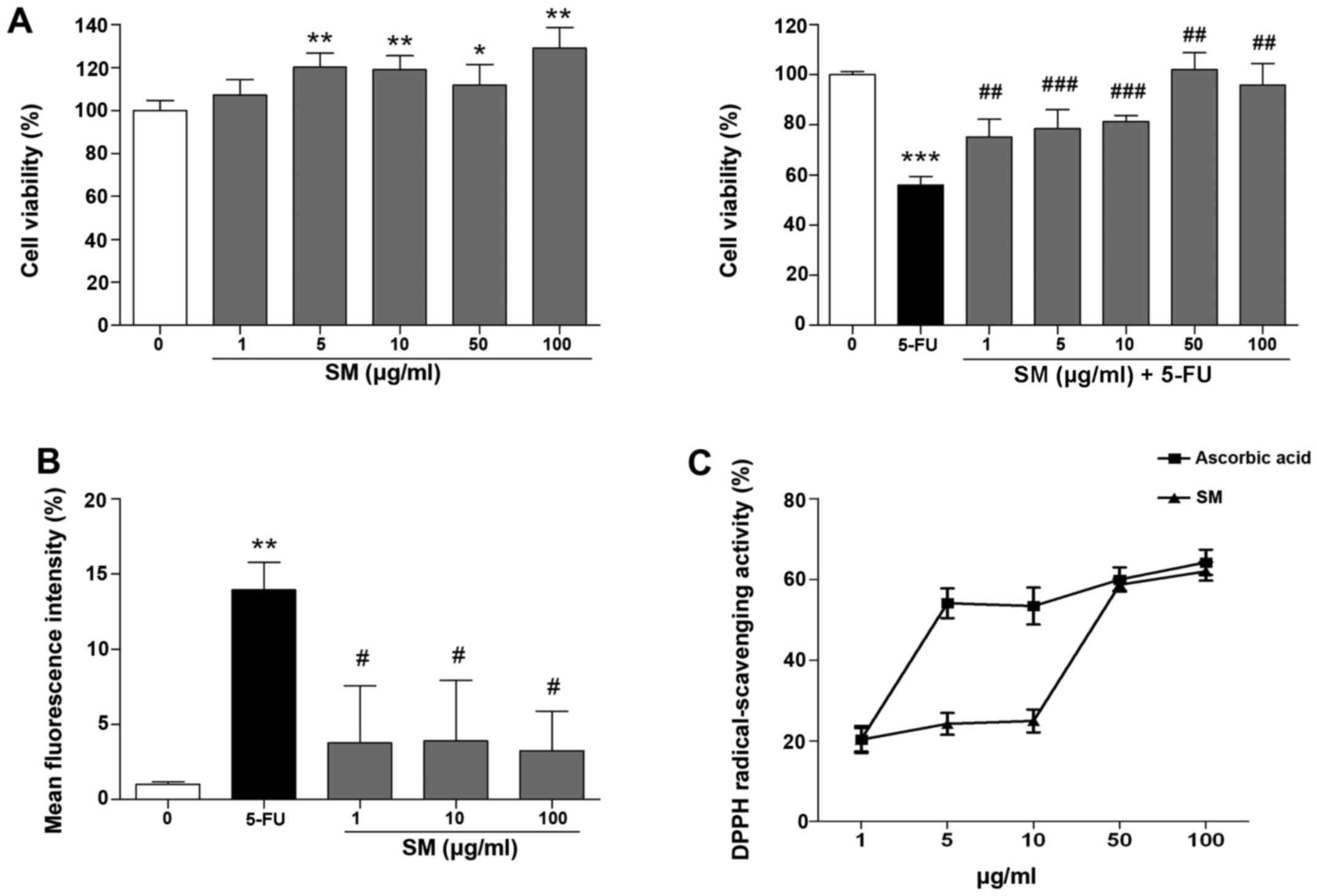
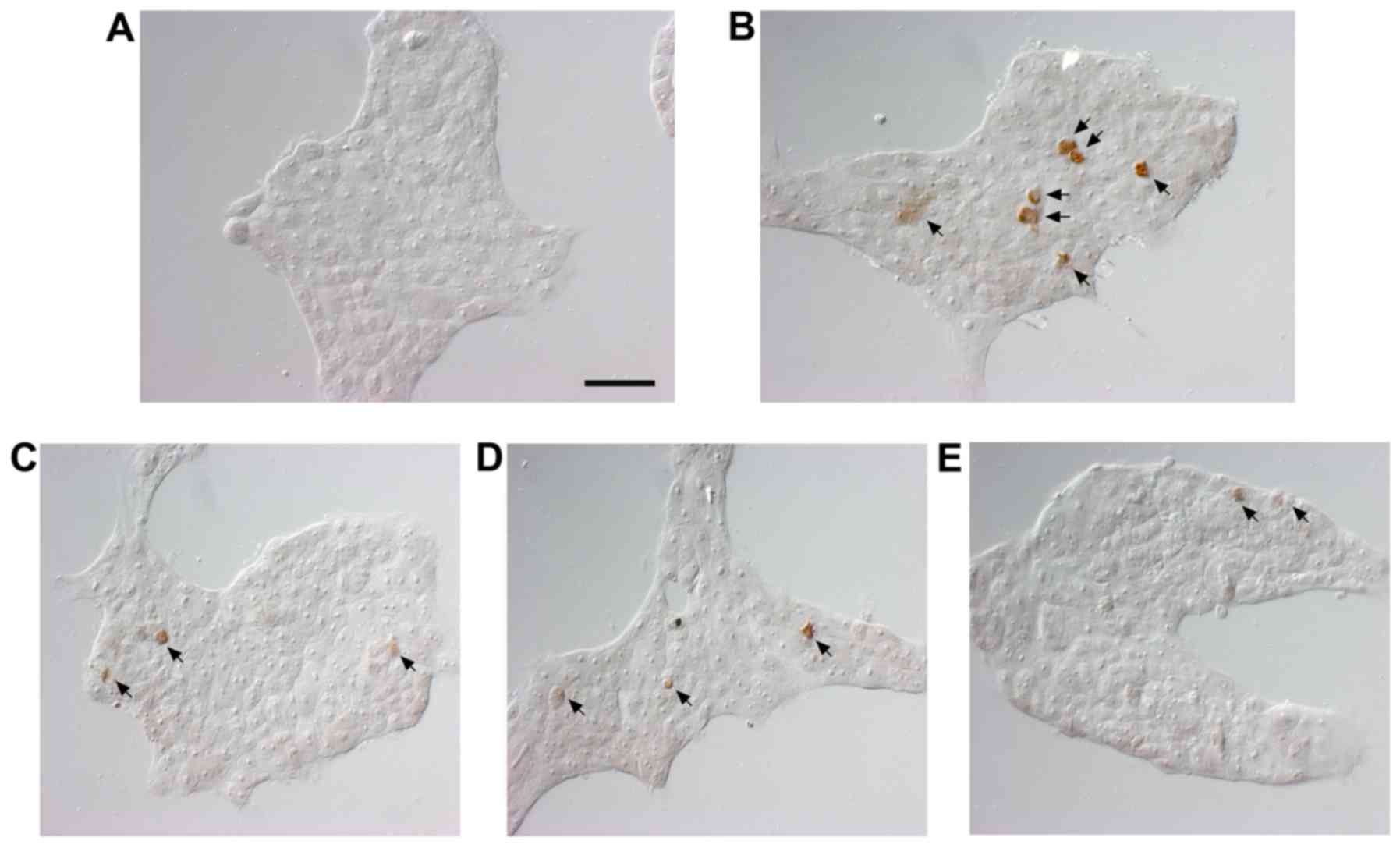
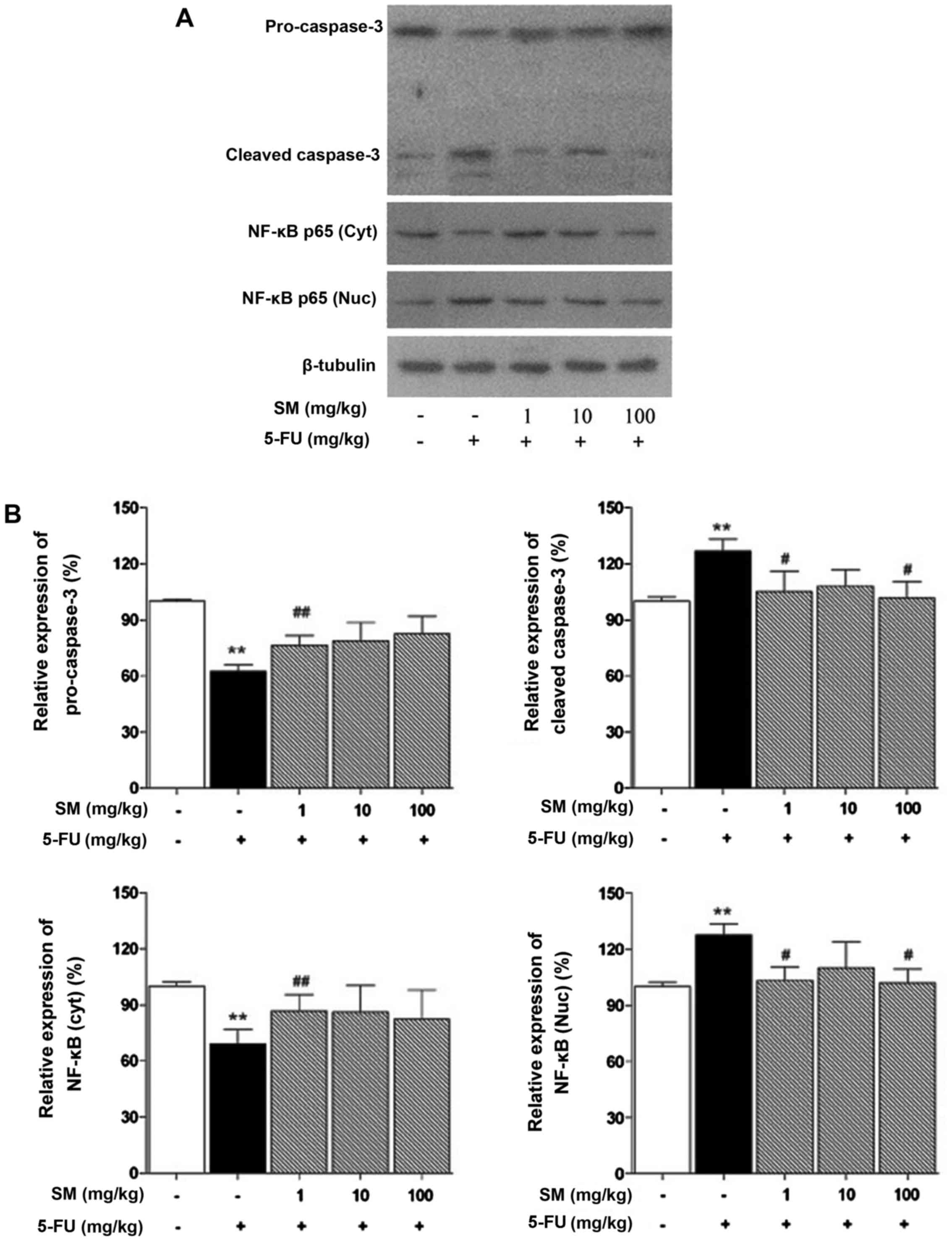
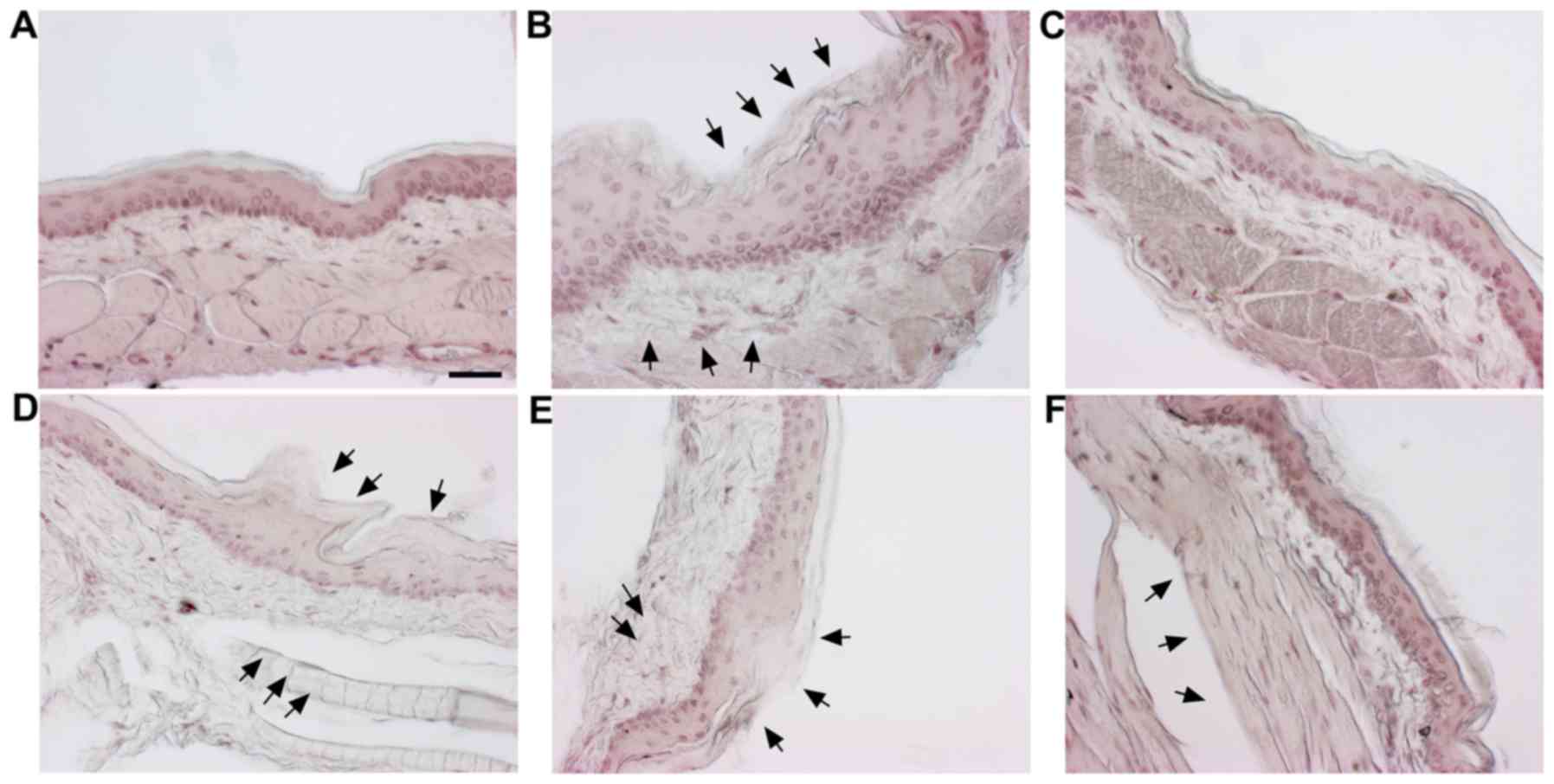
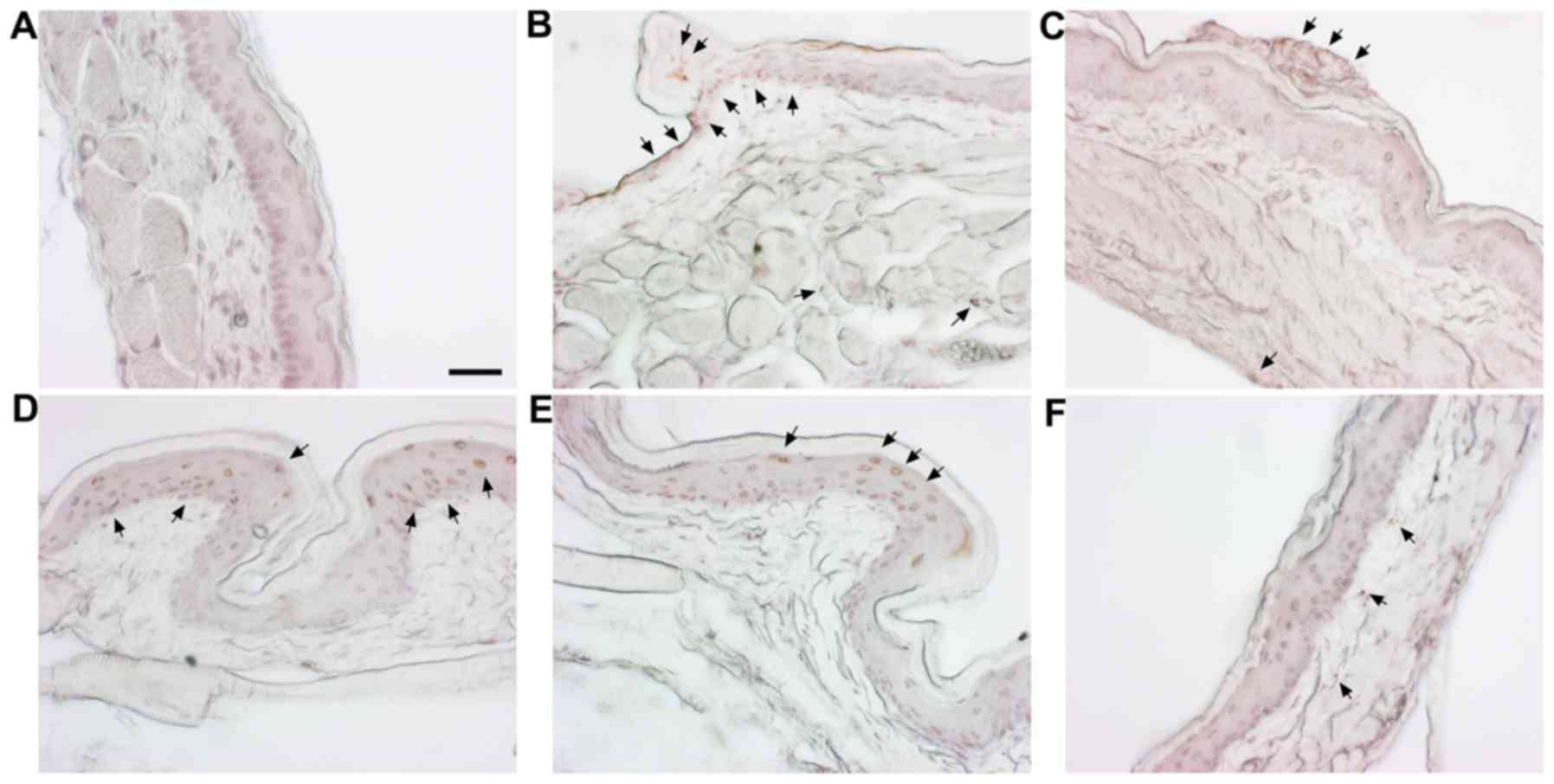
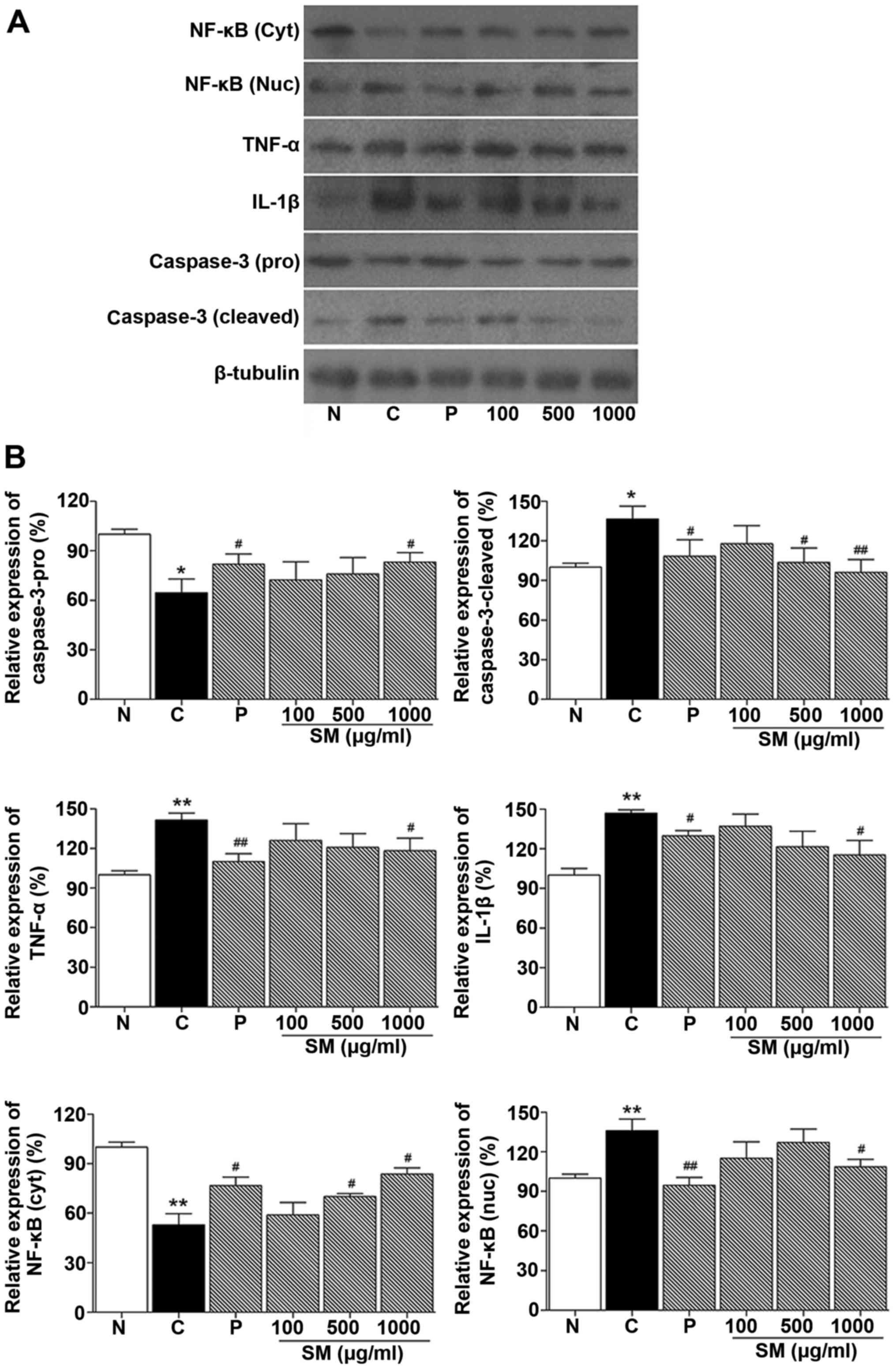
|
1
|
Sonis ST and Fey EG: Oral complications of
cancer therapy. Oncology (Williston Park). 16:680–686. 2002.
|
|
2
|
Pico JL, Avila-Garavito A and Naccache P:
Mucositis: its occurrence, consequences, and treatment in the
Oncology setting. Oncologist. 3:446–451. 1998.
|
|
3
|
Bowen JM and Keefe DM: New pathways for
alimentary mucositis. J Oncol. 2008:9078922008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loury D, Embree JR, Steinberg DA, Sonis ST
and Fiddes JC: Effect of local application of the antimicrobial
peptide IB-367 on the incidence and severity of oral mucositis in
hamsters. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
87:544–551. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sonis ST, Elting LS, Keefe D, Peterson DE,
Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly
JP and Rubenstein EB; Mucositis Study Section of the Multinational
Association for Supportive Care in Cancer; International Society
for Oral Oncology: Perspectives on cancer therapy-induced mucosal
injury: pathogenesis, measurement, epidemiology, and consequences
for patients. Cancer. 100(Suppl 9): 1995–2025. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raber-Durlacher JE, Elad S and Barasch A:
Oral mucositis. Oral Oncol. 46:452–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng KK, Goggins WB, Lee VW and Thompson
DR: Risk factors for oral mucositis in children undergoing
chemotherapy: a matched case-control study. Oral Oncol.
44:1019–1025. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mantovani G, Macciò A, Madeddu C, Mura L,
Massa E, Gramignano G, Lusso MR, Murgia V, Camboni P and Ferreli L:
Reactive oxygen species, antioxidant mechanisms and serum cytokine
levels in cancer patients: impact of an antioxidant treatment. J
Cell Mol Med. 6:570–582. 2002. View Article : Google Scholar
|
|
9
|
Sonis ST, Peterson RL, Edwards LJ, Lucey
CA, Wang L, Mason L, Login G, Ymamkawa M, Moses G, Bouchard P, et
al: Defining mechanisms of action of interleukin-11 on the
progression of radiation-induced oral mucositis in hamsters. Oral
Oncol. 36:373–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sonis ST: The pathobiology of mucositis.
Nat Rev Cancer. 4:277–284. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beaven AW and Shea TC: Palifermin: a
keratinocyte growth factor that reduces oral mucositis after stem
cell transplant for haematological malignancies. Expert Opin
Pharmacother. 7:2287–2299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kazemian A, Kamian S, Aghili M, Hashemi FA
and Haddad P: Benzydamine for prophylaxis of radiation-induced oral
mucositis in head and neck cancers: a double-blind
placebo-controlled randomized clinical trial. Eur J Cancer Care
(Engl). 18:174–178. 2009. View Article : Google Scholar
|
|
13
|
Su CY, Ming QL, Rahman K, Han T and Qin
LP: Salvia miltiorrhiza: traditional medicinal uses, chemistry, and
pharmacology. Chin J Nat Med. 13:163–182. 2015.PubMed/NCBI
|
|
14
|
Yu XY, Lin SG, Chen X, Zhou ZW, Liang J,
Duan W, Chowbay B, Wen JY, Chan E, Cao J, et al: Transport of
cryptotanshinone, a major active triterpenoid in Salvia
miltiorrhiza Bunge widely used in the treatment of stroke and
Alzheimer's disease, across the blood-brain barrier. Curr Drug
Metab. 8:365–378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang XZ, Qian SS, Zhang YJ and Wang RQ:
Salvia miltiorrhiza: a source for anti-Alzheimer's disease drugs.
Pharm Biol. 54:18–24. 2016. View Article : Google Scholar
|
|
16
|
Ren B, Zhang YX, Zhou HX, Sun FW, Zhang
ZF, Wei Z, Zhang CY and Si DW: Tanshinone IIA prevents the loss of
nigrostriatal dopaminergic neurons by inhibiting NADPH oxidase and
iNOS in the MPTP model of Parkinson's disease. J Neurol Sci.
348:142–152. 2015. View Article : Google Scholar
|
|
17
|
Hu L, Yu T and Jia Z: Experimental study
of the protective effects of Astragalus and Salvia miltiorrhiza
bunge on glycerol induced acute renal failure in rabbits. Zhonghua
Wai Ke Za Zhi. 34:311–314. 1996.In Chinese. PubMed/NCBI
|
|
18
|
Sun XG, Fu XQ, Cai HB, Liu Q, Li CH, Liu
YW, Li YJ, Liu ZF, Song YH and Lv ZP: Proteomic analysis of
protective effects of polysaccharides from Salvia miltiorrhiza
against immunological liver injury in mice. Phytother Res.
25:1087–1094. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Guo J, Bao J, Lu J and Wang Y: The
anticancer properties of Salvia miltiorrhiza Bunge (Danshen): a
systematic review. Med Res Rev. 34:768–794. 2014. View Article : Google Scholar
|
|
20
|
Cui L, Li T, Liu Y, Zhou L, Li P, Xu B,
Huang L, Chen Y, Liu Y, Tian X, et al: Salvianolic acid B prevents
bone loss in prednisone-treated rats through stimulation of
osteogenesis and bone marrow angiogenesis. PLoS One. 7:e346472012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Y, Wang L, Zhang L, Wang T, Zhou Y,
Ding C, Yang R, Wang X and Yu L: Optimization of extraction and
antioxidant activity of polysaccharides from Salvia miltiorrhiza
Bunge residue. Int J Biol Macromol. 79:533–541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiang G, Yang X, Shi L, Zhang H, Chen B,
Zhao Y, Zu M, Zhou D, Guo J, Yang H, et al: Antidiabetic effect of
salvianolic acid A on diabetic animal models via AMPK activation
and mitochondrial regulation. Cell Physiol Biochem. 36:395–408.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin Q, Jiang S, Wu YL, Bai T, Yang Y, Jin
X, Lian LH and Nan JX: Hepatoprotective effect of cryptotanshinone
from Salvia miltiorrhiza in
D-galactosamine/lipopolysaccharide-induced fulminant hepatic
failure. Phytomedicine. 21:141–147. 2014. View Article : Google Scholar
|
|
24
|
Lv H, Wang L, Shen J, Hao S, Ming A, Wang
X, Su F and Zhang Z: Salvianolic acid B attenuates apoptosis and
inflammation via SIRT1 activation in experimental stroke rats.
Brain Res Bull. 115:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sonis ST, Tracey C, Shklar G, Jenson J and
Florine D: An animal model for mucositis induced by cancer
chemotherapy. Oral Surg Oral Med Oral Pathol. 69:437–443. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Florin A, Maire M, Bozec A, Hellani A,
Chater S, Bars R, Chuzel F and Benahmed M: Androgens and
postmeiotic germ cells regulate claudin-11 expression in rat
Sertoli cells. Endocrinology. 146:1532–1540. 2005. View Article : Google Scholar
|
|
27
|
Blijlevens NM: Cytotoxic treatment-induced
gastrointestinal symptoms. Curr Opin Support Palliat Care. 1:16–22.
2007. View Article : Google Scholar
|
|
28
|
Stringer AM and Logan RM: The role of oral
flora in the development of chemotherapy-induced oral mucositis. J
Oral Pathol Med. 44:81–87. 2015. View Article : Google Scholar
|
|
29
|
Nonzee NJ, Dandade NA, Patel U, Markossian
T, Agulnik M, Argiris A, Patel JD, Kern RC, Munshi HG, Calhoun EA,
et al: Evaluating the supportive care costs of severe
radiochemotherapy-induced mucositis and pharyngitis: results from a
Northwestern University. Costs of Cancer program pilot study with
head and neck and nonsmall cell lung cancer patients who received
care at a county hospital, a Veterans administration hospital, or a
comprehensive cancer care center. Cancer. 113:1446–1452. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahmed KM: The effect of olive leaf extract
in decreasing the expression of two pro-inflammatory cytokines in
patients receiving chemotherapy for cancer. A randomized clinical
trial. Saudi Dent J. 25:141–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Evrard A, Cuq P, Robert B, Vian L,
Pèlegrin A and Cano JP: Enhancement of 5-fluorouracil cytotoxicity
by human thymidine-phosphorylase expression in cancer cells: in
vitro and in vivo study. Int J Cancer. 80:465–470. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kodach LL, Bos CL, Durán N, Peppelenbosch
MP, Ferreira CV and Hardwick JC: Violacein synergistically
increases 5-fluorouracil cytotoxicity, induces apoptosis and
inhibits Akt-mediated signal transduction in human colorectal
cancer cells. Carcinogenesis. 27:508–516. 2006. View Article : Google Scholar
|
|
33
|
Criswell T, Leskov K, Miyamoto S, Luo G
and Boothman DA: Transcription factors activated in mammalian cells
after clinically relevant doses of ionizing radiation. Oncogene.
22:5813–5827. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mendoza G, Alvarez AI, Pulido MM, Molina
AJ, Merino G, Real R, Fernandes P and Prieto JG: Inhibitory effects
of different antioxidants on hyaluronan depolymerization. Carbohydr
Res. 342:96–102. 2007. View Article : Google Scholar
|
|
35
|
Kaji H, Inukai Y, Maiguma T, Ono H,
Teshima D, Hiramoto K and Makino K: Radical scavenging activity of
bisbenzylisoquinoline alkaloids and traditional prophylactics
against chemotherapy-induced oral mucositis. J Clin Pharm Ther.
34:197–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Erstad DJ and Cusack JC Jr: Targeting the
NF-κB pathway in cancer therapy. Surg Oncol Clin N Am. 22:705–746.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lappas M, Permezel M and Rice GE:
N-acetyl-cysteine inhibits phospholipid metabolism, proinflammatory
cytokine release, protease activity, and nuclear factor-kappaB
deoxyribonucleic acid-binding activity in human fetal membranes in
vitro. J Clin Endocrinol Metab. 88:1723–1729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bamba S, Andoh A, Yasui H, Araki Y, Bamba
T and Fujiyama Y: Matrix metalloproteinase-3 secretion from human
colonic subepithelial myofibroblasts: role of interleukin-17. J
Gastroenterol. 38:548–554. 2003.PubMed/NCBI
|
|
39
|
Curra M, Martins MA, Lauxen IS, Pellicioli
AC, Sant'Ana Filho M, Pavesi VC, Carrard VC and Martins MD: Effect
of topical chamomile on immunohistochemical levels of IL-1β and
TNF-α in 5-fluorouracil-induced oral mucositis in hamsters. Cancer
Chemother Pharmacol. 71:293–299. 2013. View Article : Google Scholar
|