Introduction
Low back pain (LBP) is a major threat to the health
of middle-aged and elderly individuals, among whom nearly one-third
require surgery. LBP causes a heavy socio-economical burden.
Intervertebral disc degeneration (IDD) is widely recognized as a
main contributor to LBP. The etiological factors of IDD include
aging, smoking, abnormal mechanical loading, occupational exposure
and trauma (1–6). IDD is characterized by decreased
disc height, nucleus pulposus (NP) dehydration, annulus fibrosus
(AF) tear, immune cell infiltration, neovas cularization and
neuronal ingrowth (7,8). Therapeutic mea sures based on
preventing and delaying the initiation and progression of IDD are
considered to be effective measures for LBP treatment.
The pro-inflammatory and catabolic phenotype of disc
cells has been identified in degenerative discs. Extracellular
matrix (ECM) catabolic proteases are potently associated with the
structural failure of discs. Pro-inflammatory cytokines suppress
the matrix synthesis of disc cells and upregulate the expression of
matrix proteases in disc cells, leading to an imbalance between
matrix anabolism and catabolism in IVDs. Moreover, pro-inflammatory
cytokines are able to induce the senescence, apoptosis and
autophagy of disc cells (9,10).
Therefore, the pro-inflammatory and catabolic phenotype of disc
cells is detrimental to the structural and functional homeostasis
of discs. Noticeably, the viability and function of disc cells are
regulated by the microenvironment of discs. The microenvironment of
degenerative IVDs is characterized by low pH, high osmotic
pressure, hypoxia and low nutrition. The harsh microenvironment of
degenerative discs disrupts the homeostasis of disc cells, which
plays a crucial role in the pathogenesis of IDD (11). However, the regulatory effect of
the microenvironment on the pro-inflammatory and catabolic
phenotype of disc cells has not been elucidated.
N-acetylated proline-glycine-proline
(N-Ac-PGP) is a tripeptide generated from collagens degraded
by matrix metalloproteinase 8 (MMP8), MMP9 and prolyl endopeptidase
(PE) (12). Previous studies have
reported the wide role of N-Ac-PGP in neutrophilic
inflammatory diseases, such as chronic obstructive pulmonary
disease and inflammatory bowel disease (13,14). Recently, we identified the
presence of N-Ac-PGP in human NP tissues. The level of
N-Ac-PGP in NP tissues is positively correlated with the
grade of IDD, suggesting the involvement of N-Ac-PGP in the
pathogenesis of IDD. Moreover, N-Ac-PGP promotes the
migration of cartilage endplate stem cells (CESCs) and induces the
differentiation of CESCs toward a pro-inflammatory and catabolic
phenotype via CXCR1/2. Thus, we conclude that N-Ac-PGP is a
pro-inflammatory and catabolic matrikine in the microenvironment of
IVDs (15). Nevertheless, the
effect of N-Ac-PGP on human functional disc cells and the
molecular mechanism underlying this effect remain unknown.
In this study, the Milliplex MAP assay (Millipore,
Billerica, MA, USA) was used to measure the level of
pro-inflammatory cytokines in conditioned culture medium of human
NP cells treated with N-Ac-PGP. We also analyzed the
expression of pro-inflammatory cytokines and matrix catabolic
proteases in NP cells using reverse transcription-quantitative PCR
(RT-qPCR). Lastly, we investigated the activation of the nuclear
factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK)
signaling path ways in NP cells treated with N-Ac-PGP. The
role of the NF-κB and MAPK signaling pathways in mediating the
effect of N-Ac-PGP on the phenotype of NP cells was
investigated using specific signaling inhibitors. Our study, for
the first time, elucidated the effect of N-Ac-PGP on the
catabolic and pro-inflammatory signaling of human NP cells via the
NF-κB and MAPK pathways, further revealing the roles of
N-Ac-PGP in the pathogenesis of IDD. The present study
provides a novel insight into the mechanism underlying the
establishment and progression of IDD.
Materials and methods
Ethics statement
The present study was performed complying with the
ethical standards set forth by the Declaration of Helsinki, and was
approved by the Ethics Committee of Xinqiao Hospital (Chonqing,
China).
Reagents and antibodies
N-Ac-PGP was provided by Sigma-Aldrich (St.
Louis, MO, USA). N-Ac-PGP was diluted with
phosphate-buffered saline (PBS; 1 mg/ml). The rabbit monoclonal
anti-human p38 (no. 9212), phos pho-p38 (p-p38; no. 9211), JNK (no.
9252), p-JNK (no. 9251), ERK (no. 9102) and p-ERK (no. 9101)
antibodies were all obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). The mouse monoclonal anti-human GAPDH
(sc-47724) antibody was provided by Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The rabbit polyclonal anti-human p65
antibody (ab16502) and the rabbit monoclonal anti-human p-p65
antibody (ab76302) were both purchased from Abcam (Cambridge, MA,
USA). The goat polyclonal anti-mouse IgG (H+L) horseradish
peroxidase (HRP)-conjugated seco ndary antibody (ZB2305) and the
goat anti-rabbit IgG (H+L) HRP-conjugated secondary antibody
(ZB2301) were both purchased from ZSGB-BIO (Beijing, China). The
p38 (SB202190, SB), JNK (SP600125, SP) and ERK (U0126, U) signaling
inhibitors were provided by MedChem Express (Princeton, NJ, USA).
The NF-κB inhibitor (PDTC) was purchased from Beyotime Institute of
Biotechnology (Shanghai, China).
NP cell culture
Human NP tissues were collected from 8 patients
undergoing lumbar discectomy and a fusion procedure in Xinqiao
Hospital (Chongqing, China) (Table
I). Pre-operative MRI was performed to evaluate the Pfirrmann
grade of the intervertebral discs (IVDs) (16). IVD specimens were preserved in
sterile saline solution once dissected from the human body. NP
tissues, a gel-like tissue, were carefully isolated from IVD
specimens and minced into thin tissue blocks (1 mm3).
After washing with PBS three times, NP tissues were incubated with
0.2% type II collagenase (Sigma-Aldrich) in Dulbecco's modified
Eagle's medium (DMEM)/F-12 medium (Invitrogen Life Technologies,
Carlsbad, CA, USA) at 37°C for 12 h. Next, the suspension was
collected and centrifuged at 1,200 rpm for 6 min. The isolated NP
cells were cultured in DMEM/F-12 medium containing 10% fetal bovine
serum (FBS) and 1% penicillin/streptomycin (Invitrogen Life
Technologies), and grew as a monolayer in 5% CO2 at
37°C. The medium was replaced by fresh medium twice a week. When
the cells reached about 80% confluence, they were sub-cultured. NP
cells from different patients were pooled at passage 1 and were
used at passage 2 or 3. The marker genes of NP cells used in this
study were analyzed by semi-quantitative reverse transcription PCR.
Three NP cell-positive gene markers [CA12, KRT19 and CDH2 (17)] and a NP cell-negative gene marker
[IBSP (17)] were validated (data
not shown).
 | Table IDemographic information of the NP
tissue donors. |
Table I
Demographic information of the NP
tissue donors.
| Donors | Sex | Age (years) | Pfirrmann
grade |
|---|
| Patient 1 | Male | 43 | II |
| Patient 2 | Male | 36 | II |
| Patient 3 | Male | 48 | III |
| Patient 4 | Male | 52 | III |
| Patient 5 | Male | 57 | III |
| Patient 6 | Female | 42 | II |
| Patient 7 | Female | 41 | III |
| Patient 8 | Female | 37 | II |
Milliplex MAP assay
NP cells were trypsinized and seeded into 6-well
culture plates (Corning Inc., Corning, NY, USA). They were then
treated with various concentrations of N-Ac-PGP (0.1, 1, 10
and 100 µg/ml) for different durations (1, 3, 5 and 7 days)
at 37°C with 5% CO2. The conditioned media were
harvested for Milliplex MAP Human Cytokine/Chemokine Multiplex
Immunoassay (Millipore). The cytokines and chemokines included
interleukin-1β (IL-1β), IL-6, IL-17, C-C motif ligand 2 (CCL2) and
tumor necrosis factor-α (TNF-α). In brief, 25 µl standard
solution, 25 µl mixed beads and 200 µl wash water
were incubated in one well for 17 h at 4°C and for 2 h at room
temperature. Then, after washing with wash buffer three times, 25
µl antibodies was added to each well and incubation was
carried out at 25°C for 1 h. After adding 25 µl of
streptavidin-phycoerythrin to each well, the solution was incubated
for 1 h at 25°C. Lastly, the wells were washed with wash buffer
three times and were treated with 150 µl of sheath fluid. An
automated immunoassay analyzer (Luminex 200™; Millipore) was used
to detect the fluorescence intensity of each well. NP cells without
any treatment served as the blank control. NP cells treated with
PBS served as the negative control. Median fluorescent intensity
(MFI) data were analyzed using a 5-parameter logistic or
spline-fitting method for calculating cytokine or chemokine
concentrations in the conditioned culture media.
Treatment with MAPK and NF-κB signaling
inhibitors
To analyze the effect of N-Ac-PGP on the
phosphorylation of ERK, JNK, p38 and p65, NP cells were treated
with N-Ac-PGP (100 µg/ml) for 15, 30, 45 and 60 min.
The activation of these signaling pathways was investigated via
western blot assays. In order to determine the role of the MAPK and
NF-κB signaling pathways in mediating the catabolic and
pro-inflammatory effect of N-Ac-PGP, NP cells were
pre-treated with U (1 µg/ml), SP (1 µg/ml), SB (1
µg/ml) and PDTC (1 µg/ml) for 30 min followed by
N-Ac-PGP treatment (100 µg/ml) for 3 days. After
that, the total RNA of the NP cells was isolated to perform RT-qPCR
analysis. NP cells without any treatment served as the control.
RT-qPCR
The RNeasy Mini kit (Qiagen, Valencia, CA, USA) was
used to extract total RNA of the NP cells. RNA (1 µg) was
used to perform reverse transcription using the Omniscript Reverse
Transcription kit (Qiagen) according to the protocol provided the
manufacturer. RT-qPCR was performed using a ViiA7 Real-Time PCR
system (Applied Biosystems, Foster City, CA, USA) and the
QuantiNova™ SYBR-Green PCR kit (Qiagen) according to the
manufacturer's instructions. The 20 µl reaction volume was
applied. Reaction parameters were as follows: initial heat
activation for 2 min at 95°C, followed by 40 cycles of 5 sec at
95°C for template denaturation and 34 sec at 60°C for annealing and
extension. All samples were amplified in triplicate, and the
results are presented as a Ct value, which is the cycle number at
which the amplified product is first detected. The expression level
of the targeted genes was normalized to that of GAPDH. Relative
mRNA expression levels of target genes were calculated using the
2−ΔΔCt method (18).
The primers of the target genes investigated in the present study
are listed in Table II.
 | Table IIPrimer sequences used in the RT-qPCR
analysis. |
Table II
Primer sequences used in the RT-qPCR
analysis.
| Genes | Primer
sequences |
|---|
| MMP3 | F:
CACGGAACCTGTCCCTCCAGAA |
| R:
GCATCCACGCCTGAAGGAAGAG |
| MMP13 | F:
CTGGCTGCCTTCCTCTTCTTGA |
| R:
GTCATGGAGCTTGCTGCATTCT |
| ADAMTS4 | F:
AAGAGTCCTGCCAGCGGTCAA |
| R:
ATCTGCCACCACCAGTGTCTCC |
| ADAMTS5 | F:
TGTCCTGCCAGCGGATGTGT |
| R:
GATGCCGTCACAGCCAGTTCTC |
| IL-6 | F:
AGCCAGAGCTGTGCAGATGAGT |
| R:
GGCATTTGTGGTTGGGTCAGGG |
| CCL2 | F:
ACCAGCAGCAAGTGTCCCAAAG |
| R:
GGGTTTGCTTGTCCAGGTGGTC |
| CCL5 | F:
CAGCAGTCGTCCACAGGTCAAG |
| R:
GGCACACACTTGGCGGTTCTT |
| CXCL10 | F:
GGAACCTCCAGTCTCAGCACCA |
| R:
GCTGATGCAGGTACAGCGTAC |
| GAPDH | F:
CCAGCAAGAGCACAAGAGGAAGAG |
| R:
GGTCTACATGGCAACTGTGAGGAG |
Western blot analysis
NP cells were lysed with radio-immunoprecipitation
assay lysis buffer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing proteinase inhibitor on ice for 30 min. A BCA kit
(Beyotime Institute of Biotechnology) was used to measure the
concentration of the proteins. The proteins were mixed with loading
buffer (Invitrogen Life Technologies). Proteins (50 µg) were
separated by 10% (w/v) sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and were then transferred to
polyvinylidene fluoride (PVDF) membranes (Millipore). PVDF
membranes were blocked with 5% non-fat milk in Tris-buffered saline
(TBS) for 1 h at 37°C and then were incubated with primary
antibodies against GAPDH (1:1,000 dilution), p65 (1:1,000
dilution), p-p65 (1:1,000 dilution), p38 (1:1,000 dilution), p-p38
(1:1,000 dilution), ERK (1:1,000 dilution), p-ERK (1:1,000
dilution), JNK (1:1,000 dilution) and p-JNK (1:100 dilution)
overnight at 4°C. After rinsing with TBS three times, PVDF
membranes were incubated with HRP-conjugated secondary antibodies
respectively at 37°C for 1 h. Lastly, the target proteins were
detected using an enhanced chemiluminescence system (Millipore,
Bedford, MA, USA) and scanned using an ImagineQuant LAS 4000 (GE
Healthcare, Fairfield, CT, USA). The optical density (OD) of the
western blot bands was measured using ImageJ software (National
Institutes of Health, Bethesda, MD, USA). The phosphorylation level
of each protein = OD of the phospho-protein/OD of total protein.
The relative phosphorylation level (RPL) of proteins in each group
= the phosphorylation level of proteins in each group/the
phosphorylation level of proteins in the control group.
Statistical analysis
All experiments were independently performed at
least three times. The data are presented as the mean ± standard
error of the mean (SEM). For multiple comparisons among three or
more groups, the one-way analysis of variance (ANOVA) and the least
significant difference (LSD) comparison were performed. The data of
RT-qPCR were analyzed by Kruskal-Wallis non-parametric analysis and
Mann-Whitney U post hoc tests as previously mentioned (19). GraphPad Prism 6 (GraphPad
Software, Inc., La Jolla, CA, USA) and SPSS version 22.0
statistical software (International Business Machines Corporation,
Armonk, NY, USA) were used to analyze and display the data of this
study. P<0.05 was regarded as indicative of statistical
significance.
Results
N-Ac-PGP increases the secretion of
pro-inflammatory cytokines by NP cells in a dose-dependent
manner
The conditioned culture media of NP cells treated
with N-Ac-PGP (0.1, 1, 10 and 100 µg/ml) for various
durations (1, 3, 5 and 7 days) were collected to measure the level
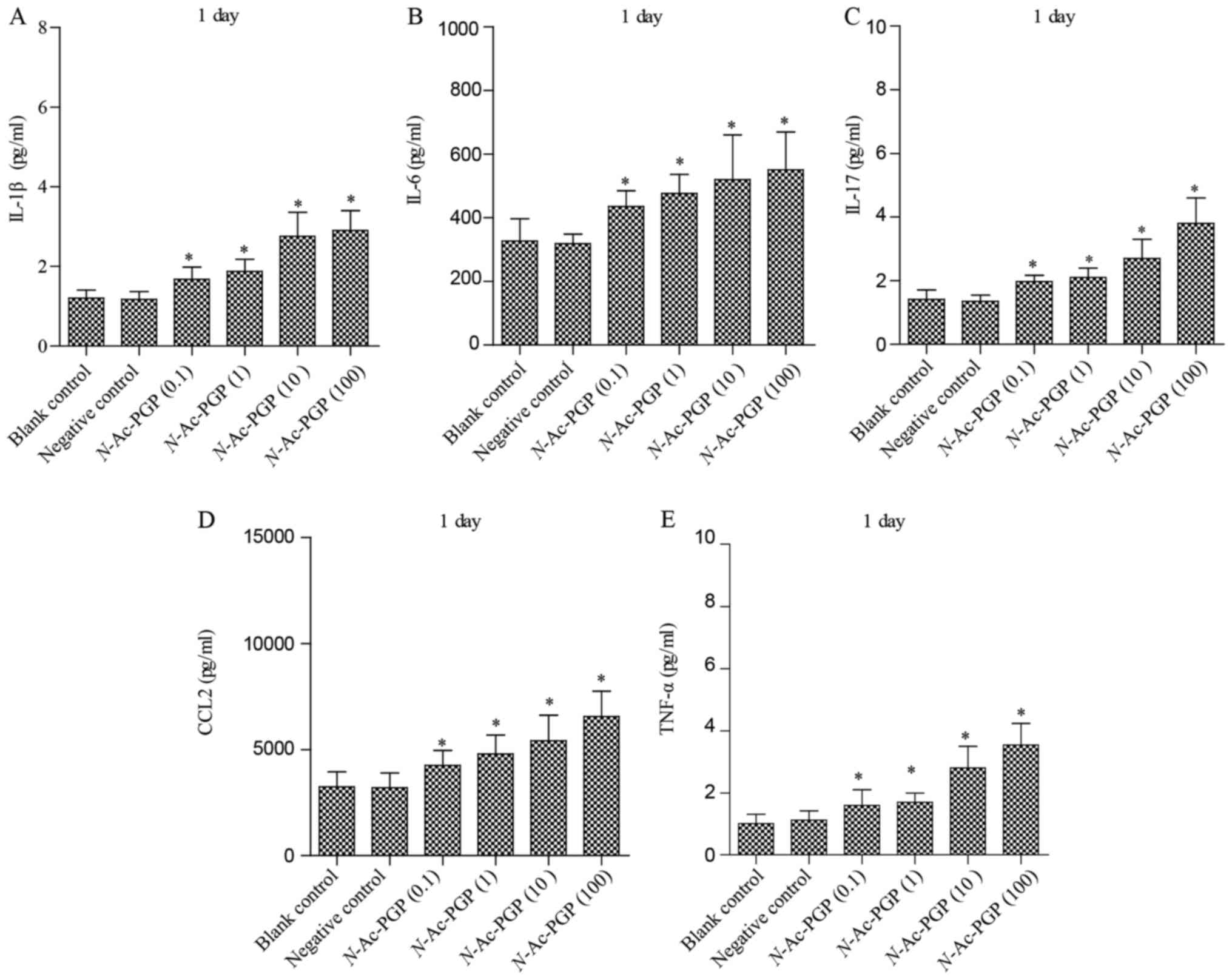
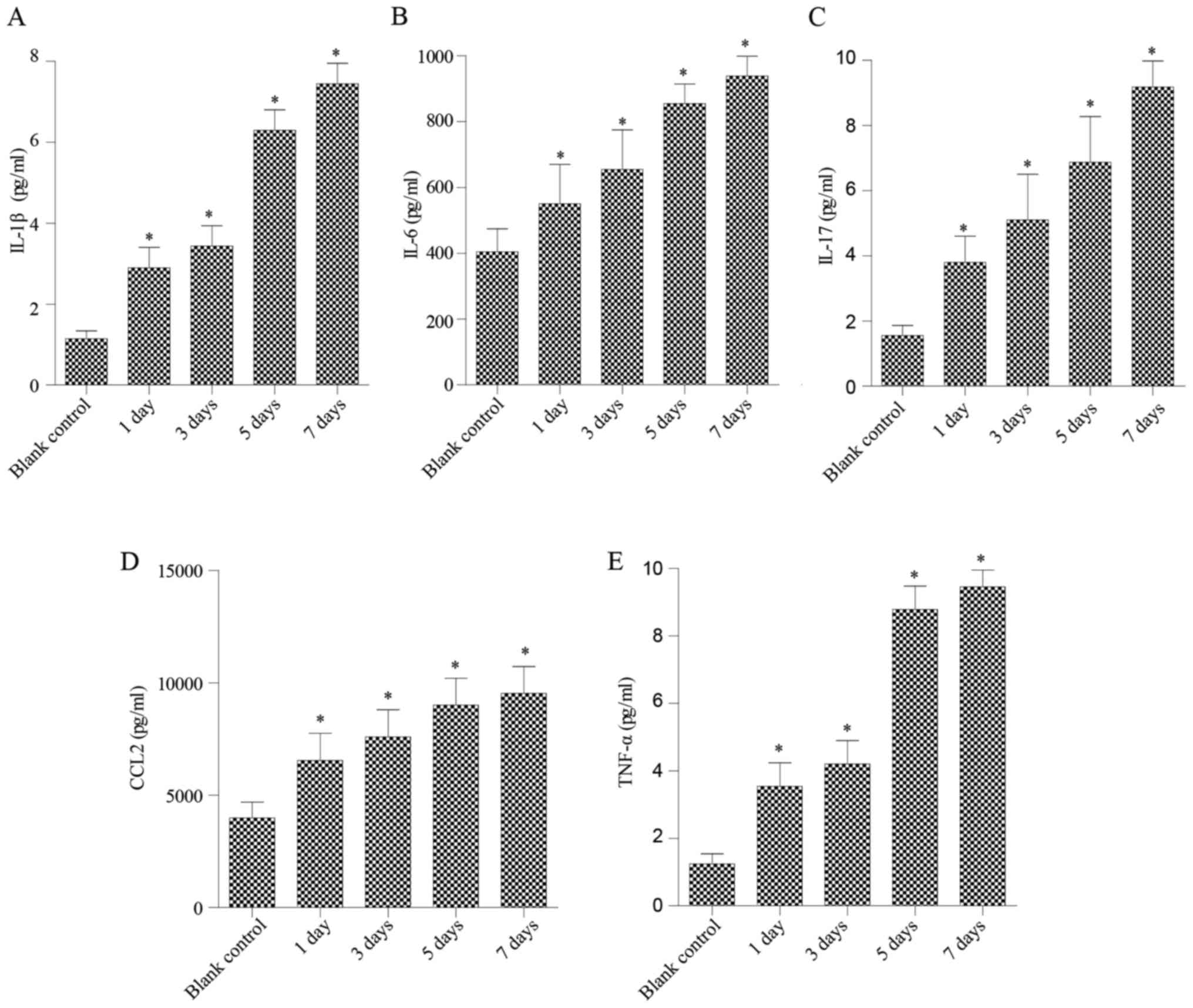
of pro-inflammatory cytokines secreted by NP cells. As shown in
Fig. 1, after treatment with
various concentrations of N-Ac-PGP for 1 day, increases in
the secretion of IL-1β, IL-6, IL-17, CCL2 and TNF-α by NP cells
were observed regardless of the concentration of N-Ac-PGP.
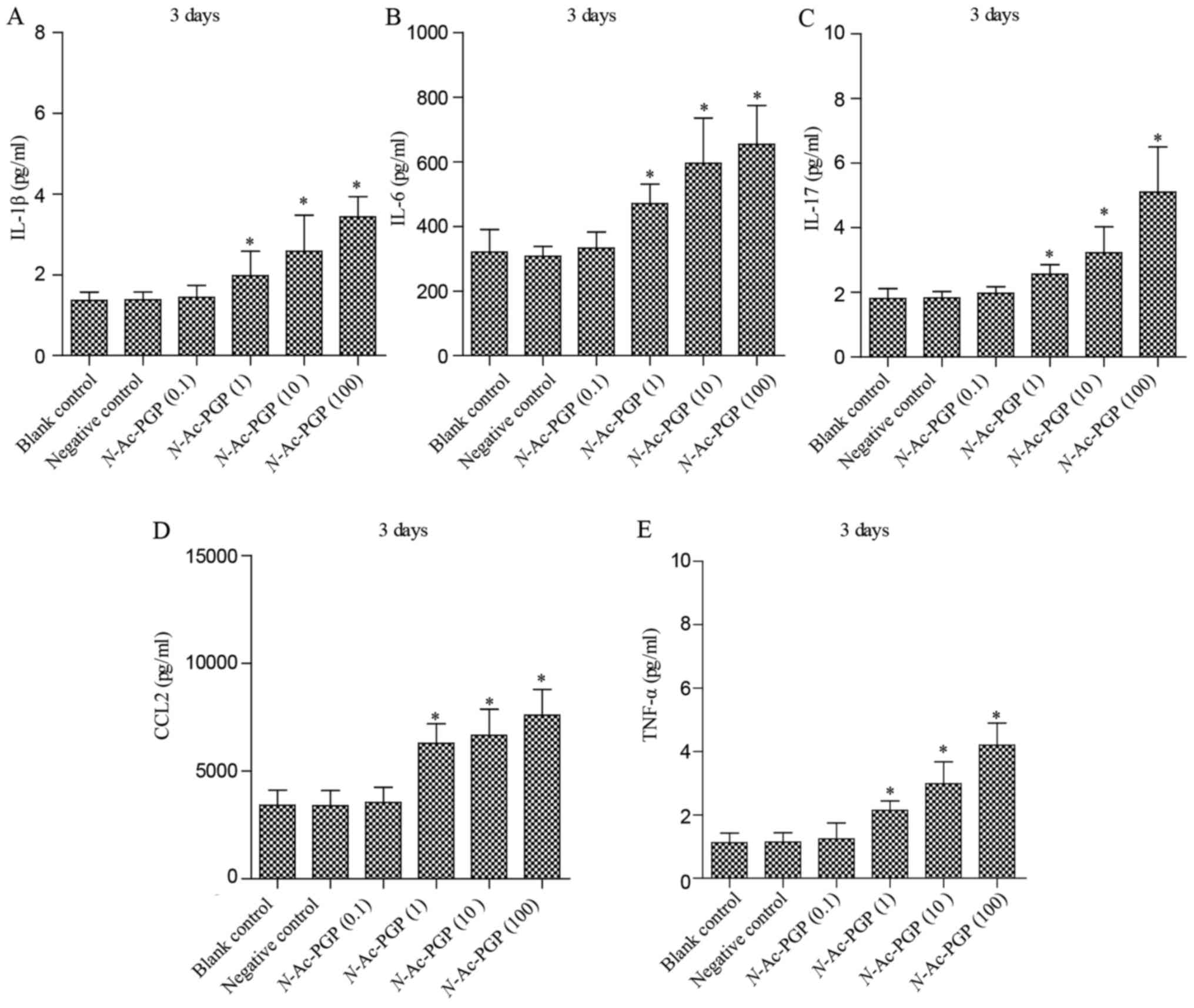
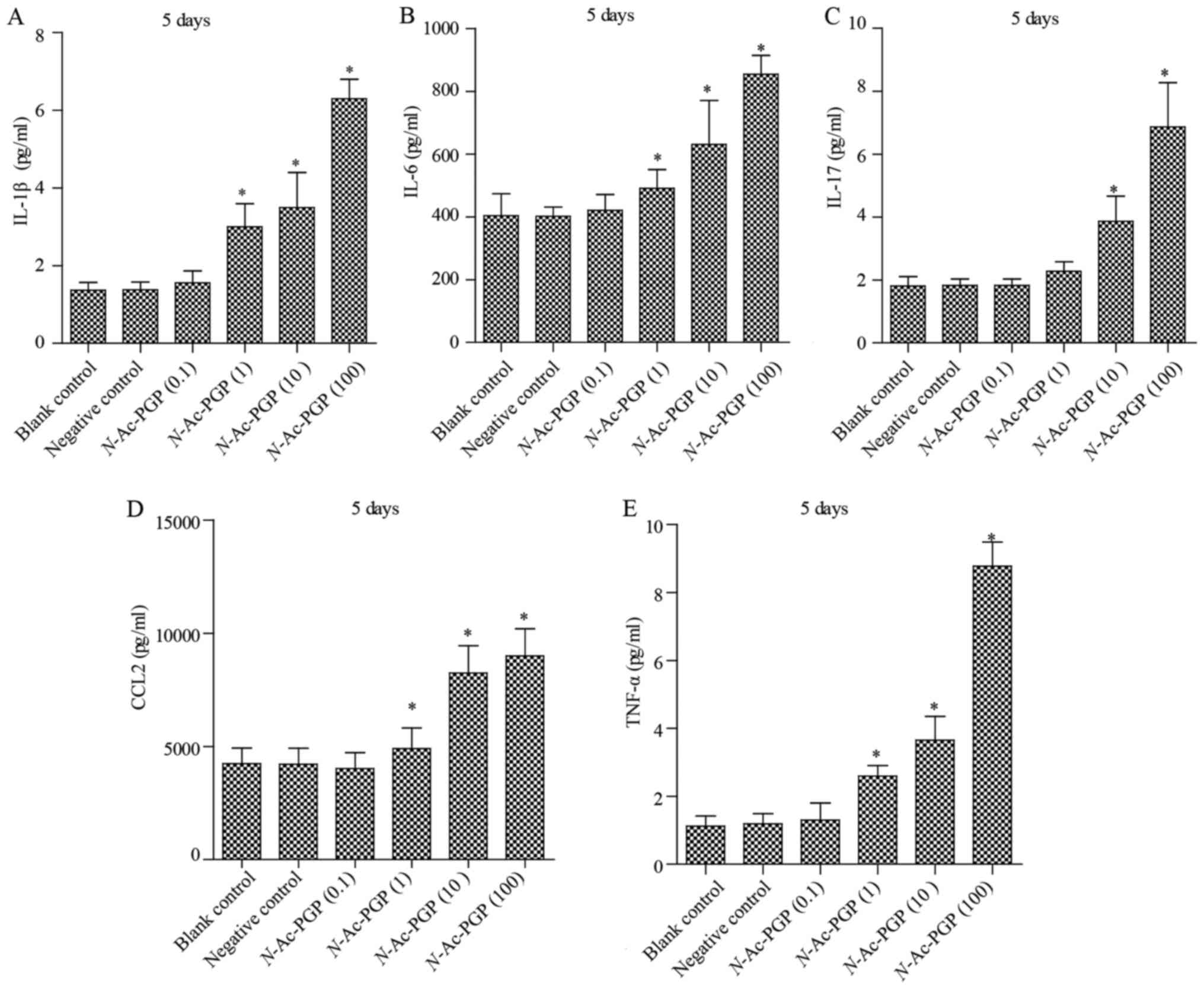
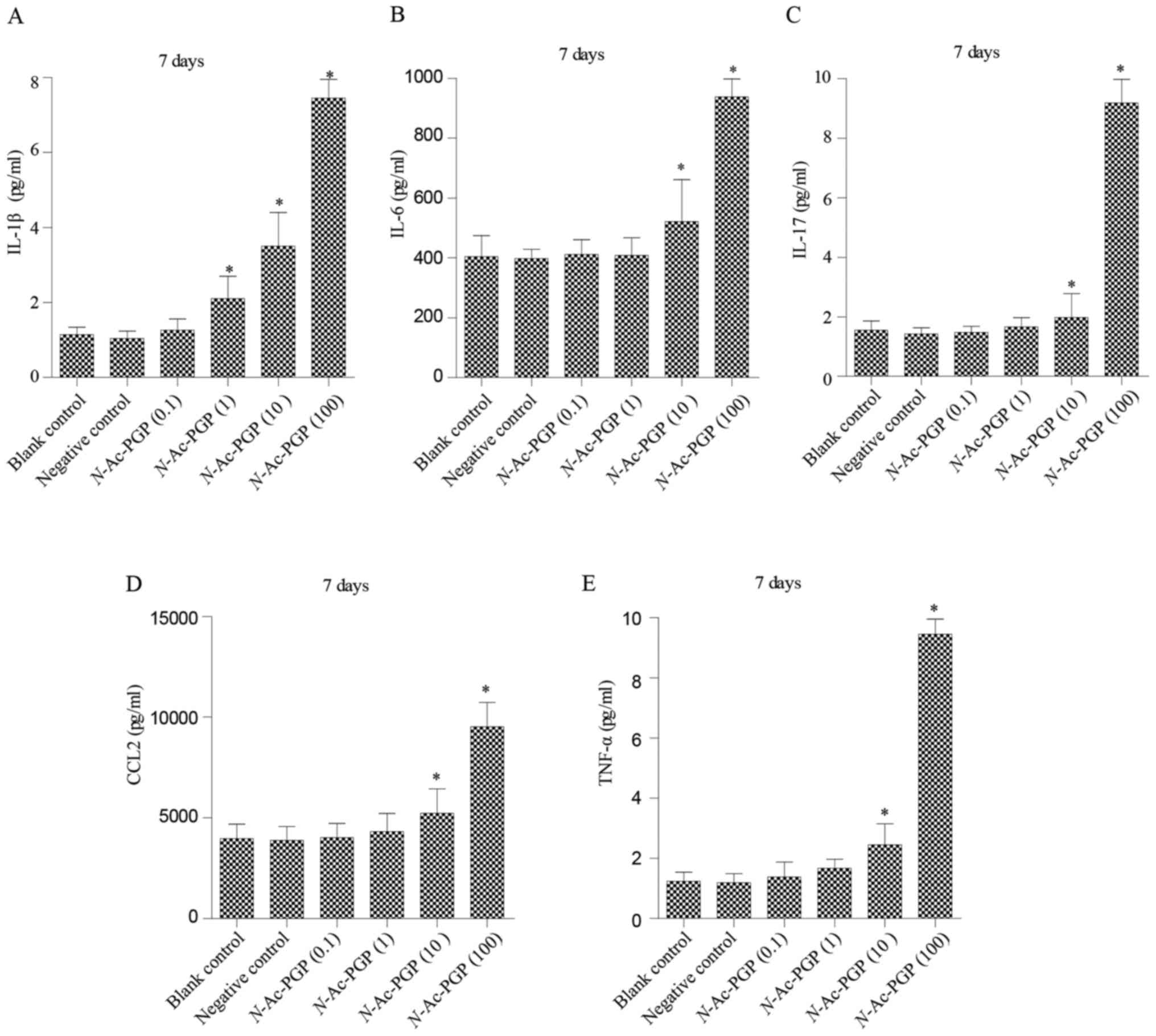
With increasing duration of N-Ac-PGP treatment, the
inductive effect of N-Ac-PGP on the secretion of IL-1β,
IL-6, IL-17, CCL2 and TNF-α by NP cells was observed (Figs. 2Figure 3–4), indicating the potent
pro-inflammatory effect of N-Ac-PGP on human NP cells.
Furthermore, the pro-inflammatory effect of N-Ac-PGP was
shown to be dose-dependent. Taking Fig. 2 as an example, the secretion of
IL-1β started to increase after 1 µg/ml of N-Ac-PGP
treatment while there was no significant change in NP cells treated
with 0.1 µg/ml of N-Ac-PGP. With increasing
concentrations of N-Ac-PGP (10 and 100 µg/ml), a
higher magnitude of increased IL-1β secretion induced by
N-Ac-PGP was determined (Fig.
2). Noticeably, the inductive effect of N-Ac-PGP on the
secretion of IL-6, IL-17, CCL2 and TNF-α by NP cells showed a
similar dose-dependent trend (Figs.
1Figure 2Figure 3–4). Generally speaking, N-Ac-PGP
at 10 and 100 µg/ml showed a significant pro-inflammatory
effect on NP cells. Thus, N-Ac-PGP is a potent
pro-inflammatory matrikine in the microenvironment of discs.
The temporal pattern of the
pro-inflammatory effect of N-Ac-PGP on NP cells
As mentioned above, N-Ac-PGP enhanced the
secretion of pro-inflammatory cytokines by NP cells in a
dose-dependent manner. Herein, we investigated the relationship
between the duration of N-Ac-PGP treatment and the
pro-inflammatory effect of N-Ac-PGP on NP cells. We found
that the level of pro-inflammatory cytokines in culture media
significantly increased after N-Ac-PGP treatment (100
µg/ml) for 1 day (Fig. 5).
With the prolonged duration of N-Ac-PGP treatment, the
production of cytokines by NP cells was gradually increased
(Fig. 5), indicating that
N-Ac-PGP induces the secretion of pro-inflammatory cytokines
by NP cells in a time-dependent manner.
N-Ac-PGP upregulates the expression of
matrix degradation proteases in NP cells
In addition to the pro-inflammatory phenotype, the
matrix catabolic phenotype of NP cells is a main threat to the ECM
homeostasis of discs and is crucial to the pathogenesis of IDD.
However, the influence of N-Ac-PGP on the expression of
matrix catabolic genes in human NP cells is unknown. To elucidate
this issue, NP cells were incubated with N-Ac-PGP (100
µg/ml) for 3 days. RT-qPCR analysis was then performed to
detect the expression of various matrix degradation proteases in
the NP cells, including MMP3, MMP13, a disintegrin and
metalloproteinase with thrombospondin motif 4 (ADAMTS4) and ADAMTS5
(Fig. 6). As a result, the
expression of matrix proteases was markedly upregulated by
N-Ac-PGP. In contrast, a significant upregulation of IL-6,
CCL2, CCL5 and C-X-C motif ligand 10 (CXCL10) in NP cells was also
induced by N-Ac-PGP (Fig.
6), which was consistent with the results of the Milliplex MAP
assays. In conclusion, N-Ac-PGP is able to regulate both
inflammatory and catabolic signaling in NP cells, confirming the
pro-inflammatory and catabolic effect of N-Ac-PGP in the
microenvironment of discs.
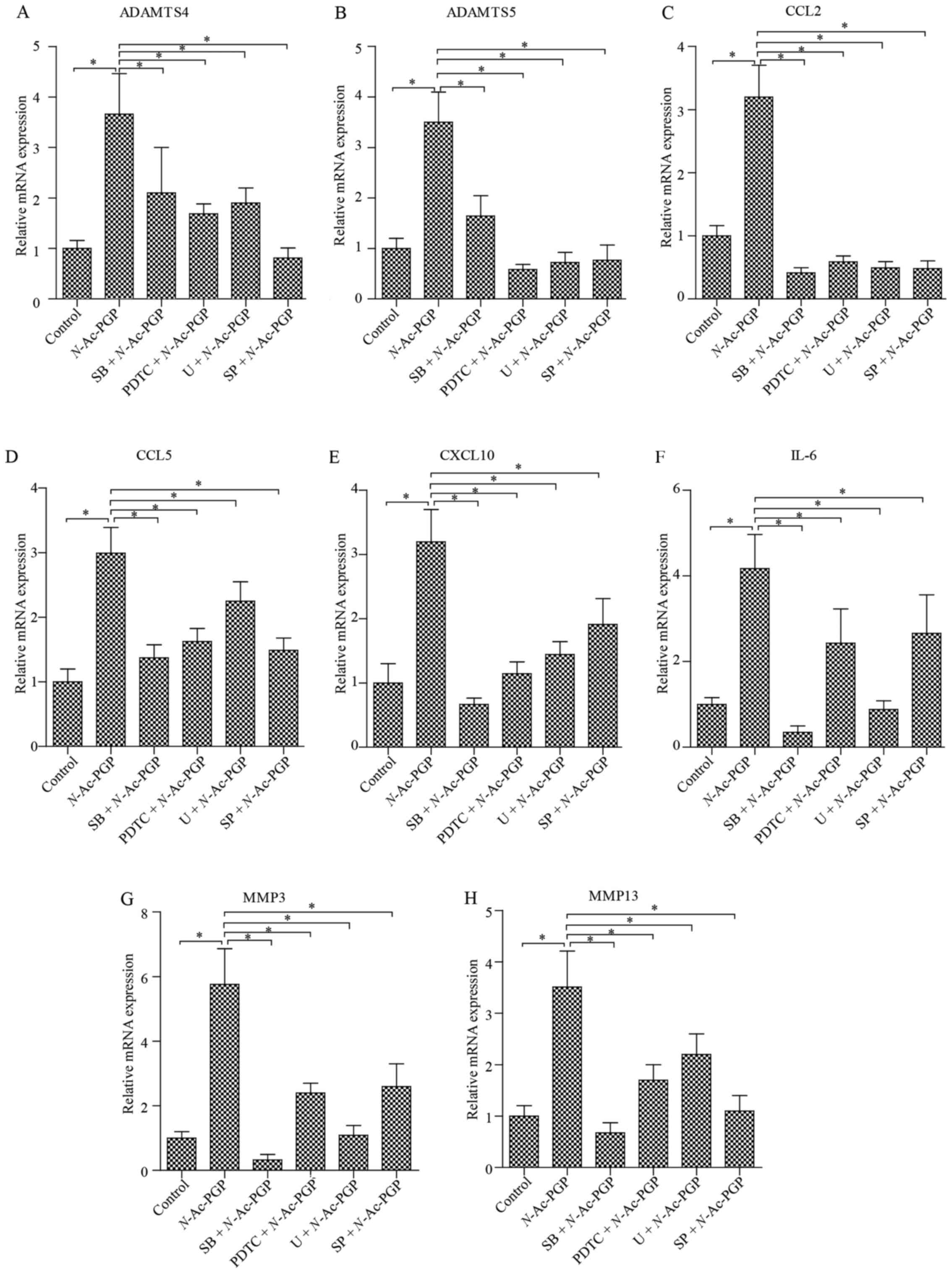 | Figure 6(A–H) Quantitative PCR analysis of
matrix degradation enzymes and pro-inflammatory cytokines in NP
cells treated with N-acetylated proline-glycine-proline
(N-Ac-PGP, 100 µg/ml) for 3 days. NP cells were
pretreated with p38 inhibitor (SB202190, SB), JNK inhibitor
(SP600125, SP), ERK inhibitor (U0126, U) or nuclear factor-κB
(NF-κB) inhibitor (PDTC) for 30 min followed by N-Ac-PGP
treatment for signaling inhibition. The results are presented as
the mean ± SEM. *P<0.05. NP, nucleus pulposus;
ADAMTS, a disintegrin and metalloproteinase with thrombospondin
motifs; CCL, C-C motif ligand; CXCL10, C-X-C motif chemokine ligand
10; IL, interleukin; MMP, matrix metalloproteinase. |
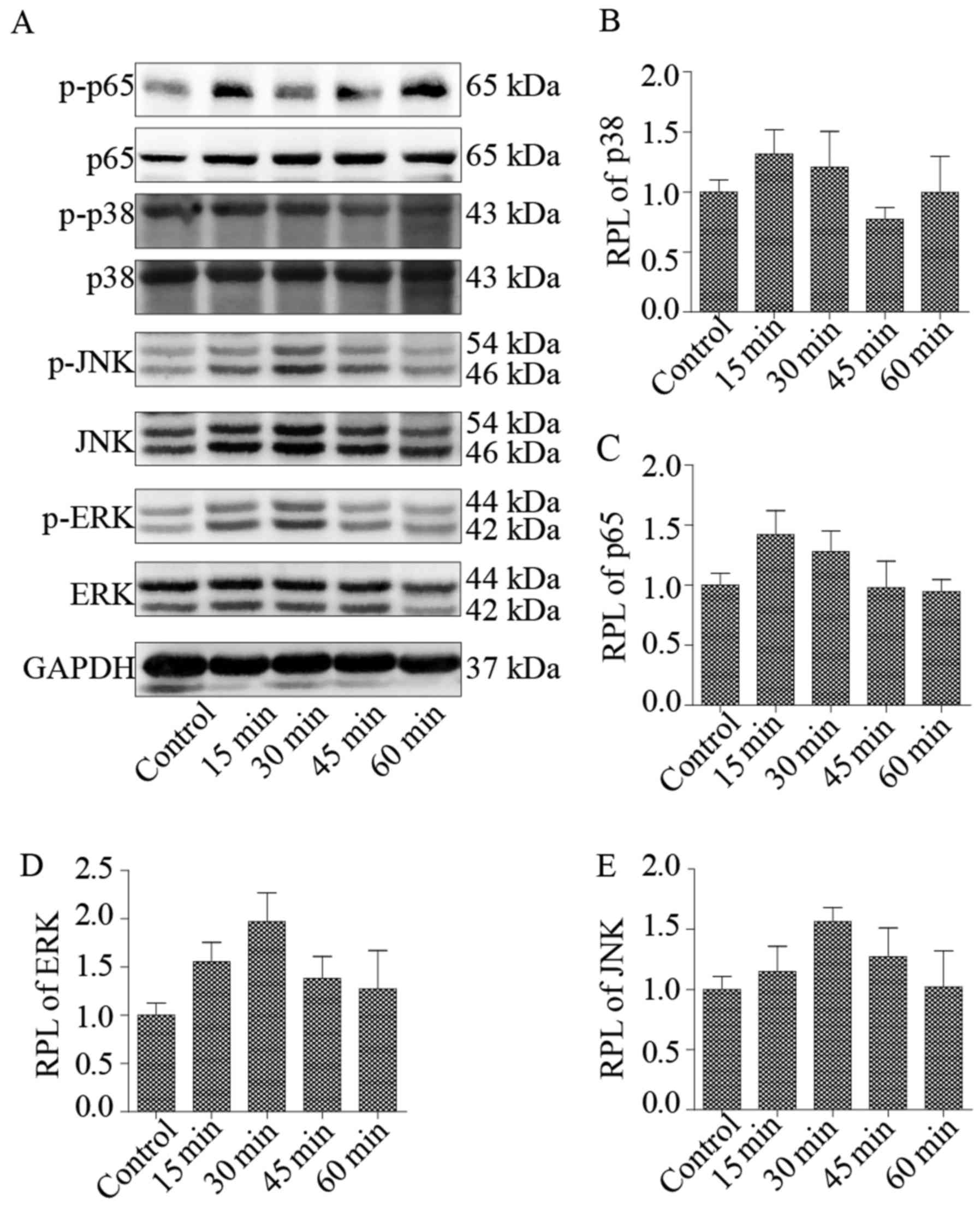
NF-κB and MAPK signaling pathways are
involved in the pro-inflammatory and catabolic effect of N-Ac-PGP
on NP cells
NF-κB and MAPK signaling pathways are crucial to the
regulation of inflammatory and catabolic signaling in IVDs
(20). To investigate whether
N-Ac-PGP regulates the pro-inflammatory and matrix catabolic
phenotype of NP cells through NF-κB and MAPK pathways, the
phosphorylation of ERK, JNK, p38 and p65 in NP cells treated with
N-Ac-PGP (100 µg/ml) was analyzed. Two signaling
pathways of MAPK (ERK and JNK) were maximally phosphorylated after
N-Ac-PGP treatment for 30 min while p38 and p65 were
maximally phosphorylated after N-Ac-PGP treatment for 15 min
(Fig. 7). After N-Ac-PGP
treatment for 15 or 30 min, the phosphorylation levels of ERK, JNK,
p38 and p65 were gradually decreased and returned to normal
(Fig. 7), indicating that the
NF-κF and MAPK signaling pathways in NP cells were activated at the
early stage of N-Ac-PGP treatment. In order to investigate
the efficacy of the specific signaling inhibitors on the
phosphorylation of p65, ERK, JNK, p38 in NP cells at the early
stage of N-Ac-PGP treatment, NP cells were pre-treated with
U (1 µg/ml), SP (1 µg/ml), SB (1 µg/ml) and
PDTC (1 µg/ml) for 30 min followed by N-Ac-PGP
treatment (100 µg/ml) for 30 min, 30 min, 15 min and 15 min
respectively. As a result, the specific signaling inhibitors
suppressed the phosphorylation of p65, ERK, JNK and p38 at the
early stage of N-Ac-PGP treatment (Fig. 8). Furthermore, the results of
RT-qPCR revealed that the inductive effect of N-Ac-PGP
treatment for 3 days on the expression of CCL2, CCL5, IL-6, CXCL10,
MMP3, MMP13, ADAMTS4 and ADAMTS5 in NP cells was retarded by the
specific signaling inhibitors (Fig.
6), suggesting that the pro-inflammatory and matrix catabolic
effect of N-Ac-PGP on NP cells is mediated by the activation
of NF-κB and MAPK signaling pathways at the early stage of
N-Ac-PGP treatment.
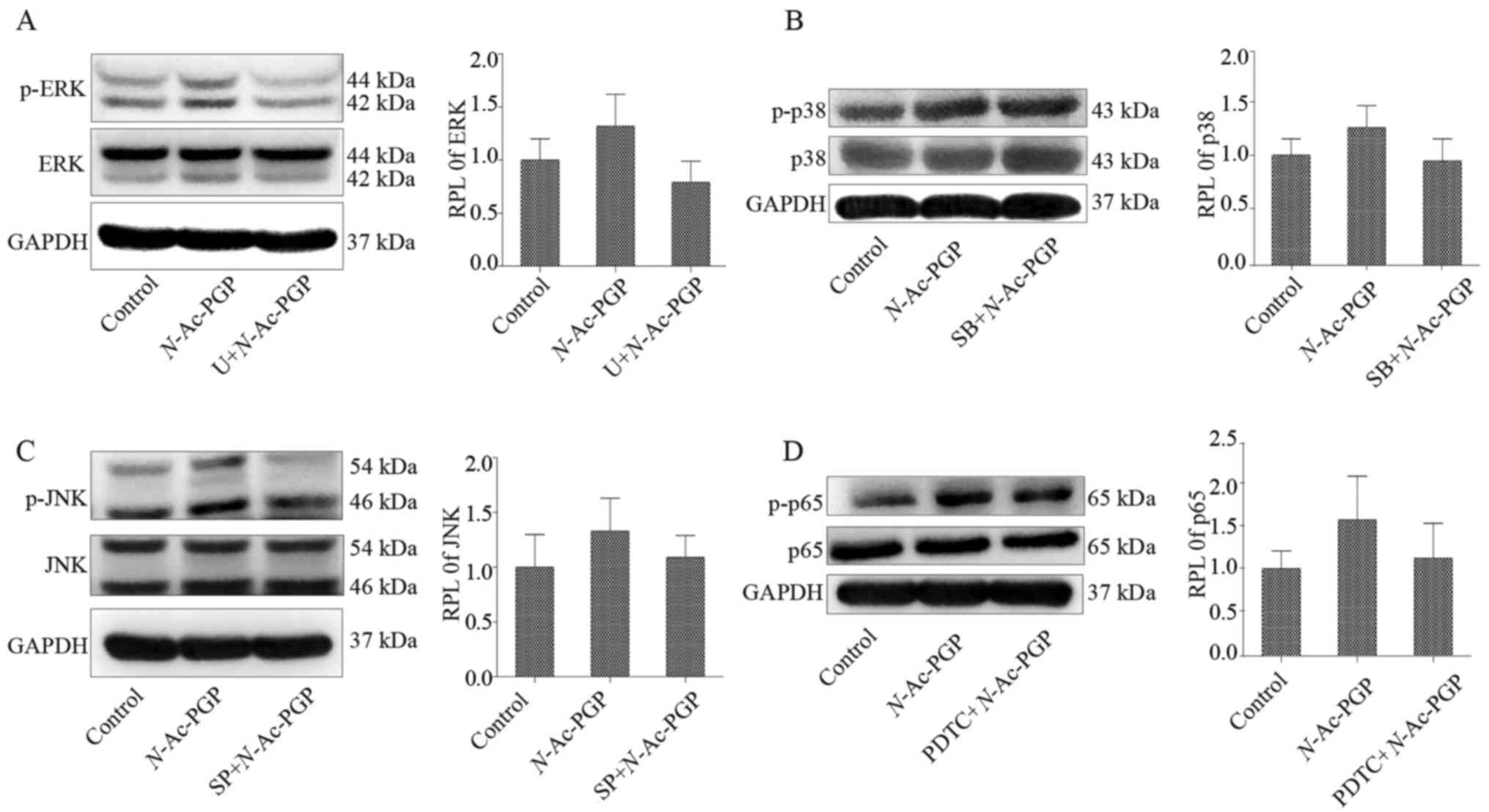 | Figure 8(A–D) Western blot analysis of the
phosphorylation of ERK, p38, JNK and p65 in nucleus pulposus (NP)
cells. NP cells were pre-treated with the ERK (U0126, U, 1
µg/ml), JNK (SP600125, SP, 1 µg/ml), p38 (SB202190,
SB, 1 µg/ml) and nuclear factor-κB (NF-κB) (PDTC, 1
µg/ml) signaling inhibitors for 30 min followed by
N-acetylated proline-glycine-proline (N-Ac-PGP)
treatment (100 µg/ml) for 30, 30, 15 and 15 min
respectively. The relative phosphorylation level (RPL) of p38, p65,
ERK, JNK in NP cells was calculated. NP cells without any treatment
served as the control. |
Discussion
In the present study, we demonstrated that
N-Ac-PGP induces the expression of pro-inflammatory
cytokines and ECM proteases in human NP cells, which was consistent
with the pro-inflammatory and catabolic effect of N-Ac-PGP
on human CESCs (15). This
suggests that N-Ac-PGP is involved in the pathogenesis of
IDD by enhancing inflammation and promoting ECM degradation in the
microenvironment of IVDs. N-Ac-PGP is detrimental to the
structural and functional homeostasis of IVDs. Furthermore, the
NF-κB and MAPK signaling pathways were shown to mediate the
catabolic and pro-inflammatory effect of N-Ac-PGP on NP
cells. To our knowledge, this is the first study to investigate the
effect of N-Ac-PGP on the phenotype of human NP cells and
the molecular pathways underlying this effect, which provides
further insight into the pathogenesis of IDD.
The structural and functional maintenance of IVDs
depends on a balance between the ECM anabolism and catabolism of
disc cells (21,22). This balance is disturbed in
degenerative discs. Various matrix catabolic proteases accumulate
in the microenvironment of discs with IDD progression, including
MMP3, MMP13, ADAMTS4 and ADAMTS5. These proteases degrade ECM to
cause structural failure of IVDs (9). On the other hand, IDD is a
pro-inflammatory cytokine-mediated pathological process. Various
pro-inflammatory cytokines including TNF-α, IL-6, IL-17, IL-1β and
CCL2 produced by disc cells upregulate the expression of matrix
proteases and suppress matrix anabolism in disc cells via autocrine
and paracrine processes. Moreover, pro-inflammatory mediators
induce the senescence, autophagy and apoptosis of disc cells
(10,23). In short, pro-inflammatory
cytokines and matrix proteases are crucial to the establishment and
progression of IDD. Therefore, the matrix catabolic and
pro-inflammatory secretory phenotype of disc cells plays an
important role in the pathogenesis of IDD. Noticeably, the
phenotype of resident disc cells is regulable for the adaption to
the microenvironment of IDD. In addition, the function and
viability of disc cells depend on microenvironment homeostasis. The
microenvironment of degenerative discs is harsh and is
characterized by an acidic pH, high osmotic pressure, low oxygen
and nutrition deficiency. With IDD progression, the
microenvironment of discs becomes harsher due to excessive
cytokines, chemokines, proteases and growth factors (11,24). Investigation of the effects of
microenvironmental factors on the matrix catabolic and
pro-inflammatory phenotype of disc cells facilitates the
development of strategies to suppress the production of cytokines
and matrix proteases by disc cells and consequently prevents or
delays the establishment and progression of IDD.
N-Ac-PGP is a matrikine derived from collagen
through a proteolytic cascade involving MMP8, MMP9 and PE (12). It is a chemokine via CXCR1/2
(25). Previous studies have
reported the involvement of N-Ac-PGP in chronic obstructive
pulmonary disease and inflammatory bowel disease via inducing the
migration of neutrophils (13,14). We reported that the levels of
MMP8, MMP9 and PE in human NP tissues were significantly increased
with increasing Pfirrmann grade of the discs, suggesting an
enhanced collagen degradation in degenerative discs that forms a
suitable microenvironment for N-Ac-PGP generation. In fact,
we confirmed the presence of N-Ac-PGP in human NP tissues
and found that the level of N-Ac-PGP in NP tissues is
positively correlated with the Pfirrmann grade of IVDs.
Furthermore, N-Ac-PGP was shown to recruit CESCs from
cartilage endplate into NP and induce the differentiation of CESCs
toward a pro-inflammatory phenotype (15). Herein, the roles of
N-Ac-PGP in the microenvironment of NP were extended.
N-Ac-PGP potently induced the expression of matrix catabolic
and pro-inflammatory genes in resident NP cells, suggesting that
N-Ac-PGP is detrimental to the functional and structural
homeostasis of IVDs via enhancing inflammation and matrix
catabolism in the microenvironment of discs. N-Ac-PGP is a
pro-degenerative matrikine for IVDs.
The NF-κB and MAPK signaling pathways are regarded
as the major pathways mediating musculoskeletal disorders,
including osteoarthritis, osteoporosis muscular dystrophy and
rheumatoid arthritis (26–31).
NF-κB plays a central role in the cellular response to various
stimuli, such as inflammation, cytokines, mechanical stress,
oxidative stress and genotoxic stress. Previous studies have
reported the roles of NF-κB in IDD. Nerlich et al reported
the activation of NF-κB along with oxidative stress in human IVDs
in vivo. The activity of NF-κB in IVDs was found to increase
with aging and progression of IDD (32). NF-κB activation is associated with
oxidative stress-induced senescence of NP cells (33). Moreover, MMPs, ADAMTS proteases
and cytokines are the target genes of NF-κB in NP cells (20,34,35), indicating that the NF-κB pathway
is involved in the matrix catabolism and inflammation of IVDs. On
the other hand, MAPK is a highly conserved signal transduction
pathway that is activated by environmental stress (36). The ECM metabolism and inflammation
of IVDs are also associated with MAPK activation in disc cells. The
inhibition of the MAPK pathway by specific signaling inhibi-tors
can suppress the enhanced ECM catabolism in disc cells induced by
inflammatory cytokines (20,37–39). In conclusion, both NF-κB and MAPK
pathways are tightly associated with inflammatory and matrix
catabolic signaling in IVDs. Therefore, we propose that they also
play a role in mediating the pro-inflammatory and catabolic effect
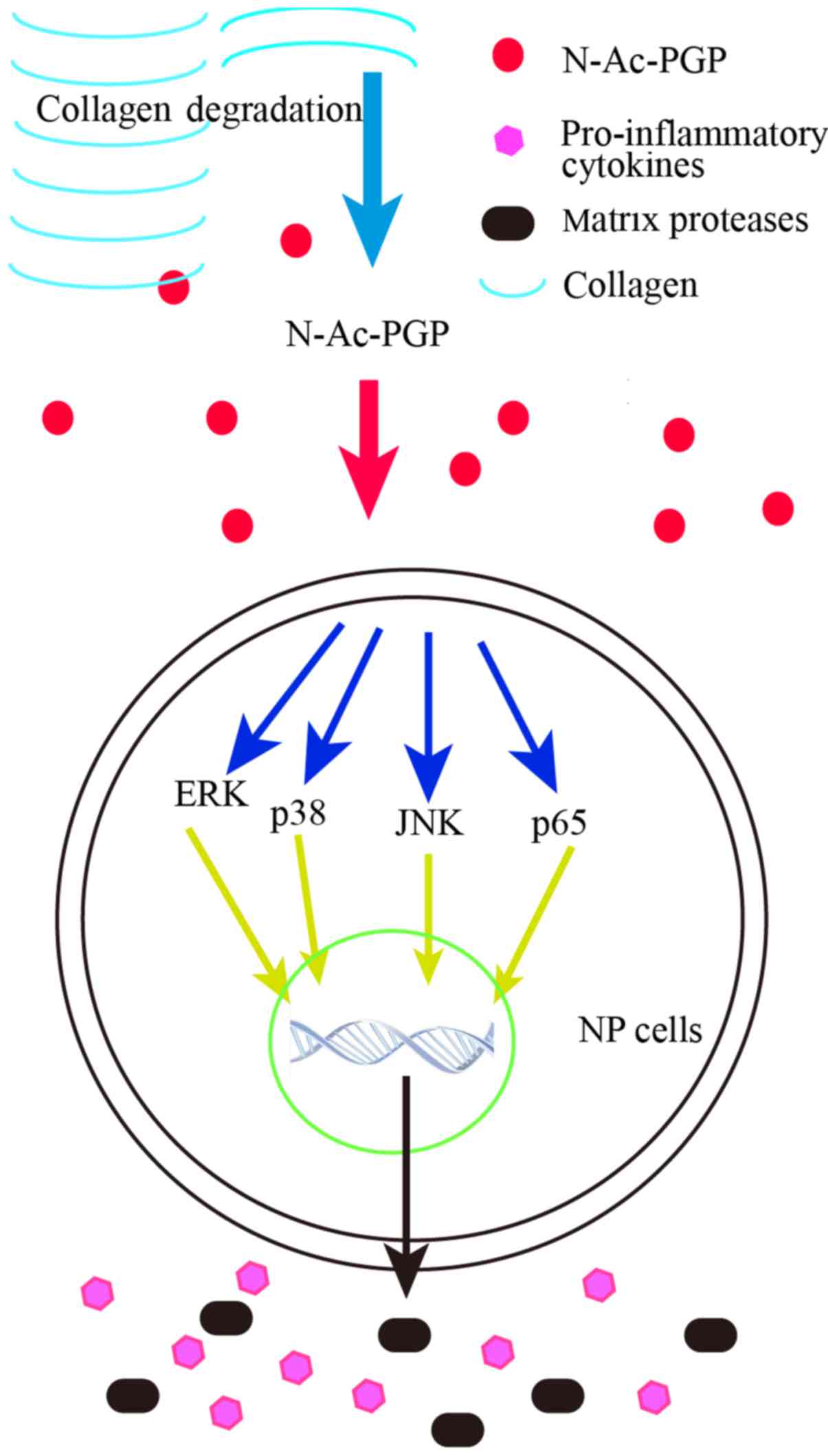
of N-Ac-PGP on NP cells. According to our results, the
phosphorylation of the NF-κB and MAPK pathways in NP cells was
enhanced at the early stage of N-Ac-PGP treatment. Moreover,
when the activation of NF-κB and MAPK pathways at the early stage
of N-Ac-PGP treatment was inhibited by specific signaling
inhibitors, the pro-inflammatory and catabolic effects of
N-Ac-PGP on NP cells was markedly counteracted, suggesting
that N-Ac-PGP upregulates the expression of matrix catabolic
and pro-inflammatory genes in NP cells via the activation of the
NF-κB and MAPK signaling pathways at the early stage of
N-Ac-PGP treatment (Fig.
9).
Actually, there are several limitations of this
study. Firstly, the mechanism underlying the interaction between
N-Ac-PGP and NP cells remains unknown. Our previous study
revealed that N-Ac-PGP affects CESCs by binding to
N-Ac-PGP receptors (CXCR1 and CXCR2) (15). More studies are required to
confirm that the effect of N-Ac-PGP on NP cells is also
dependent on these receptors. Second, our study clearly showed that
N-Ac-PGP affects NP cells via the NF-κB and MAPK signaling
pathways. However, the crosstalk between the two pathways is
unclear. This is a new issue which warrants further investigation.
In addition, we pooled NP cells from different patients at passage
1 and used them at passage 2 or 3. In this situation, the effect of
different degeneration grade on the response of NP cells to
N-Ac-PGP treatment was unable to be revealed. This should be
investigated in the future.
In conclusion, N-Ac-PGP upregulates the
expression of pro-inflammatory and ECM catabolic genes in a
dose-dependent and time-dependent manner. This effect was mediated
by the activation of the NF-κB and MAPK signaling pathways at the
early stage of N-Ac-PGP treatment. Our findings contribute
to a better understanding of the roles of N-Ac-PGP in the
pathogenesis of IDD and facilitates the development of a novel
therapeutic approach targeting N-Ac-PGP to prevent or retard
the initiation and progression of IDD.
Abbreviations:
|
IVD
|
intervertebral disc
|
|
IDD
|
intervertebral disc degeneration
|
|
NP
|
nucleus pulposus
|
|
AF
|
annulus fibrosus
|
|
N-Ac-PGP
|
N-acetylated
proline-glycine-proline
|
|
ECM
|
extracellular matrix
|
|
LBP
|
low back pain
|
|
MMP
|
matrix metalloproteinase
|
|
PE
|
prolyl endopeptidase
|
|
CESCs
|
cartilage endplate stem cells
|
|
CEP
|
cartilage endplate
|
|
NF-κB
|
nuclear factor-κB
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ADAMTS
|
a disintegrin and metalloproteinase
with thrombospondin motifs
|
|
PBS
|
phosphate-buffered saline
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
|
CCL
|
C-C motif ligand
|
|
CXCL10
|
C-X-C motif chemokine ligand 10
|
|
RPL
|
relative phosphorylation level
|
References
|
1
|
Roberts S, Evans H, Trivedi J and Menage
J: Histology and pathology of the human intervertebral disc. J Bone
Joint Surg Am. 88(Suppl 2): 10–14. 2006.PubMed/NCBI
|
|
2
|
Battié MC, Videman T, Kaprio J, Gibbons
LE, Gill K, Manninen H, Saarela J and Peltonen L: The twin spine
study: contributions to a changing view of disc degeneration. Spine
J. 9:47–59. 2009. View Article : Google Scholar
|
|
3
|
Xing QJ, Liang QQ, Bian Q, Ding DF, Cui
XJ, Shi Q and Wang YJ: Leg amputation accelerates senescence of rat
lumbar intervertebral discs. Spine. 35:E1253–E1261. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang D, Nasto LA, Roughley P, Leme AS,
Houghton AM, Usas A, Sowa G, Lee J, Niedernhofer L, Shapiro S, et
al: Spine degeneration in a murine model of chronic human tobacco
smokers. Osteoarthritis Cartilage. 20:896–905. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stirling A, Worthington T, Rafiq M,
Lambert PA and Elliott TS: Association between sciatica and
Propionibacterium acnes. Lancet. 357:2024–2025. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park EY and Park JB: Dose- and
time-dependent effect of high glucose concentration on viability of
notochordal cells and expression of matrix degrading and fibrotic
enzymes. Int Orthop. 37:1179–1186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams MA and Roughley PJ: What is
intervertebral disc degeneration, and what causes it? Spine.
31:2151–2161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Binch AL, Cole AA, Breakwell LM, Michael
AL, Chiverton N, Creemers LB, Cross AK and Le Maitre CL: Nerves are
more abundant than blood vessels in the degenerate human
intervertebral disc. Arthritis Res Ther. 17:3702015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vo V, Hartman RA, Yurube T, Jacobs LJ,
Sowa GA and Kang JD: Expression and regulation of
metalloproteinases and their inhibitors in intervertebral disc
aging and degeneration. Spine J. 13:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar
|
|
11
|
Huang YC, Leung VY, Lu WW and Luk KD: The
effects of microenvironment in mesenchymal stem cell-based
regeneration of intervertebral disc. Spine J. 13:352–362. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gaggar A, Jackson PL, Noerager BD,
O'Reilly PJ, McQuaid DB, Rowe SM, Clancy JP and Blalock JE: A novel
proteolytic cascade generates an extracellular matrix-derived
chemoattractant in chronic neutrophilic inflammation. J Immunol.
180:5662–5669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Reilly P, Jackson PL, Noerager B, Parker
S, Dransfield M, Gaggar A and Blalock JE: N-alpha-PGP and PGP,
potential biomarkers and therapeutic targets for COPD. Respir Res.
10:382009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koelink PJ, Overbeek SA, Braber S, Morgan
ME, Henricks PA, Abdul Roda M, Verspaget HW, Wolfkamp SC, te Velde
AA, Jones CW, et al: Collagen degradation and neutrophilic
infiltration: a vicious circle in inflammatory bowel disease. Gut.
63:578–587. 2014. View Article : Google Scholar :
|
|
15
|
Feng C, Zhang Y, Yang M, Huang B and Zhou
Y: Collagen-derived N-acetylated proline-glycine-proline in
intervertebral discs modulates CXCR1/2 expression and activation in
cartilage endplate stem cells to induce migration and
differentiation toward a pro-inflammatory phenotype. Stem Cells.
33:3558–3568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine. 26:1873–1878. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lv F, Leung VY, Huang S, Huang Y, Sun Y
and Cheung KM: In search of nucleus pulposus-specific molecular
markers. Rheumatology (Oxford). 53. pp. 600–610. 2014, View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Hiyama A, Sakai D, Risbud MV, Tanaka M,
Arai F, Abe K and Mochida J: Enhancement of intervertebral disc
cell senescence by WNT/β-catenin signaling-induced matrix
metalloproteinase expression. Arthritis Rheum. 62:3036–3047. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wuertz K, Vo N, Kletsas D and Boos N:
Inflammatory and catabolic signalling in intervertebral discs: the
roles of NF-κB and MAP kinases. Eur Cell Mater. 23:103–119.
2012.
|
|
21
|
Feng C, Liu H, Yang Y, Huang B and Zhou Y:
Growth and differentiation factor-5 contributes to the structural
and functional maintenance of the intervertebral disc. Cell Physiol
Biochem. 35:1–16. 2015. View Article : Google Scholar
|
|
22
|
Kepler CK, Ponnappan RK, Tannoury CA,
Risbud MV and Anderson DG: The molecular basis of intervertebral
disc degeneration. Spine J. 13:318–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding F, Shao ZW and Xiong LM: Cell death
in intervertebral disc degeneration. Apoptosis. 18:777–785. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang YC, Urban JP and Luk KD:
Intervertebral disc regeneration: do nutrients lead the way? Nat
Rev Rheumatol. 10:561–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weathington NM, van Houwelingen AH,
Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G,
Nijkamp FP and Blalock JE: A novel peptide CXCR ligand derived from
extracellular matrix degradation during airway inflammation. Nat
Med. 12:317–323. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marcu KB, Otero M, Olivotto E, Borzi RM
and Goldring MB: NF-kappaB signaling: multiple angles to target OA.
Curr Drug Targets. 11:599–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HJ, Chang EJ, Kim HM, Lee SB, Kim HD,
Su Kim G and Kim HH: Antioxidant alpha-lipoic acid inhibits
osteoclast differentiation by reducing nuclear factor-kappaB DNA
binding and prevents in vivo bone resorption induced by receptor
activator of nuclear factor-kappaB ligand and tumor necrosis
factor-alpha. Free Radic Biol Med. 40:1483–1493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Acharyya S, Villalta SA, Bakkar N,
Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S,
Sahenk Z, et al: Interplay of IKK/NF-kappaB signaling in
macrophages and myofibers promotes muscle degeneration in Duchenne
muscular dystrophy. J Clin Invest. 117:889–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dai S, Hirayama T, Abbas S and Abu-Amer Y:
The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide,
blocks osteoclastogenesis and bone erosion in inflammatory
arthritis. J Biol Chem. 279:37219–37222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang P, Han J and Hui L: MAPK signaling
in inflammation-associated cancer development. Protein Cell.
1:218–226. 2010. View Article : Google Scholar
|
|
31
|
Zarubin T and Han J: Activation and
signaling of the p38 MAP kinase pathway. Cell Res. 15:11–18. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nerlich AG, Bachmeier BE, Schleicher E,
Rohrbach H, Paesold G and Boos N: Immunomorphological analysis of
RAGE receptor expression and NF-kappaB activation in tissue samples
from normal and degenerated intervertebral discs of various ages.
Ann NY Acad Sci. 1096:239–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dimozi A, Mavrogonatou E, Sklirou A and
Kletsas D: Oxidative stress inhibits the proliferation, induces
premature senescence and promotes a catabolic phenotype in human
nucleus pulposus intervertebral disc cells. Eur Cell Mater.
30:89–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Séguin CA, Bojarski M, Pilliar RM,
Roughley PJ and Kandel RA: Differential regulation of matrix
degrading enzymes in a TNFalpha-induced model of nucleus pulposus
tissue degeneration. Matrix Biol. 25:409–418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wako M, Ohba T, Ando T, Arai Y, Koyama K,
Hamada Y, Nakao A and Haro H: Mechanism of signal transduction in
tumor necrosis factor-like weak inducer of apoptosis-induced matrix
degradation by MMP-3 upregulation in disc tissues. Spine.
33:2489–2494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001.PubMed/NCBI
|
|
37
|
Kim JS, Ellman MB, An HS, Yan D, van
Wijnen AJ, Murphy G, Hoskin DW and Im HJ: Lactoferricin mediates
anabolic and anti-catabolic effects in the intervertebral disc. J
Cell Physiol. 227:1512–1520. 2012. View Article : Google Scholar
|
|
38
|
Kim JH, Studer RK, Vo V, Sowa GA and Kang
JD: p38 MAPK inhibition selectively mitigates inflammatory
mediators and VEGF production in AF cells co-cultured with
activated macrophage-like THP-1 cells. Osteoarthritis and
cartilage/OARS. Osteoarthritis Res Soc. 17:1662–1669. 2009.
View Article : Google Scholar
|
|
39
|
Séguin CA, Pilliar RM, Madri JA and Kandel
RA: TNF-alpha induces MMP2 gelatinase activity and MT1-MMP
expression in an in vitro model of nucleus pulposus tissue
degeneration. Spine. 33:356–365. 2008. View Article : Google Scholar : PubMed/NCBI
|