Introduction
Type 2 diabetes, a chronic metabolic disorder, has
become a serious public health issue worldwide. In spite of this,
the prevalence of the disease still continues to increase along
with changes in lifestyle and escalating obesity rates, especially
in developing countries in Asia which have suffered from a rapidly
emerging epidemic of diabetes (1). In addition to insulin resistance,
pancreatic β cell dysfunction, characterized by decreased insulin
secretion, is another hallmark of type 2 diabetes. It is well know
that type 2 diabetes often coexists with dyslipidemia, indicating
elevated plasma free fatty acids (FFAs) (2,3).
Increased levels of FFAs not only decrease insulin-induced glucose
disposal by inhibiting insulin signaling, thus leading to insulin
resistance (4), but also affect
insulin secretion and insulin content in pancreatic β cells
(5). Although FFAs acutely
increase insulin output under physiological conditions,
constitutive exposure to physiological or higher concentrations of
FFAs cause detrimental effects on pancreatic β cells. Elevated
levels of FFAs induce an inflammatory response by activating
Toll-like receptor-4 (TLR4)/myeloid differentiation factor 88
(MyD88) signaling (6), trigger
endoplasmic reticulum (ER) stress and apoptosis (7,8),
inhibit adenosine monophosphate-activated protein kinase-α (AMPKα)
(9) and insulin biosynthesis
(10), thereby leading to a loss
of pancreatic β cells and a reduction in glucose-stimulated insulin
secretion (GSIS) (5,8,9),
which cause progressive function failure of pancreatic β cells and
exacerbate type 2 diabetes. Moreover, elevated FFAs do not need to
enter into the cells to exert the effects, but sustained binding
occurs to the cell surface receptor called G-protein-coupled
receptor 40 (GPR40), highly expressed in rodent and human
pancreatic β cells, and impairs GSIS in pancreatic β cells
(11). In fact, the gene
expression of GPR40 is significantly decreased in islets from type
2 diabetic donors and chronic elevated FFA-incubated islets, which
is related to reduced GSIS (11),
implying dysfunction of GPR40 signaling.
Pollen Typhae, a Chinese herbal medicine,
contains flavonoids such as naringenin, kaempferol, isorhamnetin,
isorhamnetin-3-O-neohesperidoside, typhaneoside, and other
constituents (12,13). According to previous reports,
Pollen Typhae has anticoagulant activity (14) and functions to treat trauma,
coronary heart diseases and myocardial infarction (15). Recently, Pollen Typhae has
been used to treat type 2 diabetes in clinical practice in China.
We previously reported that Pollen Typhae total flavone
(PTF), an extract from Pollen Typhae mainly containing
typhaneoside and other components (16), decreases blood glucose and
improves insulin resistance and dyslipidemia in type 2 diabetic
rats (17). Further research
revealed that FFAs contribute to insulin resistance involving
dysfunction of β-arrestin-2-mediated signaling (18), and that PTF has beneficial effects
on this signaling, thus ameliorating insulin-induced glucose uptake
and palmitic acid (PA)-induced insulin resistance (16,19). The underlying molecular mechanisms
of PTF in the treatment of type 2 diabetes, however, remain to be
fully elucidated. Because of the action of PTF in improving
dyslipidemia, we hypothesized that PTF would ameliorate FFA-induced
impairment of GSIS in pancreatic β cells.
This study aimed to observe the effects of PTF on
GSIS induced by PA in INS-1 cells and to explore the potential
mechanisms.
Materials and methods
Reagents
RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from Gibco (Grand Island, NY, USA). FFA-free bovine serum
albumin (FFA-free BSA), PA,
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)
carbonyl]-2H-tetrazolium hydroxide (XTT), streptozotocin (STZ),
sodium pyruvate, HEPES, GW9508, U-73122, nifedipine (NIF), and
phenazine methosulfate (PMS) were obtained from Sigma (St. Louis,
MO, USA). Culture plates were purchased from Corning (New York, NY,
USA). Xi'an Salao Biotechnology Co., Ltd. (Xi'an, China) provided
and confirmed PTF. The BCA protein assay kit and RIPA cell lysis
buffer were from Beyotime Institute of Biotechnology Co., Ltd.
(Shanghai, China). Rat insulin enzyme-linked immunosorbent assay
(ELISA) kit was provided by Mercodia (Uppsala, Sweden). Rat
inositol 1,4,5,-trisphosphate (IP3) ELISA kit was obtained from
CusaBio (Wuhan, China). Rat diacyl glycerol (DAG) ELISA kit was
purchased from Shanghai Tongwei Biological Technology Co., Ltd.
(Shanghai, China). IP-One ELISA kit was obtained from Cisbio
Bioassays (Codolet, France). Protein kinase C (PKC) kinase activity
kit was purchased from Enzo Life Sciences (Farmingdale, NY, USA).
Antibody directed against GPR40 (cat. no. sc-32905; dilution,
1:200) was purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Antibodies to phospholipase C (PLC)β1 (cat. no.
ab140746; dilution, 1:1,000) and PKC (cat. no. ab23511; dilution,
1:1,000) were from Abcam (Cambridge, MA, USA). Anti-PLCβ3 antibody
(cat. no. 14247; dilution, 1:1,000), secondary HRP-conjugated
antibodies (cat. nos. 7074S and 7076S; dilution, 1:3,000) and
staurosporine (STA) were purchased from Cell Signaling Technology
(Danvers, MA, USA). ECL Plus detection reagents were provided by
Hanbio Biotechnology Co., Ltd. (Shanghai, China).
Cell culture
INS-1 rat insulin-secreting cells (Shanghai Meixuan
Biological Science and Technology Co., Ltd., Shanghai, China) were
cultured in RPMI-1640 medium (containing 11.1 mM glucose),
supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml
streptomycin, 10 mM HEPES, 1 mM sodium pyruvate, and 50 µM
β-mercaptoethanol. The cells were maintained for growth at 37°C
under a humidified atmosphere containing 5% CO2. Culture
medium was changed every other day.
PA preparation
PA/BSA conjugates were prepared according to a
previously described procedure (18). In brief, PA was added into 0.1 N
NaOH followed by a shaking water bath at 70°C until the PA was
dissolved; the concentration of PA solution was 75 mM. Then, 10%
FFA-free BSA-RPMI-1640 medium was used to dilute the solution to a
stock concentration of 5 mM at 55°C. The solution was sterilized by
filtration and then kept at −80°C in a refrigerator. After being
placed in a water bath at 55°C for 15 min, the PA solution was
cooled to room temperature and diluted with 1% FFA-free
BSA-RPMI-1640 medium. The solution containing the same
concentration of NaOH and 10% FFA-free BSA-RPMI-1640 medium was
taken as a control solution.
Viability assay
The viability was determined by XTT assay (16). Briefly, INS-1 cells were grown in
96-well culture plates. When the cells reached confluency, the
cells were treated with PA (0 and 0.5 mM) or various concentrations
of PTF (0, 0.03125, 0.0625, 0.125, 0.25, 0.5 and 1.0 mg/ml), which
were diluted with 1% FFA-free BSA-RPMI-1640 medium and then added
to the cells (100 µl each well). After incubation for the
indicated time, 50 µl of 0.10% XTT dissolved in serum-free
RPMI-1640 medium was added to the cells in each well. The cells
were incubated for 4 h at 37°C, and then the optical absorbance was
measured using a microplate reader (BioTek, Epoch; BioTek
Instruments, Inc., Winooski, VT, USA) at 490 nm.
Insulin secretion assay
Insulin secretion assay was conducted according to a
previously described procedure with some modifications (20). INS-1 cells were grown in 24-well
plates and reached confluency. After treatment with PA or/and PTF
and inhibitors, the cells were washed with phosphate-buffered
saline (PBS) twice, and incubated with Krebs-Ringer buffer (KRB:
120 mM NaCl, 5 mM NaHCO3, 5 mM KCl, 1.2 mM
KH2PO4, 2.5 mM CaCl2, 1.2 mM
MgSO4, 10 mM HEPES, and 0.2% BSA, pH 7.2) for 30 min.
Then, the cells were incubated with KRB containing 2.8 or/and 16.7
mM glucose for 60 min. The supernatants were collected for insulin
analysis using an ELISA kit according to the manufacturer's
instructions. The attached cells were lysed in cell lysis buffer
followed by centrifugation to remove the debris, and the protein
concentrations of the supernatants were determined using a BCA
protein assay kit. Final insulin concentrations per well were
normalized by the protein content.
Animal trial design
Hunan Slaccas Jingda Laboratory Animal Co., Ltd.
(Changsha, China) provided male Sprague-Dawley rats weighing
160–200 g, and Shanghai Slaccas Laboratory Animal Co., Ltd.
(Shanghai, China) provided chow diet: normal pellet diet (NPD) with
4.6% fat (~10% of calories as fat) and high-fat diet (HFD, ~40% of
calories as fat). Type 2 diabetes was induced in the rats by a
high-fat diet and low-dose STZ as previously described (17). The diabetic rats were randomly
divided into 2 groups: the diabetes model group (MOD, n=8) and the
PTF group (PTF, n=8). The rats in the former group received an
equal volume of distilled water by intragastric administration per
day and free access to HFD, while the rats in the latter group were
administered PTF (0.20 g/kg/day) by oral gavage each day and
received HFD freely. The NPD-fed rats were taken as a control group
(CON, n=8, non-diabetic rats) receiving an equal volume of
distilled water by intragastric administration each day and NPD
feeding. The treatment course lasted for 4 weeks. Blood was
collected before and after treatment, and insulin secretion
function of pancreatic β cells was evaluated by Homeostasis model
assessment of β cell function (HOMA-β). HOMA-β = 20 × fasting
insulin/(fasting glucose − 3.5) (21). This study was approved by the
Ethics Committee of Guangxi University of Chinese Medicine. All
procedures were in accordance with internationally accepted
principles for laboratory animal use and care.
Determination of IP3 and DAG
After treatment with PA or/and PTF and U-73122 for
24 h, the cells were incubated with KRB containing 2.5 mM glucose
for 1 h followed by treatment with 16.7 mM glucose for 5 min. Then,
the cells were collected and stored overnight at −20°C. After two
freeze-thaw cycles to break up the cell membranes, the cell lysates
were centrifuged (5,000 × g, 4°C, 5 min for IP3; 1900 × g, 4°C, 20
min for DAG) to remove the debris. The supernatants were used to
analyze IP3 and DAG levels using ELISA kits according to the
manufacturer's instructions. The final concentrations of IP3 and
DAG were normalized by the protein content, respectively.
PLC activity assay
IP-One ELISA kit was used to analyze the activity of
PLC by quantification of inositol phosphate (IP1) according to the
manufacturer's instructions. The cells were preincubated with PA
or/and PTF and U-73122 for 24 h. The cells were then exposed to
GW9508 (20 µM). After stimulation for 60 min, the cells were
lysed and centrifuged. The supernatants were transferred to the
ELISA plate followed by an addition of IP1-HRP conjugate and
anti-IP1 monoclonal antibodies. After incubation for 3 h at room
temperature, the plate was washed and TMB was added and incubation
was carried out for 30 min at room temperature in a dark
environment. Finally, stop solution was added to the plate to stop
the reaction and the optical absorbance was read at 450 nm with
optical correction at 620 nm using a microplate reader
(Infinite® 200 Pro NanoQuant; Tecan, Männedorf,
Switzerland). The concentration of IP1 was calculated according to
the standard curve and normalized by the protein content.
Determination of PKC activity
The activity of PKC was determined using a
commercial ELISA kit. The cells were preincubated with PA or/and
PTF and STA for 24 h. The cells were then stimulated with or
without GW9508 (20 µM) for 60 min. After cell lysis and
subsequent centrifugation, the supernatants of cell lysates were
transferred to the ELISA plate for determination of PKC activity
according to the manufacturer's instructions. Finally, the plate
was read at 450 nm using a microplate reader (Infinite®
200 Pro NanoQuant; Tecan). The PKC activity was normalized by the
protein content.
Western blotting
The protein expression in INS-1 cells was analyzed
by western blotting as previously described with certain
modifications (16). After
treatment for 24 h, the cells were washed twice with ice-cold PBS,
and then lysed in RIPA cell lysis buffer for 15 min on ice. The
lysates were centrifugated (12,000 × g for 5 min, 4°C) to remove
the insoluble material. The supernatants were collected and then
the protein concentrations were determined by the BCA method. The
proteins were subjected to sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and then electrophoretically
transferred to polyvinylidene difluoride membranes. The membranes
were incubated with the appropriate primary antibodies overnight at
4°C and then with the HRP-conjugated secondary antibodies for 1 h
at room temperature. Immunoreactivity was visualized by incubation
with ECL reagents.
Statistical analysis
The data are presented as the means ± standard
deviation (SD). Student's t-test was used to identify the
significant differences between two groups. When multiple
comparisons were performed, the significance was analyzed by
one-way analysis of variance (ANOVA). All data were analyzed with
SPSS 16.0 for Windows. A value of P<0.05 was regarded as
statistically significant.
Results
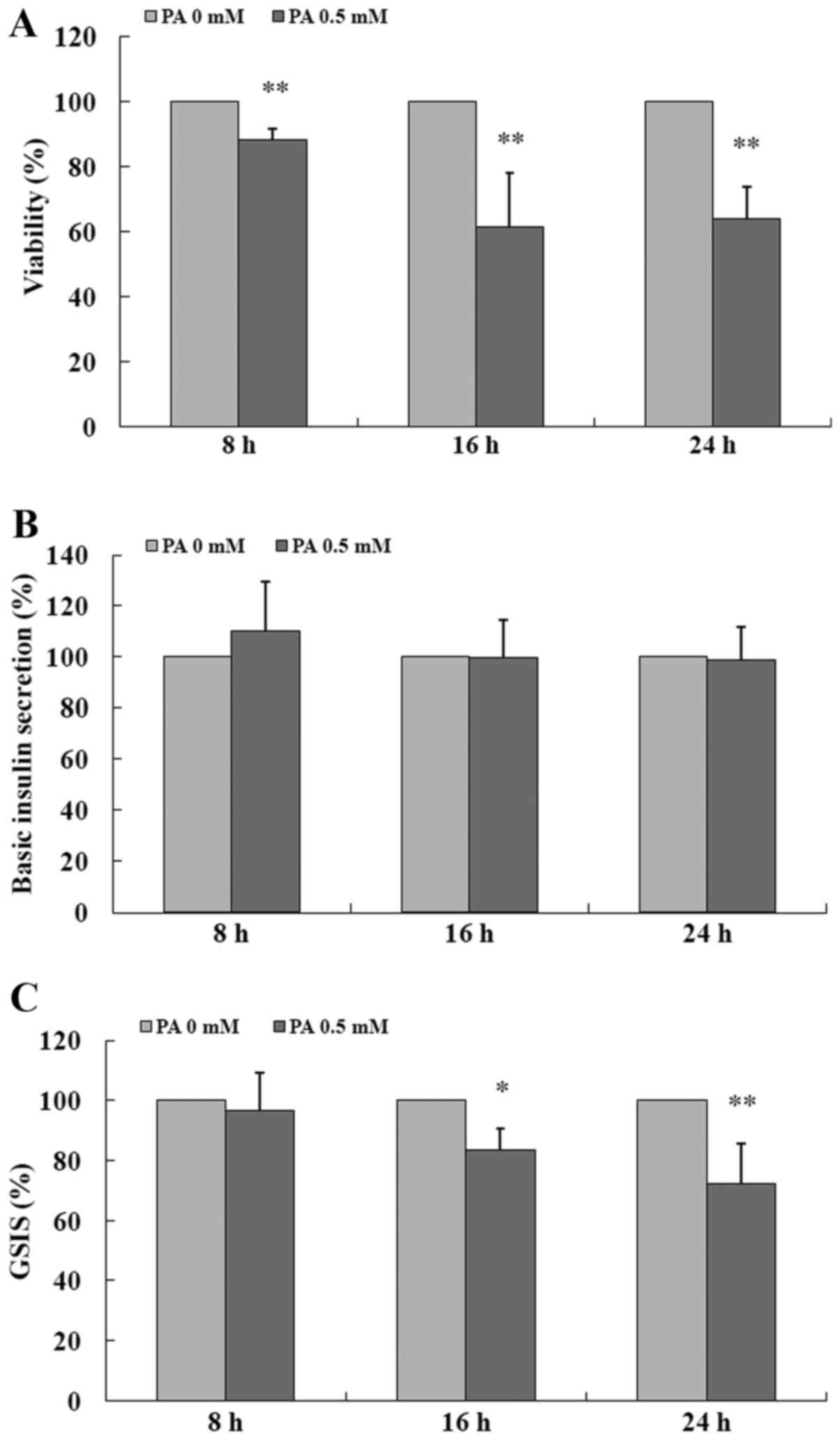
PA impairs GSIS in INS-1 cells
As shown in Fig.
1, exposure to 0.5 mM PA of the INS-1 cells for 8, 16 and 24 h
significantly impaired the viability (all P<0.01) (Fig. 1A) as compared to the control (0.0
mM PA), respectively, but the insulin secretion in response to 2.8
mM glucose (basic insulin secretion) was not markedly changed by PA
(Fig. 1B). In the presence of
high glucose (16.7 mM) stimulation, PA pretreatment for 8 h only
reduced GSIS by 3.54% without statistical significance when
compared to the control (P>0.05) (Fig. 1C), but administration of PA for 16
and 24 h obviously decreased GSIS by 16.65% (P<0.05) (Fig. 1C) and 27.61% (P<0.01) (Fig. 1C), respectively. The data indicate
that PA impairs GSIS in a time-dependent manner in INS-1 cells.
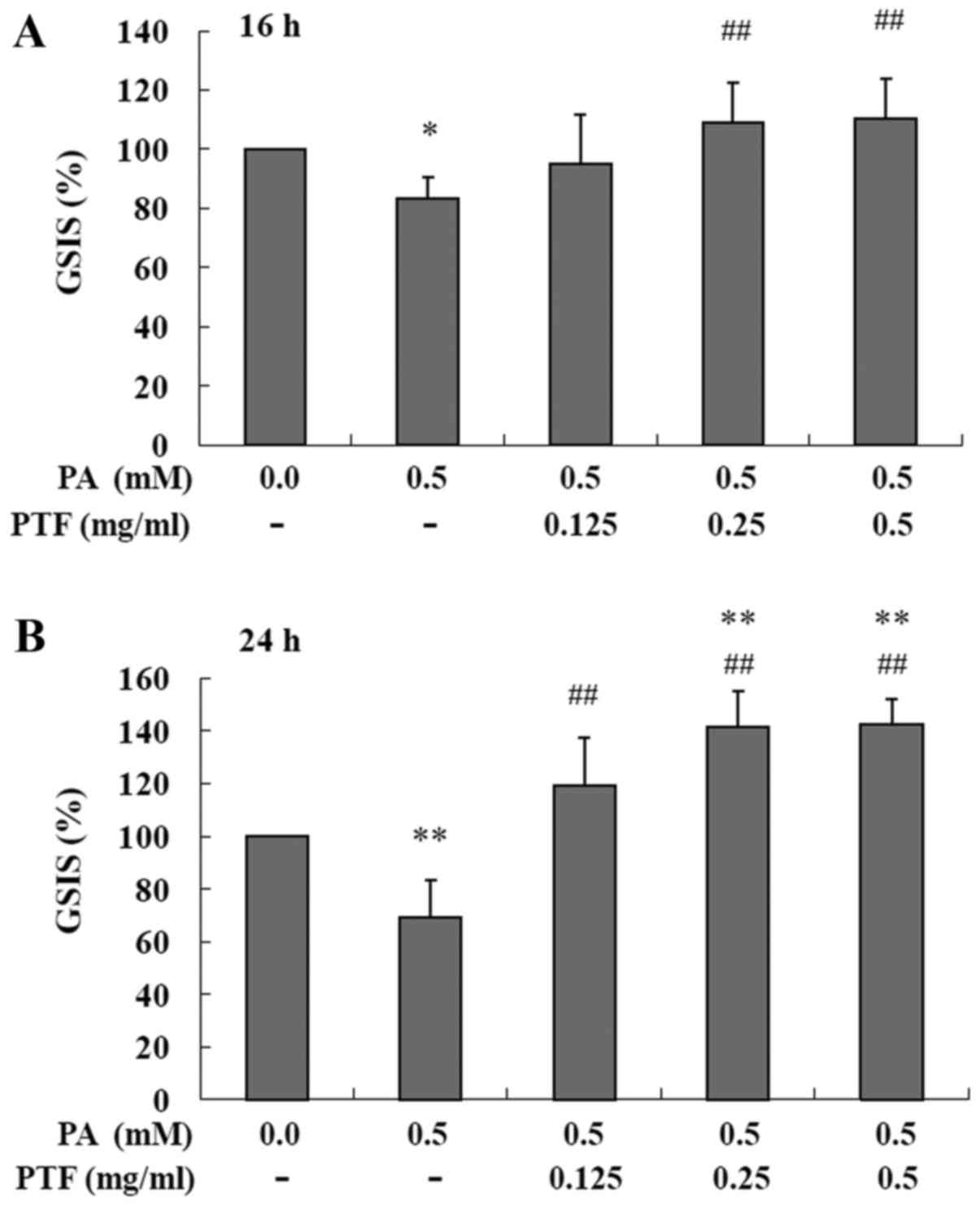
PTF prevents the impairment of GSIS
induced by PA in INS-1 cells
As shown in Fig.
2, treatment with PA (0.5 mM) for 16 (Fig. 2A) and 24 h (Fig. 2B) markedly reduced GSIS in INS-1
cells, respectively. Moreover, administration of PTF for 16 h at
the concentrations of 0.25 and 0.5 mg/ml significantly prevented
the reduction in GSIS induced by PA (all P<0.01). Moreover,
PA-induced impairment of GSIS was also blocked by PTF treatment for
24 h at all concentrations (0.125, 0.25 and 0.5 mg/ml, all
P<0.01). Notably, the improvement by PTF was dependent on the
dose of PTF, meaning that PTF dose dependently potentiated GSIS
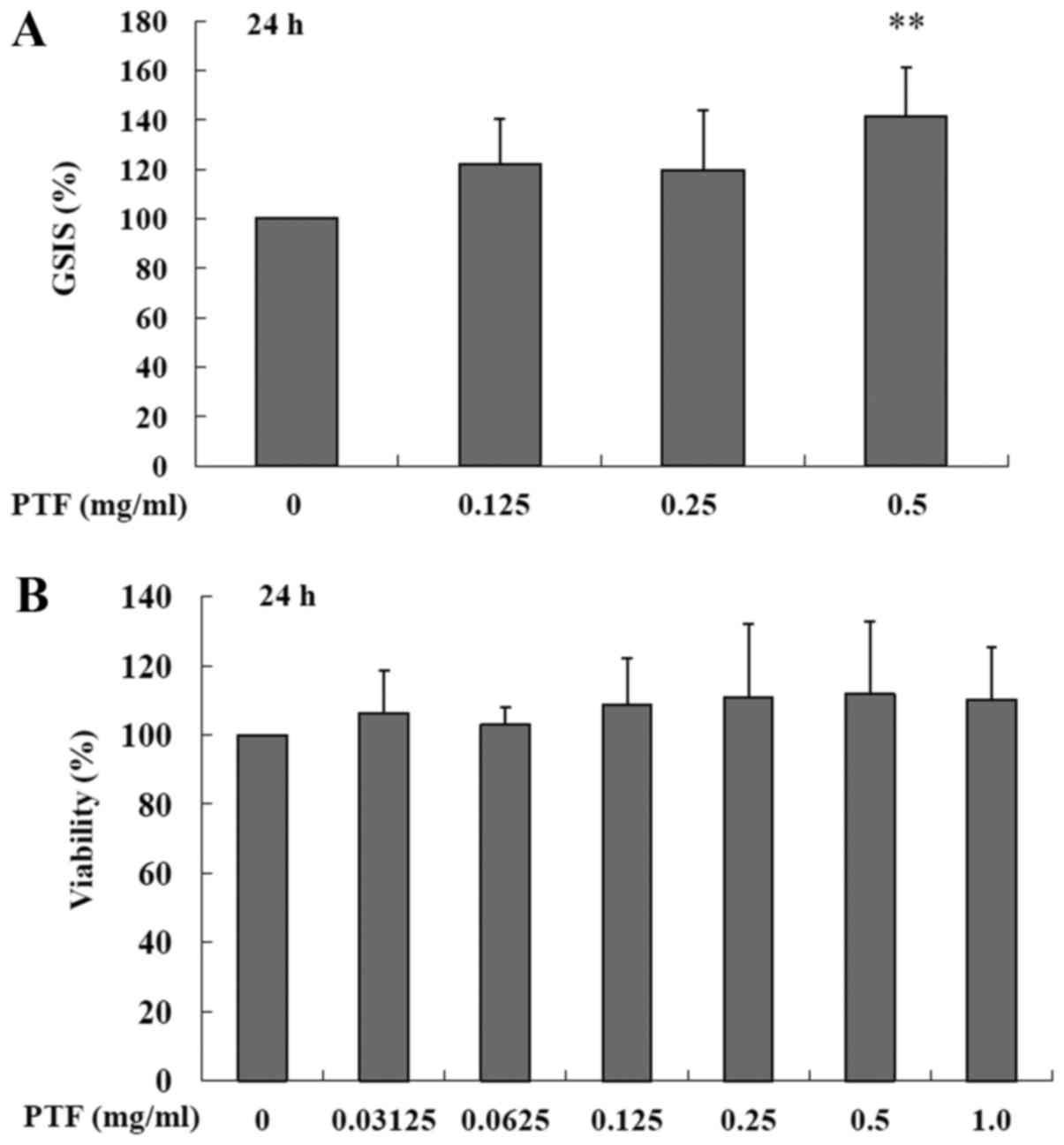
induced by PA in the INS-1 cells. Additionally, treatment with PTF
for 24 h at the concentration of 0.5 mg/ml also increased GSIS
(P<0.01) (Fig. 3A) in the
absence of PA when compared to the control (0.0 mg/ml PTF). In
addition, PTF at the concentrations of 0.03125–1.0 mg/ml did not
obviously affect the viability of the INS-1 cells (Fig. 3B). The results suggest that PTF
prevents PA-induced impairment of GSIS in a dose-dependent manner
in INS-1 cells.
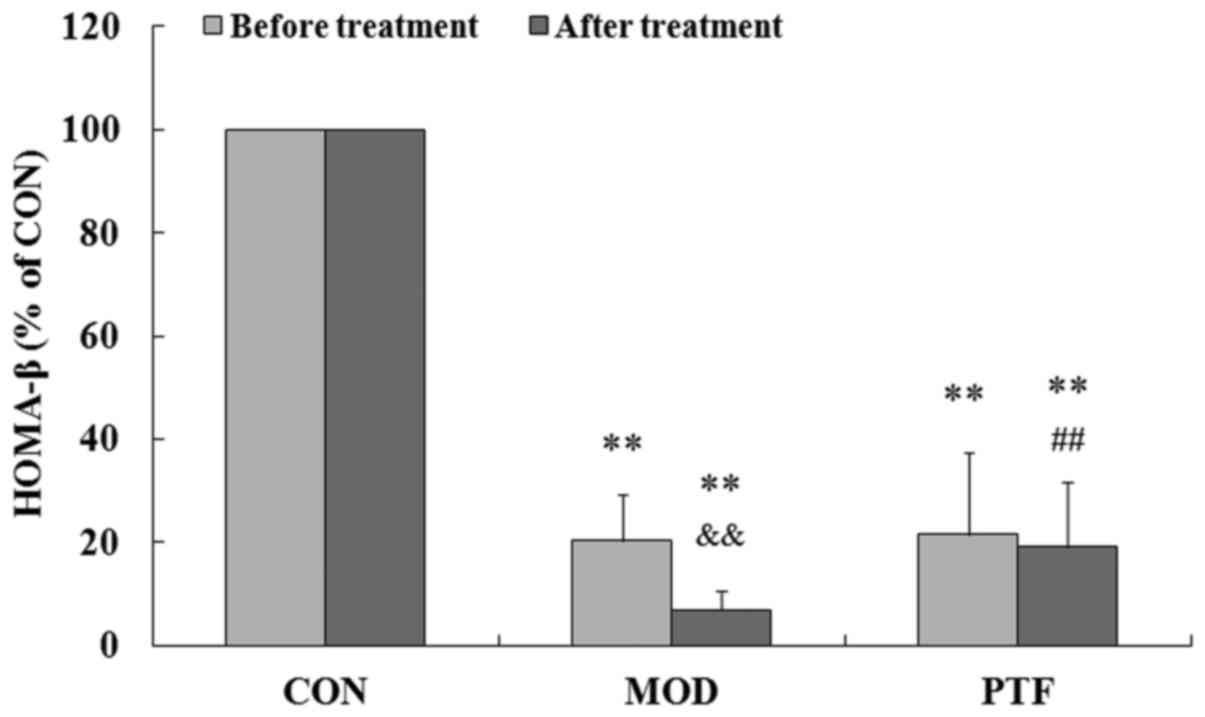
PTF ameliorates the insulin secretion
function in type 2 diabetic rats
To further confirm the effects of PTF on insulin
secretion in vivo, type 2 diabetes was induced in rats by a
high-fat diet and low-dose STZ. We previously reported that type 2
diabetic rats are characterized by hyperglycaemia, insulin
resistance, and dyslipidemia (17). In this study, the insulin
secretion function of pancreatic β cells was severely impaired in
type 2 diabetic rats, showing much lower HOMA-β than that in the
control group (P<0.01) (Fig.
4). Administration of PTF for 4 weeks significantly inhibited a
further reduction in HOMA-β in the type 2 diabetic rats, indicating
that HOMA-β in the PTF group was higher than that in the diabetes
model group (P<0.01). The results provide convincing evidence
for PTF regulation of PA-induced insulin secretion in INS-1
cells.
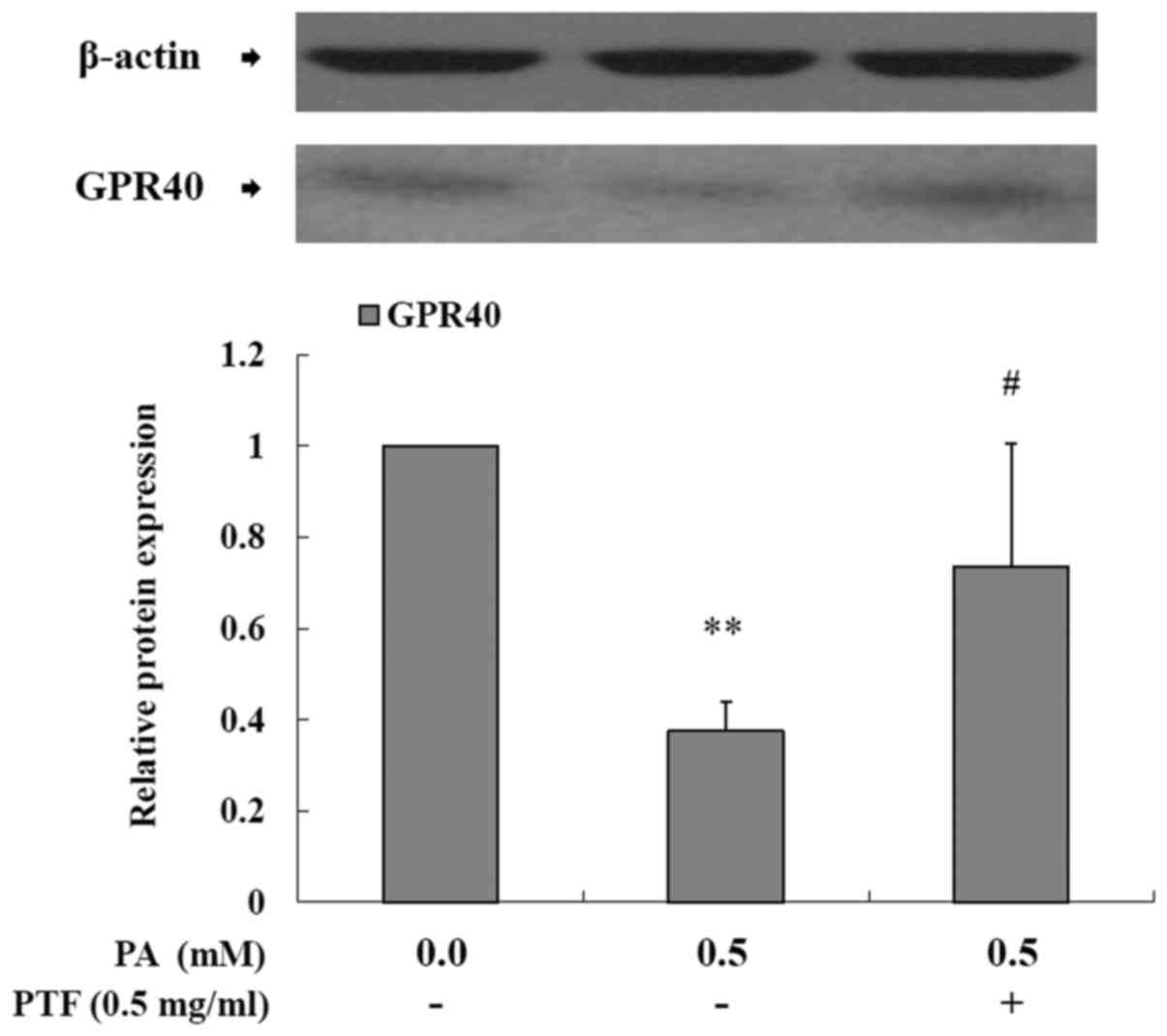
PTF enhances the protein expression of
GPR40 reduced by PA in INS-1 cells
GPR40 is a membrane-bound receptor for medium- and
long-chain FFAs. Acute FFA exposure promotes GSIS through GPR40,
but chronic elevated FFAs lead to a reduction in GPR40 expression
with decreased GSIS (5,11). In this study, PA exposure to INS-1
cells for 24 h decreased the protein expression of GPR40
(P<0.01) (Fig. 5) as compared
to the control (0.0 mM PA), implying that PA impaired GSIS
involving GPR40 and the subsequent cascade reaction in INS-1 cells.
Administration of PTF inhibited the reduction of GPR40 protein
expression (P<0.05).
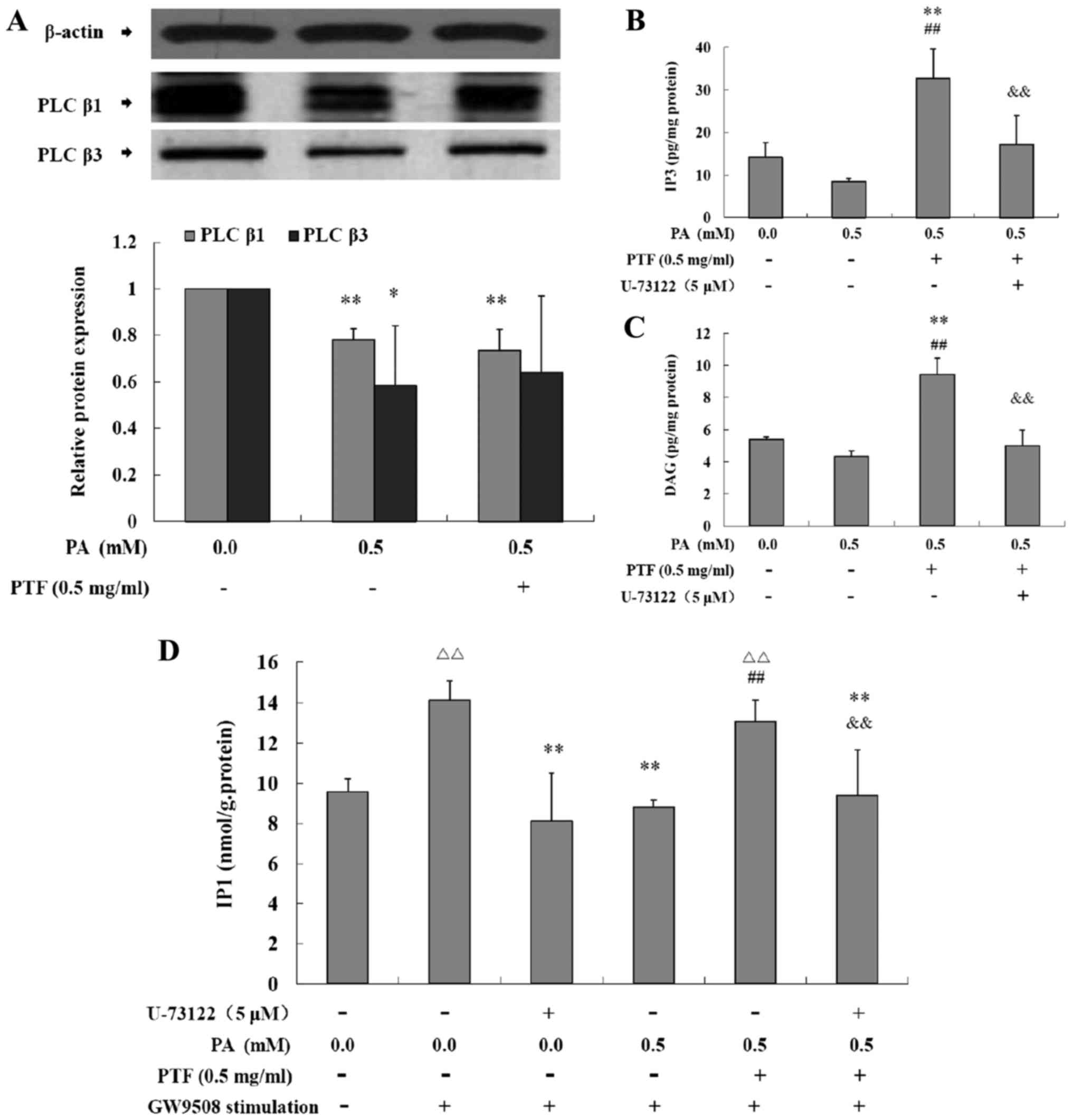
Effects of PTF on the protein expression
and the activity of PLC induced by PA in INS-1 cells
GW9508, a selective GPR40 agonist, binds GPR40 and
subsequently activates the β subtype of PLC, which promotes
phosphatidylinositol-4,5-bisphosphate (PIP2) to produce the second
messenger molecules IP3 and DAG, thus potentiating GSIS in
pancreatic β cells (22,23). IP3 can be transformed into IP2 and
IP1. PA exposure for 24 h significantly decreased the protein
expression of PLCβ1 and PLCβ3 (P<0.05 or P<0.01) (Fig. 6A) when compared to the control
(0.0 mM PA), while PTF treatment did not inhibit the reduction in
the protein expression in the INS-1 cells. Moreover, PA incubation
for 24 h reduced IP3 (Fig. 6B)
and DAG levels (Fig. 6C), and PTF
significantly increased IP3 (P<0.01) and DAG levels (P<0.01)
induced by PA in the INS-1 cells when compared to the control or
0.5 mM PA, which were blocked by U-73122, an inhibitor of PLC.
Additionally, GW9508 exposure to INS-1 cells for 60 min acutely
increased IP1 levels (P<0.01) (Fig. 6D), and PA preincubation for 24 h
significantly decreased GW9508-stimulated IP1 levels (P<0.01),
which was similar to U-73122, suggesting decreased activity of PLC
induced by PA. Notably, PTF markedly prevented this reduction
(P<0.01), which was also inhibited by U-73122. Together, PTF
promotes GW9508-stimulated activity of PLC induced by PA in INS-1
cells.
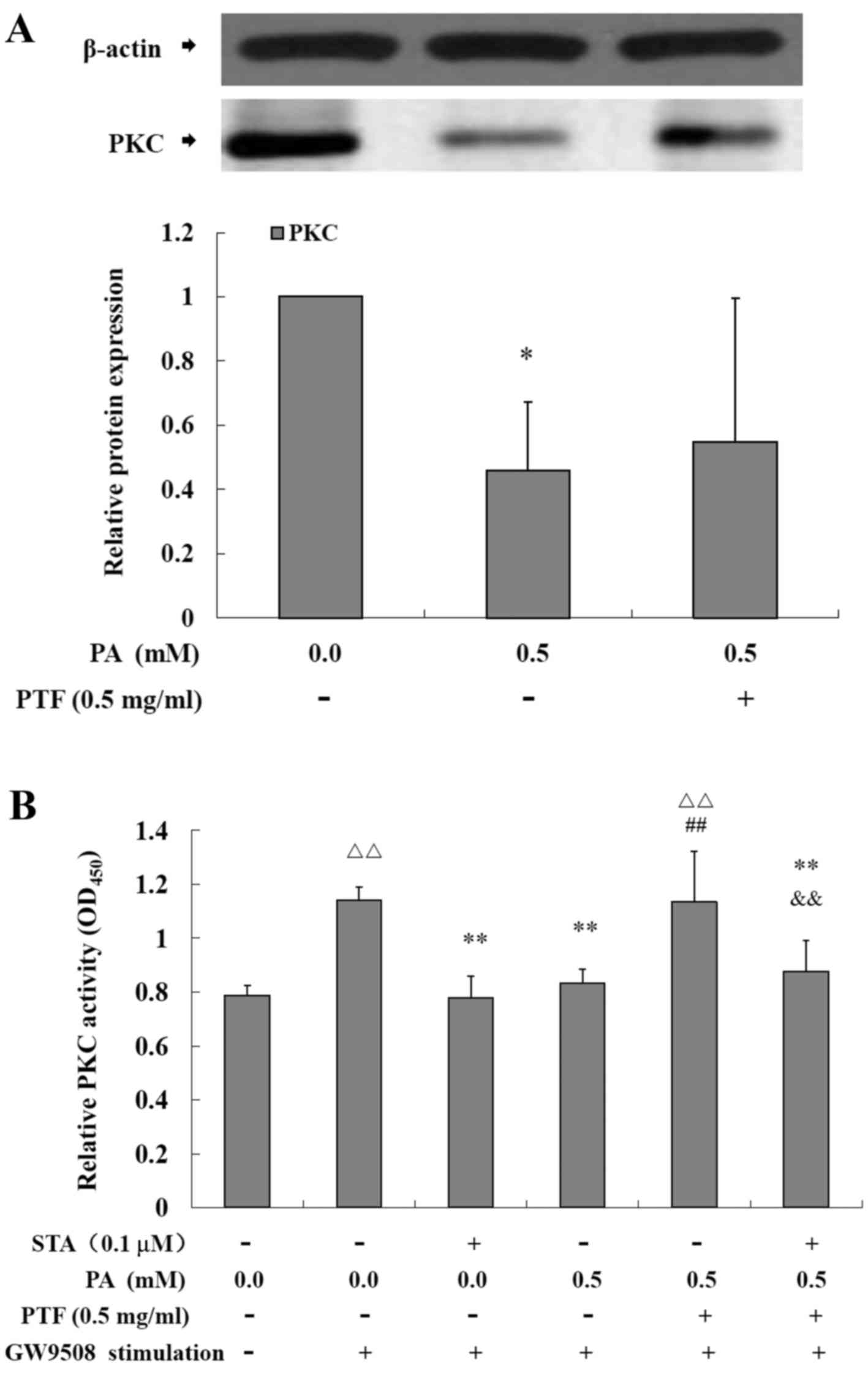
Effects of PTF on PKC protein expression
and activity induced by PA in INS-1 cells
As shown in Fig.
7, compared with the control (0.0 mM PA), PA significantly
reduced the protein expression of PKC (P<0.05) (Fig. 7A), and PTF to a certain extent
strengthened the protein expression reduced by PA in INS-1 cells.
Moreover, acute GW9508 exposure markedly increased the activity of
PKC (P<0.01) (Fig. 7B), but PA
pretreatment for 24 h led to a significant reduction (P<0.01) in
the activity of PKC stimulated by GW9508 in the INS-1 cells, which
was similar to STA, a specific inhibitor of PKC. Moreover, PTF
intervention prevented the reduction (P<0.01), which was
inhibited by STA.
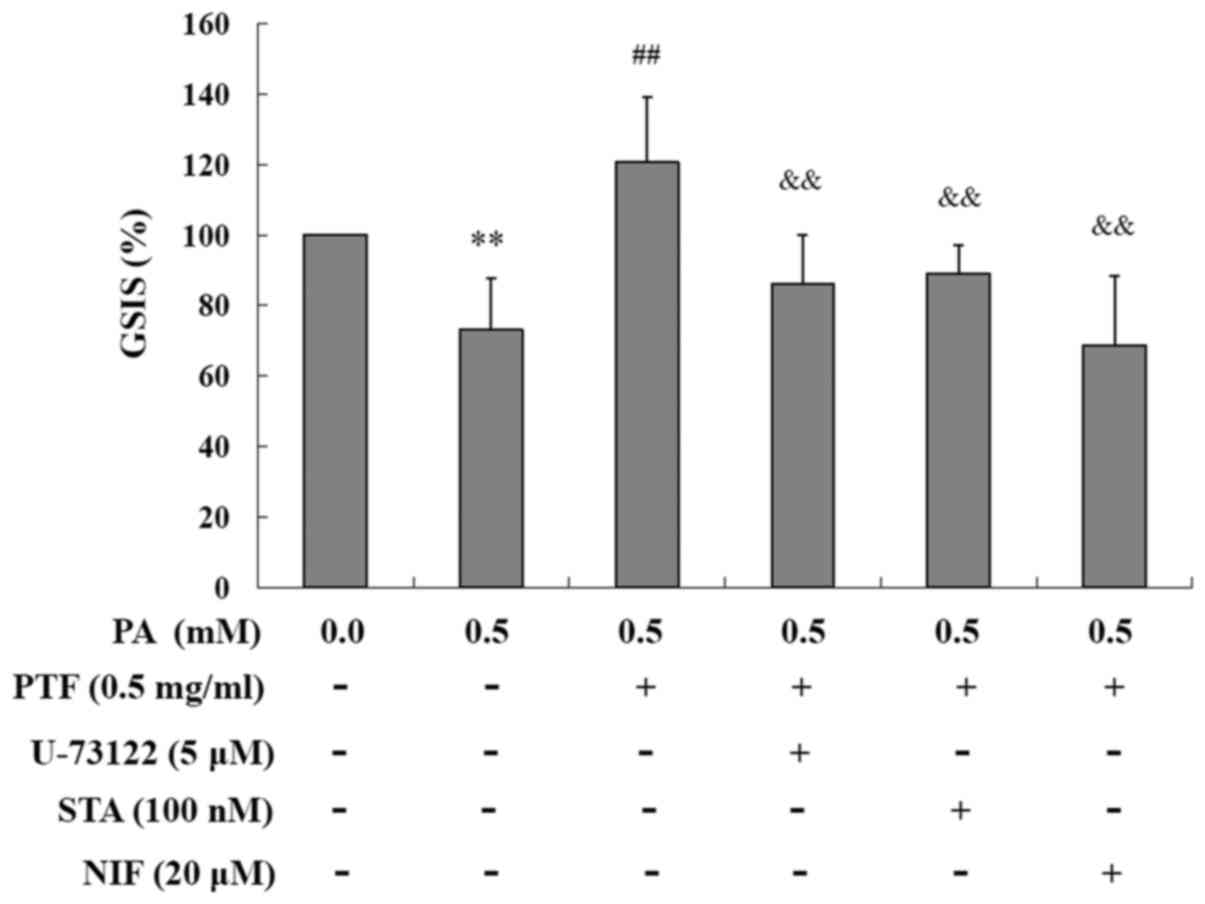
Preventive effect of PTF on PA-induced
impairment of GSIS is abolished by several inhibitors in INS-1
cells
In line with the above state, PA reduced GSIS in the
INS-1 cells, which was prevented by PTF. The preventive effect of
PTF was abolished by U-73122, STA and NIF, respectively (all
P<0.01) (Fig. 8), known as PLC
inhibitor, PKC inhibitor, and calcium antagonist, respectively. The
results imply that PTF prevents PA-induced impairment of GSIS
involving GPR40 signaling.
Discussion
In addition to providing an important energy source,
FFAs, such as saturated fatty acid PA and unsaturated fatty acid
oleic acid (OA), also play a key role in mediating insulin
secretion of pancreatic β cells. Studies have shown that acute FFA
exposure increases insulin output in pancreatic β cells (5,24),
whereas chronic elevated FFAs reduce GSIS (8,25).
In the present study, PA exposure to INS-1 cells severely impaired
cell viability. Although PA did not affect the basic insulin
secretion, PA significantly reduced GSIS in a time-dependent
manner, which was compatible with previous reports (8,25).
In addition, administration of PTF prevented PA-induced impairment
of GSIS in a dose-dependent manner, and potentiated GSIS in INS-1
cells. Moreover, PTF ameliorated the insulin secretion function in
HFD and low-dose STZ-induced type 2 diabetic rats. It is generally
accepted that insulin secretion dysfunction is a hallmark of type 2
diabetes. Moreover, dyslipidaemia is a characteristic of type 2
diabetes, and continuous elevated blood lipids contribute to
insulin secretion dysfunction, thus exacerbating type 2 diabetes
(26). We previously reported
that PTF reduces triglyceride (TG) and low-density lipoprotein
cholesterol (LDL-c), decreases blood glucose, and improves insulin
resistance in diabetic rats (17). Together, these results indicate
the protective action of PTF against PA-induced impairment of GSIS
in INS-1 cells, suggesting the anti-diabetic action of PTF.
GPR40, known as free fatty acid receptor 1 (FFAR1),
is a cell-surface receptor and is predominantly expressed in
pancreatic β cells. GPR40 has a high affinity with endogenous
medium- and long-chain fatty acids including PA and OA. Activated
GPR40 by FFAs or agonist amplifies GSIS from pancreatic β cells
(24,27,28). In fact, GPR40 expression is
decreased in type 2 diabetic islets with reduced GSIS (11,29), and GPR40 knockout impairs GSIS in
mice (30,31). On the contrary, overexpression of
GPR40 potentiates GSIS and ameliorates glucose tolerance in normal
and diabetic mice (32).
Therefore, GPR40 is a potential therapeutic target for the
development of anti-diabetic drugs (33,34). In this study, elevated PA exposure
for 24 h led to a significant reduction in GPR40 protein expression
in INS-1 cells. Del Guerra et al also reported that
increased FFA preexposure for 24 h significantly decreased GPR40
mRNA in islets from multiorgan donors (11), consistent with this study,
implying that chronic elevated FFAs result in a reduction in GPR40
involving impaired GSIS. In addition, PTF treatment prevented GPR40
reduction induced by PA. Additionally, similar to the above state,
PTF ameliorates insulin resistance in type 2 diabetic rats
(17) in addition to augmenting
GSIS in INS-1 cells and improving insulin secretion function in
type 2 diabetic rats, which is similar to rosiglitazone, a
peroxisome proliferator-activated receptor (PPAR)-γ agonist
functioning to improve insulin resistance. Rosiglitazone has been
reported to upregulate GPR40 expression and promote insulin
secretion in pancreatic islets (29,35), which was blocked by a PLC
inhibitor (35). The data suggest
that PTF prevented PA-induced impairment of GSIS involving GPR40
signaling in INS-1 cells.
Upon stimulation, GPR40 activates the β subtype of
PLC, which catalyzes PIP2 to produce IP3 and DAG. Increased IP3
transfers to the ER and promotes the release of stored
Ca2+, and elevated cytoplasmic Ca2+ levels
augment GSIS (24,34). In addition, DAG activates PKC and
then amplifies GSIS (34,36). The acute promotion of GSIS by FFAs
is dependent on GPR40 signaling, which is attenuated by GPR40
knockout, PLC or L-type Ca2+ channel inhibitor (24,30,37). Activated GPR40 by fasiglifam
(TAK-875) was also found to promote the activation of GPR40
substrate cascade signal transduction, thus augmenting GSIS
(38,39). Contrary to the acute effects of
FFAs, chronic FFA exposure to islets decreases GPR40 mRNA
expression and GSIS (11),
implying impairment of the GPR40 signaling pathway. To further
reveal the mechanisms, we observed the effects of PTF on GPR40
signaling induced by PA in INS-1 cells. In the present study, PA
exposure for 24 h significantly decreased the protein expression of
PLCβ1, PLCβ3 and PKC in the INS-1 cells; moreover, PA reduced
intracellular IP3 and DAG levels, and markedly inhibited
GW9508-stimulated activity of PLC and PKC. Acute exposure to
GW9508, a GPR40 agonist, increased the activity of GPR40 signaling
in INS-1 cells, indicating elevated activity of PLC and PKC, which
were inhibited by PLC and PKC inhibitors, respectively.
Interestingly, this inhibition was similar to PA. Studies have
reported that GW9508 amplifies GSIS involving PKC activation
(23), and that TAK-875 enhances
GSIS through IP3-mediated Ca2+ release and DAG/PKC
mechanisms (38), which were
consistent with the findings of the present study. The data
revealed that elevated PA caused inactivation of the GPR40 pathway
in INS-1 cells. Although PTF did not obviously change the protein
expression of PLCβ1, PLCβ3 or PKC, PTF significantly increased
intracellular IP3 and DAG levels, and potentiated GW9508-stimulated
activity of PLC and PKC induced by PA in the INS-1 cells, which
were similar to GW9508 and blocked by PLC and PKC inhibitors,
respectively. Furthermore, the preventive effect of PTF on
PA-induced impairment of GSIS was abolished by PLC and PKC
inhibitors, and calcium antagonist, respectively. Glucagon-like
peptide-1 (GLP-1), an endogenous glucose-lowering hormone, also
stimulates insulin secretion through the PLC/PKC-dependent pathway
in isolated islets (40). The
extract from Corydalis edulis Maxim., a widely grown plant
in China, was found to enhance insulin secretion by the activation
of PKC in pancreatic β cells (41). The results provide evidence for
the protective role of PTF against PA-induced impairment of GSIS
involving GPR40 signaling in INS-1 cells.
In addition to the beneficial effects of PTF on
GPR40 signaling in vitro, PTF was found to decrease TG and
LDL-c in type 2 diabetic rats (17). This study showed that PTF improved
insulin secretion function in type 2 diabetic rats. Type 2 diabetes
often coexists with dyslipidemia; it is likely that PTF reduces
plasma FFA levels in vivo, thus decreasing the negative
influence of FFAs on pancreatic β cells and improving insulin
secretion. Furthermore, chronic elevated FFAs accumulate in
pancreatic β cells, thereby leading to pancreatic lipotoxicity.
Chronic exposure to high FFAs triggers oxidative stress (42,43), inflammatory response (6,44),
and apoptosis (45,46), and inhibits preproinsulin gene
expression (10), thus impairing
pancreatic β cell function. Lou et al reported that PTF
inhibits IL-6 mRNA expression and IL-6 secretion via the NF-κB
pathway in PA-induced C2C12 skeletal muscle cells (47). We propose that PTF may ameliorate
pancreatic lipotoxicity through inhibition of lipid accumulation
and inflammation, and subsequently protects insulin secretion of
pancreatic β cells, which warrants further research.
We previously reported that PTF contains
typhaneoside and other components (16). Moreover, Pollen Typhae
extract was further determined to contain several flavonoids
including typhaneoside, isorhamnetin, naringenin, kaempferol,
quercetin and isorhamnetin-3-O-neohesperidoside (12,13,48). Studies indicate that naringenin
and quercetin enhance GSIS in INS-1E cells (49), while kaempferol improves the
impairment of GSIS induced by palmitate in INS-1E cells and human
islets (50), which are in line
with the findings of the present study. Further studies revealed
that naringenin and quercetin promote the gene expression of
glucose transporter-2 (GLUT-2), glucokinase (GCK), INS-1, and
duodenal homeobox-1 (PDX-1), which involve an increase in insulin
secretion, and that naringenin enhances the Bcl-2 mRNA level and
reduces the caspase-3 mRNA level, whereas quercetin promotes the
Bcl-2 mRNA level, thereby inhibiting β cell apoptosis (49). Additionally, naringenin inhibits
the activity of dipeptidyl peptidase IV (DPP-IV) (51), which plays a role in insulin
secretion. Quercetin increased Ca2+ influx by activating
L-type Ca2+ channels, thus inducing insulin secretion,
which were nearly abolished by the Ca2+ channel
antagonist nifedipine (52).
Similarly, the improvement in PA-induced impairment of GSIS by PTF
was also blocked by nifedipine in this study. Moreover, kaempferol
was found to protect INS-1E cells and human islets against
palmitate-induced apoptosis and improve palmitate-induced
dysfunction via upregulation of the PDX-1/cAMP/PKA/CREB signaling
cascade (50). It was confirmed
that increased FFAs induce oxidative stress to impair GSIS in
pancreatic islets (42,43), and typhaneoside and
isorhamnetin-3-O-neohesperidoside have antioxidant capacity against
lipopolysaccharide in human umbilical vein endothelial cells
(12). These two compounds may
mediate insulin secretion in pancreatic β cells. Therefore, it is
necessary to ascertain whether other flavonoids including
typhaneoside and isorhamnetin-3-O-neohesperidoside can regulate
insulin secretion and to reveal the underlying mechanisms.
In conclusion, the present study demonstrated that
PTF exerts a protective role against PA-induced impairment of GSIS
involving GPR40 signaling in INS-1 cells. The study provides
evidence for the treatment of type 2 diabetes using Pollen
Typhae in clinical practice.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81202677) and the Guangxi
Key Laboratory of Chinese Medicine Foundation Research (no.
15-140-32-05), Guangxi University of Chinese Medicine.
References
|
1
|
Chan JC, Malik V, Jia W, Kadowaki T,
Yajnik CS, Yoon KH and Hu FB: Diabetes in Asia: Epidemiology, risk
factors, and pathophysiology. JAMA. 301:2129–2140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang H, Tantiwong P, Sriwijitkamol A,
Shanmugasundaram K, Mohan S, Espinoza S, Defronzo RA, Dubé JJ and
Musi N: Effect of a sustained reduction in plasma free fatty acid
concentration on insulin signalling and inflammation in skeletal
muscle from human subjects. J Physiol. 591:2897–2909. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daniele G, Eldor R, Merovci A, Clarke GD,
Xiong J, Tripathy D, Taranova A, Abdul-Ghani M and DeFronzo RA:
Chronic reduction of plasma free fatty acid improves mitochondrial
function and whole-body insulin sensitivity in obese and type 2
diabetic individuals. Diabetes. 63:2812–2820. 2014. View Article : Google Scholar :
|
|
4
|
Belfort R, Mandarino L, Kashyap S, Wirfel
K, Pratipanawatr T, Berria R, Defronzo RA and Cusi K: Dose-response
effect of elevated plasma free fatty acid on insulin signaling.
Diabetes. 54:1640–1648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ayvaz G, Balos Törüner F, Karakoç A,
Yetkin I, Cakir N and Arslan M: Acute and chronic effects of
different concentrations of free fatty acids on the insulin
secreting function of islets. Diabetes Metab. 28:3S7–3S112.
2002.
|
|
6
|
Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi
M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, et al:
Saturated fatty acid and TLR signaling link β cell dysfunction and
islet inflammation. Cell Metab. 15:518–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kharroubi I, Ladrière L, Cardozo AK,
Dogusan Z, Cnop M and Eizirik DL: Free fatty acids and cytokines
induce pancreatic beta-cell apoptosis by different mechanisms: Role
of nuclear factor-kappaB and endoplasmic reticulum stress.
Endocrinology. 145:5087–5096. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Y, Tong Y, Gong M, Lu Y, Wang C, Zhou
M, Yang Q, Mao T and Tong N: Activation of PPARβ/δ protects
pancreatic β cells from palmitate-induced apoptosis by upregulating
the expression of GLP-1 receptor. Cell Signal. 26:268–278. 2014.
View Article : Google Scholar
|
|
9
|
Sun Y, Ren M, Gao GQ, Gong B, Xin W, Guo
H, Zhang XJ, Gao L and Zhao JJ: Chronic palmitate exposure inhibits
AMPKalpha and decreases glucose-stimulated insulin secretion from
beta-cells: Modulation by fenofibrate. Acta Pharmacol Sin.
29:443–450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ritz-Laser B, Meda P, Constant I, Klages
N, Charollais A, Morales A, Magnan C, Ktorza A and Philippe J:
Glucose-induced preproinsulin gene expression is inhibited by the
free fatty acid palmitate. Endocrinology. 140:4005–4014. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Del Guerra S, Bugliani M, D'Aleo V, Del
Prato S, Boggi U, Mosca F, Filipponi F and Lupi R:
G-protein-coupled receptor 40 (GPR40) expression and its regulation
in human pancreatic islets: The role of type 2 diabetes and fatty
acids. Nutr Metab Cardiovasc Dis. 20:22–25. 2010. View Article : Google Scholar
|
|
12
|
Chen P, Cao Y, Bao B, Zhang L and Ding A:
Antioxidant capacity of Typha angustifolia extracts and two active
flavonoids. Pharm Biol. 55:1283–1288. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao S, Ni B, Feng L, Yin X, Dou H, Fu J,
Lin L and Ni J: Simultaneous determination of typhaneoside and
isorhamnetin-3-O-neohesperidoside in rats after oral administration
of Pollen Typhae extract by UPLC-MS/MS. J Chromatogr Sci.
53:866–871. 2015. View Article : Google Scholar
|
|
14
|
Ohkura N, Tamura K, Tanaka A, Matsuda J
and Atsumi G: Experimental study on the hemostatc activity of
Pollen Typhae: A traditional folk medicine used by external and
oral application. Blood Coagul Fibrinolysis. 22:631–636. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Zhang CY, Xu DM, Huang GQ, Xu YL,
Wang ZY, Fang SD, Chen Y and Gu YL: The antiatherogenic effects of
components isolated from pollen typhae. Thromb Res. 57:957–966.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng XT, Wang TZ, Chen Y, Liu JB, Liu Y
and Wang WJ: Pollen Typhae total flavone improves insulin-induced
glucose uptake through the β-arrestin-2-mediated signaling in C2C12
myotubes. Int J Mol Med. 30:914–922. 2012.PubMed/NCBI
|
|
17
|
Feng XT, Chen Q, Xie Z, Liang X, Jiang ZH,
Zhao W and Leng J: Pollen Typhae total flavone improves insulin
resistance in high-fat diet and low-dose streptozotocin-induced
type 2 diabetic rats. Biosci Biotechnol Biochem. 78:1738–1742.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng XT, Wang TZ, Leng J, Chen Y, Liu JB,
Liu Y and Wang WJ: Palmitate contributes to insulin resistance
through down-regulation of the Src-mediated phosphorylation of Akt
in C2C12 myotubes. Biosci Biotechnol Biochem. 76:1356–1361. 2012.
View Article : Google Scholar
|
|
19
|
Feng XT, Zhai LN, Wang CL, Zhao W, Chen Q
and Huang XQ: Effects of Pollen Typhae total flavone on
β-arrestin-2/Src/Akt signaling in adipose tissues of type 2
diabetic rats. Afr J Tradit Complement Altern Med. 12:74–78. 2015.
View Article : Google Scholar
|
|
20
|
Borg J, Klint C, Wierup N, Ström K,
Larsson S, Sundler F, Lupi R, Marchetti P, Xu G, Kimmel A, et al:
Perilipin is present in islets of Langerhans and protects against
lipotoxicity when overexpressed in the beta-cell line INS-1.
Endocrinology. 150:3049–3057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bas AL, Demirci S, Yazihan N, Uney K and
Ermis Kaya E: Nerium oleander distillate improves fat and glucose
metabolism in high-fat diet-fed streptozotocin-induced diabetic
rats. Int J Endocrinol. 2012:9471872012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yaluri N, Modi S, López Rodríguez M,
Stančáková A, Kuusisto J, Kokkola T and Laakso M: Simvastatin
impairs insulin secretion by multiple mechanisms in MIN6 cells.
PLoS One. 10:e01429022015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Graciano MF, Valle MM, Curi R and
Carpinelli AR: Evidence for the involvement of GPR40 and NADPH
oxidase in palmitic acid-induced superoxide production and insulin
secretion. Islets. 5:139–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujiwara K, Maekawa F and Yada T: Oleic
acid interacts with GPR40 to induce Ca2+ signaling in
rat islet beta-cells: Mediation by PLC and L-type Ca2+
channel and link to insulin release. Am J Physiol Endocrinol Metab.
289:E670–E677. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barlow J, Jensen VH, Jastroch M and
Affourtit C: Palmitate-induced impairment of glucose-stimulated
insulin secretion precedes mitochondrial dysfunction in mouse
pancreatic islets. Biochem J. 473:487–496. 2016. View Article : Google Scholar
|
|
26
|
Zheng S, Zhou H, Han T, Li Y, Zhang Y, Liu
W and Hu Y: Clinical characteristics and beta cell function in
Chinese patients with newly diagnosed type 2 diabetes mellitus with
different levels of serum triglyceride. BMC Endocr Disord.
15:212015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kristinsson H, Smith DM, Bergsten P and
Sargsyan E: FFAR1 is involved in both the acute and chronic effects
of palmitate on insulin secretion. Endocrinology. 154:4078–4088.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yashiro H, Tsujihata Y, Takeuchi K, Hazama
M, Johnson PR and Rorsman P: The effects of TAK-875, a selective G
protein-coupled receptor 40/free fatty acid 1 agonist, on insulin
and glucagon secretion in isolated rat and human islets. J
Pharmacol Exp Ther. 340:483–489. 2012. View Article : Google Scholar
|
|
29
|
Meidute Abaraviciene S, Muhammed SJ,
Amisten S, Lundquist I and Salehi A: GPR40 protein levels are
crucial to the regulation of stimulated hormone secretion in
pancreatic islets. Lessons from spontaneous obesity-prone and
non-obese type 2 diabetes in rats. Mol Cell Endocrinol.
381:150–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alquier T, Peyot ML, Latour MG, Kebede M,
Sorensen CM, Gesta S, Ronald Kahn C, Smith RD, Jetton TL, Metz TO,
et al: Deletion of GPR40 impairs glucose-induced insulin secretion
in vivo in mice without affecting intracellular fuel metabolism in
islets. Diabetes. 58:2607–2615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferdaoussi M, Bergeron V, Zarrouki B,
Kolic J, Cantley J, Fielitz J, Olson EN, Prentki M, Biden T,
MacDonald PE, et al: G protein-coupled receptor (GPR)40-dependent
potentiation of insulin secretion in mouse islets is mediated by
protein kinase D1. Diabetologia. 55:2682–2692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nagasumi K, Esaki R, Iwachidow K, Yasuhara
Y, Ogi K, Tanaka H, Nakata M, Yano T, Shimakawa K, Taketomi S, et
al: Overexpression of GPR40 in pancreatic β-cells augments
glucose-stimulated insulin secretion and improves glucose tolerance
in normal and diabetic mice. Diabetes. 58:1067–1076. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen C, Li H and Long YQ: GPR40 agonists
for the treatment of type 2 diabetes mellitus: The biological
characteristics and the chemical space. Bioorg Med Chem Lett.
26:5603–5612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng XT, Leng J, Xie Z, Li SL, Zhao W and
Tang QL: GPR40: A therapeutic target for mediating insulin
secretion (Review). Int J Mol Med. 30:1261–1266. 2012.PubMed/NCBI
|
|
35
|
Kim HS, Hwang YC, Koo SH, Park KS, Lee MS,
Kim KW and Lee MK: PPAR-γ activation increases insulin secretion
through the up-regulation of the free fatty acid receptor GPR40 in
pancreatic β-cells. PLoS One. 8:e501282013. View Article : Google Scholar
|
|
36
|
Straub SG and Sharp GW: Massive
augmentation of stimulated insulin secretion induced by fatty
acid-free BSA in rat pancreatic islets. Diabetes. 53:3152–3158.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schnell S, Schaefer M and Schöfl C: Free
fatty acids increase cytosolic free calcium and stimulate insulin
secretion from beta-cells through activation of GPR40. Mol Cell
Endocrinol. 263:173–180. 2007. View Article : Google Scholar
|
|
38
|
Sakuma K, Yabuki C, Maruyama M, Abiru A,
Komatsu H, Negoro N, Tsujihata Y, Takeuchi K, Habata Y and Mori M:
Fasiglifam (TAK-875) has dual potentiating mechanisms via
Gαq-GPR40/FFAR1 signaling branches on glucose-dependent insulin
secretion. Pharmacol Res Perspect. 4:e002372016. View Article : Google Scholar
|
|
39
|
Yamada H, Yoshida M, Ito K, Dezaki K, Yada
T, Ishikawa SE and Kakei M: Potentiation of glucose-stimulated
insulin secretion by the GPR40-PLC-TRPC pathway in pancreatic
β-cells. Sci Rep. 6:259122016. View Article : Google Scholar
|
|
40
|
Shigeto M, Cha CY, Rorsman P and Kaku K: A
role of PLC/PKC-dependent pathway in GLP-1-stimulated insulin
secretion. J Mol Med (Berl). 95:361–368. 2017. View Article : Google Scholar
|
|
41
|
Zheng J, Zhao Y, Lun Q, Song Y, Shi S, Gu
X, Pan B, Qu C, Li J and Tu P: Corydalis edulis Maxim. promotes
insulin secretion via the activation of protein kinase Cs (PKCs) in
mice and pancreatic β cells. Sci Rep. 7:404542017. View Article : Google Scholar
|
|
42
|
Zhang X, Bao Y, Ke L and Yu Y: Elevated
circulating free fatty acids levels causing pancreatic islet cell
dysfunction through oxidative stress. J Endocrinol Invest.
33:388–394. 2010. View Article : Google Scholar
|
|
43
|
Piro S, Rabuazzo AM, Renis M and Purrello
F: Effects of metformin on oxidative stress, adenine nucleotides
balance, and glucose-induced insulin release impaired by chronic
free fatty acids exposure in rat pancreatic islets. J Endocrinol
Invest. 35:504–510. 2012.
|
|
44
|
Yin J, Peng Y, Wu J, Wang Y and Yao L:
Toll-like receptor 2/4 links to free fatty acid-induced
inflammation and β-cell dysfunction. J Leukoc Biol. 95:47–52. 2014.
View Article : Google Scholar
|
|
45
|
Xiong X, Sun X, Wang Q, Qian X, Zhang Y,
Pan X and Dong XC: SIRT6 protects against palmitate-induced
pancreatic β-cell dysfunction and apoptosis. J Endocrinol.
231:159–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Litwak SA, Wali JA, Pappas EG, Saadi H,
Stanley WJ, Varanasi LC, Kay TW, Thomas HE and Gurzov EN: Lipotoxic
stress induces pancreatic β-cell apoptosis through modulation of
Bcl-2 proteins by the ubiquitin-proteasome system. J Diabetes Res.
2015:2806152015. View Article : Google Scholar
|
|
47
|
Lou SY, Liu Y, Chen WH, Ying J, He YM and
Wang WJ: Pollen Typhae total flavones inhibit expression of
interleukin-6 in C2C12 skeletal muscle cells cultured with
palmitate. Zhong Xi Yi Jie He Xue Bao. 6:488–492. 2008.In Chinese.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu XA, Azietaku JT, Li J, Cao J, An M, He
J, Gao XM and Chang YX: Simultaneous determination of eight
flavonoids in plasma using LC-MS/MS and application to a
pharmacokinetic study after oral administration of Pollen Typhae
extract to rats. J Chromatogr B Analyt Technol Biomed Life Sci.
1044–1045:158–165. 2017. View Article : Google Scholar
|
|
49
|
Bhattacharya S, Oksbjerg N, Young JF and
Jeppesen PB: Caffeic acid, naringenin and quercetin enhance
glucose-stimulated insulin secretion and glucose sensitivity in
INS-1E cells. Diabetes Obes Metab. 16:602–612. 2014. View Article : Google Scholar
|
|
50
|
Zhang Y, Zhen W, Maechler P and Liu D:
Small molecule kaempferol modulates PDX-1 protein expression and
subsequently promotes pancreatic β-cell survival and function via
CREB. J Nutr Biochem. 24:638–646. 2013. View Article : Google Scholar
|
|
51
|
Bower AM, Real Hernandez LM, Berhow MA and
de Mejia EG: Bioactive compounds from culinary herbs inhibit a
molecular target for type 2 diabetes management, dipeptidyl
peptidase IV. J Agric Food Chem. 62:6147–6158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bardy G, Virsolvy A, Quignard JF, Ravier
MA, Bertrand G, Dalle S, Cros G, Magous R, Richard S and Oiry C:
Quercetin induces insulin secretion by direct activation of L-type
calcium channels in pancreatic beta cells. Br J Pharmacol.
169:1102–1113. 2013. View Article : Google Scholar : PubMed/NCBI
|