Introduction
Obstructive sleep apnea/hypopnea syndrome (OSAHS) is
a common disorder characterized by repetitive narrowing or collapse
of the upper airway (UA) during sleep, resulting in a recurrent
reduction or cessation of airflow. As periods of apnea/hypopnea
occur intermittently, OSAHS is associated with chronic intermittent
hypoxia (CIH). It is a multifactorial syndrome, which is mainly
caused by narrow anatomic structure and defective functioning of UA
(1). However, the pathophysiology
of OSAHS remains incompletely understood. Although most patients
with OSAHS have a narrow UA, for some individuals who have an
anatomical predisposition to UA collapse, their UAs may still be
maintained opened by UA dilator muscles. The state of UA depends on
the balance between positive intraluminal pressure to open the
airway and negative surface tension to keep it closed (2). There is substantial evidence
supporting that UA dilator muscles play important roles in
maintaining airway patency (3).
Exposure to CIH, a result of repetitive narrowing or collapse of
UA, promotes the activation of UA dilator muscles, causes a
transition from slow to fast in muscle fiber types (4), and reduces the endurance of dilator
muscles (5). Genioglossus, an
important UA dilator muscle, is the main tongue muscle which exerts
forward propulsion to the tongue and contracts in coordination
before the diaphragm contracts. As for the important role of the
genioglossus in maintaining UA patency, it is referred to as the
safeguard of the UA (6).
Several studies have suggested that intermittent
hypoxia promotes the expression of hypoxia-inducible factor-1
(HIF-1) and activates serial reactions to hypoxia (7–10).
HIF-1 is a heterodimeric transcription factor composed of a
hypoxia-inducible HIF-1α subunit and a consistently expressed
HIF-1β subunit, which is well known for regulating a wide range of
genes in response to hypoxia. The activation of HIF-1 depends on
HIF-1α, which is normally kept low in cells by proteosomal
degradation but is stabilized and transferred into the nucleus
under hypoxia. HIF-1α was found to exhibit higher expression in
skeletal muscles than other non-muscle tissues in normoxic
conditions, indicating that it plays an important role in skeletal
muscles (11). In our previous
study, HIF-1α was verified to have regulating ability in
genioglossus myogenesis in hypoxia (12). We also found that 17β-estradiol
(E2) exerts protective effects of fatigue resistance on
the genioglossus in CIH rats (13,14), and these effects were coincident
with the downregulation of HIF-1α (12,13). HIF-1α was found to be destabilized
by estrogen treatment even under hypoxic conditions. This raises
the question of how estrogen downregulates the expression of
HIF-1α.
The genomic effect of estrogen is mediated by two
estrogen receptor (ER) isoforms, ERα and ERβ, which belong to the
family of nuclear receptors. The varying tissue-specific
distribution and expression level of ERα and ERβ is the basis of
the different biological effects of estrogen (15,16). A series of investigators found
clear expression of ERα but weak or undetectable expression of ERβ
in skeletal muscle in mice (17,18). It can be inferred that ERα may be
of great significance in skeletal muscle.
To date, expanding the upper airway is the main
method in the clinical treatment and management of OSAHS, including
continuous positive airway pressure (CPAP), oral appliance and
surgical treatment. However, each of these methods is associated
with many defects and complications. Some researchers have
discovered the possibility of medical therapy for OSAHS in recent
years (19), but there are still
no effective pharmacotherapies for individuals with OSAHS. Several
researchers have indicated that female hormones may possess
protective effects against OSAHS (20,21). A previous study confirmed that
estrogen may protect the function of the upper airway (22). However, estrogen cannot be used
for patients with OSAHS due to its side effects such as coronary
heart disease, stroke and breast cancer. To limit the side effects
of estrogen, estrogenic compounds are needed. Phytoestrogens, due
to their similar structure with estrogens, have estrogenic effects
via ERs and exhibit fewer side effects and long-term health
benefits (prevention of osteoporosis, cardiovascular disease and
breast cancer) (23). Resveratrol
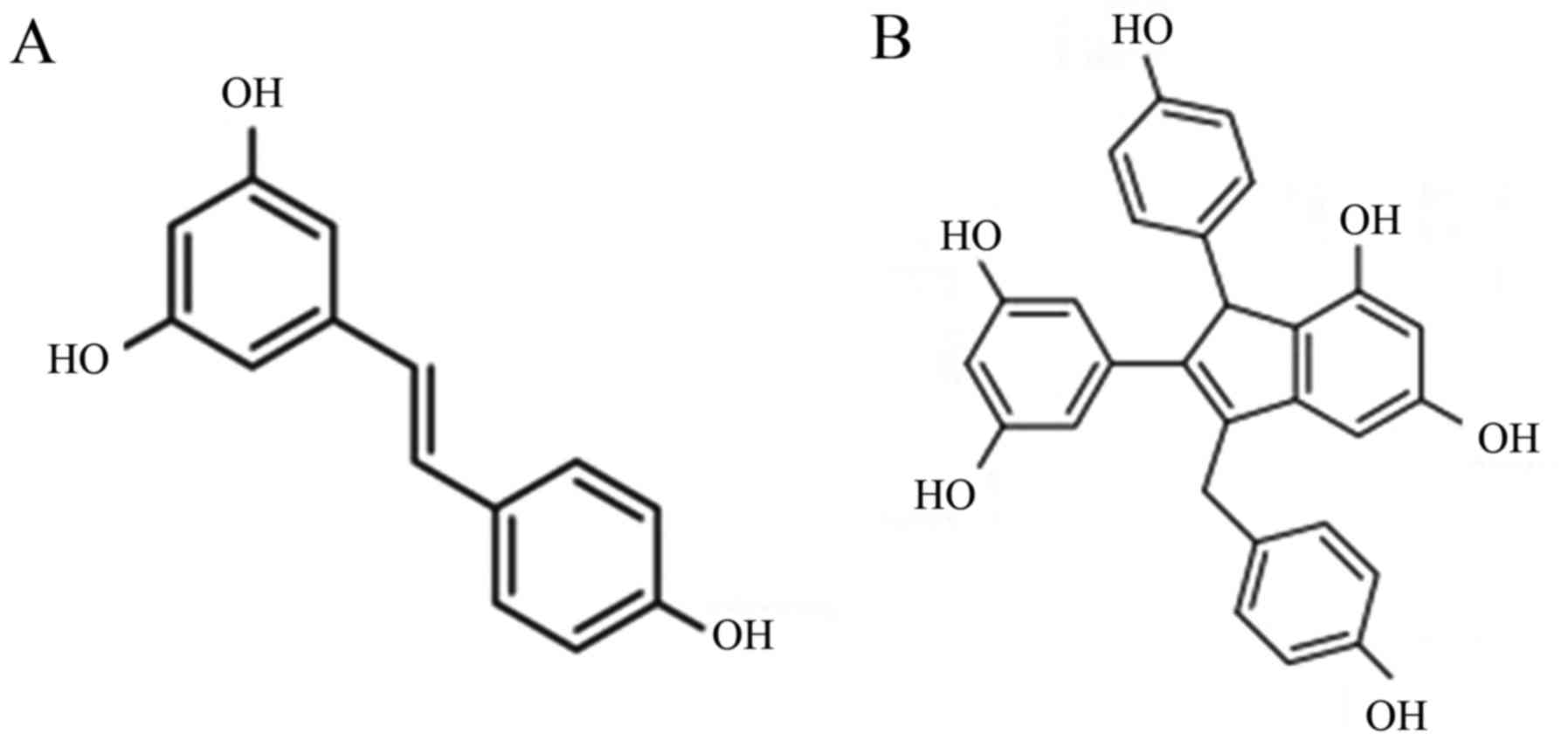
(Fig. 1A), a polyphenol found in
a variety of plants, is such a type of phytoestrogen. Recently,
Zhong et al reported a new approach with which to synthesize
derivatives of resveratrol, which have similar pharmacological
activities as resveratrol. These derivatives have increased
availability than resveratrol and may be alternatives to estrogen
in various therapies (24). One
type of resveratrol dimer (RD), an endo-shifted olefin isomer of
parthenocissin A (Fig. 1B), is
testified to have considerable estrogenic properties and minimum
cytoxia in pre-experiments. Studying the effects of RD on HIF-1α
and comparing the results with those obtained from E2
may be the first move to explore the possibility of RD replacing
E2 in the medical therapy for OSAHS.
In the present study, we isolated genioglossus
myoblasts and silenced ERα to investigate the effect of
E2 and RD on HIF-1α and the underlying mechanism. Our
study may aid to elucidate the molecular mechanism of E2
and RD involved in the effects on the physical properties of the
genioglossus and contribute to our future study of medical therapy
for OSAHS.
Materials and methods
Materials
17β-estradiol, SB203580 and MTT solution were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Resveratrol
dimer was a kind gift of Professor X. Sun and coworkers (School of
Pharmacy, Fudan University, Shanghai, China). The bicinchoninic
acid (BCA) protein assay kit was obtained from Beyotime Institute
of Biotechnology (Jiangsu, China). TRIzol reagent, expression
vector kit with GFP and packaging mix were obtained from Invitrogen
(Carlsbad, CA, USA). Lipofectamine 2000, Dulbecco's modified
Eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained
from Gibco-BRL (Grand Island, NY, USA). Monoclonal anti-HIF-1α
antibody, anti-ERα antibody and anti-ERβ antibody were from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-p38 antibody
and anti-p-p38 antibody were purchased from Cell Signaling
Technology (Boston, MA, USA). IRDye800-conjugated secondary
antibodies were procured from Rockland Immunochemicals
(Gilbertsville, PA, USA).
Primary cultivation and identification of
genioglossus myoblasts
Genioglossus myoblasts were isolated and cultured as
previously described (14). All
procedures were approved by the Animal Care Committee of Tongji
University. Under sterile conditions, the genioglossus of 2- to
3-day-old C57BL/6J mice was excised and minced with surgical
scissors and forceps. After being transferred to a 15-ml centrifuge
tube, the muscle slurry was enzymatically digested with 0.05% type
II collagenase at 37°C for 40 min, and centrifuged at 200 × g for 1
min. Further digestion was initiated in 0.25% trypsin-EDTA at 37°C
for 30 min, and stopped by the addition of 20% FBS (HyClone, Logan,
UT, USA). Then, a 75-µm sieve (Millipore, Billerica, MA,
USA) was used to filtrate the dissociated cells, which was followed
by 200 × g centrifugation for 1 min. The sediment was resuspended
in DMEM supplemented with 25% FBS. Cells were plated on culture
dishes after twice repeated differential attachment treatment.
After reaching 80% confluence, the growth medium was replaced with
normal medium (10% FBS in DMEM).
To assess whether the putative myoblasts have the
capacity of differentiation, the culture medium was replaced with
differentiation medium (2% horse serum in DMEM) when cell fusion
reached 80%. The cells were observed and photographed 4 days after
the induction of differentiation to evaluate their morphological
appearance.
After the second passage, the putative myoblasts
were seeded onto 6-well plates at a density of 2×104
cells/ml. After reaching 60% confluence, plated cells were fixed
with paraformaldehyde for 15 min, then blocked with 5% bovine serum
albumin [BSA, dissolved in phosphate-buffered saline (PBS)] for 1 h
at room temperature, and incubated with 1:500 α-sarcomeric actin
monoclonal antibody overnight at 4°C, and then HRP-conjugated goat
anti-mouse secondary antibody for 1 h. After being washed with PBS,
the cells were incubated using the SABC kit for 30 min and then
stained using a diaminobenzidine (DAB; Beyotime Institute of
Biotechnology) kit according to the manufacturer's instructions.
Fibroblasts were treated in the same way as the negative
control.
Lentivirus production, ERα gene silencing
and selection by flow cytometry
The ERα-knockdown shRNA was constructed by inserting
the ERα shRNA fragment into empty plasmid pLKO.1. The DNA fragment
for ERα was obtained with oligonucleotide forward,
5′-GGAGAATGTTGAAGCACAAGC-3′ and reverse,
5′-GCTTGTGCTTCAACATTCTCC-3′ sequences. The scrambled fragment was
inserted as the control: ERα scrambled (ERα-NS) forward,
5′-GTTCTCCGAACGTGTCACG-3′ and reverse, 5′-ACGTGACACGTTCGGAGAAC-3′.
293T cells (obtained from the Type Culture Collection of the
Chinese Academy of Sciences, Shanghai, China) were seeded onto
6-well plates at a density of 9×104 cells/ml. After
reaching 80% confluence, 293T cells were transfected with a mixture
containing 2 µg pLKO.1 shRNA vector, packing plasmids (2
µg psPAX2 and 2 µg pMD2.G), 12 µl transfection
agent Lipofectamine™ 2000 (Invitrogen) and 600 µl DMEM.
After 48 h, the supernatant was harvested and filtered through
0.45-µm filters and the virus supernatants were obtained and
then stored at 20°C. For ERα gene silencing, the genioglossus
myoblasts were plated onto 6-well plates and medium was replaced
with virus supernatant 12 h afterward. Twenty-four hours later, the
virus supernatant was removed and fresh medium was added. After
reaching 80% confluence, transfected myoblasts (ERα-KD and ERα-NS)
were digested with 0.25% trypsin-EDTA at 37°C for 1.5 min,
centrifuged at 200 × g for 5 min and resuspended in Hank's
solution. Then, a 40-µm sieve was used to filtrate the
cells, followed by 200 × g centrifugation for 5 min. The
supernatant was discarded and the cells were resuspended in DMEM,
and then sorted by flow cytometry. GFP-positive myoblasts were
obtained and used for further experiments. The effectiveness of ERα
gene silencing was verified by mRNA and protein expression.
Cell treatment and hypoxic
conditions
The myoblasts, ERα-knockdown myoblasts (KD group)
and ERα-scrambled myoblasts (NS group) were cultured at an
atmospheric oxygen concentration (21% O2, 5%
CO2; balance N2) in an incubator. Myoblasts
exposed to hypoxia were cultured in a hypoxia chamber (1%
O2, 5% CO2; balance N2). Cells in
different groups were treated with vehicle-dimethyl sulfoxide
(DMSO), or 1 µmol/l E2, or 1 µmol/l RD, or
1 µmol/l E2 and 10 µmol/l SB203580
(according to MTT assay and preliminary experiments), and then
incubated under normoxia or hypoxia for 24 h.
MTT-based cytotoxicity assay of
E2 and RD
The effects of E2 and RD on the myoblasts
in normoxia or hypoxia were measured by MTT assay. Third passage
myoblasts were seeded onto 96-well plates at 2×104
cells/ml. Various concentrations of E2 and RD
(10−5, 10−6, 10−7, 10−8
and 10−9 mol/l) were added and the cells were incubated
in a normoxic or hypoxic condition after reaching 40% confluence.
The vehicles of the same concentration were added into fresh
culture medium as control. After 24, 48 and 72 h, the plated cells
were incubated with MTT solution (5 mg/ml) at 37°C for 4 h, and
then DMSO (100 µl/well) (both from Sigma-Aldrich) to
dissolve the formazan precipitate. After been mixed for 30 min,
viable cells were detected by measuring the absorbance at 595 nm
with a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
The mean optical density of 6 wells in the same group was used to
assess the viability of the cells.
RNA isolation, RT-PCR, and real time
PCR
RT-qPCR was employed to detect the mRNA expression
of ERα and HIF-1α. Total RNA was isolated from the cells using
TRIzol reagent, and then reverse transcribed to cDNA using
PrimeScript RT reagent kit (Takara, Shiga, Japan) in a GeneAmp PCR
System 9700 (Applied Biosystems, Foster City, CA, USA). Isolated
RNA and cDNA were both quantified using the NanoDrop ND-1000
spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
Real-time PCR was performed using SYBR Green as detection reagent
on a 7500 real-time PCR system (7500; Applied Biosystems). The 20
µl PCR mixture contained 2 µl cDNA product, 10
µl SYBR Premix Ex Taq, 0.4 µl ROX Reference Dye II,
6.8 µl RNase-free water, and 0.4 µl each of the
forward and reverse primers. Gene-specific primers for ERα and
HIF-1α are described in Table I.
The first step of the PCR protocol was 95°C for 30 sec, followed by
45 cycles of 95°C for 5 sec, and 60°C for 34 sec as the second
step. A melting curve analysis was performed to ensure specificity
of the PCR products. β-actin was used as a control, and the
relative expression of the target genes was evaluated by a
comparative CT method and normalized to the control. The average
values were obtained from five repeated experiments.
 | Table INucleotide sequences of primers used
for PCR amplification. |
Table I
Nucleotide sequences of primers used
for PCR amplification.
| Gene | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| ERα |
AGGCGGCATACGGAAAGAC |
CATTTCGGCCTTCCAAGTCA |
| HIF-1α |
GACAATAGCTTCGCAGAATGC |
TCGTAACTGGTCAGCTGTGG |
| β-actin |
CCTCATGAAGATCCTGACCG |
TGCCAATAGTGATGACCTGG |
Western blot analysis
Cell lysates were obtained using ice-cold RIPA
buffer (Pierce, Rockford, IL, USA) with phenylmethanesulfonyl
fluoride (1 mmol/l; Beyotime, Shanghai, China). Protein was
quantified using the BCA protein assay. An equal amount of protein
was denatured with sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) sample buffer at 95°C for 5 min,
separated on SDS-PAGE gels using a Bio-Rad apparatus, and then
transferred to PVDF membranes (Millipore). After blocking with 5%
bovine serum albumin (dissolved in TBST), the membranes were
incubated with anti-HIF-1α antibody (1:500), anti-ERα antibody
(1:1,000), anti-ERβ antibody (1:500), anti-p38 MAPK antibody
(1:1,000), anti-p-p38 MAPK antibody (1:1,000), anti-β-actin
antibody (1:2,500) or anti-glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) antibody (1:2,500) at 4°C overnight. After 3×10 min washes
in TBST, the membranes were incubated with IRDye800-conjugated
secondary antibodies (1:10,000) away from light for 20 min at room
temperature. The immunoblots were imaged using the LI-COR Odyssey
Infrared Imaging system (Li-COR, Lincoln, NE, USA). Anti-β-actin or
anti-GAPDH was used as the loading control in the western blot
analyses.
Data analysis
All quantitative results were obtained from
triplicate samples. Data are expressed as mean ± standard deviation
(SD). The independent samples t-test was used for the cell
proliferation assay. One-way ANOVA was used to assess the
difference between the control and experimental groups. A P-value
<0.05 was considered to indicate a statistically significant
result. The statistical program used was SPSS version 17.0 (SPSS
Inc. Chicago, IL, USA).
Results
Characterization of mouse genioglossus
myoblasts
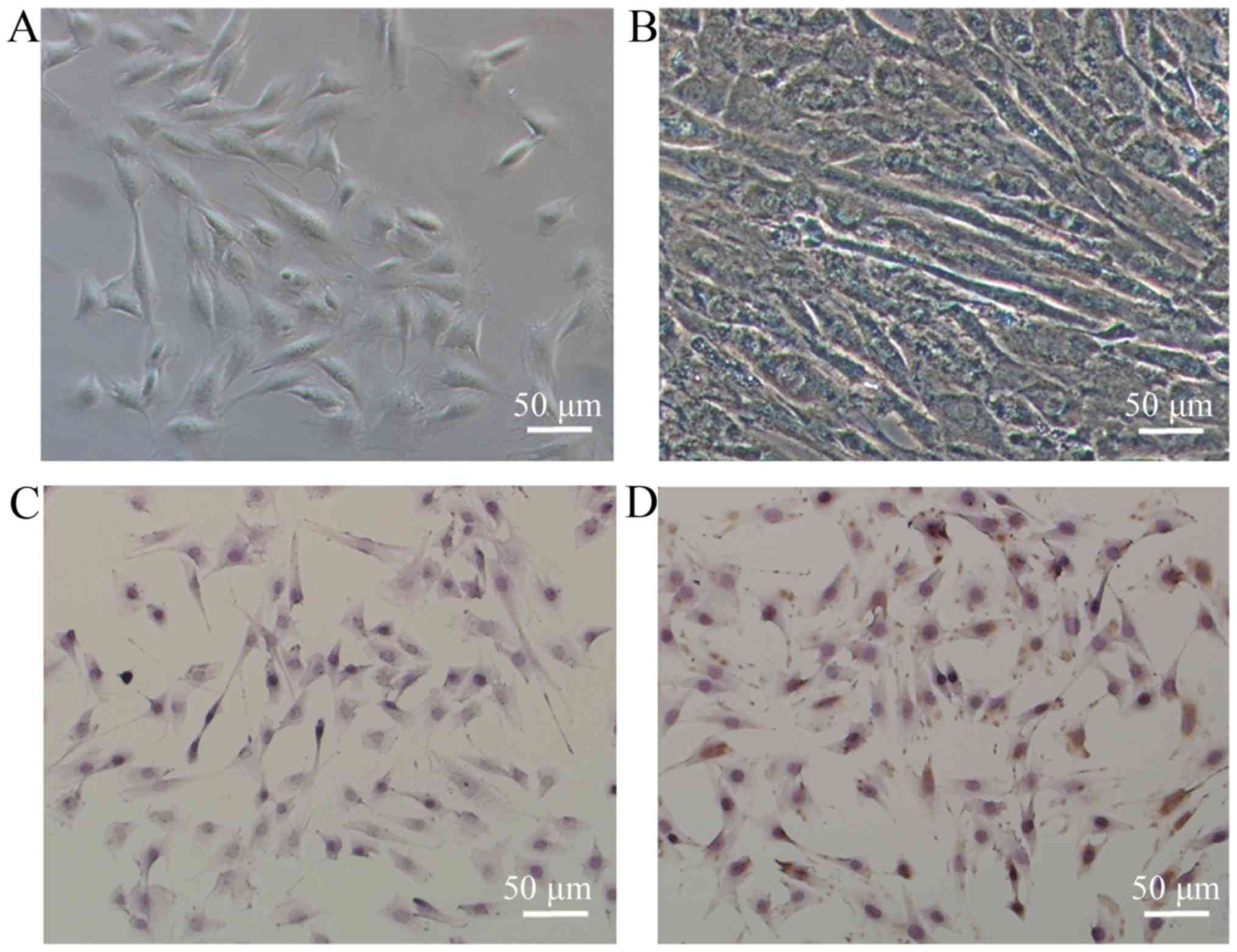
The newly separated cells were spherical and
displayed strong refractivity. Once the cells adhered, they became
grossly fibroblast-like in shape and had a spindle form (Fig. 2A). After being induced with 2%
horse serum, the genioglossus myoblasts were found to fuse into
myotubes (Fig. 2B). More than 95%
of isolated cells demonstrated positive immunostaining for
α-sarcomeric actin, as assessed by immunocytochemical staining
(Fig. 2D), while the fibroblasts
(control) exhibited negativity (Fig.
2C).
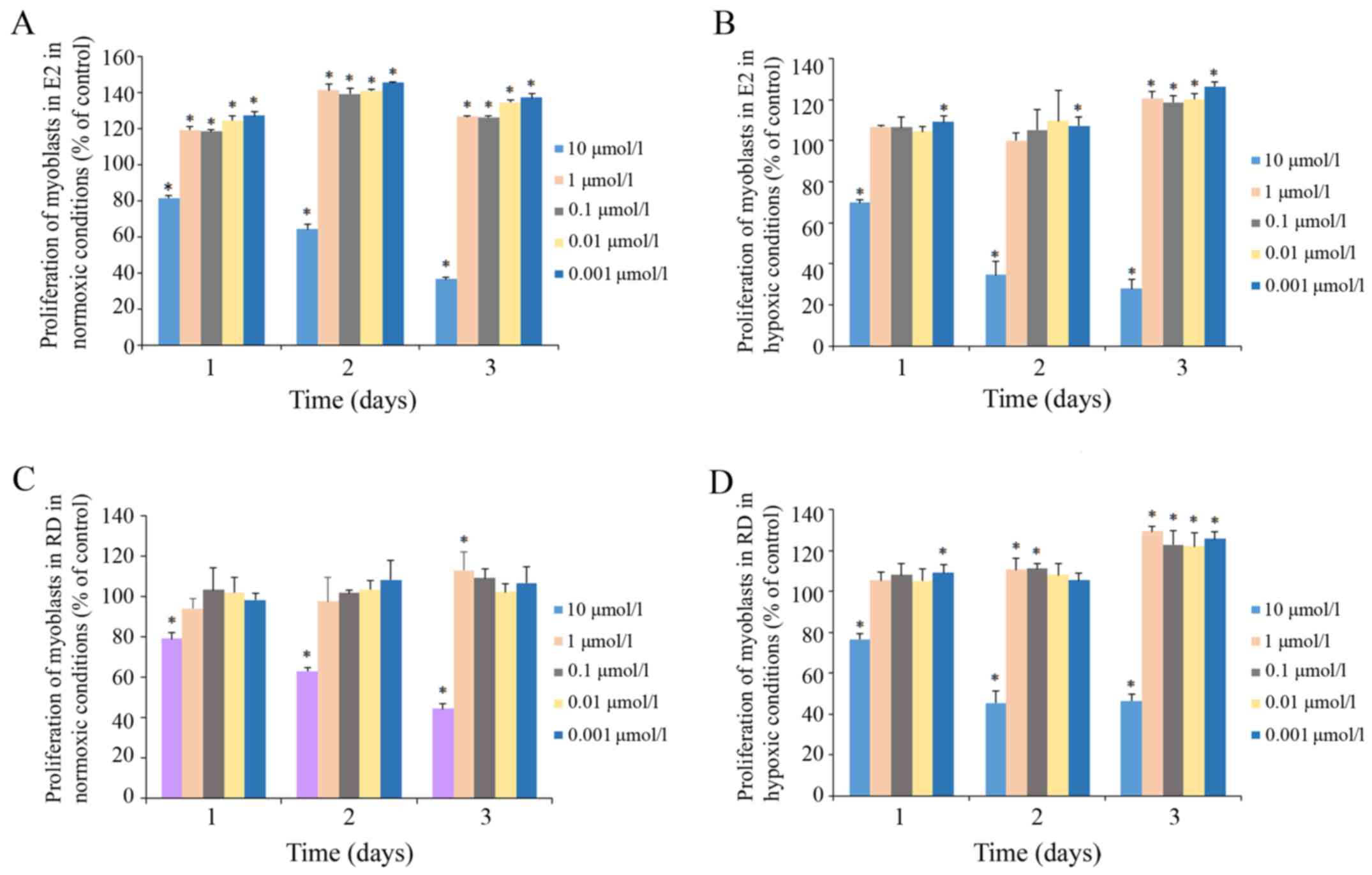
The effects of E2 and RD on genioglossus
myoblast viability were determined by MTT assay (Fig. 3). The exposure of myoblasts to 10
µM E2 resulted in a significant decrease in cell
viability under both normoxic and hypoxic conditions, while at
other lower concentrations E2 had no inhibitory effect
within 72 h. The same trend was found in myoblasts treated with RD.
In addition, E2 at 1, 0.1, 0.01 and 0.001 µM
significantly improved the proliferation under a normoxic condition
within 72 h. Therefore, concentrations of 1 µM were used for
further experiments.
Role of ERα in the effects of
E2 and RD on the expression of HIF-1α
HIF-1α expression at the mRNA
level
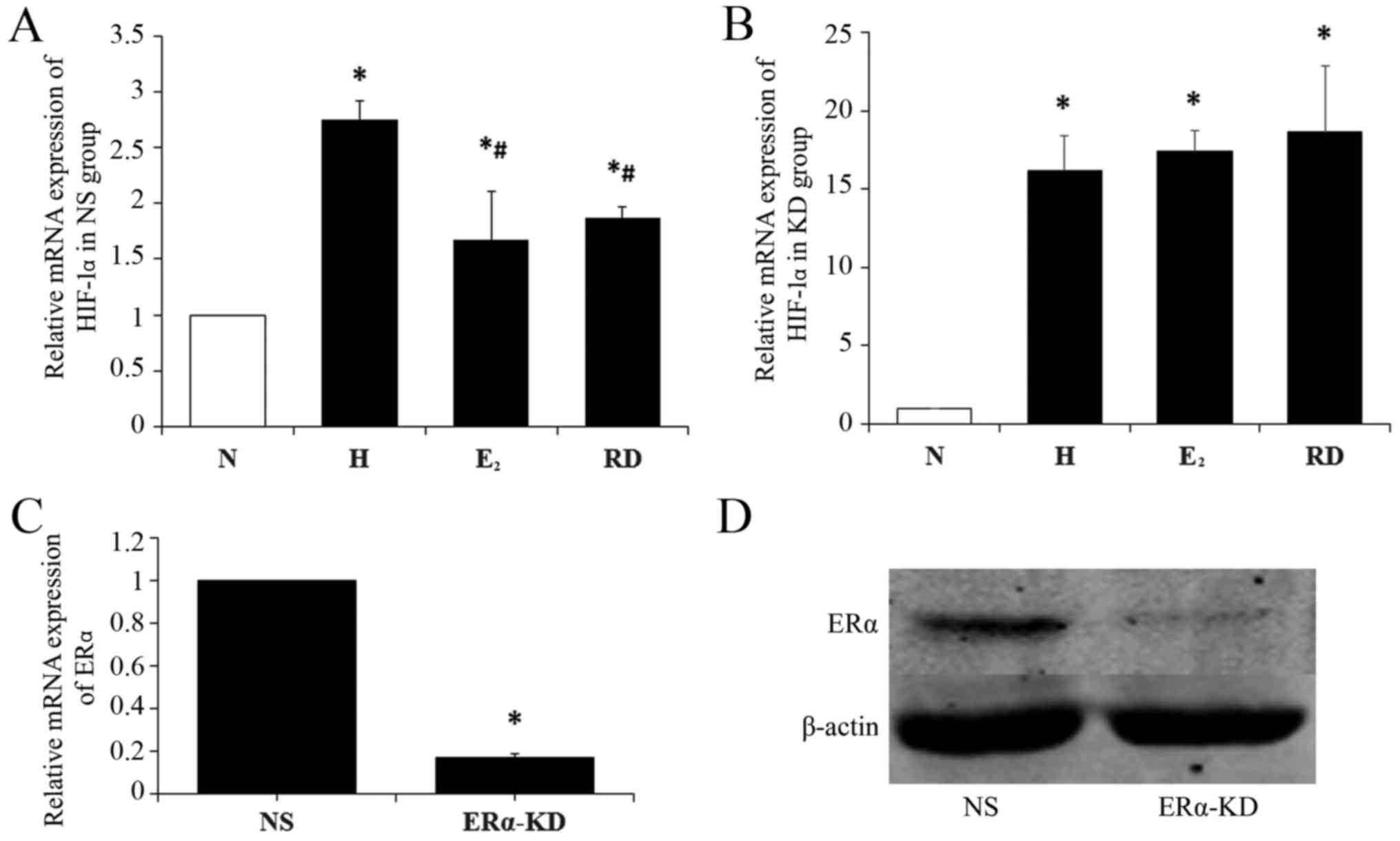
HIF-1α mRNA expression in the genioglossus myoblasts
in hypoxia was significantly higher than that in normoxia
(P<0.05). After the myoblasts were treated with E2,
HIF-1α mRNA was significantly lower than in mere hypoxia
(P<0.05) but still higher than in normoxia (P<0.05). It was
noteworthy that no significant difference was observed in HIF-1α
mRNA between the RD group and E2 group (P>0.05)
(Fig. 4A).
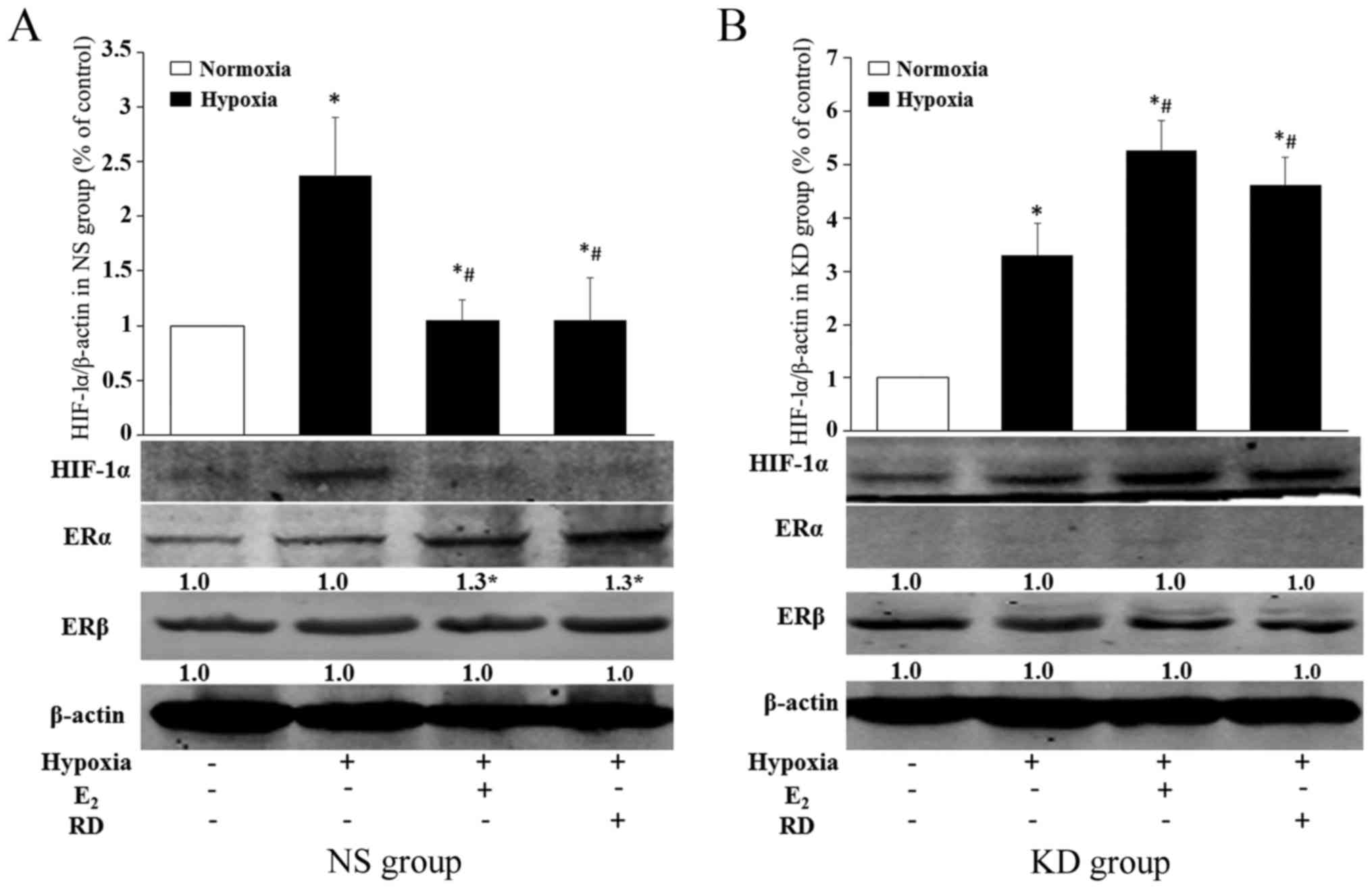
To determine the role of ERα on HIF-1α expression in
myoblasts, HIF-1α mRNAs were analyzed after ERα knockdown (KD
group). The silencing efficiency of ERα was validated by RT-qPCR
(Fig. 4C) and western blot
analysis (Fig. 4D). As shown in
Fig. 4B, hypoxia induced HIF-1α
mRNA expression in myoblasts in the KD group (P<0.05) as in the
NS group, while the inhibitory effects of E2 and RD on
hypoxia induced-HIF-1α were blocked by ERα knockdown, which implies
that the inhibitory effect of E2 and RD on HIF-1α is
mediated by ERα.
HIF-1α expression at the protein
level
The western blot results of HIF-1α expression were
generally in accordance with those of RT-qPCR. In consideration of
the possible effects of E2 or RD on the expression of
ERs, ERα and ERβ protein expression was detected as control. ERα
expression in myoblasts in normoxia and hypoxia was at a similar
level (P>0.05), but both E2 and RD induced ERα
expression notably (Fig. 5A).
Weak expression of ERα was detected in the KD group (Fig. 5B). No significant difference in
ERβ expression was observed in both the NS group and KD group
(Fig. 5). As for HIF-1α
expression, hypoxia induced the HIF-1α protein expression of
myoblasts both in the NS group (P<0.05) and KD group
(P<0.05). As shown in Fig. 5A,
the HIF-1α protein level was significantly lower in the myoblasts
treated with E2 (P<0.05) or RD (P<0.05) than that
in mere hypoxia. After silencing of ERα, however, the level was
higher in E2- or RD-treated myoblasts than under hypoxia
(P<0.05) (Fig. 5B).
Taken together, these results indicated that RD, as
well as E2, inhibited the overexpression of HIF-1α in
hypoxia; ERα knockdown prevented E2- or RD-dependent
HIF-1α suppression.
Role of p38 MAPK pathways in the
effects of E2 on the expression of HIF-1α
Several studies have demonstrated that estrogen can
activate the MAPK pathway in a variety of cell types (25–27). Since the MAPK pathways are widely
linked to the activation of HIF-1α, we aimed to ascertain whether
these pathways are essential in the inhibitory effect of estrogen
on HIF-1α in genioglossus myoblasts.
E2 activates p38 MAPK
pathways in hypoxia via ERα
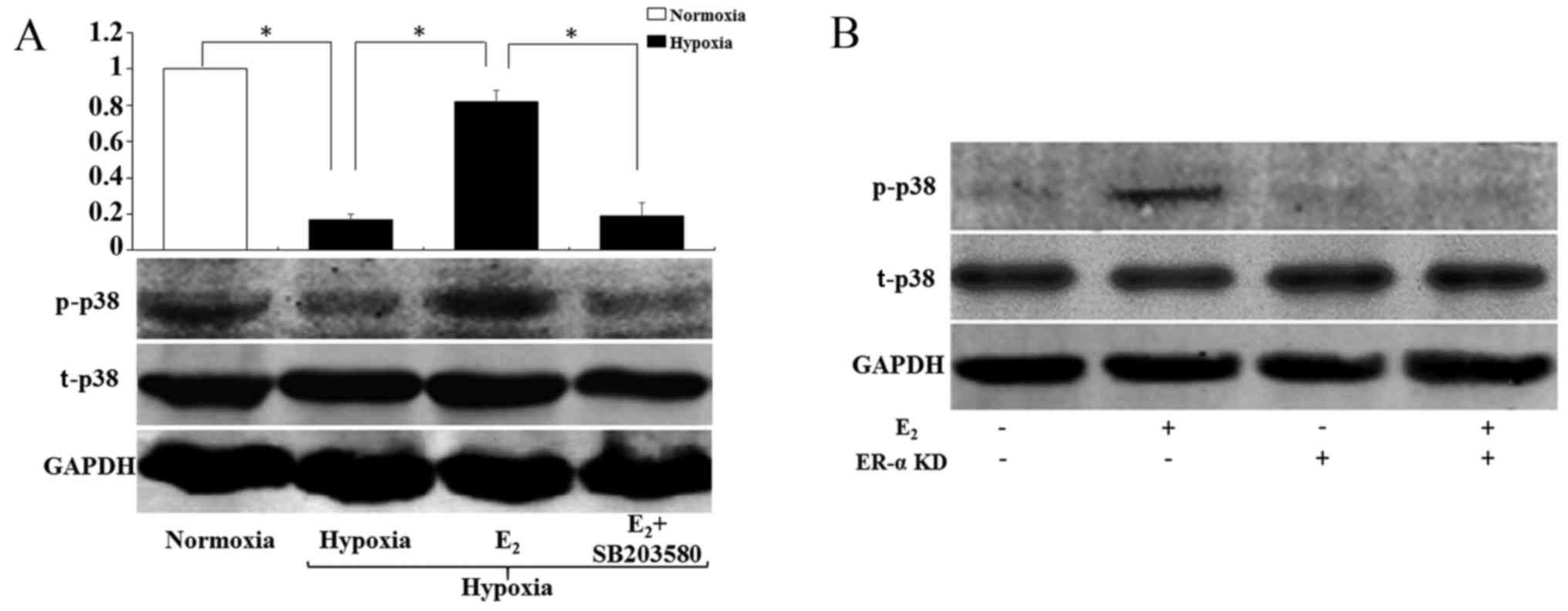
Phosphorylated p38 MAPK (p-p38) (Fig. 6A) expression was significantly
lower in hypoxia than in normoxia (P<0.05), while it was
increased after E2 treatment (P<0.05). As expected,
the effects of E2 were blocked by p38 MAPK inhibitor
SB203580 (P<0.05). Total p38 MAPK expression was not affected by
the different treatments.
To ascertain the role of ERα in
E2-initiated p38 MAPK activation, we knocked down ERα by
siRNA and found that E2 treatment increased
phosphorylation of p38 MAPK in the genioglossus myoblasts, which
was blocked by ERα knockdown (Fig.
6B).
Following inhibition of p38 MAPK,
E2 upregulates HIF-1α expression
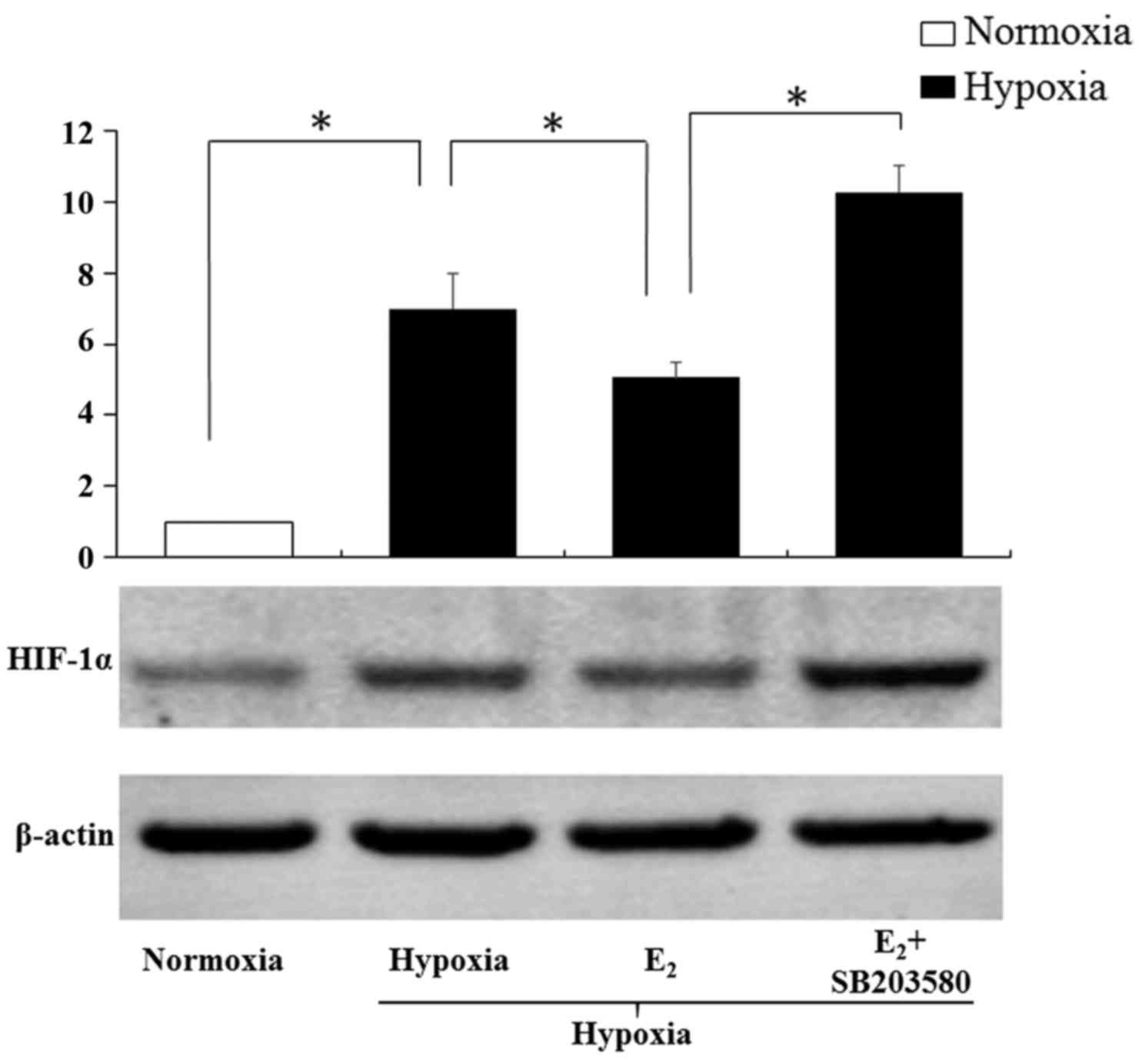
As shown in the Fig.
7, hypoxia induced the HIF-1α protein expression in the
myoblasts (P<0.05), and E2 reverted it partly. The
downregulatory effects of E2 on HIF-1α coincided with
the former results (Fig. 5A).
However, after co-treatment with E2 and SB203580, HIF-1α
protein expression was increased significantly (P<0.05). These
results indicated that p38 MAPK pathways were essential in the
inhibitory effect of E2 on HIF-1α in the genioglossus
myoblasts.
Discussion
It is documented that the biological effect of
estrogen is mediated by ERs. Conventional ERs are transcription
factors regulating gene expression, and these mechanisms may be
mediated by a series of signaling molecules in several cell types.
HIF-1α has been reported to be involved in the hormonal regulation
of genes (28,29). In addition, our previous studies
demonstrated that downregulation of HIF-1α may be a pivotal
explanation for the protective effects of estrogen on the
genioglossus in CIH rats (12,13). Yet, hormonal HIF-1α regulation has
not been clarified. A clear expression of ERα and weak expression
of ERβ in rat genioglossus muscles were detected in our previous
study (unpublished data). Furthermore, the expression of ERα, but
not ERβ, was found to be regulated by E2 in the
genioglossus of rats (30). In
the present study, we revealed that the inhibitory effect of
E2 or RD on HIF-1α expression was ERα-dependent and
extended the association of E2/ERα with HIF-1α. The
discovery of E2- or RD-mediated HIF-1α regulation
contributes to our future understanding of the molecular mechanisms
underlying OSAHS.
Previous studies have described an association
between E2/ERα and HIF-1 expressions in various organs
or cell types. For example, Xu et al reported that estrogen
treatment reduced the expression levels of HIF-1α and vascular
endothelial growth factor (VEGF) and improved the metabolic
syndrome in periaortic and intra-abdominal fat in ovariectomized
rats (31). Miyauchi et al
suggested that HIF-1α protein was stabilized in the absence of
estrogen but destabilized by treatment with E2 under
hypoxia in osteoclasts, and ERα was required for
E2-dependent HIF-1α destabilization (32). Rzemieniec et al confirmed a
pivotal involvement of ERα, but not ERβ or the recently identified
membrane ER G-protein-coupled receptor 30 (GPER), in the
neuroprotective potential of raloxifene, a type of selective
estrogen receptor modulator (SERM), against hypoxia-induced damage
of mouse hippocampal cells (33).
Our results showed that both E2 and RD inhibited HIF-1α
expression in genioglossus myoblasts in a hypoxic condition, and
these effects were eliminated by ERα knockdown. Therefore, we
showed that ERα plays a crucial role in the downregulatory effect
of E2 and RD on HIF-1α. As for RD, although it exhibited
higher binding affinity for ERβ than ERα in our previous study, the
downregulatory effect on HIF-1α in hypoxia is also mediated by
ERα.
The expression of hypoxia-regulated HIF-1α has been
described to be altered along with time (34,35). Therefore, we detected the HIF-1α
expression after 24, 48 and 72 h in the present study and observed
a similar variation pattern of HIF-1α expression at those three
time-points (data not shown). In our study, the protein expression
of ERα and ERβ was detected as control. In the NS group, both
E2 and RD markedly activated ERα expression. It can be
deduced that the transcriptionally active state of ERα increased
through binding to ligands. As shown in Figs. 4 and 5, E2 or RD inhibited the
hypoxia-induced overexpression of HIF-1α, while ERα knockdown
prevented this E2- or RD-dependent HIF-1α suppression.
Actually, the expression of HIF-1α was slightly upregulated in the
absence of ERα, which may be explained by the roles of other ERs.
ERβ is acknowledged to bind to the same nucleotides for DNA
contacts, estrogen-responsive element (ERE), with ERα, but induce
different structural changes of DNA with the help of ligands and
cofactors, and thus display different transcriptional responses
(36). In addition, some studies
suggest that GPER activates a network of transduction pathways
involving the epidermal growth factor receptor (EGFR), the
intracellular cyclic AMP (cAMP), the mitogen-activated protein
kinase (MAPK) cascade, and calcium mobilization (37–39). De Francesco et al reported
that E2 upregulated the expression of HIF-1 at both the
mRNA and protein levels via GRER and the EGFR/ERK/c-fos
transduction pathway in breast cancer cells and cancer-associated
fibroblasts (40). The expression
of GPER was not monitored in our studies, but invariable expression
of ERβ was observed in both the NS and KD group. As such, it is not
unreasonable to suspect that ERβ or GPER could make a contribution
to inducing HIF-1α expression in the absence of ERα in myoblasts.
Clearly, further study on the relationship of HIF-1α and ERs is
required.
Some investigators support that HIF-1α expression
following estrogen excess or deficiency is tissue-specific. Studies
involving E2/ERα and HIF-1 expression in skeletal
muscles, to our knowledge, are much less than those regarding
estrogen reproductive tissues, such as breast, ovary and uterus.
This is the first study to show that ERα is responsible for the
inhibitory effects of E2 and RD on HIF-1α in
genioglossus myoblasts.
To shed more light on the mechanism underlying the
inhibitory effect of E2 on HIF-1α, we extended the
association of E2/ERα with HIF-1α to include an
interaction with p38 MAPK signaling pathways. E2 is
known to be involved in the activation of p38 MAPK signaling
pathways (28,41–43). There are many studies that have
indicated a role of these two pathways in the regulation of HIF-1α
transactivity and synthesis (43,44). We observed that exposure of
genioglossus myoblasts to hypoxia for 24 h significantly inhibited
the phosphorylation of p38 MAPK, illustrating that inactivity of
p38 MAPK signaling pathways may be related to HIF-1α expression. In
the present study, treatment of myoblasts with E2 led to
the generation of phosphorylated p38 MAPK, but the phosphorelation
effect was blunted in the absence of ERα. To determine whether the
signaling pathways lie on the upstream of HIF-1, we observed the
effects of p38 MAPK inhibitor SB203580 on the expression of HIF-1α.
The induction of p38 MAPK phosphorylation by E2 was
blocked by SB203580. Moreover, p38 MAPK inhibitor induced the
expression of HIF-1α even in the existence of E2,
indicating that these signaling molecules were involved in the
inhibitory effect of E2/ERα on HIF-1α expression. To sum
up, these results suggest that activation of the p38 MAPK pathways
plays an important role in the inhibitory effect of E2
on HIF-1α expression.
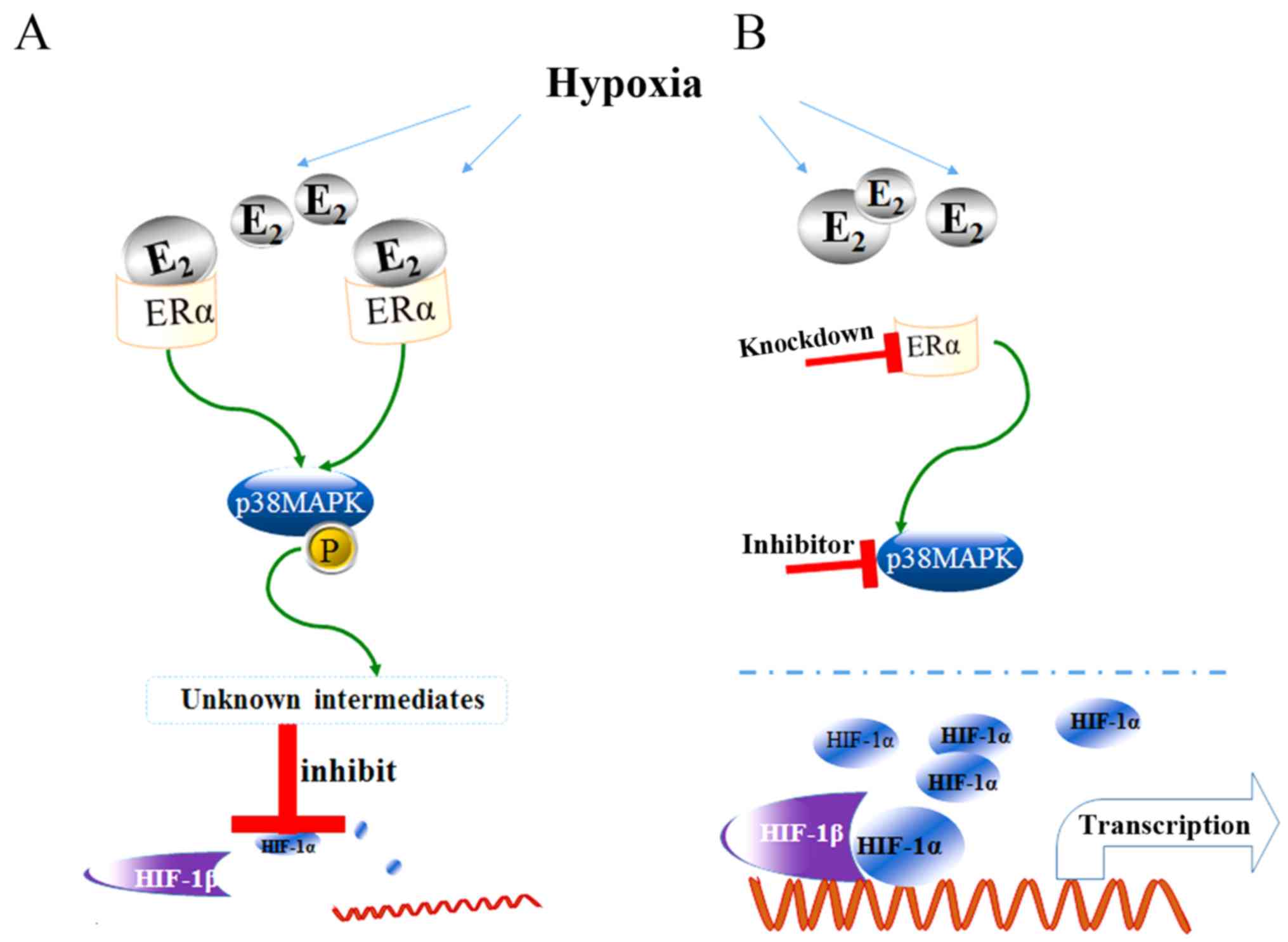
In conclusion, the present study demonstrated that
both E2 and RD inhibited the overexpression of HIF-1α in
genioglossus myoblasts under hypoxic condition. ERα knockdown
prevented the suppression, indicating that ERα may be responsible
for these inhibitory effects. Moreover, activation of the p38 MAPK
pathways may play an important role in the inhibitory effect of
E2 on HIF-1α expression (Fig. 8).
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China, 81271192 (2012). We
are grateful to Professor X. Sun and Dr C. Zhong (School of
Pharmacy, Fudan University, Shanghai, China) for supply of
resveratrol dimers.
References
|
1
|
Eckert DJ and Malhotra A: Pathophysiology
of adult obstructive sleep apnea. Proc Am Thorac Soc. 5:144–153.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Douglas NJ and Polo O: Pathogenesis of
obstructive sleep apnoea/hypopnoea syndrome. Lancet. 344:653–655.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Owens RL, Eckert DJ, Yeh SY and Malhotra
A: Upper airway function in the pathogenesis of obstructive sleep
apnea: A review of the current literature. Curr Opin Pulm Med.
14:519–524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sériès FJ, Simoneau SA, Pierre S St and
Marc I: Characteristics of the genioglossus and musculus uvulae in
sleep apnea hypopnea syndrome and in snorers. Am J Respir Crit Care
Med. 153:1870–1874. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bradford A, McGuire M and O'Halloran KD:
Does episodic hypoxia affect upper airway dilator muscle function?
Implications for the pathophysiology of obstructive sleep apnoea.
Respir Physiol Neurobiol. 147:223–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malhotra A, Pillar G, Fogel RB, Beauregard
J, Edwards JK, Slamowitz DI, Shea SA and White DP: Genioglossal but
not palatal muscle activity relates closely to pharyngeal pressure.
Am J Respir Crit Care Med. 162:1058–1062. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai Z, Manalo DJ, Wei G, Rodriguez ER,
Fox-Talbot K, Lu H, Zweier JL and Semenza GL: Hearts from rodents
exposed to intermittent hypoxia or erythropoietin are protected
against ischemia-reperfusion injury. Cir culation. 108:79–85.
2003.
|
|
8
|
Yuan G, Nanduri J, Bhasker CR, Semenza GL
and Prabhakar NR: Ca2+/calmodulin kinase-dependent
activation of hypoxia inducible factor 1 transcriptional activity
in cells subjected to intermittent hypoxia. J Biol Chem.
280:4321–4328. 2005. View Article : Google Scholar
|
|
9
|
Pae EK, Wu J, Nguyen D, Monti R and Harper
RM: Geniohyoid muscle properties and myosin heavy chain composition
are altered after short-term intermittent hypoxic exposure. J Appl
Physiol (1985). 98:889–894. 2005. View Article : Google Scholar
|
|
10
|
Chen L, Zhang J, Gan TX, Chen-Izu Y,
Hasday JD, Karmazyn M, Balke CW and Scharf SM: Left ventricular
dysfunction and associated cellular injury in rats exposed to
chronic intermittent hypoxia. J Appl Physiol (1985). 104:218–223.
2008. View Article : Google Scholar
|
|
11
|
Stroka DM, Burkhardt T, Desbaillets I,
Wenger RH, Neil DA, Bauer C, Gassmann M and Candinas D: HIF-1 is
expressed in normoxic tissue and displays an organ-specific
regulation under systemic hypoxia. FASEB J. 15:2445–2453.
2001.PubMed/NCBI
|
|
12
|
Zhou J and Liu Y: Effects of genistein and
estrogen on the genioglossus in rats exposed to chronic
intermittent hypoxia may be HIF-1α dependent. Oral Dis. 19:702–711.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia SS and Liu YH: Down-regulation of
hypoxia inducible factor-1 alpha: A possible explanation for the
protective effects of estrogen on genioglossus fatigue resistance.
Eur J Oral Sci. 118:139–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Y, Liu Y and Li Y: Comparison of
natural estrogens and synthetic derivative on genioglossus function
and estrogen receptors expression in rats with chronic intermittent
hypoxia. J Steroid Biochem Mol Biol. 140:71–79. 2014. View Article : Google Scholar
|
|
15
|
Solakidi S, Psarra AM and Sekeris CE:
Differential distribution of glucocorticoid and estrogen receptor
isoforms: Localization of GRbeta and ERalpha in nucleoli and
GRalpha and ERbeta in the mitochondria of human osteosarcoma SaOS-2
and hepatocarcinoma HepG2 cell lines. J Musculoskelet Neuronal
Interact. 7:240–245. 2007.PubMed/NCBI
|
|
16
|
Aschim EL, Saether T, Wiger R, Grotmol T
and Haugen TB: Differential distribution of splice variants of
estrogen receptor beta in human testicular cells suggests specific
functions in spermatogenesis. J Steroid Biochem Mol Biol.
92:97–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Couse JF, Lindzey J, Grandien K,
Gustafsson JA and Korach KS: Tissue distribution and quantitative
analysis of estrogen receptor-alpha (ERalpha) and estrogen
receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type
and ERalpha-knockout mouse. Endocrinology. 138:4613–4621. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalbe C, Mau M, Wollenhaupt K and Rehfeldt
C: Evidence for estrogen receptor alpha and beta expression in
skeletal muscle of pigs. Histochem Cell Biol. 127:95–107. 2007.
View Article : Google Scholar
|
|
19
|
Veasey SC, Guilleminault C, Strohl KP,
Sanders MH, Ballard RD and Magalang UJ: Medical therapy for
obstructive sleep apnea: A review by the Medical Therapy for
Obstructive Sleep Apnea Task Force of the Standards of Practice
Committee of the American Academy of Sleep Medicine. Sleep.
29:1036–1044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bixler EO, Vgontzas AN, Lin HM, Ten Have
T, Rein J, Vela-Bueno A and Kales A: Prevalence of sleep-disordered
breathing in women: Effects of gender. Am J Respir Crit Care Med.
163:608–613. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pickett CK, Regensteiner JG, Woodard WD,
Hagerman DD, Weil JV and Moore LG: Progestin and estrogen reduce
sleep-disordered breathing in postmenopausal women. J Appl Physiol
(1985). 66:1656–1661. 1989.
|
|
22
|
Popovic RM and White DP: Upper airway
muscle activity in normal women: Influence of hormonal status. J
Appl Physiol (1985). 84:1055–1062. 1998.
|
|
23
|
Patisaul HB and Jefferson W: The pros and
cons of phytoestrogens. Front Neuroendocrinol. 31:400–419. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhong C, Zhu J, Chang J and Sun X: Concise
total syntheses of (±)isopaucifloral F, (±)quadrangularin A, and
(±)pallidol. Tetrahedron Lett. 52:2815–2817. 2011. View Article : Google Scholar
|
|
25
|
Bi R, Broutman G, Foy MR, Thompson RF and
Baudry M: The tyrosine kinase and mitogen-activated protein kinase
pathways mediate multiple effects of estrogen in hippocampus. Proc
Natl Acad Sci USA. 97:3602–3607. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song RX, McPherson RA, Adam L, Bao Y,
Shupnik M, Kumar R and Santen RJ: Linkage of rapid estrogen action
to MAPK activation by ERalpha-Shc association and Shc pathway
activation. Mol Endocrinol. 16:116–127. 2002.PubMed/NCBI
|
|
27
|
Chen CC, Lee WR and Safe S: Egr-1 is
activated by 17beta-estradiol in MCF-7 cells by mitogen-activated
protein kinase-dependent phosphorylation of ELK-1. J Cell Biochem.
93:1063–1074. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kazi AA and Koos RD: Estrogen-induced
activation of hypoxia-inducible factor-1alpha, vascular endothelial
growth factor expression, and edema in the uterus are mediated by
the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology.
148:2363–2374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yun SP, Lee MY, Ryu JM, Song CH and Han
HJ: Role of HIF-1alpha and VEGF in human mesenchymal stem cell
proliferation by 17beta-estradiol: Involvement of PKC, PI3K/Akt,
and MAPKs. Am J Physiol Cell Physiol. 296:C317–C326. 2009.
View Article : Google Scholar
|
|
30
|
Hou YX, Jia SS and Liu YH:
17beta-Estradiol accentuates contractility of rat genioglossal
muscle via regulation of estrogen receptor alpha. Arch Oral Biol.
55:309–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu J, Xiang Q, Lin G, Fu X, Zhou K, Jiang
P, Zheng S and Wang T: Estrogen improved metabolic syndrome through
down-regulation of VEGF and HIF-1α to inhibit hypoxia of periaortic
and intra-abdominal fat in ovariectomized female rats. Mol Biol
Rep. 39:8177–8185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyauchi Y, Sato Y, Kobayashi T, Yoshida
S, Mori T, Kanagawa H, Katsuyama E, Fujie A, Hao W, Miyamoto K, et
al: HIF1α is required for osteoclast activation by estrogen
deficiency in postmenopausal osteoporosis. Proc Natl Acad Sci USA.
110:16568–16573. 2013. View Article : Google Scholar
|
|
33
|
Rzemieniec J, Litwa E, Wnuk A, Lason W,
Gołas A, Krzeptowski W and Kajta M: Neuroprotective action of
raloxifene against hypoxia-induced damage in mouse hippocampal
cells depends on ERα but not ERβ or GPR30 signalling. J Steroid
Biochem Mol Biol. 146:26–37. 2015. View Article : Google Scholar
|
|
34
|
Camps C, Saini HK, Mole DR, Choudhry H,
Reczko M, Guerra-Assunção JA, Tian YM, Buffa FM, Harris AL,
Hatzigeorgiou AG, et al: Integrated analysis of microRNA and mRNA
expression and association with HIF binding reveals the complexity
of microRNA expression regulation under hypoxia. Mol Cancer.
13:282014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chung HY, Lee SJ, Lee JM, Huh S, Kim HK,
Kwon OH, Lim HJ, Oh EJ, Kim TJ, O TM, et al: Expression patterns of
HIF-1alpha under hypoxia in vascular smooth muscle cells of venous
malformations. Ann Plast Surg. 75:332–7. 2015. View Article : Google Scholar
|
|
36
|
Yi P, Driscoll MD, Huang J, Bhagat S, Hilf
R, Bambara RA and Muyan M: The effects of estrogen-responsive
element- and ligand-induced structural changes on the recruitment
of cofactors and transcriptional responses by ER alpha and ER beta.
Mol Endocrinol. 16:674–693. 2002.PubMed/NCBI
|
|
37
|
Filardo EJ, Quinn JA, Bland KI and
Frackelton AR Jr: Estrogen-induced activation of Erk-1 and Erk-2
requires the G protein-coupled receptor homolog, GPR30, and occurs
via trans-activation of the epidermal growth factor receptor
through release of HB-EGF. Mol Endocrinol. 14:1649–1660. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thomas P, Pang Y, Filardo EJ and Dong J:
Identity of an estrogen membrane receptor coupled to a G protein in
human breast cancer cells. Endocrinology. 146:624–632. 2005.
View Article : Google Scholar
|
|
39
|
Revankar CM, Cimino DF, Sklar LA,
Arterburn JB and Prossnitz ER: A transmembrane intracellular
estrogen receptor mediates rapid cell signaling. Science.
307:1625–1630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Francesco EM, Pellegrino M, Santolla
MF, Lappano R, Ricchio E, Abonante S and Maggiolini M: GPER
mediates activation of HIF1α/VEGF signaling by estrogens. Cancer
Res. 74:4053–4064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guzeloglu Kayisli O, Kayisli UA, Luleci G
and Arici A: In vivo and in vitro regulation of Akt activation in
human endometrial cells is estrogen dependent. Biol Reprod.
71:714–721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Linford NJ, Yang Y, Cook DG and Dorsa DM:
Neuronal apoptosis resulting from high doses of the isoflavone
genistein: Role for calcium and p42/44 mitogen-activated protein
kinase. J Pharmacol Exp Ther. 299:67–75. 2001.PubMed/NCBI
|
|
43
|
Salceda S, Beck I, Srinivas V and Caro J:
Complex role of protein phosphorylation in gene activation by
hypoxia. Kidney Int. 51:556–559. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mazure NM, Chen EY, Laderoute KR and
Giaccia AJ: Induction of vascular endothelial growth factor by
hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt
signaling pathway in Ha-ras-transformed cells through a hypoxia
inducible factor-1 transcriptional element. Blood. 90:3322–3331.
1997.PubMed/NCBI
|