Introduction
The cyclooxygenases (COXs), also known as
prostaglandin endoperoxide H synthases (PGHSs), are 67–72 kDa
integral membrane proteins located on the endoplasmic reticulum
(ER) and the nuclear envelope. COXs are fatty acid oxygenases and
members of the myeloperoxidase superfamily (1–5).
COXs are bifunctional enzymes and sequence homodimers; each monomer
has COX (or bis-dioxygenase) activity and peroxidase (POX) activity
via physically distinct COX and POX active sites (1,3,5).
COXs catalyze the conversion of arachidonic acid (AA) to
PGH2, which is the initial rate-limiting step in
prostaglandin (PG) biosynthesis (1–6).
The production of PGH2 is a two-step reaction: AA binds
inside the COX tunnel and reacts to form the intermediate
PGG2 and PGG2 is bound and modified within
the peroxidase active site to form the final product,
PGH2 (3–7).
All vertebrates investigated to date possess two COX
isoforms, COX-1 and COX-2. In most cases, COX-1 is expressed
constitutively to produce PGs that mediate 'housekeeping'
functions, whereas the expression of COX-2 is highly inducible in
response to growth factors, tumor promoters or cytokines (6,8).
COX-2-derived PGs participate in a number of pathophysiological
responses, such as inflammation, carcinogenesis and modulation of
cell growth and survival (9).
Increasing evidence has indicated that the induced expression and
activation of COX-2 are observed in many types of tumor cells, and
are involved in tumor progression and aggressiveness (10,11). In addition, the expression of
COX-2 was observed to be induced in cancer cells during anticancer
chemoradiotherapies, resulting in drug resistance (11–13). Thus, the inhibition of COX-2 may
provide a very significant therapy which may benefit a large
proportion of the patient population (10). While broad spectrum
COX-2-inhibiting non-steroidal anti-inflammatory drugs (NSAIDs),
and COX-2-specific inhibitors have been successfully established
(11,12), both of these are known to cause
side-effects, such as myocardial infarction (11). Therefore, there still remains an
urgent need to develop anti-COX-2 therapies with reduced or no
side-effects.
The preparation of COX-2 protein is the initial step
for the development of COX-2 inhibitors. A eukaryotic
heme-containing and membrane-bound protein, COX-2 is expressed at a
rather low level in native hosts. Heterologous expression is the
only efficient strategy with which to obtain a large amount of
human COX-2 protein. Generally, the most frequently used
heterologous expression systems include prokaryotic, yeast,
plant-based, insect/baculovirus and mammalian expression systems,
as well as expression in eukaryotic organisms (14,15). With the progression of COX-2
structural studies (3–6), the insect/baculovirus expression
system has become the most widespread method for acquiring high
quality functional products (16–19). However, several limitations of the
insect/baculovirus system, including a relatively high cost,
methodological challenges and relatively low yields obtained using
this system, limit its use for large-scale fermentation and more
widespread application. Moreover, protein synthesis rates are
generally much faster in prokaryotes than in eukaryotes (20). Thus, bacterial hosts are
preferred, due to their rapid growth rate, their capacity for
continuous fermentation, high-level post-induction target protein
expression and a relatively low cost (14,20–26). However, to date, and at least to
the best of our knowledge, limited research has been carried out to
characterize and purify human COX-2 expressed in prokaryotic cells
(27).
In this study, a truncated form of human COX-2,
containing 257 residues of the C-terminus was cloned, and it
exhibited high-level heterologous expression in Escherichia coli
(E. coli) BL21(DE3) cells using the pET28b(+) expression vector
system. In addition, the antigenicity and the COX activity of
truncated human COX-2 (trCOX-2) products were validated and these
results demonstrate the reliability of this method to obtain
functional COX-2 products from a prokaryotic expression system.
Materials and methods
Materials
BamHI, HindIII and T4 DNA ligase were
all purchased from Takara Biotechnology Co., Ltd. (Dalian, China).
A Ni2+-NTA Superflow Cartridge was purchased from Qiagen
(Valencia, CA, USA). PD-10 desalting columns were obtained from
Amersham Pharmacia Biotech, Inc. (Piscataway, NJ, USA). Coomassie
Brilliant Blue R-250 was obtained from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Anti-COX-1 (sc-166573) and anti-COX-2
antibodies (sc-166475) were both purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Mouse anti-His
monoclonal antibody (M0812-3) was purchased from Hangzhou HuaAn
Biotechnology Co., Ltd. (Hangzhou, China). Horseradish peroxidase
(HRP)-conjugated anti-mouse immunoglobulin G (IgG; SA00001-1) was
purchased from Proteintech (Chicago, IL, USA). AA was purchased
from Alfa Aesar (Ward Hill, MA, USA). All other chemicals and
reagents used were of highest purity.
Homology modeling and molecular
docking
The three dimensional structure of trCOX-2 was
modeled through homology modeling using a published murine COX-2
structure (PDB ID: 4RRW) as the template (28). The homology modeling and
calibration of models was conducted online using the SWISS-MODEL
server (29–32). The molecular docking was conducted
online using the SwissDock server (33,34) with AA (PubChem CID: 444899)
designated as the ligand and the trCOX-2 modeled structure
designated as the receptor. All structure files were visualized
using PyMOL (installed on an Ubuntu Linux system provided by
Canonical Ltd.). Protein-ligand interactions were analyzed with the
help of the PyMOL viewer.
Construction of pET28b-trCOX-2
The 771 bp stretch of sequence at the 3′-end of
full-length human COX-2 gene was amplified to obtain trCOX-2
using primers designed using Primer Premier 5.0 with the following
sequences: forward, 5′-TAACGTGGATCCGGACCCAGAACTACTTT-3′ and
reverse, 5′-GACCCCAAGCTTATACAGTTCAGT-3′. The DNA fragment coding
for trCOX-2 was cloned into the pET28b(+) vector (Novagen, Madison,
WI, USA), containing 6 histidines at both the amino terminus and
the C-terminus. The recombinant plasmid, pET28b-trCOX-2, was
produced in the E. coli strain JM109 and sequenced by Sangon
Biotech Co., Ltd. (Shanghai, China). This plasmid expresses a 305
amino acid stretch of trCOX-2, which contains 257 amino acids of
the C-terminus residue of human COX-2 and additional histidine
tags.
Expression of trCOX-2 in E. coli strain
BL21(DE3)
The pET28b-trCOX-2 plasmid was transformed into
E. coli BL21(DE3) cells and induced to express trCOX-2
according to our previous studies (25,26). Briefly, E. coli BL21(DE3)
cells were transformed with pET28b-trCOX-2 to obtain E. coli
trCOX-2/BL21(DE3) that could express trCOX-2. E. coli
trCOX-2/BL21(DE3) were grown in Luria-Bertani (LB) medium with 30
µg/ml kanamycin at 37°C with shaking until the optical
density at 600 nm reached 0.6. The cells were then stimulated with
1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 2, 3, 4, 6
and 8 h at 30°C with shaking. E. coli trCOX-2/BL21(DE3)
cells were harvested by centrifugation at 8,000 rpm for 15 min at
4°C and lysed by sonication in buffer A containing 20 mM sodium
phosphate, pH 7.4, 500 mM NaCl, 10 mM imidazole, 0.1 mM
phenylmethylsulfonyl fluoride (PMSF) and 1 mM β-mercaptoethanol.
The lysates were fractionated by centrifugation at 15,000 rpm for
15 min at 4°C. The supernatant and precipitate were separately
analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and stained with Coomassie Brilliant
Blue R-250 to visualize the expression of trCOX-2. The average gray
value of each band was detected and quantified using BandScan 5.0
software (Glyko Inc., Novato, CA, USA), and the results were
expressed as the ratio of trCOX-2 to total proteins.
Denaturation of inclusion bodies
Inclusion bodies were washed sequentially with
buffer B (0.5% Triton X-100, 500 mM NaCl, 20 mM sodium phosphate,
pH 7.4) and buffer C (2 M urea, 500 mM NaCl, 20 mM sodium
phosphate, pH 7.4). The washed inclusion bodies were subsequently
denatured in binding buffer D (8 M urea, 20 mM sodium phosphate, pH
7.4, 500 mM NaCl, 0.1 mM PMSF, 1 mM β-mercaptoethanol and 10 mM
imidazole) overnight at 4°C. The soluble denatured inclusion body
proteins were carefully collected by centrifugation at 15,000 rpm
at 4°C for 20 min.
Purification and renaturation of
inclusion body proteins
The soluble inclusion body proteins were applied to
a Ni2+-NTA Superflow Cartridge (Qiagen) equilibrated
with binding buffer. The column was next washed sequentially with
binding buffer D followed by washing buffer (8 M urea, 20 mM sodium
phosphate, pH 7.4, 500 mM NaCl, 0.1 mM PMSF, 1 mM β-mercaptoethanol
and 40 mM imidazole) and then eluted with elution buffer (8 M urea,
20 mM sodium phosphate, pH 7.4, 500 mM NaCl, 0.1 mM PMSF, 1 mM
β-mercaptoethanol and 500 mM imidazole). The purification of
denatured trCOX-2 was monitored by analyzing aliquots of the
collected samples using 12% SDS-PAGE and then stained with
Coomassie Brilliant Blue R-250. The desired eluted proteins were
refolded as previously described (26). Briefly, the purified denatured
trCOX-2 products were diluted 1:10 in refolding buffer E (42 mM
Tris-HCl, pH 8.0, 62 mM HEPES, 2.5 mM DTT, 0.1 mM CaCl2,
0.5 M arginine) and slowly stirred on ice for 4 h to allow COX-2
renaturation to occur. The renatured trCOX-2 was stored at −80°C
following determination of protein concentration using the Bradford
assay.
Western blot analysis
The samples were subjected to SDS-PAGE followed by
electrophoretic transfer onto polyvinylidene difluoride (PVDF)
membranes. Non-specific binding was blocked with blocking buffer
containing PBST [0.05% Tween-20 in phosphate-buffered saline (PBS)]
with 5% non-fat milk for 1 h at room temperature. The membranes
were then incubated overnight at 4°C with antibodies specific
either for the His-tag or COX-2 in PBST containing 5% non-fat milk
at the dilutions specified by the manufacturers. After washing 3
times with PBST, the membranes were incubated with HRP-conjugated
secondary antibodies at a dilution of 1:5,000 in PBST containing 5%
non-fat milk for 1 h at room temperature. The membranes were
subsequently washed 3 times with PBST and the protein bands were
detected using a western blot detection system.
Enzyme-linked immunosorbent assay
(ELISA)
For ELISA, the purified trCOX-2 (1–10 µg/ml)
was coated onto the surface of wells of a 96-well ELISA plate
overnight at 4°C. The wells were then blocked with PBST containing
3% non-fat milk for 1 h at 37°C. Following sequential incubation
with a primary antibody (antibodies against COX-1 or COX-2) and
HRP-conjugated IgG, the reaction was developed by the addition of
o-phenylenediamine (OPD) and monitored using a microplate reader
(Thermo Labsystems, Waltham, MA, USA) at a wavelength of 492 nm.
Wells coated with the same amount of BSA instead of trCOX-2 served
as the negative control (NC).
COX assay
For measurement of COX activity, O2
consumed in the assay mixture was monitored using a dissolved
oxygen detector (OXY5401S; Puyang, China) at 37°C (5,6).
The standard assay mixture contained 6 ml of 100 mM Tris, pH 7.4, 2
mM phenol, 10 µM hematin and 100 µM of AA substrate.
Reactions were initiated by the addition of up to 50 µg of
protein. The variation of the O2 concentration between
the initiation and completion of the reaction was monitored and
compared with the assay mixture without the enzyme sample. All of
the above reactions were monitored in an anaerobic workstation.
Results
Design strategy for the expression of
human COX-2 at a high level in E. coli
Full-length human COX-2 contains 604 amino acids,
beginning with the signal peptide, followed by an epidermal growth
factor (EGF)-like domain, a membrane binding domain, a dimerization
domain and the catalytic domain at the carboxyl terminus (1). Our preliminary data showed that it
was very difficult to express full-length human COX-2 in E.
coli after our group made several failed attempts to purify the
full-length human COX-2 (data not shown). We surmised that these
difficulties were due to the known phenomenon of heterogeneous
membrane polarization observed in membrane proteins, as well as to
the large size of the COX-2 target protein. Based on these factors,
subsequent attempts were made to remove the non-catalytic domain
using published knowledge of protein structures and function
(34–38). As previous results have shown that
the deletion of the N-terminal signal peptide could significantly
increase protein expression levels in E. coli (19), to obtain a high yield of
functional human COX-2 in E. coli, we designed a strategy to
prepare a trCOX-2 possessing catalytic activity. Following the
deletion of the N-terminal 347 amino acid residues from full-length
human COX-2, the remainder of the trCOX-2 should still possess the
core catalytic portion of full-length COX-2, including all
important binding and catalytic sites (6).
Computer simulation of trCOX-2
Homology modeling and structure
alignment of trCOX-2
To verify our design strategy of human trCOX-2, we
conducted homology modeling of trCOX-2 with partial human COX-2
catalytic domain containing 257 residues of the C-terminus. Human
COX-2 and murine COX-2 share 85% sequence identity and share highly
conserved crystal structures. The trCOX-2 structure with 305 amino
acids (containing some amino acids from the vector) was depicted
according to SWISS-MODEL using a database of the PDB entry 4RRW, as
shown in Fig. 1A. The newly
published crystal structure of murine COX-2 (PDB ID: 4RRW) was
selected as the template, with a sequence similarity of 94% and a
coverage of 79%. It contains 242 amino acids aligning to the
Arg-363 to Leu-604 stretch of trCOX-2. The modeled structure had a
reasonable QMEAN4 score (|QMEAN4| <1). The trCOX-2 is a monomer,
while 4RRW is a homotetramer. The alignment between the trCOX-2 and
one monomer of 4RRW, which are oriented in the same direction with
homologous residue positions aligned are shown in Fig. 1B. According to the modeling
structure, 3 α-helices in trCOX-2 form the catalytic pocket, while
5 α-helices form the catalytic pocket in murine COX-2. Important
residues, including Phe-381, Tyr-385, Trp-387, Val-523, Glu-524,
Ser-530 and Leu-531, possess almost the same relative spatial
relationships in both trCOX-2 and the template. Although the larger
pocket of trCOX-2 may lead to weaker interactions and impaired
enzyme activity, the remaining helices, especially the key
residues, mainly adopt the same conformation compared with the
template. These results indicate that the major catalytic domain is
conserved in trCOX-2.
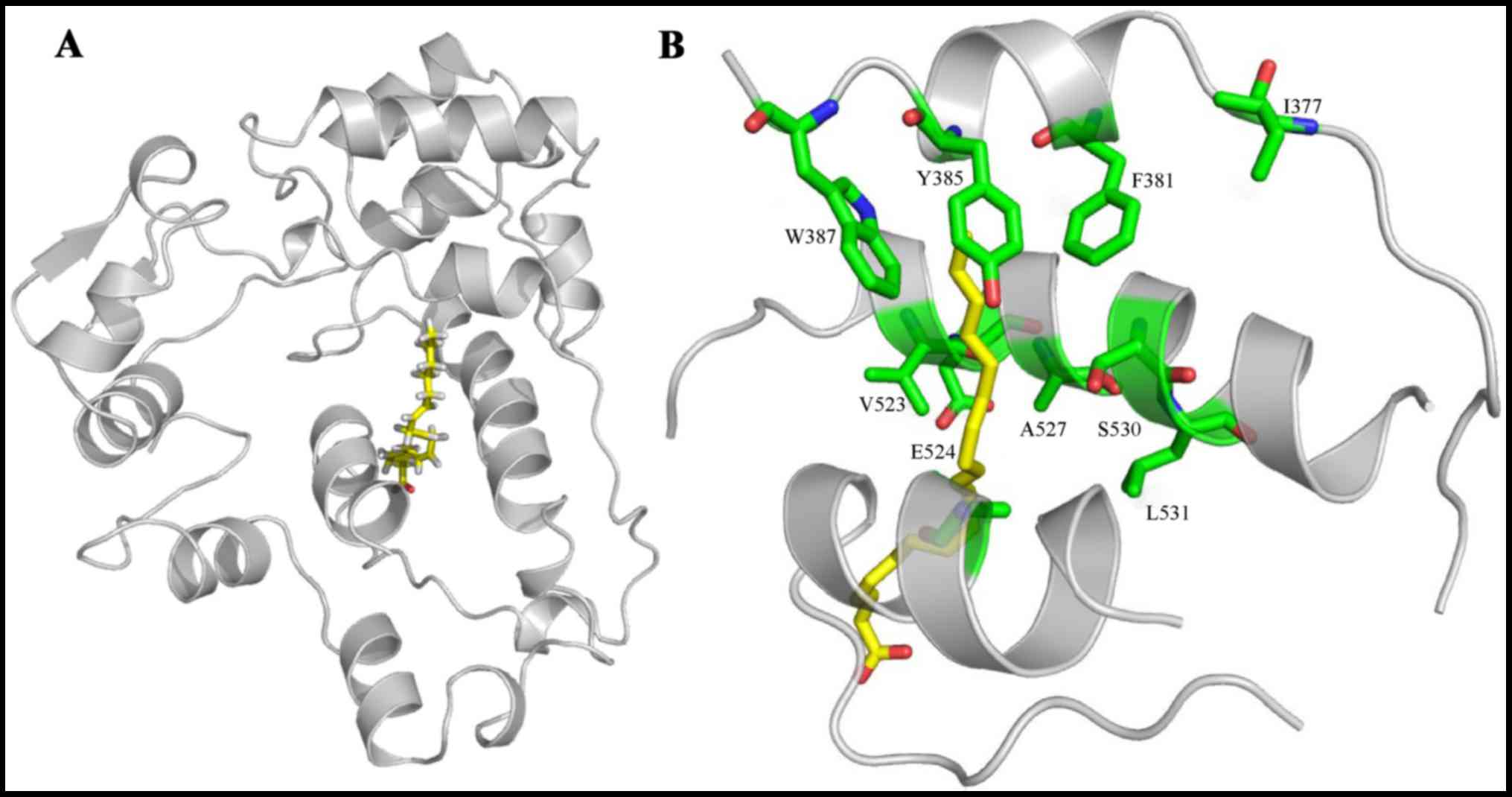
Docking of AA to trCOX-2
We then conducted molecular docking between AA and
trCOX-2. The docking results (Fig.
2A) revealed that AA bound in the COX channel of trCOX-2,
further elucidating the important catalytic residues of trCOX-2
which may exhibit enzyme activity. As there is no significant
structural differences between the core-binding pockets of muCOX-2
and trCOX-2, their similar binding structures raise the possibility
that trCOX-2 retains enzyme activity (4,6).
As depicted in Fig. 2B, AA is
oriented with its carboxylate moiety proximal to the COX-2 channel
opening. Specifically, the AA ω-end is located within the
hydrophobic groove proximal to the Tyr-385 and Ser-530 residues
positioned at the channel apex. Polar interactions are indicated
between Tyr-385 and AA, Glu-524 and AA. Taken together, these
results indicate that the hydrophobic groove and polar groups
interact together to stabilize AA when it is bound within the COX
channel.
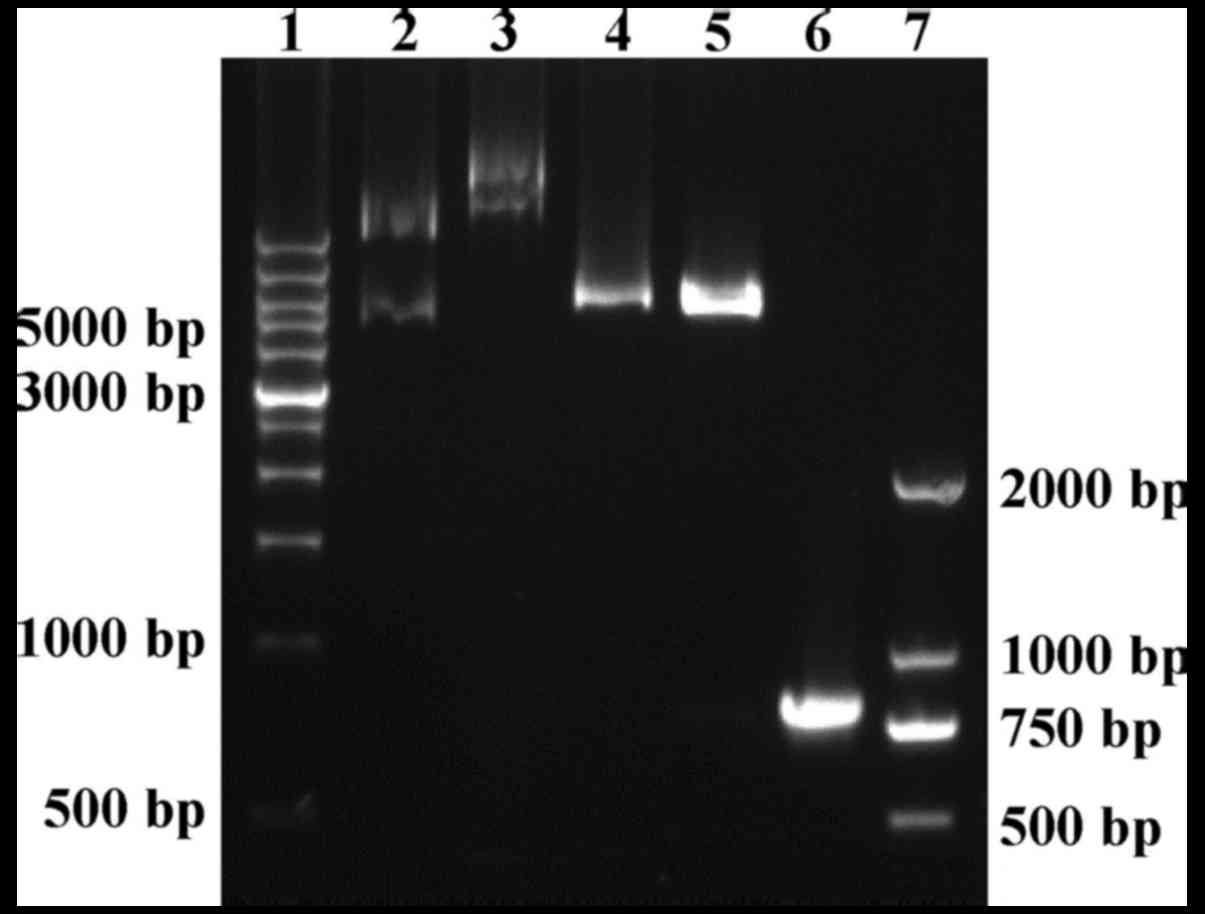
Recombinant pET28b-trCOX-2 expression
plasmid was constructed successfully
To prepare functional trCOX-2 expressed in a
prokaryotic expression system, we cloned trCOX-2 and constructed a
prokaryotic expression plasmid. As shown in Fig. 3, the 771 bp PCR product encoding
the C-terminal segment of human COX-2 (including 257 amino acid
residents) was cloned successfully and inserted into the
prokaryotic expression vector pET28b(+). Positive recombinant
plasmids were confirmed with digestion using BamHI and
HindIII enzymes (Fig. 3).
The sequencing results provided further evidence of successful
construction of the recombinant pET28b-trCOX-2 expression plasmid
and confirmed the presence of two 6xHis-tags, located at both the
N- and C-terminus of trCOX-2. The full-length of the fusion protein
with His-tags, trCOX-2, was 305 amino acids (34.4 kDa).
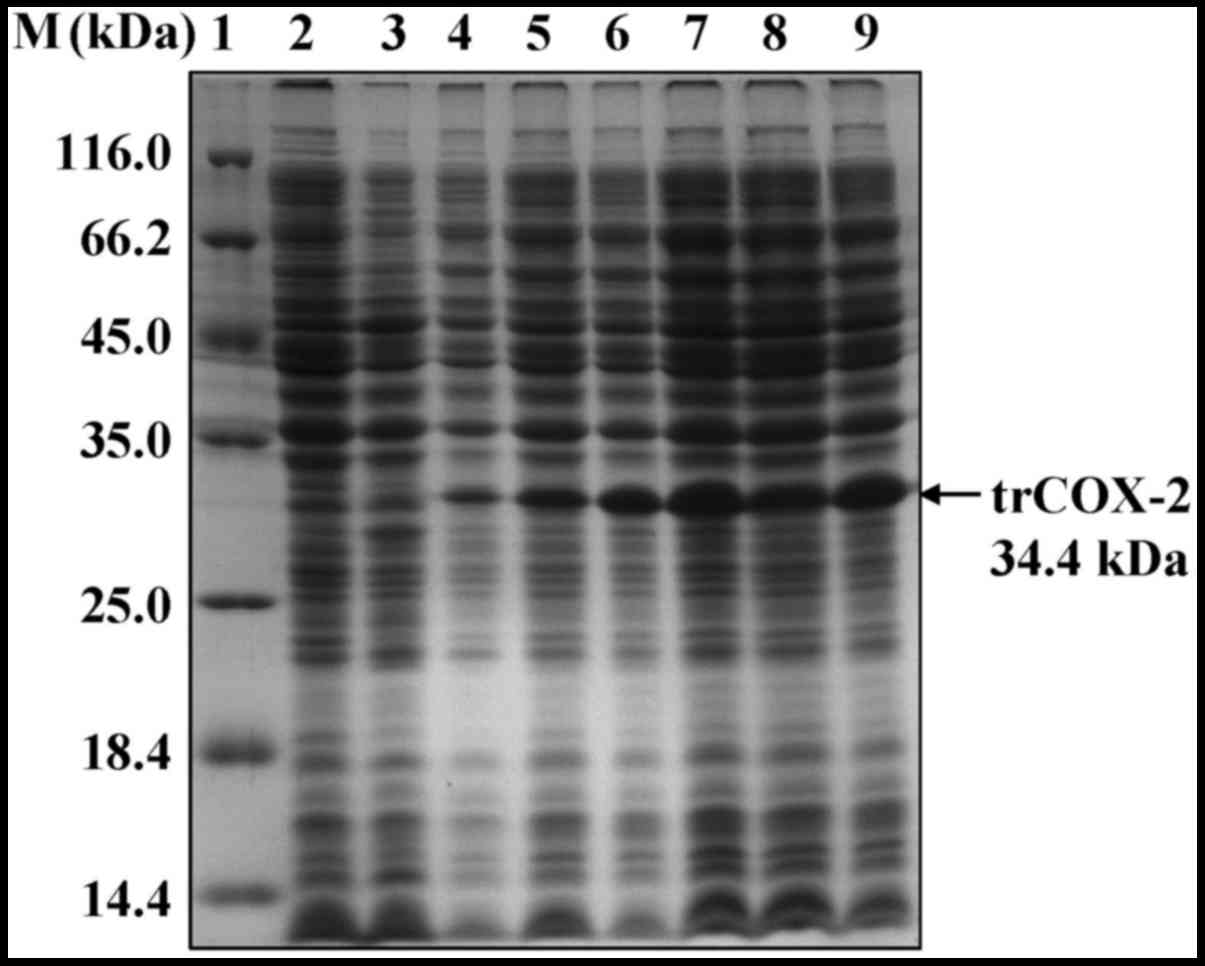
Expression and purification of
trCOX-2
To obtain human trCOX-2 protein, competent E.
coli BL21(DE3) cells were transformed with pET28b-trCOX-2 to
prepare E. coli trCOX-2/BL21(DE3) that could express human
trCOX-2. We found that the expression level of the trCOX-2 protein
was very high after IPTG induction, as detected by SDS-PAGE
(Fig. 4). In addition, the
expression of target proteins reached the highest level (up to 31%
of the total E. coli protein) at 4 h after IPTG induction
(Fig. 4), but they were expressed
as inclusion bodies as they were found in the pellets of cell
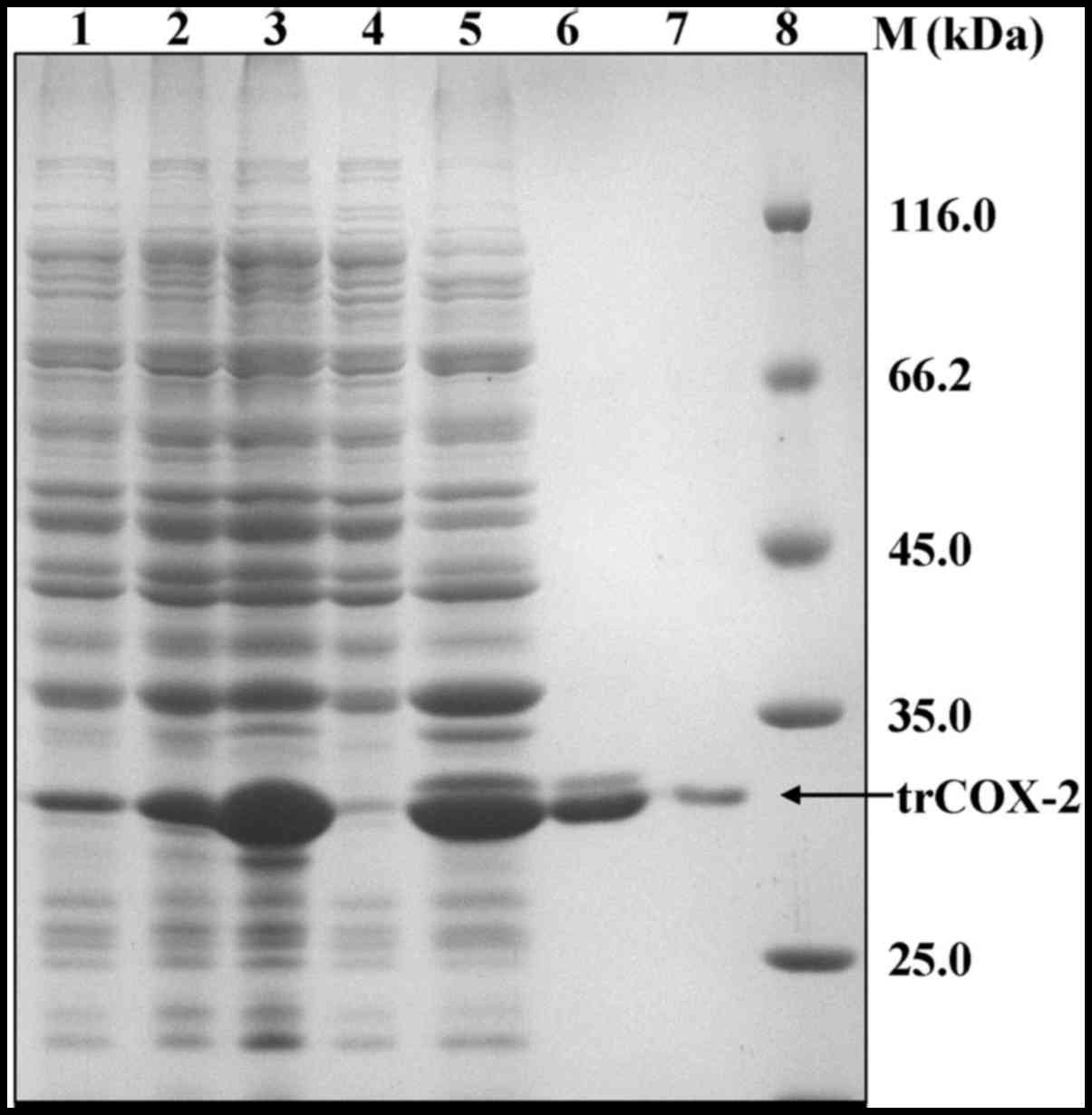
lysates (Fig. 5). In order to
purify trCOX-2, the pellets containing the inclusion bodies were
first washed with Triton X-100 and 2 M urea to obtain crude
inclusion bodies, which were then solubilized using
urea-denaturation. The soluble inclusion body proteins with
His-tags were then subjected to affinity purification. SDS-PAGE
analysis of the eluted fractions revealed that a single band of
approximately 34 kDa was detected (Fig. 5). The purity of the products was
>95%, as estimated by gel analysis using Bandscan software. The
eluted fractions containing the protein of interest were refolded
by dilution in refolding buffer to prepare purified active trCOX-2.
The purification of human trCOX-2 is summarized in Table I. This process yielded
approximately 35 mg of purified protein from 1 liter of E.
coli culture.
 | Table IPurification of trCOX-2 from E.
coli BL21(DE3). |
Table I
Purification of trCOX-2 from E.
coli BL21(DE3).
| Steps | Total products
(mg/l)a | Yield rate (%) |
|---|
| Crude inclusion
bodies | 800 | 100 |
| After
Ni2+-NTA purification | 75 | 9.4 |
| Renaturation
protein | 35 | 4.4 |
Identification of purified
trCOX-2
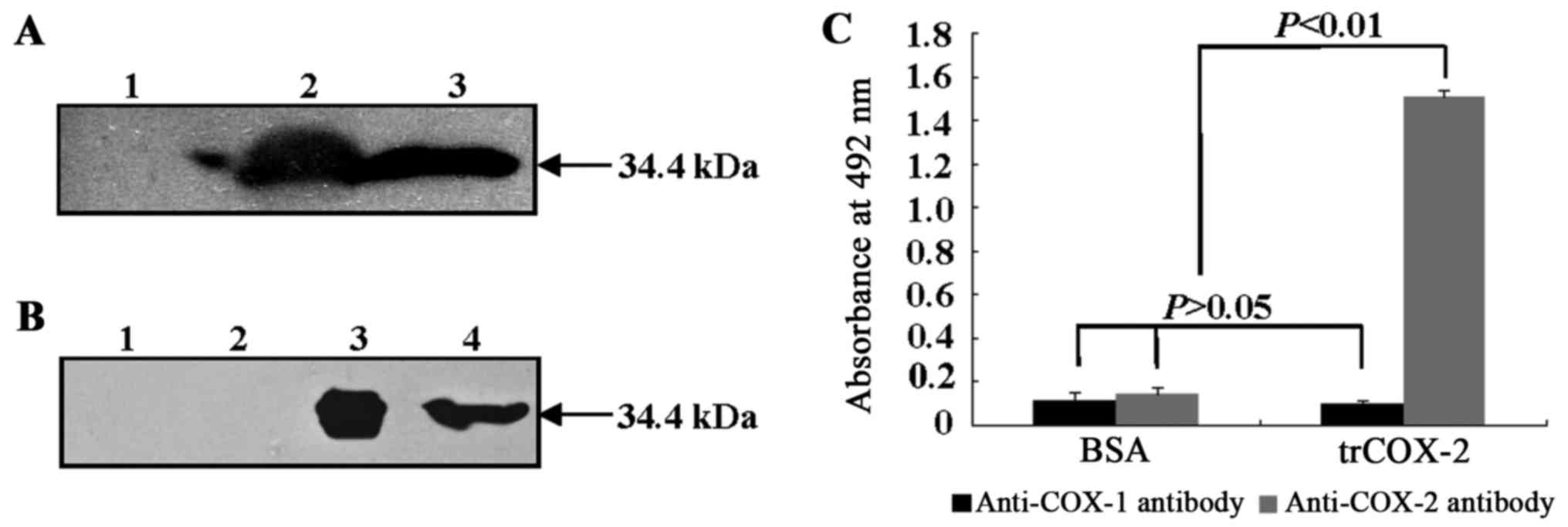
Western blot analyses using anti-His-tag antibody
(Fig. 6A) and anti-COX-2 antibody
(Fig. 6B) were used to further
identify recombinant trCOX-2, because recombinant trCOX-2 contains
His-tags at both the N- and C-terminus. As shown in Fig. 6, both the cell lysate of E.
coli pET28b-trCOX-2/BL21(DE3) induced by IPTG for 4 h and the
trCOX-2 protein showed a clear single band at approximately 34 kDa
after incubation with anti-His-tag antibody (Fig. 6A) and anti-COX-2 antibody
(Fig. 6B), respectively. Protein
bands were not found in samples without IPTG induction. These
results suggested trCOX-2 with His-tags was expressed successfully
in E. coli pET28b-trCOX-2/BL21(DE3) cells induced by IPTG
for 4 h and that purified trCOX-2 was obtained.
In order to examine the antigenicity and binding
activity of prepared trCOX-2 to anti-COX-2 or anti-COX-1 antibody,
an ELISA assay was performed. As shown in Fig. 6C, the ELISA assay results
demonstrated that the recombinant trCOX-2 showed selective binding
to anti-COX-2 antibody. The absorbance at 490 nm due to binding
between recombinant trCOX-2 and anti-COX-2 antibody was
significantly higher (P<0.01) than the absorbance observed for
each of three controls: the binding of BSA, an irrelevant antigen,
to anti-COX-2 antibody; BSA binding to anti-COX-1 antibody;
recombinant trCOX-2 binding to anti-COX-1 antibody. Moreover, only
low absorbance readings were obtained for anti-COX-1 antibody
binding to either BSA or trCOX-2 and these results were not
significantly different from the results for BSA binding to
anti-COX-2 antibody (P>0.05). These results suggest that
purified recombinant trCOX-2 retains the same antigenicity as human
COX-2 and has selective binding affinity to anti-COX-2
antibody.
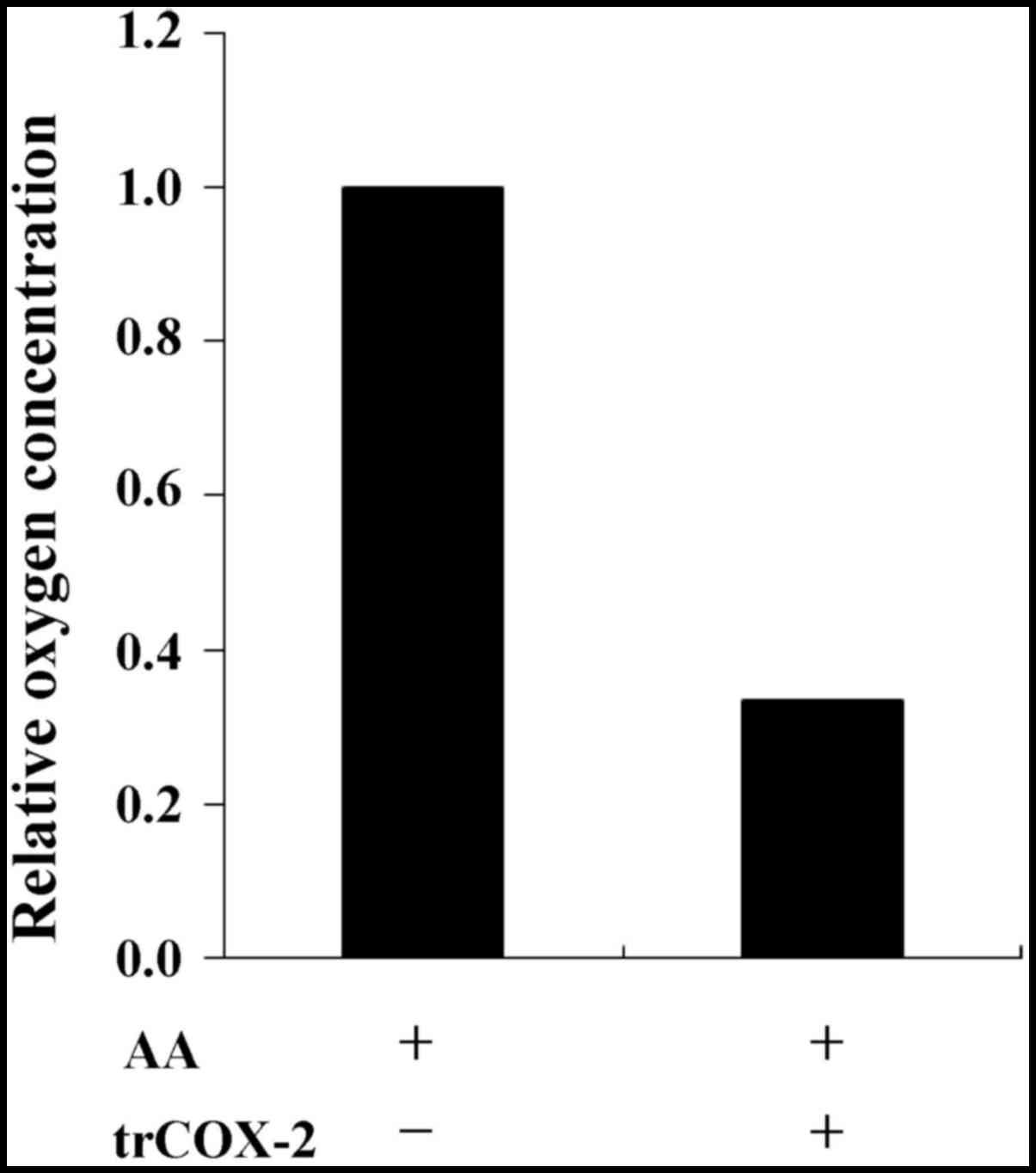
Purified trCOX-2 exhibits COX
activity
To verify the catalytic activity of trCOX-2, COX
activity was conducted by recording oxygen consumption. The
oxygenation and cyclization of AA within the COX active site during
formation of PGG2 consumes oxygen in the reaction
whereby COX-2 catalyzes the conversion of AA to PGs. The oxygen
consumption of COX activity can be monitored using a detector to
measure dissolved oxygen and recorded as the relative variation in
oxygen concentration. As shown in Fig. 7, compared with the control group
without trCOX-2 protein, the final oxygen concentration was
apparently lower in the test group after addition of trCOX-2
protein. This result demonstrates that the purified trCOX-2 still
exhibited COX activity, further confirming our speculation that
trCOX-2 possessed COX activity.
Discussion
In this study, we report a strategy with which to
express trCOX-2 in E. coli BL21(DE3) cells. For the purpose
of achieving high-level expression in E. coli cells, human
COX-2 was truncated to remove the stretch of residues from the
N-terminus, with the aim to reduce the size and structural
complexity of human COX-2, while maintaining enzyme activity.
Homology modeling and molecular docking results predicted that
trCOX-2 retained the active site in its 3D structure and that AA
could still bind to the hydrophobic groove, as shown in Figs. 1 and 2. The fusion trCOX-2 protein with
His-tags was efficiently expressed and was localized to inclusion
bodies after IPTG induction. Through denaturation, purification and
renaturation, we successfully obtained soluble trCOX-2 proteins
that were recognized specifically by anti-COX-2 antibody but not by
anti-COX-1 antibody. Moreover, the COX assays indicated that the
trCOX-2 maintained COX activity. This human COX-2 preparation
strategy provides a reliable method to obtain functional products
and is a valuable guide for prokaryotic expression of eukaryotic
membrane protein.
COX-2 is a rate-limiting key enzyme which catalyzes
the conversion of AA into PGs. The expression of COX-2 is
intimately involved in a number of pathologies, such as
inflammation, pain and various epithelial tumors (39,40). In addition, COX-2 closely
correlates with and is widely involved in most processes giving
rise to malignant tumor development, including the formation of
carcinogens, tumor promotion, inhibition of apoptosis, stimulation
of angiogenesis, invasion, metastasis and drug-resistance (11–13). COX-2 overexpression has been
regarded as an early event in carcinogenesis (10–12). Therefore, COX-2 is an important
target for anti-inflammation and anticancer therapies. To develop
these therapies, an effective and inexpensive expression strategy
to obtain bioactive and functional human COX-2 would be a key
step.
Although different types of recombinant proteins
have been successfully isolated in various expression systems,
including E. coli cells (14,15), previous studies have shown that
functional COX-2 has been most often expressed in
insect/baculovirus expression systems for structure determination
and function analysis in vitro (16–19). However, several advantages of
prokaryotic systems over insect/baculovirus expression systems
favor use of a prokaryotic system for high yield production of
COX-2. E. coli is one of the most widely used expression
hosts, coupled with the fact that techniques for protein
overexpression in E. coli are well developed. Because
protein synthesis rates are generally much faster in prokaryotes
than in eukaryotes (20), for
large-scale production of proteins, bacterial expression hosts such
as E. coli are preferred due to its rapid growth rate,
capacity for continuous fermentation, high-level expression of
target protein after induction and relatively low cost (14,20–23). In this study, E. coli
BL21(DE3) and pET28b(+) were used to achieve overexpression of
functional truncated human COX-2. We obtained approximately 350 mg
of renatured trCOX-2 from 10 liters of culture using this
prokaryotic expression system (Table
I). Previous studies have shown that 10 liters of fermentation
cultures of insect cells only yielded 35 mg of COX-2 (17), showing that COX-2 was extracted
almost 10-fold more efficiently in our prokaryotic expression
system than using an insect/baculovirus expression system.
Therefore, the expression system described in this study guarantees
a high yield of human COX-2 protein.
The smaller size and simpler protein structure of
human recombinant COX-2 protein has permitted its effective
expression in prokaryotic expression systems (20–23,36,37). Information from the crystal
structure of COX-2 has revealed that key active residues (Tyr-385,
Phe-381, Val-523, Glu-524 and Ser-530) are found in the catalytic
domain within the C-terminus. In order to obtain high-level
expression of human COX-2 in E. coli cells, the truncated
form lacking the N-terminus containing 257 residues of the
C-terminus was prepared to maximally reduce the size and structural
complexity of human COX-2 while maintaining its enzyme activity. As
a result, trCOX-2 protein with COX activity was expressed
successfully at a high-level in E. coli cells.
In our E. coli expression system, the
eukaryotic membrane proteins are inclined to be expressed in
insoluble forms known as inclusion bodies (20). Inclusion bodies are aggregations
of proteins which are largely protected from proteolytic
degradation by host cell enzymes (14,20). Through proper denaturant and
efficient renaturant methods, high-purity target proteins may be
retrieved in large amounts (20–26). The key step to obtaining a large
quantity of functional protein is the establishment of an
economical and highly effective method to dissolve and renature the
inclusion bodies (24–26). For the first time, to the best of
our knowledge, we obtained functional trCOX-2 using this
prokaryotic expression system through denaturation and renaturation
with buffer D and E, respectively (see Materials and methods).
In conclusion, our study describes a prokaryotic
functional expression strategy to generate high yields of truncated
and enzymatically active human COX-2. The trCOX-2 product is useful
for designing COX-2 inhibitors and anti-COX-2 antibodies.
Furthermore, this method provides a practical foundation to achieve
overexpression of eukaryotic membrane proteins in an E. coli
expression system.
Abbreviations:
|
COXs
|
cyclooxygenases
|
|
PGs
|
prostaglandins
|
|
AA
|
arachidonic acid
|
|
PGE2
|
prostaglandin E2
|
|
trCOX-2
|
truncated human COX-2
|
Acknowledgments
This study was partly supported by the National
Natural Science Foundation of China (nos. 31170882, 31570934,
81428006), the S&T Development Planning Program of Jilin
Province (nos. 20111806, 20150414027GH, 20160101213JC) and the
Fundamental Research Funds for the Central Universities (no.
451160306023).
References
|
1
|
Chandrasekharan V and Simmons DL: The
cyclooxygenases. Genome Biol. 5:2412004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rowlinson SW, Crews BC, Lanzo CA and
Marnett LJ: The binding of arachidonic acid in the cyclooxygenase
active site of mouse prostaglandin endoperoxide synthase-2 (COX-2).
A putative L-shaped binding conformation utilizing the top channel
region. J Biol Chem. 274:23305–23310. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vecchio AJ and Malkowski MG: The
structural basis of endocannabinoid oxygenation by
cyclooxygenase-2. J Biol Chem. 286:20736–20745. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vecchio AJ, Orlando BJ, Nandagiri R and
Malkowski MG: Investigating substrate promiscuity in
cyclooxygenase-2: the role of Arg-120 and residues lining the
hydrophobic groove. J Biol Chem. 287:24619–24630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong L, Vecchio AJ, Sharma NP, Jurban BJ,
Malkowski MG and Smith WL: Human cyclooxygenase-2 is a sequence
homodimer that functions as a conformational heterodimer. J Biol
Chem. 286:19035–19046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vecchio AJ, Simmons DM and Malkowski MG:
Structural basis of fatty acid substrate binding to
cyclooxygenase-2. J Biol Chem. 285:22152–22163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kudalkar SN, Nikas SP, Kingsley PJ, Xu S,
Galligan JJ, Rouzer CA, Banerjee S, Ji L, Eno MR, Makriyannis A, et
al: 13-Methylarachidonic acid is a positive allosteric modulator of
endocannabinoid oxygenation by cyclooxygenase. J Biol Chem.
290:7897–7909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanabe T and Tohnai N: Cyclooxygenase
isozymes and their gene structures and expression. Prostaglandins
Other Lipid Mediat. 68–69:95–114. 2002. View Article : Google Scholar
|
|
9
|
Li G, Han C, Xu L, Lim K, Isse K and Wu T:
Cyclooxygenase-2 prevents Fas-induced liver injury through
up-regulation of epidermal growth factor receptor. Hepatology.
50:834–843. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghosh N, Chaki R, Mandal V and Mandal SC:
COX-2 as a target for cancer chemotherapy. Pharmacol Rep.
62:233–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan Z, Khan N, Tiwari RP, Sah NK, Prasad
GB and Bisen PS: Biology of Cox-2: an application in cancer
therapeutics. Curr Drug Targets. 12:1082–1093. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Misra S and Sharma K: COX-2 signaling and
cancer: new players in old arena. Curr Drug Targets. 15:347–359.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurtova AV, Xiao J, Mo Q, Pazhanisamy S,
Krasnow R, Lerner SP, Chen F, Roh TT, Lay E, Ho PL, et al: Blocking
PGE2-induced tumour repopulation abrogates bladder
cancer chemoresistance. Nature. 517:209–213. 2015. View Article : Google Scholar
|
|
14
|
Yin J, Li G, Ren X and Herrler G: Select
what you need: a comparative evaluation of the advantages and
limitations of frequently used expression systems for foreign
genes. J Biotechnol. 127:335–347. 2007. View Article : Google Scholar
|
|
15
|
Wu Y, Zou D, Cao Y, Yao N, Wang J, Wang W,
Jiang H and Li G: Expression and purification of a human
anti-cyclin D1 single-chain variable fragment antibody AD5 and its
characterization. Int J Mol Med. 32:1451–1457. 2013.PubMed/NCBI
|
|
16
|
Percival MD, Bastien L, Griffin PR,
Kargman S, Ouellet M and O'Neill GP: Investigation of human
cyclooxygenase-2 glycosylation heterogeneity and protein expression
in insect and mammalian cell expression systems. Protein Expr
Purif. 9:388–398. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gierse JK: Purification of recombinant
human COX-1 and COX-2. Methods Mol Biol. 644:21–29. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gierse JK, McDonald JJ, Hauser SD,
Rangwala SH, Koboldt CM and Seibert K: A single amino acid
difference between cyclooxygenase-1 (COX-1) and -2 (COX-2) reverses
the selectivity of COX-2 specific inhibitors. J Biol Chem.
271:15810–15814. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cromlish WA, Payette P, Culp SA, Ouellet
M, Percival MD and Kennedy BP: High-level expression of active
human cyclooxygenase-2 in insect cells. Arch Biochem Biophys.
314:193–199. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gul N, Linares DM, Ho FY and Poolman B:
Evolved Escherichia coli strains for amplified, functional
expression of membrane proteins. J Mol Biol. 426:136–149. 2014.
View Article : Google Scholar
|
|
21
|
Liu B, Li G, Sui X, Yin J, Wang H and Ren
X: Expression and functional analysis of porcine aminopeptidase N
produced in prokaryotic expression system. J Biotechnol. 141:91–96.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arbabi-Ghahroudi M, Tanha J and MacKenzie
R: Prokaryotic expression of antibodies. Cancer Metastasis Rev.
24:501–519. 2005. View Article : Google Scholar
|
|
23
|
Sheibani N: Prokaryotic gene fusion
expression systems and their use in structural and functional
studies of proteins. Prep Biochem Biotechnol. 29:77–90. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim CS and Lee EK: Effects of operating
parameters in in vitro renaturation of a fusion protein of human
growth hormone and glutathione S transferase from inclusion body.
Process Biochem. 36:111–117. 2000. View Article : Google Scholar
|
|
25
|
Li GY, Zou DS, Zhou LH and Cao YH:
Expression and purification of recombinant human cyclin D1 in E.
coli BL21. J Jilin Univ. 44:839–843. 2006.
|
|
26
|
Cao YH, Xu JJ, Chen Y, Wang Q, Feng J, Hao
DY and Li GY: Prokaryotic expression, purification and renaturation
of recombinant human CDK4. J Jilin Univ. 46:992–996. 2008.
|
|
27
|
Xiang Y, Wang HY, Lei W and Sun M: The
expression and purification of COX-2 in Escherichia coli. J
Southwest Univ. 12:121–125. 2008. View Article : Google Scholar
|
|
28
|
Blobaum AL, Xu S, Rowlinson SW, Duggan KC,
Banerjee S, Kudalkar SN, Birmingham WR, Ghebreselasie K and Marnett
LJ: Action at a distance: mutations of peripheral residues
transform rapid reversible inhibitors to slow, tight binders of
cyclooxygenase-2. J Biol Chem. 290:12793–12803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arnold K, Bordoli L, Kopp J and Schwede T:
The SWISS-MODEL workspace: a web-based environment for protein
structure homology modelling. Bioinformatics. 22:195–201. 2006.
View Article : Google Scholar
|
|
30
|
Guex N, Peitsch MC and Schwede T:
Automated comparative protein structure modeling with SWISS-MODEL
and Swiss-PdbViewer: a historical perspective. Electrophoresis.
30(Suppl 1): S162–S173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kiefer F, Arnold K, Künzli M, Bordoli L
and Schwede T: The SWISS-MODEL repository and associated resources.
Nucleic Acids Res. 37(Database): D387–D392. 2009. View Article : Google Scholar :
|
|
32
|
Biasini M, Bienert S, Waterhouse A, Arnold
K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M,
Bordoli L, et al: SWISS-MODEL: modelling protein tertiary and
quaternary structure using evolutionary information. Nucleic Acids
Res. 42:W252–W258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grosdidier A, Zoete V and Michielin O:
Fast docking using the CHARMM force field with EADock DSS. J Comput
Chem. 32:2149–2159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grosdidier A, Zoete V and Michielin O:
SwissDock, a protein-small molecule docking web service based on
EADock DSS. Nucleic Acids Res. 39:W270–W277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nandana V, Singh S, Singh AN and Dubey VK:
Procerain B, a cysteine protease from Calotropis procera, requires
N-terminus pro-region for activity: cDNA cloning and expression
with pro-sequence. Protein Expr Purif. 103:16–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Doray B, Chen CD and Kemper B: N-terminal
deletions and His-tag fusions dramatically affect expression of
cytochrome 450 2C2 in bacteria. Arch Biochem Biophys. 393:143–153.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Housaindokhta MR, Bozorgmehr MR, Hosseini
HE, Jalal R, Asoodeh A, Saberi M, Haratipour Z and Monhemi H:
Structural properties of the truncated and wild types of
Taka-amylase: a molecular dynamics simulation and docking study. J
Mol Catal B Enzym. 95:36–40. 2013. View Article : Google Scholar
|
|
38
|
Wilkins MR, Gasteiger E, Bairoch A,
Sanchez JC, Williams KL, Appel RD and Hochstrasser DF: Protein
identification and analysis tools in the ExPASy server. Methods Mol
Biol. 112:531–552. 1999.PubMed/NCBI
|
|
39
|
Kim SF, Huri DA and Snyder SH: Inducible
nitric oxide synthase binds, S-nitrosylates, and activates
cyclooxygenase-2. Science. 310:1966–1970. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gierse JK, Hauser SD, Creely DP, Koboldt
C, Rangwala SH, Isakson PC and Seibert K: Expression and selective
inhibition of the constitutive and inducible forms of human
cyclooxygenase. Biochem J. 305:479–484. 1995. View Article : Google Scholar
|