Introduction
Oral cancer is the most prevalent type of cancer
affecting the head and neck (1).
Worldwide in 2012 there were 300,400 new cases of oral cancer and
145,400 individuals succumbed to the disease (2). It was estimated that in 2016 there
would be 48,330 new cases of oral cancer in the USA and 9,570
individuals would succumb to the disease (3). Chen et al (4) projected that in 2015 in China, there
would be ~48,000 newly diagnosed cases of oral cancer and ~22,000
individuals would suffer mortality as a result. These estimates
proved accurate. The majority of cases of oral cancer (~90%) are
comprised of squamous cell carcinomas; the tongue is the most
common site for oral squamous cell carcinomas (1). Although notable advancements in
treatment have been achieved through combined therapies, including
surgery, radiotherapy and neo-adjuvant chemotherapy, the prognosis
and 5-year survival rate for individuals with tongue squamous cell
carcinoma (TSCC) have not been significantly improved over the past
several decades, remaining at ~50% (5). Treatment failure is primarily due to
frequent local and regional recurrences, and lymph node metastases
(6). Previous epidemiological
studies have suggested that there has been an increase in the
incidence of TSCC worldwide (7,8).
Therefore, the identification of novel effective chemotherapeutic
agents is required.
A previous epidemiological study has indicated that
the dietary intake of fresh fruits and vegetables reduces the risk
of oral cancer (9). This
preventive action has been attributed to the polyphenols contained
in fruits and vegetables (10,11). A number of polyphenolic compounds,
including curcumin (12,13), green tea polyphenol
epigallocatechin-3-gallate (14)
and resveratrol (15) have
previously demonstrated promising chemopreventive efficacy on oral
cancer. Proanthocyanidins are the principal polyphenols in grapes
and are abundant in grape seeds (16,17). Grape seed proanthocyanidins (GSPs)
have been revealed to possess chemopreventive and chemotherapeutic
potential against several types of cancer in vitro and in
vivo (18–20). Studies have demonstrated that GSPs
may inhibit the growth and invasiveness of oral tumor cells
(21–25). A recent study by Shrotriya et
al (26) revealed that grape
seed extract and resveratrol significantly inhibited tumor
promotion and progression in a 4-nitroquinoline 1-oxide-induced
oral tumorigenesis model in mice. However, the chemopreventive
potential and the underlying mechanisms of GSPs against TSCC are
not well understood.
In the present study, the effects of GSPs on the
proliferation, migration and invasion, and matrix
metalloproteinase-2 (MMP-2) and MMP-9 secretion of TSCC Tca8113
cells was investigated. In addition, the underlying mechanisms by
which GSPs function was also examined. The present study aimed to
provide scientific evidence supporting GSPs as chemopreventive and
chemotherapeutic agents against TSCC.
Materials and methods
Materials
GSPs containing ≥95% proanthocyanidins, ≥1.8%
proanthocyanidins B2 and ≥60% oligomers were obtained
from Tianjin Jianfeng Natural Product R&D Co., Ltd. (Tianjin,
China). Dulbecco's modified Eagle's medium (DMEM) was obtained from
Gibco, Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Fetal
bovine serum (FBS) was purchased from National HyClone
Bio-Engineering Co., Ltd. (Lanzhou, China). Sulforhodamine B (SRB)
and gelatin from porcine skin were obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). The Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit
was purchased from MultiSciences Biotech Co., Ltd. (Hangzhou,
China). RNAiso Plus and the PrimeScript reverse
transcription-polymerase chain reaction (RT-PCR) kit were purchased
from Takara Bio, Inc. (Otsu, Japan). Millicell Cell Culture Inserts
were obtained from EMD Millipore (Billerica, MA, USA). Matrigel was
purchased from BD Biosciences (Franklin Lakes, NJ, USA). Primary
antibodies directed against protein kinase B (Akt; cat. no.
sc-8312), phosphorylated (p) Akt (cat. no. sc-7985-R) and β-actin
(cat. no. sc-130656) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Primary antibodies directed against IκB
kinase (IKK; cat. no. ab178870) and pIKK (cat. no. ab55341) were
purchased from Abcam (Cambridge, MA, USA). Horseradish
peroxidase-conjugated goat anti-rabbit IgG (cat. no. ZB-2301) was
purchased from OriGene Technologies, Inc. (Beijing, China). The
nuclear factor-κB (NF-κB). Activation, nuclear translocation assay
kit (cat. no. SN368) was purchased from Beyotime Institute of
Biotechnology (Haimen, China).
Cell culture
Human TSCC Tca8113 cells were obtained from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China), and cultured in DMEM supplemented with 10% FBS at 37°C and
5% CO2.
Cell viability assay
The viability of Tca8113 cells was determined using
an SRB assay as previously described (27), with certain modifications. Tca8113
cells were plated into 96-well plates at a density of
5×103 cells/well in 100 µl DMEM. Following
incubation at 37°C overnight, the cells were treated with GSPs at
varying concentrations (0–200 µg/ml) for 24, 48 or 72 h.
Subsequently, the cultures were fixed with cold 10% trichloroacetic
acid at 4°C for 1 h and washed with water. Next, the plates were
air-dried and the fixed cells were stained with 0.4% SRB at room
temperature for 10 min and washed repeatedly with 0.1% acetic acid
to remove the unbound dye. The bound SRB was dissolved in 1% Tris
(pH 10.5). The optical density was measured at 515 nm using a
microplate reader.
Annexin V-FITC/PI staining
The amount of apoptotic Tca8113 cells was determined
using an Annexin V-FITC/PI apoptosis detection kit according to the
manufacturer's protocol. Following treatment with varying
concentrations (0–200 µg/ml) of GSPs for 24 h, the Tca8113
cells were collected and washed twice with cold phosphate-buffered
saline (PBS). Subsequently, ~5×105 cells were
resuspended in binding buffer, and stained with Annexin V-FITC and
PI. The stained cells were detected by flow cytometry using
CellQuest software (version 3.3; BD Biosciences).
RT-semi-qPCR (RT-sqPCR) analysis
Tca8113 cells were treated with different
concentrations (0–100 µg/ml) of GSPs for 24 h. Total RNA was
prepared using RNAiso Plus according to the manufacturer's
protocol. RT-PCR was performed using the PrimeScript RT-PCR kit
according to the manufacturer's protocol. The primers used were as
follows: Apoptosis regulator BAX (Bax) forward, 5′-CCC TTT TGC TTC
AGG GTT TC-3′ and reverse, 5′-GCC ACT CGG AAA AAG ACC TC-3′;
apoptosis regulator Bcl-2 (Bcl-2) forward, 5′-TGT TGG CCT TCT TTG
AGT TCG-3′ and reverse, 5′-TCA CTT GTG GCC CAG ATA GG-3′; β-actin
forward, 5′-CCA CAC CTT CTA CAA TGA GC-3′ and reverse, 5′-TGA GGT
AGT CAG TCA GGT CC-3′. The thermocycling conditions were as
follows: 30 cycles of 98°C for 10 sec, 55°C for 30 sec and 72°C for
1 min, followed by incubation at 72°C for 5 min. β-actin was used
as the internal control. The PCR products were run on 2% agarose
gels and visualized by ethidium bromide staining. Images were
captured under ultraviolet light. Densitometric analysis was
performed using Adobe Photoshop CS5 (Adobe Systems, Inc., San Jose,
CA, USA). The values of target mRNA expression were normalized to
that of the β-actin mRNA expression.
Cell migration assay
Cell migration was assayed in Millicell Cell Culture
Inserts as previously described (28). Tca8113 cells were trypsinized and
suspended in serum-free DMEM. A total of 2×105 cells in
0.4 ml medium were seeded into the cell culture inserts in the
upper chamber, with various concentrations of GSPs (0–50
µg/ml). The culture inserts were placed into 24-well plates
filled with 0.6 ml DMEM supplemented with 20% FBS as a
chemoattractant. Following incubation at 37°C for 24 h, the
non-migrated cells on the upper surface of the membrane were wiped
off with a cotton swab. Cells that had crossed the inserts were
fixed with methanol for 30 min and then stained with 0.1% crystal
violet for 10 min at room temperature. Images were captured using
light microscopy (magnification, ×100). The number of cells that
had migrated was counted using ImageJ software (version 1.51j8;
National Institutes of Health, Bethesda, MD, USA).
Cell invasion assay
A cell invasion assay was performed as previously
described (29). Briefly, the
upper chamber of Millicell Cell Culture Inserts was coated with 50
µl Matrigel diluted 1:8 with PBS. Subsequently,
4×105 Tca8113 cells in 0.4 ml serum-free DMEM with or
without GSPs (0–50 µg/ml) were added to the upper chamber.
The lower chamber was filled with 0.6 ml DMEM supplemented with 20%
FBS as a chemoattractant to induce invasion. Following incubation
at 37°C for 24 h, the culture inserts were removed and the
non-invasive cells on the upper surface of culture inserts were
scraped away with a cotton swab. The cells that invaded through the
Matrigel were fixed with methanol for 30 min and then stained with
0.1% crystal violet for 10 min at room temperature. Images were
captured by light microscopy (magnification, ×100) and the number
of cells was counted using ImageJ software.
Gelatin zymography
The enzymatic activities of MMP-2 and MMP-9 were
examined by gelatin zymography as described previously (28). Subconfluent Tca8113 cells were
treated with GSPs (0–50 µg/ml) for 24 h in serum-free DMEM.
Following treatment, the conditioned medium was collected and
centrifuged at 300 x g for 10 min at 4°C to remove cellular debris.
The supernatants were subjected to 7.5% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10 µg
protein/lane) in a gel containing 1% gelatin. Following
electrophoresis, the gels were washed with a washing buffer [50 mM
Tris-HCl (pH 7.5), 100 mM NaCl and 2.5% Triton X-100] and incubated
in a reaction buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl and 10
mM CaCl2] at 37°C for 36 h. Subsequently, the gels were
stained with 0.25% Coomassie Blue R250 for 30 min, and destained
for 10 min with 25% methanol and 7.5% acetic acid at room
temperature. Enzyme activity was visualized as a bright band on a
blue background. The band intensities were measured using Adobe
Photoshop CS5 software.
Western blot analysis
Tca8113 cells were treated with GSPs (0–100
µg/ml) for 24 h and lysed using radioimmunoprecipitation
assay buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology). Total protein was collected and then quantified
using the Bradford assay. Protein was separated via 10% SDS-PAGE
(40 µg protein/lane). Following electrophoresis, the
separated proteins were transferred to polyvidyline fluoride
membranes. The membranes were subsequently probed with the primary
antibodies directed against AKT (1:800), pAKT (1:800), IKK
(1:1,000), pIKK (1:1,000) and β-actin (1:400) at 4°C overnight.
Following this, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibodies at
4°C for 2 h. The secondary antibodies were diluted 1:8,000 for AKT,
1:8,000 for p-AKT, 1:10,000 for IKK, 1:10,000 for p-IKK and 1:4,000
for β-actin. Immunoreactivity was detected using SuperSignal West
Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Bands were quantified
using Adobe Photoshop CS5.
NF-κB activation and nuclear
translocation assay
NF-κB activation and nuclear translocation were
examined using a NF-κB. Activation, nuclear translocation assay kit
according to the manufacturer's protocol. Briefly, Tca8113 cells
were treated with various concentrations of GSPs (0–100
µg/ml) for 24 h. Using the reagents provided in the kit, the
cells were washed and fixed, and then incubated with blocking
solution for 1 h at room temperature. Cells were incubated with
rabbit anti-NF-κB p65 antibodies (provided in the kit) overnight at
4°C. Following washing, the cells were further incubated with
Cy3-conjugated secondary antibodies (provided in the kit) for 1 h
and 4′,6-diamidino-2-phenylindole (DAPI) for 5 min at room
temperature. Images were captured by fluorescence microscopy
(magnification, ×200).
Statistical analysis
Data are expressed as the mean ± standard error.
Statistical significance between the control and the GSP treated
groups was determined by one-way analysis of variance followed by a
post hoc Dunnett's test using SPSS software (version 12; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
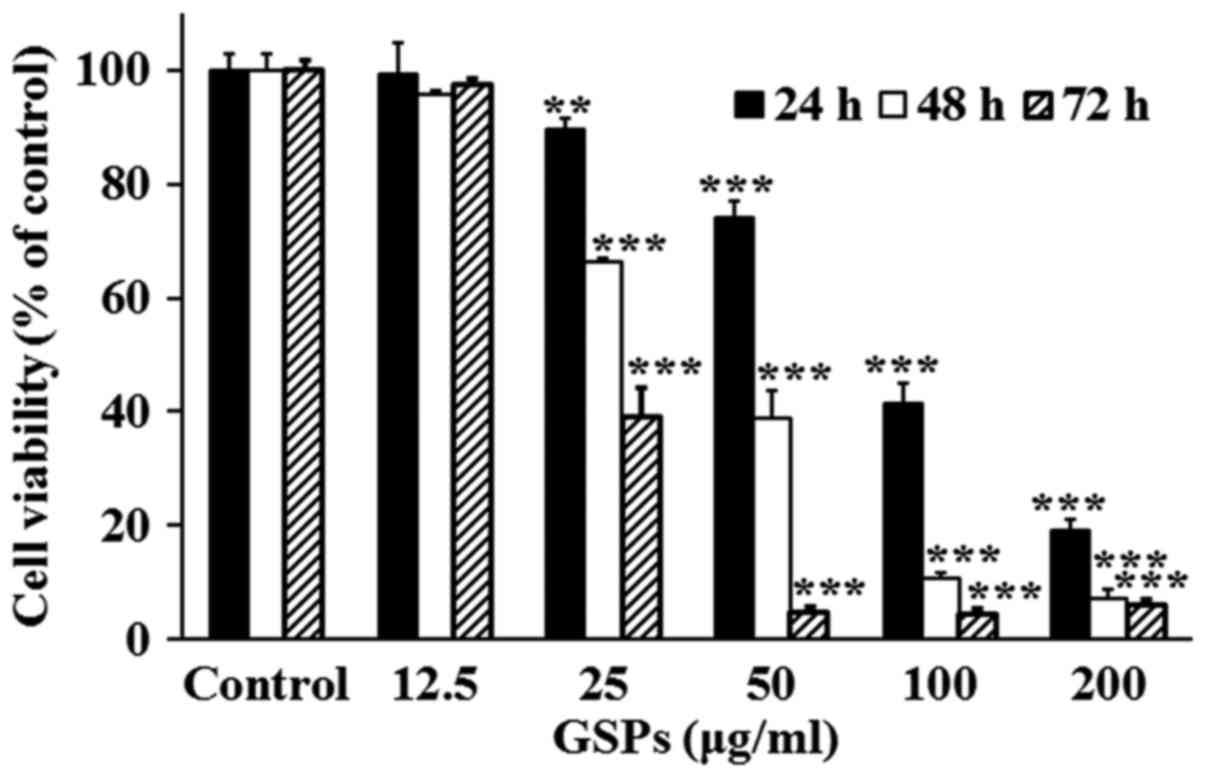
GSPs decrease the viability of Tca8113
cells
The effect of GSPs on the viability of Tca8113 cells
was evaluated using an SRB assay (Fig. 1). The results demonstrated that
treatment with 25–200 µg/ml GSPs significantly inhibited the
viability of Tca8113 cells compared with the control group in a
dose-dependent manner. The half maximal inhibitory concentration
values were 86.36, 43.65 and 31.17 µg/ml for 24, 48 and 72
h, respectively.
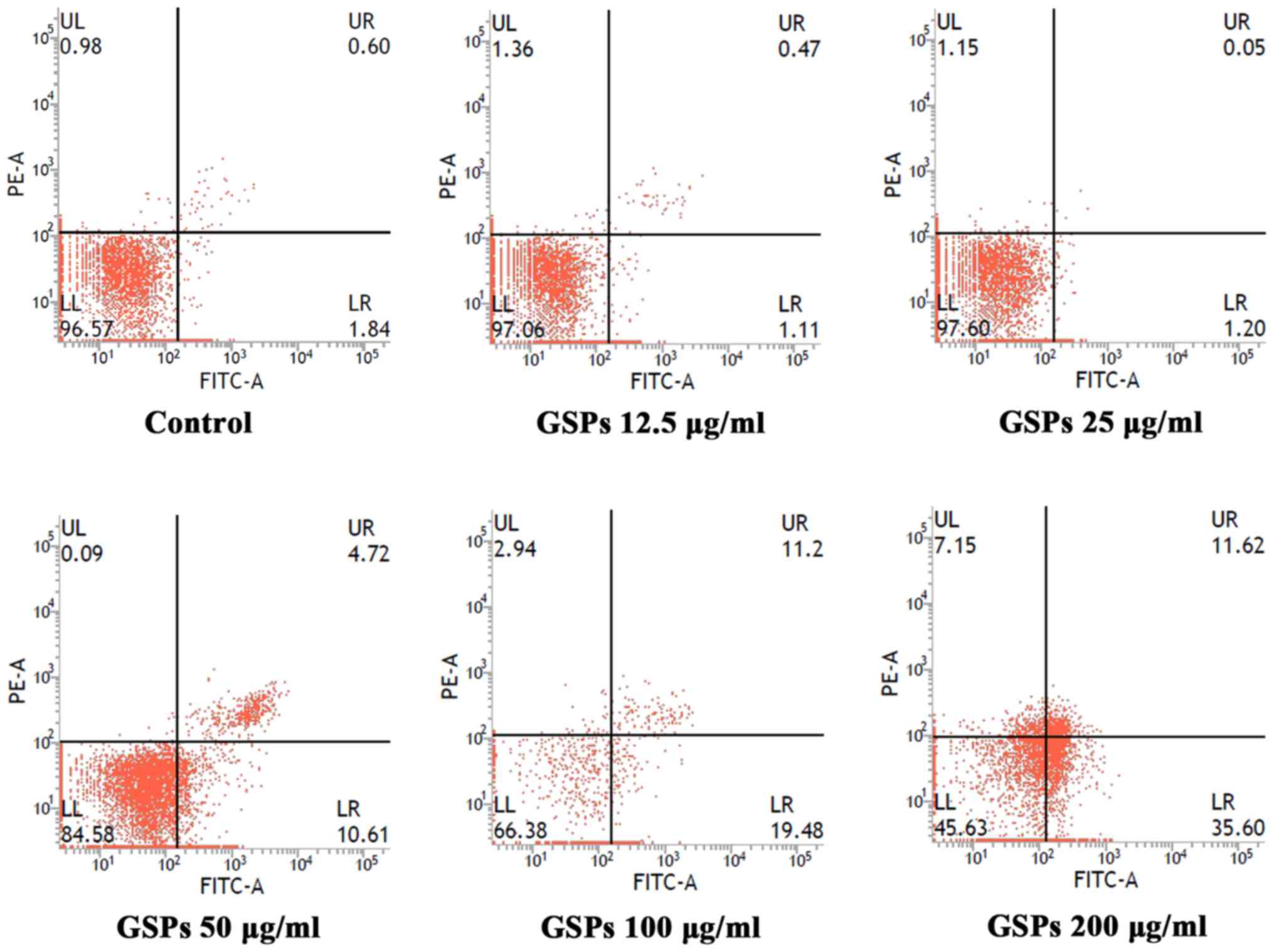
GSPs induce the apoptosis of Tca8113
cells
To determine whether the growth inhibitory effect of
GSPs was associated with apoptosis, the effect of GSPs on the
apoptosis of Tca8113 cells was analyzed by Annexin V-FITC/PI double
staining (Fig. 2). Apoptotic
cells may be divided into early-stage apoptotic cells and
late-stage apoptotic cells. The Annexin V+ and
PI− cells in the lower right quadrant of flow cytometry
histograms are early-stage apoptotic cells, while the Annexin
V+ and PI+ cells in the upper right quadrant
of the histograms are late-stage apoptotic cells. Treatment with
low concentrations (12 and 25 µg/ml) of GSP had no notable
effect on early or late-stage apoptosis. When the GSP concentration
was increased, early and late-stage apoptosis were induced. The
total apoptotic rates for cells treated with GSPs at concentrations
of 0, 12.5, 25, 50, 100 and 200 µg/ml were 2.64, 1.58, 1.25,
15.33, 30.68 and 47.22%, respectively.
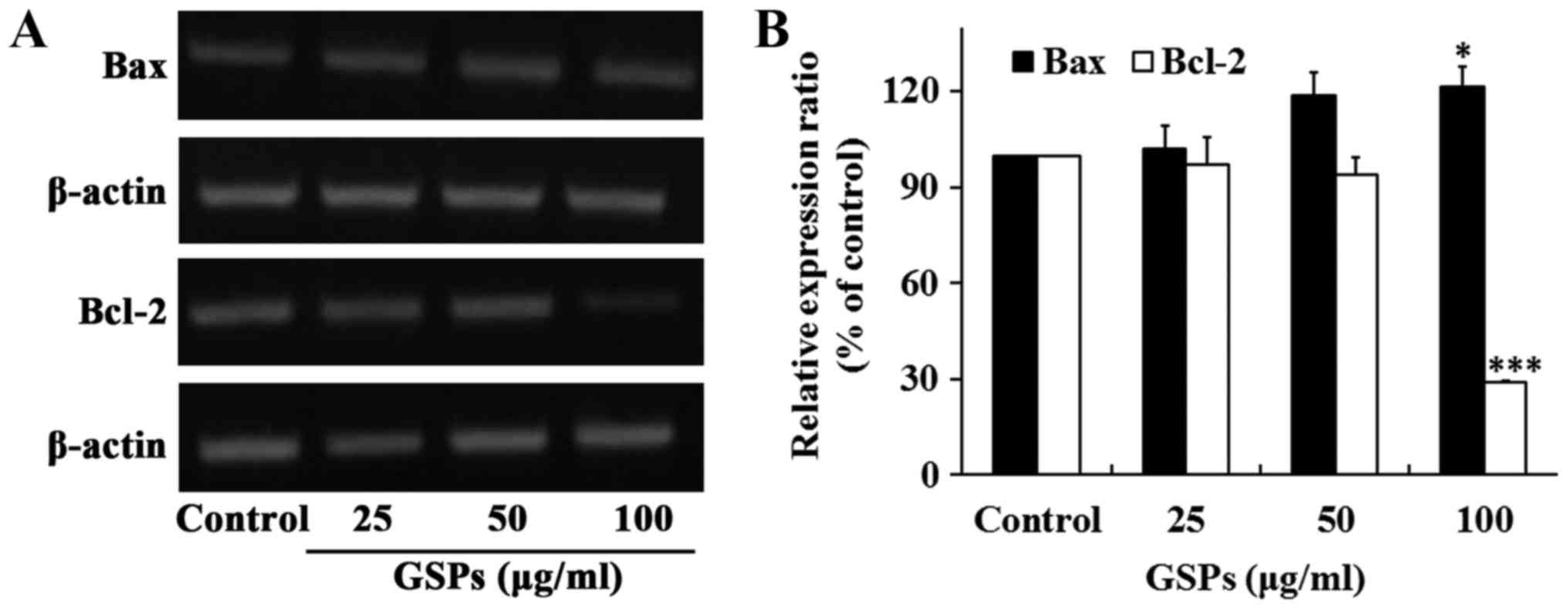
The proteins Bax and Bcl-2 serve crucial roles in
the regulation of apoptosis. To determine whether these two
proteins were associated with the GSP-induced apoptosis of Tca8113
cells, the expression of Bax and Bcl-2 was examined by RT-sqPCR.
The results revealed that the treatment of Tca8113 cells with 100
µg/ml GSPs for 24 h significantly increased the expression
of Bax, whereas it significantly decreased the expression of Bcl-2
(Fig. 3). No notable differences
were observed when lower concentrations of GSP were
administered.
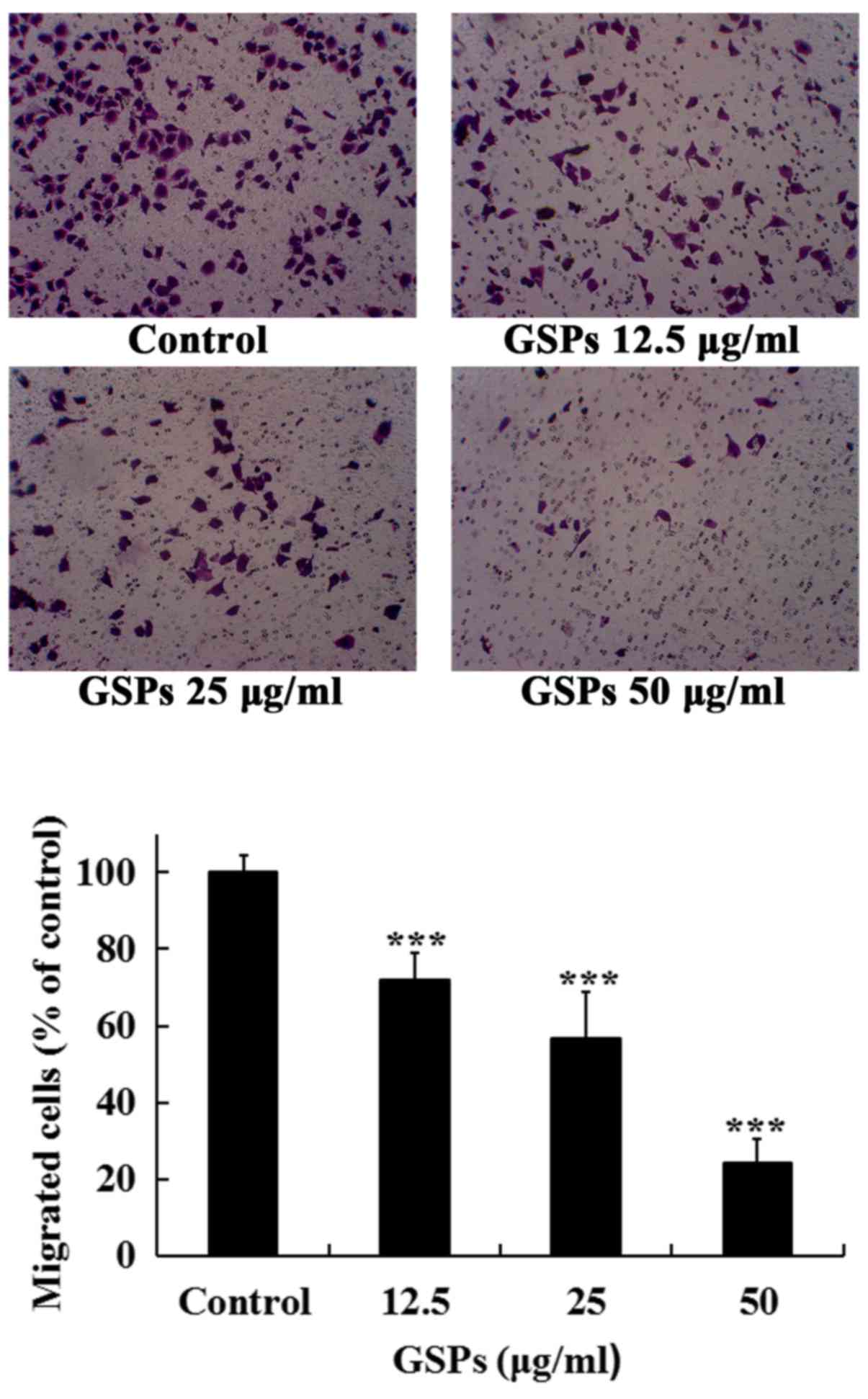
GSPs inhibit the migration of Tca8113
cells
The migration of cancer cells and their invasion
through the extracellular matrix are important steps during the
process of cancer metastasis. The effects of GSPs on the migration
and invasion, and MMP-2 and MMP-9 secretion of Tca8113 cells were
examined.
As treatment with 50 µg/ml GSPs for 24 h
induced an apoptotic rate of 15.33%, the maximal concentration of
50 µg/ml was selected to detect whether low concentrations
of GSPs decreased the migration and invasion, and secretion of
MMP-2 and MMP-9 in Tca8113 cells. As revealed in Fig. 4, treatment with GSPs significantly
inhibited the serum-induced migration of Tca8113 cells compared
with the control group in a dose-dependent manner. The migration
inhibition rates of GSPs at 12.5, 25 and 50 µg/ml were 28.0,
43.4 and 75.9%, respectively.
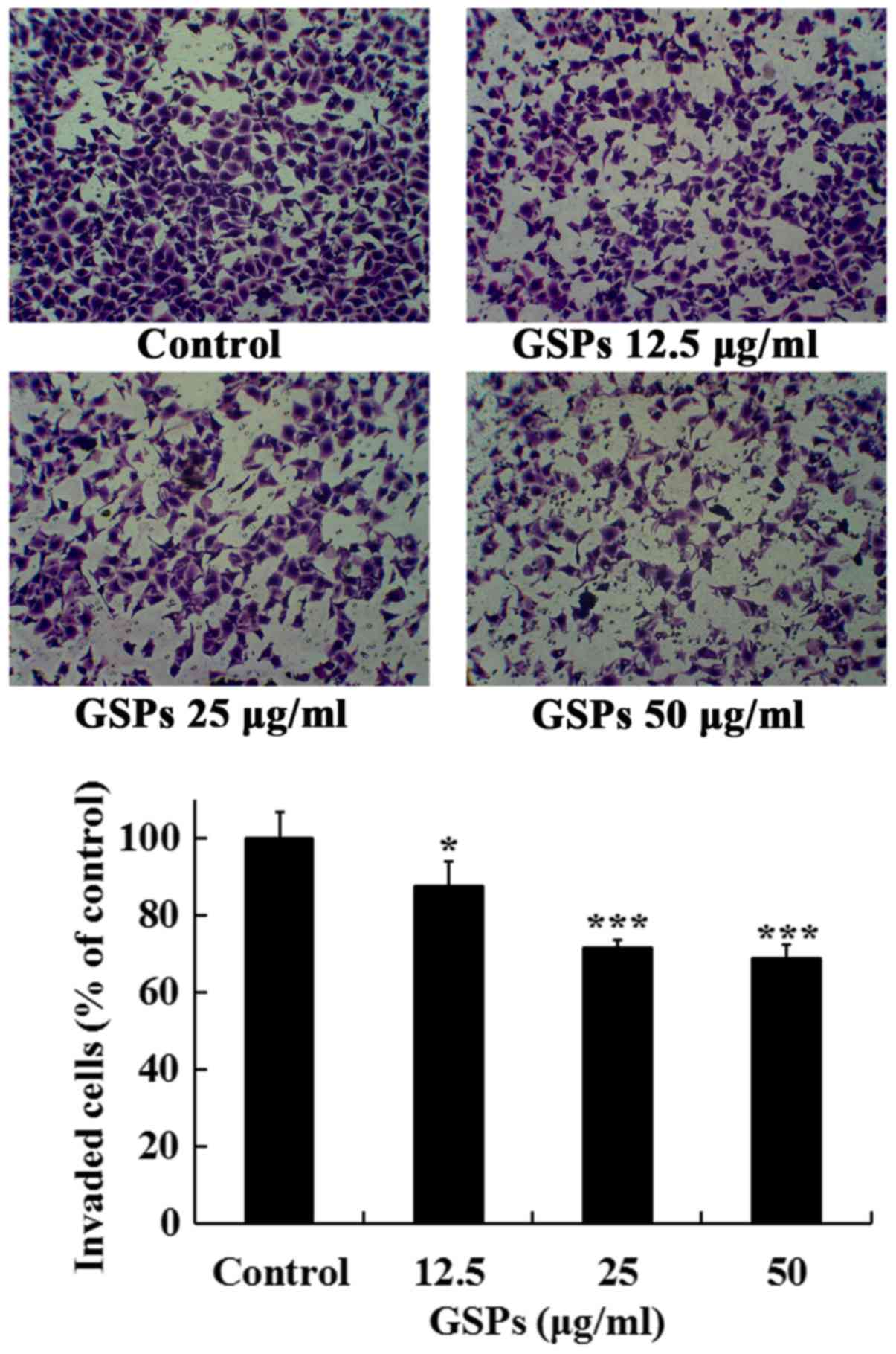
GSPs inhibit the invasion of Tca8113
cells
The effects of GSPs on the invasive capacity of
Tca8113 cells were further investigated. As revealed in Fig. 5, treatment of the Tca8113 cells
with different concentrations of GSPs (10–50 µg/ml) for 24 h
significantly inhibited the serum-induced invasion of Tca8113 cells
compared with the control group. The invasion inhibition rates of
GSPs at 12.5, 25 and 50 µg/ml were 12.5, 28.4 and 31.3%,
respectively.
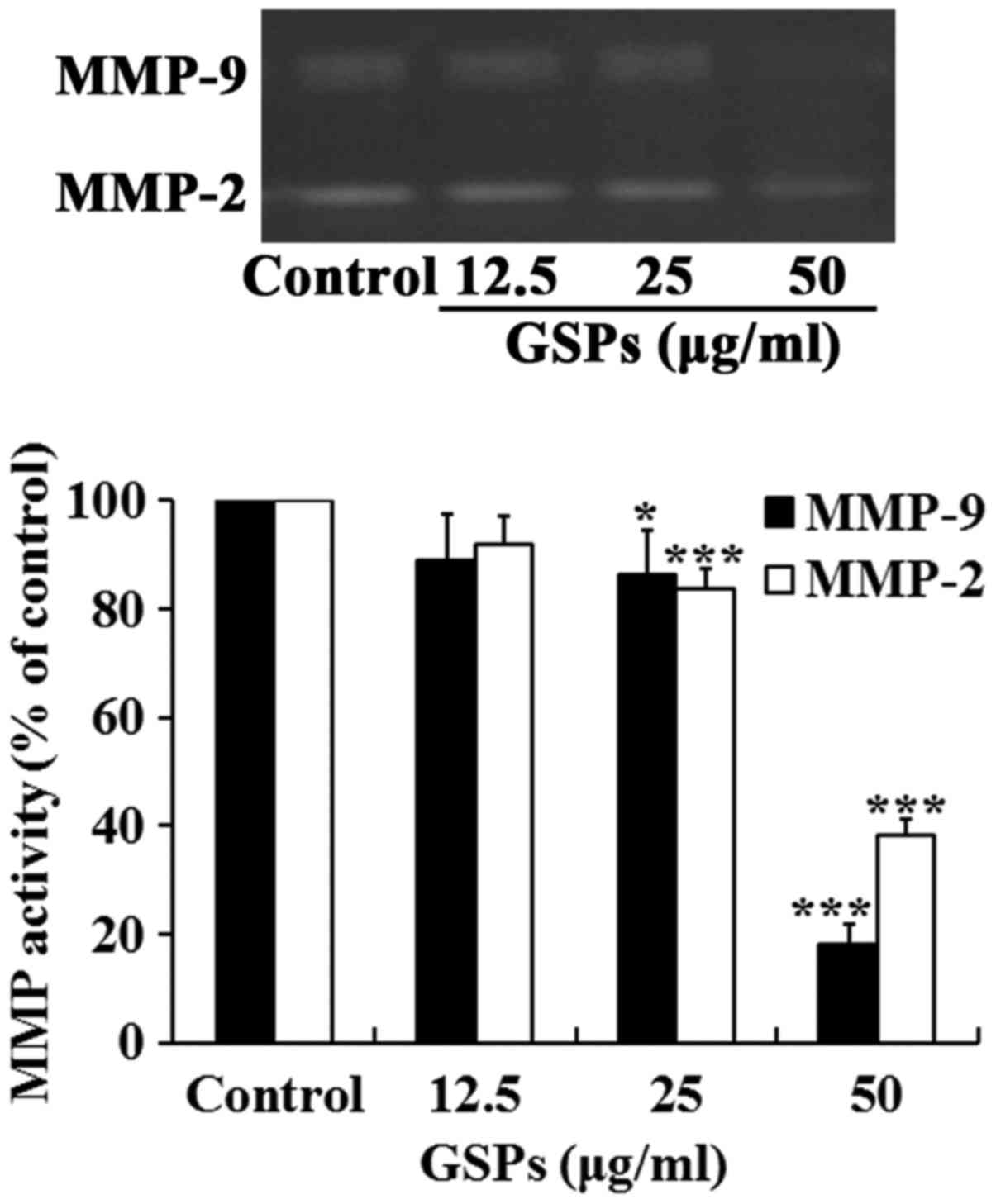
GSPs inhibit the secretion of MMP-2 and
MMP-9 by Tca8113 cells
Degradation of the extracellular matrix by MMP-2 and
MMP-9 is a key event in the process of tumor metastasis. The effect
of GSP on the secretion of MMP-2 and MMP-9 by Tca8113 cells was
examined. As demonstrated in Fig.
6, treatment with GSPs reduced the secretion of MMP-2 and MMP-9
in a dose-dependent manner. Treatment with 25 and 50 µg/ml
GSPs significantly reduced the secretion of MMP-2 and MMP-9
compared with the control group.
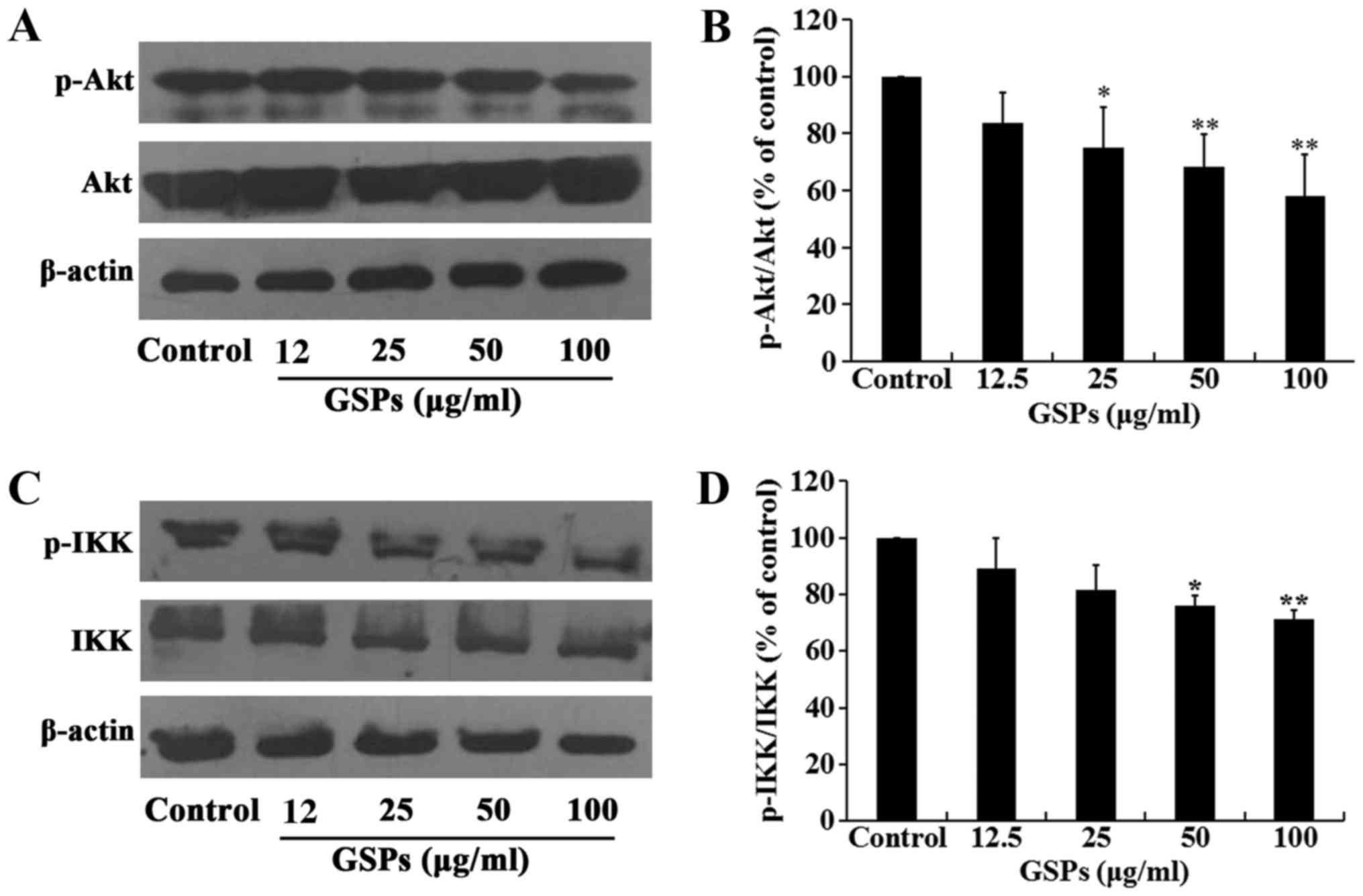
GSPs inhibit the phosphorylation of Akt
in Tca8113 cells
The Akt signaling pathway regulates a number of
cellular processes, including the proliferation, survival and
metastasis of cancer cells. The effect of GSPs on the expression
and phosphorylation of Akt in Tca8113 cells was investigated by
western blot analysis. The results demonstrated that treatment of
Tca8113 cells with 25–100 µg/ml GSPs significantly inhibited
the phosphorylation of Akt compared with the control group in a
dose-dependent manner, but did not affect the total Akt expression
level (Fig. 7A and B).
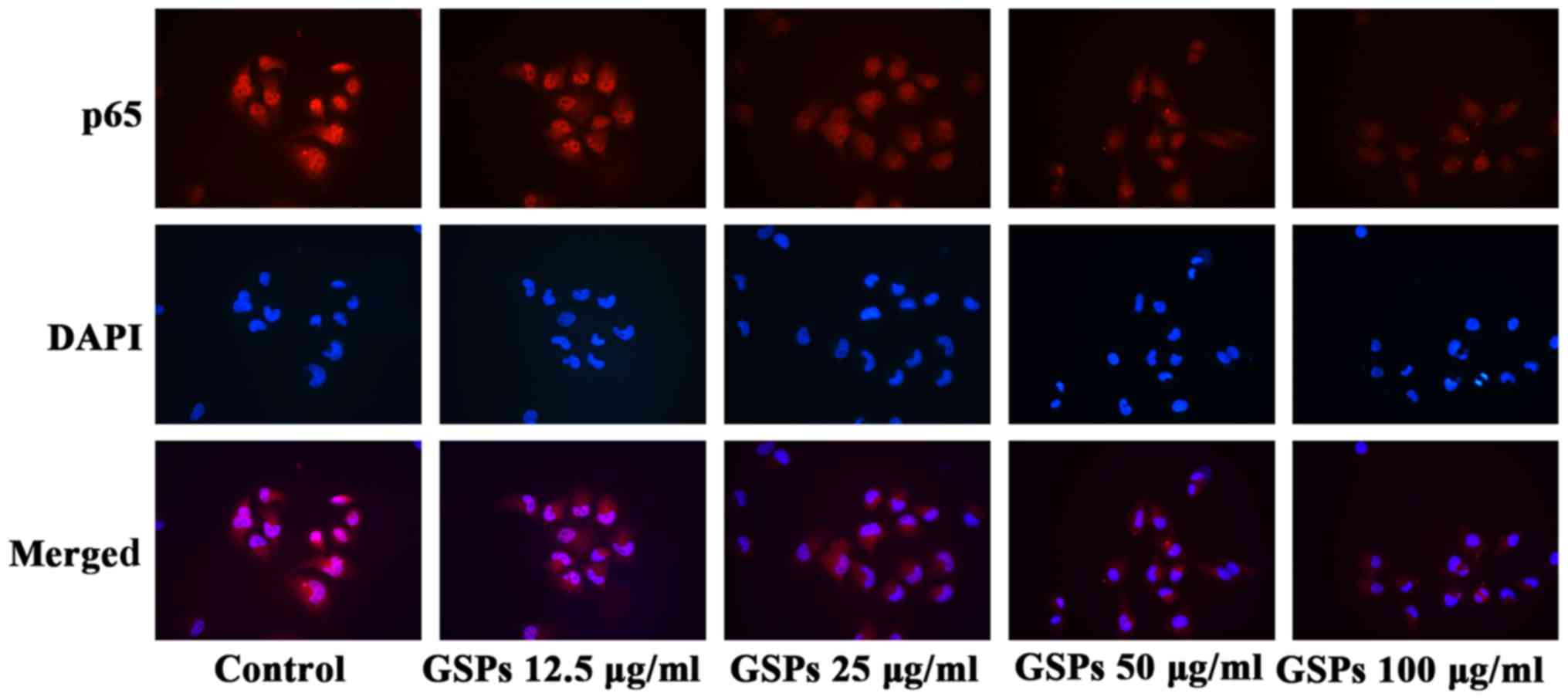
GSPs inhibit NF-κB activation and nuclear
translocation in Tca8113 cells
The NF-κB signaling pathway is one of the downstream
targets of activated Akt. The effect of GSPs on the expression and
phosphorylation of IKK in Tca8113 cells was examined. As
demonstrated in Fig. 7C and D,
GSPs administered at a concentration of 50 and 100 µg/ml
significantly inhibited the phosphorylation of IKK compared with
the control group. However, there was no clear effect on the total
IKK expression levels. The results of the NF-κB activation and
nuclear translocation assay (Fig.
8) further indicated that GSPs inhibited the translocation of
NF-κB into the nucleus in a dose-dependent manner in Tca8113 cells,
as when higher concentrations of GSP were administered there was
less NF-κB p65 visible in the nucleus.
Discussion
TSCC is the most common type of oral cancer
(1). Despite significant advances
in diagnosis and therapy, the 5-year survival rate of patients with
TSCC remains poor due to the frequent local and regional
recurrences, and neck lymph node metastases (30). Therefore, innovative and more
effective treatment strategies are required for the prevention and
treatment of TSCC. Previous studies have revealed that GSPs have
anticancer effects on various types of human cancer whilst
exhibiting no apparent toxicity in vivo (31,32). In the present study, the antitumor
activity of GSPs on TSCC was investigated and their underlying
mechanisms of action were explored. The results revealed that the
treatment of Tca8113 cells with GSPs decreased viability and
induced apoptosis in a dose-dependent manner. GSPs also inhibited
the migration, invasion and MMP secretion of Tca8113 cells at
non-cytotoxic concentrations. The mechanisms of action of these
effects were associated with the inhibition of the Akt/NF-κB
signaling pathway.
Bcl-2 and Bax are key regulators of cell growth and
apoptosis. Overexpression of Bcl-2 inhibits cell apoptosis, whereas
overexpression of Bax promotes cell death (33,34). In the present study, GSPs were
revealed to downregulate the expression of Bcl-2 and upregulate the
expression of Bax in Tca8113 cells, suggesting that Bcl-2 and Bax
serve roles in the GSP-induced apoptosis of Tca8113 cells.
The secretion of MMP for extracellular matrix
degradation, allowing the migration and invasion of cancer cells,
is key for tumor metastasis (35). The results of the present study
revealed that GSPs inhibited the migration, invasion and MMP
secretion of Tca8113 cells when administered at low concentrations.
These results suggest that GSPs may inhibit the metastatic
capabilities of Tca8113 cells.
The phosphoinositide 3-kinase/Akt pathway is a major
signaling pathway that controls cell proliferation, apoptosis,
angiogenesis and metastasis during cancer progression (36). This pathway is hyperactivated in
the majority of types of cancer (37). Akt is a serine/threonine kinase;
once activated through phosphorylation by other kinases, Akt
proceeds to phosphorylate a series of protein substrates that are
important to cell proliferation, migration and invasion, including
Bcl-2-associated agonist of cell death, glycogen synthase kinase 3β
and IKK (38,39). NF-κB is a heterodimer composed of
a p65 and a p50 subunit. Typically, NF-κB is sequestered in the
cytoplasm by an inhibitor named IκB. Upon activation by the
phosphorylation by a variety of receptor signals, including Akt,
IKK phosphorylates IκB, resulting in the ubiquitination and
degradation of IκB. Therefore, NF-κB is liberated from the control
of IκB (40). NF-κB subsequently
migrates into the nucleus and proceeds to activate the expression
of a number of genes that are important for cell survival and
mobility, such as Bcl-2 and MMP (41). In oral squamous cell carcinoma,
NF-κB, IKK-α and Akt have been identified to be constitutively
activated, which is associated with their malignant behavior and
antiapoptotic activity (42). In
the present study, treatment with GSPs inhibited the
phosphorylation of Akt and IKK, as well as the translocation of
NF-κB into the nucleus in Tca8113 cells.
The results of the present study indicate that GSPs
inhibit the viability, migration and invasion of Tca8113 cells
through inhibition of the Akt/NF-κB signaling pathway. These
results suggest that GSPs may be developed as a potential
chemopreventive agent against TSCC.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30700142 and
81260330) and the Program of Innovation and Entrepreneurship for
Talents of Lanzhou City (grant no. 2015-RC-26).
References
|
1
|
Chi AC, Day TA and Neville BW: Oral cavity
and oropharyngeal squamous cell carcinoma–an update. CA Cancer J
Clin. 65:401–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
6
|
Sano D and Myers JN: Metastasis of
squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev.
26:645–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ng JH, Iyer NG, Tan MH and Edgren G:
Changing epidemiology of oral squamous cell carcinoma of the
tongue: A global study. Head Neck. 39:297–304. 2017. View Article : Google Scholar
|
|
8
|
Patel SC, Carpenter WR, Tyree S, Couch ME,
Weissler M, Hackman T, Hayes DN, Shores C and Chera BS: Increasing
incidence of oral tongue squamous cell carcinoma in young white
women, age 18 to 44 years. J Clin Oncol. 29:1488–1494. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pavia M, Pileggi C, Nobile CG and
Angelillo IF: Association between fruit and vegetable consumption
and oral cancer: A meta-analysis of observational studies. Am J
Clin Nutr. 83:1126–1134. 2006.PubMed/NCBI
|
|
10
|
Khan N, Afaq F and Mukhtar H: Cancer
chemoprevention through dietary antioxidants: Progress and promise.
Antioxid Redox Signal. 10:475–510. 2008. View Article : Google Scholar
|
|
11
|
Weng CJ and Yen GC: Chemopreventive
effects of dietary phytochemicals against cancer invasion and
metastasis: Phenolic acids, monophenol, polyphenol, and their
derivatives. Cancer Treat Rev. 38:76–87. 2012. View Article : Google Scholar
|
|
12
|
Gonçalves VP, Ortega AA, Guimarães MR,
Curylofo FA, Rossa Junior C, Ribeiro DA and Spolidorio LC:
Chemopreventive activity of systemically administered curcumin on
oral cancer in the 4-nitroquinoline 1-oxide model. J Cell Biochem.
116:787–796. 2015. View Article : Google Scholar
|
|
13
|
Zlotogorski A, Dayan A, Dayan D, Chaushu
G, Salo T and Vered M: Nutraceuticals as new treatment approaches
for oral cancer: I. Curcumin. Oral Oncol. 49:187–191. 2013.
View Article : Google Scholar
|
|
14
|
Iriti M and Varoni EM: Chemopreventive
potential of flavonoids in oral squamous cell carcinoma in human
studies. Nutrients. 5:2564–2576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zlotogorski A, Dayan A, Dayan D, Chaushu
G, Salo T and Vered M: Nutraceuticals as new treatment approaches
for oral cancer: II. Green tea extracts and resveratrol. Oral
Oncol. 49:502–506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Silva RC, Rigaud J, Cheynier V and Chemina
A: Procyanidin dimers and trimers from grape seeds. Phytochemistry.
30:1259–1264. 1991. View Article : Google Scholar
|
|
17
|
Prieur C, Rigaud J, Cheynier V and
Moutounet M: Oligomeric and polymeric procyanidins from grape
seeds. Phytochemistry. 36:781–789. 1994. View Article : Google Scholar
|
|
18
|
Nandakumar V, Singh T and Katiyar SK:
Multi-targeted prevention and therapy of cancer by
proanthocyanidins. Cancer Lett. 269:378–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaur M, Agarwal C and Agarwal R:
Anticancer and cancer chemopreventive potential of grape seed
extract and other grape-based products. J Nutr. 139:1806S–1812S.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katiyar SK and Athar M: Grape seeds: Ripe
for cancer chemoprevention. Cancer Prev Res (Phila). 6:617–621.
2013. View Article : Google Scholar
|
|
21
|
Chatelain K, Phippen S, McCabe J, Teeters
CA, O'Malley S and Kingsley K: Cranberry and grape seed extracts
inhibit the proliferative phenotype of oral squamous cell
carcinomas. Evid Based Complement Alternat Med. 2011:4676912011.
View Article : Google Scholar :
|
|
22
|
Lin YS, Chen SF, Liu CL and Nieh S: The
chemoadjuvant potential of grape seed procyanidins on p53-related
cell death in oral cancer cells. J Oral Pathol Med. 41:322–331.
2012. View Article : Google Scholar
|
|
23
|
Aghbali A, Hosseini SV, Delazar A, Gharavi
NK, Shahneh FZ, Orangi M, Bandehagh A and Baradaran B: Induction of
apoptosis by grape seed extract (Vitis vinifera) in oral squamous
cell carcinoma. Bosn J Basic Med Sci. 13:186–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Q, Prasad R, Rosenthal E and Katiyar
SK: Grape seed proanthocyanidins inhibit the invasiveness of human
HNSCC cells by targeting EGFR and reversing the
epithelial-to-mesenchymal transition. PLoS One. 7:e310932012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yen CY, Hou MF, Yang ZW, Tang JY, Li KT,
Huang HW, Huang YH, Lee SY, Fu TF, Hsieh CY, et al: Concentration
effects of grape seed extracts in anti-oral cancer cells involving
differential apoptosis, oxidative stress, and DNA damage. BMC
Complement Altern Med. 15:942015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shrotriya S, Tyagi A, Deep G, Orlicky DJ,
Wisell J, Wang XJ, Sclafani RA, Agarwal R and Agarwal C: Grape seed
extract and resveratrol prevent 4-nitroquinoline 1-oxide induced
oral tumorigenesis in mice by modulating AMPK activation and
associated biological responses. Mol Carcinog. 54:291–300. 2015.
View Article : Google Scholar
|
|
27
|
Vichai V and Kirtikara K: Sulforhodamine B
colorimetric assay for cytotoxicity screening. Nat Protoc.
1:1112–1116. 2006. View Article : Google Scholar
|
|
28
|
Huang S, Yang N, Liu Y, Hu L, Zhao J, Gao
J, Li Y, Li C, Zhang X and Huang T: Grape seed proanthocyanidins
inhibit angiogenesis via the downregulation of both vascular
endothelial growth factor and angiopoietin signaling. Nutr Res.
32:530–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Albini A, Iwamoto Y, Kleinman HK, Martin
GR, Aaronson SA, Kozlowski JM and McEwan RN: A rapid in vitro assay
for quantitating the invasive potential of tumor cells. Cancer Res.
47:3239–3245. 1987.PubMed/NCBI
|
|
30
|
Dogan E, Cetinayak HO, Sarioglu S, Erdag
TK and Ikiz AO: Patterns of cervical lymph node metastases in oral
tongue squamous cell carcinoma: Implications for elective and
therapeutic neck dissection. J Laryngol Otol. 128:268–273. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fraga CG and Oteiza PI: Dietary
flavonoids: Role of (−)-epicatechin and related procyanidins in
cell signaling. Free Radic Biol Med. 51:813–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bagchi D, Swaroop A, Preuss HG and Bagchi
M: Free radical scavenging, antioxidant and cancer chemoprevention
by grape seed proanthocyanidin: An overview. Mutat Res. 768:69–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Delbridge AR, Grabow S, Strasser A and
Vaux DL: Thirty years of BCL-2: Translating cell death discoveries
into novel cancer therapies. Nat Rev Cancer. 16:99–109. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wan L, Pantel K and Kang Y: Tumor
metastasis: Moving new biological insights into the clinic. Nat
Med. 19:1450–1464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tokunaga E, Oki E, Egashira A, Sadanaga N,
Morita M, Kakeji Y and Maehara Y: Deregulation of the Akt pathway
in human cancer. Curr Cancer Drug Targets. 8:27–36. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oeckinghaus A, Hayden MS, Ghosh S and
Matias-Guiu X: Crosstalk in NF-κB signaling pathways. Nat Immunol.
12:695–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nakayama H, Ikebe T, Beppu M and Shirasuna
K: High expression levels of nuclear factor kappaB, IkappaB kinase
alpha and Akt kinase in squamous cell carcinoma of the oral cavity.
Cancer. 92:3037–3044. 2001. View Article : Google Scholar : PubMed/NCBI
|