Introduction
The arteriovenous fistula (AVF) is the preferred
hemodialysis vascular access for patients with end-stage renal
disease (ESRD) (1). However, it
has been reported that early AVF failure occurs in 28–60% of AVFs,
in which the AVF fails to mature sufficiently to support dialysis
therapy (2,3). Early AVF failure is defined by
venous neointimal hyperplasia (VNH) in combination with a fistula
with inadequate blood flow to support dialysis and of insufficient
size to allow for repetitive cannulation, caused by inadequate
vascular dilatation (4). Although
the molecular mechanisms underlying VNH development are
multifactorial, previous studies have suggested that hypoxia plays
a key role (5,6). Hypoxia is known as the primary
stimulus enhancing the expression of hypoxia inducible factor
(HIF)-1α, which is a transcription factor regulating numerous
genes, including vascular endothelial growth factor (VEGF) and
matrix metalloproteinases (MMPs) (7). The interaction between VEGF and the
VEGF receptor is an essential step in angiogenesis, cellular
proliferation, sprouting and migration. MMPs are key enzymes that
cause the breakdown of extracellular matrix proteins, such as
collagen and elastin, which facilitate the migration of vascular
smooth muscle cells (VSMCs) in VNH (8). Misra et al found that the
protein levels of HIF-1α, pro-MMP-2 and pro-MMP-9 were
significantly increased in specimens removed from patients with
failed hemodialysis vascular accesses and in a porcine model of
hemodialysis graft failure. They also found that HIF-1α, VEGF-A and
MMP-2 gene expression was significantly upregulated at the venous
stenosis in a mouse model of AVF with renal insufficiency (5,6).
Collectively, these observations suggest that hypoxia and HIF-1α
play an important role in neointimal formation in early AVF
failure.
Therapy aimed at improving oxygen tension in the
vascular wall may prevent early AVF failure by reducing HIF-1α
expression. Previous studies have demonstrated that artery wall
hypoxia, neointimal formation and VSMC proliferation can be
inhibited by the administration of 40% supplemental oxygen in an
artery-to-prosthetic graft anastomosis model. Moreover, 42 days of
30% supplemental oxygen was shown to attenuate intimal hyperplasia
and VSMC proliferation in a rabbit model of AVF (9,10).
Oxygen dissolved in plasma is the most bioavailable for tissues.
More oxygen can be delivered deeper into the tissues by increasing
the partial pressure of oxygen (paO2) in arterial blood.
Compared with the effects of normobaric oxygen, the concentration
of dissolved oxygen in the plasma can be increased greatly with
hyperbaric therapy. It was previously suggested that the oxygen
dissolved in plasma increases approximately 3-fold if the patient
is breathing room air when pressure was increased from one
atmosphere absolute (ATA) to 2–2.5 ATA. If the inhaled oxygen
concentration was increased to 100% under pressure, the plasma
oxygen concentration increased by almost 17-fold. In theory, with
100% oxygen at 2.5 ATA, enough oxygen can be dissolved in the
plasma to meet the normal requirements of the body at rest without
the need for hemoglobin (Hb) (11). Therefore, hyperbaric oxygen (HBO)
therapy may prevent early AVF failure.
HBO therapy was defined as the intermittent
inhalation of 100% oxygen inside a chamber with pressure greater
than 1 ATA (12). Rits et
al found that HBO can decrease intimal thickness following
carotid artery balloon injury in a rat model (13). Sharifi et al concluded that
HBO inhibits human coronary artery restenosis following
interventional surgery (14).
However, these studies primarily focused on arterial disease, while
the effect of HBO on venous disease, specifically on early AVF
failure, has not yet been investigated, at least to the best of our
knowledge.
The primary aim of this study was to examine the
hypothesis that HBO therapy may inhibit the expression of HIF-1α
and proliferating cell nuclear antigen (PCNA), attenuate VNH
development, and further increase blood flow in the venous segment
in a rabbit model of AVF with chronic renal failure (CRF).
Materials and methods
Study design
Study approval was obtained from the Institutional
Animal Care and Use Committee of Chongqing Medical University,
Chongqing, China before any procedures were performed on the
animals. A total of 96 adult New Zealand white rabbits, with an
average weight of 3,000 g, were maintained in separate cages at
constant humidity and temperature. The animal room was kept on a
12-h light/dark cycle. The rabbits were randomly divided into 3
groups according to postoperative treatment as follows: the sham +
HBO group, in which 32 CRF rabbits underwent sham operation and
were treated with HBO; the AVF alone group, in which 32 CRF rabbits
were subjected to arteriovenous fistulization, but received no
further treatment; and the AVF + HBO group, in which 32 CRF rabbits
were subjected to arteriovenous fistulization and were treated with
HBO. In the sham operation, the animals received only a skin
incision. These groups were further subdivided into 4 subgroups of
8 rabbits each, according to a pre-determined euthanasia time-point
at 1, 7, 14 or 28 days post-operatively.
Creation of a rabbit model of CRF
The model of CRF was established by the oral
administration of adenine. Adenine is metabolized to
2,8-dihydroxyadenine, which crystallizes in tubular fluid, leading
to chronic tubulointerstitial injury, renal insufficiency and
metabolic abnormalities characteristic of CRF. All rabbits were
provided daily with standard pelleted rabbit chow containing
adenine, which they ate ad libitum. The concentration of
adenine in the chow was 0.6% for the first 4 weeks, followed by
0.3% for 2 weeks (induction phase), and 0.15% (maintenance phase)
thereafter, until the termination of the study. Rabbits had free
access to tap water throughout the experiment. Blood samples were
obtained from the auricular vein before (week 0) and every week
after the first adenine feeding until the end of the experiment.
Red blood cell (RBC) and Hb values were determined using a
hematology analyzer (Sysmex XE-5000; Sysmex, Kobe, Japan). The
levels of serum creatinine (SCr) and blood urea nitrogen (BUN) were
measured using an automatic biochemical analyzer (Roche, Basel,
Switzerland).
Creation of a carotid-jugular model of
AVF
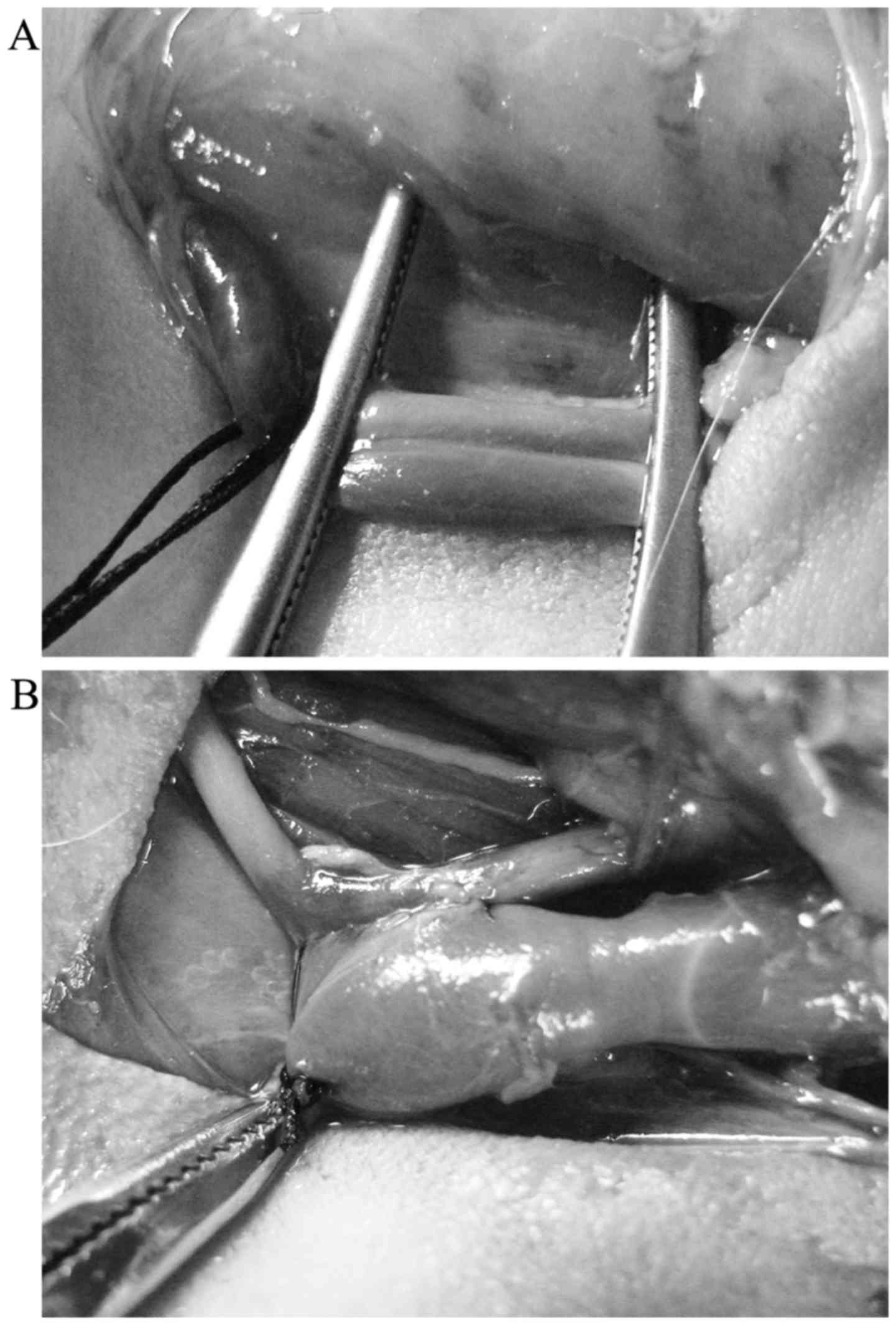
In the sixth week, arteriovenous fistulization was
performed at the left neck. Under ×10-loupe magnification, the
operative field was sterilized with povidone-iodine and isolated
with sterile field cloths. A 3-cm skin incision was made along the
left mandibular angle to the left medial clavicle. The areas
proximal and distal to the left common carotid artery (LCCA) and
the left common jugular vein (LCJV) were exposed and clamped with
two vascular clamps. An arteriotomy was subsequently performed at
the LCCA (~3-mm incision). The LCJV was used for a side-to-side
anastomosis to the LCCA using a 9-0 Prolene suture, and the distal
side of the LCJV was permanently ligated using a 5-0 silk suture.
The AVF was created when the anastomotic vein exhibited stable
tremors and pulsation (Fig.
1).
HBO therapy and blood gas analysis
A total of 5 days of HBO treatment followed by 2
days of rest was defined as the course of treatment. Four such
courses were performed, totaling 28 days. The first session began
on the first post-operative day with 8 animals at a time. A
hyperbaric chamber was pressurized to 2.5 ATA over a period of 20
min. The inlet and vent valves were adjusted to maintain the oxygen
pressure (2.5 ATA) and concentration (97–100%) for 60 min and then
slowly depressurized over a period of 20 min. Blood pH,
paO2, paCO and HCO3− values were
determined using a blood-gas analyzer (Ciba Corning Diagnostics
Corp., East Walpole, CA, USA). Blood samples were drawn via an ear
artery catheter during HBO treatment sessions.
High-frequency ultrasonography
A high-frequency duplex ultrasonography system (GE
LOGIQ-P5; GE Healthcare Piscataway, NJ, USA) and a 7.5-MHz
transducer were used to detect changes in vascular morphology and
hemodynamics in the proximal vein after surgery (on days 0, 1, 7,
14 and 28). The proximal vein was imaged within ~1 cm of the
fistula in the longitudinal plane. After the vascular longitudinal
and transverse axes views were scanned, the average diameter of the
vessels was measured using two-dimensional echocardiography, the
velocity time integral (VTI) of flow and heart rate (HR) were
recorded using the pulsed Doppler technique, and blood flow
(ml/min) was calculated using the formula π(AD/2)2 × VTI × HR.
Tissue harvesting
The rabbits were anesthetized with pentobarbital,
and vessels were dissected free of the surrounding soft tissue and
perfused with 0.9% NaCl solution at 80 mmHg for 2 min via an
abdominal aortic cannulation and cut off 1 cm from the fistula in
the longitudinal plane. Subsequently, after being separated from
the arteries, the venous segments were cut into two segments, one
of which was frozen in liquid nitrogen for western blot analysis
and the other fixed in 4% paraformaldehyde for immunohistochemical
analysis and histological staining.
Hematoxylin and eosin (H&E)
staining
The fixed venous segments in each group were
embedded in paraffin, cut into 5-µm-thick sections, and
stained with H&E. VNH was assessed using Image Pro Plus (IPP)
6.0 (Media Cybernetics, Atlanta, GA, USA). VNH was reported as the
average intima thickness, intima area, and the ratio of the intima
and media areas. Ten sections from each animal were analyzed by 3
experimentally blinded investigators, and the values were
averaged.
Immunohistochemistry
PCNA highly correlates with cell division (15). Immunohistochemical staining for
PCNA was used to assess cell proliferation in neointimal formation.
Slides of the veins from each group were deparaffinized with
xylene, rehydrated with a gradient of ethanol, blocked in 3%
H2O2 at 37°C for 15 min, and then rinsed 3
times with phosphate-buffered saline (PBS) for 5 min each. Antigen
retrieval was performed by bringing the slides to a boil in a
sodium citrate buffer, then maintaining a sub-boiling temperature
for 20 min. The cooled slides were blocked by serum albumin in an
incubator at 37°C for 30 min, incubated with mouse polyclonal PCNA
antibody (1:200 dilution; ab19166; Abcam, Cambridge, UK) at 4°C
overnight, washed with PBS, incubated with biotin-labeled secondary
antibody (ab150077; Abcam) at 37°C for 30 min, and later stained
with 3,3′-diaminobenzidine. Samples were counterstained with
hematoxylin, dehydrated with gradient ethanol, vitrified with
xylene and sealed with neutral resins. The percentage of PCNA was
used to represent cellular proliferation. All nuclei (denominator)
and PCNA-stained nuclei (numerator) were counted using IPP software
to determine the percentage of PCNA-positive cells.
Western blot analysis
The total protein from the isolated veins was
extracted in a radioimmunoprecipitation assay buffer and quantified
using a bicinchoninic acid kit. Equivalent amounts of sample were
loaded into each well, separated by sodium dodecyl sulfate
polyacrylamide gel electrophoresis, electro-transferred onto
nitrocellulose membranes, blocked for 1 h with 5% dry milk/bovine
serum albumin, and immuno-blotted with mouse monoclonal anti-HIF-1α
primary antibody (1:1,000 dilution; ab1; Abcam) overnight at 4°C.
Afterward, the membranes were washed with 0.1% Tris-buffered saline
solution with 0.05% Tween-20 (TBST) 3 times for 10 min each and
incubated with HRP-conjugated anti-mouse secondary antibody
(ab205719; Abcam) at 37°C for 1 h and then washed 3 times prior to
band detection using enhanced chemiluminescence on a UVP gel
imaging system (UVP, Upland, CA, USA). The relative abundance of
protein was quantified using Quantity One software (Bio-Rad,
Hercules, CA, USA).
Statistical analysis
All values are expressed as the means ± standard
deviation. An analysis of variance was used for comparisons among
the 3 groups, and a two-tailed Student's t-test was used for
comparisons between 2 groups or in the same subgroup. A
significance level of α=0.05 was used. All statistical analyses
were performed using SPSS software version 19.0 (SPSS Inc.,
Chicago, IL, USA).
Results
Models of CRF and AVF
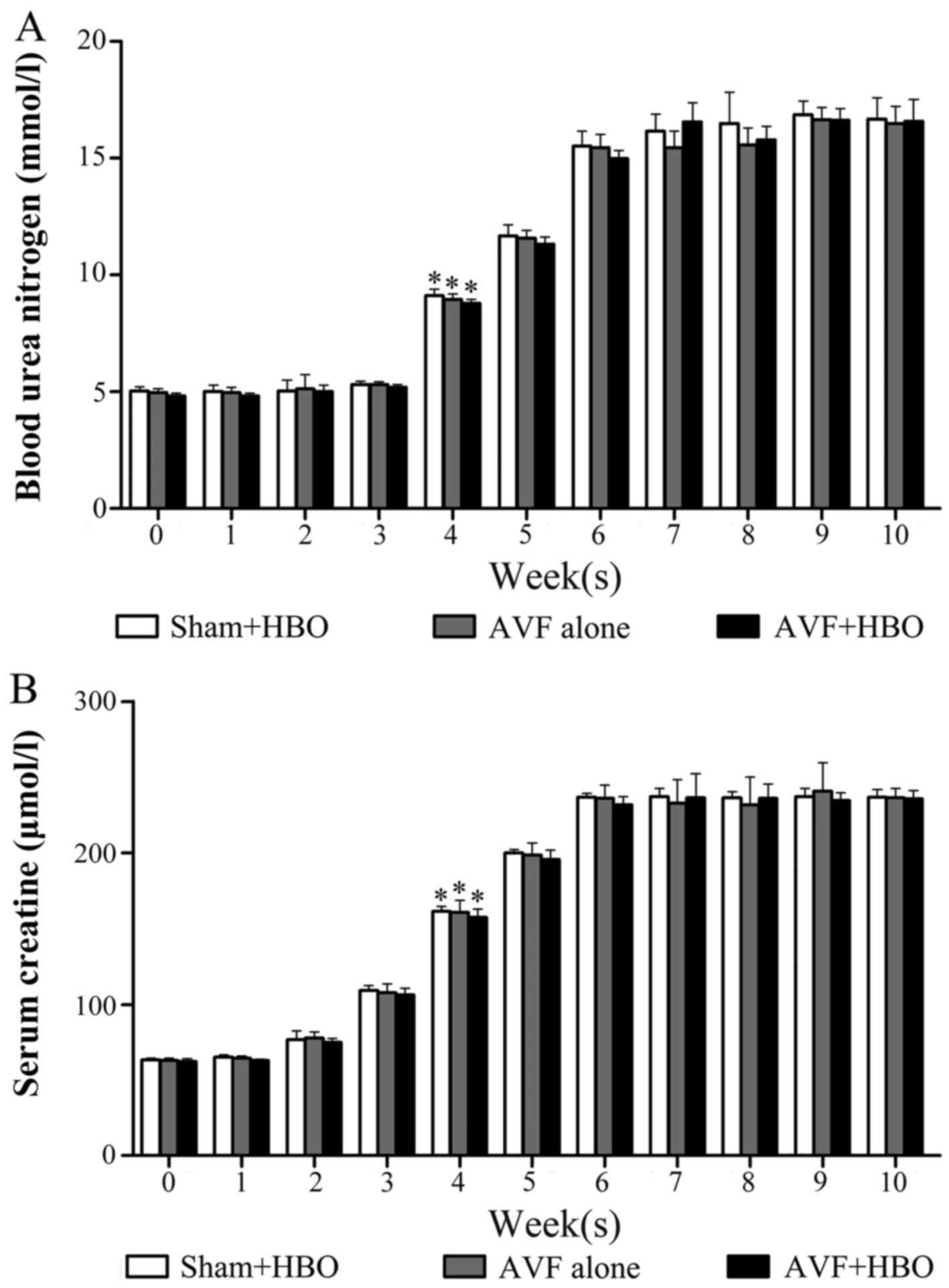
There was a significant increase in the levels of
SCr and BUN during the induction phase; however, these levels
stabilized and were essentially unaltered during the maintenance
phase. Specifically, the levels of SCr and BUN were significantly
increased at the fourth week, and they continually increased to
~3-fold greater than the normal range at the sixth week, suggesting
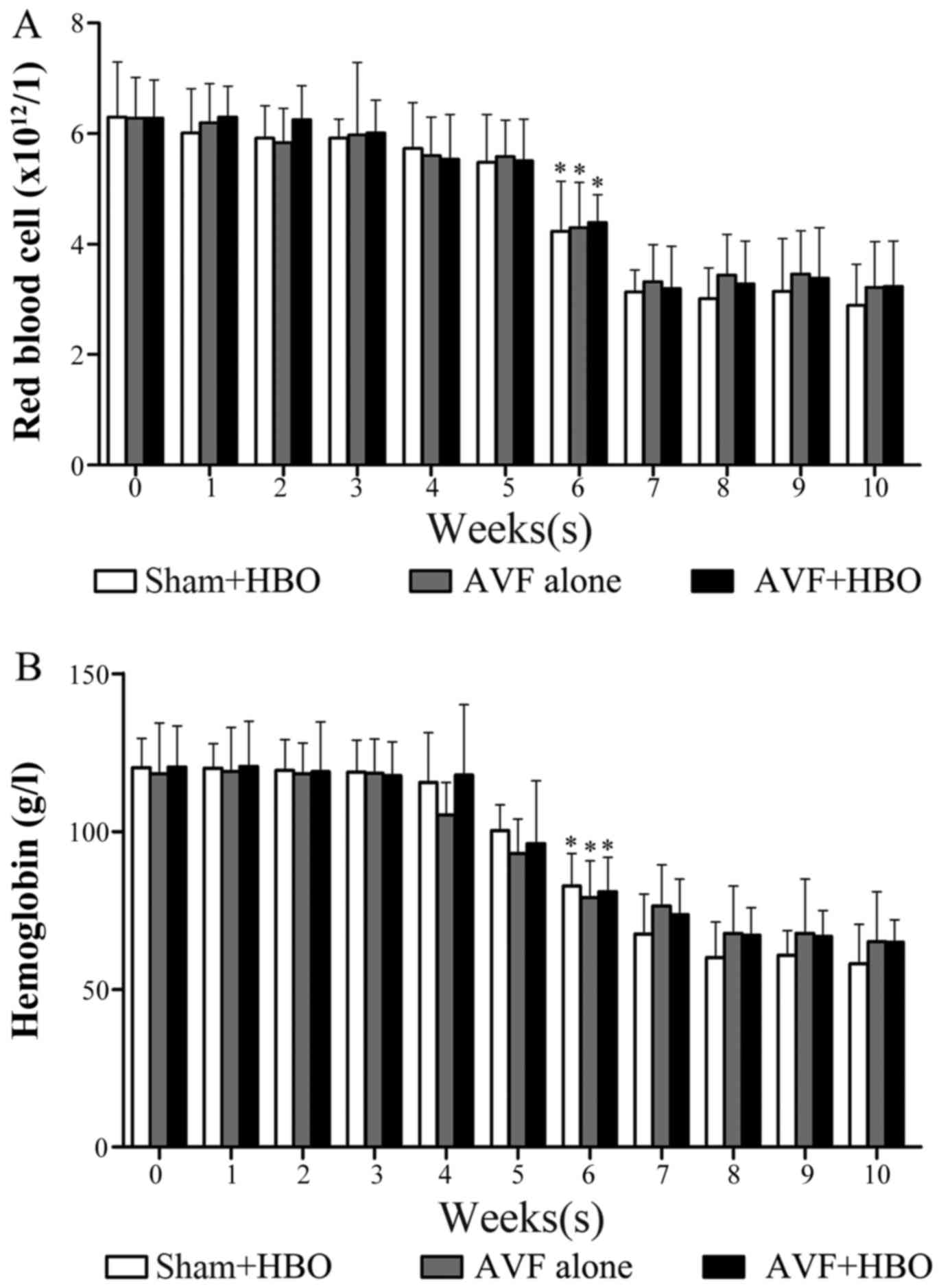
that a successful model of CRF was developed (Fig. 2). The average levels of RBCs and
Hb were notably decreased at the sixth week and were stabilized by
the end of the experiment (Fig.
3). No rabbits died due to adenine administration or HBO
treatment.
HBO improves paO2
During HBO administration, paO2 and
paCO2 in both HBO-treated groups were significantly
higher than those in the non-HBO treated group. Obvious changes in
blood pH and HCO3− values were not observed
during the experiment (Table
I).
 | Table IArterial blood gas parameters in
rabbits during hyperbaric oxygen treatment. |
Table I
Arterial blood gas parameters in
rabbits during hyperbaric oxygen treatment.
| Group | pH | PaO2
(mmHg) | PaCO2
(mmHg) |
HCO3− (mmol/l) |
|---|
| Sham + HBO
(n=32) | 7.25±0.02 |
995.29±59.57a | 37.94±7.00a | 19.19±1.11 |
| AVF alone
(n=32) | 7.24±0.05 | 81.18±12.35 | 29.04±5.40 | 19.48±3.20 |
| AVF + HBO
(n=32) | 7.24±0.02 |
1001.46±53.53a | 36.25±7.71a | 19.20±1.96 |
| Reference
values | 7.31±0.09 | 96.31±1.24 | 33.05±2.18 | 20.60±9.14 |
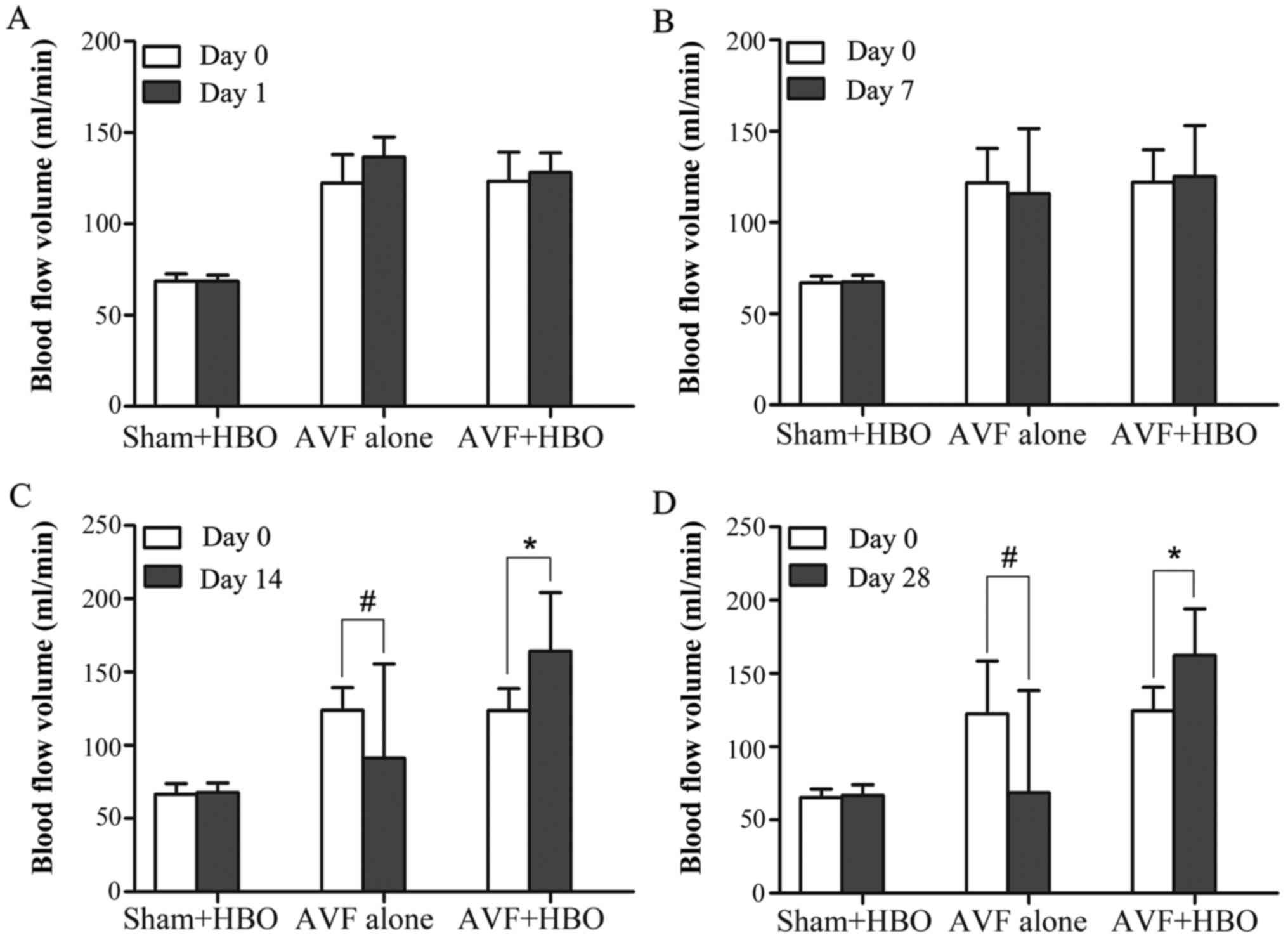
HBO increases blood flow in the proximal
vein
The comparison of blood flow was performed within
subgroups before and after HBO treatment. At 14 and 28 days after
HBO treatment, blood flow in the AVF + HBO group was increased
significantly compared with blood flow on day 0 (day 14,
164.32±39.98 vs. 123.55±15.02 ml/min; day 28, 162.42±31.60 vs.
124.47±16.11 ml/min; both P<0.05). Moreover, we observed that
the blood flow in the non-HBO treated subgroups was notably
decreased on days 14 and 28 compared with that on day 0 (day 14,
91.06±64.37 vs. 123.86±15.45 ml/min, day 28, 68.71±69.5 vs.
122.44±36.06 ml/min, both P<0.05), but it did not differ for the
sham-operated group (Fig. 4).
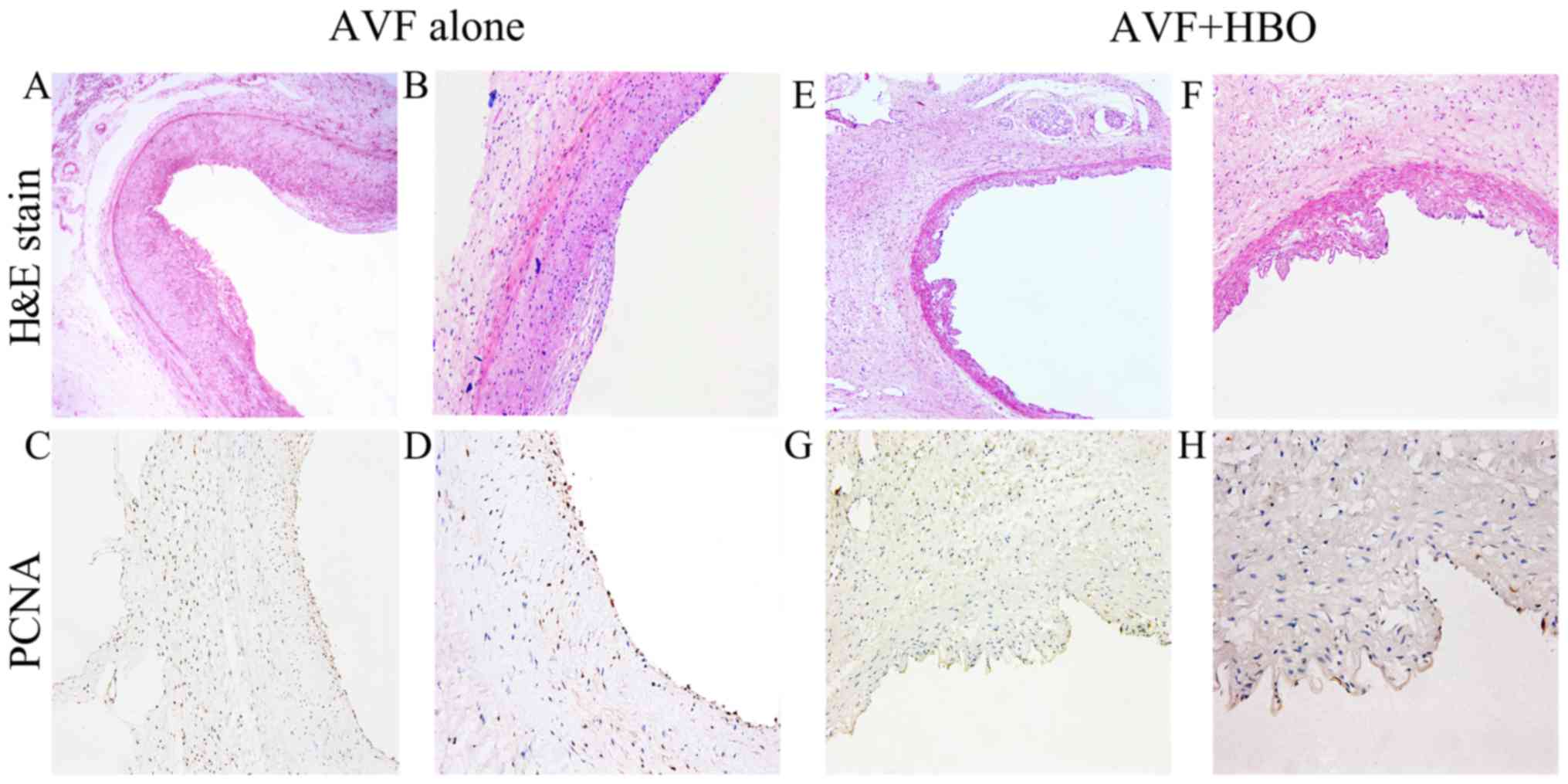
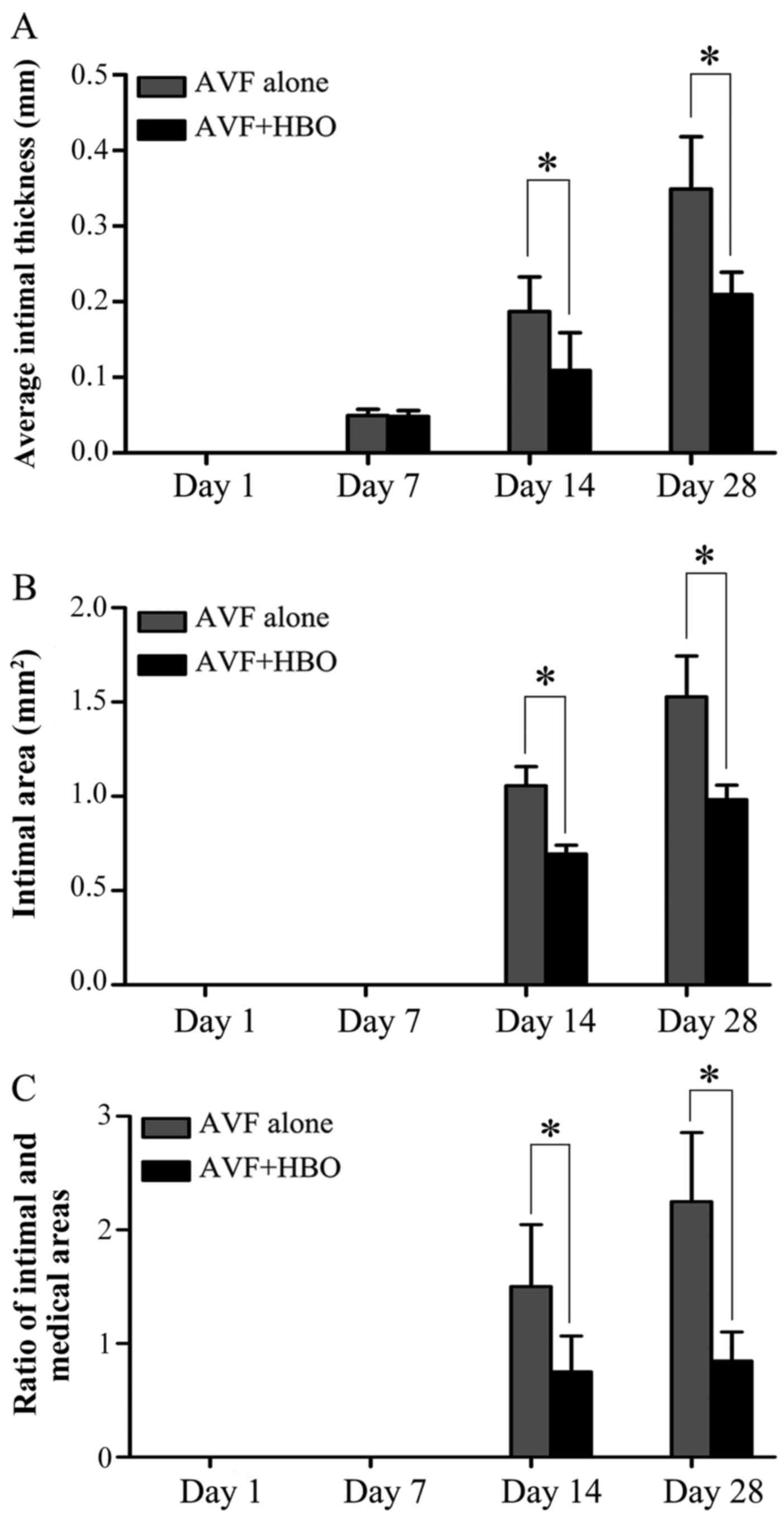
HBO attenuates neointimal formation
There was no neointimal formation in the veins of
the animals in the sham-operated group throughout the experiment.
On day 1, a change in intimal thickness was not measurable with IPP
software. The average intimal thickness was 0.0495±0.0081 mm in the
AVF alone group and 0.0482±0.0077 mm in the AVF + HBO group on day
7. On day 14, the average intimal thickness was 0.1869±0.0456 mm in
the AVF alone group and 0.1089±0.0499 mm in the AVF + HBO group
(P<0.05). By contrast, on day 28, the average intimal thickness
was 0.2091±0.0296 mm in the AVF + HBO group compared to
0.3488±0.0693 mm in the AVF alone group (P<0.05), which
indicates a reduction of 40.1% in the AVF + HBO group (Figs. 5 and 6A).
On day 7, a change in intimal area was not
measurable with IPP software. On day 14, the intimal area was 1.06
mm2 in the AVF alone group compared to 0.69
mm2 in the AVF + HBO group (P<0.05). By contrast, on
day 28, there was a statistically significant 35.3% decrease in the
intimal area in the HBO group when compared to the intimal area in
the AVF alone group (0.99±0.077 vs. 1.53±0.22 mm2,
P<0.05) (Figs. 5 and 6B).
On days 14 and 28 after HBO treatment, the ratio of
the intima and media areas in the AVF + HBO group was significantly
decreased compared to that in the non-HBO treated group (Figs. 5 and 6C).
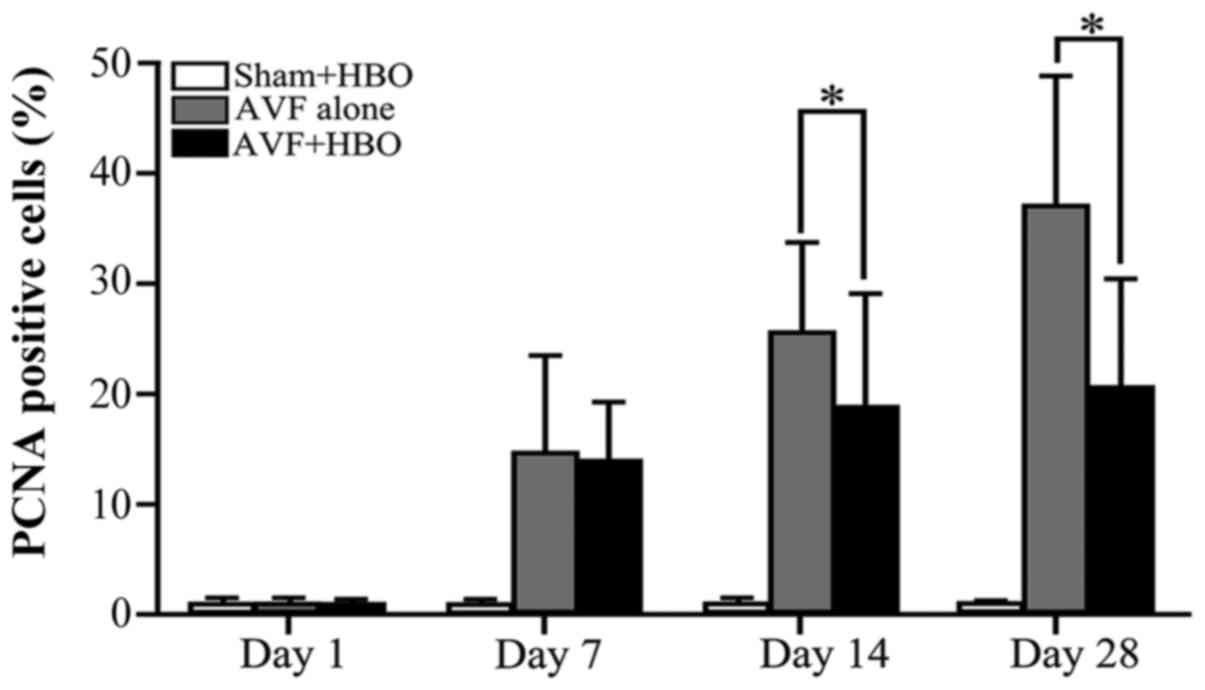
HBO downregulates PCNA expression
In the sham-operated group, there was a very weak
nuclear expression of PCNA throughout the experiment, with no
statistically significant differences. On day 1, there was no
statistically significant difference in the percentage of
PCNA-positive cells among the 3 groups. However, on day 7, compared
with the sham-operated group, the number of PCNA-positive cells
increased in the 2 AVF groups, with no significant difference. On
days 14 and 28, compared with the non-HBO treated group, the
percentage of PCNA-positive cells was decreased in the AVF + HBO
group (Figs. 5 and 7).
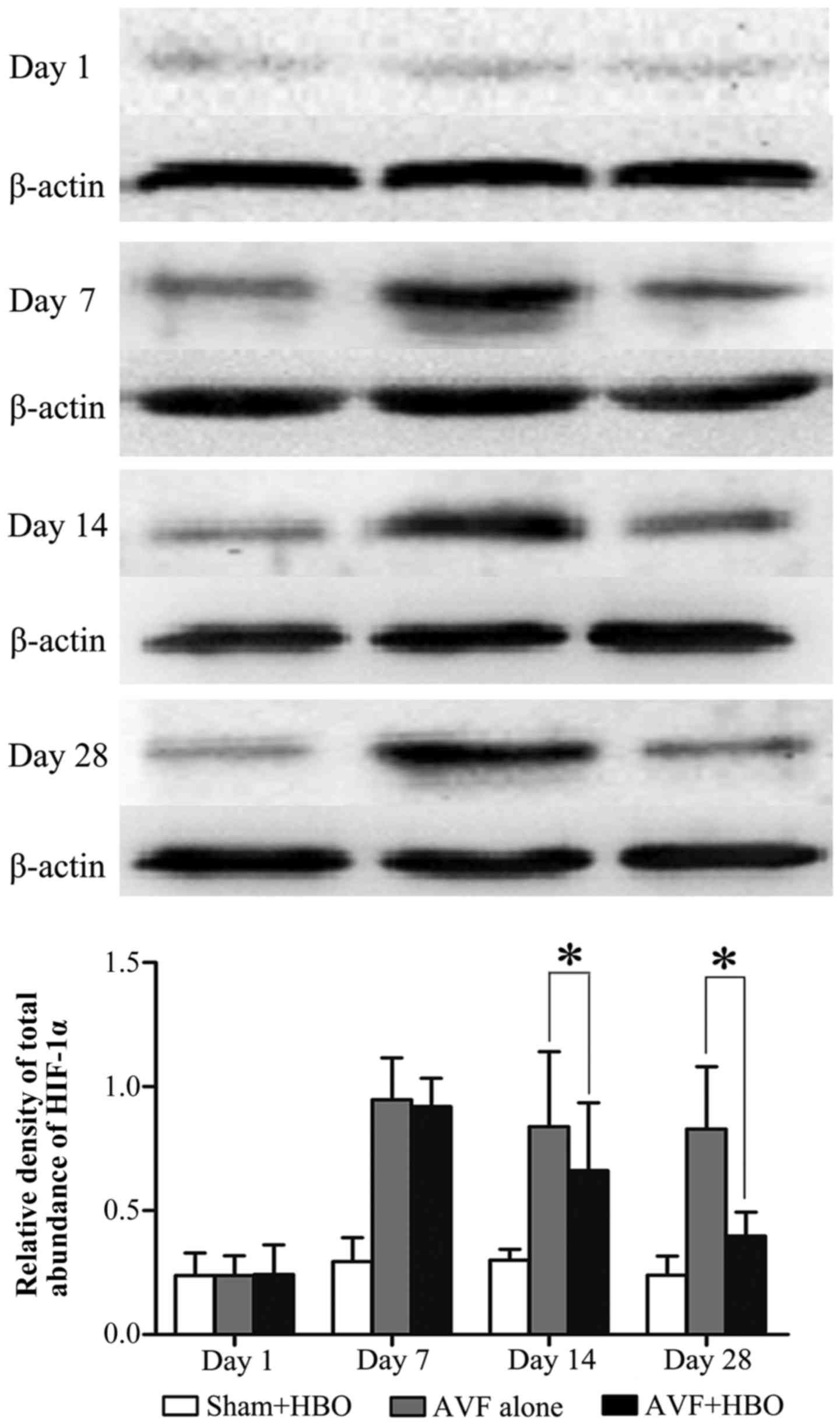
HBO downregulates the protein expression
of HIF-1α
In the next experiment, we further examined HIF-1α
protein expression by western blot analysis. Arteriovenous
fistulization significantly upregulated HIF-1α protein expression
in the 2 AVF groups. However, on days 7, 14 and 28 after HBO
treatment, the HIF-1α protein level was significantly decreased in
the AVF + HBO group (Fig. 8).
Discussion
AVF has been referred to as the 'lifeline' of
patients with ESRD. However, due to early AVF failure resulting
from VNH, reduced blood flow, and even thrombosis and occlusion,
dialysis therapy cannot be carried out on a regular treatment
schedule for many patients. The effective treatment and prevention
of VNH in clinical practice have continued to elude physicians.
Strategies to prevent VNH, including pharmacological therapy
(16), brachytherapy (17) and even gene-directed therapy
(18), have yet to be widely used
clinically. This study demonstrated that HBO inhibited VNH and
increased blood flow in the AVF in a rabbit model of CRF.
Previously, the majority of studies on hemodialysis
vascular access were performed on animal models with normal kidney
function, which does not adequately simulate the clinical scenario.
It is well recognized that ESRD is associated with increased
oxidative stress and chronic inflammation. Nitrotyrosine and
peroxynitrate, products of oxidative stress, increase the
expression of VEGF and MMPs (19). Inflammatory blood markers, such as
high-sensitivity C-reactive protein, interleukin-6 and tumor
necrosis factor-α, are associated with the magnitude of VNH and the
development of thrombosis in early AVF failure (20). A previous study by Kokubo et
al concluded that chronic kidney disease accelerated the
development of neointimal hyperplasia at the anastomotic site of an
AVF (21). In this study, the SCr
and BUN levels in all rabbits increased at the fourth week after
adenine administration. At the sixth week, the SCr and BUN levels
continued to increase, to ~3-fold greater than the normal range,
and they then plateaued and remained stable until 10 weeks.
Moreover, decreases in the RBC and Hb counts were observed. These
pathological changes occurred in rabbits that successfully modeled
the clinical course of progressive human kidney disease. We
consider that this model is particularly suitable for testing HBO
treatment methods, due to the fact that the reduction in
paO2 in animals with CRF may be ameliorated by HBO
without the need for Hb. Additional advantages of our model include
small inter-individual variation in renal function and zero
mortality, limiting the number of animals required for
induction.
There are two factors required for AVF maturation.
First, the AVF should have adequate blood flow to support dialysis;
second, it should have sufficient size to allow successful repeated
cannulation. Accordingly, increasing the blood flow and vascular
diameter of the AVF as treatment targets will improve the
maturation rate of AVF. VNH is the common factor influencing both
blood flow and vascular diameter, and it plays an important role in
early AVF failure, which results from the abnormal migration and
proliferation of VSMCs together with matrix deposition in the
venous segments. HIF-1α is expressed in all nucleated cells and
functions as a master regulator of gene transcription, mediating
cellular homeostatic responses to altered oxygenation (22), whose expression increases
exponentially as oxygen concentration declines. HIF-1α has been
reported to be a potent regulator of VEGF, MMPs and other genes
that have been implicated in VNH (5,7).
In our study, HIF-1α protein expression was observed in the AVF
alone and AVF + HBO group on days 7, 14 and 28 after AVF placement,
with no obvious expression observed in the sham-operated group.
These data suggest that surgical trauma will result in hypoxia at
the venous wall, consistent with previous findings showing
increased hypoxic levels in the vascular segments in animal models
of prosthetic vascular graft to artery anastomosis (23). The outer two-thirds of the
vascular wall is supplied with oxygen via the vasa vasorum,
although the inner one-third of the vascular wall is supplied via
the luminal diffusion of oxygen (24). One possible reason for vascular
hypoxia is the inevitable damage to vascular endothelial cells and
the vasa vasorum due to the incision and suture of the vascular
wall and adventitia dissected in the surgical process, which
prevents the diffusion of blood from the luminal side and
adventitial surface. Additionally, under conditions of uremia,
occlusive diseases of the vasa vasorum and endothelial cell
dysfunction further exacerbate hypoxia in the vascular wall
(25). The present study also
demonstrated that the number of PCNA-positive cells in venous
segments increased between days 7 and 28, when the venous wall is
most hypoxic, and the greatest change in intimal area occurred
between days 14 and 28. Previous studies have reported that the
release of VEGF, MMPs, platelet-derived growth factor, and
fibroblast growth factor increased when endothelial cells or VSMCs
were under hypoxic conditions (9,26,27). Further research revealed that the
expression of these factors may be upregulated by the
HIF-1α-dependent signaling pathway, causing cellular proliferation
and migration (28,29). A cascade of events then occurs,
whereby vascular wall hypoxia can continue to incite cellular
activity, such that the vessel becomes occluded secondary to
intimal hyperplasia.
The studies by Wan et al (9), Lata et al (10) and Santilli et al (30) demonstrated that improvement in
inhaled oxygen concentration under normal atmospheric pressure can
reverse hypoxia and intimal hyperplasia of the vascular wall in
animal models of AVF with normal renal function (9,10,30). However, the CRF model has lower
arterial oxygen capacity due to anemia, which differs from the
models with normal renal function. Therefore, HBO may have an
advantage in reversing tissue hypoxia due to its ability to
increase the arterial oxygen content and paO2 by
increasing the dissolved oxygen concentration in the plasma. The
increased paO2 improves the driving force and the
distance of diffusion in tissues. In this study, the RBC count and
Hb level were decreased in adenine-induced CRF, resembling the
clinical characteristics of uremic patients. HBO treatment notably
increased the paO2 ~10-fold higher than that in the
non-HBO treated group. The AVF-induced expression of HIF-1α and
PCNA was significantly attenuated by treatment with HBO for 28
days. These results suggest that the administration of HBO may
improve oxygen levels, resulting in the inhibition of HIF-1α
expression and reduced cellular proliferation in the proximal
venous segments. Moreover, HBO therapy did result in a
statistically significant decrease in average intimal thickening by
40.1% and intimal area by 35.3% following AVF placement. Blood flow
in the venous segments increased along with the reduction of VNH.
These results confirm our hypothesis that continuous exposure to
HBO can significantly inhibit VNH and improve blood flow following
AVF placement.
There are a few limitations to the present study
that should be mentioned. Our model primarily reflects a
tubulointerstitial disease, whereas the most common cause of CRF in
humans is glomerular scarring secondary to vascular damage. Thus,
our model should not be regarded as a model of CRF per se, but
rather as a complementary model of renal failure. Furthermore, to
quantify precisely the hypoxic changes following AVF placement by
HBO treatment, the oxygen partial pressure in each layer of the
venous wall should be further measured.
Collectively, this study demonstrates that HBO
treatment protects against the development of VNH in the proximal
vein of the AVF by suppressing cellular proliferation and
attenuating hypoxia, thus ameliorating early AVF failure, which may
suggest an insightful therapeutic intervention for patients
suffering from such afflictions. However, although the beneficial
effects of HBO treatment were demonstrated in the present study,
the underlying molecular mechanisms that mediate these protective
effects remain to be elucidated and warrant further clarification
in future studies.
Abbreviations:
|
AVF
|
arteriovenous fistula
|
|
VNH
|
venous neointimal hyperplasia
|
|
HBO
|
hyperbaric oxygen
|
|
CRF
|
chronic renal failure
|
Acknowledgments
This study was supported by the National Key
Clinical Specialties Construction Program of China.
References
|
1
|
Vascular Access Work Group: Clinical
practice guidelines for vascular access. Am J Kidney Dis. 48(Suppl
1): S248–S273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allon M, Robbin ML, Young CJ, Deierhoi MH,
Goodman J, Hanaway M, Lockhart ME and Litovsky S: Preoperative
venous intimal hyperplasia, postoperative arteriovenous fistula
stenosis, and clinical fistula outcomes. Clin J Am Soc Nephrol.
8:1750–1755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dember LM, Beck GJ, Allon M, Delmez JA,
Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene
T, et al Dialysis Access Consortium Study Group: Effect of
clopidogrel on early failure of arteriovenous fistulas for
hemodialysis: A randomized controlled trial. JAMA. 299:2164–2171.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asif A, Roy-Chaudhury P and Beathard GA:
Early arteriovenous fistula failure: A logical proposal for when
and how to intervene. Clin J Am Soc Nephrol. 1:332–339. 2006.
View Article : Google Scholar
|
|
5
|
Misra S, Shergill U, Yang B, Janardhanan R
and Misra KD: Increased expression of HIF-1alpha, VEGF-A and its
receptors, MMP-2, TIMP-1, and ADAMTS-1 at the venous stenosis of
arteriovenous fistula in a mouse model with renal insufficiency. J
Vasc Interv Radiol. 21:1255–1261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Misra S, Fu AA, Rajan DK, Juncos LA,
McKusick MA, Bjarnason H and Mukhopadhyay D: Expression of hypoxia
inducible factor-1 alpha, macrophage migration inhibition factor,
matrix metalloproteinase-2 and -9, and their inhibitors in
hemodialysis grafts and arteriovenous fistulas. J Vasc Interv
Radiol. 19:252–259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang B, Janardhanan R, Vohra P, Greene EL,
Bhattacharya S, Withers S, Roy B, Nieves Torres EC, Mandrekar J,
Leof EB, et al: Adventitial transduction of lentivirus-shRNA-VEGF-A
in arteriovenous fistula reduces venous stenosis formation. Kidney
Int. 85:289–306. 2014. View Article : Google Scholar
|
|
9
|
Wan J, Lata C, Santilli A, Green D, Roy S
and Santilli S: Supplemental oxygen reverses hypoxia-induced smooth
muscle cell proliferation by modulating HIF-alpha and VEGF levels
in a rabbit arteriovenous fistula model. Ann Vasc Surg. 28:725–736.
2014. View Article : Google Scholar
|
|
10
|
Lata C, Green D, Wan J, Roy S and Santilli
SM: The role of short-term oxygen administration in the prevention
of intimal hyperplasia. J Vasc Surg. 58:452–459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edwards ML: Hyperbaric oxygen therapy.
Part 1: History and principles. J Vet Emerg Crit Care San Antonio.
20:284–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shah J: Hyperbaric oxygen therapy. J Am
Col Certif Wound Spec. 2:9–13. 2010.PubMed/NCBI
|
|
13
|
Rits Y, Uzieblo M and Shanley CJ:
Hyperbaric oxygen decreases intimal thickness and area after
carotid artery balloon injury in a rat model. Ann Vasc Surg.
27:785–790. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharifi M, Fares W, Abdel-Karim I, Koch
JM, Sopko J and Adler D; Hyperbaric Oxygen Therapy in Percutaneous
Coronary Interventions Investigators: Usefulness of hyperbaric
oxygen therapy to inhibit restenosis after percutaneous coronary
intervention for acute myocardial infarction or unstable angina
pectoris. Am J Cardiol. 93:1533–1535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ducoux M, Urbach S, Baldacci G, Hübscher
U, Koundrioukoff S, Christensen J and Hughes P: Mediation of
proliferating cell nuclear antigen (PCNA)-dependent DNA replication
through a conserved p21(Cip1)-like PCNA-binding motif present in
the third subunit of human DNA polymerase delta. J Biol Chem.
276:49258–49266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Janardhanan R, Yang B, Vohra P, Roy B,
Withers S, Bhattacharya S, Mandrekar J, Kong H, Leof EB,
Mukhopadhyay D, et al: Simvastatin reduces venous stenosis
formation in a murine hemodialysis vascular access model. Kidney
Int. 84:338–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun S, Beitler JJ, Ohki T, Calderon TM,
Schechner R, Yaparpalvi R, Berman JW, Tellis VA and Greenstein SM:
Inhibitory effect of brachytherapy on intimal hyperplasia in
arteriovenous fistula. J Surg Res. 115:200–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nieves Torres EC, Yang B, Roy B,
Janardhanan R, Brahmbhatt A, Leof E, Mukhopadhyay D and Misra S:
Adventitial delivery of lentivirus-shRNA-ADAMTS-1 reduces venous
stenosis formation in arteriovenous fistula. PLoS One.
9:e945102014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee T and Roy-Chaudhury P: Advances and
new frontiers in the pathophysiology of venous neointimal
hyperplasia and dialysis access stenosis. Adv Chronic Kidney Dis.
16:329–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wasse H, Huang R, Naqvi N, Smith E, Wang D
and Husain A: Inflammation, oxidation and venous neointimal
hyperplasia precede vascular injury from AVF creation in CKD
patients. J Vasc Access. 13:168–174. 2012. View Article : Google Scholar :
|
|
21
|
Kokubo T, Ishikawa N, Uchida H, Chasnoff
SE, Xie X, Mathew S, Hruska KA and Choi ET: CKD accelerates
development of neointimal hyperplasia in arteriovenous fistulas. J
Am Soc Nephrol. 20:1236–1245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Semenza GL: Hypoxia-inducible factor 1 and
cardiovascular disease. Annu Rev Physiol. 76:39–56. 2014.
View Article : Google Scholar
|
|
23
|
Santilli SM, Wernsing SE and Lee ES:
Transarterial wall oxygen gradients at a prosthetic vascular graft
to artery anastomosis in the rabbit. J Vasc Surg. 31:1229–1239.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Santilli SM, Kronson J and Payne WD: The
effect of hypercholesterolemia on the rabbit transarterial wall
oxygen gradient. Ann Vasc Surg. 12:418–423. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morris ST and Jardine AG: The vascular
endothelium in chronic renal failure. J Nephrol. 13:96–105.
2000.PubMed/NCBI
|
|
26
|
Chanakira A, Dutta R, Charboneau R, Barke
R, Santilli SM and Roy S: Hypoxia differentially regulates arterial
and venous smooth muscle cell proliferation via PDGFR-β and VEGFR-2
expression. Am J Physiol Heart Circ Physiol. 302:H1173–H1184. 2012.
View Article : Google Scholar
|
|
27
|
Schultz K, Fanburg BL and Beasley D:
Hypoxia and hypoxia-inducible factor-1α promote growth
factor-induced proliferation of human vascular smooth muscle cells.
Am J Physiol Heart Circ Physiol. 290:H2528–H2534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schultz K, Murthy V, Tatro JB and Beasley
D: Prolyl hydroxylase 2 deficiency limits proliferation of vascular
smooth muscle cells by hypoxia-inducible factor-1{alpha}-dependent
mechanisms. Am J Physiol Lung Cell Mol Physiol. 296:L921–L927.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hughes D, Fu AA, Puggioni A, Glockner JF,
Anwer B, McGuire AM, Mukhopadhyay D and Misra S: Adventitial
transplantation of blood outgrowth endothelial cells in porcine
haemodialysis grafts alleviates hypoxia and decreases neointimal
proliferation through a matrix metalloproteinase-9-mediated pathway
- a pilot study. Nephrol Dial Transplant. 24:85–96. 2009.
View Article : Google Scholar
|
|
30
|
Santilli SM, Wernsing SE and Lee ES: The
effect of supplemental oxygen on the transarterial wall oxygen
gradients at a prosthetic vascular graft to artery anastomosis in
the rabbit. Ann Vasc Surg. 15:435–442. 2001. View Article : Google Scholar : PubMed/NCBI
|