Introduction
Pterygium is an inflammatory and degenerative ocular
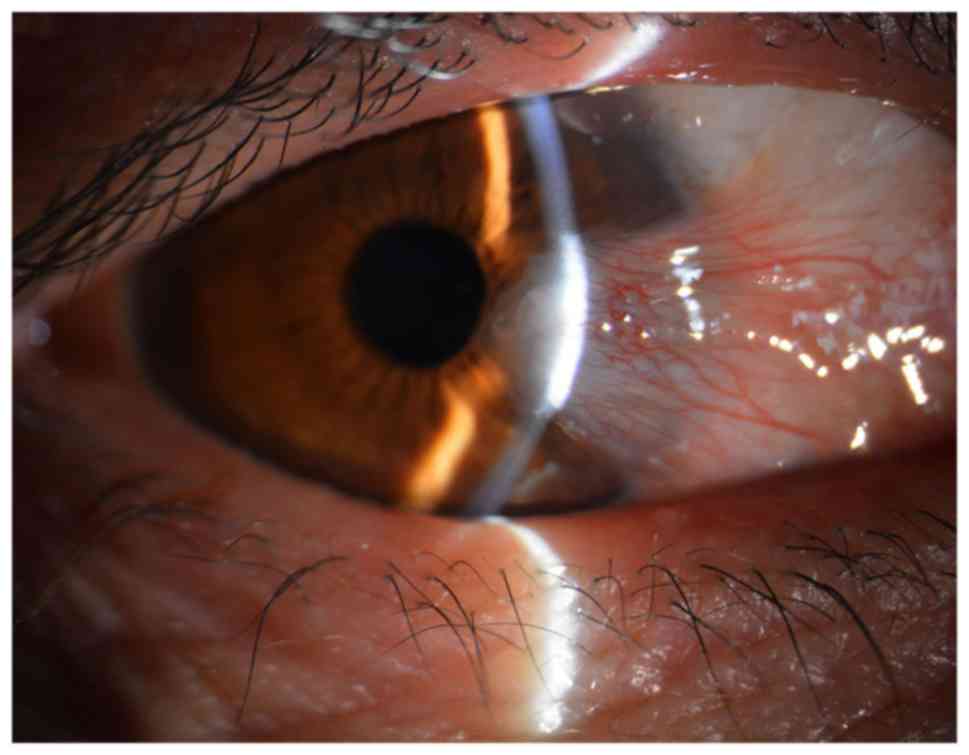
surface disease in which the conjunctiva on the cornea grows to
form fibrous tissue in the shape of a triangle (Fig. 1). Pterygium has a worldwide
distribution though it is considered more common in warm, dry
climates with a reported prevalence as high as 22% in equatorial
areas and less than 2% in latitudes above 40 degrees (1). It is speculated to be associated
with corneal and conjunctival microtrauma from exposure to sunlight
and/or dust. A higher prevalence of pterygium has been recorded
among outdoor workers as compared to indoor workers in Nigeria,
South India and Southwest China (2–4).
The pathogenesis of the disease is not yet completely understood;
however, recent evidence suggests that pterygium is histologically
composed of proliferating fibrovascular tissue and is correlated
strongly with exposure to ultraviolet (UV) radiation (5–7).
Inflammation and fibrovascular proliferation may be other factors
associated with the occurrence of pterygium. DNA damage has been
reported to initiate pterygium development. Hereditary
predisposition may be the underlying factor in the occurrence of
pterygium (8). UV light has been
shown to induce the activation of pro-inflammatory cytokines,
chronic inflammatory cells and growth factors. It may also damage
DNA in predisposed individuals. However, the integration of factors
associated with the occurrence of pterygium has not yet been
reported (9,10). Furthermore, evidence indicates
that vascular endothelial growth factor (VEGF) and basic fibroblast
growth factor (bFGF) expression is increased in pterygium (11–13). Therefore, these growth factors may
be directly or indirectly involved in the pathogenesis of
pterygium. Pterygium fibroblasts are tumour-like transformed cells
that grow much more rapidly in medium without high concentrations
of serum and can grow in a semisolid agar in contrast to normal
fibroblasts (14). Surgical
excision is the first-choice treatment for pterygium; however, the
high recurrence rate is a burden for patients. Therefore, the
treatment of pterygium remains quite controversial. The
identification of effective drugs for the treatment of pterygium is
urgently required.
Curcumin is a yellow-coloured polyphenol that is
isolated from the plant, Curcuma longa, and is the principal
curcuminoid of the popular spice, turmeric. Curcumin has been
widely studied for its antioxidant, anti-inflammatory,
anti-angiogenic and wound-healing effects (15,16). Curcumin has been shown to exert
antitumour effects that are mediated by a wide variety of
mechanisms both in vitro and in vivo. Curcumin
inhibits the proliferation and induces the apoptosis of a variety
of cancer cell types in vitro, including cells from cancers
of the breast, prostate, lung, pancreas, ovary, bladder, cervix,
head and neck, brain, kidney and skin (16–26). A previous study demonstrated that
curcumin significantly inhibited the proliferation of HPFs, which
resulted in the arrest of HPFs in the G0/G1 phase (27). However, the apoptosis of HPFs and
the VEGF pathway was not examined. The purpose of the present study
was to investigate the inhibitory effects of curcumin on human
pterygium fibroblasts (HPFs) in vitro. To the best of our
knowledge, this study is the first to examine the inhibitory
effects of curcumin on the expression of VEGF in HPFs. The two
studies coordinately investigate the effects of curcumin on the
inhibition of HPFs.
Materials and methods
Reagents
Curcumin, RNase and MTT were purchased from
Sigma-Aldrich (St Louis, MO, USA). Dulbecco's modified Eagle's
medium (DMEM), trypsin, fetal bovine serum (FBS), PBS, penicillin
and streptomycin were obtained from Gibco-BRL (Carlsbad, CA, USA).
Propidium iodide (PI), Annexin V-FITC were purchased from BD
Biosciences (San Jose, CA, USA).
Preparation of curcumin
Curcumin was dissolved in 0.05% DMSO to prepare a
stock solution at a concentration of 10 mmol/l that was stored at
−20°C. DMEM complete medium was added to dilute the curcumin to the
appropriate concentrations prior to use.
Culture and passage of HPFs
The pterygium specimens were obtained from 5 female
and 3 male patients, aged 54±8 years, who underwent pterygium
removal after they had provided written informed consent. The
representative morphology of pterygium on the cornea shown in
Figs. 1 and 2 was from a 52-year-old female patient.
The present study was reviewed and approved by the Institutional
Review Board of the First Hospital of Jilin University, Changchun,
China. HPFs were cultured from explants of the fresh pterygium
tissue from the surgical excision patients via a previously
reported method (28). Briefly,
the head of a fresh pterygium specimen was cut into small sections,
washed in Hanks solution, and placed in a culture dish. DMEM with
15% FBS and gentamicin (50 g/ml) was added to cover the explants
(all from Gibco-BRL). The culture dish was placed in a
CO2-regulated incubator in humidified 95% air/5%
CO2 atmosphere overnight. Culture medium (identical to
the first medium, but the concentration of FBS was reduced to 10%)
was added after the explants had adhered. In some cultures,
epithelial cells also migrated from the explants in the early
stage. However, epithelial cells rapidly became terminally
differentiated and lost their viability in medium with high levels
of serum and Ca2. Furthermore, during subculture, the
fibroblasts detached from the well more easily compared to the
epithelial cells. After the detachment of the fibroblasts was
almost complete, serum was added to block the effect of the enzyme
so that the epithelial cells remained in the well and could not be
passed to the subculture. Therefore, only fibroblasts were present
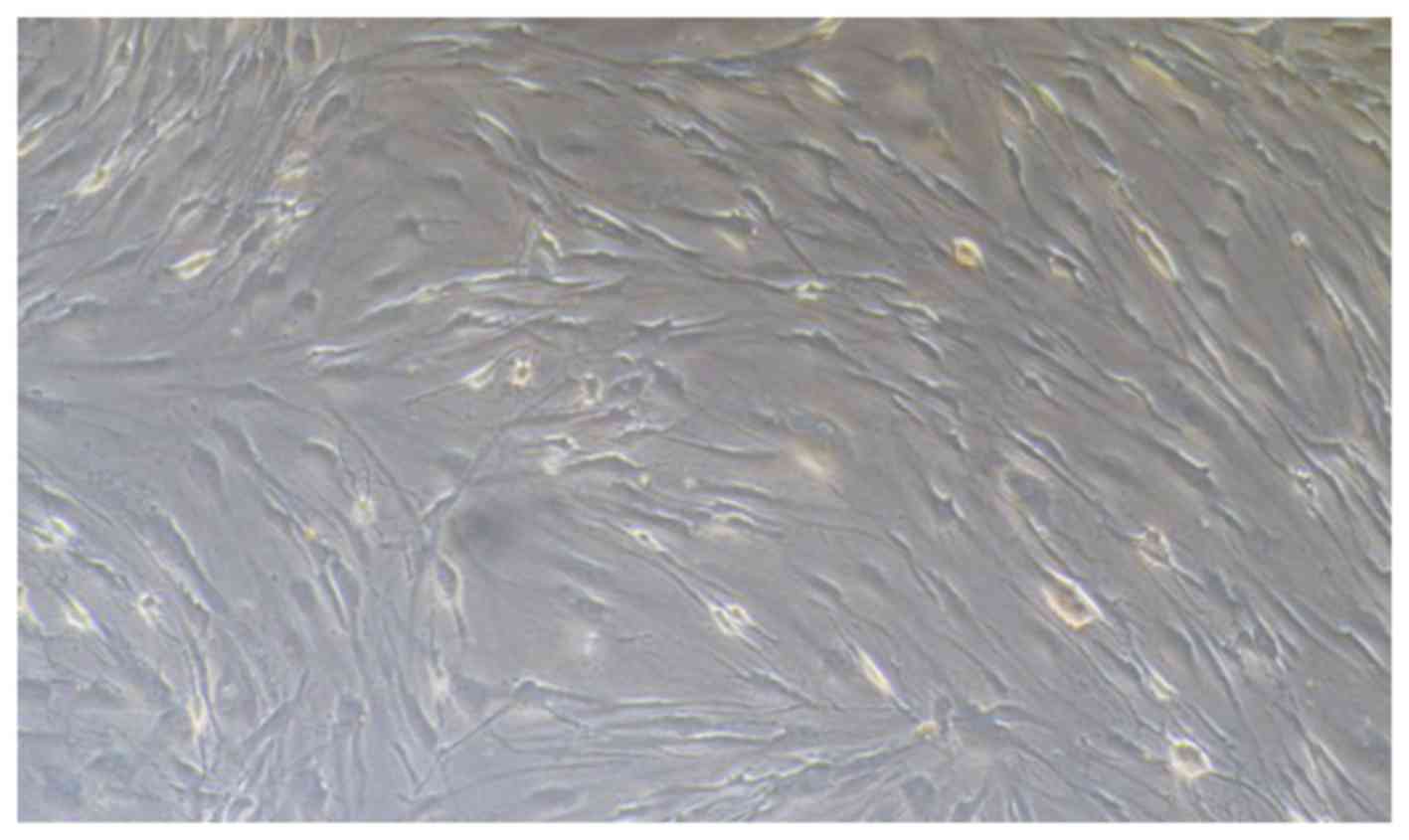
in the subcultures. The morphology of HPFs was examined using an
Olympus microscope (serial no. 36048; provided by Olympus, Tokyo,
Japan). The cells were cultured in DMEM complete medium and
passaged for 3 to 7 generations. The stable cells were used in the
following experiments.
Inhibitory effect of curcumin on HPF
proliferation
The inhibitory effects of curcumin on the
proliferation of HPFs were detected by MTT assays. All experiment
steps were performed following the instructions of the kit
instructions. Briefly, the cells were seeded on 96-well plates at a
density of 5×103/ml at a volume of 200 µl/well.
All groups without or with curcumin (5, 20, 80 or 200
µmol/l) were incubated for 24 to 72 h. MTT (1 mg/ml) was
added to each well, and the cells were incubated for 4 h. The MTT
solution was then aspirated, and 100 µl of DMSO was then
added. The 96-well plates were read using a microplate
spectrophotometer (BioTek Synergy H1; BioTek, Winooski, VT, USA) at
540 nm. The experiments were repeated in triplicate. The inhibition
percentage was calculated as follows: (1 - the value in
experimental group/the value in the control group) ×100%.
Flow cytometry (FCM) for cell
apoptosis
Annexin V-FITC and PI double staining FCM analyses
was carried out. The HPFs were plated in 96-well plates containing
200 µl medium at a density of 5×103 cells/well.
The induction of apoptosis in the HPFs was examined for the
presence or absence of curcumin (80 µmol/l). After 48 h in
culture, the HPFs were collected in 1.5 ml centrifuge tubes, washed
3 times with cold PBS and binding buffer, and then stained with
Annexin V-FITC and PI (Annexin V-FITC apoptosis detection kit; BD
Biosciences) for apoptosis detection. Briefly, the HPFs in
centrifuge tubes were first re-suspended in binding buffer.
Subsequently, 5 µl of Annexin V-FITC was added to the tubes,
which were incubated for 10 min followed by the addition of 5
µl PI. The samples were then incubated with PI for a further
15 min and immediately analysed using a flow cytometer (FACScan; BD
Biosciences) with the FlowJo FACS analysis software. The cells in
the different portions represented the different cell states as
follows: the late apoptotic cells were present in the upper right
portion, the viable cells were present in the lower left portion
and the early apoptotic cells were present in the lower right
portion.
qPCR
Total RNA was isolated from the HPFs using a Qiagen
RNeasy Mini kit (Qiagen China Co., Ltd., Shanghai, China).
Following the RNeasy Mini Kit instructions, 70% ethanol was added
to the cell lysates or the homogenates and the samples were mixed
by pipetting prior to being transferred to columns. Following
centrifugation for 2 min at 4°C and 12,000 × g, the flow-through
extract was retained and stored on ice. RNA quality was assessed
with an Agilent 2100 Bioanalyzer (Agilent Technologies GmbH,
Waldbronn, Germany). cDNA was synthesized with SuperMix II reverse
transcriptase using random hexamer primers (TransGen Biotech,
Beijing, China) following the manufacturer's instructions. The
primers for VEGF and GAPDH were designed and synthesized by the
company, Sangon Biological Engineering Co., Ltd. (Shanghai, China).
An Applied Biosystems StepOnePlus™ real-time PCR system was used to
determine the mRNA levels of VEGF and GAPDH (internal control). The
primer sequences for VEGF were as follows: sense,
5′-TGCCCACTGAGGAGTCCAAC-3′ and antisense,
5′-TGGTTCCCGAAACGCTGAG-3′. The GAPDH primers were as follows:
sense, 5′-CCAGGTGGTCTCCTCTGACTT-3′ and antisense,
5′-GTTGCTGTAGCCAAATTCGTTGT-3′. The reactions were performed using 3
µl of cDNA in a 20 µl reaction volume and the
following thermal cycles profile: 10 sec for pre-denaturation at
94°C, 5 sec for denaturation at 94°C and 30 sec for extension at
60°C, for 40 cycles. The PCR products were 200 bp in length.
Enzyme-linked immunosorbent assay
(ELISA)
The HPFs were plated at appropriate densities
(5×103 cells/well) in 96-well plates. The culture
supernatants were collected 48 h following incubation under each
experimental condition without or with curcumin (5, 20, 80 or 200
µmol/l). VEGF production was assessed using an ELISA kit
(Abcam, Cambridge, MA, USA). The optical densities were measured at
450 nm on a microplate spectrophotometer (Multiskan MCC/340; Thermo
Fisher Scientific, Inc., Pittsburgh, PA, USA) and the VEGF
concentrations were determined based on a standard curve.
Statistical analysis
All data were analysed and assessed for significance
using the D'Agostino-Pearson omnibus normality test. All data are
presented as the means ± the standard error of the mean. Mean
values were compared using paired t-tests (2 groups) followed by
the Bonferroni's correction for multiple comparison tests. P-
values <0.05 were considered to indicate statistically
significant differences. All statistical tests were performed using
Prism software (GraphPad Software Inc., San Diego, CA, USA).
Results
Effects of curcumin on the proliferation
of HPFs
Pterygium specimens were obtained from patients who
underwent pterygium removal. HPFs were cultured from explants of
fresh pterygium tissue from each surgical excision patient. The
cells were cultured in DMEM complete medium and passaged for 3 to 6
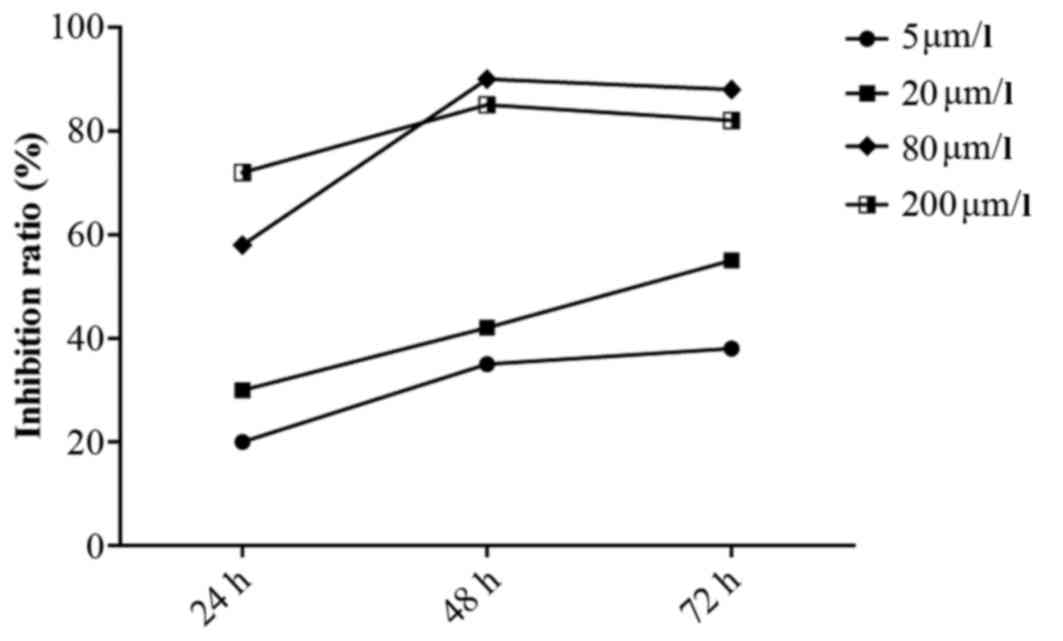
generations (Fig. 2). MTT assays
revealed that curcumin significantly inhibited the proliferation of
HPFs. The inhibitory effects of curcumin on HPFs were dose- and
time- dependent within the ranges of 5–80 µmol/l and 24–72
h. Treatment with curcumin at 80 µmol/l for 48 h elicited
the greatest inhibitory effect; this treatment inhibited the
proliferation of HPFs with an inhibitory rate exceeding 90%
compared with that of the blank control group (P<0.01). The
time-effect curve is illustrated in Fig. 3.
Effects of curcumin on the expression of
VEGF in HPFs
Based on the results of MTT assay, the cell pellets
and cell culture supernatants were collected to detect the VEGF
gene and protein concentrations 48 h following incubation under
each experimental condition, either without or with curcumin (5,
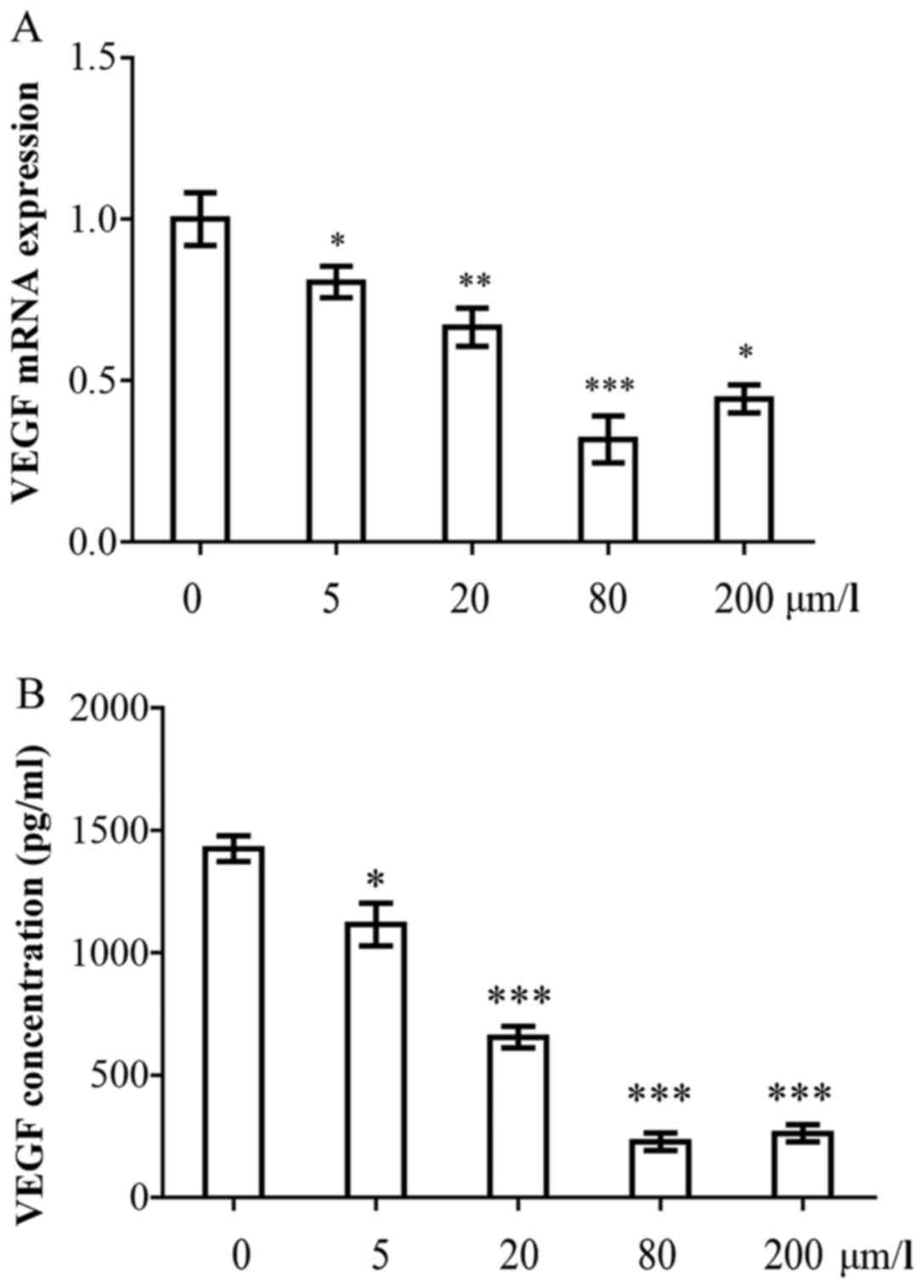
20, 80 and 200 µmol/l). As illustrated in Fig. 4A, the mRNA expression of VEGF in
the curcumin-treated groups decreased by 0.80–0.42-fold compared
with the control group (P<0.05). The inhibitory effects were
dose-dependent. Subsequently, the VEGF concentrations in the cell
culture supernatant following curcumin treatment, were
investigated. The HPFs secreted large amounts of VEGF
(1,394.5±102.5 pg/ml). However, the concentration of VEGF decreased
significantly following treatment with curcumin. The VEGF
concentrations were 1,120.5±75.5, 720.2±64.5, 180.3±35.2 and
280.6±59.5 pg/ml following treatment with 5, 20, 80 and 200
µmol/l curcumin, respectively (Fig. 4B). The inhibitory effects were
dose-dependent, and the 80 µmol/l curcumin concentration
elicited the most potent inhibitory effect.
Annexin V-FITC and PI double staining
assay
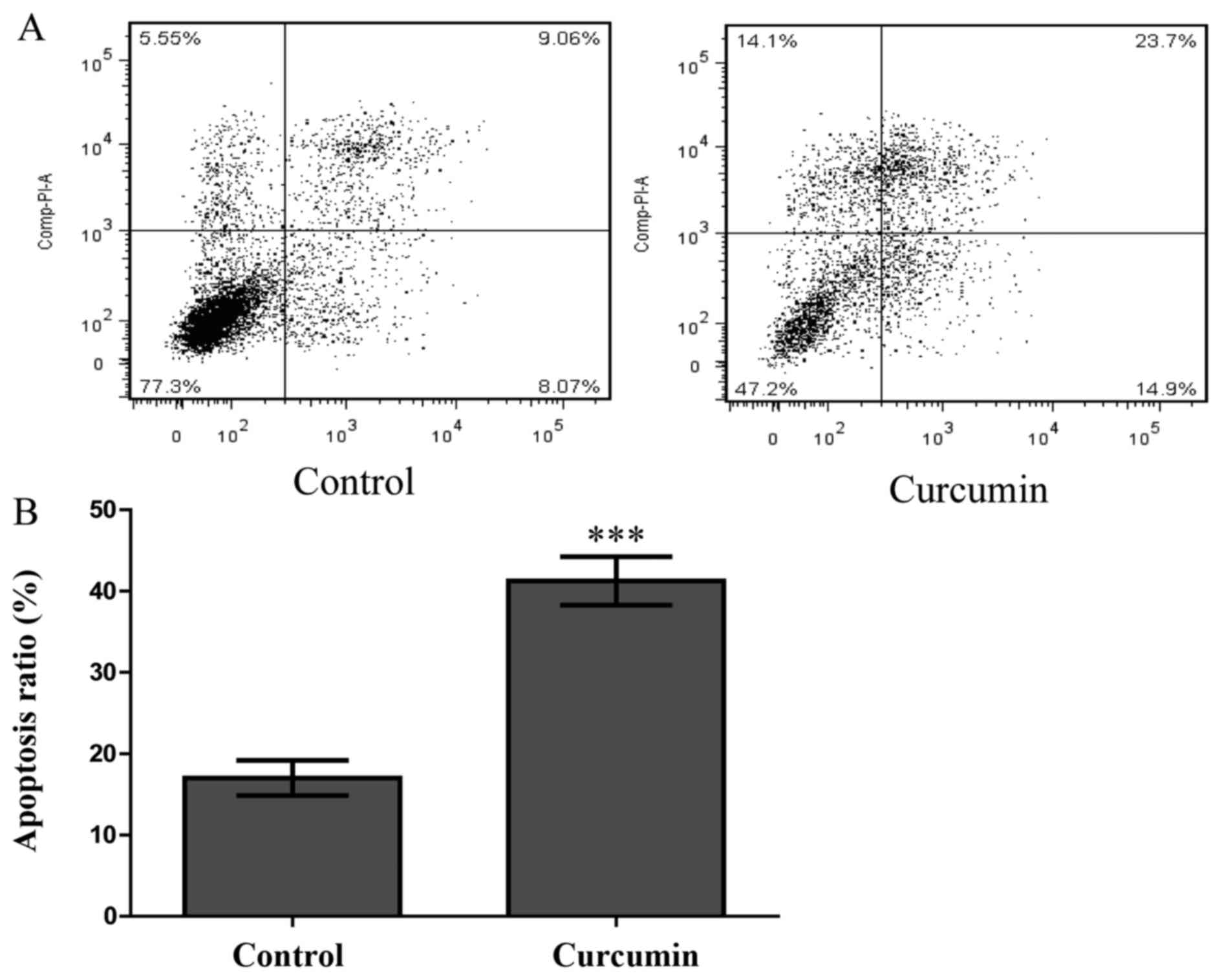
To explore whether curcumin also induces HPF
apoptosis, an Annexin V-FITC and PI double staining assay was
performed. HPFs were treated with curcumin at a concentration of 80
µmol/l for 48 h and were then analysed by FCM. As shown in
Fig. 5A, compared with the
control group, the numbers of early and late apoptotic cells
increased significantly in the treated group. The proportion of
early and late apoptotic cells in the curcumin-treatment group
reached 38.6%, which was greater than the proportion observed in
the control group (17.1%, P<0.001; Fig. 5B). This finding indicated that
curcumin significantly induced HPF apoptosis.
Discussion
Pterygium is a common ocular surface disease that
can cause loss of vision (28).
The disorder may be characterized by cell proliferation,
inflammatory processes, fibrosis, angiogenesis and the destruction
of the extracellular matrix (29). Pterygium exhibits features that
are similar to those of tumours, such as local invasion, metaplasia
of epithelial cells, the presence of oncogenic viruses, the
inactivation of tumour suppressor genes, and the loss of
heterozygosity (30). Pterygium
fibroblasts represent tumour-like transformed cells. Pterygium
fibroblasts grow much more rapidly in medium without high
concentrations of serum and can grow in a semisolid agar (11,28). The pathogenesis of pterygium and
its recurrence following surgical excision are not yet completely
understood. The identification of a new drug that inhibits HPF
proliferation would be of significance for the treatment of
pterygium.
Curcumin is also known as diferuloylmethane and is
obtained from Curcuma longa. Curcumin is regarded as a
potent anticancer drug in relation to various types of tumour
(31–34). Curcumin is extensively used in
Ayurveda, Unani, Siddha and Chinese medicine for the management of
various diseases and conditions, such as wounds, inflammation and
cancer (35). The inhibition of
tumour progression by curcumin primarily results from the
downregulation of the expression of cancer formation and
progression genes, such as p53, Egr-1 and c-Myc (36). Due to its ability to activate
caspase-3 and cytochrome c release, curcumin induces the
apoptosis of seeral tumour cell lines, including HepG2, CRC, A549,
HL60 and others (37).
Angiogenesis is an important mechanism of tumour formation.
Angiogenic factors include angiotensin, epidermal growth factor,
bFGF, transforming growth factor (TGF) and VEGF. These factors play
critical roles in tumour angiogenesis via their actions in
cancerous tumour cells (38). The
present study demonstrated that curcumin significantly inhibited
the proliferation of HPFs based on the results of MTT assay. FCM
revealed that curcumin increased the proportions of numbers of
early and late apoptotic HPFs from 17.1 to 38.6%. Our results
revealed that curcumin suppressed cell proliferation via the
apoptosis-inducing pathway.
VEGF is a prominent pro-angiogenic and tumour
growth-promoting hormone that is expressed in many types of tumour
cells. The expression of VEGF is often obligatory for tumour
angiogenesis; thus, the inhibition of the expression or biological
function of VEGF has been fervently pursued as a cancer treatment
(39). Tumorigenesis is a
multistep process that is affected by tumour genes, cytokines, and
the host immune system (40).
During tumour development, VEGF is among the important factors that
are involved in the growth, invasion and metastasis of the tumour
(41). VEGF is secreted in
greater amounts by HPFs than conjunctival fibroblasts (42). Since 2001, when the first
hypothesis about the potential benefits of anti-VEGF therapy in
human pterygium was proposed, controversies have continuously
arisen based on published articles that have reported the use of
bevacizumab as an adjuvant therapy for human pterygium (43,44). Our findings demonstrate that the
mRNA expression of VEGF in the curcumin-treated groups decreased by
0.8–0.42-fold compared with the control group (P<0.05) and that
the inhibitory effects were dose-dependent. HPFs can secrete large
amounts of VEGF; however, the concentration of VEGF significantly
decreased following treatment with curcumin. The inhibitory effects
were dose-dependent, and the 80 µmol/l curcumin
concentration elicited the most potent inhibitory effect.
Curcumin modulates the expression of VEGF via the
inhibition of the JAK2/STAT3 pathway in laryngeal squamous cell
carcinomas (45). Curcumin
specifically targets the PI3K/Akt/IKK signalling axis, which
consequently leads to the concurrent, but independent suppression
of both the NF-κB and mTOR pathways, the concomitant activation of
caspases, and the downregulation of VEGF. These events result in
the induction of apoptosis, the prevention of angiogenesis, and
ultimately the inhibition of adenoid cystic carcinoma progression
(46). These mechanisms may
represent novel targets for therapies. Therefore, our findings
suggest that curcumin suppresses the proliferation of pterygium by
inducing HPF apoptosis and inhibiting VEGF expression. To the best
of our knowledge, this is the first study to demonstrate the
inhibition of the expression of VEGF by HPFs by curcumin. However,
additional studies on the molecular regulatory mechanisms are
warranted to fully determine and understand the effects.
Acknowledgments
The present study was supported in part by grants
from the Jilin Provincial Natural Science Foundation of China (no.
20140520014JH) and the 4th Young Scientists Fund of Jilin
University (no. 2013068).
References
|
1
|
Viso E, Gude F and Rodríguez-Ares MT:
Prevalence of pinguecula and pterygium in a general population in
Spain. Eye (Lond). 25:350–7. 2011. View Article : Google Scholar
|
|
2
|
Achigbu E and Ezepue UF: Prevalence and
severity of pterygium among commercial motorcycle riders in South
Eastern Nigeria. Ghana Med J. 48:153–157. 2014. View Article : Google Scholar
|
|
3
|
Asokan R, Venkatasubbu RS, Velumuri L,
Lingam V and George R: Prevalence and associated factors for
pterygium and pinguecula in a South Indian population. Ophthalmic
Physiol Opt. 32:39–44. 2012. View Article : Google Scholar
|
|
4
|
Zhong H, Chen Q, Li J, Shen W, Sheng X,
Niu Z, Zhou H, Wei T, Yuan Y and Pan CW: Ethnic variations in
pterygium in a rural population in southwestern China: The Yunnan
minority eye studies. Ophthalmic Epidemiol. 23:116–121. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coroneo MT, Di Girolamo N and Wakefield D:
The pathogenesis of pterygia. Curr Opin Ophthalmol. 10:282–288.
1999. View Article : Google Scholar
|
|
6
|
Threlfall TJ and English DR: Sun exposure
and pterygium of the eye: a dose-response curve. Am J Ophthalmol.
128:280–287. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chui J, Di Girolamo N, Wakefield D and
Coroneo MT: The pathogenesis of pterygium: current concepts and
their therapeutic implications. Ocul Surf. 6:24–43. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anguria P, Kitinya J, Ntuli S and
Carmichael T: The role of heredity in pterygium development. Int J
Ophthalmol. 7:563–573. 2014.PubMed/NCBI
|
|
9
|
Bianchi E, Scarinci F, Grande C, Plateroti
R, Plateroti P, Plateroti AM, Fumagalli L, Capozzi P, Feher J and
Artico M: Immunohistochemical profile of VEGF, TGF-β and
PGE2 in human pterygium and normal conjunctiva:
experimental study and review of the literature. Int J Immunopathol
Pharmacol. 25:607–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Young CH, Lo YL, Tsai YY, Shih TS, Lee H
and Cheng YW: CYP1A1 gene polymorphisms as a risk factor for
pterygium. Mol Vis. 16:1054–1058. 2010.PubMed/NCBI
|
|
11
|
Kria L, Ohira A and Amemiya T: Growth
factors in cultured pterygium fibroblasts: immunohistochemical and
ELISA analysis. Graefes Arch Clin Exp Ophthalmol. 236:702–708.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Powers MR, Qu Z, O'Brien B, Wilson DJ,
Thompson JE and Rosenbaum JT: Immunolocalization of bFGF in
pterygia: association with mast cells. Cornea. 16:545–549. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee DH, Cho HJ, Kim JT, Choi JS and Joo
CK: Expression of vascular endothelial growth factor and inducible
nitric oxide synthase in pterygia. Cornea. 20:738–742. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen JK, Tsai RJ and Lin SS: Fibroblasts
isolated from human pterygia exhibit transformed cell
characteristics. In Vitro Cell Dev Biol Anim. 30A:243–248. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maheshwari RK, Singh AK, Gaddipati J and
Srimal RC: Multiple biological activities of curcumin: a short
review. Life Sci. 78:2081–2087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang N, Li H, Jia J and He M:
Anti-inflammatory effect of curcumin on mast cell-mediated allergic
responses in ovalbumin-induced allergic rhinitis mouse. Cell
Immunol. 298:88–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Collaborative Ocular Melanoma Study Group:
The COMS randomized trial of iodine 125 brachytherapy for choroidal
melanoma: V. Twelve-year mortality rates and prognostic factors:
COMS report No. 28. Arch Ophthalmol. 124:1684–1693. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bimonte S, Barbieri A, Palma G, Rea D,
Luciano A, D'Aiuto M, Arra C and Izzo F: Dissecting the role of
curcumin in tumour growth and angiogenesis in mouse model of human
breast cancer. BioMed Res Int. 2015:8781342015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan N and Mukhtar H: Dietary agents for
prevention and treatment of lung cancer. Cancer Lett. 359:155–164.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen A, Xu J and Johnson AC: Curcumin
inhibits human colon cancer cell growth by suppressing gene
expression of epidermal growth factor receptor through reducing the
activity of the transcription factor Egr-1. Oncogene. 25:278–287.
2006.
|
|
22
|
Gupta SC, Patchva S and Aggarwal BB:
Therapeutic roles of curcumin: lessons learned from clinical
trials. AAPS J. 15:195–218. 2013. View Article : Google Scholar :
|
|
23
|
Amin AR, Haque A, Rahman MA, Chen ZG,
Khuri FR and Shin DM: Curcumin induces apoptosis of upper
aerodigestive tract cancer cells by targeting multiple pathways.
PLoS One. 10:e01242182015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi M, Cai Q, Yao L, Mao Y, Ming Y and
Ouyang G: Antiproliferation and apoptosis induced by curcumin in
human ovarian cancer cells. Cell Biol Int. 30:221–226. 2006.
View Article : Google Scholar
|
|
25
|
Hong JH, Ahn KS, Bae E, Jeon SS and Choi
HY: The effects of curcumin on the invasiveness of prostate cancer
in vitro and in vivo. Prostate Cancer Prostatic Dis. 9:147–152.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu C, Song E, Hu DN, Chen M, Xue C, Rosen
R and McCormick SA: Curcumin induces cell death in human uveal
melanoma cells through mitochondrial pathway. Curr Eye Res.
35:352–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang M, Bian F, Wen C and Hao N:
Inhibitory effect of curcumin on proliferation of human pterygium
fibroblasts. J Huazhong Univ Sci Technolog Med Sci. 27:339–342.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang SF, Lin CY, Yang PY, Chao SC, Ye YZ
and Hu DN: Increased expression of gelatinase (MMP-2 and MMP-9) in
pterygia and pterygium fibroblasts with disease progression and
activation of protein kinase C. Invest Ophthalmol Vis Sci.
50:4588–4596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ribatti D, Nico B, Perra MT, Maxia C,
Piras F, Murtas D, Crivellato E and Sirigu P: Correlation between
NGF/TrkA and microvascular density in human pterygium. Int J Exp
Pathol. 90:615–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dos Reis GM, de P R Júnior A, E Silva KS,
Rodrigues DA, Gomes MC, Martins JV, da Costa IR, Freitas GA and
Moura KK: Pterygium in patients from Goiânia, Goiás, Brazil. Genet
Mol Res. 14:6182–6188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shehzad A, Lee J and Lee YS: Curcumin in
various cancers. Biofactors. 39:56–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Witkin JM and Li X: Curcumin, an active
constiuent of the ancient medicinal herb Curcuma longa L.: some
uses and the establishment and biological basis of medical
efficacy. CNS Neurol Disord Drug Targets. 12:487–497. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y and Zhang T: Targeting cancer stem
cells by curcumin and clinical applications. Cancer Lett.
346:197–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prasad S, Gupta SC, Tyagi AK and Aggarwal
BB: Curcumin, a component of golden spice: from bedside to bench
and back. Biotechnol Adv. 32:1053–1064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rahmani AH, Al Zohairy MA, Aly SM and Khan
MA: Curcumin: a potential candidate in prevention of cancer via
modulation of molecular pathways. Biomed Res Int. 2014:7616082014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han SS, Chung ST, Robertson DA, Ranjan D
and Bondada S: Curcumin causes the growth arrest and apoptosis of B
cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-κB, and
p53. Clin Immunol. 93:152–161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mukherjee Nee Chakraborty S, Ghosh U,
Bhattacharyya NP, Bhattacharya RK, Dey S and Roy M:
Curcumin-induced apoptosis in human leukemia cell HL-60 is
associated with inhibition of telomerase activity. Mol Cell
Biochem. 297:31–39. 2007. View Article : Google Scholar
|
|
38
|
Shishodia S, Chaturvedi MM and Aggarwal
BB: Role of curcumin in cancer therapy. Curr Probl Cancer.
31:243–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Zhang D, Stoner GD and Huang C:
Differential effects of black raspberry and strawberry extracts on
BaPDE-induced activation of transcription factors and their target
genes. Mol Carcinog. 47:286–294. 2008. View
Article : Google Scholar
|
|
40
|
Coussens LM and Werb Z: Matrix
metalloproteinases and the development of cancer. Chem Biol.
3:895–904. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsai CH, Chiang YC, Chen HT, Huang PH, Hsu
HC and Tang CH: High glucose induces vascular endothelial growth
factor production in human synovial fibroblasts through reactive
oxygen species generation. Biochim Biophys Acta. 1830:2649–2658.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu W, Sha X, Wen Y, Zhao W, Luo W and Hua
Z: Effect of Avastin on the migration and invasion of pterygium
fibroblasts. Eye Sci. 29:214–218. 2014.
|
|
43
|
Hosseini H, Nejabat M and Khalili MR:
Bevacizumab (Avastin) as a potential novel adjunct in the
management of pterygia. Med Hypotheses. 69:925–927. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bahar I, Yeung SN, Sella R and Slomovic A:
Anterior segment uses of bevacizumab. Curr Opin Ophthalmol.
23:303–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu A, Huang JJ, Jin XJ, Li JP, Tang YJ,
Huang XF, Cui HJ, Xu WH and Sun GB: Curcumin suppresses
invasiveness and vasculogenic mimicry of squamous cell carcinoma of
the larynx through the inhibition of JAK-2/STAT-3 signaling
pathway. Am J Cancer Res. 5:278–288. 2014.
|
|
46
|
Sun ZJ, Chen G, Zhang W, Hu X, Liu Y, Zhou
Q, Zhu LX and Zhao YF: Curcumin dually inhibits both mammalian
target of rapamycin and nuclear factor-κB pathways through a
crossed phosphatidylinositol 3-kinase/Akt/IκB kinase complex
signaling axis in adenoid cystic carcinoma. Mol Pharmacol.
79:106–118. 2011. View Article : Google Scholar
|