Introduction
Inflammatory bowel disease (IBD), including Crohn's
disease and ulcerative colitis, is characterized by chronic
intestinal inflammation resulting from a complex interaction of
genetically susceptible hosts, disruption of mucosal barriers, an
abnormal immune response to environmental factors, and disturbance
of the intestinal flora (1). The
pathogenesis of IBD remains unclear, although studies in IBD animal
models and IBD patients have identified a common pathologic
outcome: structural and functional impairment of the intestinal
mucosal barrier (2-4). The changes in intestinal epithelial
mucosal barrier function are important in the occurrence,
development and prognosis of IBD. Dysfunction of intestinal
epithelial tight junctions leads to immune activation and promotes
the occurrence of colitis (5).
Clinically, the degree of mucosal repair has become the standard
for evaluating the therapeutic effect of IBD (2).
The key phenomenon in the early repair of epithelial
cells following injury is cell migration, whereby normal epithelial
cells surrounding the damaged area migrate to the injured area, and
subsequently reconstruct and maintain epithelial integrity, which
is referred to as the rapid repair pathway (6). Slow repair predominantly refers to
the process of cell proliferation. Cell proliferation primarily
repairs the damaged mucosa by increasing the number of mitotic
cells (7). Trefoil factor 3
(TFF3) is a small molecule polypeptide that is secreted by
intestinal goblet cells and significantly contributes to the
protection of intestinal mucosal integrity, the reconstruction and
repair of intestinal mucosal injury and the anti-apoptosis of
intestinal mucous cells (8,9).
Numerous studies have indicated that TFF3 promotes
the migration of various types of cells in vitro and in
vivo, and that the migration mechanism may be closely
associated with the APC protein, β-catenin, E-cadherin, the
epidermal growth factor (EGF) receptor complex and extracellular
signal-regulated kinase (ERK). It has been found that repair of the
epithelium by TFF3 is independent of transforming growth factor-β
and that the epithelium is precisely repaired by promoting the
migration of cells surrounding the injured area to the damaged site
(10). In addition, TFF3 promotes
the migration of epithelial cells by affecting the expression and
localization of catenin in epithelial cells, and by inducing
phosphorylation of catenin, leading to a decrease in tight
junctions between cells (11,12). The EGF and Ras signalling pathways
may also be involved in the mechanism through which TFF3 promotes
intestinal epithelial cell migration. However, the upstream
regulatory mechanism of TFF3 remains unclear.
In a previous study, TFF3 was demonstrated to be a
novel target of miR-7-5p (13).
miR-7 has been investigated primarily in tumour diseases. Numerous
reports have indicated that miR-7 affects the functions of cell
metabolism, growth, proliferation and apoptosis through acting on
different target proteins (14,15). Therefore, we were interested in
further examining the effects of miR-7-5p on TFF3 and the
relationship among miR-7-5p, TFF3 and the proliferation and
migration of intestinal epithelial cells. In the present study, we
hope to provide a theoretical basis for the mechanism and treatment
of IBD.
Materials and methods
Cell culture experiments
The human colonic epithelial cell line LS174T was
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). The cell line was grown to near confluence in Dulbecco's
modified eagle's medium supplemented with 10% fetal bovine serum
(FBS; all from HyClone Laboratories, GE Healthcare Life Sciences,
Logan, UT, USA), 50 U/ml penicillin and 50 mg/ml streptomycin at
37°C. The cells were subcultured following partial digestion with
0.25% trypsin and 0.9 mmol/l EDTA in Ca2+-free and
Mg2+-free phosphate-buffered saline (PBS).
TaqMan assays
To evaluate the expression level of miR-7-5p in the
LS174T cells, total RNA was isolated from LS174T cells using the
mirVana miRNA isolation kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Reverse transcription (RT) was performed using
the TaqMan miRNA reverse transcription kit (Thermo Fisher
Scientific, Inc.) with a stem loop-specific RT primer, followed by
TaqMan PCR analysis with small RNA-specific primers for miR-7-5p
and U6 small nucleolar RNA (snRNA; Thermo Fisher Scientific, Inc.).
Briefly, RT was performed as follows: the RT Master Mix included
100 nM dNTPs with 0.15 µl dTTP, MutiScribeTM Reverse
Transcription (50 U/µl; 1 µl), 10× reverse buffer
(1.5 µl), 0.19 µl RNase inhibitor (20 U/µl)
and 4.16 µl nuclease-free water. RT Master Mix (7 µl)
was combined with 5 µl total RNA (1-10 ng), and 3 µl
RT primer (5X) was added, mixed and centrifuged at 1000 × g at 4°C
for 5 sec. The following parameter values were used to program the
thermal cycler: 16°C temperature bath for 30 min, 42°C 30 min for
RT reaction, 85°C heating for 5 min to terminate the reaction, and
setting the temperature at 4°C for running qPCR. According to the
manufacturer's instructions, qPCR was performed to measure the
expression levels of miR-7-5p and U6 using the Universal PCR Master
Mix kit (Thermo Fisher Scientific, Inc.) and specific TaqMan probes
(Thermo Fisher Scientific, Inc.). The PCR protocol was as follows:
20 µl reaction volume, including 10 µl TaqMan
Universal PCR Master Mix II no UNg+ (Thermo Fisher
Scientific, Inc.; cat. no. 4440049), 7.67 µl nuclease-free
water, 1.0 µl TaqMan Small RNA assay (20X) and 1.33
µl product from the RT reaction. The thermocycling
conditions were as follows: optional Amperase UNg activity, 50°C
for 2 min; enzyme activation, 95°C for 10 min and PCR was performed
for 40 cycles. Denaturing was performed at 95°C for 15 sec and
extension was performed at 60°C for 60 sec. The relative expression
levels of miR-7-5p were normalized to the expression of U6 RNA via
the 2−∆∆Ct method (16). The measurements were performed in
triplicate.
Plasmid construction and
transfection
The miR-7-5p mimic, miRNA negative control (NC),
miR-7-5p inhibitor and miR-7-5p inhibitor NC were purchased from
Guangzhou RiboBio Co., Ltd. (guangzhou, China). The sequences were
as follows: 5′-UGGAAGACUAGUGAUUUUGUUGU-3′ for miR-7-5p mimic;
5′-UUUGUACUACACAAAAGUACUG-3′ for miR-7-5p mimic NC;
5′mAmCmAmAmCmAmAmAmAmUmCmAmCmUmAmGmUmCmUmUmCmCmA3′ for miR-7-5p
inhibitor; and 5′-mCmAmGmUmAmCmUmUmUmUmGmUmGmUmAmGmUmAmCmAmAmA-3′
for miR-7-5p inhibitor NC. The LS174T cells were seeded at a
density of 4×105 cells/well in 6-well plates at 37°C and
transfected with 100 nM miR-7-5p mimic, miRNA NC, miR-7-5p
inhibitor NC, and miR-7-5p inhibitor of each at a ratio of 1:2 and
2 µg TFF3 (545.36 µg/ml). The TFF3 plasmid was
purchased from SinoBiological, Inc. (Beijing, China). Transfection
was performed using Lipofectamine 3000 (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Cells were
harvested after 48 h and subjected to various assays.
Western blot analysis
Proteins from the transfected cells were harvested
at 48 h post-transfection using radioimmune precipitation assay
buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium
deoxycholate and 0.1% SDS and inhibitors, including sodium
orthovanadate, sodium fluoride, EDTA and leupeptin, as well as
proteinase/phosphatase inhibitors) for 30 min on ice. Equal
quantities of protein (10–20 µg) were separated on 8–15%
SDS-PAGE gels and transferred to polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA) at 80 v, 1.5 h. The protein
expression levels of phosphoinositide 3-kinase (PI3K), Akt,
phosphorylated (p)-Akt, TFF3 and glyceraldehydes-3-phosphate
dehydrogenase (GAPDH) were detected using specific antibodies of
anti-PI3K (rabbit polyclonal antibody; dilution, 1:1,000; cat. no.
WL01169; Wanleibio, Shenyang, China), anti-AKT (rabbit polyclonal
antibody; dilution, 1:1,000; cat. no. WL0003b; Wanleibio),
anti-p-AKT (rabbit polyclonal antibody; dilution, 1:1,000; cat. no.
WLP001; Wanleibio), anti-TFF3 (rabbit monoclonal antibody;
dilution, 1:1,000; cat. no. ab108599; Abcam, Cambridge, MA, USA)
and anti-GAPDH (rabbit monoclonal antibody; dilution, 1:5,000; cat.
no. ab9485; Abcam). After washing in Tris-buffered saline with
Tween-20 (0.1%) (Sigma-Aldrich, St. Louis, MO, USA), membranes were
incubated with the secondary antibody [horseradish
peroxidase-labeled goat anti-rabbit immunoglobulin G (H+L);
dilution, 1:2,000; cat. no. A0208; Beyotime Institute of
Biotechnology, Haimen, China] at room temperature for 2 h. Bands
were visualized using the enhanced chemiluminescence method
(Pierce™ ECL Western Blotting Substrate; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Semi-quantification
was performed using the ImageJ v1.48u software (National Institutes
of Health, Bethesda, Maryland, USA).
Cell proliferation assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to measure cell proliferation. At 0, 24, 48 and 72 h
post-transfection, the transfection medium was replaced with 150
µl of fresh serum-free medium containing 0.5 g/l MTT in each
well. Following incubation at 37°C for 4 h, the MTT medium was
removed via aspiration, and 50 µl dimethyl sulfoxide (DMSO)
was added to each well. Subsequent to incubation at 37°C for a
further 10 min, the absorbance (490 nm) of each sample was measured
using a plate reader (BioTek Instruments, Inc., Winooski, VT, USA).
This experiment was repeated 3 times.
Cell migration assay
The migration ability of LS174T cells was evaluated
using 24-well Transwell chambers (EMD Millipore, Boston, MA, USA).
For all groups, LS174T cells were diluted in serum-free medium at
the logarithmic growth phase. The concentration of the cell
suspension was adjusted to 5×104 cells/ml, and 200
µl of the suspension was then inoculated into the upper
Transwell chamber. The cells were subsequently incubated for 24 h
at 37°C and 5% Co2 incubator, fixed with 4%
paraformaldehyde at room temperature for 20 min and stained with
0.5% crystal violet dye for 5 min at room temperature. After
washing various times with water, the number of cells that had
migrated through the filter into the lower wells was counted using
an inverted microscope, and 5 fields were selected and calculated
the arithmetic mean value.
Experimental grouping
To illustrate the interaction of miR-7-5p, TFF3 and
the PI3K/Akt signalling pathway in the regulation of LS174T cell
proliferation and migration by miR-7-5p, a PI3K/Akt signalling
pathway inhibitor (LY294002) and a TFF3-overexpressing plasmid were
used. The LS174T cells were divided into six groups as follows: i)
miRNA NC group, when the LS174T cells were 60–80% confluent, they
were transfected with the miRNA mimic NC for 48 h and harvested;
ii) miR-7-5p mimic experimental group, when the LS174T cells were
60–80% confluent, the cells were harvested after transfection with
miR-7-5p mimics for 48 h; iii) miR-7-5p mimic + TFF3 overexpression
vector transfection group, when the LS174T cells were 60–80%
confluent, the cells were transfected with the TFF3 plasmid
together with miR-7-5p mimics and were harvested following
co-culture for 48 h; iv) miRNA mimic NC + LY294002 treatment group,
24 h after the cells were transfected with the miRNA mimic NC, they
were treated with the PI3K inhibitor, LY294002 (50 µM) for
24 h; v) miR-7-5p mimic + LY294002 treatment group, 24 h after the
cells were transfected with miR-7-5p mimics, they were treated with
50 µM LY294002 for 24 h; and vi) miR-7-5p mimics + TFF3
overexpression plasmid + LY294002 treatment group, the cells were
transfected with miR-7-5p mimics and TFF3 plasmids for 24 h and
treated with 50 µM LY294002 for 24 h.
Statistical analysis
All values were expressed as means ± standard error
of the mean. Differences between the groups were analysed via the
one-way ANOVA, followed by the Bonferroni post hoc analyses, as
appropriate. A P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using the SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Effect of miR-7-5p on proliferation and
migration of LS174T cells
The proliferation and migration functions of
intestinal epithelial cells are significant following intestinal
damage. The effect of miR-7-5p in the proliferation of LS174T cells
was evaluated using the MTT assay, and the effect of miR-7-5p on
the migration of LS174T cells was evaluated in Transwell chambers.
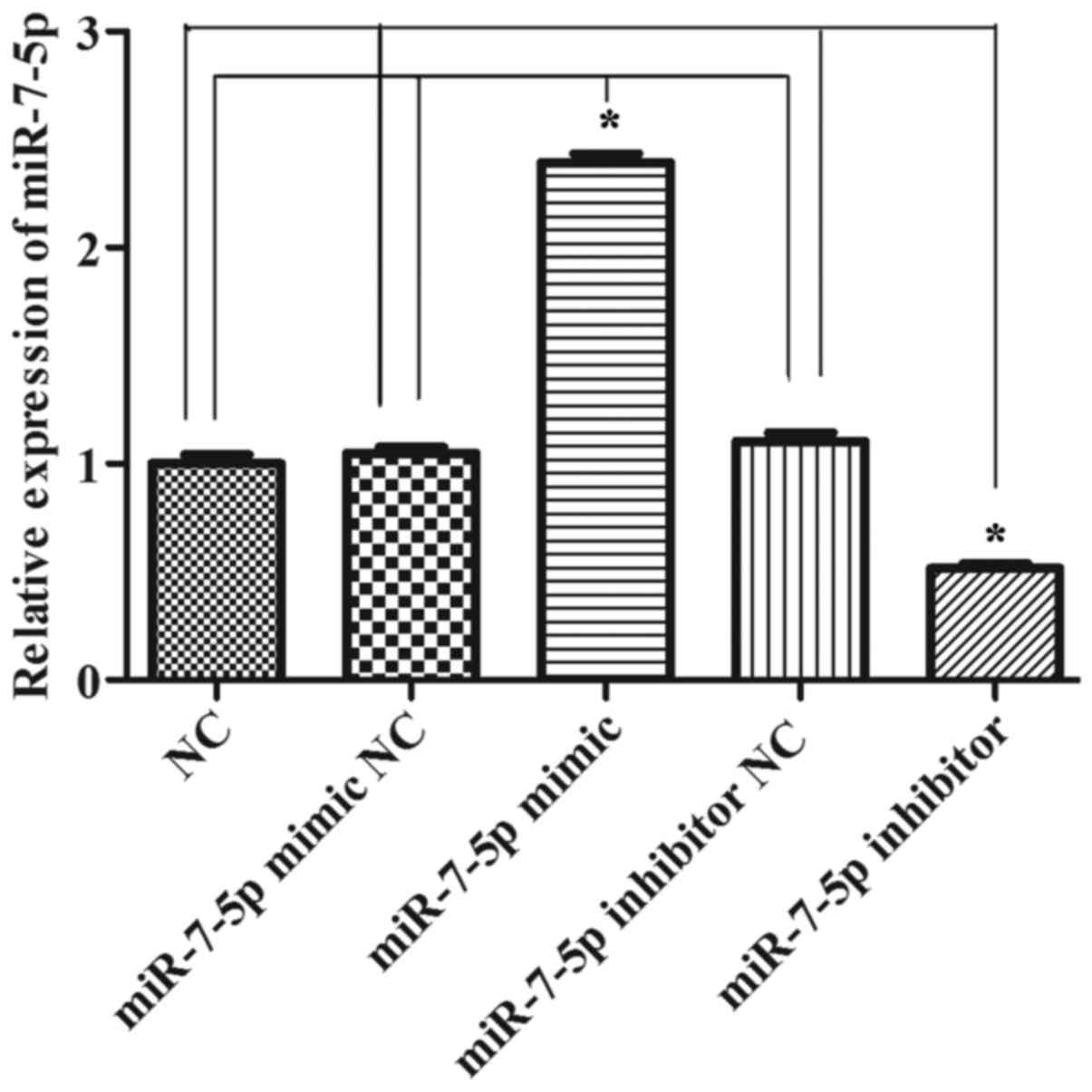
Fig. 1 demonstrates that
successful results were obtained 24 h after transfecting miR-7-5p
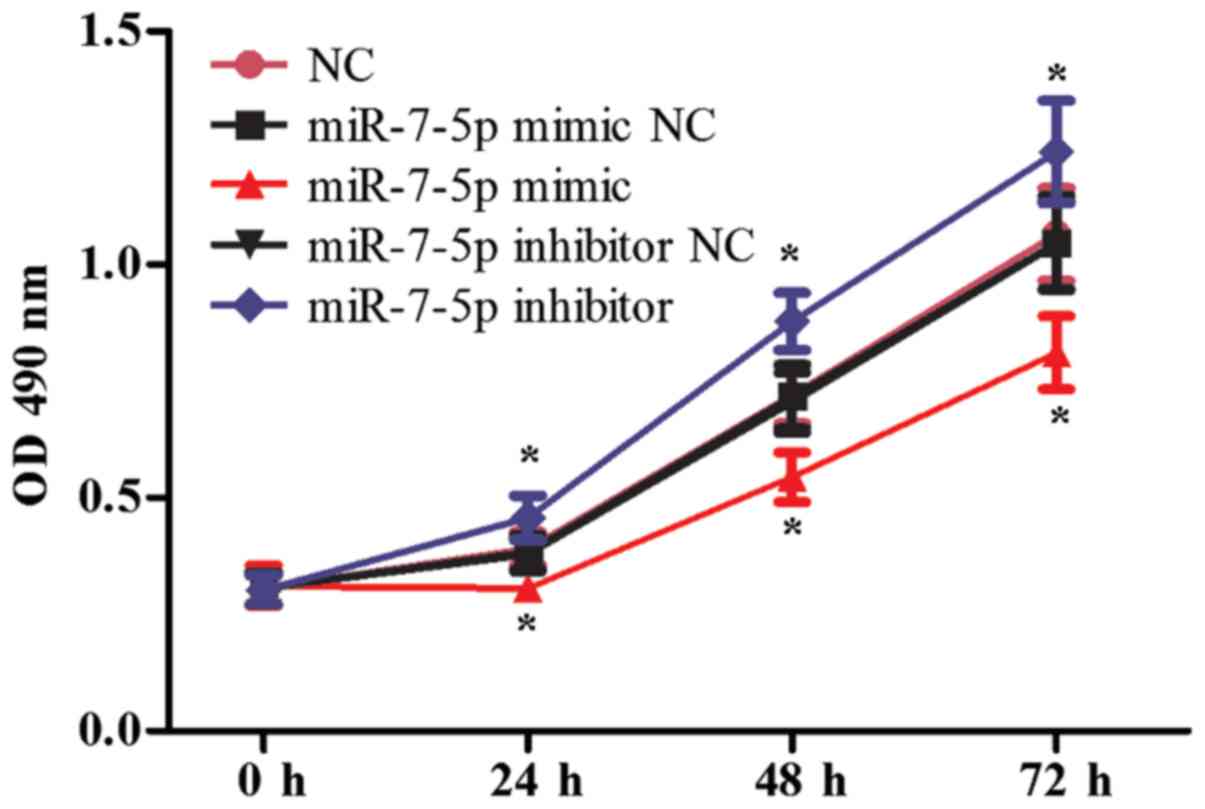
mimics and a miR-7-5p inhibitor. As presented in Fig. 2, compared with the NC group, the
miRNA mimic NC group and the miRNA inhibitor NC group, the
overexpression of miR-7-5p decreased the cell proliferation ability
(P<0.05), whereas inhibiting the expression of miR-7-5p
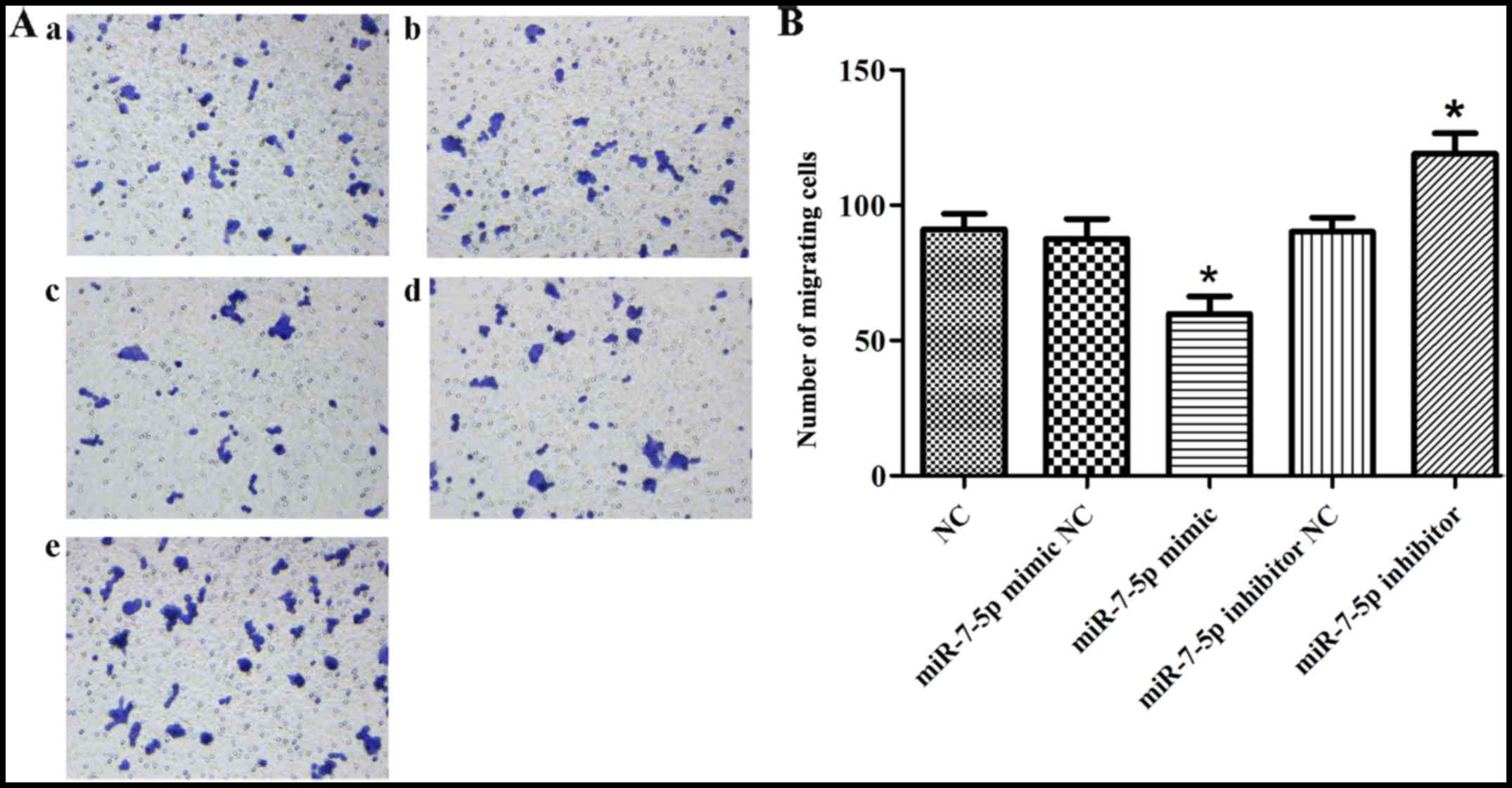
increased cell proliferation. Fig.
3 demonstrates that, compared with the NC group, the miRNA
mimic NC group and the miRNA inhibitor NC group, the overexpression
of miR-7-5p decreased, whereas inhibiting the expression level of
miR-7-5p increased the number of cells that migrated through the
chamber (P<0.05).
Effect of miR-7-5p on the expression
levels of TFF3, PI3K, Akt and p-Akt protein
As shown in Fig.
4, compared with the NC group, the miRNA mimic NC group and the
miRNA inhibitor NC group, the overexpression of miR-7-5p decreased
the levels of TFF3, PI3K and p-Akt protein expression, whereas
inhibiting the expression level of miR-7-5p increased TFF3, PI3K
and p-Akt protein expression levels and the difference was
statistically significant (P<0.05). However, no effect of
miR-7-5p was observed on the expression level of the Akt protein.
In a previous study, miR-7-5p was demonstrated to bind to the 3′UTR
of TFF3. Thus, these results indicated that miR-7-5p influences the
proliferation and migration of LS174T cells by targeting TFF3, with
the possible participation of the PI3K/Akt signalling pathway.
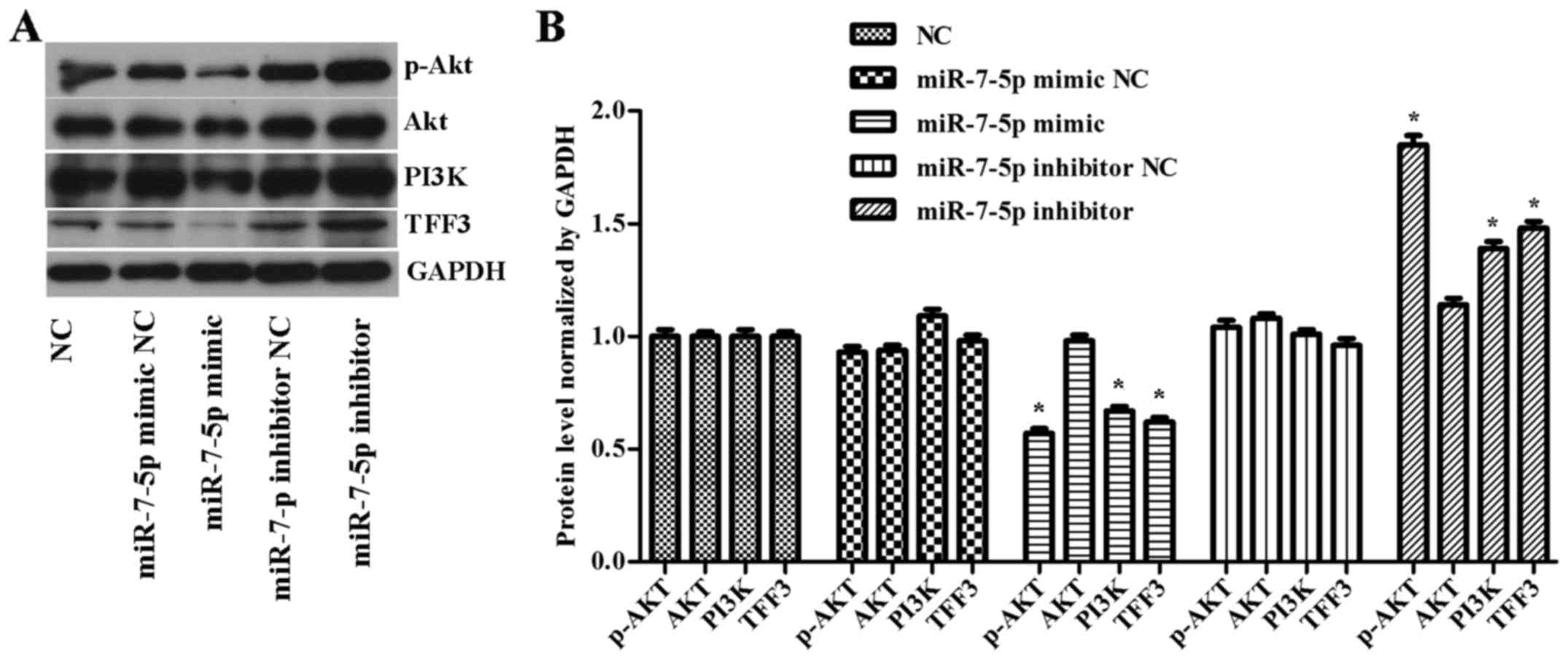 | Figure 4(A) Western blot detection of TFF3,
PI3K, Akt and p-Akt expression levels in cells with overexpressed
or downregulated miR-7-5p levels. GAPDH served as the loading
control. (B) Quantitative analysis of TFF3, PI3K, Akt and p-Akt
protein levels normalized to GAPDH levels. The assays were
performed in triplicate. All values are presented as the means ±
standard deviation of three replicates. *P<0.05
compared with NC, miR-7-5p mimic NC and miR-7-5p inhibitor NC
groups. TFF3, trefoil factor 3; PI3K, phosphoinositide 3-kinase; p,
phosphorylated; miR, microRNA; GAPDH, glyceraldehydes-3-phosphate
dehydrogenase. |
Effect of miR-7-5p, TFF3 and LY294002 on
the protein expression levels of TFF3, PI3K, Akt and p-Akt
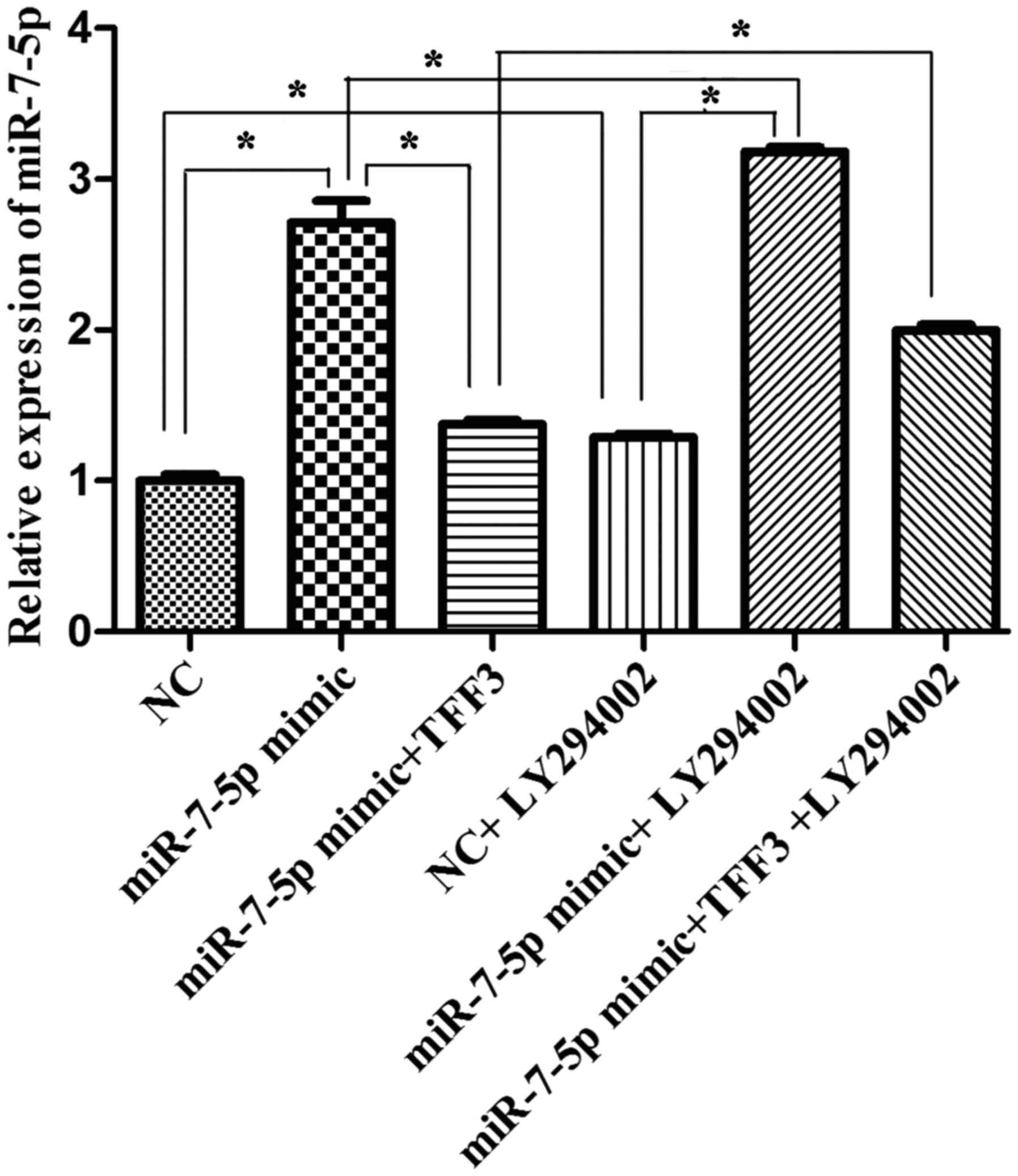
According to the experimental groups, the
transfected cells were harvested 48 h after transfection, and the
mRNA expression level of miR-7 was detected via qPCR. As
demonstrated in Fig. 5, the
expression level of miR-7-5p in the miR-7-5p mimic group was
significantly higher than that in the miRNA NC group. Compared with
the miR-7-5p mimic group, the expression level of miR-7-5p in the
miR-7-5p mimic + TFF3 plasmid group was significantly decreased,
while compared with the miR-7-5p mimic + TFF3 plasmid group, the
expression level of miR-7-5p in the miR-7-5p mimic + TFF3 plasmid +
LY294002 group was significantly increased, and this difference was
statistically significant (P<0.05). These findings indicated
that the TFF3 overexpression plasmid was able to inhibit the
expression level of miR-7-5p, but that LY294002 inhibited this
inhibitory effect of the TFF3 plasmid on miR-7-5p. The expression
level of miR-7-5p in the miRNA NC + LY294002 group, the miR-7-5p
mimic + LY294002 group, and the miR-7-5p mimic + TFF3 plasmid +
LY294002 group was greater than that in the miRNA NC group, the
miR-7-5p mimic group, and the miR-7-5p mimic + TFF3 plasmid group,
respectively, and these differences were statistically significant
(P<0.05). Thus, these findings indicated that the expression
level of miR-7-5p was upregulated by the PI3K/Akt signalling
pathway inhibitor, indicating that inhibition of the PI3K/Akt
signalling pathway may result in feedback upregulation of miR-7-5p
expression.
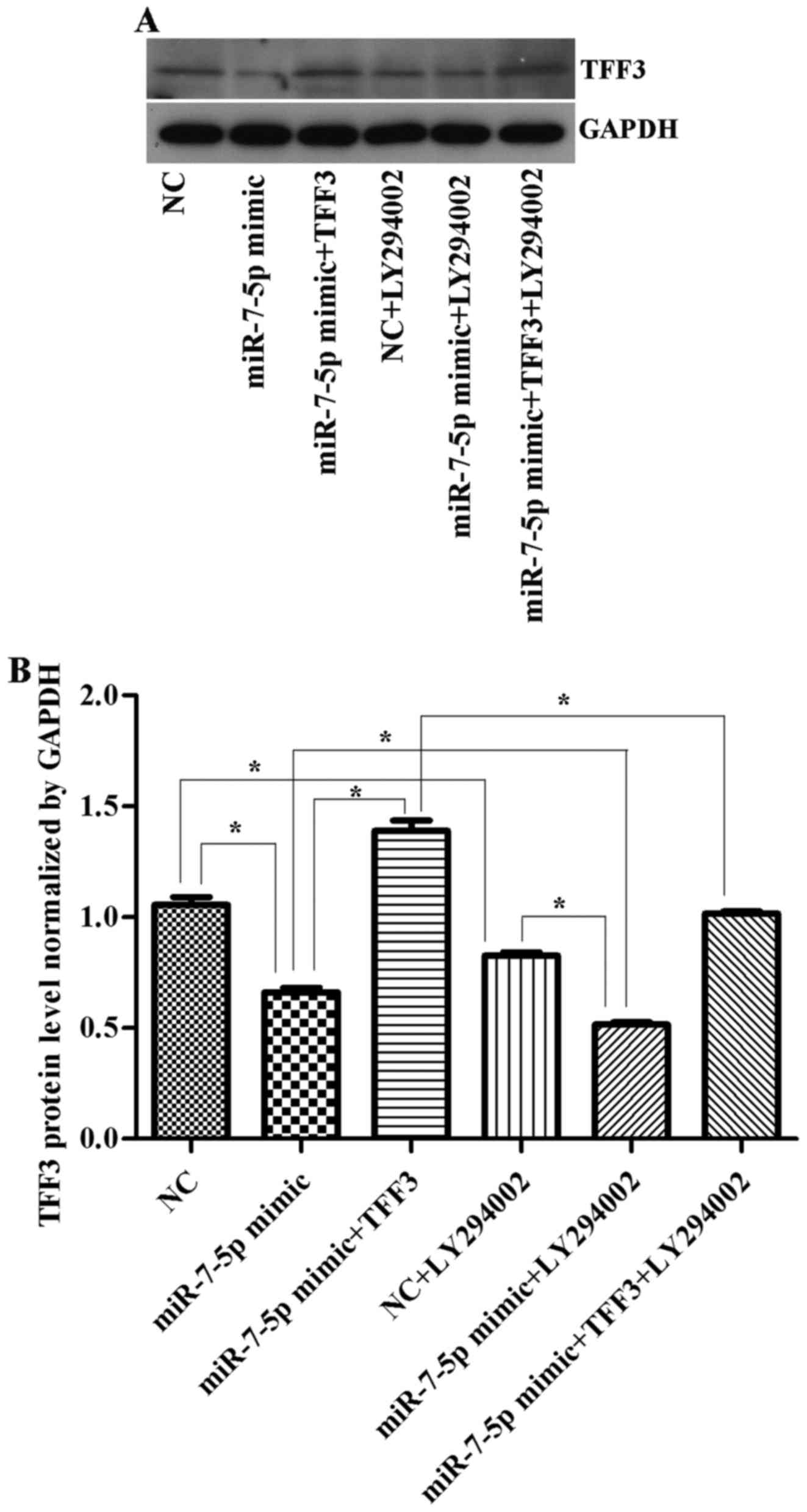
The TFF3 protein was detected by western blot
analysis (Fig. 6). The results
(Figs. 6Figure 7–8) showed that the expression levels of
the TFF3, PI3K, and p-Akt proteins in the miR-7-5p mimic group were
significantly lower than those in the NC group, whereas the
expression levels of the TFF3, PI3K and p-Akt proteins in the
miR-7-5p mimic + TFF3 plasmid group were higher than those in the
miR-7-5p mimic group. The difference between these groups was
significant (P<0.05), indicating that miR-7-5p downregulates the
protein expression of its target, TFF3. The TFF3 promotes PI3K
protein expression, and miR-7-5p inhibits the activation of the
PI3K/Akt signalling pathway while downregulating TFF3 protein
expression levels. However, following treatment of the miR-7-5p
mimic + TFF3 plasmid group with LY294002, the expression levels of
the TFF3, PI3K and p-Akt proteins decreased, indicating that
blocking the PI3K/Akt signalling pathway further promotes the
effect of miR-7-5p on TFF3. Compared with the NC group, the
expression level of the TFF3 protein in the NC + LY294002 group was
significantly decreased (P<0.05). Compared with the miR-7-5p
mimic group, the expression level of the TFF3 protein in miR-7-5p
mimics + LY294002 group was significantly decreased (P<0.05).
Compared with the miR-7-5p mimic + TFF3 plasmid group, the
expression level of the TFF3 protein in the miR-7-5p mimic + TFF3
plasmid + LY294002 group was also significantly decreased
(P<0.05). Taken together, these findings demonstrated that
subsequent to adding the PI3K/Akt signalling pathway inhibitor, the
expression level of miR-7-5p was upregulated and the expression
level of TFF3 was downregulated, which indicated that inhibiting
the PI3K/Akt signalling pathway may exert a feedback effect leading
to the upregulation of miR-7-5p expression and the inhibition of
TFF3 expression.
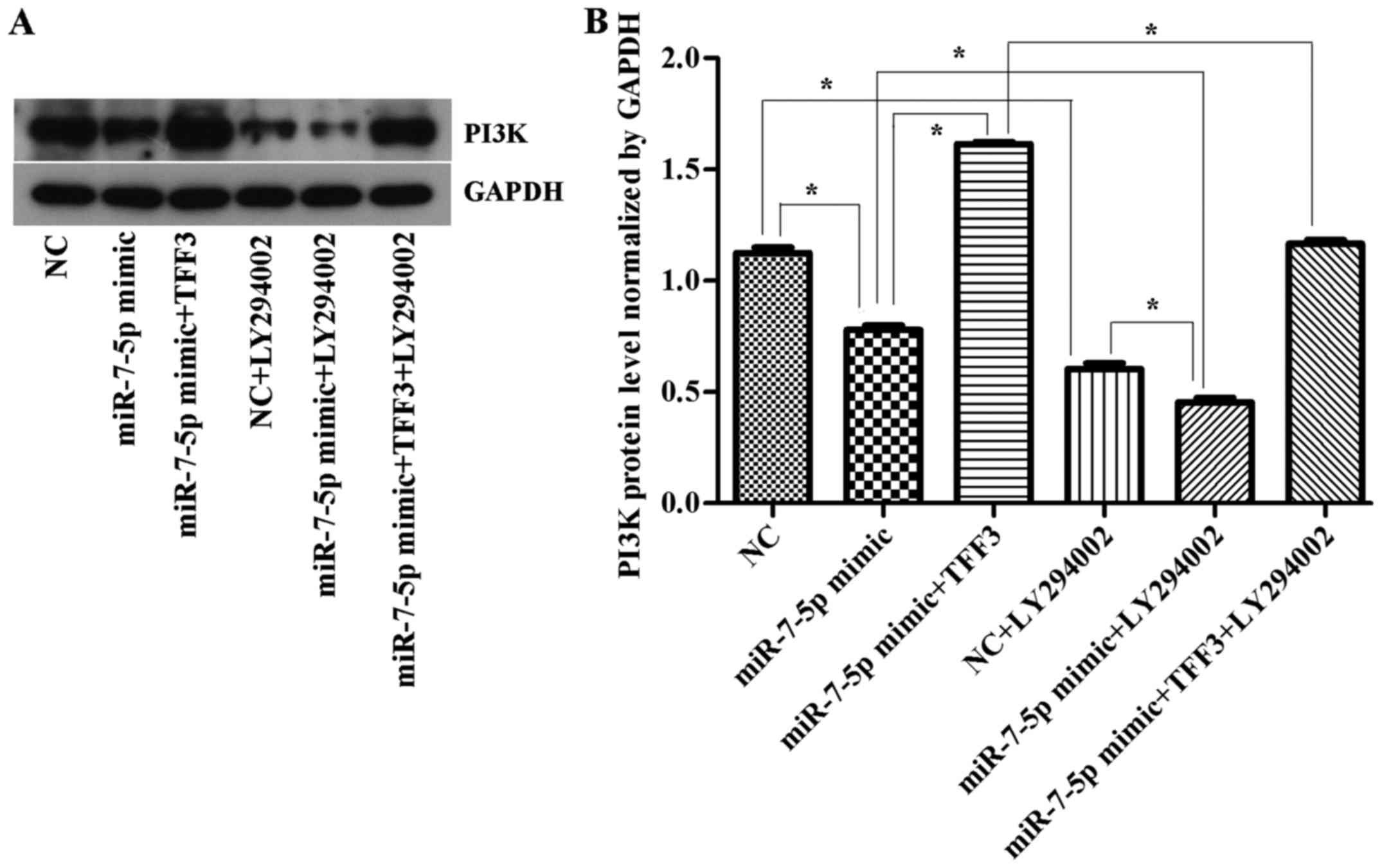
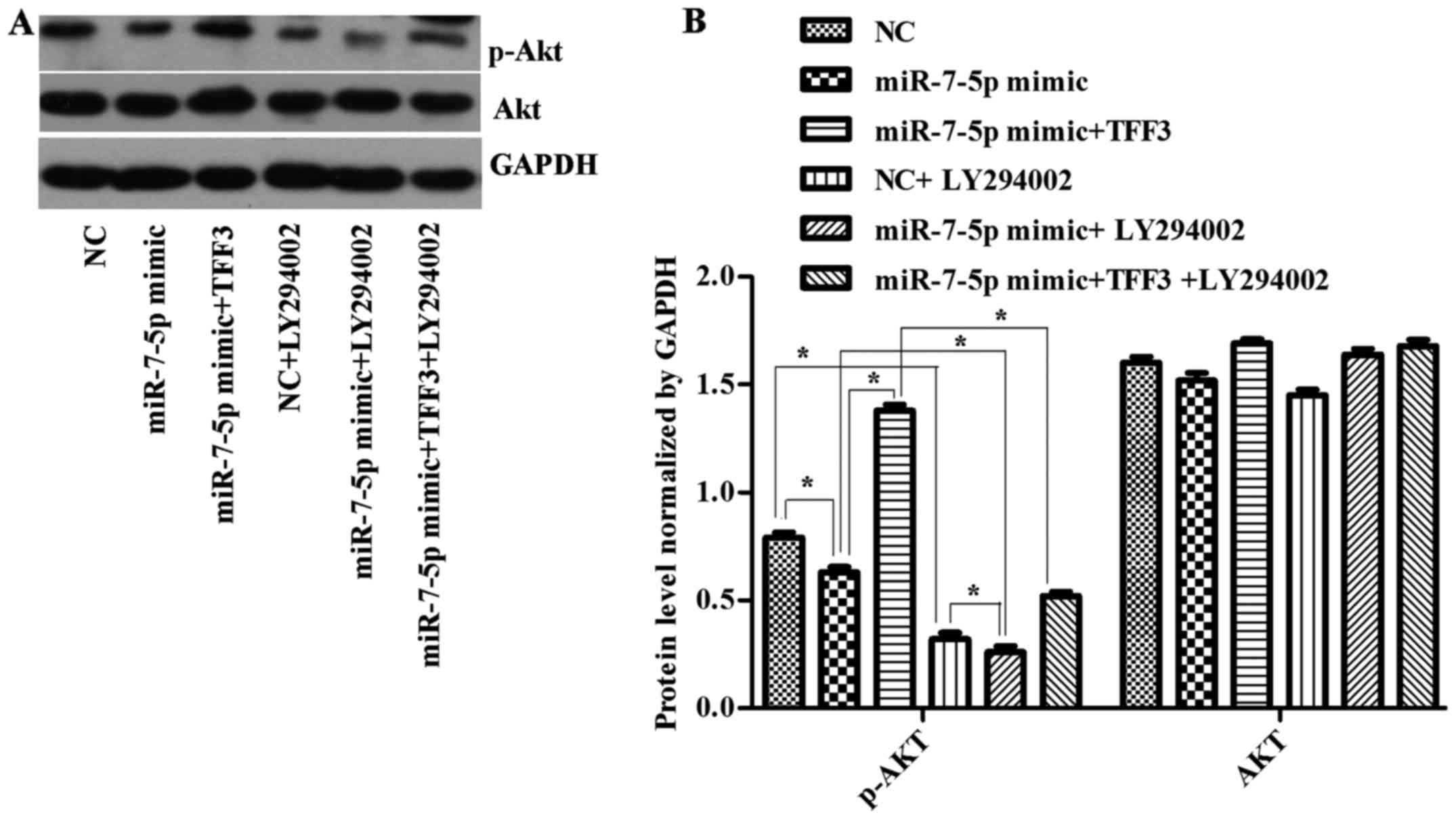
The expression levels of the PI3K and p-Akt protein
were consistent with the trend of TFF3 protein expression (Figs. 7 and 8), although there was no significant
difference in the expression levels of the AKT protein between the
groups (Fig. 8B), suggesting that
the primary mechanism by which miR-7-5p regulates the expression
level of TFF3 and affects the PI3K/Akt signalling pathway occurs
via modulation of Akt protein phosphorylation.
Effect of miR-7-5p, TFF3 and LY294002 on
the proliferation and migration of LS174T cells
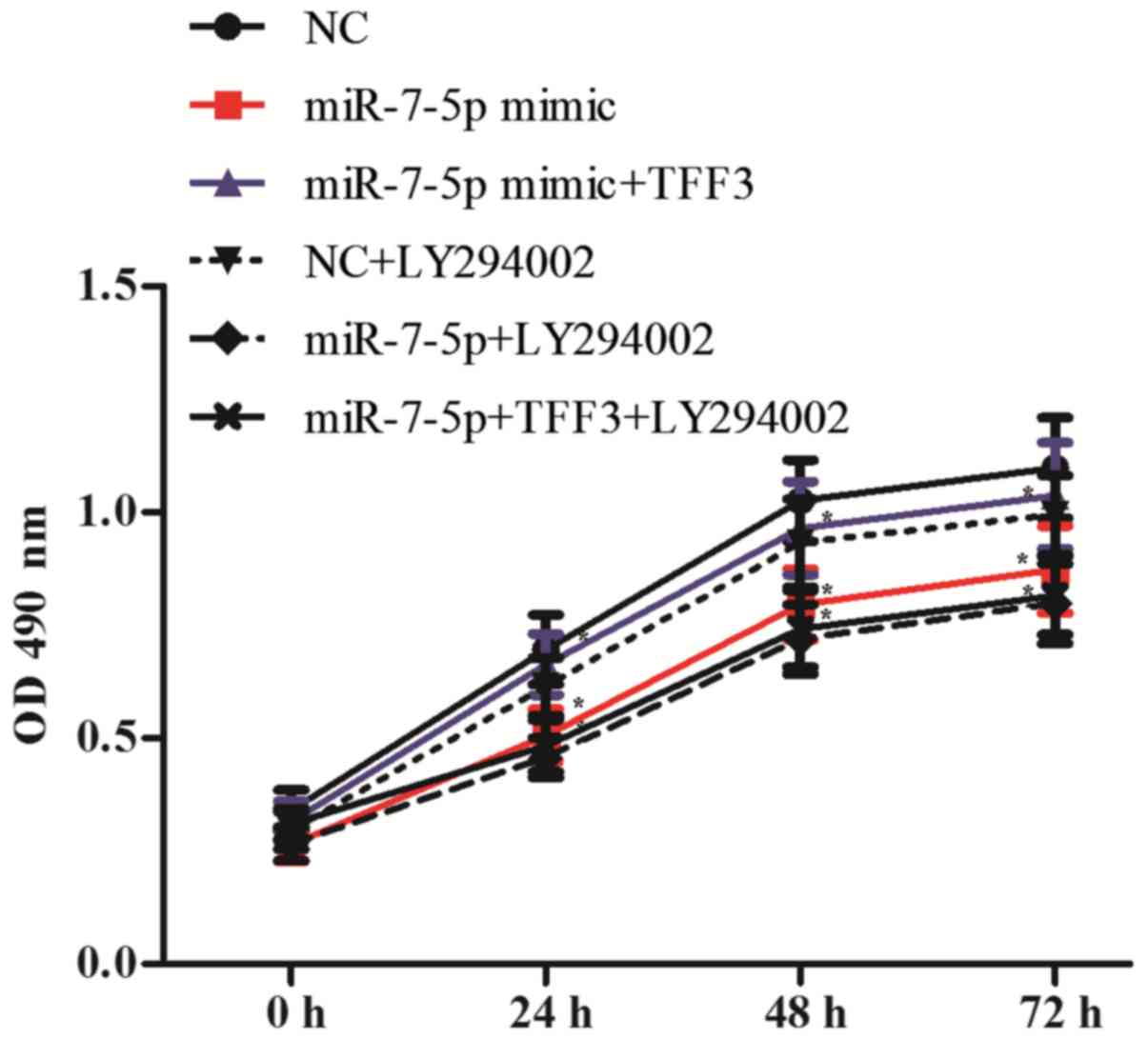
To detect the cell proliferation of each group, the
cell optical density (OD) at 490 nm was detected at 0, 24, 48 and
72 h after transfection. As shown in Fig. 9, compared with the NC group, the
OD value of the miR-7 mimic group decreased significantly,
indicating a decreased cell proliferation ability; the difference
between the groups was statistically significant (P<0.05).
Compared with the miR-7-5p mimic group, the miR-7-5p mimic + TFF3
plasmid group showed a significantly increased OD value and
enhanced cell proliferation ability, with a significant difference
observed between the groups (P<0.05). Compared with the miR-7-5p
mimic group, the miR-7-5p mimic + LY294002 group exhibited a slight
decrease in the OD value, although the difference between the
groups was not significant (P>0.05). This result indicates that
in addition to the PI3K/Akt signalling pathway, miR-7-5p affects
cell proliferation via pathways other than regulating TFF3.
However, compared with the miR-7-5p mimic + TFF3 plasmid group, the
miR-7-5p mimic + TFF3 plasmid + LY294002 group exhibited a
decreased OD value and decreased cell proliferation ability. These
results indicated that the PI3K/Akt signalling pathway is involved
in the regulation of TFF3 by miR-7-5p, subsequently affecting the
proliferation of LS174T cells, and that blocking the PI3K/Akt
signalling pathway further promotes the inhibitory effect of
miR-7-5p on cell proliferation.
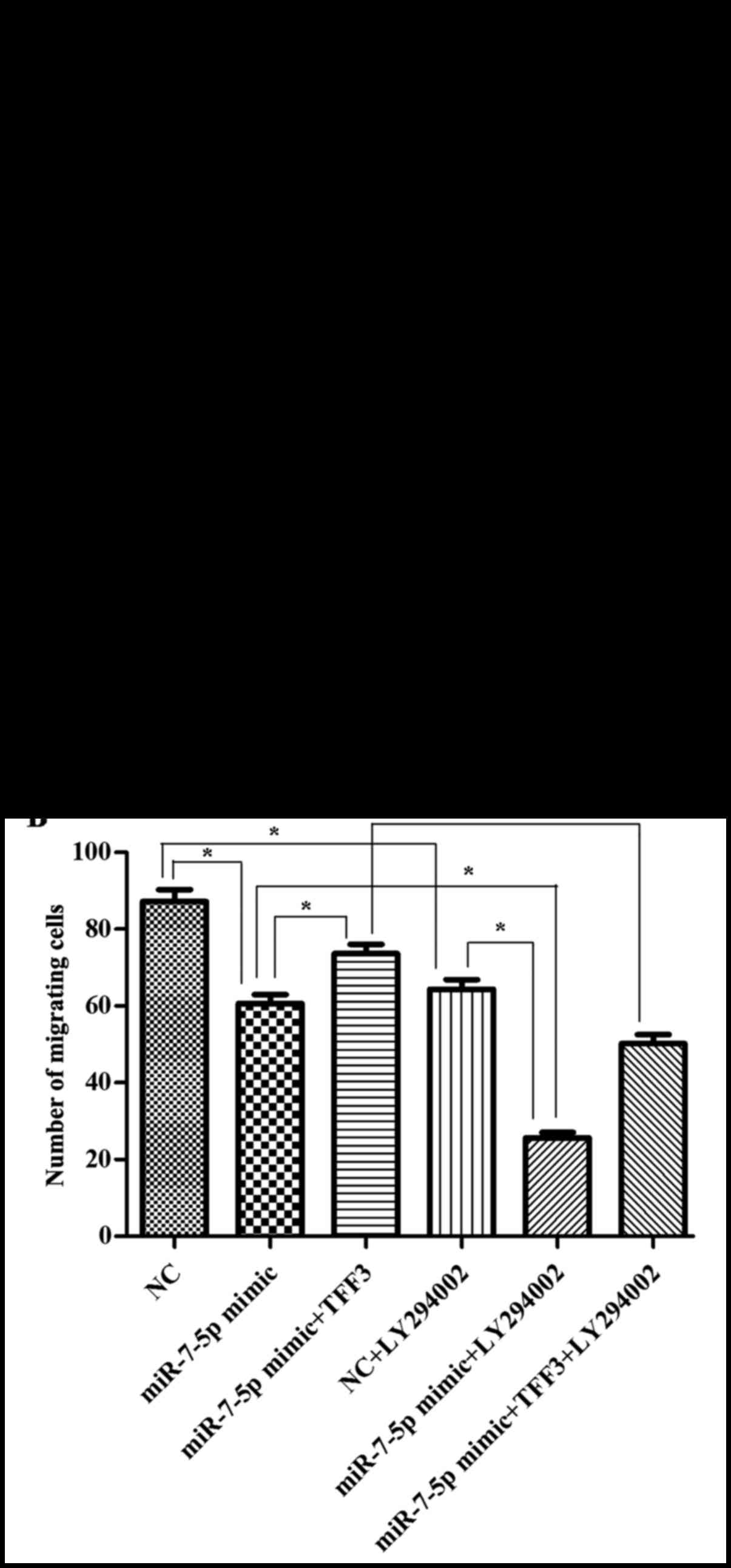
The present study illustrated the effect of
miR-7-5p, TFF3 and LY294002 on the migration of LS174T cells via
Transwell chamber assays. The number of cells that migrated through
the Transwell chamber was counted following 24 h of incubation. As
presented in Fig. 10, compared
with the NC group, the cell migration ability of the miR-7-5p high
expression group was significantly decreased. Compared with the
miR-7-5p mimic group, the number of cells passing through the
Transwell membrane was increased in the miR-7-5p mimic + TFF3
plasmid group. This finding indicated that the cell migration
ability was increased, indicating that the TFF3 plasmid weakened
the inhibitory effect of miR-7-5p on the migration ability of the
cells, and supported the effect of miR-7-5p by targeting TFF3 and
mediating cell migration. Compared with the miR-7-5p mimic group,
the miR-7-5p mimic + LY294002 group exhibited a decrease in the
number of cells passing through the Transwell membrane, suggesting
that LY294002 further downregulated the migration ability of LS174T
cells. The number of cells passing through the Transwell membrane
in the miR-7-5p mimic group decreased, whereas it increased in the
miR-7-5p mimic + TFF3 plasmid group. However, when the miR-7-5p
mimic + TFF3 overexpression plasmid group was treated with
LY294002, the number of cells passing through the Transwell
membrane decreased once more, indicating that LY294002 blocked the
promoting effect of the TFF3 overexpression plasmid on cell
migration. Taken together, these findings indicate that the effect
of miR-7-5p on the migration of LS174T cells via TFF3 regulation
was mediated by the PI3K/Akt signalling pathway, and blocking this
pathway may further promote the inhibitory effect of miR-7-5p on
cell migration.
Discussion
miRNAs are a type of snRNA that lead to mRNA
degradation and translational repression by binding to the 3′UTR of
target mRNAs. miRNAs are significant in processes, such as cell
proliferation, apoptosis, growth and development, cell
differentiation and metabolism (17).
miR-7 is one type of mature miRNA that has been
investigated in tumour diseases. Numerous reports have suggested
that miR-7 affects the functions of cell metabolism, growth,
proliferation and apoptosis by acting on different target proteins
(14,15,18–20). Fang et al (18) proposed that miR-7 inhibits the
growth and metabolism of hepatocellular carcinoma by regulating the
PI3K/Akt signalling pathway. Meza-Sosa et al (19) demonstrated that miR-7 promotes
epithelial cell transdifferentiation by targeting Kruppel like
factor 4. Xu et al (20)
revealed that miR-7 regulates XRCC2, and thereby inhibits the
proliferation of colorectal cancer cells and induces apoptosis
(20). In our previous study,
miR-7-5p was found to be differentially expressed in IBD lesions
and normal tissues. In addition, it was confirmed that miR-7-5p
binds to the TFF3 3′UTR and regulates the expression level of TFF3
at the post-transcriptional level (13).
The TFF3 proteins are a group of small molecular
peptides that are predominantly secreted by goblet cells (21). TFF3 exhibits a special three-lobed
structure. This structure exhibits high stability, which causes the
TFF to present strong anti-protease hydrolysis, acid digestibility
and heat-resistant properties. Therefore, TFF3 maintains biological
activity in the complex environment of the digestive tract
(22). Investigation of the
response of TFF3 to mucosal damage and its response in patients
with IBD has indicated that TFF3 is significant in injury repair
and mucosal protection, and the underlying mechanisms include the
promotion of cell migration, cell proliferation and anti-apoptosis
effects (23-25).
The present study demonstrates that miR-7 may
regulate the function of tumour cells by regulating multiple
pathways, such as the PI3K/Akt and mitogen-activated protein kinase
kinase/ERK signalling pathways (26,27). Li et al (28) found that miR-7 inhibits the
proliferation and invasion of human colorectal cancer cells, and
that PI3K signalling pathways are involved in this process. Zhang
et al (29) showed that
miR-21 could regulate the tight junctional permeability of
intestinal epithelial cells, which was mediated by the PI3K/Akt
signalling pathway. It has also been suggested that TFF3 and the
PI3K/Akt pathway play important roles in cell migration and
proliferation. Dise et al (30) demonstrated that EGF promoted
intestinal epithelial cell migration by activating PI3K and causing
Rac activation. Langlois et al (31) showed that PTEN inhibits tumour
progression by controlling cell polarity, the establishment of
cell-cell junctions, paracellular permeability, migration and
metabolic potential and that PTEN phosphorylation is mediated by
the activation of PI3K. Sun et al (32) demonstrated that intestinal trefoil
factor (ITF) can promote the proliferation and migration of gastric
epithelial cells. ITF protects the integrity of gastric epithelial
cells from damage by activating PI3K/Akt cell signalling pathways.
Lin et al (33) revealed
that TFF3 overexpression may promote the expression of zonula
occludens-1, occludin and claudin-1, but this effect was inhibited
after suppressing the PI3K/Akt signalling pathway.
Therefore, the aim of the present study was to
elucidate the association between miR-7-5p, TFF3 and PI3K/Akt in
the process, whereby miR-7-5p regulates the proliferation and
migration of intestinal epithelial cell. The current results
revealed that miR-7-5p decreased the expression level of TFF3, and
inhibited LS174T cell proliferation and migration, accompanied by
decreased expression of PI3K and p-Akt. Furthermore, it was
identified that miR-7-5p expression levels were decreased following
combined treatment with the TFF3 plasmid and miR-7-5p mimics,
compared with treatment with the miR-7-5p mimics alone. This effect
was accompanied by increased expression levels of TFF3, PI3K and
p-Akt, and by enhanced LS174T cell proliferation and migration.
Furthermore, the expression level of miR-7-5p in the miRNA NC +
LY294002 group, the miR-7-5p mimic + LY294002 group, and the
miR-7-5p mimic + TFF3 plasmid + LY294002 group was higher than in
the NC group, the miR-7-5p mimic group, and the miR-7-5p mimic +
TFF3 plasmid group, respectively. Accordingly, the expression level
of TFF3 was downregulated, and cell proliferation and migration
were downregulated simultaneously. These data indicate that
miR-7-5p regulates TFF3 and inhibits the proliferation and
migration of LS174T cells. The PI3K/Akt signalling pathway is
involved in the regulation of cell proliferation and migration via
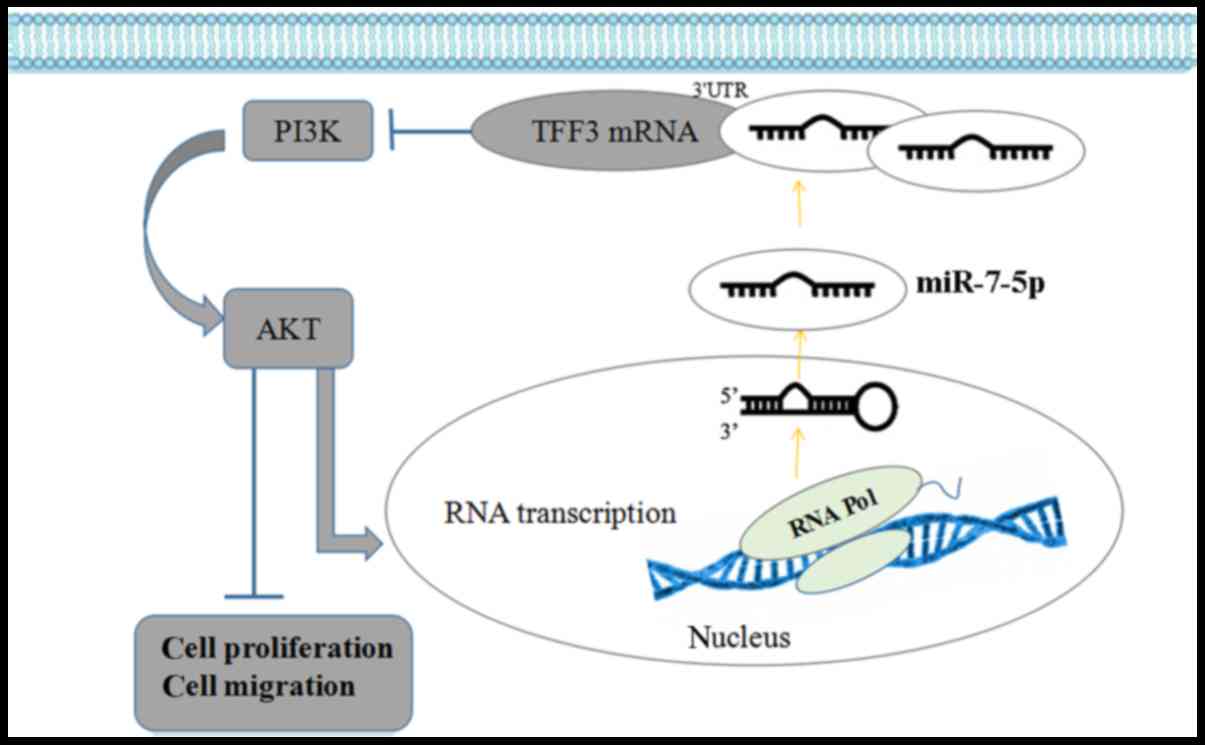
miR-7-5p targeting TFF3 (Fig.
11). The PI3K/Akt signalling pathway may exert a feedback
regulation effect on miR-7-5p; inhibition of the activity of this
pathway may enhance miR-7-5p expression levels and further enhance
the effect of miR-7-5p on cell proliferation and migration.
In conclusion, miR-7-5p inhibits the proliferation
and migration of LS174T cells by targeting TFF3 via inhibiting the
PI3K/Akt signalling pathway. Additionally, the PI3K/Akt signalling
pathway may have a feedback regulation effect on miR-7-5p.
Therefore, miR-7-5p may serve as a therapeutic target for
protection of intestinal epithelial barrier integrity.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China Youth Foundation
(grant no. 81400585) and the Natural Science Foundation of Liaoning
Province, China (grant no. 2014021042).
References
|
1
|
Peloquin JM, Goel G, Villablanca EJ and
Xavier RJ: Mechanisms of pediatric inflammatory bowel disease. Annu
Rev Immunol. 34:31–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neurath MF and Travis SP: Mucosal healing
in inflammatory bowel diseases: A systematic review. Gut.
61:1619–1635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hollander D, Vadheim CM, Brettholz E,
Petersen GM, Delahunty T and Rotter JI: Increased intestinal
permeability in patients with Crohn's disease and their relatives.
A possible etiologic factor. Ann Intern Med. 105:883–885. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petersson J, Schreiber O, Hansson GC,
Gendler SJ, Velcich A, Lundberg JO, Roos S, Holm L and Phillipson
M: Importance and regulation of the colonic mucus barrier in a
mouse model of colitis. Am J Physiol Gastrointest Liver Physiol.
300:G327–G333. 2011. View Article : Google Scholar
|
|
5
|
Su L, Shen L, Clayburgh DR, Nalle SC,
Sullivan EA, Meddings JB, Abraham C and Turner JR: Targeted
epithelial tight junction dysfunction causes immune activation and
contributes to development of experimental colitis.
Gastroenterology. 136:551–563. 2009. View Article : Google Scholar :
|
|
6
|
Rhoads JM, Niu X, Odle J and Graves LM:
Role of mTOR signaling in intestinal cell migration. Am J Physiol
Gastrointest Liver Physiol. 291:G510–G517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sukhotnik I, Shteinberg D, Ben Lulu S,
Bashenko Y, Mogilner JG, Ure BM, Shaoul R, Shamian B and Coran AG:
Transforming growth factor-alpha stimulates enterocyte
proliferation and accelerates intestinal recovery following
methotrexate-induced intestinal mucositis in a rat and a cell
culture model. Pediatr Surg Int. 24:1303–1311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoffmann W: Trefoil factors TFF (trefoil
factor family) peptide-triggered signals promoting mucosal
restitution. Cell Mol Life Sci. 62:2932–2938. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kjellev S: The trefoil factor family -
small peptides with multiple functionalities. Cell Mol Life Sci.
66:1350–1369. 2009. View Article : Google Scholar
|
|
10
|
Sturm A and Dignass AU: Epithelial
restitution and wound healing in inflammatory bowel disease. World
J Gastroenterol. 14:348–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perrais M, Chen X, Perez-Moreno M and
Gumbiner BM: E-cadherin homophilic ligation inhibits cell growth
and epidermal growth factor receptor signaling independently of
other cell interactions. Mol Biol Cell. 18:2013–2025. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oloumi A, McPhee T and Dedhar S:
Regulation of E-cadherin expression and beta-catenin/Tcf
transcriptional activity by the integrin-linked kinase. Biochim
Biophys Acta. 1691:1–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo J, Sun M, Teng × and Xu L:
MicroRNA-7-5p regulates the expression of TFF3 in inflammatory
bowel disease. Mol Med Rep. 16:1200–1206. 2017.PubMed/NCBI
|
|
14
|
Giles KM, Brown RA, Ganda C, Podgorny MJ,
Candy PA, Wintle LC, Richardson KL, Kalinowski FC, Stuart LM and
Epis MR: microRNA-7-5p inhibits melanoma cell proliferation and
metastasis by suppressing RelA/NF-κB. Oncotarget. 7:31663–31680.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kefas B, Godlewski J, Comeau L, Li Y,
Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S, et
al: microRNA-7 inhibits the epidermal growth factor receptor and
the Akt pathway and is down-regulated in glioblastoma. Cancer Res.
68:3566–3572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang Y, Xue JL, Shen Q, Chen J and Tian L:
MicroRNA-7 inhibits tumor growth and metastasis by targeting the
phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma.
Hepatology. 55:1852–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meza-Sosa KF, Pérez-García EI,
Camacho-Concha N, López-Gutiérrez O, Pedraza-Alva G and
Pérez-Martínez L: MiR-7 promotes epithelial cell transformation by
targeting the tumor suppressor KLF4. PLoS One. 9:e1039872014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu K, Chen Z, Qin C and Song X: miR-7
inhibits colorectal cancer cell proliferation and induces apoptosis
by targeting XRCC2. Onco Targets Ther. 7:325–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meyer zum Büschenfelde D, Hoschützky H,
Tauber R and Huber O: Molecular mechanisms involved in TFF3
peptide-mediated modulation of the E-cadherin/catenin cell adhesion
complex. Peptides. 25:873–883. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buda A, Jepson MA and Pignatelli M:
Regulatory function of trefoil peptides (TFF) on intestinal cell
junctional complexes. Cell Commun Adhes. 19:63–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoffmann W: Trefoil factor family (TFF)
peptides: Regulators of mucosal regeneration and repair, and more.
Peptides. 25:727–730. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wright NA, Poulsom R, Stamp G, Van Norden
S, Sarraf C, Elia G, Ahnen D, Jeffery R, Longcroft J, Pike C, et
al: Trefoil peptide gene expression in gastrointestinal epithelial
cells in inflammatory bowel disease. Scand J gastroenterol Suppl.
193(sup193): 76–82. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Renes IB, Verburg M, Van Nispen DJ, Büller
HA, Dekker J and Einerhand AW: Distinct epithelial responses in
experimental colitis: Implications for ion uptake and mucosal
protection. Am J Physiol Gastrointest Liver Physiol. 283:G169–G179.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou X, Hu Y, Dai L, Wang Y, Zhou J, Wang
W, Di W and Qiu L: MicroRNA-7 inhibits tumor metastasis and
reverses epithelial-mesenchymal transition through AKT/ERK1/2
inactivation by targeting EGFR in epithelial ovarian cancer. PLoS
One. 9:e967182014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Z, Jiang Z, Huang J, Huang S, Li Y, Yu
S, Yu S and Liu X: miR-7 inhibits glioblastoma growth by
simultaneously interfering with the PI3K/ATK and Raf/MEK/ERK
pathways. Int J Oncol. 44:1571–1580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Li Y, Liu Y, Xie P, Li F and Li G:
PAX6 a novel target of microRNA-7, promotes cellular proliferation
and invasion in human colorectal cancer cells. Dig Dis Sci.
59:598–606. 2014. View Article : Google Scholar
|
|
29
|
Zhang L, Shen J, Cheng J and Fan X:
MicroRNA-21 regulates intestinal epithelial tight junction
permeability. Cell Biochem Funct. 33:235–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dise RS, Frey MR, Whitehead RH and Polk
DB: epidermal growth factor stimulates Rac activation through Src
and phosphatidylinositol 3-kinase to promote colonic epithelial
cell migration. Am J Physiol Gastrointest Liver Physiol.
294:G276–G285. 2008. View Article : Google Scholar
|
|
31
|
Langlois MJ, Bergeron S, Bernatchez G,
Boudreau F, Saucier C, Perreault N, Carrier JC and Rivard N: The
PTEN phosphatase controls intestinal epithelial cell polarity and
barrier function: Role in colorectal cancer progression. PLoS One.
5:e157422010. View Article : Google Scholar
|
|
32
|
Sun Z, Liu H, Yang Z, Shao D, Zhang W, Ren
Y, Sun B, Lin J, Xu M and Nie S: Intestinal trefoil factor
activates the PI3K/Akt signaling pathway to protect gastric mucosal
epithelium from damage. Int J Oncol. 45:1123–1132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin N, Xu LF and Sun M: The protective
effect of trefoil factor 3 on the intestinal tight junction barrier
is mediated by toll-like receptor 2 via a PI3K/Akt dependent
mechanism. Biochem Biophys Res Commun. 440:143–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|