Introduction
Oral mucositis is inflammation of the oral and
oropharyngeal mucosa and usually occurs as a side-effect of cancer
chemotherapy or radiotherapy in the head and neck region (1). This condition affects 10–40% of
patients receiving the standard dose of chemotherapy for tumors and
60–100% of patients undergoing myeloablative chemotherapy for
hematopoietic stem cell transplant and high-dose radiation therapy
(2,3). Symptoms include pain, vomiting, dry
mouth and diarrhea, and sequentially secondary infection of the
oral mucosa leading to decreased quality of life (3,4).
Oral mucositis can reduce the effectiveness of chemotherapy as a
result of the alteration or discontinuation of the chemotherapy
(5). Therefore, the development
of therapeutic agents that can be combined with chemotherapy to
prevent oral mucositis as a side-effect of chemotherapy is
crucial.
The incidence of oral mucositis is divided into five
biological steps: initiation, upregulation and generation of
messenger signals, signal amplification, ulceration and healing
(6). Chemotherapy-induced damage
to cells or tissues is caused by reactive oxygen species (ROS)
(7), thus ROS play a main role in
the initiation phase of oral mucositis and activate several
transcription factors such as nuclear factor-κB (NF-κB), a key
transcription factor involved in the development of mucositis.
NF-κB increases the production of proinflammatory cytokines, such
as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and
induces apoptosis (8,9). Upregulation of proinflammatory
cytokines amplifies the primary damage (10). Recently, mechanistically based
oral mucositis drugs were evaluated and developed such as
keratinocyte growth factor (KGF), benzydamine HCl and COX-2
inhibitors (10–12).
The dried root of Salvia miltiorrhiza Bunge
(SM) (Lamiaceae), has been used in Korea, China and Japan for the
treatment of various diseases, including coronary heart disease
(13), cerebrovascular disease
(14), Alzheimer's disease
(15), Parkinson's disease
(16), renal deficiency (17), hepatocirrhosis (18), cancer (19) and bone loss (20). The antioxidant (21), antidiabetic (22) and hepatoprotective (23) effects of SM on apoptosis and
inflammation were investigated in a rat model of stroke (24). However, knowledge regarding the
effects of SM extract on oral mucositis is limited. The effects of
SM on molecular mechanisms in vitro and in vivo need
to be elucidated. In the present study, we examined whether SM
could be used in the development of a novel therapeutic agent for
the treatment of mucositis induced by 5-fluorouracil (5-FU). To
identify the effects of SM on human pharyngeal cells and hamster,
we performed scavenging of free radical activities against
2,2-diphenyl-1-picrylhydrazyl (DPPH), cell viability assay, ROS
level measurements, TUNEL assay and immunoblotting.
Materials and methods
Preparation of SM extract
SM was purchased from Kyung Hee University Medical
Center (Seoul, Korea). A 300 g sample of dried medicinal herb was
boiled in 3 l water for 2 h at 100°C, and the suspension was
filtered and evaporated under reduced pressure. The filtrate was
lyophilized and yielded 69.1 g powder. The dried extract was
dissolved in distilled deionized water (Millipore, Billerica, MA,
USA) and vortexed for 2 min at room temperature.
In vitro studies
Human pharyngeal cell culture
Human pharyngeal cell line (Detroit 562, ATCC
CCL-138) was purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The Detroit 562 cell line was cultured
in modified Eagle's medium (MEM) supplemented with 10% fetal bovine
serum (FBS; both from Gibco-BRL, Grand Island, NY, USA), 100 U/ml
penicillin and 100 μg/ml streptomycin at 37°C in a
humidified atmosphere of 5% CO2.
Cell viability and proliferation
assays
Cell proliferation was determined using the MTT
assay. The Detroit 562 cells were starved for 24 h and
simultaneously treated with several concentrations of SM (1, 5, 10,
50 or 100 μg/ml) only or with several concentrations of SM
plus 10 μM 5-FU. After 48 h, the medium was removed, and the
cells were incubated with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
to measure metabolic activity. Spectrophotometric analysis at 450
nm to measure metabolic activity was performed using a microtiter
plate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
ROS assay
ROS production was performed according to the
protocol of the intracellular ROS assay kit (Cell Biolabs, Inc.,
San Diego, CA, USA). Cells were cultured in a 96-well cell culture
plate and treated with several concentrations of SM (1, 5, 10, 50
or 100 μg/ml) with 10 μM 5-FU for 48 h. Next, the
cells pretreated with 1 mM 2′,7′-dichlorofluorescein diacetate
(DCFH-DA) were incubated for 60 min at 37°C. After a brief
incubation, the cell fluorescence was read on a fluorometric plate
reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
480/530 nm. ROS production was determined by comparison with the
predetermined 2′,7′-dichlorofluorescein (DCF) standard curve.
DPPH assay
Mixtures of 0.1 mM 2,2-diphenyl-1-picrylhydrazyl
(DPPH) solution with methanol and SM (1, 5, 10, 50 or 100
μg/ml) were incubated in the dark for 30 min at room
temperature. After 30 min, the absorbance at 517 nm was read for
each sample on a microtiter plate reader (Molecular Devices, LLC).
Radical scavenging activity was calculated using the following
formula: DPPH radical scavenging activity (%) = [(AB-AT)/AB] × 100;
where AB is the absorbance of the blank sample and AT is the
absorbance of the tested extract solution.
In vivo studies
Animals and experimental protocol
Seven-week-old male golden Syrian hamsters (SLC,
Inc., Hamamatsu, Japan) weighing 100–110 g each were used. The
animals were housed in a specific pathogen-free environment with a
12 h light/dark cycle at the Center for Laboratory Animal Care and
Use at Kyung Hee University. Animal care and experimental
procedures conformed to the 'Guide for the Care and Use of
Laboratory Animals'. The protocol for the induction of oral
mucositis was modified on the basis of a previously published
protocol (25). The protocols for
the use of hamsters in this study were approved by the
Institutional Animal Care and Use Committee (IACUC) of Kyung Hee
University. Briefly, all the animals received intraperitoneal
(i.p.) administration of 80 mg/kg of the chemotherapy drug 5-FU on
day 0, followed by i.p. administration of 60 mg/kg 5-FU on day 2.
The cheek pouch of the animals was everted and the mucosa was
irritated by superficial scratching with the tip of an 18-gauge
needle by the same operator on days 3 and 4.
Experimental groups
The hamsters were randomly divided into six groups:
normal group (vehicle-treated, n=6), control (5-FU 80 mg/kg, i.p.
n=6), positive control (0.15% benzydi-mine HCl, o.p. n=6) and three
groups treated with different concentrations of SM with 80 mg/kg of
5-FU (5-FU + SM: 100, 500 and 1,000 mg/kg of SM and 5-FU, o.p.
n=6/each group). 5-FU was administered on days 1 and 2 from the
beginning of the study and SM and benzydimine HCl were administered
for 5 days a week for 2 weeks. The animals were weighed weekly in
order to adjust the gavage volume and to monitor their general
health.
Histological evaluation of oral
mucositis
For histological studies, the cheek pouches were
fixed overnight in Bouin's solution, dehydrated in 70, 80, 95 and
100% ethanol, xylene and embedded in paraffin. Tissue sections (5
μm) were prepared in order to perform hematoxylin and eosin
(H&E) staining. The sections were deparaffinized and rehydrated
in xylene, 100, 95, 80 and 70% ethanol. The sections were
over-stained with hematoxylin, usually 3–5 min and excess stain was
rinsed off in deionized water. Then the sections were destained for
a few seconds in acidic alcohol until the sections appeared red in
color (usually 4–5 dips) and then rinsed briefly in deionized water
to remove the acid. Hematoxylin-stained slides from the last tap
water were rinsed and placed in 70% ethanol for 3 min. Slides were
placed in eosin for 2 min and then slides were put through 95 and
100% ethanol and xylene. After H&E staining, the slides were
mounted with Canada balsam.
TUNEL assay
5-FU-induced cell death was investigated on the day
14 using the terminal deoxynucleotidyltransferase (TdT)-mediated
dUTP nick end labelling (TUNEL) method (ApopTag®, no.
S7101; Merck Millipore, Darmstadt, Germany). Briefly, after
deparaffinizing, the samples were rehydrated and incubated with 20
mg/ml proteinase K for 15 min at room temperature. Endogenous
peroxidases were blocked by treatment with 3% (v/v) hydrogen
peroxide in phosphate-buffered saline (PBS) for 5 min at room
temperature. After washing, the sections were then incubated in a
humidified chamber at 37°C for 1 h with TdT buffer containing TdT
enzyme and reaction buffer. Specimens were incubated for 10 min at
room temperature with a stop/wash buffer and then incubated in a
humidified chamber for 30 min with anti-digoxigenin peroxidase
conjugate at room temperature. After a series of PBS washes, the
slides were covered with peroxidase substrate for color development
and then washed in three changes of dH2O and
counterstained in 0.5% (w/v) methyl green for 10 min at room
temperature. The TUNEL-positive cells were counted (10
fields/slide; magnification, ×1,000) for statistical
comparisons.
Western blotting
Proteins from homogenized cheek pouches of hamsters
were separated using a nuclear extraction kit following a
modification of the manufacturer's instructions (Active Motif,
Carlsbad, CA, USA). SDS-PAGE and western blotting were performed as
previously described (26).
Samples for protein extraction were half of the same cheek pouches
of hamsters used for RNA extractions. Equivalent amount (50
μg) of protein extracts was separated on 10% Tris-glycine
gels by SDS-PAGE and transferred to nitrocellulose membranes using
25 mM Tris and 250 mM glycine buffer containing 20% methanol, pH
8.3. Transfer was performed at a constant voltage of 120 mA for 1
h. After transfer, the membranes were blocked in PBS containing
0.05% Tween-20 (PBS-T) with 5% skim milk for 2 h at room
temperature and incubated with the primary antibodies (1:1,000) for
IL-1β (sc-12742), TNF-α (sc-1350), NF-κB (sc-109) and IL-4
(sc-32242) (all from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) in PBS-T overnight at 4°C. Following overnight incubation, the
membranes were rinsed with 1X PBS three times and incubated with
conjugated goat anti-rabbit IgG for 1 h at room temperature,
followed by three additional washes with 1X PBS.
Statistical analysis
Statistical analysis was performed using GraphPrism
4.0.3 software (GraphPad Software, Inc., San Diego, CA, USA). Data
are presented as means with standard deviation (SD) and analyzed
using the statistical software SPSS, version 12.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
SM enhances proliferation of the human
pharyngeal cells
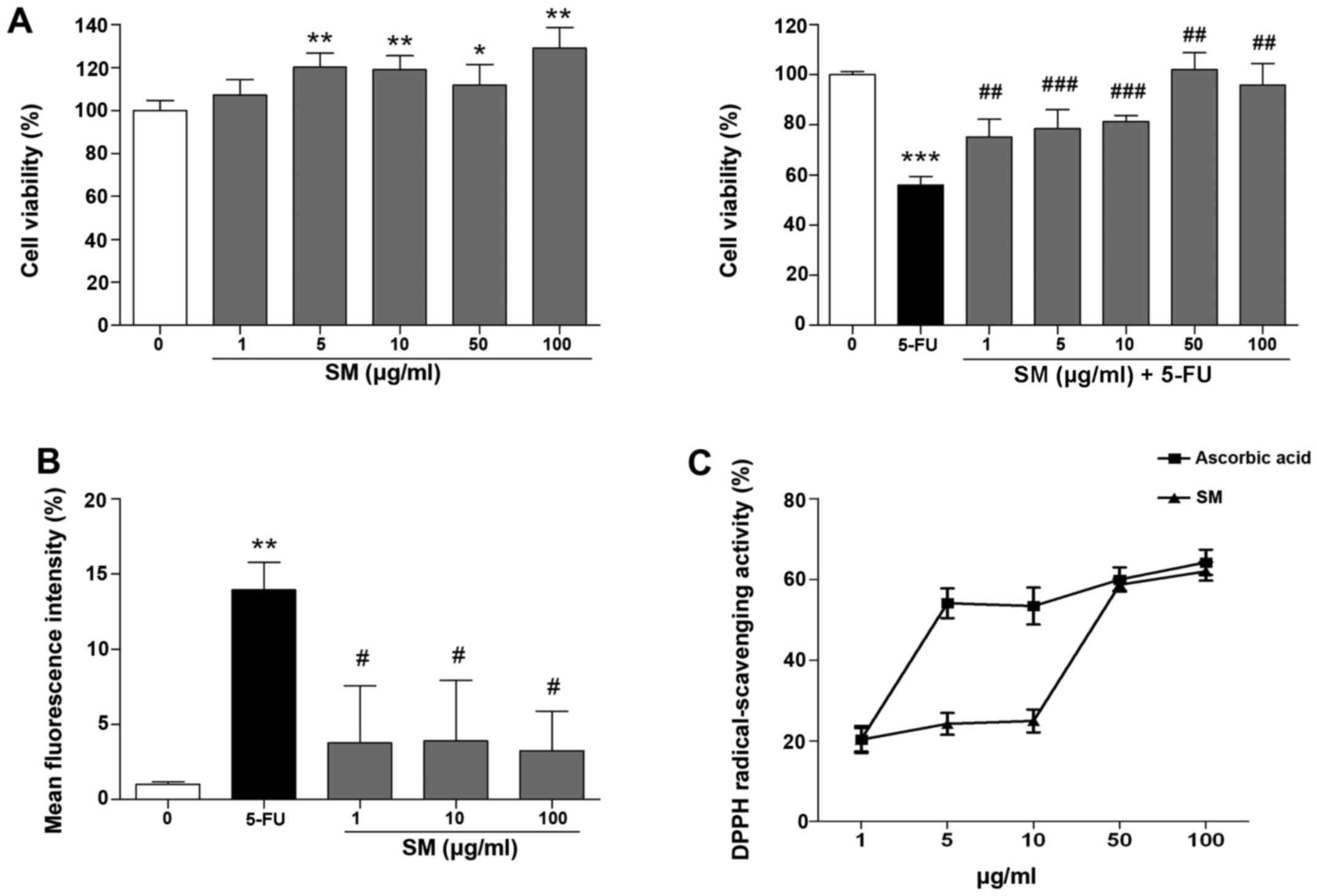
The effects of SM on proliferation of the Detroit
562 human pharyngeal cells were determined from cell growth
kinetics using the MTT assay, which measures the metabolic activity
of viable cells. Growth-arrested Detroit 562 cells were cultured in
starvation medium for 24 h prior to the experiment and incubated
for 48 h with SM and 10 μM 5-FU. SM activated cell
proliferation; specifically, the number of cells treated with 100
μg/ml SM increased by 128.97±9.7% compared with that noted
in the untreated cells (p<0.01; Fig. 1A). The cell viability in the 5-FU
and SM-treated groups (1, 5, 10, 50 or 100 μg/ml) was
significantly increased compared with the cell viability of the
control (10 μM 5-FU-only treated) group (75.19±7.01,
78.43±7.0, 81.33±2.41, 102.1±6.8 and 95.97±8.54 vs. 56.01±3.48%,
respectively; p<0.01, Fig.
1A), indicating that SM enhanced the proliferation rate of
Detroit 562 cells and exerted protective effects against
5-FU-induced cytotoxicity.
SM decreased intracellular ROS production
and increases the DPPH-scavenging activity
As shown in Fig.
1B, the production of superoxide anions in the 10 μM
5-FU-treated cells was decreased markedly by co-treatment of 1, 10
and 100 μg/ml of SM compared with the (10 μM
5-FU-only treated) control. Next, the effects of SM on
DPPH-scavenging activity were assessed. The DPPH-scavenging
activity increased to 20.30, 24.21, 24.95, 58.65 and 61.96% at 1,
5, 10, 50 and 100 μg/ml of SM treatment, respectively. The
IC50 value of SM was 42.6 μg/ml. As a positive
control, we measured the effects of ascorbic acid (AC) on
DPPH-scavenging activity, which increased to 20.35, 54.08, 53.39,
59.95 and 64.23% at 1, 5, 10, 50 and 100 μg/ml SM
concentrations, respectively. The IC50 value of AC was
4.6 μg/ml (Fig. 1C).
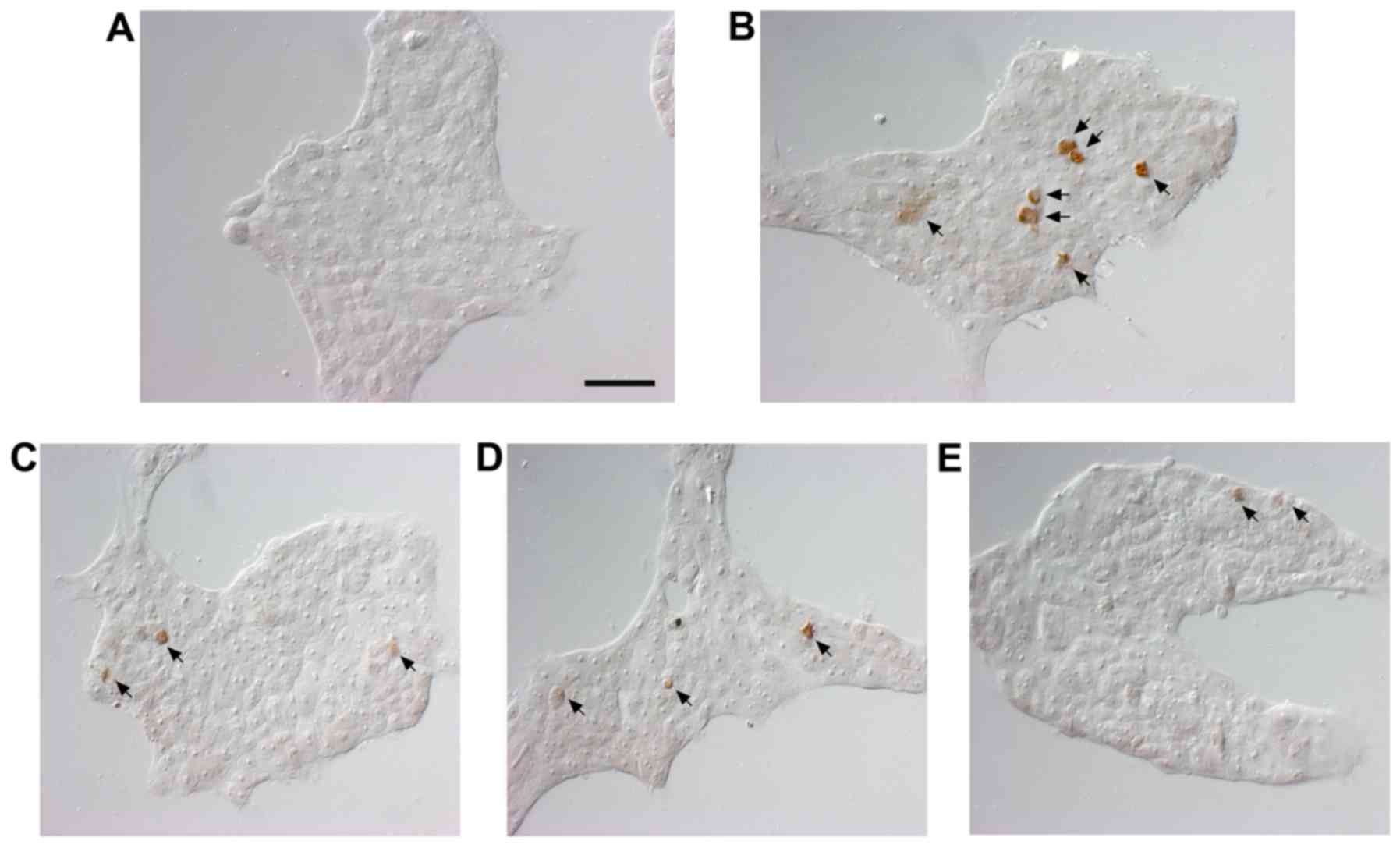
SM reduces apoptosis in the human
pharyngeal cell line
Nuclear DNA breaks in human pharyngeal cells were
detected using TUNEL staining to estimate the extent of apoptosis
and assessed under magnification, ×400. The results of the TUNEL
staining showed positive staining for DNA fragmentation in the
5-FU-treated compared with the untreated cells (Fig. 2). The number of TUNEL-positive
cells was reduced by 1, 10 and 100 μg/ml following SM
treatment.
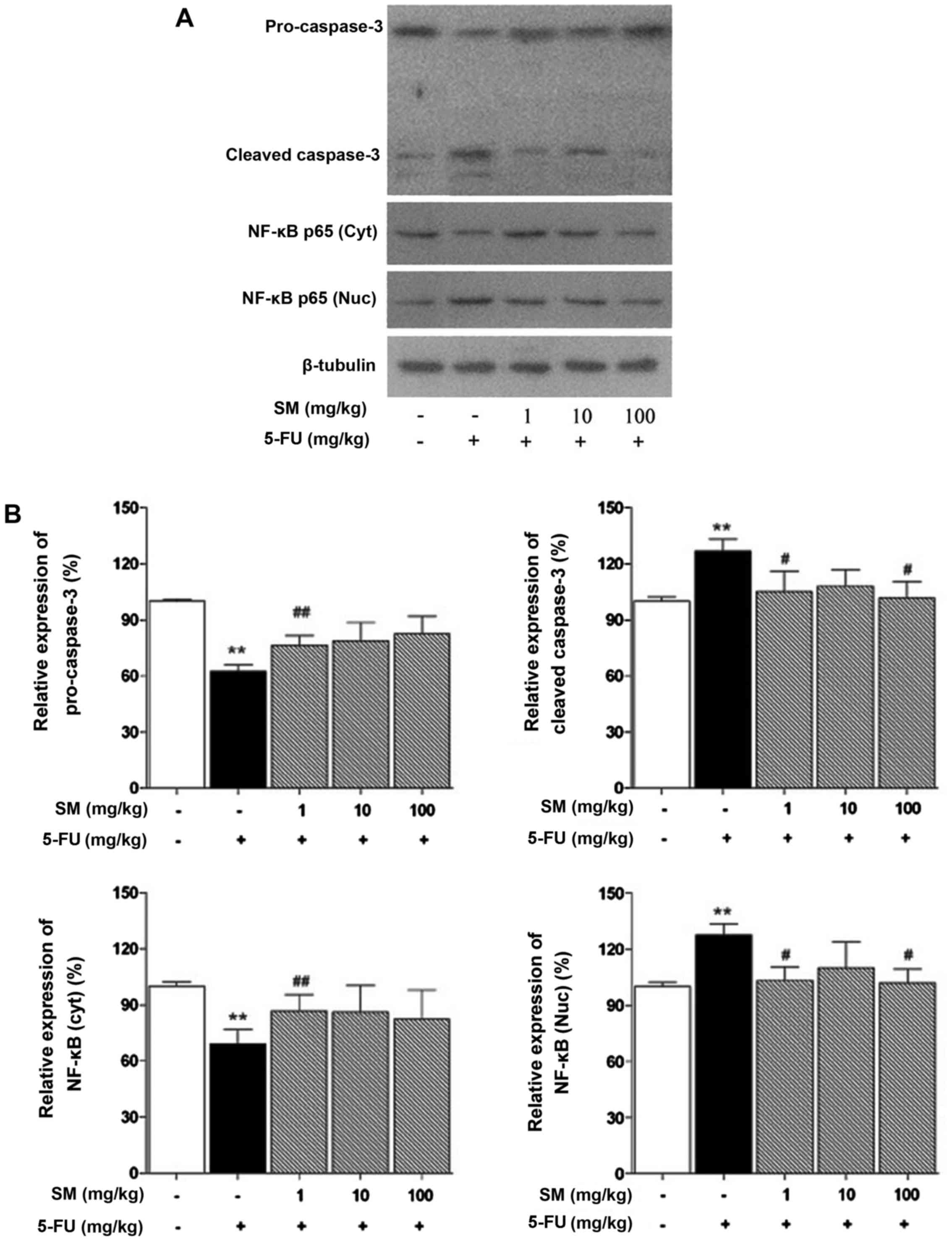
SM regulates apoptosis-related signaling
pathways
We investigated whether SM is associated with NF-κB
and caspase-dependent apoptosis. The NF-κB and caspase-3 signaling
pathways were activated by 5-FU treatment but were decreased by all
concentrations of SM (Fig.
3).
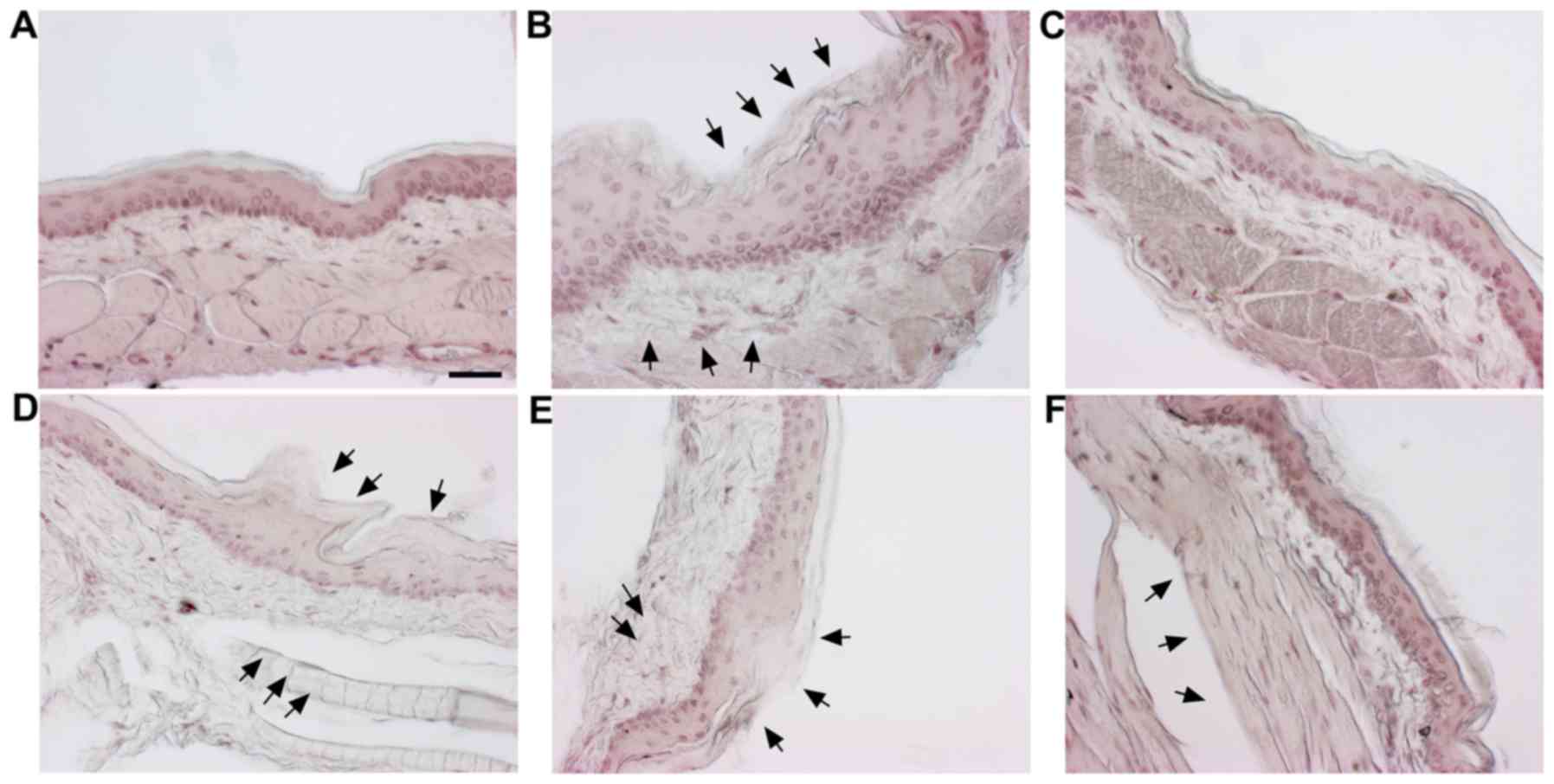
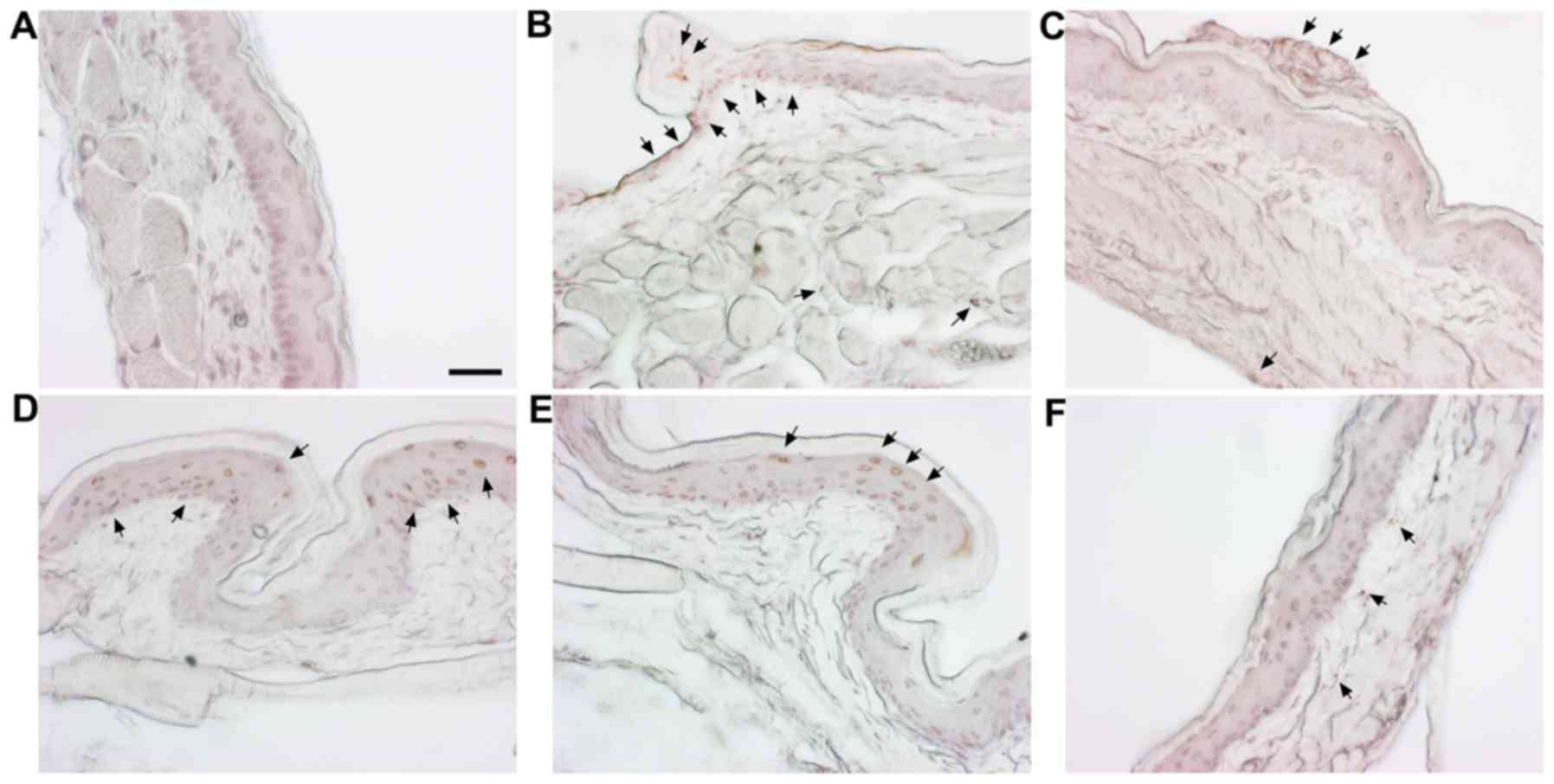
Histological effects of SM on cheek
pouches of hamsters
H&E staining was carried out to observe the
histological changes in the cheek pouches. The normal group is the
vehicle-treated group and the control is the 5-FU (80 mg/kg, i.p.)
only treated group. Positive control is the benzydimine HCl-treated
group. In addition, there were three co-treatment groups with SM
(100, 500 and 1,000 mg/kg) and 5-FU (80 mg/kg, i.p.). Histological
examination demonstrated a normal arrangement of cellular
components in the cheek pouches of the hamsters (Fig. 4A). Stratum corneum exfoliation,
degradation of the epithelial layer and ulcers were observed in the
control (Fig. 4B). The observed
damages were recovered in the positive control (Fig. 4C) and the 500, 1,000 mg/kg of
SM-treated groups (Fig. 4E and
F); epithelial layers were recovered.
Detection of apoptosis in cheek pouches
of hamsters
To examine the apoptosis in cheek pouches of the
hamsters, TUNEL staining was carried out. The cheek pouches of
hamsters showed a significant increase in apoptotic changes in the
group treated with 5-FU (Fig. 5B,
arrow) when compared with the normal group (Fig. 5A), while the positive control
group (Fig. 5C) and SM and
5-FU-treated groups showed epithelial layer and granular layer
(Fig. 5D–F).
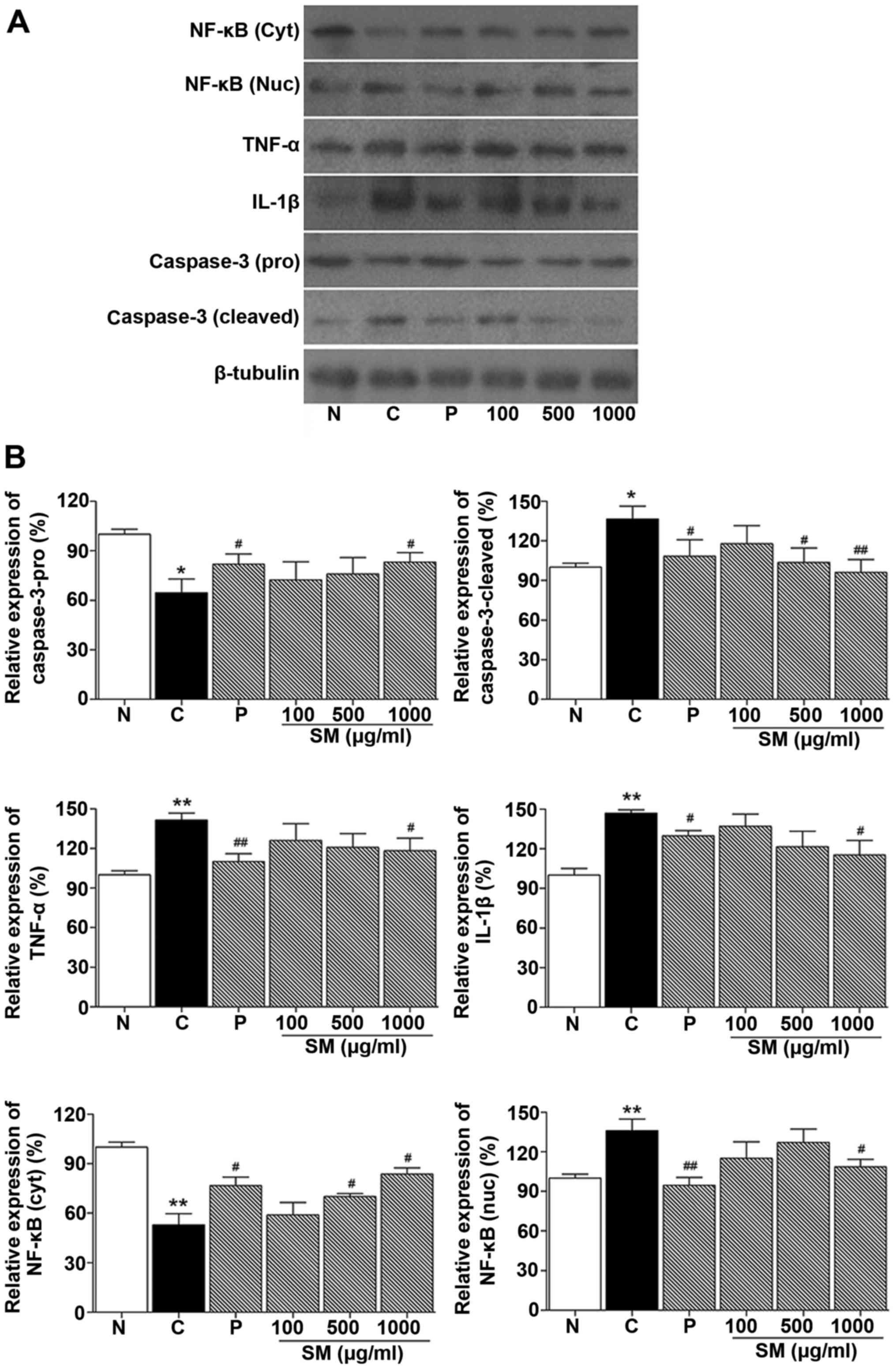
Effect of SM on the expression of IL-1β,
TNF-α, NF-κB and caspase-3 in cheek pouches of hamsters
Western blotting was performed to determine the
effect of SM on proinflammatory cytokines (IL-1β and TNF-α), NF-κB
and caspase-3 expression level in cheek pouches of hamsters. As
shown in Fig. 6, NF-κB, TNF-α and
IL-1β protein expression levels in the control group were increased
compared to the normal group (136.01%, p<0.05; 141.5%,
p<0.05; 147.0%, p<0.01, respectively). NF-κB, TNF-α and
IL-1β, protein levels in the positive control (benzydimine
HCl-treated) group were decreased compared to the control group
(94.7 vs. 136.0%, p<0.01; 110.1 vs. 141.5%, p<0.01 and 129.9
vs. 147.0%, p<0.01, respectively). In contrast, in the 1,000
mg/kg SM and 5-FU-treated group, NF-κB, TNF-α and IL-1β protein
expression levels were significantly decreased when compared to
these levels in the control group (108.7 vs. 136.0%; 118.19 vs.
141.5%; 115.3 vs. 147.0%, p<0.05, respectively; Fig. 6).
Caspase-3 cleaved protein level in the control group
was incre ased compared to that noted in the normal group (136.62,
p<0.05, respectively). This level was decreased in the positive
control (benzydimine HCl) group compared to the control group
(108.3 vs. 136.6%, p<0.05, respectively). In addition, in the SM
and 5-FU-treated groups, caspase-3 protein expression was
significantly decreased when compared with that noted in the
control group (103.7%, p<0.05 and 96.1%, p<0.01 vs. 136.6%,
respectively; Fig. 6).
Discussion
Cancer chemotherapy targets rapidly dividing cancer
cells but also interferes with DNA, RNA and protein synthesis
(27). Therefore, normal tissue
cells as well as cancer cells are damaged. Oral mucositis is an
adverse effect of cancer chemotherapeutic drugs (28). Ulceration of the oral mucosa and
oropharynx can lead to decreased quality of life and modification
with highly expensive cancer treatments (29). The use of current medications for
mucositis such as palifermin and benzydamine is limited due to
their high cost (30). Therefore,
the development of new oral mucositis therapeutic drugs that can be
universally used to promote effectiveness is necessary. Salvia
miltiorrhiza Bunge (SM) is known for its antioxidative and
anti-inflammatory effects (22,23). However, the molecular mechanisms
of SM are poorly understood, and its effects on oral mucositis have
yet to be determined. In the present study, we investigated the
effects of SM on 5-FU-induced oral mucositis.
5-FU is an anticancer drug which is used most
frequently for carcinomas of the breast, colon, and skin (31). 5-FU inhibits thymidylate synthase
or incorporation of nucleic acid into RNA and DNA and causes cell
death. But it can induce oral mucositis. Therefore, inhibition or
the prevention of the cytotoxic effects of 5-FU on the mucosa is a
reasonable strategy (32).
In this study, we examined cell viability using an
MTT assay, DPPH and ROS reproduction to determine the antioxidant
activities of SM. Generation of ROS linked to increased oxidative
stress causes oxidative damage and the pathogenesis of several
diseases (33). ROS are an
important mediator of downstream biological of oral mucositis
(34). The antioxidant ability of
natural products is crucial in oral mucositis treatment. SM
promoted proliferation of human pharyngeal cells without cytotoxic
effects. Additionally, SM showed protective effects against
5-FU-induced cytotoxicity and, as expected, stimulated cell
growth.
The DPPH assay is widely used to assess free
radical-scavenging abilities of natural products reflecting their
antioxidant properties (35). SM
reduced the stable radical DPPH to yellow-colored diphenylpicryl
hydrazine dose-dependently. Based on this result, SM showed
antioxidant activities against scavenging DPPH free radicals.
Additionally, ROS production was effectively suppressed at all
concentrations in the SM-treated cells. To further understand the
antioxidant effects of SM on the human pharyngeal cell line, we
examined ROS production. SM-treated cells showed significantly
lowered ROS production implying that SM may protect mucosal injury
initiated by ROS generation.
DNA strand breaks caused by ROS activate
transcription factors such as NF-κB (36). Activation of the NF-κB pathway
leads to the expression of antiapoptotic genes and induces
proinflammatory cytokines (37).
Additionally, these reactions cause oral mucositis. The biological
steps of oral mucositis involve various pathways including those
associated with mitogen-activated protein kinase (MAPK), ceramide
and matrix metalloproteinases (MMPs) (38). Thus, therapeutic drugs for oral
mucositis will help reduce apoptosis and recover epithelial cells
in the oral mucositis. To examine the regulation of apoptosis by SM
in our study, the NF-κB and caspase-independent apoptotic pathways
were examined by immunoblotting using 5-FU-treated human pharyngeal
cells and hamster cheek pouches. In the TUNEL assay, the number of
apoptosis-positive cells in the SM-treated groups was decreased
compared with the control, and SM treatment also reduced the
expression of NF-κB and cleaved caspase-3 both in vitro and
in vivo. Several transcription factors and proinflammatory
cytokines such as IL-1β and TNF-α are known to be involved in the
development of mucositis (39).
SM treatment for 14 days led to a decrease in the expression level
of proinflammatory cytokines (IL-1β and TNF-α) and NF-κB in the
5-FU-induced oral mucositis.
In conclusion, the present study demonstrated that
SM promoted cell proliferation and had protective effects against
oxidative stress. Additionally, SM inhibited apoptotic cell death
mediated by the NF-κB-caspase-3 signaling pathways. Moreover,
changes in NF-κB and proinflammatory cytokine expression following
SM treatment suggest that SM reduces inflammation during the
development of mucositis. Although other molecular mechanisms
remain to be elucidated in further studies, this study showed the
possible use of SM as a therapeutic agent for oral mucositis.
References
|
1
|
Sonis ST and Fey EG: Oral complications of
cancer therapy. Oncology (Williston Park). 16:680–686. 2002.
|
|
2
|
Pico JL, Avila-Garavito A and Naccache P:
Mucositis: its occurrence, consequences, and treatment in the
Oncology setting. Oncologist. 3:446–451. 1998.
|
|
3
|
Bowen JM and Keefe DM: New pathways for
alimentary mucositis. J Oncol. 2008:9078922008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loury D, Embree JR, Steinberg DA, Sonis ST
and Fiddes JC: Effect of local application of the antimicrobial
peptide IB-367 on the incidence and severity of oral mucositis in
hamsters. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
87:544–551. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sonis ST, Elting LS, Keefe D, Peterson DE,
Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly
JP and Rubenstein EB; Mucositis Study Section of the Multinational
Association for Supportive Care in Cancer; International Society
for Oral Oncology: Perspectives on cancer therapy-induced mucosal
injury: pathogenesis, measurement, epidemiology, and consequences
for patients. Cancer. 100(Suppl 9): 1995–2025. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raber-Durlacher JE, Elad S and Barasch A:
Oral mucositis. Oral Oncol. 46:452–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng KK, Goggins WB, Lee VW and Thompson
DR: Risk factors for oral mucositis in children undergoing
chemotherapy: a matched case-control study. Oral Oncol.
44:1019–1025. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mantovani G, Macciò A, Madeddu C, Mura L,
Massa E, Gramignano G, Lusso MR, Murgia V, Camboni P and Ferreli L:
Reactive oxygen species, antioxidant mechanisms and serum cytokine
levels in cancer patients: impact of an antioxidant treatment. J
Cell Mol Med. 6:570–582. 2002. View Article : Google Scholar
|
|
9
|
Sonis ST, Peterson RL, Edwards LJ, Lucey
CA, Wang L, Mason L, Login G, Ymamkawa M, Moses G, Bouchard P, et
al: Defining mechanisms of action of interleukin-11 on the
progression of radiation-induced oral mucositis in hamsters. Oral
Oncol. 36:373–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sonis ST: The pathobiology of mucositis.
Nat Rev Cancer. 4:277–284. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beaven AW and Shea TC: Palifermin: a
keratinocyte growth factor that reduces oral mucositis after stem
cell transplant for haematological malignancies. Expert Opin
Pharmacother. 7:2287–2299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kazemian A, Kamian S, Aghili M, Hashemi FA
and Haddad P: Benzydamine for prophylaxis of radiation-induced oral
mucositis in head and neck cancers: a double-blind
placebo-controlled randomized clinical trial. Eur J Cancer Care
(Engl). 18:174–178. 2009. View Article : Google Scholar
|
|
13
|
Su CY, Ming QL, Rahman K, Han T and Qin
LP: Salvia miltiorrhiza: traditional medicinal uses, chemistry, and
pharmacology. Chin J Nat Med. 13:163–182. 2015.PubMed/NCBI
|
|
14
|
Yu XY, Lin SG, Chen X, Zhou ZW, Liang J,
Duan W, Chowbay B, Wen JY, Chan E, Cao J, et al: Transport of
cryptotanshinone, a major active triterpenoid in Salvia
miltiorrhiza Bunge widely used in the treatment of stroke and
Alzheimer's disease, across the blood-brain barrier. Curr Drug
Metab. 8:365–378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang XZ, Qian SS, Zhang YJ and Wang RQ:
Salvia miltiorrhiza: a source for anti-Alzheimer's disease drugs.
Pharm Biol. 54:18–24. 2016. View Article : Google Scholar
|
|
16
|
Ren B, Zhang YX, Zhou HX, Sun FW, Zhang
ZF, Wei Z, Zhang CY and Si DW: Tanshinone IIA prevents the loss of
nigrostriatal dopaminergic neurons by inhibiting NADPH oxidase and
iNOS in the MPTP model of Parkinson's disease. J Neurol Sci.
348:142–152. 2015. View Article : Google Scholar
|
|
17
|
Hu L, Yu T and Jia Z: Experimental study
of the protective effects of Astragalus and Salvia miltiorrhiza
bunge on glycerol induced acute renal failure in rabbits. Zhonghua
Wai Ke Za Zhi. 34:311–314. 1996.In Chinese. PubMed/NCBI
|
|
18
|
Sun XG, Fu XQ, Cai HB, Liu Q, Li CH, Liu
YW, Li YJ, Liu ZF, Song YH and Lv ZP: Proteomic analysis of
protective effects of polysaccharides from Salvia miltiorrhiza
against immunological liver injury in mice. Phytother Res.
25:1087–1094. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Guo J, Bao J, Lu J and Wang Y: The
anticancer properties of Salvia miltiorrhiza Bunge (Danshen): a
systematic review. Med Res Rev. 34:768–794. 2014. View Article : Google Scholar
|
|
20
|
Cui L, Li T, Liu Y, Zhou L, Li P, Xu B,
Huang L, Chen Y, Liu Y, Tian X, et al: Salvianolic acid B prevents
bone loss in prednisone-treated rats through stimulation of
osteogenesis and bone marrow angiogenesis. PLoS One. 7:e346472012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Y, Wang L, Zhang L, Wang T, Zhou Y,
Ding C, Yang R, Wang X and Yu L: Optimization of extraction and
antioxidant activity of polysaccharides from Salvia miltiorrhiza
Bunge residue. Int J Biol Macromol. 79:533–541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiang G, Yang X, Shi L, Zhang H, Chen B,
Zhao Y, Zu M, Zhou D, Guo J, Yang H, et al: Antidiabetic effect of
salvianolic acid A on diabetic animal models via AMPK activation
and mitochondrial regulation. Cell Physiol Biochem. 36:395–408.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin Q, Jiang S, Wu YL, Bai T, Yang Y, Jin
X, Lian LH and Nan JX: Hepatoprotective effect of cryptotanshinone
from Salvia miltiorrhiza in
D-galactosamine/lipopolysaccharide-induced fulminant hepatic
failure. Phytomedicine. 21:141–147. 2014. View Article : Google Scholar
|
|
24
|
Lv H, Wang L, Shen J, Hao S, Ming A, Wang
X, Su F and Zhang Z: Salvianolic acid B attenuates apoptosis and
inflammation via SIRT1 activation in experimental stroke rats.
Brain Res Bull. 115:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sonis ST, Tracey C, Shklar G, Jenson J and
Florine D: An animal model for mucositis induced by cancer
chemotherapy. Oral Surg Oral Med Oral Pathol. 69:437–443. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Florin A, Maire M, Bozec A, Hellani A,
Chater S, Bars R, Chuzel F and Benahmed M: Androgens and
postmeiotic germ cells regulate claudin-11 expression in rat
Sertoli cells. Endocrinology. 146:1532–1540. 2005. View Article : Google Scholar
|
|
27
|
Blijlevens NM: Cytotoxic treatment-induced
gastrointestinal symptoms. Curr Opin Support Palliat Care. 1:16–22.
2007. View Article : Google Scholar
|
|
28
|
Stringer AM and Logan RM: The role of oral
flora in the development of chemotherapy-induced oral mucositis. J
Oral Pathol Med. 44:81–87. 2015. View Article : Google Scholar
|
|
29
|
Nonzee NJ, Dandade NA, Patel U, Markossian
T, Agulnik M, Argiris A, Patel JD, Kern RC, Munshi HG, Calhoun EA,
et al: Evaluating the supportive care costs of severe
radiochemotherapy-induced mucositis and pharyngitis: results from a
Northwestern University. Costs of Cancer program pilot study with
head and neck and nonsmall cell lung cancer patients who received
care at a county hospital, a Veterans administration hospital, or a
comprehensive cancer care center. Cancer. 113:1446–1452. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahmed KM: The effect of olive leaf extract
in decreasing the expression of two pro-inflammatory cytokines in
patients receiving chemotherapy for cancer. A randomized clinical
trial. Saudi Dent J. 25:141–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Evrard A, Cuq P, Robert B, Vian L,
Pèlegrin A and Cano JP: Enhancement of 5-fluorouracil cytotoxicity
by human thymidine-phosphorylase expression in cancer cells: in
vitro and in vivo study. Int J Cancer. 80:465–470. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kodach LL, Bos CL, Durán N, Peppelenbosch
MP, Ferreira CV and Hardwick JC: Violacein synergistically
increases 5-fluorouracil cytotoxicity, induces apoptosis and
inhibits Akt-mediated signal transduction in human colorectal
cancer cells. Carcinogenesis. 27:508–516. 2006. View Article : Google Scholar
|
|
33
|
Criswell T, Leskov K, Miyamoto S, Luo G
and Boothman DA: Transcription factors activated in mammalian cells
after clinically relevant doses of ionizing radiation. Oncogene.
22:5813–5827. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mendoza G, Alvarez AI, Pulido MM, Molina
AJ, Merino G, Real R, Fernandes P and Prieto JG: Inhibitory effects
of different antioxidants on hyaluronan depolymerization. Carbohydr
Res. 342:96–102. 2007. View Article : Google Scholar
|
|
35
|
Kaji H, Inukai Y, Maiguma T, Ono H,
Teshima D, Hiramoto K and Makino K: Radical scavenging activity of
bisbenzylisoquinoline alkaloids and traditional prophylactics
against chemotherapy-induced oral mucositis. J Clin Pharm Ther.
34:197–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Erstad DJ and Cusack JC Jr: Targeting the
NF-κB pathway in cancer therapy. Surg Oncol Clin N Am. 22:705–746.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lappas M, Permezel M and Rice GE:
N-acetyl-cysteine inhibits phospholipid metabolism, proinflammatory
cytokine release, protease activity, and nuclear factor-kappaB
deoxyribonucleic acid-binding activity in human fetal membranes in
vitro. J Clin Endocrinol Metab. 88:1723–1729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bamba S, Andoh A, Yasui H, Araki Y, Bamba
T and Fujiyama Y: Matrix metalloproteinase-3 secretion from human
colonic subepithelial myofibroblasts: role of interleukin-17. J
Gastroenterol. 38:548–554. 2003.PubMed/NCBI
|
|
39
|
Curra M, Martins MA, Lauxen IS, Pellicioli
AC, Sant'Ana Filho M, Pavesi VC, Carrard VC and Martins MD: Effect
of topical chamomile on immunohistochemical levels of IL-1β and
TNF-α in 5-fluorouracil-induced oral mucositis in hamsters. Cancer
Chemother Pharmacol. 71:293–299. 2013. View Article : Google Scholar
|