Introduction
Atherosclerotic cardiovascular diseases are among
the major causes of health issues in the past decades. Macrophages,
particularly foam cells, play a pivotal role in the development and
progression of early- and late-stage atherosclerotic lesions
(1). Foam cell development is
related to the imbalance between lipid uptake and efflux (2). Promoting cholesterol efflux from
foam cells is important to decrease the size of atherosclerotic
plaques and protect the cells from atherosclerosis. Multiple
mechanisms are involved in cholesterol efflux, including free
diffusion, membrane adenosine triphosphate (ATP)-binding cassette
(ABC) transporters and lipoprotein receptors (3,4).
ABCA1 is a key transporter which mediates cellular
cholesterol and phospholipid efflux to lipid-poor apolipoprotein
A-I (apoA-I) in high-density lipoprotein (HDL) synthesis and
reverses cholesterol transport, which is underscored by the marked
accumulation of lipids in peripheral tissues observed in Tangier
disease (5). Furthermore,
loss-of-function mutations in the ABCA1 gene in humans positively
correlate with aortic intima thickness (6). ABCA1 mutations block the capacity of
ABCA1 to induce macrophage cholesterol efflux and have been shown
to promote atherosclerosis (7).
Thus, ABCA1 is thought to be a promising therapeutic target for the
prevention of atherosclerosis.
The expression of the ABCA1 gene in macrophages is
transcriptionally regulated by ligand-dependent nuclear receptors
(8). Peroxisome
proliferator-activated receptor γ (PPARγ) (a member of the nuclear
receptor superfamily) enhances cholesterol efflux by inducing the
transcription of the liver X receptor α (LXRα) gene and ABCA1
(9). LXRα binds heterodimers with
retinoid X receptor and follows a connection of specific DNA
response elements in the ABCA1 promoter to stimulate the ABCA1 gene
transcription. This stimulation increases ABCA1-dependent
cholesterol efflux to apoA-I (10). Thus, PPARγ/LXRα/ABCA1 signaling
represents a powerful means of stimulating cholesterol efflux in
macrophages and strongly affects the development of atherosclerotic
plaques.
Allicin is an essential anti-atherosclerotic that
has been studied for its cardioprotective properties with very
promising results (11). A recent
study reported that allicin exerts powerful effects, protecting
HUVECs from apoptosis and suggested that the protection occurs via
a mechanism involving the protection from
H2O2-mediated oxidative stress (12). In addition, allicin reduces
cholesterol levels and inhibits macrophage cytokine production
induced by lipopolysaccharide (LPS), and alters the composition of
fatty acids in mice or rats fed a high-fat acid diet (13,14). However, the role of allicin in
lipid accumulation in foam cells remains unclear.
The present study explored the effects of allicin on
lipid accumulation and cholesterol metabolism-related gene
expression in order to examine the effects of cholesterol efflux on
THP-1 macrophage-derived foam cells. Our findings demonstrate that
allicin reduces lipid accumulation through the upregulation of
ABCA1 expression via PPARγ/LXRα signaling in THP-1
macrophage-derived foam cells. Overall, the results provide a new
direction for the prevention of atherosclerosis.
Materials and methods
Materials and reagents
RPMI-1640 medium (SH30809) and fetal bovine serum
(FBS; SH30088.03HI) were acquired from (GE Healthcare Life Sciences
HyClone, Logan, UT, USA); TRIzol reagent (15596026),
Lipofectamine® 2000 transfection reagent (11668030) and
the cDNA synthesis kit (N8080234) were purchased from
Invitrogen/Thermo Fisher Scientific, Inc. (Waltham, MA, USA);
antibodies to allicin (sc-480646), phorbol myristate acetate (PMA;
sc-3576) and GW9662 (PPARγ antagonist; sc-202641), rabbit
monoclonal antibody against ABCA1 (sc-53482), and β-actin
(sc-7210), LXRα (sc-1000) and PPARγ (sc-9000) antibodies were all
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA); cell lysis buffer (P0013) was purchased from Beijing ComWin
Biotech Co., Ltd. (Beijing, China); streptomycinc and penicillin
(ST488-1 and ST488-2) were purchased from Beyotime (Shanghai,
China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; C0009) was obtained from Beyotime.
Construction of foam cell model
THP-1 cells (purchased from the Cell Culture Center,
Institute of Biochemistry and Cell Biology, Chinese Academy of Life
Sciences, Shanghai, China; cat. no. CBD11410) were cultured in
RPMI-1640 (10% FBS, 100 µg/ml streptomycin and 100 U/ml
penicillin) at 37°C in a 5% CO2 humidified atmosphere,
and then treated 100 nM PMA for 24 h. Subsequently, the medium was
replaced with fresh medium and the cells were incubated with 50
mg/ml oxydized low-density lipoprotein (ox-LDL) for 48 h to
establish the model of THP-1 macrophage-derived foam cells.
High-performance liquid chromatography
(HPLC) assays
HPLC analysis was conducted as previously described
(15). Sterol analyses were
performed using a HPLC system (2790; controlled with Empower Pro
software; Waters Corp., Milford, MA, USA). Sterols were detected
using a photodiode array detector equipped with a 4−1
liter cell (996; Waters Corp.). The analysis of cholesterol and
cholesterol esters was performed following elution with
acetonitrile (sc-477507)-isopropanol (sc-489314) (both from Santa
Cruz Biotechnology, Inc.) at 30:70 (v/v) and detected by absorbance
at 210 nm.
Cellular cholesterol efflux
experiments
Cellular cholesterol efflux analysis was conducted
as previously described (15). In
brief, the THP-1 cells were cultured with 0.2 µl Ci/ml of
[3H]cholesterol and ox-LDL (50 µg/ml) for 48 h,
followed by treatment with allicin. The cells were then washed with
phosphate-buffered saline (PBS) 3 times and incubated with
RPMI-1640 medium containing 0.1% BSA and 20 µg/ml human
plasma apoA-1 (sc-111827; Santa Cruz Biotechnology, Inc.)
overnight. Liquid scintillation counting was used to measure
[3H]cholesterol in the medium and cells. Percentage
efflux was calculated using the following equation: [total medium
counts/(total cellular counts + total medium counts)] ×100%.
Evaluation of lipid accumulation by Oil
Red O staining
THP-1 macrophage-derived foam cells were seeded in
6-well plates (4×105 cells/well). The cells were treated
with allincin, alone or together with small interfering RNA (siRNA)
or GW9662 for an additional 6 h. After 6 h, the cells were washed
with PBS 3 times (15 sec each time), and incubated with 10%
formalin 5 min. After rinsing with 60% isopropanol, the cells
incubated with fresh filtered Oil Red O solution for 15 min and
then washed with isopropanol (60%) (sc-489314), followed by
counterstaining with hematoxylin (sc-396328) (both from Santa Cruz
Biotechnology, Inc.) for 4 min and the cells were then observed and
photographed using a microscope.
MTT assay
The THP-1 macrophage-derived foam cells
(8×103/ml) were seeded in 96-well microtiter plates
(CW0543; ComWin Biotech Co., Ltd., Beijing, China). The cells were
then incubated with various concentrations of allicin (2.5, 5, 10,
20 and 40 g/l) for 24 h or were incubated with 5 g/l allicin for
different time preiods of time (3, 6, 12, 24 and 48 h).
Subsequently, 10 µl MTT solution was added to each well,
followed by incubation for 4 h at 37°C. The absorbance at the 490
nm wavelength was measured using a 2104 EnVision Multilabel Reader
(cat. no. 2104-0010; PerkinElmer, Inc., Waltham, MA, USA). Cell
viability was calculated as follows: cell viability (%) = [(allicin
A value - untreated control A value)]/[(control group A value -
untreated control A value)] ×100%.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
reagent (cat. no. 15596026; Invitrogen/Thermo Fisher Scientific,
Inc.). Subsequently, complemetary DNA was synthesized using a
reverse transcriptase kit (cat. no. N8080234; Invitrogen/Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The relative mRNA expression levels of were
determined using a SYBR-Green real-time PCR kit (cat. no. 4367659;
Agilent Technologies, Inc., Santa Clara, CA, USA) and normalized to
glyceraldehyde 3-phosphate dehydrogenase (GAPDH). qPCR was
performed using the ABI 7500 Fast Real-Time PCR system (cat. no.
4406985; Applied Biosystems/Thermo Fisher Scientific, Inc.) and the
following gene-specific primers: GAPDH sense, 5′-TGCCATCAACGA
CCCCTTCA-3′ and antisense, 5′-TGACCTTGCCCACAGCC TTG-3′; ABCA1
sence, 5′-TCCAGGCCAGTACGGAATTC-3′ and antisense,
5′-ACTTTCCTCGCCAAACCAGTAG-3′; LXRα sense,
5′-TCTGCGGTGGAGCTGTGGAA-3′ and anti-sense,
5′-TGACGCTGGGCGGAAGAAT-3′; PPARγ sense,
5′-CCTCCCTGATGAATAAAGATGG-3′ and antisense,
5′-GCAAACTCAAACTTAGGCTCCA-3′. All primers were designed using the
National Center for Biotechnology Information Primer-BLAST tool
(http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=Blast
Home). PCR was performed under the following conditions:
denaturation at 50°C for 2 min, followed by 38 cycles of 95°C for
15 sec and 60°C for 1 min. Gene expression was normalized to
internal controls and fold changes were calculated using relative
quantification (2−ΔΔCq).
Western blot analysis
The cells were lysed in RIPA buffer (cat. no. P0013;
Beyotime) and 1 mmol/l phenyl methyl sulfonyl fluoride (PMSF; cat.
no. ST506-2; Beyotime) at 94:6. The protein concentration was
determined using a BCA protein assay kit (cat. no. 23227; Thermo
Fisher Scientific, Inc.), following the manufacturer's
instructions. Proteins were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gels (cat. no. P0012A)
(10%) and then transferred onto a polyvinylidene difluoride
membranes (PVDF) (cat. no. FFP39) (both from Beyotime). The
membranes were immunoblotted with anti-β-actin (1:1,000),
anti-ABCA1 (1:500), anti-PPARγ (1:250), anti-LXRα (1:250)
antibodies at 4°C overnight. Subsequently, the corresponding
secondary antibody (1:1,000) conjugated with peroxidase and
enhanced chemiluminence reagents (cat. no. P0018; Beyotime) were
applied to visualize the targeted antigens. The protein contents
were assessed using LabWork image analysis software (cat. no.
P2403; Biomagin Systems Pvt., Ltd., Battaramulla, Sri Lanka).
Transfection with siRNA
siRNA targeting ABCA1 (Q000000019-1-B) were
purchased from RiboBio Co., Ltd. (Guangzhou, China), PPARγ
(sc-29455) and LXRα (sc-38829) were all purchased from Santa Cruz
Biotechnology, Inc. A control siRNA specific for the red
fluorescent protein (CCACTACCTGAGCACCCAG) was used as a negative
control (sc-37007; Santa Cruz Biotechnology, Inc.). The cells
(2×106 cells/well) were transfected using Lipofectamine
2000 (Invitrogen) as previously described (16). The effeciency of transfection was
examined bys by RT-qPCR and western blot analysis.
Statistical analysis
The experiments were performed in 3 or more
different repetitions. The data are presented as the means ±
standard deviation (SD). The statistical significance of
differences between groups was analyzed with the Student's t-test
using SPSS 11.0 and GraphPad Prism 5.0 software. Values of P≤0.05
were considered to indicate statistically significant
differences.
Results
Effect of allicin on the viability of
THP-1 macrophage-derived foam cells
First, we examined the effect of allicin on THP-1
macrophage-derived foam cells. THP-1 macrophage-derived foam cells
were stimulated with PMA and ox-LDL (50 mg/l), and the cells were
then maintained in fresh serum-free medium for 4 h to synchronize
their growth. The medium was replaced with fresh serum-free medium
containing various concentrations of allicin (2.5, 5, 10, 20 and 40
g/l) and the cells were then incubated for 24 h. The results of MTT
assay indicated that the viability of THP-1 macrophage-derived foam
cells decreased with the increasing concentrations of allicin, with
the most promiment effect observed at the concentration of 40 g/l;
cell viability was not altered at a low concentration of allicin (5
g/l) (Fig. 1A). The THP-1
macrophage-derived foam cells were incubated with 5 g/l allicin for
0, 6, 12, 24 and 48 h to investigate whether allicin reduces cell
viability in a time-dependent manner. The results of MTT assay
indicated that the viability of the THP-1 macrophage-derived foam
cells was not altered when incubated with 5 g/l allicin for
different periods of time (Fig.
1B).
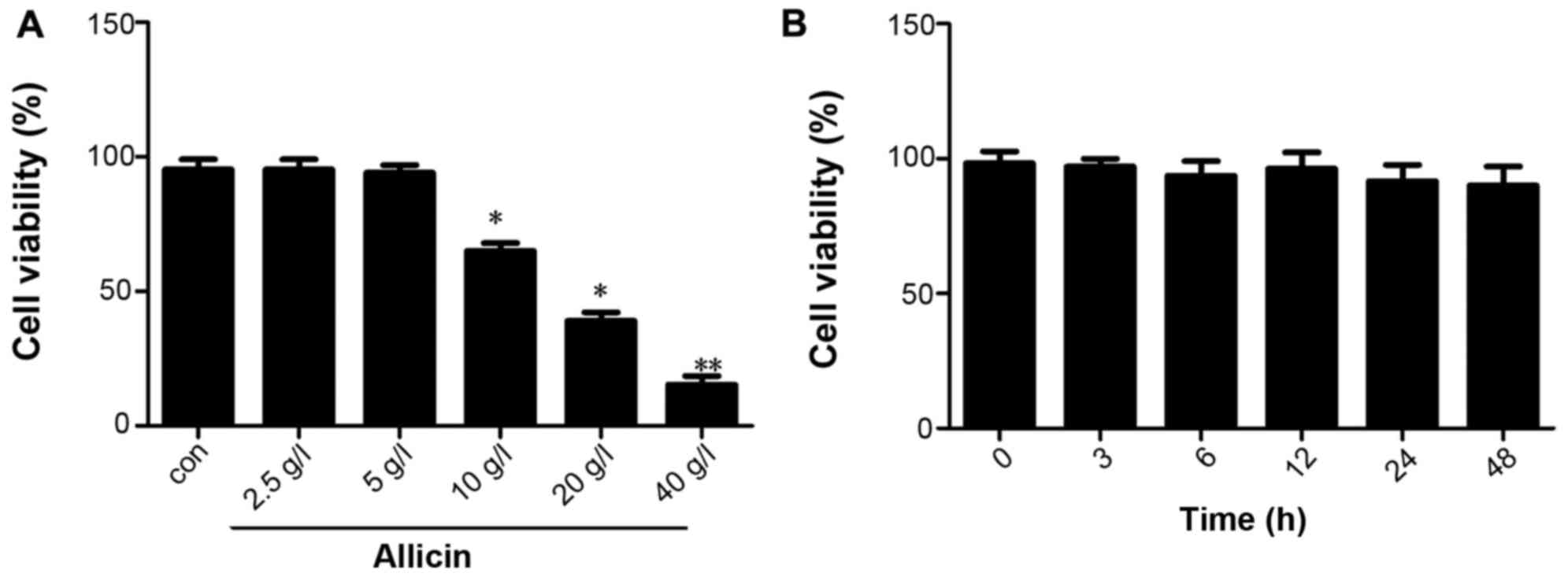 | Figure 1Effect of allicin at various
concentrations on the viability of THP-1 macrophage-derived foam
cells. (A) THP-1 macrophage-derived foam cells were stimulated with
PMA and ox-LDL (50 mg/l), and then treated with 2.5, 5, 10, 20 and
40 g/l allicin for 24 h and were subjected to MTT assay to examine
cell viability. (B) THP-1 macrophage-derived foam cells were
stimulated with PMA and ox-LDL (50 mg/l), and then treated with 5
g/l allicin for 3, 6, 12, 24 and 48 h and were subjected to MTT
assay to examine cell viability. The results are representative of
3 independent experiments. Data are expressed as the means ± SD
(n=3), *P<0.05 and **P<0.01 vs.
control. ox-LDL, oxygenized low density lipoprotein; PMA, phorbol
myristate acetate. |
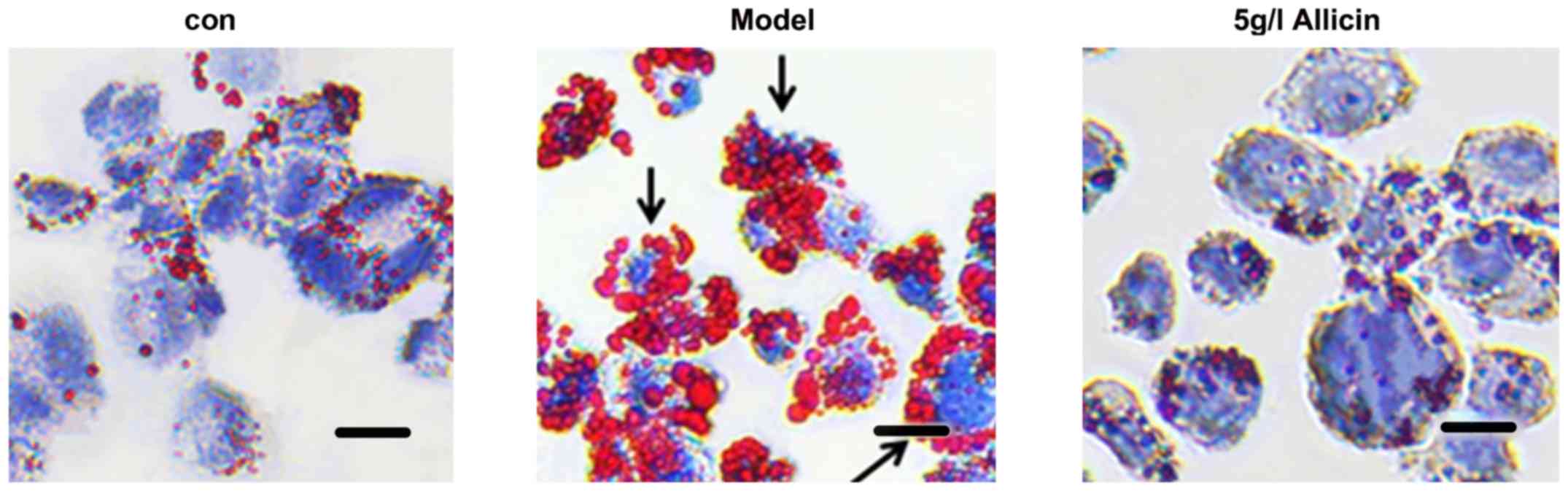
Allicin reduces lipid accumulation in
THP-1 macrophage-derived foam cells
the THP-1 macrophage-derived foam cells were
incubated with 5 g/l of allicin for 24 h to investigate whether
allicin reduces lipid accumulation. The cells were stained with Oil
Red O. Compared with the model group, 5 g/l allicin significantly
decreased intracellular lipid droplet accumulation (Fig. 2). Moreover, we detected the total
cholesterol (TC), free cholesterol (FC), and cholesterol ester (CE)
levels in THP-1 macrophage-derived foam cells following incubation
with 5 g/l allicin for 24 h. The results revealed that allicin
significantly decreased the levels of TC, FC and CE in the THP-1
macrophage-derived foam cells (Table
I). These findings demonstrated that allicin reduced lipid
accumulation in THP-1 macrophage-derived foam cells.
 | Table IEffects of allicin on free
cholesterol and cholesterol esters in in THP-1 macrophage-derived
foam cells. |
Table I
Effects of allicin on free
cholesterol and cholesterol esters in in THP-1 macrophage-derived
foam cells.
| Group | TC | FC | CE | CE/TC (%) |
|---|
| Control | 152.32±11.54 | 98.73±7.29 | 55.57±5.61 | 36.48 |
| Model group |
513.75±31.46a |
186.48±21.16a |
327.23±23.12a | 63.69a |
| Allicin (5
g/l) |
176.98±20.56b | 91.18±25.62b | 85.92±24.39b | 48.54b |
Allicin increases cholesterol efflux by
upregulating ABCA1 expression in THP-1 macrophage-derived foam
cells
Reverse cholesterol transport (RCT) is the key to
inhibit the formation of foam cells. ABCA1, a membrane transporter,
plays a critical role in cholesterol efflux, HDL metabolism and
macrophage RCT (16). Therefore,
in this study, we first examined the effects of allicin on
cholesterol efflux. The results revealed that allicin increased
cholesterol efflux in THP-1 macrophage-derived foam cells (Table II). The mRNA and protein
expression patterns of ABCA1 were detected by RT-qPCR and western
blot analysis. As shown in Fig.
3, allicin significantly upregulated ABCA1 expression at both
the mRNA and protein level.
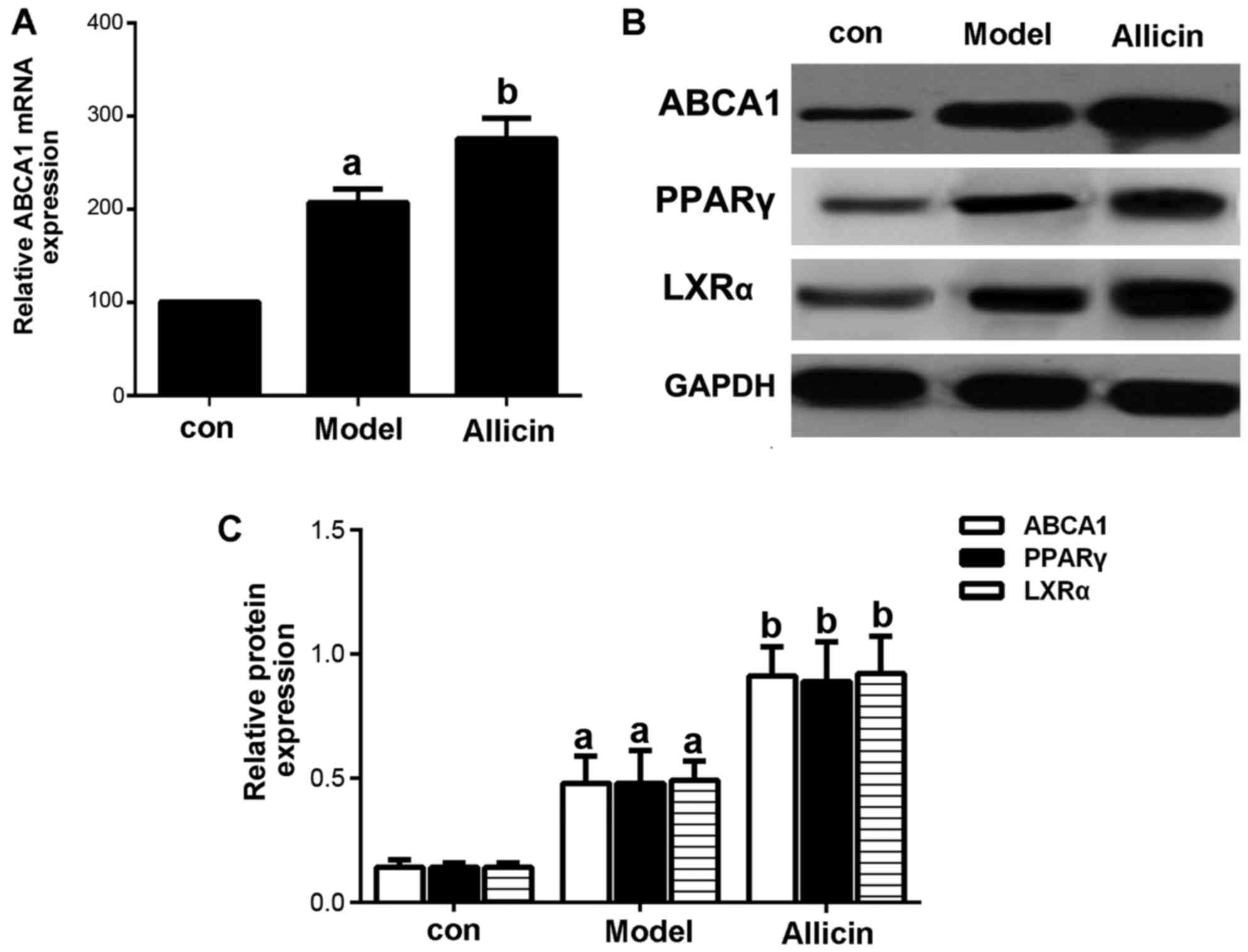 | Figure 3Allicin upregulates the expression of
ABCA1 in THP-1 macrophage-derived foam cells. (A) THP-1
macrophage-derived foam cells were stimulated with PMA and ox-LDL
(50 mg/l), and were then incubated with 50 µmol/l ABCA1
siRNA for 6 h, followed by treatment with 5 g/l allicin for 24 h.
ABCA1 mRNA expression was analyzed by RT-qPCR. (B and C) THP-1
macrophage-derived foam cells were stimulated with PMA and ox-LDL
(50 mg/l), and then treated with 5 g/l allicin for 24 h. The
protein expression of ABCA1, LXRα and PPARγ was determined by
western blot analysis. The results are representative of 3
independent experiments. aP<0.05 was used to compare
the control group; bP<0.05 was used to compare the
model group. con, control.ABCA1, adenosine triphosphate
(ATP)-binding cassette transporters A1; LXRα, liver X receptor α;
PPARγ, peroxisome proliferator-activated receptor γ; ox-LDL,
oxygenized low density lipoprotein; PMA, phorbol myristate
acetate. |
 | Table IIEffect of allicin on cholesterol
efflux in THP-1 macrophage-derived foam cells. |
Table II
Effect of allicin on cholesterol
efflux in THP-1 macrophage-derived foam cells.
| Group | Cholesterol efflux
(%) |
|---|
| Control | 8.92±1.64 |
| Model group | 18. 65±1.75a |
| Allicin (5
g/l) | 43.36±2.56b |
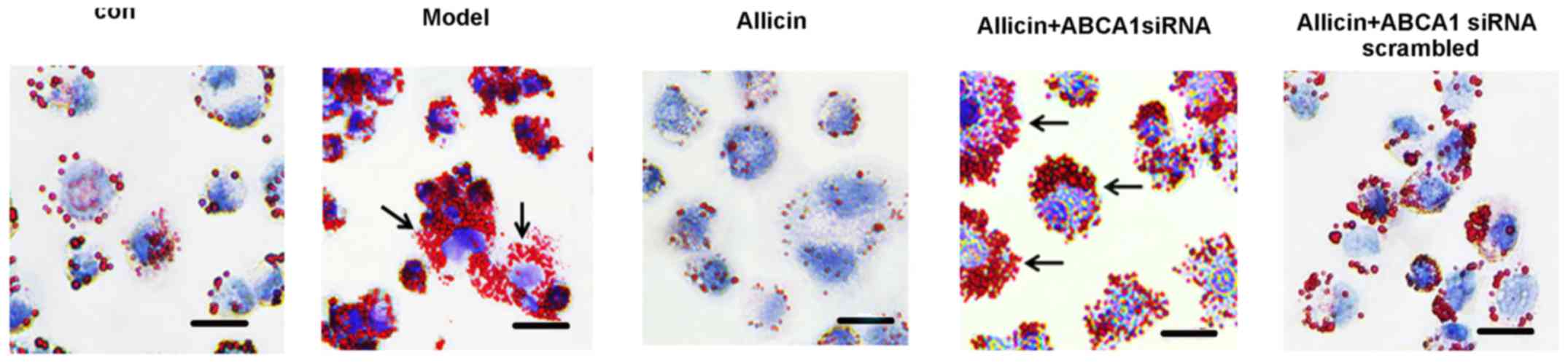
Subsequently, we further investigated whether
allicin increases cholesterol efflux by upregulating the ABCA1
expression in THP-1 macrophage-derived foam cells. Transfection
with ABCA1 siRNA eliminated the effects of allicin, thus increasing
intracellular lipid droplet accumulation, leading to higher levels
of TC, FC and CE in the transfected cells compared to the cells
treawted with allicin (Fig. 4 and
Table III). Furthermore, we
detected the protein expression of PPARγ and LXRα. As shown in
Fig. 3B, allicin increased PPARγ
and LXRα protein expression, indicating that the upregulation of
ABCA1 expression occurred via PPARγ/LXRα signaling.
 | Table IIIEffect of allicin on free cholesterol
and cholesterol esters in in THP-1 macrophage-derived foam
cells |
Table III
Effect of allicin on free cholesterol
and cholesterol esters in in THP-1 macrophage-derived foam
cells
| Group | TC | FC | CE | CE/TC (%) |
|---|
| Control | 148.32±10.48 | 97.73±6.29 | 51.57±5.72 | 36.48 |
| Model group | 543.15±22.36 | 194.48±21.16 | 349.23±33.12 | 63.69 |
| Allicin(5 g/l) |
167.68±23.36a | 97.18±21.62a | 70.82±22.39a | 42.23a |
| Allicin(5 g/l) +
ABCA1 scrambled | 163.28±23.36 | 98.38±21.62 | 64.90±22.39 | 39.74 |
| Allicin(5 g/l) +
ABCA1 siRNA |
553.15±23.36b |
198.48±27.16b |
355.23±31.12b | 64.22b |
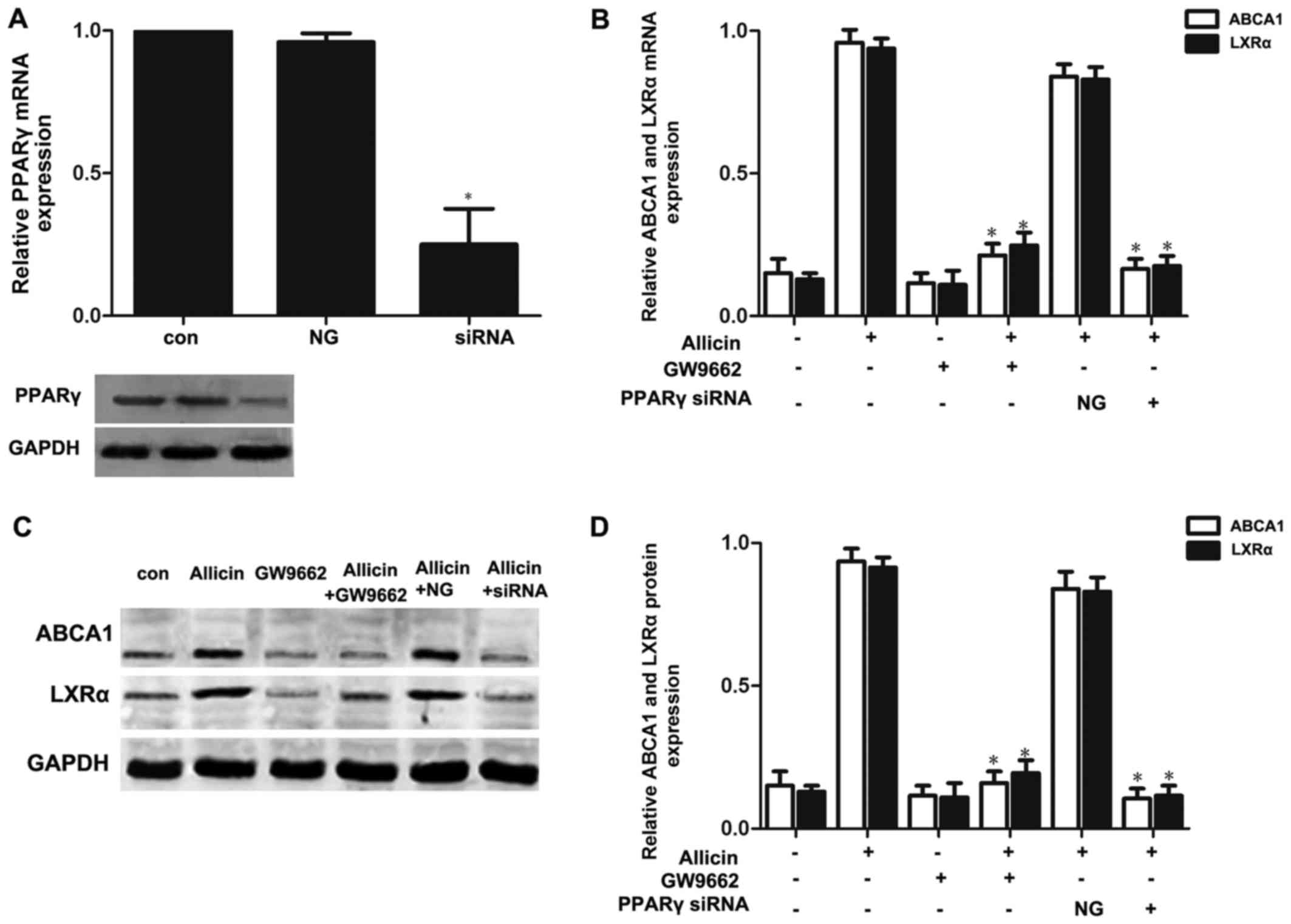
PPARγ-LXRα signaling is involved in the
allicin-induced upregulation of ABCA1 expression in THP-1
macrophage-derived foam cells
Previous studies have reported that PPARγ/LXRα
signaling is the key to upregulating ABCA1 expression (7,10).
Thus, we wished to further confirm whether allicin upregulates the
expression of ABCA1 via PPARγ/LXRα signaling in THP-1
macrophage-derived foam cells. Firstly, the THP-1
macrophage-derived foam cells were treated with PPARγ siRNA or
GW9662 (a PPARγ antagonist; 10 mmol/l) prior to exposure to 5 g/l
allicin. As shown in Fig. 5,
pre-treatment of the cells with PPARγ siRNA or GW9662 markedly
abolished the effects of allicin, leading to a decrease in the
expression of LXRα and ABCA1. These results indicate that PPARγ is
involved in the allicin-induced upregulation of ABCA1 expression,
and that LXRα may play a role in the regulation of ABCA1 expression
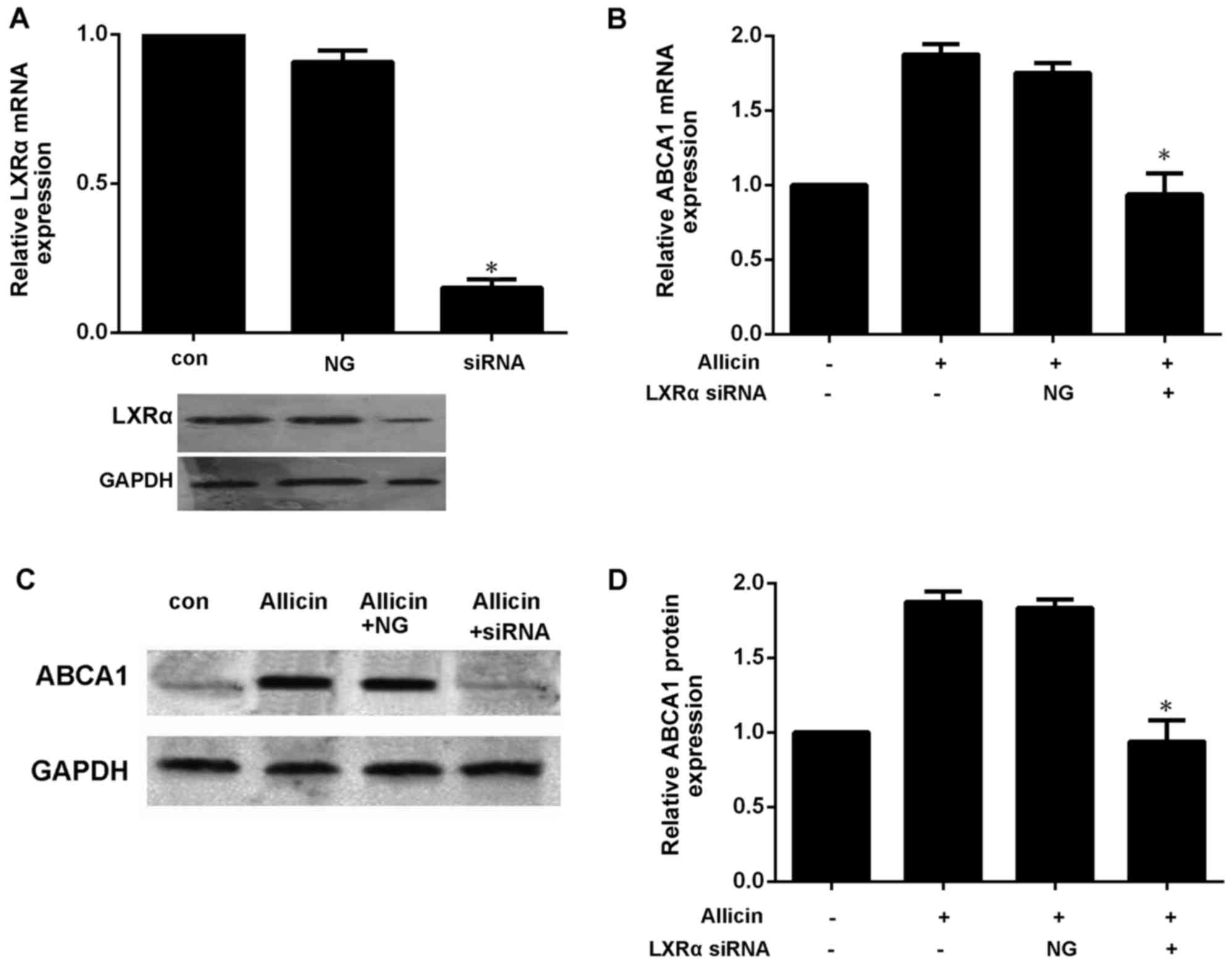
by allicin. Moreover, transfection of the THP-1 macrophage-derived
foam cells with LXRα siRNA significantly decreased the expression
of ABCA1 (Fig. 6). These results
thus indicate that allicin upregulates ABCA1 expression via
PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells.
Discussion
Both clinical and reference studies have reported
that allicin may help in delaying the progression of cardiovascular
diseases. Atherosclerosis is the basis of cardiovascular diseases;
cellular cholesterol accumulates in lipid-engorged macrophage foam
cells, thereby driving lipid deposition to the core of
atherosclerosis (1). Thus,
inhibiting the formation of foam cells is an important therapeutic
strategy for atherosclerosis. ABCA1-mediated cholesterol efflux is
a process through which excess cell cholesterol of foam cells is
picked up by HDL particles and delivered to the liver for final
excretion (3). In the present
study, our data supported the finding that allicin upregulates the
expression of ABCA1 to increase cholesterol efflux and reduce
cellular cholesterol and CE via PPARγ/LXRα signaling in THP-1
macrophage-derived foam cells.
Allicin is a major active component that can be
extracted from garlic samples. It has been demonstrated that
allicin exerts anti-inflammatory, antioxidant and lipid-modulating
effects (14). It has also been
shown that allicin can i) protect vascular endothelial cells by
delaying the oxidation of LDL, ii) prevent blood clots by
inhibiting platelet aggregation, and iii) increase HDL-C but reduce
TC, triglyceride and LDL levels (17). Animal tests have also revealed
that allicin reduces the atherosclerotic plaque area of
LDLR−/− and apoE−/− mice and significantly
reduces the level of cholesterol in mice (18); however, the molecular mechanisms
involved are unclear. Subsequent studies have indicated that
allicin can regulate the NF-κB signaling pathway, as well as
inhibit the expression of interleukin-6 and tumor necrosis factor-α
(17,19). Inflammation and cholesterol
metabolism disorder are considered major risk factors that give
rise to atherosclerosis; however, allicin can be potentially used
as an anti-atherosclerotic. However, studies on the effects exerted
by allicin on lipid accumulation in foam cells are relatively few.
On the basis of these studies, the current study determined the
effects of allicin on cholesterol efflux and lipid accumulation in
foam cells and proposed the pertinent mechanism by observing the
anti-atherosclerotic efects of allicin from the core process of
atherosclerosis occurrence. A new experimental basis was provided
for studies regarding the preventive effects of allicin on
atherosclerosis. In addition, this study demonstrated the effects
of allicin on the vitality of THP-1 foam cells. According to the
results, allicin can degrade cell vitality at higher
concentrations. However, cell vitality was not significantly
altered at <5 g/l allicin. Moreover, 5 g/l allicin was used to
examine the effects of various treatments durations, and cell
vitality was found to be unaltered with time. Finally, 5 g/l
allicin was used to process foam cells and observe any change in
lipid droplet accumulation in these cells. As shown in Fig. 2 and Table I, 5 g/l allicin decreased lipid
accumulation in foam cells and inhibited lipid droplet formation.
Thus, allicin exerted a direct inhibitory effect on lipid
accumulation in foam cells.
Cholesterol efflux is the main pathway for reducing
lipid accumulation in foam cells. ABCA1 is the core protein for
regulating RCT. By inducing cholesterol efflux from cells, allicin
significantly balanced the lipids in cells. Studies have indicated
that ABCA1 is the key protein for driving cholesterol efflux and
reducing lipid accumulation in foam cells. Xu et al
(20) and Liu et al
(21) reported that the
upregulated expression of ABCA1 significantly facilitated the
efflux of cholesterol from THP-1-derived foam cells; it also reduce
the levels of TC, FC and CE within foam cells, and reduced lipid
accumulation in foam cells. By contrast, the inhibition of ABCA1
expression may also hinder cholesterol efflux and facilitate lipid
accumulation in foam cells. Westerterp et al (22) and He et al (23) also confirmed this observation. On
the basis of previous results, we deduced that ABCA1 is the key
protein for reducing lipid accumulation in foam cells through the
effects of allicin. To prove the correctness of this deduction, we
observed the change in ABCA1 expression along with cholesterol
efflux from foam cells after processing these cells with 5 g/l
allicin. The results indicated that allicin indeed upregulated
ABCA1 expression in foam cells and facilitated cholesterol efflux.
Subsequently, the cells were transected with ABCA1 siRNA. Allicin
was found to facilitate cholesterol efflux from the foam cells, and
this reducing effect on lipid accumulation in foam cells was
reversed by ABCA1 siRNA. These observations confirmed our deduction
and indicated that the upregulation of ABCA1 facilitates
cholesterol efflux and decreases lipid accumulation in foam cells
through allicin treatment.
The PPARγ/LXRα pathway is the core mechanism for
regulating ABCA1 expression, and the effect of this pathway on
ABCA1 expression has been widely accepted (24). Liver X receptor, as a nuclear
transcription factor, can regulate multiple genes in the
cholesterol-transporting pathway, e.g., transcriptional regulation
of ABCA1 and ABCG1 (25). Another
study demonstrated that the LXR stimulant, T0901317, inhibited the
progression of atherosclerosis in mice (26). Cellular experiments have confirmed
that T0901317 upregulates ABCA1 and ABCG1 expression by activating
LXRα in macrophages, thereby driving cholesterol within the cells
to flow to apoAI and HDL and inhibiting the formation of foam cells
(27). However, another study
indicated that LXRα expression was regulated by other nuclear
transcription factors, e.g., PPAR (28). PPAR, a type of nuclear
transcription factor, has 3 subtypes: PPARα, PPARβ and PPARγ. These
nuclear transcription factors are combined with their respective
ligands to alter spatial conformation and subsequently combine with
the PPAR response element within the target gene promoter to
regulate the transcription of the target gene. These nuclear
transcription factors can also activate LXRα and combine with AX
receptor to form a heterodimer, which regulates the transcription
of the target gene. Among the 3 PPAR subtypes, PPARγ can regulate
glucose and lipid metabolism, as well as inflammatory response and
immunity (29,30). Hence, these transcription factors
are the key to the transcriptional regulation of cell
differentiation and lipid metabolism. It has been demonstrated that
PPARγ upregulates LXRα expression (31) and that allicin upregulates PPARγ
expression and regulates the inflammatory response of endothelial
cells (17). Therefore, the
PPARγ/LXRα pathway contributes to the promoting effect of allicin
on ABCA1 expression. As shown in Fig.
3, that allicin upregulated PPARγ and LXRα protein expression
in THP-1 foam cells. Furthermore, to determine the participation of
the PPARγ/LXRα pathway in the upregulation of ABCA1 expression by
allicin, we transfected the THP-1 foam cells with PPARγ siRNA or an
inhibitor, along with LXRα siRNA. Subsequently, we observed any
change in ABCA1 expression. The results indicated that following
transfection with PPARγ siRNA or an inhibitor along with LXRα
siRNA, the upregulatory effect of allicin on ABCA1 expression was
significantly reversed. Therefore, allicin upregulates ABCA1
expression, facilitates cholesterol efflux, and reduces lipid
accumulation in THP-1 foam cells by activating the PPARγ/LXRα
pathway.
In conclusion, this study proves that allicin
reduces lipid accumulation through the upregulation of ABCA1
expression via PPARγ/LXRα signaling in THP-1 macrophage-derived
foam cells. Treatment with allicin in the field of cardiovascular
disease and the use of arsenic drug research and development may
provide a novel strategy for the prevention and/or treatment of
atherosclerosis. These findings offer a new perspective on the use
of allicin for the treatment of atherosclerosis.
Acknowledgments
The present study was supported by grants from the
Medical Foundation of Huizhou (no. 2015Y134); the Medical Research
Foundation of Guangdong (no. A2015620); the Graduate Student
Research Innovation Project of Hunan (no. CX2013B396); and the
Natural Science Foundation of China (grant no. 81600342).
References
|
1
|
Zeller I and Srivastava S: Macrophage
functions in atherosclerosis. Circ Res. 115:e83–e85. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu XH, Fu YC, Zhang DW, Yin K and Tang CK:
Foam cells in atherosclerosis. Clin Chim Acta. 424:245–252. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Westerterp M, Bochem AE, Yvan-Charvet L,
Murphy AJ, Wang N and Tall AR: ATP-binding cassette transporters,
atherosclerosis, and inflammation. Circ Res. 114:157–170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heinecke JW: Small HDL promotes
cholesterol efflux by the ABCA1 pathway in macrophages:
Implications for therapies targeted to HDL. Circ Res.
116:1101–1103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Negi SI, Brautbar A, Virani SS, Anand A,
Polisecki E, Asztalos BF, Ballantyne CM, Schaefer EJ and Jones PH:
A novel mutation in the ABCA1 gene causing an atypical phenotype of
Tangier disease. J Clin Lipidol. 7:82–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pervaiz MA, Gau G, Jaffe AS, Saenger AK,
Baudhuin L and Ellison J: A Non-classical presentation of tangier
disease with three ABCA1 mutations. JIMD Rep. 4:109–111. 2012.
View Article : Google Scholar
|
|
7
|
Fitzgerald ML, Mujawar Z and Tamehiro N:
ABC transporters, atherosclerosis and inflammation.
Atherosclerosis. 211:361–370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang CX and Zhang YL: The target of
regulating the ATP-binding cassette A1 protein (ABCA1): Promoting
ABCA1-mediated cholesterol efflux in different cells. Curr Pharm
Biotechnol. 14:623–631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chinetti G, Lestavel S, Bocher V, Remaley
AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, et
al: PPAR-alpha and PPAR-gamma activators induce cholesterol removal
from human macrophage foam cells through stimulation of the ABCA1
pathway. Nat Med. 7:53–58. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmitz G and Langmann T: Transcriptional
regulatory networks in lipid metabolism control ABCA1 expression.
Biochim Biophys Acta. 1735:1–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borlinghaus J, Albrecht F, Gruhlke MC,
Nwachukwu ID and Slusarenko AJ: Allicin: Chemistry and biological
properties. Molecules. 19:12591–12618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen S, Tang Y, Qian Y, Chen R, Zhang L,
Wo L and Chai H: Allicin prevents
H2O2-induced apoptosis of HUVECs by
inhibiting an oxidative stress pathway. BMC Complement Altern Med.
14:321–329. 2014. View Article : Google Scholar
|
|
13
|
Sung J, Harfouche Y, De La Cruz M, Zamora
MP, Liu Y, Rego JA and Buckley NE: Garlic (Allium sativum)
stimulates lipopolysaccharide-induced tumor necrosis factor-alpha
production from J774A.1 murine macrophages. Phytother Res.
29:288–294. 2015. View
Article : Google Scholar
|
|
14
|
Gonen A, Harats D, Rabinkov A, Miron T,
Mirelman D, Wilchek M, Weiner L, Ulman E, Levkovitz H, Ben-Shushan
D, et al: The antiatherogenic effect of allicin: Possible mode of
action. Pathobiology. 72:325–334. 2005. View Article : Google Scholar
|
|
15
|
Lin XL, Liu MH, Hu HJ, Feng HR, Fan XJ,
Zou WW, Pan YQ, Hu XM and Wang Z: Curcumin enhanced cholesterol
efflux by upregulating ABCA1 expression through AMPK-SIRT1-LXRα
signaling in THP-1 macrophage-derived foam cells. DNA Cell Biol.
34:561–572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin X, Freeman SR, Vaisman B, Liu Y, Chang
J, Varsano N, Addadi L, Remaley A and Kruth HS: ABCA1 contributes
to macrophage deposition of extracellular cholesterol. J Lipid Res.
56:1720–1726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mo SJ, Son EW, Rhee DK and Pyo S:
Modulation of TNF-alpha-induced ICAM-1 expression, NO and
H2O2 production by alginate, allicin and
ascorbic acid in human endothelial cells. Arch Pharm Res.
26:244–251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li RK, Li JF, Zhou XR, Yin M, Zhang L and
Pan J: Effects of allicin on plasma lipid metabolism of
atherosclerotic mice. Chin J Clin (Electronic Version). 1:29–33.
2007.
|
|
19
|
Li C, Lun W, Zhao X, Lei S, Guo Y, Ma J
and Zhi F: Allicin alleviates inflammation of
trinitrobenzenesulfonic acid-induced rats and suppresses p38 and
JNK pathways in Caco-2 cells. Mediators Inflamm. 2015:4346922015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu X, Li Q, Pang L, Huang G, Huang J, Shi
M, Sun X and Wang Y: Arctigenin promotes cholesterol efflux from
THP-1 macrophages through PPAR-γ/LXR-α signaling pathway. Biochem
Biophys Res Commun. 441:321–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu XY, Lu Q, Ouyang XP, Tang SL, Zhao GJ,
Lv YC, He PP, Kuang HJ, Tang YY, Fu Y, et al: Apelin-13 increases
expression of ATP-binding cassette transporter A1 via activating
protein kinase C α signaling in THP-1 macrophage-derived foam
cells. Atherosclerosis. 226:398–407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Westerterp M, Murphy AJ, Wang M, Pagler
TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X,
Abramowicz S, et al: Deficiency of ATP-binding cassette
transporters A1 and G1 in macrophages increases inflammation and
accelerates atherosclerosis in mice. Circ Res. 112:1456–1465. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He Y, Zhang L, Li Z, Gao H, Yue Z, Liu Z,
Liu X, Feng X and Liu P: RIP140 triggers foam-cell formation by
repressing ABCA1/G1 expression and cholesterol efflux via liver X
receptor. FEBS Lett. 589:455–460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chinetti-Gbaguidi G, Baron M, Bouhlel MA,
Vanhoutte J, Copin C, Sebti Y, Derudas B, Mayi T, Bories G,
Tailleux A, et al: Human atherosclerotic plaque alternative
macrophages display low cholesterol handling but high phagocytosis
because of distinct activities of the PPARγ and LXRα pathways. Circ
Res. 108:985–995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen SG, Xiao J, Liu XH, Liu MM, Mo ZC,
Yin K, Zhao GJ, Jiang J, Cui LB, Tan CZ, et al: Ibrolipim increases
ABCA1/G1 expression by the LXRα signaling pathway in THP-1
macrophage-derived foam cells. Acta Pharmacol Sin. 31:1343–1349.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kappus MS, Murphy AJ, Abramowicz S, Ntonga
V, Welch CL, Tall AR and Westerterp M: Activation of liver X
receptor decreases atherosclerosis in Ldlr−/− mice in
the absence of ATP-binding cassette transporters A1 and G1 in
myeloid cells. Arterioscler Thromb Vasc Biol. 34:279–284. 2014.
View Article : Google Scholar
|
|
27
|
Ma AZ, Song ZY and Zhang Q: Cholesterol
efflux is LXRα isoform-dependent in human macrophages. BMC
Cardiovasc Disord. 14:802014. View Article : Google Scholar
|
|
28
|
Parikh M, Patel K, Soni S and Gandhi T:
Liver X receptor: A cardinal target for atherosclerosis and beyond.
J Atheroscler Thromb. 21:519–531. 2014.PubMed/NCBI
|
|
29
|
Monsalve FA, Pyarasani RD, Delgado-Lopez F
and Moore-Carrasco R: Peroxisome proliferator-activated receptor
targets for the treatment of metabolic diseases. Mediators Inflamm.
2013:5496272013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng X, Liu X, Song L, He Y, Li X and
Zhang H: Atorvastatin inhibits macrophage-derived foam cell
formation by suppressing the activation of PPARγ and NF-κB pathway.
Nan Fang Yi Ke Da Xue Xue Bao. 34:896–900. 2014.In Chinese.
PubMed/NCBI
|
|
31
|
Baranowski M, Blachnio-Zabielska AU,
Zabielski P, Harasim E, Harasiuk D, Chabowski A and Gorski J: Liver
X receptor agonist T0901317 enhanced peroxisome
proliferator-activated receptor-delta expression and fatty acid
oxidation in rat skeletal muscle. J Physiol Pharmacol. 64:289–297.
2013.PubMed/NCBI
|