Introduction
Ischemic cerebrovascular disease, such as ischemic
stroke, is a leading cause of death in developed countries and can
severely impact affected individuals (1,2).
Ischemic stroke is primarily caused by deprivation of blood flow to
regions of the brain, and results in deficiencies in oxygen and
glucose supply (3). During
ischemic stroke, mitochondrial dysfunction and calcium-activated
proteolysis, accompanied by a complex cascade of damaging events
including the generation of reactive oxygen species (ROS),
ultimately led to the death of neurons (4–7).
Therefore, targeting of the oxidant signaling pathway and
antioxidant treatments may represent a novel therapeutic strategy
for the treatment of ischemic cerebrovascular disease.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is a basic leucine zipper protein and a redox-sensitive member of
the cap'n'collar family of transcription factors (8,9).
It has been demonstrated in previous studies that Nrf2 and the
antioxidant response element (ARE) pathway exerted protective
effects against the generation of oxidative stress in mammalian
neurons (10,11). The activity of Nrf2 is negatively
regulated by kelch-like ECH-associated protein 1 (Keap1). Under
basal conditions, Nrf2 is primarily localized in the cytoplasm and
is associated with Keap1 (12).
This Keap1/Nrf2 complex is targeted for ubiquitination and
proteasomal degradation, thereby maintaining quiescence of Nrf2
activity (13). In response to
stress, Nrf2 is released from Keap1 via phosphorylation and
translocates to the nucleus to carry out its transcriptional
activities (14). Therefore, the
discovery of new molecules that modulate the Nrf2/ARE pathway may
aid in designing new strategies for the treatment of oxidative
stress-related diseases.
The flavanone naringenin (NAR) is a major
antioxidant present in citrus fruits, and has been found to exert
antioxidant, anticarcinogenic and antimutagenic effects (15). Several studies have documented
that NAR inhibits the growth of various types of cancer cells
through the inhibition of cell proliferation and activation of
apoptosis (16,17). Regarding its antioxidant effect,
research has indicated that NAR may reduce lactate dehydrogenase
leakage and ROS generation, increase mitochondrial membrane
potential, and reduce caspase-3/7 activity and DNA damage (18,19). However, the effects of NAR on
ischemic cerebrovascular disease, particularly ischemic stroke,
remain unclear. In the present study, the authors demonstrated that
NAR could reduce oxidative stress and improve the mitochondrial
dysfunction in vitro via activation of the Nrf2/ARE
signaling pathway in neurons.
Materials and methods
Cell culture and treatments
A total of 10 neonatal Sprague-Dawley (SD) rats
(weighing, 5–6 g, uncertain gender, fed by breastfeeding, kept in
an environment at 21±2°C) were obtained from the Experimental
Animal Center of Southern Medical University, (Guangzhou, China).
The institutional review boards of all participating centers
approved the protocols used in the study, and all experimental
protocols were approved by the Review Committee for the Use of
Human or Animal Subjects of Southern Medical University (Guangzhou,
China; permit number: 2016213). Neurons were isolated through
enzymatic digestion of the brain tissue of the rats, as previously
described (20). Briefly, the
rats were sacrificed by an overdose of 1% sodium pentobarbital (40
mg/kg per SD rat) administered via intraperitoneal injection, and
then the cerebral cortices from neonatal SD rats (0–2 days old)
were dissected and trypsinized with 0.125% trypsin for 30 min at
37°C. Samples were passed through a 200-μm mesh sieve and
cells were collected by centrifugation (500 × g for 5 min). Cells
were resuspended in Dulbecco's modified Eagle's medium (DMEM) with
10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml streptomycin and 100 U/ml penicillin,
then seeded onto poly-D-lysine-coated 25-mm dishes and cultured at
37°C in a 5% CO2 atmosphere. Culture medium was
replenished twice a week with fresh complete DMEM.
At 24 h after plating, neurons were divided into the
following five groups: Control, model, NAR-L (low-dose), NAR-M
(medium-dose) and NAR-H (high-dose). Neurons in the control group
were cultured under normal conditions for 6 days. Neurons in the
model group were cultured under normal conditions for 4 days,
followed by oxygen deprivation (90% N2, 5% O2
and 5% CO2) for 12 h and oxygen restoration (95% air and
5% CO2) for 36 h. In the NAR treatment groups, neurons
were cultured under normal conditions for 4 days, then co-cultured
with different concentrations of NAR (20 μM for NAR-L, 40
μM for NAR-M and 80 μM for NAR-H) for 8 h, followed
by oxygen deprivation for 12 h and oxygen restoration for 36 h. In
the time-course assay, neurons were co-cultured with 80 μM
NAR for 0, 2, 4 and 8 h prior to oxygen deprivation/reoxygenation,
and assessed by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blot analysis.
Measurement of ROS
The generation of ROS was measured by
chloromethyl-2′,7′dichlorodihydro fluorescein diacetate
(CM-H2DCFDA) staining. For CM-H2DCFDA
staining, cells were washed with ice cold PBS and treated with 10
μM CM-H2DCFDA (Invitrogen, Thermo Fisher
Scientific) in DMEM for 45 min. Cells were then trypsinized and
suspended in phosphate-buffered saline (PBS) and fluorescence was
measured at 538 nm. Furthermore, the activities of superoxide
dismutase 1 (SOD1) and glutathione (GSH), and the content of
malondialdehyde (MDA), in the different groups were determined to
assess the anti-oxidative effects of NAR using commercial kits
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China).
Determination of mitochondrial adenine
nucleotide trans-locase (ANT) transport activity in neurons
The authors determined mitochondrial ANT transport
activity using an inhibitor termination method. A total of 20
μl 3H-ADP solution (0.3 μmol/l) was added
into 50 μl of prepared mitochondrial suspension for 10 sec,
then 50 μl of the ANT inhibitor atrac-tyloside (3.2 nmol/l)
was added and mixed immediately to terminate ANT transporter
function. The mixture was centrifuged for 20 min at 3,000 × g and
4°C and the supernatant was discarded. After resuspension and
washing with 1 ml of separation medium, 400 μl
H2O2 (8.8 mol/l) was added to the
precipitate, which was subsequently incubated in a 70°C water bath
for 40 min. ANT transport activity was measured using the liquid
scintillation counting method (Triathler liquid scintillation
counter; Triathler, Turku, Finland). ANT transport activity is
positive proportional to the number of scintillation counts
(dpm/sec), and the number of scintillation counts can reflect the
change in ANT transport activity of different groups.
Measurement of mitochondrial membrane
potential (Δψm)
The authors used JC-1 staining to measure
mitochondrial depolarization in neurons of the control, model,
NAR-L, NAR-M and NAR-H groups. Following treatment, 200 μl
cells were collected and incubated for 20 min with an equal volume
of JC-1 staining solution at 37°C, then rinsed twice with PBS. Δψm
was determined by measuring the relative amount of dual emissions
from mitochondrial JC-1 monomers or aggregates using flow cytometry
(BD Biosciences, Franklin Lakes, NJ, USA). Δψm was presented in
units of fluorescence intensity.
Analysis of adenylate levels in
neurons
The levels of adenine nucleotides in the neurons of
each group were determined by high-performance liquid
chromatography (HPLC), according to the method described by Yang
et al (21). The following
conditions were used for HPLC: wavelength, 254 nm; sensitivity,
0.01 AUFS; mobile phase, 2 mmol/l PBS (pH 5.5); and speed, 1
ml/min. A YWG-ODS C18 (10 μm) column (4.6×250 nm) was
used.
Cell transfection
Cells were harvested during the logarithmic growth
phase. Small interfering RNAs (siRNAs) against Nrf2 (siNfr2) and
negative control siRNAs (siRNA-NC) were purchased from Shanghai
GenePharma Co., Ltd. (Shanghai, China) and transfected into cells
using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific),
according to the manufacturer's protocol. The sequences of siNrf2
and siRNA-NC are presented in Table
I. Following transfection, western blotting was used to assess
the transfection efficiency, and model and NAR treatment groups
were established prior to further experiments.
 | Table IThe sequences of siRNAs used in the
present study. |
Table I
The sequences of siRNAs used in the
present study.
| Gene | Name | Sequence
(5′-3′) |
|---|
| siNrf2 | siNrf2-sense |
TGGAGCAAGTTTGGCAGGAGCTATT |
|
siNrf2-antisense |
AATAGCTCCTGCCAAACTTGCTCCA |
| siRNA-NC | Control sense |
GGAAAUCGAGUUCGCCGUU |
| Control
antisense |
AACGGCGAACUCGAUUUCC |
Measurement of cell proliferation
Cell viability was determined using an MTT assay.
Briefly, neurons were plated onto 96-well plates at a density of
1×104 cells/well. Following treatment, 20 μl MTT
(5 mg/ml) was added to each well and incubated for 4 h. The medium
was then removed and the formazan crystals were dissolved with
dimethyl sulfoxide (DMSO). Absorbance was read at 570 nm with a
microplate reader (Multiskan Spectrum; Thermo Fisher
Scientific).
Flow cytometry
Apoptotic cells were recognized and distinguished
from normal cells using an Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis kit for flow cytometric
analysis, according to the manufacturer's instructions (Invitrogen,
Thermo Fisher Scientific). After treatment, neurons were harvested
and washed twice with cold PBS, then incubated with 5 μl
FITC-Annexin V and 5 μl PI working solution (100
μg/ml) for 15 min in the dark at room temperature. Cellular
fluorescence was measured by flow cytometric analysis (BD Accuri
C6; BD Biosciences) (22).
Western blotting
Neurons were lysed in a radioimmunoprecipitation
assay buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40]
containing a protease inhibitor cocktail (Roche Diagnostics GmbH,
Basel, Switzerland) using standard procedures. The total proteins
concentration was determined using a Bio-Rad protein assay system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). A total of 50
μg proteins were subjected to 12% SDS-PAGE, and then
transferred onto nitrocellulose membranes (Millipore, Billerica,
MA, USA). The membranes were blocked with 5% (w/v) skimmed milk in
0.05% Tris-buffered saline with Tween-20 at room temperature for 2
h. Immunoblotting was performed with primary antibodies against
Nrf2 (ab31163, dilution 1:1,000; Abcam, Cambridge, MA, USA) and its
downstream targets heme oxygenase 1 (HO-1, ab1324, dilution 1:250;
Abcam) and NAD(P)H quinone dehydrogenase 1 (NQO1, ab28947, dilution
1:1,000; Abcam). β-actin levels were used for normalization. The
protein bands were scanned using ECL western blotting detection
reagents (Pierce, Rockford, IL, USA) and quantified using a
ChemiDoc image analysis system (Bio-Rad Laboratories).
RNA isolation and RT-qPCR analysis
Total RNA was extracted from the cultured neurons of
each group using TRIzol reagent (Invitrogen, Thermo Fisher
Scientific). A cDNA Synthesis kit (Takara Biotechnology Co., Ltd.,
Dalian, China) was used to synthesize cDNA according to the
manufacturer's instructions. qPCR was performed using an iCycleriQ
Detection System (Bio-Rad Laboratories) and interaction dye
SYBR-Green. The sequences of the primers used are listed in
Table II. GAPDH mRNA levels were
used for normalization. DNA was amplified with an initial
denaturation at 95°C for 5 min, followed by 39 cycles of 95°C (15
sec) and 60°C (15 sec). RT-qPCR data were analyzed and expressed as
relative mRNA levels using CT values (23) and were subsequently converted to
fold changes.
 | Table IIPrimers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table II
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Sequence
(5′-3′) |
|---|
| Cytochrome
c | F:
CAACAAGAACAAAGGTATCACC |
| R:
GGTGATACCTTTGTTCTTGTTG |
| Caspase-3 | F:
CACTGGAATGTCAGCTCGCAATG |
| R:
CAGGTCCACAGGTCCGTTCGTTC |
| Bax | F:
CAAGAAGCTGAGCGAGTGTCTCA |
| R:
GTCTGCAAACATGTCAGCTGCCAC |
| Bcl-2 | F:
TGAAGTACATCCATTATAAGCTG |
| R:
CAGGTGGACCACAGGTGGCACA |
| Nrf2 | F:
GCAACTCCAGAAGGAACAGG |
| R:
AGGCATCTTGTTTGGGAATG |
| HO-1 | F:
AAGATTGCGCAGAAGGCCATGG |
| R:
GTCTTTGTGTTCCTCTGTCAGCAG |
| NQO1 | F:
GCAGCGGCTCCATGTACTC |
| R:
CCAGTTGAGGTTCTAAGAC |
| β-actin | F:
GTCCACACCCGCCACCAGTTC |
| R:
TCCCACCATCACACCCTGGTG |
Statistical analysis
All the original experimental data were analyzed
using the SPSS software (version 19.0; IBM SPSS, Armonk, NY, USA).
Comparisons among the groups were performed by one-way analysis of
variance (ANOVA), and Fisher's least significant difference test
was used for comparisons between two groups. P<0.05 was
considered to indicate statistically significant differences.
Results
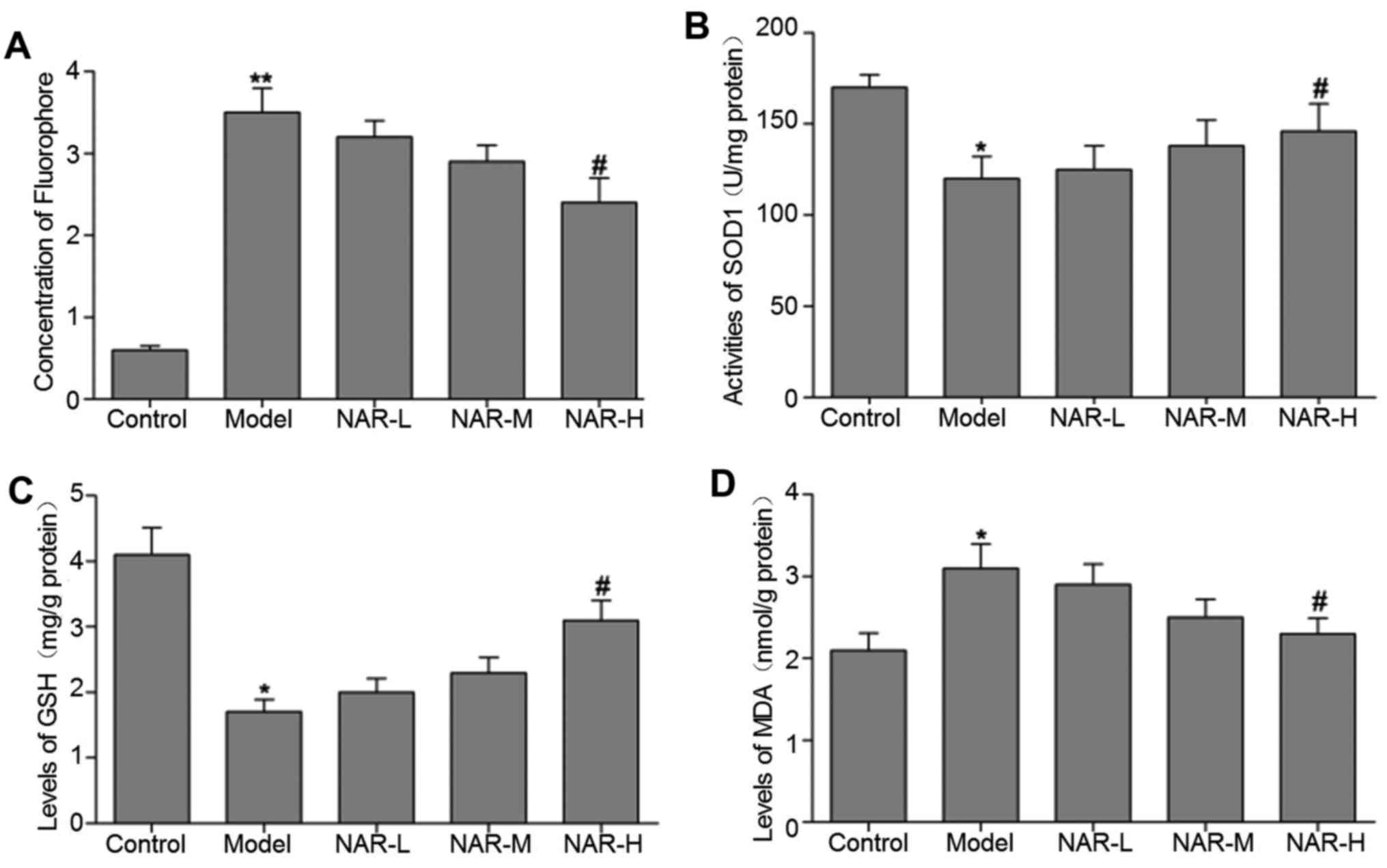
NAR reduces oxidative stress
To assess the antioxidant effect of NAR, ROS levels
in the neurons of the different cell groups were analyzed by
CM-H2DCFDA staining. As presented in Fig. 1A, the authors observed an
increased accumulation of ROS in the model group when compared with
the control group (P<0.01). In turn, the levels of ROS were
significantly decreased in the NAR-H group when compared with the
model group (P<0.05). Additionally, compared with the model
group, the high-dose NAR group exhibited significant increases in
the activities of SOD1 and GSH (P<0.05; Fig. 1B and C). Furthermore, the level of
MDA was significantly decreased in the NAR-H group when compared
with the model group (P<0.05; Fig.
1D). The levels of MDA and ROS, and activities of SOD1 and GSH,
did not differ significantly between the NAR-L, NAR-M and model
groups (P>0.05).
NAR attenuates mitochondrial
dysfunction
Mitochondria are considered to be a major producer
of ROS in mammalian cells, and overproduction of ROS may be an
indicator of mitochondrial dysfunction (24). Thus, the authors initially
measured the concentration of adenylate in the different cell
groups using a HPLC method. The data indicated that, compared with
the control group, the levels of ATP, ADP and AMP decreased
significantly in the model group, whereas the NAR-H groups
exhibited notable improvements in adenylate levels when compared
with the model group (Table
III; P<0.05).
 | Table IIIEffect of naringenin on the
concentration of adenylate. |
Table III
Effect of naringenin on the
concentration of adenylate.
| Group | ATP
(nmol/mg) | ADP
(nmol/mg) | AMP
(nmol/mg) |
|---|
| Control | 2.84±0.21 | 4.48±0.31 | 4.88±0.38 |
| Model | 1.11±0.11a | 1.74±0.15a | 3.53±0.28a |
| NAR-L | 1.26±0.11 | 1.96±0.18 | 3.61±0.29 |
| NAR-M | 1.63±0.09 | 2.36±0.16 | 3.77±0.33 |
| NAR-H | 2.46±0.19b | 3.93±0.34b | 4.37±0.35b |
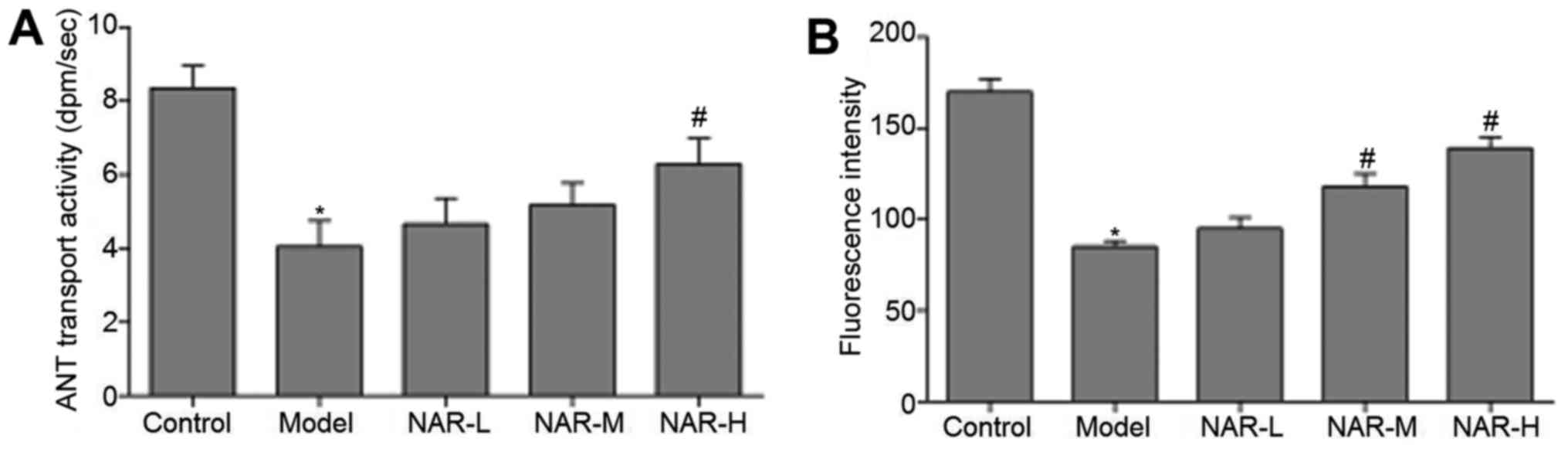
ANT is a transporter of ATP and ADP in
the mitochondrial membrane
ANT transport activity can reflect ATP synthesis and
normal cell function. Therefore, the authors subsequently detected
mitochondrial ANT transport activity in the neurons of the
different cell groups. The data indicated that mitochondrial ANT
transport activity was significantly decreased in the model group
when compared with the control group (P<0.05), and significantly
increased in NAR-H group when compared with the model group
(P<0.05). However, ANT transport activity did not differ
significantly between the NAR-L, NAR-M and model groups
(P>0.05). These results are presented in Fig. 2A.
Δψm was also analyzed by JC-1
staining
As in Fig. 2B, Δψm
was significantly reduced in the model group when compared with the
control group (P<0.05), while Δψm was markedly increased in the
NAR-M and NAR-H groups when compared with the model group
(P<0.05). Δψm did not differ significantly in the NAR-L group
(P>0.05).
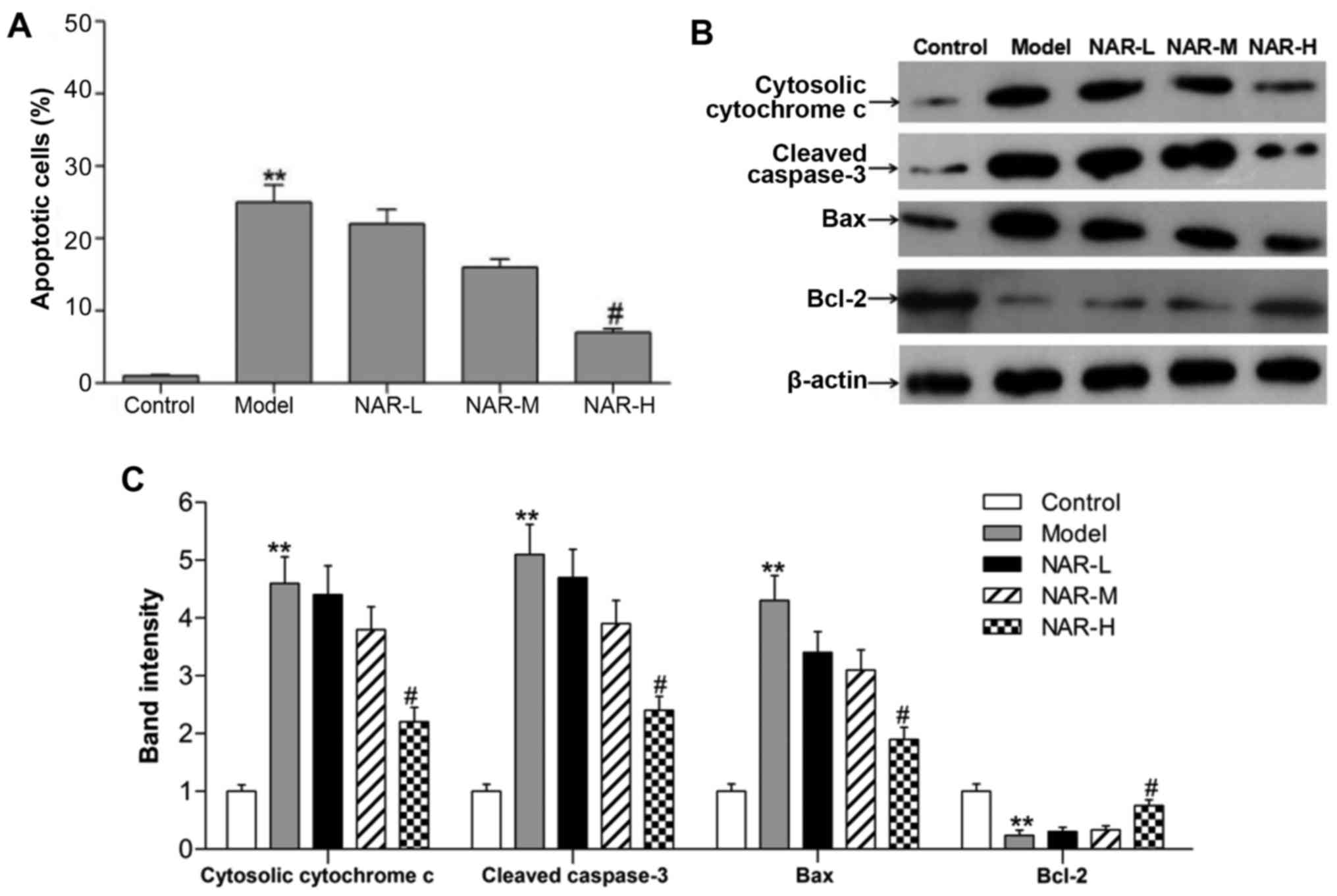
NAR increases cell viability and
decreases cell apoptosis
It is well established that intracellular oxidative
stress and mitochondrial dysfunction cause cell injury and
neurotoxicity (25). Therefore,
flow cytometry was performed to assess cell apoptosis in the
different groups. As shown in Fig.
3A, the number of apoptotic cells was significantly increased
in the model group when compared with the control group
(P<0.01), while the number of apoptotic cells was significantly
decreased in the NAR-H group when compared with the model group
(P<0.05). To verify results of the apoptosis assay, western blot
analysis was performed to detect markers of apoptosis, namely
B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax),
cytosolic cytochrome c and cleaved caspase-3 (Fig. 3B and C). A strong increase was
observed in the expression of Bax and reduced expression of Bcl-2
in the model group (P<0.01). Furthermore, the expression levels
of cytosolic cytochrome c and cleaved caspase-3 were
markedly increased in the model group (P<0.01). However,
high-dose NAR could reverse these expression changes (P<0.05).
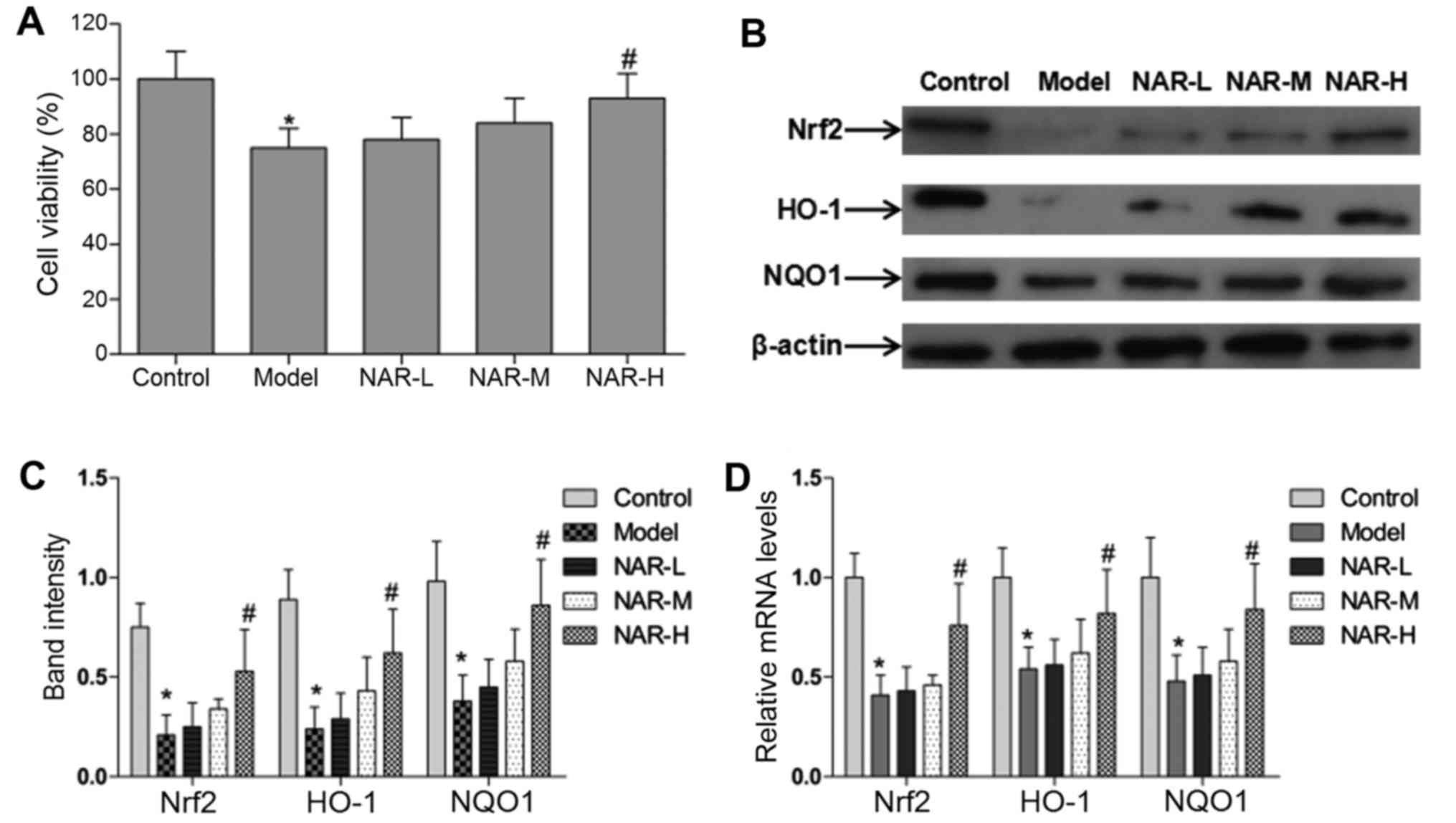
An MTT method was subsequently used to measure cell viability in
the different groups. The data indicated that the viability of
cells was significantly decreased in the model group and increased
in the NAR-H group (P<0.05; Fig.
4A). However, neuronal viability, along with the rate and
markers of apoptosis, did not differ significantly in the NAR-L and
NAR-M groups (P>0.05).
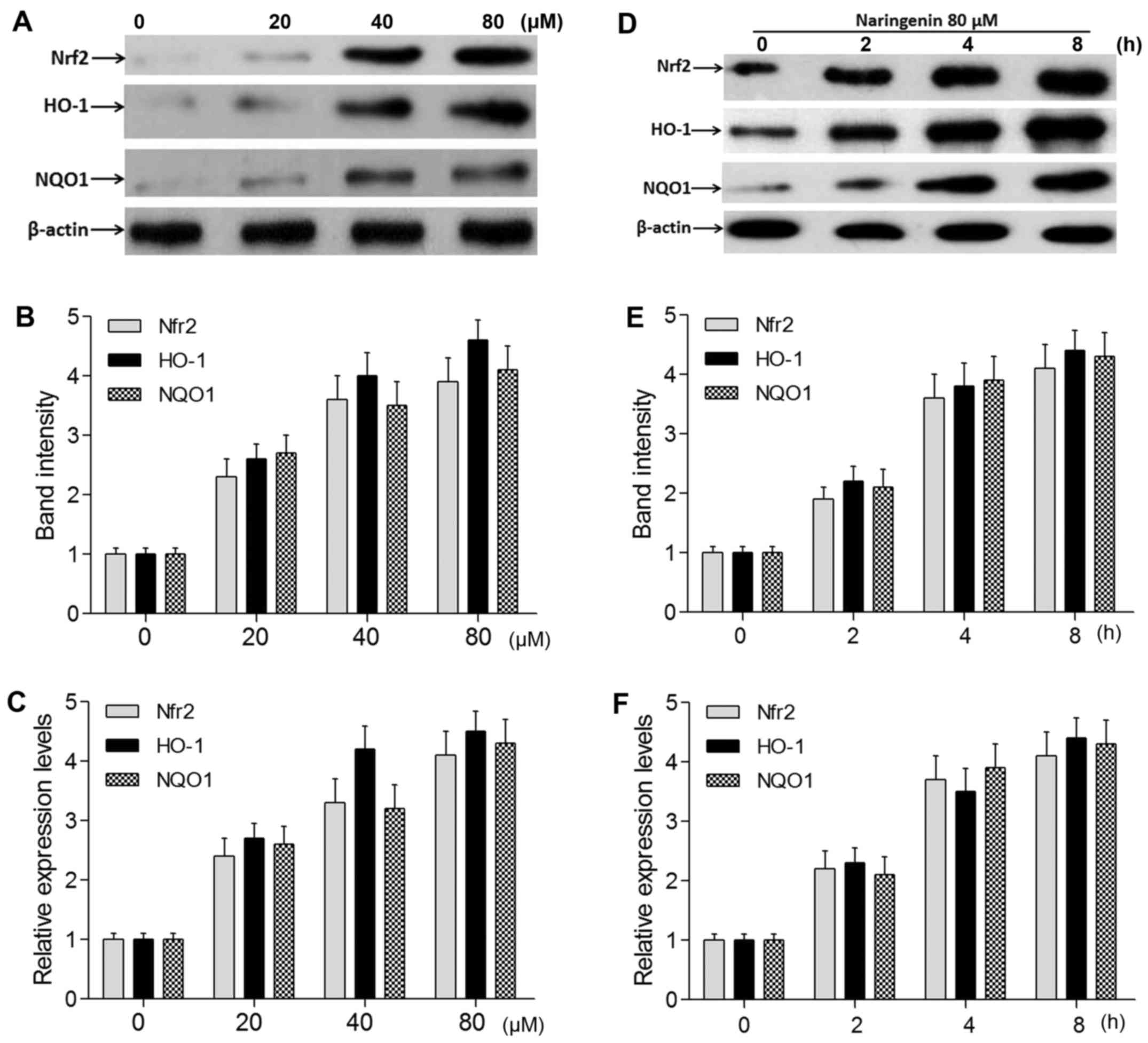
NAR increases the gene expression of Nrf2 and its
target genes. The authors performed RT-qPCR and western blot
analysis to measure the expression level of Nrf2 and its target
genes in oxidative stressed neurons following treatment with
different concentrations of NAR. The experiments demonstrated that
the protein and mRNA levels of Nrf2 and its target genes HO-1 and
NQO1 were significantly decreased in the model group and increased
in the NAR-H group (P<0.05; Fig.
4B–D). In addition, NAR induced the transcription of Nrf2, HO-1
and NQO1 in an apparent dose- and time-dependent manner (Fig. 5).
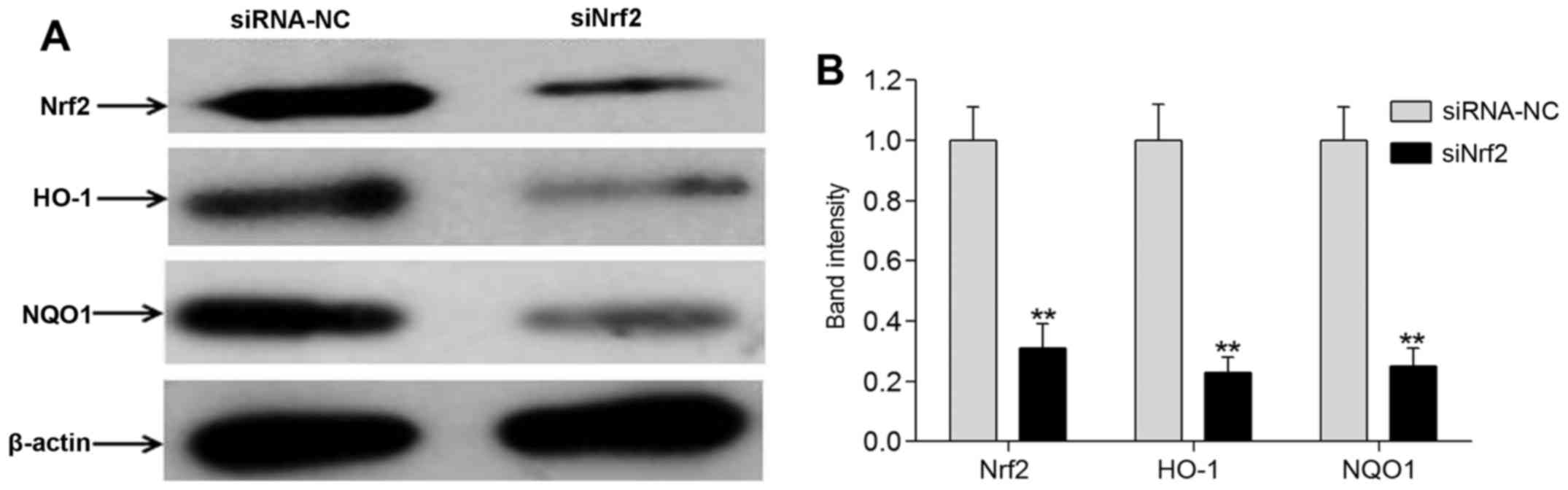
To further explore the effect of NAR on neurons,
Nrf2 was knocked down using RNA interference. Compared with the
siRNA-NC cell group, the expression levels of Nrf2 and its target
genes HO-1 and NQO1 were markedly reduced in the siNrf2 group, as
demonstrated by western blot analysis (P<0.01; Fig. 6). These data indicated that
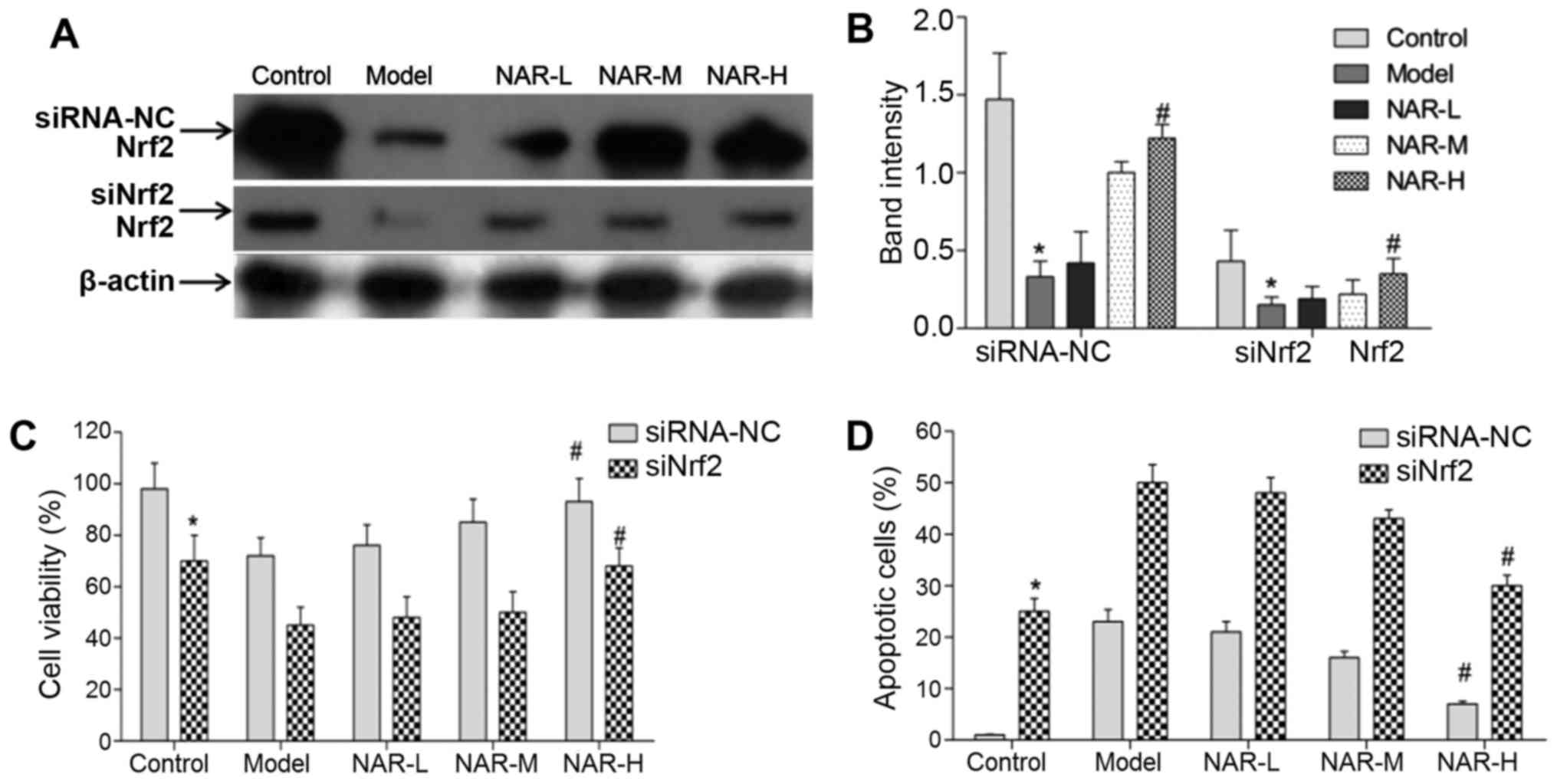
knockdown of Nrf2 using RNA interference was successful. Moreover,
in both the siRNA-NC and siNrf2 cell groups, NAR-H treatment could
increase the expression levels of Nrf2 when compared with
transfected model neurons (P<0.05; Fig. 7).
In addition, the authors assessed the viability and
apoptosis of neurons following Nrf2 knockdown. Compared with the
siRNA-NC group, the viability of neurons was significantly
decreased and the apoptosis of neurons was markedly increased in
the siNrf2 cell group (P<0.05). These results indicated that
Nrf2 could regulate the viability and apoptosis of neurons.
Following NAR treatment, the data indicated that NAR could rescue
the viability of neurons in the siRNA-NC and siNrf2 cells groups.
Notably, NAR-H treatment significantly rescued the viability of
transfected model neurons (P<0.05; Fig. 7C). In addition, the data indicated
that NAR treatment could inhibit the apoptosis of neurons in the
siRNA-NC and siNrf2 cell groups; NAR-H treatment significantly
rescued the apoptosis of transfected model neurons (P<0.05;
Fig. 7D).
Taken together, these data suggested that NAR could
activate the Nrf2/ARE signaling pathway and regulate the gene
expression of Nrf2 and its downstream targets.
Discussion
Evidence indicates that excessive formation of ROS
and the resulting oxidative stress are key contributing factors in
the aggravation of neuronal damage following ischemic insult
(26). Under normal conditions,
ROS serve roles in various crucial physiological processes, and can
be rapidly scavenged by many endogenous antioxidant enzymes
(27). During ischemia, ROS are
over-produced to a level that exceeds the reductive capabilities of
endogenous antioxidant systems, leading to neurotoxicity and,
ultimately, neuronal cell death by necrosis and apoptosis (28,29). Therefore, reducing oxidative
stress could effectively increase cell viability and decrease cell
apoptosis, and is a potential therapeutic strategy for the
treatment of ischemic insult.
Mitochondria serve essential roles in energy
production through the oxidative phosphorylation pathway, which
supplies ATP for numerous intracellular biological processes
(30,31). Increasing evidence suggests that
mitochondria are an important source of ROS, due to ROS leakage
from the electron transport chain, while also being susceptible to
oxidative damage, which leads to mitochondrial dysfunction
(32,33). In turn, mitochondrial dysfunction
can result in mitochondrial fission, energy depletion and cell
apoptosis (34,35). Notably, mitochondrial dysfunction
is considered to be a major contributing factor in the development
and progression of many neurodegenerative conditions, such as
Parkinson's, Huntington's and Alzheimer's disease, traumatic brain
injury and ischemia-reperfusion injury (36,37). Thus, decreasing mitochondrial
dysfunction and improving mitochondrial function may be another
promising target for the treatment of ischemic insult.
Flavonoids are major components of many edible
plants and medicinal herbs, and have attracted a great deal of
attention in previous years due to their reported antioxidative and
antitumor effects in various chronic diseases (38,39). Among them is NAR, an abundant
flavanone in citrus fruits (40),
and previous evidence has indicated that NAR offers protection
against oxidative stress through its strong antioxidant activity
(41). A previous study also
reported that NAR reduced the necrosis of myocytes induced by ROS
and exerted cytoprotective effects through its antioxidant
activities (42). In the present
study, the authors observed increased ROS accumulation in the model
group and a lower ROS content in the NAR-H group. Moreover,
high-dose NAR significantly increased the activities of SOD1 and
GSH, and decreased the production of MDA. These data demonstrated
that oxidative stress occurred during neuronal ischemic injury, and
that a high concentration of NAR could effectively reduce this
oxidative stress through the activation of endogenous antioxidant
enzymes.
The authors further explored the effect of NAR on
mitochondrial dysfunction. First, the levels of intracellular
adenylate in each group were determined. The data indicated that
ATP, ADP and AMP were significantly decreased in the model group,
whereas adenylate levels in the NAR-H group were notably improved.
In addition, ANT transport activity and Δψm were analyzed. The
results indicated that ANT transport activity and Δψm were
significantly reduced in the model group, but were markedly
increased in the NAR-H group. These observations revealed that NAR
could attenuate mitochondrial dysfunction by enhancing ANT
transport activity and Δψm and restoring energy production.
It is well established that mitochondrial
dysfunction can lead to cell death in various cell types (43). The viability and apoptosis of
cells in each group were further investigated, and detected a
decrease in viability and increase in apoptosis in cells of the
model group. By contrast, cells in the NAR-H group exhibited
increased viability and decreased apoptosis. Taken together, these
results indicated that NAR can effectively reduce oxidative stress
and ameliorate mitochondrial dysfunction to ultimately attenuate
cell death.
Therapies targeting the Nrf2 pathway have previously
become a promising strategy for the treatment of stroke (44). Nrf2 is a major regulator of
several cytoprotective factors, including antioxidative enzymes and
anti-inflammatory factors (45,46). Activation of Nrf2 serves a pivotal
role in enhancing the endogenous defense mechanisms that protect
the brain against progressive ischemic damage and promote its
recovery after stroke (47,48). Moreover, activation of Nrf2 can
upregulate the expression of several antioxidative enzymes,
including HO-1 and NQO1. The present study verified that NAR-H
treatment enhanced the relative gene expression of Nrf2 and its
targets genes. In addition, NAR-H treatment could significantly
rescue the viability of neurons following siRNA knockdown of
Nrf2.
In conclusion, the results demonstrated that
administration of NAR prior to oxygen deprivation/reoxygenation
reduced oxidative stress and improved mitochondrial dysfunction via
activation of the Nrf2/ARE signaling pathway in neurons. In future
studies, in order to simulate the clinical status of ischemic
cerebrovascular disease as much as possible, neurons could be
exposed to hypoxia and then re-supplied with oxygen and NAR for
different periods of time. After re-supplying oxygen and NAR, the
cell viability, the levels of ROS generation, Nrf2 and its
downstream genes and mitochondrial functions should be assayed.
Abbreviations:
|
NAR
|
naringenin
|
|
ROS
|
reactive oxygen species
|
|
GSH
|
glutathione
|
|
MDA
|
malondialdehyde
|
|
SOD1
|
superoxide dismutase 1
|
|
ANT
|
adenine nucleotide translocase
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
ARE
|
antioxidant response element
|
|
Keap1
|
kelch-like ECH-associated protein
1
|
|
CM-H2DCFDA
|
chloromethyl-2′,7′dichlorodihydro
fluorescein diacetate
|
|
Δψm
|
mitochondrial membrane potential
|
|
HPLC
|
high performance liquid
chromatography
|
|
siRNA
|
small interfering RNA
|
|
siNfr2
|
anti-Nrf2 siRNA
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
HO-1
|
heme oxygenase 1
|
|
NQO1
|
NAD(P)H quinone dehydrogenase 1
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
Bax
|
Bcl-2-associated X protein
|
Acknowledgments
The present study was supported by a class general
financial grant from the Guangxi Natural Science Foundation (grant
no. 2012GXNSFAA053076); the Youth Fund Project of the Guangxi
Natural Science Foundation (grant nos. 2012GXNSFBA053082 and
2013GXNSFBA019153); and the Guangxi Scientific and Technological
Research Projects of Universities (grant no. KY2015ZD062).
References
|
1
|
Zhang L, Zhang X, Zhang C, Bai X, Zhang J,
Zhao X, Chen L, Wang L, Zhu C, Cui L, et al: Nobiletin promotes
antioxidant and anti-inflammatory responses and elicits protection
against ischemic stroke in vivo. Brain Res. 1636:130–141. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gopinath K and Sudhandiran G: Naringin
modulates oxidative stress and inflammation in 3-nitropropionic
acid-induced neurodegeneration through the activation of nuclear
factor-erythroid 2-related factor-2 signalling pathway.
Neuroscience. 227:134–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sahota P and Savitz SI: Investigational
therapies for ischemic stroke: Neuroprotection and neurorecovery.
Neurotherapeutics. 8:434–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albarracin SL, Stab B, Casas Z, Sutachan
JJ, Samudio I, Gonzalez J, Gonzalo L, Capani F, Morales L and
Barreto GE: Effects of natural antioxidants in neurodegenerative
disease. Nutr Neurosci. 15:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdul-Muneer PM, Chandra N and Haorah J:
Interactions of oxidative stress and neurovascular inflammation in
the pathogenesis of traumatic brain injury. Mol Neurobiol.
51:966–979. 2015. View Article : Google Scholar
|
|
6
|
Antoniou X, Falconi M, Di Marino D and
Borsello T: JNK3 as a therapeutic target for neurodegenerative
diseases. J Alzheimers Dis. 24:633–642. 2011.PubMed/NCBI
|
|
7
|
Ashrafi G and Schwarz TL: The pathways of
mitophagy for quality control and clearance of mitochondria. Cell
Death Differ. 20:31–42. 2013. View Article : Google Scholar
|
|
8
|
Alfieri A, Srivastava S, Siow RC, Cash D,
Modo M, Duchen MR, Fraser PA, Williams SC and Mann GE: Sulforaphane
preconditioning of the Nrf2/HO-1 defense pathway protects the
cerebral vasculature against blood-brain barrier disruption and
neurological deficits in stroke. Free Radic Biol Med. 65:1012–1022.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen PC, Vargas MR, Pani AK, Smeyne RJ,
Johnson DA, Kan YW and Johnson JA: Nrf2-mediated neuroprotection in
the MPTP mouse model of Parkinson's disease: Critical role for the
astrocyte. Proc Natl Acad Sci USA. 106:2933–2938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cuadrado A, Moreno-Murciano P and
Pedraza-Chaverri J: The transcription factor Nrf2 as a new
therapeutic target in Parkinson's disease. Expert Opin Ther
Targets. 13:319–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Vries HE, Witte M, Hondius D,
Rozemuller AJ, Drukarch B, Hoozemans J and van Horssen J:
Nrf2-induced antioxidant protection: A promising target to
counteract ROS-mediated damage in neurodegenerative disease? Free
Radic Biol Med. 45:1375–1383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du Y, Villeneuve NF, Wang XJ, Sun Z, Chen
W, Li J, Lou H, Wong PK and Zhang DD: Oridonin confers protection
against arsenic-induced toxicity through activation of the
Nrf2-mediated defensive response. Environ Health Perspect.
116:1154–1161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jazwa A, Rojo AI, Innamorato NG, Hesse M,
Fernández-Ruiz J and Cuadrado A: Pharmacological targeting of the
transcription factor Nrf2 at the basal ganglia provides disease
modifying therapy for experimental parkinsonism. Antioxid Redox
Signal. 14:2347–2360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaidery NA, Banerjee R, Yang L, Smirnova
NA, Hushpulian DM, Liby KT, Williams CR, Yamamoto M, Kensler TW,
Ratan RR, et al: Targeting Nrf2-mediated gene transcription by
extremely potent synthetic triterpenoids attenuate dopaminergic
neurotoxicity in the MPTP mouse model of Parkinson's disease.
Antioxid Redox Signal. 18:139–157. 2013. View Article : Google Scholar :
|
|
15
|
Chen D, Chen MS, Cui QC, Yang H and Dou
QP: Structure-proteasome-inhibitory activity relationships of
dietary flavonoids in human cancer cells. Front Biosci.
12:1935–1945. 2007. View
Article : Google Scholar
|
|
16
|
Kawaii S, Tomono Y, Katase E, Ogawa K and
Yano M: HL-60 differentiating activity and flavonoid content of the
readily extractable fraction prepared from citrus juices. J Agric
Food Chem. 47:128–135. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanno S, Tomizawa A, Ohtake T, Koiwai K,
Ujibe M and Ishikawa M: Naringenin-induced apoptosis via activation
of NF-kappaB and necrosis involving the loss of ATP in human
promyeloleukemia HL-60 cells. Toxicol Lett. 166:131–139. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Youdim KA, Qaiser MZ, Begley DJ,
Rice-Evans CA and Abbott NJ: Flavonoid permeability across an in
situ model of the blood-brain barrier. Free Radic Biol Med.
36:592–604. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prasain JK, Carlson SH and Wyss JM:
Flavonoids and age-related disease: Risk, benefits and critical
windows. Maturitas. 66:163–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu ZL, Huang C, Fu H, Jin Y, Wu WN, Xiong
QJ, Xie N, Long LH, Chen JG and Wang F: Disruption of PICK1
attenuates the function of ASICs and PKC regulation of ASICs. Am J
Physiol Cell Physiol. 299:C1355–C1362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y, Zhang H, Li X, Yang T and Jiang Q:
Effects of PPARα/PGC-1α on the myocardial energy metabolism during
heart failure in the doxorubicin induced dilated cardiomyopathy in
mice. Int J Clin Exp Med. 7:2435–2442. 2014.
|
|
22
|
Schmid I, Uittenbogaart CH and Giorgi JV:
Sensitive method for measuring apoptosis and cell surface phenotype
in human thymocytes by flow cytometry. Cytometry. 15:12–20. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Chaturvedi RK and Flint Beal M:
Mitochondrial diseases of the brain. Free Radic Biol Med. 63:1–29.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciccone S, Maiani E, Bellusci G, Diederich
M and Gonfloni S: Parkinson's disease: A complex interplay of
mitochondrial DNA alterations and oxidative stress. Int J Mol Sci.
14:2388–2409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cookson MR: Parkinsonism due to mutations
in PINK1, parkin, and DJ-1 and oxidative stress and mitochondrial
pathways. Cold Spring Harb Perspect Med. 2:a0094152012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boveris A, Oshino N and Chance B: The
cellular production of hydrogen peroxide. Biochem J. 128:617–630.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dhalla NS, Temsah RM and Netticadan T:
Role of oxidative stress in cardiovascular diseases. J Hypertens.
18:655–673. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abdelmegeed MA and Song BJ: Functional
roles of protein nitration in acute and chronic liver diseases.
Oxid Med Cell Longev. 2014:1496272014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai Z, Wu Z, Yang Y, Wang J, Satterfield
MC, Meininger CJ, Bazer FW and Wu G: Nitric oxide and energy
metabolism in mammals. Biofactors. 39:383–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dashdorj A, Jyothi KR, Lim S, Jo A, Nguyen
MN, Ha J, Yoon KS, Kim HJ, Park JH, Murphy MP, et al:
Mitochondria-targeted antioxidant MitoQ ameliorates experimental
mouse colitis by suppressing NLRP3 inflammasome-mediated
inflammatory cytokines. BMC Med. 11:1782013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wey MC, Fernandez E, Martinez PA, Sullivan
P, Goldstein DS and Strong R: Neurodegeneration and motor
dysfunction in mice lacking cytosolic and mitochondrial aldehyde
dehydrogenases: Implications for Parkinson's disease. PLoS One.
7:e315222012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Winklhofer KF: Parkin and mitochondrial
quality control: Toward assembling the puzzle. Trends Cell Biol.
24:332–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Winterbourn CC: Reconciling the chemistry
and biology of reactive oxygen species. Nat Chem Biol. 4:278–286.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu W, Xu H, Wang Z, Mao Y, Yuan L, Luo W,
Cui Z, Cui T, Wang XL and Shen YH: PINK1-Parkin-mediated mitophagy
protects mitochondrial integrity and prevents metabolic
stress-induced endothelial injury. PLoS One. 10:e01324992015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang J and Ney PA: Reticulocyte
mitophagy: Monitoring mitochondrial clearance in a mammalian model.
Autophagy. 6:405–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu K, Puchowicz MA, Sun X and LaManna JC:
Mitochondrial dysfunction in aging rat brain following transient
global ischemia. Adv Exp Med Biol. 614:379–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Purushotham A, Tian M and Belury MA: The
citrus fruit flavonoid naringenin suppresses hepatic glucose
production from Fao hepatoma cells. Mol Nutr Food Res. 53:300–307.
2009. View Article : Google Scholar
|
|
39
|
Sohn E, Kim J, Kim CS, Kim YS, Jang DS and
Kim JS: Extract of the aerial parts of Aster koraiensis reduced
development of diabetic nephropathy via anti-apoptosis of podocytes
in streptozotocin-induced diabetic rats. Biochem Biophys Res
Commun. 391:733–738. 2010. View Article : Google Scholar
|
|
40
|
Rajadurai M, Prince PS and Prince M:
Preventive effect of naringin on isoproterenol-induced
cardiotoxicity in Wistar rats: An in vivo and in vitro study.
Toxicology. 232:216–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu L, Xu DM and Cheng YY: Distinct
effects of naringenin and hesperetin on nitric oxide production
from endothelial cells. J Agric Food Chem. 56:824–829. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pérez-Jiménez J, Neveu V, Vos F and
Scalbert A: Identification of the 100 richest dietary sources of
polyphenols: An application of the Phenol-Explorer database. Eur J
Clin Nutr. 64(Suppl 3): S112–S120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu J, Li Q, Wang X, Yu S, Li L, Wu X, Chen
Y, Zhao J and Zhao Y: Neuroprotection by curcumin in ischemic brain
injury involves the Akt/Nrf2 pathway. PLoS One. 8:e598432013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ren J, Fan C, Chen N, Huang J and Yang Q:
Resveratrol pretreatment attenuates cerebral ischemic injury by
upregu-lating expression of transcription factor Nrf2 and HO-1 in
rats. Neurochem Res. 36:2352–2362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fujita K, Maeda D, Xiao Q and Srinivasula
SM: Nrf2-mediated induction of p62 controls Toll-like
receptor-4-driven aggresome-like induced structure formation and
autophagic degradation. Proc Natl Acad Sci USA. 108:1427–1432.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang B, Cao W, Biswal S and Doré S: Carbon
monoxide-activated Nrf2 pathway leads to protection against
permanent focal cerebral ischemia. Stroke. 42:2605–2610. 2011.
View Article : Google Scholar : PubMed/NCBI
|