
caries. J Am Dent Assoc. 52:1–8. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dean HT: Endemic fluorosis and its
relation to dental caries. 1938. Public Health Rep. 121(Suppl 1):
213–219; discussion 212. 2006.PubMed/NCBI
|
|
28
|
Drozdzowska B, Pluskiewicz W and Tarnawska
B: Panoramic-based mandibular indices in relation to mandibular
bone mineral density and skeletal status assessed by dual energy
X-ray absorptiometry and quantitative ultrasound. Dentomaxillofac
Radiol. 31:361–367. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nordahl J, Andersson G and Reinholt FP:
Chondroclasts and osteoclasts in bones of young rats: Comparison of
ultrastructural and functional features. Calcif Tissue Int.
63:401–408. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nordahl J, Hollberg K, Mengarelli-Widholm
S, Andersson G and Reinholt FP: Morphological and functional
features of clasts in low phosphate, vitamin D-deficiency rickets.
Calcif Tissue Int. 67:400–407. 2000. View Article : Google Scholar
|
|
31
|
Chambers TJ: Regulation of the
differentiation and function of osteoclasts. J Pathol. 192:4–13.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen JQ, Heldman MR, Herrmann MA, Kedei N,
Woo W, Blumberg PM and Goldsmith PK: Absolute quantitation of
endogenous proteins with precision and accuracy using a capillary
western system. Anal Biochem. 442:97–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dragomir A and Roomans GM: Increased
chloride efflux in colchicine-resistant airway epithelial cell
lines. Biochem Pharmacol. 68:253–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen S, Wan XL and Sears M: pICln can
regulate swelling-induced Cl− currents in either layer of rabbit
ciliary epithelium. Biochem Biophys Res Commun. 246:59–63. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan D, Gurumurthy A, Wright M, Pfeiler TW,
Loboa EG and Everett ET: Genetic background influences fluoride's
effects on osteoclastogenesis. Bone. 41:1036–1044. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Linkhart TA, Linkhart SG, Kodama Y, Farley
JR, Dimai HP, Wright KR, Wergedal JE, Sheng M, Beamer WG, Donahue
LR, et al: Osteoclast formation in bone marrow cultures from two
inbred strains of mice with different bone densities. J Bone Miner
Res. 14:39–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Judex S, Garman R, Squire M, Donahue LR
and Rubin C: Genetically based influences on the site-specific
regulation of trabecular and cortical bone morphology. J Bone Miner
Res. 19:600–606. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Turner CH, Hsieh YF, Müller R, Bouxsein
ML, Baylink DJ, Rosen CJ, Grynpas MD, Donahue LR and Beamer WG:
Genetic regulation of cortical and trabecular bone strength and
microstructure in inbred strains of mice. J Bone Miner Res.
15:1126–1131. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Turner CH, Hsieh YF, Müller R, Bouxsein
ML, Rosen CJ, McCrann ME, Donahue LR and Beamer WG: Variation in
bone biomechanical properties, microstructure, and density in BXH
recombinant inbred mice. J Bone Miner Res. 16:206–213. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Szewczyk KA, Fuller K and Chambers TJ:
Distinctive subdomains in the resorbing surface of osteoclasts.
PLoS One. 8:e602852013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu H1, Xu G and Li YP: Atp6v0d2 is an
essential component of the osteoclast-specific proton pump that
mediates extracellular acidification in bone resorption. J Bone
Miner Res. 24:871–885. 2009. View Article : Google Scholar :
|
|
42
|
Väänänen K: Mechanism of osteoclast
mediated bone resorption - rationale for the design of new
therapeutics. Adv Drug Deliv Rev. 57:959–971. 2005. View Article : Google Scholar
|
|
43
|
Taranta A, Migliaccio S, Recchia I,
Caniglia M, Luciani M, De Rossi G, Dionisi-Vici C, Pinto RM,
Francalanci P, Boldrini R, et al: Genotype-phenotype relationship
in human ATP6i-dependent autosomal recessive osteopetrosis. Am J
Pathol. 162:57–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
de Vernejoul MC and Bénichou O: Human
osteopetrosis and other sclerosing disorders: Recent genetic
developments. Calcif Tissue Int. 69:1–6. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pei J, Li B, Gao Y, Wei Y, Zhou L, Yao H,
Wang J and Sun D: Fluoride decreased osteoclastic bone resorption
through the inhibition of NFATc1 gene expression. Environ Toxicol.
29:588–595. 2014. View Article : Google Scholar
|
|
46
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Teitelbaum SL and Ross FP: Genetic
regulation of osteoclast development and function. Nat Rev Genet.
4:638–649. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Neutzsky-Wulff AV, Sims NA, Supanchart C,
Kornak U, Felsenberg D, Poulton IJ, Martin TJ, Karsdal MA and
Henriksen K: Severe developmental bone phenotype in ClC-7 deficient
mice. Dev Biol. 344:1001–1010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Plans V, Rickheit G and Jentsch TJ:
Physiological roles of CLC Cl(−)/H (+) exchangers in renal proximal
tubules. Pflugers Arch. 458:23–37. 2009. View Article : Google Scholar
|