Introduction
Soft tissue defects are common injuries, which can
be caused by oncologic resections, complex traumas and congenital
abnormalities (1,2). Deficiencies in soft tissue are great
challenges in medicine (3). In
reconstructive surgery, adipose tissue engraftment offers a
promising alternative with which to fill irregular defects and
stimulates natural soft tissue regeneration. The first visible
application of adipose tissue engraftment was attempted by Neuber
in 1893 (4). The emergence of
liposuction (5), methods for
extracting autologous fat and graft procedures have promoted the
application of the technique in plastic surgery (6). Furthermore, adipose tissue is easily
harvested, and is available in abundance with less inflammation or
allergic reactions. Nevertheless, the unpredictable absorption
rates ranging, from 22% to 90% greatly influence the engraftment of
adipose tissue (7–9). Progress has been made in soft tissue
engraftment. Adipose tissue is grafted with epidermal growth factor
(EGF) (10,11), platelet rich plasma (12,13), fibrin glue (14,15), vascular endothelial growth factor
(VEGF) (16,17) and botulinum toxin A (BoNTA)
(18).
BoNTA is a strong neurotoxin produced by the
anaerobic bacterium, Clostridium botulinum (19). Chemodenervation is induced by its
effects on presynaptic neurons, and it results in the functional
denervation of muscle for 6 months by inhibiting acetylcholine
release. Consequently, the use of BoNTA in cosmetics for the
effacement of dynamic wrinkles is wide-spread (20). Aside from the removal of wrinkles,
the use of BoNTA continues to increase in a number of applications
for its face-lifting effects (21), anti-photoaging effects on the
skin, scar reduction (22) and
flap survival improvement (23).
As regards adipose tissue engraftment, clinicians
have noted more optimal aesthetic results are achieved when BoNTA
is applied prior to adipose tissue engraftment (24). A previous study reported the
injection of adipose tissue grafts on the bilateral sides of the
backs of BALB/c-nu mice. The BoNTA-treated sides exhibited a higher
engraftment level than that the control sides. The conjunction of
adipose tissue grafting with BoNTA led to an improved survival
(18). However, the underlying
mechanisms have yet not been confirmed.
In this study, our aim was to examine the effects of
BoNTA on adipose tissue in vivo and in vitro. We
aimed to shed light onto the role of BoNTA in the survival rate of
adipose tissue grafts, and to elucidate the underlying cellular and
molecular mechanisms of the combination therapy.
Materials and methods
Ethics approval
Ethics approval was provided by the Medical Ethics
Committee of West China School/Hospital of Stomatology Sichuan
University, with the following reference number:
SKLODLL2013A159.
Adipose tissue engraftment
The adipose tissue of 20 Sprague-Dawley (SD) rats
(weighing, 160–180 g) was extracted from the inguinal fat layer and
then chopped for grafting. The extracted adipose tissue was
centrifuged at 800 rpm for 5 min. The mixture in the tube contained
lipid and cell debris in the superior part, adipose tissue in the
middle layer and fluid components in the low layer. Following the
removal of the upper and lower parts, the adipose tissue was
harvested. Adipose tissue was transplanted into the backs of
another 6 SD rats. On the left sides of the SD rat back regions,
the adipose tissue was transplanted alone as the control group, and
on the right sides of the back regions, a mixture of adipose tissue
(2 ml) and BoNTA (0.2 U) (Hengli Company, Lanzhou, China) was
transplanted as the experimental group.
After 5 weeks, the SD rats were sacrificed and the
adipose tissues were dissected and photographed using a digital
camera (EOS 550D; Canon Inc., Tokyo, Japan). The weight (g) of the
adipose tissue was valued using an electronic scale. Graft volume
(ml) was valued by the liquid overflow method based on the
Archimedes principle of buoyancy. The weight and volume were
assessed by a researcher without knowing the group assignment.
Hematoxylin and eosin (H&E) staining,
immunofluorescence staining, Oil Red O staining and terminal
deoxynucleotidyl-transferase-mediated dUTP nick-end labeling
(TUNEL) staining
Adipose tissue blocks were cut into tissue slices of
9 µm thickness using a cryotome (CM3050; Leica, Wetzlar,
Germany). The frozen tissue slices were stained with H&E dye
and subjected to immunofluorescence staining using the following
primary antibodies: anti-CD31/PECAM-1 (Cat. no. bs-0195R; Bioss,
Woburn, MA, USA) and anti-VEGF (Cat. no. ab46154; Abcam, Cambridge,
UK) antibodies. The slices were also subjected to Oil Red O
staining (Sigma-Aldrich, St. Louis, MO, USA) and TUNEL staining
(KeyGen Biotech, Nanjing, China). The integrity of cellularity was
assessed in accordance with morphology. The degree of integrity was
graded according to the proportion of adipocytes with a morphology
which was similar to that of normal adipocytes on a scale of 1–5 as
follows: 1 (<5%), 2 (5–25%), 3 (25–50%), 4 (50–75%) and 5
(>75%), as previously described (25).
Isolation and culture of rat
adipose-derived stem cells (ASCs)
ASCs were isolated from the inguinal fat layer of
other SD rats (weighing, 160–180 g). The tissue was carefully cut
and washed with PBS and kept at room temperature for 5 min. The
tissue on the top layer was collected and transferred into a new
tube. Subsequently, 0.2% collagenase was added into the tube, and
the tissue was transferred into a 37°C digital heating circulating
water bath for 40 min. The tissue was then centrifuged at 1,200 rpm
for 5 min. In the tube, the supernatant layer was discarded, and
the remaining amount was resuspended in PBS. After the supernatant
layer was centrifuged at 1,200 rpm for 5 min and discarded, the
subside layer was finally incubated at 37°C in a 5 CO2
incubator. Cells at passage 3 were used in the in vitro
experiments.
Flow cytometric analysis of ASCs
Cells at passage 0 and 1 were immunolabeled at 4°C
for 3 min with the following antibodies: CD29, CD45, CD31, CD34,
CD146 and CD90. We used a BD AccuriTM C6 flow cytometer (BD
Biosciences, San Jose, CA, USA) to perform the analyses.
Cell Counting kit-8 (CCK-8) assay
We used the CCK-8 (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) to evaluate the effects of BoNTA on ASC
proliferation. The ASCs were plated in 96-well culture plates.
After the ASCs were incubated at 37°C for 1 day, BoNTA was added at
final concentrations of 0×10−2, 1×10−2,
2×10−2, 3×10−2, 4×10−2,
5×10−2, 6×10−2, 7×10−2,
8×10−2, and 1.5×10−1, 2×10−1,
3×10−1, 4×10−1 and 5×10−1 U/ml
with culture medium in the experimental groups. The control wells
were treated with culture medium alone. The culture medium was
changed every other day.
On days 1, 2, 3, 4 and 5, the CCK-8 dye solution (90
µl of α-MEM with 10% FBS plus 10 µl CCK-8) was added
into each well. Wells containing culture medium without cells were
used as blanks. Following incubation at 37°C for 3 h, the samples
were taken out and the absorbance was measured at 450 nm using a
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The result was calculated according to the formula: [1-D (λ)
treated/D (λ) control] ×100%.
Adipogenic differentiation
To determine differentiation potential, the ASCs
were plated in 6-well culture plates. When cells reached 80%
confluence, the basal medium was replaced with adipogenic medium
which was replaced every other day. The adipogenic medium contained
α-MEM (HyClone, Logan, UT, USA) supplemented with 10% FBS (Biowest,
Nuaillé, France), 0.4 µg/ml dexamethasone (Tianjin
Pharmaceutical Jiaozuo Co., Tianjin, China), 5 µg/ml insulin
(Novo Nordisk, Copenhagen, Denmark), 72 µg/ml indomethacin
and 111 µg/ml IBMX (both from Sigma-Aldrich). The
experimental group was treated with BoNTA at a final concentration
of 8×10−2 U/ml, but BoNTA was not added to the control
wells.
Fat droplets within adipocytes were stained by Oil
Red O. Finally the D (λ) was measured at 51 nm, and the adipogenic
differentiation rate was calculated according to the formula: [1-D
(λ) treated/D (λ) control] ×100%.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA samples to investigate the effect of BoNTA on
ASCs adipogenic differentiation were obtained on day 3 and day 6
from the induced ASCs and the results were analyzed by RT-qPCR.
Total RNA was extracted using RNAiso Plus (Takara
Bio, Inc., Kusatsu, Japan), and reverse transcriptase was used to
synthesize the cDNA according to the manufacturer's instructions.
Quantitative PCR (qPCR) was performed with an applied ABI Prism
7300 System (Applied Biosystems, Foster City, CA, USA), using SYBR
Premix Ex Taq (Perfect Real Time) (Takara Bio, Inc.). The ΔΔct
method was used to determine the relative quantification of mRNA
expression in the samples, and the fold change was determined as
2−ΔΔct. The specific primer sequences which represent
pluripotence and three-germ layer differentiation marker genes are
listed in Table I.
 | Table IPrimers for PCR. |
Table I
Primers for PCR.
| Gene | Primers |
|---|
| C/EBP α |
GCCAAGAAGTCGGTGGATAAGA |
|
GTCACTGGTCAACTCCAACACCT |
| PPARγ2 |
GCCCTTTGGTGACTTTATGGAG |
|
GCAGCAGGTTGTCTTGGATGT |
| Adiponectin |
CGTTCTCTTCACCTACGACCAGT |
|
ATTGTTGTCCCCTTCCCCATAC |
| Leptin |
GTTCCTGTGGCTTTGGTCCTAT |
|
GATACCGACTGCGTGTGTGAA |
| C/EBPδ |
CTGCCATGTATGACGACGAGAG |
|
CGCTTTGTGATTGCTGTTGAAG |
| FABP4 |
GTAGAAGGGGACTTGGTCGTCAT |
|
ACTTTCCTGTCATCTGGGGTGA |
| VEGF |
GCTGCTGCAATGATGAAGCC |
|
TGTGGTCACTTACTTTTCTGGC |
| GAPDH |
TATGACTCTACCCACGGCAAG |
|
TACTCAGCACCAGCATCACC |
Western blot analysis
Total proteins were extracted using the Total
Protein Extraction kit (KeyGen Biotech) on days 3 and 6. Lysates
were precipitated at 12,000 rpm for 15 min and the total protein
content was assessed using the BCA Protein Assay kit (KeyGen
Biotech). Proteins were separated by electrophoresis through
SDS-polyacrylamide gels and transferred onto a nitrocellulose
membrane, and the membranes were then blocked and incubated with
mouse monoclonal antibody [ACTN05 (C4)] to actin (Cat. no. ab3280),
rabbit monoclonal antibody [EP708Y] to C/EBPα (Cat. no. ab40761)
(both from Abcam), anti-peroxisome proliferator sctivated receptor
γ, isoform 1and 2 antibody (Cat. no. MAB3872; Millipore Corp.,
Billerica, MA, USA), rabbit polyclonal antibody to adiponectin
(Cat. no. ab62551; Abcam), fatty acid binding protein 4 (FABP4)
antibody (Cat. no. 220367; Zen-Bio, Inc., Durham, NC, USA) and
anti-VEGF antibody (Cat. no. ab46154; Abcam) at 4°C overnight. The
results were visualized with Immobilon Western Chemiluminescent HRP
Substrate (Millipore Corp.) after they were incubated with the
secondary antibody. The secondary antibodies were
peroxidase-conjugated goat anti-rabbit lgG (H+L) (Cat. no. ZB-2301)
and peroxidase-conjugated goat anti-mouse lgG (H+L) (Cat. no.
ZB-2305) (both from ZSGB-BIO, Beijing, China).
Statistical analysis
Data were measured and expressed as the means ±
standard deviation. Statistical analysis using GraphPad Prism 5.02
(GraphPad Software, La Jolla, CA, USA) was performed with a paired
Student's t tests. A two-sided value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Adipose tissue engraftment
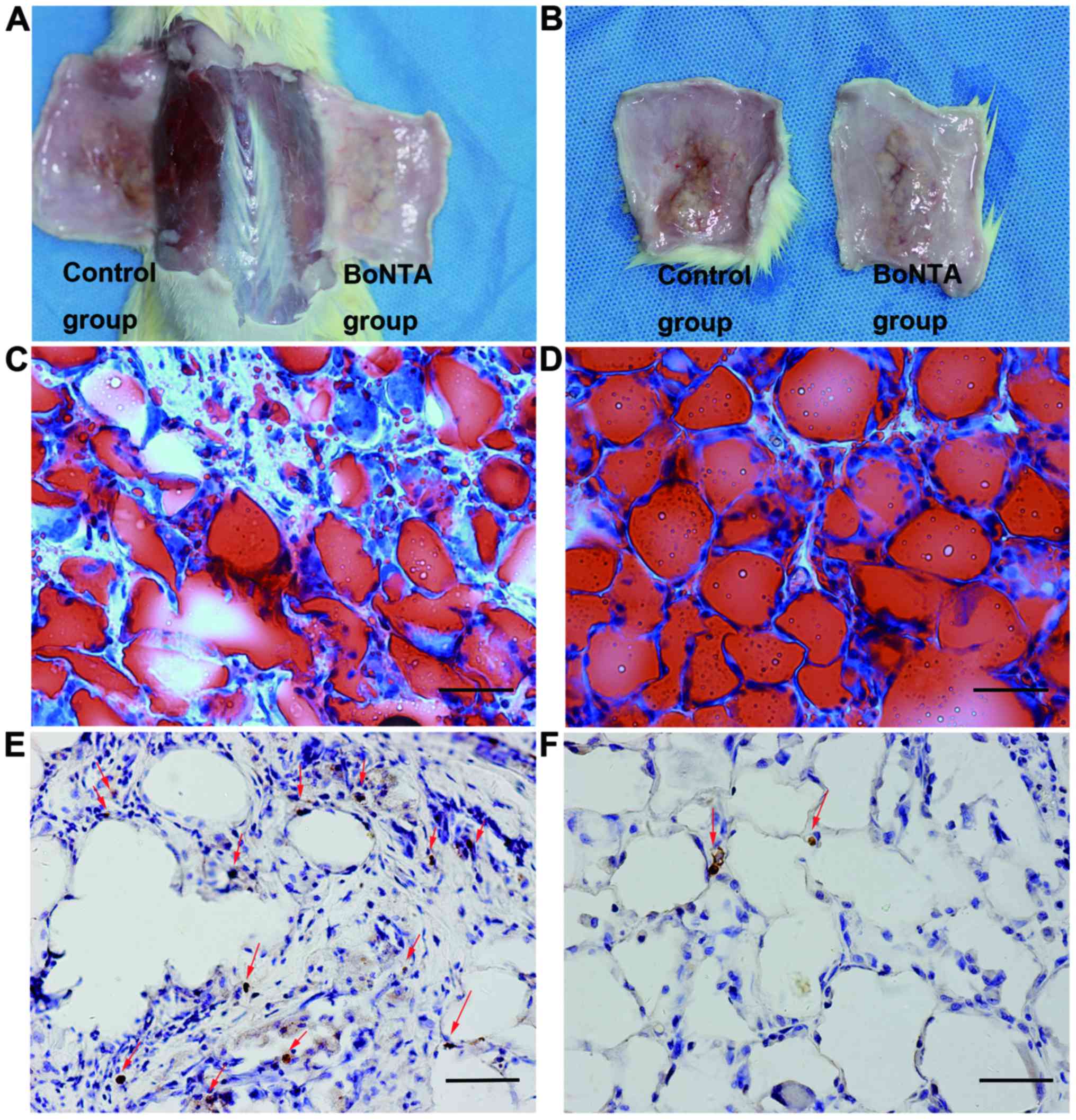
The adipose tissues were dissected and photographed
at 5 weeks after grafting (Fig. 1A
and B). The mean weight and volume of the adipose tissue grafts
were higher in the sides treated with BoNTA, with statistical
significance between the control and BoNTA sides observed in weight
(0.85±0.088 vs. 1.15±0.077, n=6, P<0.01) (Table II) and volume (0.9±0.084 vs.
1.20±0.074, n=6, P<0.01) (Table
III).
 | Table IIComparisons of weight (g) between the
two sides. |
Table II
Comparisons of weight (g) between the
two sides.
| Sprague-Dawley
rat | BoNTA side | Control side |
|---|
| 1 | 1.1007 | 0.7352 |
| 2 | 1.2021 | 0.9014 |
| 3 | 1.2435 | 0.9637 |
| 4 | 1.0352 | 0.8935 |
| 5 | 1.1368 | 0.7605 |
| 6 | 1.2032 | 0.8734 |
| Mean | 1.153583333 | 0.85461667 |
| SD | 0.077451441 | 0.08839781 |
 | Table IIIComparisons of volume (ml) between
the two sides. |
Table III
Comparisons of volume (ml) between
the two sides.
| Sprague-Dawley
rat | BoNTA side | Control side |
|---|
| 1 | 1.15 | 0.80 |
| 2 | 1.25 | 0.95 |
| 3 | 1.30 | 1.00 |
| 4 | 1.10 | 0.95 |
| 5 | 1.20 | 0.80 |
| 6 | 1.25 | 0.90 |
| Mean | 1.20 | 0.9 |
| SD | 0.073598007 | 0.083666 |
TUNEL staining confirmed that the combined technique
ameliorated apoptosis in adipose tissue. Visibly less apoptosis was
observed in the BoNTA group (Fig.
1F) than in the control group (Fig. 1E).
To further confirm the integrity of cellularity, the
results from Oil Red O staining revealed an average degree of
2.50±0.548 on the control sides (Fig.
1C) and that of 4.67±0.516 on the BoNTA sides (Fig. 1D). The data of cellular integrity
between the 2 groups reached statistical significance (P=0.000444)
(Table IV).
 | Table IVComparisons of histological cellular
integrity grades (scales) between the two sides. |
Table IV
Comparisons of histological cellular
integrity grades (scales) between the two sides.
| Sprague-Dawley
rat | BoNTA side | Control side |
|---|
| 1 | 5 | 2 |
| 2 | 4 | 3 |
| 3 | 5 | 2 |
| 4 | 5 | 3 |
| 5 | 5 | 3 |
| 6 | 4 | 2 |
| Mean | 4.666667 | 2.5 |
| SD | 0.516398 | 0.547723 |
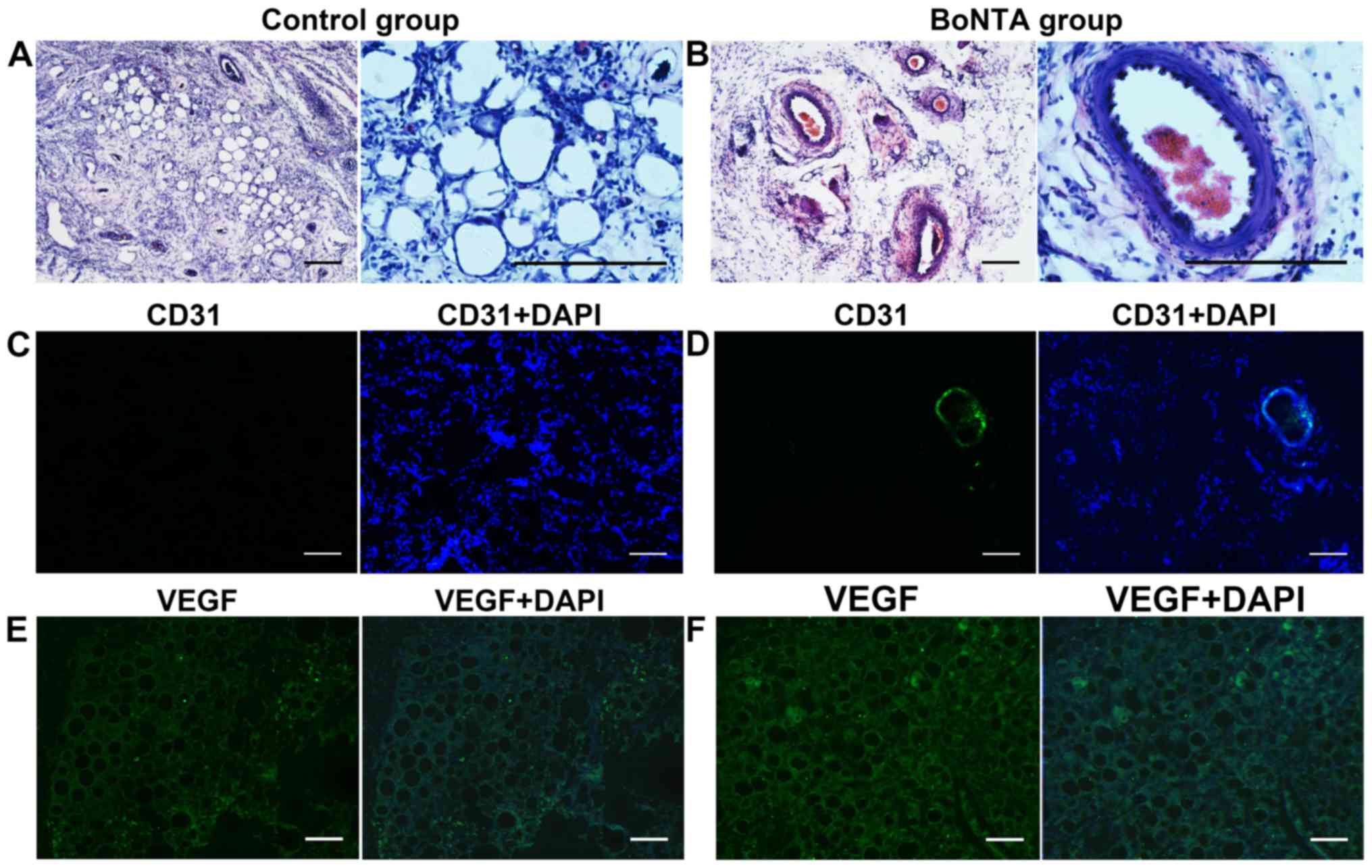
H&E staining and immunofluorescence
staining
The results of H&E staining revealed more blood
vessels in the BoNTA group in general. Fewer blood vessels were
observed in the control group morphologically (Fig. 2 A and B).
Immunofluorescence staining of the grafted adipose
tissue displayed a higher number of CD31 positively stained vessels
in the BoNTA sides compared to the control sides. In the control
sides, the expression of green fluorescent (CD31) could be rarely
observed (Fig. 2C). By contrast,
strong green fluorescent (CD31) could be easily observed in the
BoNTA sides (Fig. 2D). In the
BoNTA sides, the expression of VEGF was higher than that in the
control sides (Fig. 2E and
F).
Isolation and characterization of ASCs
from SD rats
Primary ASCs were isolated from SD rats. To verify
the properties of SD rat ASCs, cells at passage 0 (P0) and cells at
passage 1 (P1) were characterized by flow cytometric analysis. Flow
cytometric analysis of surface antigen expression in the cells at
passage 0 revealed CD29+, CD45−,
CD31−, CD146+/CD146− (weakly
positive), CD34+/CD34− (weakly positive) and
CD90+ expression (Fig.
3A), and in the cells at passage 1 it revealed
CD29+, CD45−, CD31−,
CD146−, CD34+, CD90+ expression
(Fig. 3B).
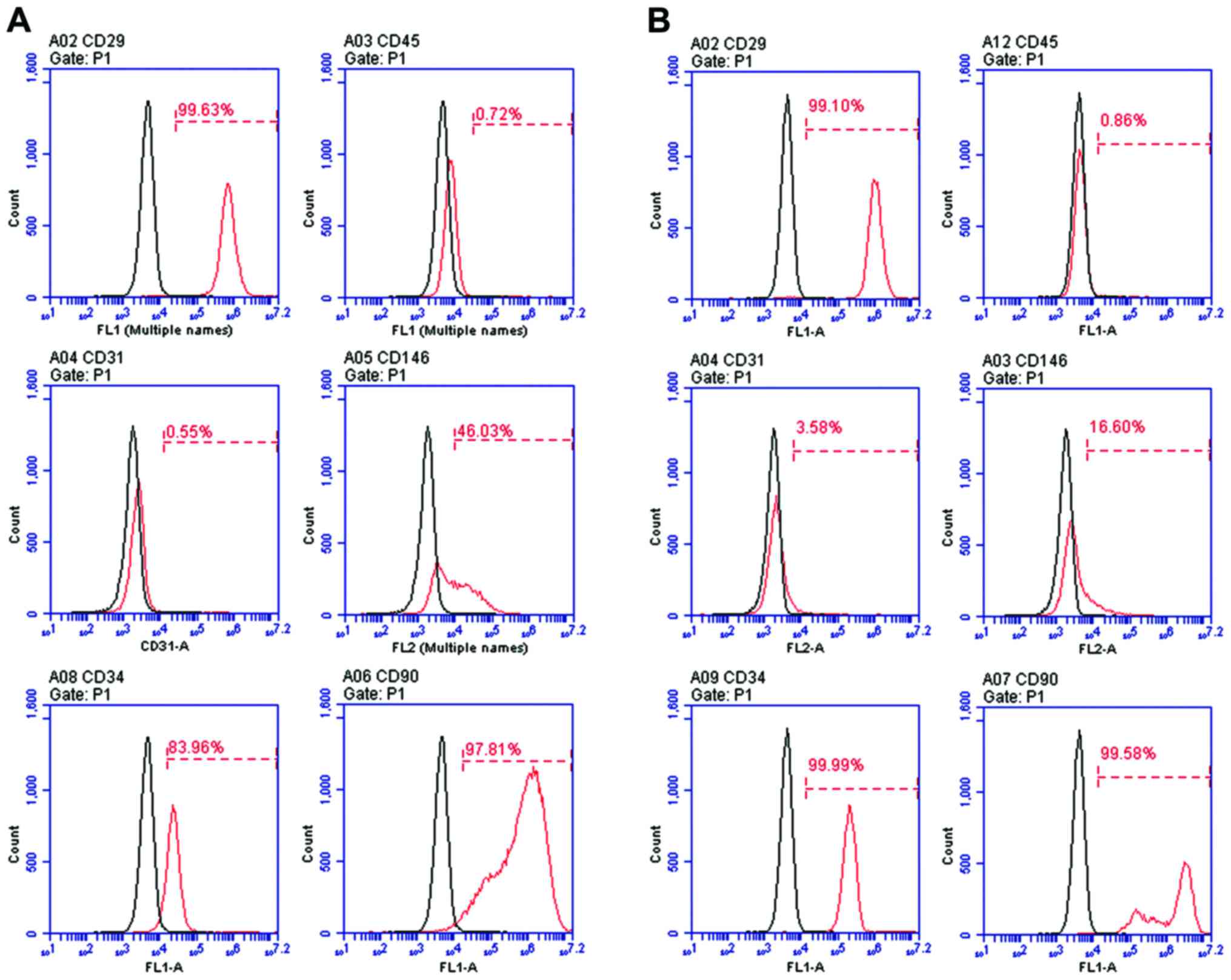 | Figure 3Flow cytometric analysis of surface
antigen expression in primary ASCs at (A) passage 0:
CD29+, CD45−, CD31−,
CD146+/CD146− (weakly positive),
CD34+/CD34− (weakly positive),
CD90+ and ASCs at passage 1: CD29+,
CD45−, CD31−, CD146−,
CD34+, CD90+ (B). The antibodies are specific
for the identification of ASCs. ASCs, adipose-derived stem
cells. |
CCK-8 assay
The CCK-8 assay allows the determination of
differences in cell proliferation; thus, we used this to determine
the effects of BoNTA on the proliferation of ASCs and to find the
optimal dose of BoNTA during treatment. BoNTA promoted cell
proliferation (P<0.01) (Fig.
4A), and the effect was dose-dependent. When the concentration
of BoNTA was >8×10−2 U/ml, cell proliferation
increased slightly (Fig. 4B). In
consideration of drug toxicity, we selected the dose of
8×10−2 U/ml as the optimal dose in the following
experiments.
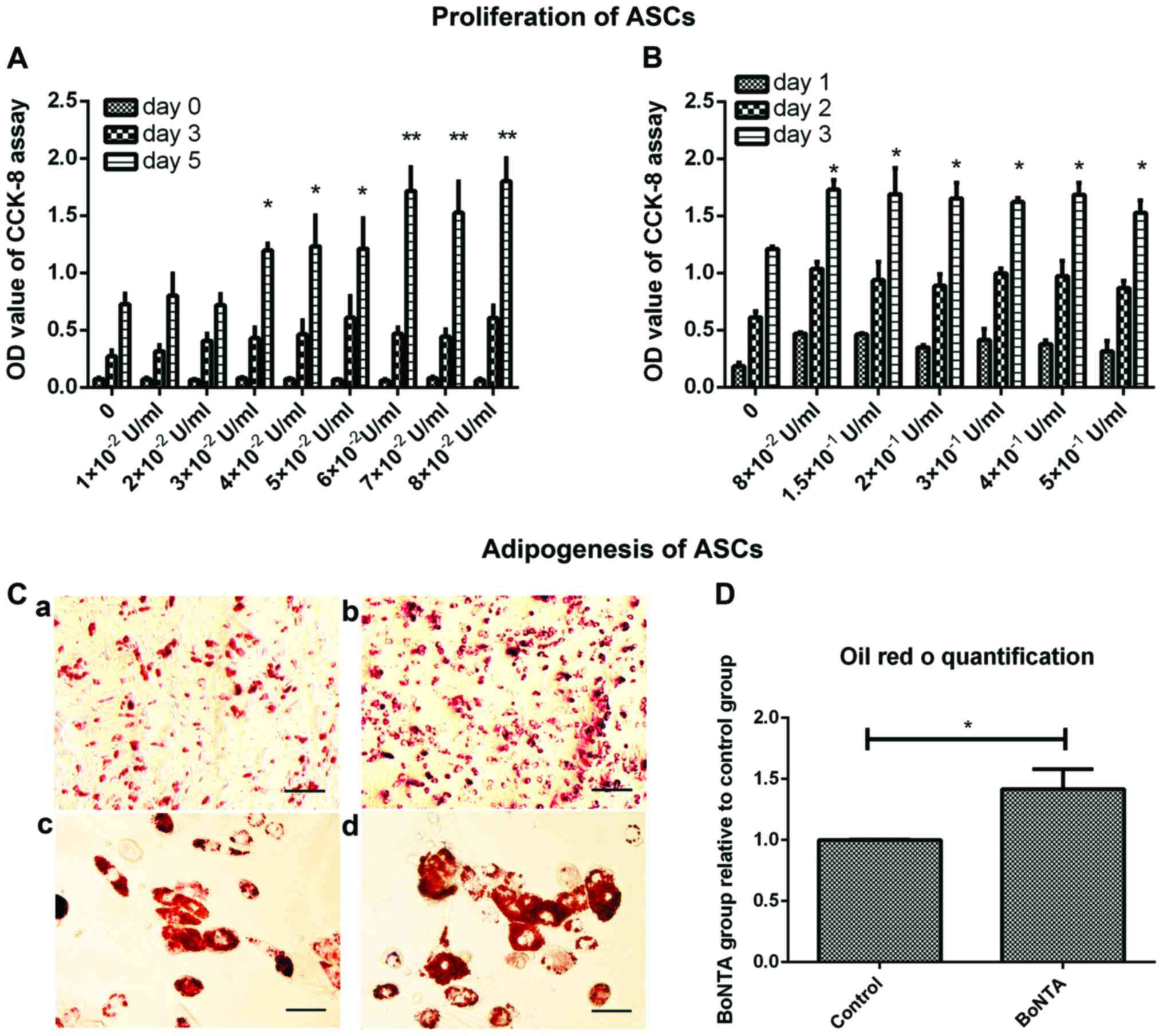 | Figure 4Proliferation and adipogenesis of
ASCs was examined. CCK-8 assay was conducted after the cells were
incubated with 1×10−2, 2×10−2,
3×10−2, 4×10−2, 5×10−2,
6×10−2, 7×10−2, 8×10−2 U/ml BoNTA
for 5 days (A); the cells were treated with 8×10−2,
1.5×10−1, 2×10−1, 3×10−1,
4×10−1, 5×10−1 U/ml BoNTA for 3 days (B). The
control group (C-a and C-c) and BoNTA group (C-b and C-d) were
stained with Oil Red O after being induced to differentiate into
adipocytes on day 8 (C-a and C-b; scale bar, 2 µm; C c and C
d; scale bar, 2 µm). Quantification of Oil Red O staining
was assessed by isopropanol leaching and absorbance at 51 nm (D).
Data are the means ± standard deviation of 3 separate experiments.
*P<0.05, **P<0.01, t-test vs. control
(no treatment). ASCs, adipose-derived stem cells; BoNTA, botulinum
toxin A. |
Adipogenic differentiation of ASCs
The level of adipogenic differentiation was
evaluated by Oil Red O staining that measured the lipid droplets
accumulated on day 8. The BoNTA group was treated with
8×10−2 U/ml BoNTA through the whole experimental
process, while the control group was not treated with BoNTA. In
general, more Oil Red O-stained cells were accumulated in the BoNTA
group than in the control group visually (Fig. 4C). The results of quantification
analysis revealed about 50% increase in the BoNTA group in Oil Red
Ostaining intensity, with statistical significance (Fig. 4D).
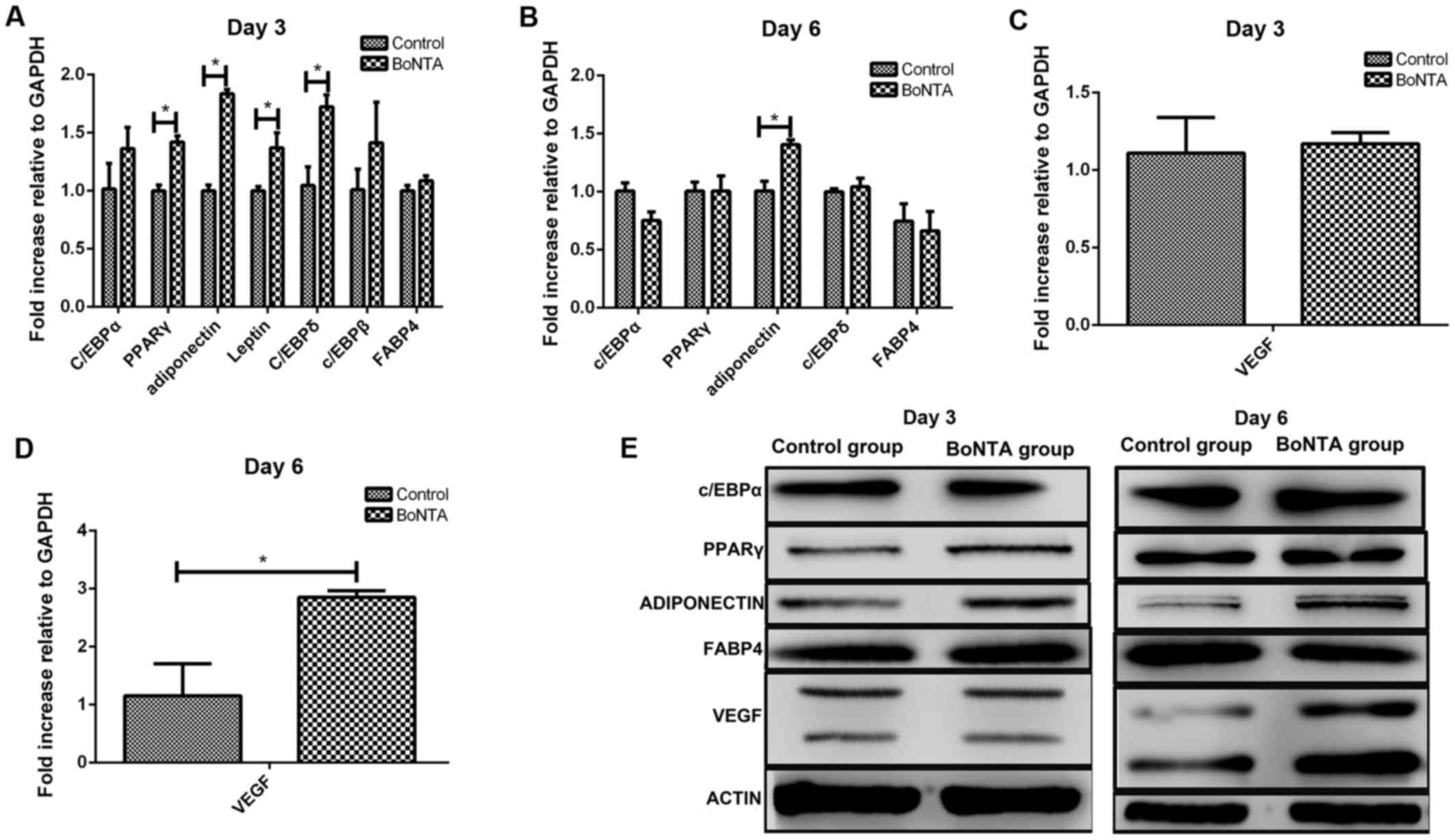
Adipogenic maker genes on days 3 and
6
The experimental group was treated with
8×10−2 U/ml BoNTA, while the control group was not
treated with BoNTA. Following adipogenic induction, the expression
levels of C/EBPα, peroxisome proliferator-activated receptor
(PPARγ), adiponectin, leptin, C/EBPδ and C/EBPβ were higher in the
BoNTA group compared with those in the control group on day 3. In
particular, the levels of PPARγ, adiponectin, leptin and C/EBPδ
reached statistical significance (Fig. 5A). On day 6, only adiponectin
expression exhibited a statistically significant increase, while
the levels of C/EBPα, PPARγ, C/EBPδ and FABP4 between the 2 groups
exhibited no statistical difference (Fig. 5B).
Following the induction of adipogenic
differentiation, the results revealed that BoNTA upregulated the
expression of VEGF on day 6, with statistical difference (Fig. 5C and D).
Western blot analysis
The protein expression levels of PPARγ, C/EBPα,
adiponectin, FABP4 and VEGF were determined by western blot
analysis on days 3 and 6 after adipogenic induction (Fig. 5E). Consistent with the results of
RT-qPCR, we found that BoNTA upregulated the expression levels of
PPARγ and adiponectin on day 3 and the expression levels of
adiponectin and VEGF on day 6 compared to those in the control
group.
Discussion
The use of adipose tissue engraftment in conjunction
with BoNTA is widespread in plastic and reconstructive surgery;
however, this combined use remains controversial. Some clinicians
state that the increased survival rate is due to the effect of
BoNTA on decreasing muscle contraction, leading to the relatively
immobile settlement of adipose tissue (18,26). However, no study to date has
reported the mechanisms underlying the effects of BoNTA on adipose
tissue grafts, particularly as regards the key factors of
successful adipose tissue engraftment.
Our results indicated that the combination of BoNTA
and adipose tissue improved graft survival. The high residual
weight, volume, less apoptosis and better cellular integrity
encouraged the possible use of BoNTA in adipose tissue engraftment.
Unlike the study by Baek et al (18) or thoughts of other clinicians, our
results demonstrated that the higher survival rate was not merely
due to the fact that BoNTA decreased the muscle contraction or led
to the relatively immobile settlement of adipose tissue. Our
results of H&E staining and immunofluorescence staining (CD31
and VEGF) displayed more induced revascularization in the BoNTA
group. In the field of adipose tissue engraftment, numerous studies
have verified the importance of revascularization in the recipient
site (27–29). The degree of induced
revascularization is essential for improving the survival of
adipose tissue grafts with the number of microvessels increasing
from day 7, decreasing slightly by day 30 and being stable
thereafter. The angiogenic cytokines, VEGF included, have been
found to promote revascularization about 7 days in parallel
(30). VEGF (31) and CD31 (platelet/endothelial cell
adhesion molecule) are crucial angiogenic factors responsible for
revascularization in the graft (32). BoNTA has been reported to improve
skin flap engraftment through revascularization (25); however, prior to our study, no
study was available on the effect of BoNTA on revascularization in
adipose tissue grafts, at least to the best of our knowledge.
The surviving adipose graft is a mixture of
adipocytes that are differentiated from ASCs and mature adipocytes
that survive. Adipocytes begin to die mostly on day 1 after
grafting (33). Recent studies
have shown that adipose tissue grafts enriched with expanded ASCs
markedly improved residual graft volume and histological appearance
in both human and animals. The ASC-enriched graft displayed higher
amounts of adipose tissue and less necrotic tissue and newly formed
connective tissue (34,35). However, some studies indicated
that after grafting, newly formed adipose tissue was more likely to
be host-derived (36). Given that
ASCs are the main components of adipose tissue (ASCs, mature
adipocytes, and fibroblast), independent of where the ASCs are
derived from (donor-derived or host-derived), our attention is
drawn to the important role that ASCs play in adipose tissue
engraftment. Thus, we further investigated the effects of BoNTA on
ASCs.
BoNTA has been proven to have no negative effect on
ASCs after adipose tissue engraftment (37). In the study by Sunaga et
al, after grafting, the proliferation of ASCs increased on day
3. ASCs proliferated in parallel with the death of adipocytes, to
restore the necrotic tissue. New adipocytes were generated at 1
week and peaked at 4 weeks in number (38). Therefore, the proliferation and
adipogenesis of ASCs are crucial factors in adipose tissue
engraftment. In this study, we found an optimal dose
(8×10−2 U/ml) to improve the proliferation of ASCs; in
addition, BoNTA promoted adipogenesis and enhanced the angiogenic
ability of the ASCs, and thus it helped to improve the survival
rate.
To the best of our knowledge, this is the first
study to demonstrate that BoNTA improves adipose tissue engraftment
through revascularization. We also assessed the effect of BoNTA on
the ASCs, particularly as regards proliferation, adipogenesis and
angiogenesis. On the whole, our study confirmed the optimal dose of
BoNTA that increased the survival of adipose tissue grafts and also
provided a reasonable explanation to support the combined use of
BoNTA and tissue engraftment as a promising therapy.
Acknowledgments
The present study was supported by Nature Science
Foundation of China (30973348) and Basic Research Program of
Sichuan Province (2012JY0077).
References
|
1
|
Locke MB and de Chalain TM: Current
practice in autologous fat transplantation: Suggested clinical
guidelines based on a review of recent literature. Ann Plast Surg.
60:98–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patrick CW Jr: Tissue engineering
strategies for adipose tissue repair. Anat Rec. 263:361–366. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gomillion CT and Burg KJ: Stem cells and
adipose tissue engineering. Biomaterials. 27:6052–6063. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ersek RA, Chang P and Salisbury MA: Lipo
layering of autologous fat: an improved technique with promising
results. Plast Reconstr Surg. 101:820–826. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Illouz YG and Illouz Y-G: Body contouring
by lipolysis: A 5-year experience with over 3000 cases. Plast
Reconstr Surg. 72:591–597. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coleman SR: Structural fat grafting.
Aesthet Surg J. 18:386–388. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Illouz YG: Present results of fat
injection. Aesthetic Plast Surg. 12:175–181. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ersek RA: Transplantation of purified
autologous fat: A 3 year follow-up is disappointing. Plast Reconstr
Surg. 87:219–228. 1991. View Article : Google Scholar
|
|
9
|
Goldwyn RM: Unproven treatment: Whose
benefit, whose responsibility? Plast Reconstr Surg. 81:946–947.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park B, Kong JS, Kang S and Kim YW: The
effect of epidermal growth factor on autogenous fat graft.
Aesthetic Plast Surg. 35:738–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saliba I, Alzahrani M, Zhu T and Chemtob
S: Growth factors expression in hyaluronic acid fat graft
myringoplasty. Laryngoscope. 124:E224–E230. 2014. View Article : Google Scholar
|
|
12
|
Serra-Mestre JM, Serra-Renom JM, Martinez
L, Almadori A and D'Andrea F: Platelet-rich plasma mixed-fat
grafting: A reasonable prosurvival strategy for fat grafts?
Aesthetic Plast Surg. 38:1041–1049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li K, Li F, Li J, Wang H, Zheng X, Long J,
Guo W and Tian W: Increased survival of human free fat grafts with
varying densities of human adipose-derived stem cells and
platelet-rich plasma. J Tissue Eng Regen Med. 11:209–219. 2017.
View Article : Google Scholar
|
|
14
|
Karaçal N, Cobanoğlu U, Ambarcioğlu O and
Kutlu N: The effect of fibrin glue on fat graft survival. J Plast
Reconstr Aesthet Surg. 60:300–303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Torio-Padron N, Baerlecken N, Momeni A,
Stark GB and Borges J: Engineering of adipose tissue by injection
of human preadipocytes in fibrin. Aesthetic Plast Surg. 31:285–293.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu FT, Li HM, Yin QS, Liu DL, Nan H, Zhao
PR and Liang SW: Human breast adipose-derived stem cells
transfected with the stromal cell-derived factor-1 receptor CXCR4
exhibit enhanced viability in human autologous free fat grafts.
Cell Physiol Biochem. 34:2091–2104. 2014. View Article : Google Scholar
|
|
17
|
Li L, Pan S, Ni B and Lin Y: Improvement
in autologous human fat transplant survival with SVF plus VEGF-PLA
nano-sustained release microspheres. Cell Biol Int. 38:962–970.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baek RM, Park SO, Jeong EC, Oh HS, Kim SW,
Minn KW and Lee SY: The effect of botulinum toxin A on fat graft
survival. Aesthetic Plast Surg. 36:680–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katona P: Botulinum toxin: Therapeutic
agent to cosmetic enhancement to lethal biothreat. Anaerobe.
18:240–243. 2012. View Article : Google Scholar
|
|
20
|
Rohrich RJ, Janis JE, Fagien S and Stuzin
JM: Botulinum toxin: Expanding role in medicine. Plast Reconstr
Surg. 112(5 Suppl): 1S–3S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kashkouli MB, Amani A, Jamshidian Tehrani
M, Yousefi S and Jazayeri AA: Eighteen-point abobotulinum toxin a
upper face rejuvenation: An eye plastic perspective on 845
subjects. Ophthal Plast Reconstr Surg. 30:219–224. 2014.PubMed/NCBI
|
|
22
|
Xiao Z, Zhang M, Liu Y and Ren L:
Botulinum toxin type a inhibits connective tissue growth factor
expression in fibroblasts derived from hypertrophic scar. Aesthetic
Plast Surg. 35:802–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chenwang D, Shiwei B, Dashan Y, Qiang L,
Bin C, Muxin Z, Pengcheng L and Senkai L: Application of botulinum
toxin type A in myocutaneous flap expansion. Plast Reconstr Surg.
124:1450–1457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wise JB and Greco T: Injectable treatments
for the aging face. Facial Plast Surg. 22:140–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim TK, Oh EJ, Chung JY, Park JW, Cho BC
and Chung HY: The effects of botulinum toxin A on the survival of a
random cutaneous flap. J Plast Reconstr Aesthet Surg. 62:906–913.
2009. View Article : Google Scholar
|
|
26
|
Centeno RF: Combination volume
rejuvenation therapy of the face: fat, fillers, and Botox. Aesthet
Surg J. 26:460–464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karacaoglu E, Kizilkaya E, Cermik H and
Zienowicz R: The role of recipient sites in fat-graft survival:
Experimental study. Ann Plast Surg. 55:63–68; discussion 68. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sezgin B, Ozmen S, Bulam H, Omeroglu S,
Yuksel S, Cayci B and Peker T: Improving fat graft survival through
preconditioning of the recipient site with microneedling. J Plast
Reconstr Aesthet Surg. 67:712–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Serra-Renom JM and Fontdevila J: Treatment
of facial fat atrophy related to treatment with protease inhibitors
by autologous fat injection in patients with human immunodeficiency
virus infection. Plast Reconstr Surg. 114:551–555; discussion
556–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Atik B, Oztürk G, Erdoğan E and Tan O:
Comparison of techniques for long-term storage of fat grafts: An
experimental study. Plast Reconstr Surg. 118:1533–1537. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hausman GJ and Richardson RL: Adipose
tissue angiogenesis. J Anim Sci. 82:925–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Basilio-de-Oliveira RP and Pannain VL:
Prognostic angiogenic markers (endoglin, VEGF, CD31) and tumor cell
proliferation (Ki67) for gastrointestinal stromal tumors. World J
Gastroenterol. 21:6924–6930. 2015.PubMed/NCBI
|
|
33
|
Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno
S and Yoshimura K: The fate of adipocytes after nonvascularized fat
grafting: Evidence of early death and replacement of adipocytes.
Plast Reconstr Surg. 129:1081–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu F, Li J, Gao J, Ogawa R, Ou C, Yang B
and Fu B: Improvement of the survival of human autologous fat
transplantation by using VEGF-transfected adipose-derived stem
cells. Plast Reconstr Surg. 124:1437–1446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kølle SF, Fischer-Nielsen A, Mathiasen AB,
Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M,
Rasmussen BS, Talman ML, et al: Enrichment of autologous fat grafts
with ex-vivo expanded adipose tissue-derived stem cells for graft
survival: A randomised placebo-controlled trial. Lancet.
382:1113–1120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Doi K, Ogata F, Eto H, Kato H, Kuno S,
Kinoshita K, Kanayama K, Feng J, Manabe I and Yoshimura K:
Differential contributions of graft-derived and host-derived cells
in tissue regeneration/remodeling after fat grafting. Plast
Reconstr Surg. 135:1607–1617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gugerell A, Kober J, Schmid M, Nickl S,
Kamolz LP and Keck M: Botulinum toxin A and lidocaine have an
impact on adipose derived stem cells, fibroblasts, and mature
adipocytes in vitro. J Plast Reconstr Aesthet Surg. 67:1276–1281.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sunaga A, Sugawara Y, Katsuragi-Tomioka Y
and Kobayashi E: The fate of nonvascularized fat grafts:
Histological and bioluminescent study. Plast Reconstr Surg Glob
Open. 1:e402013. View Article : Google Scholar
|