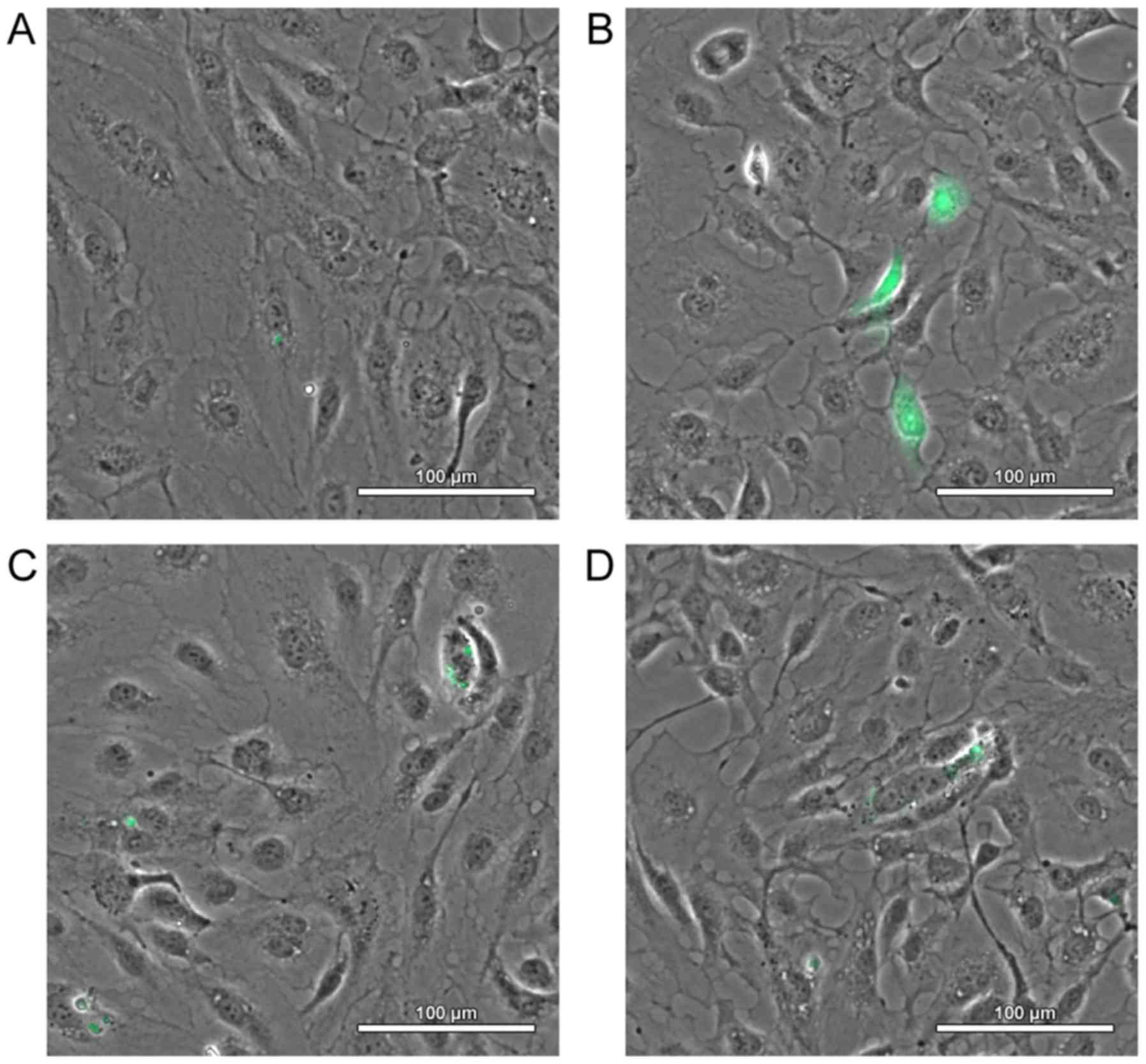
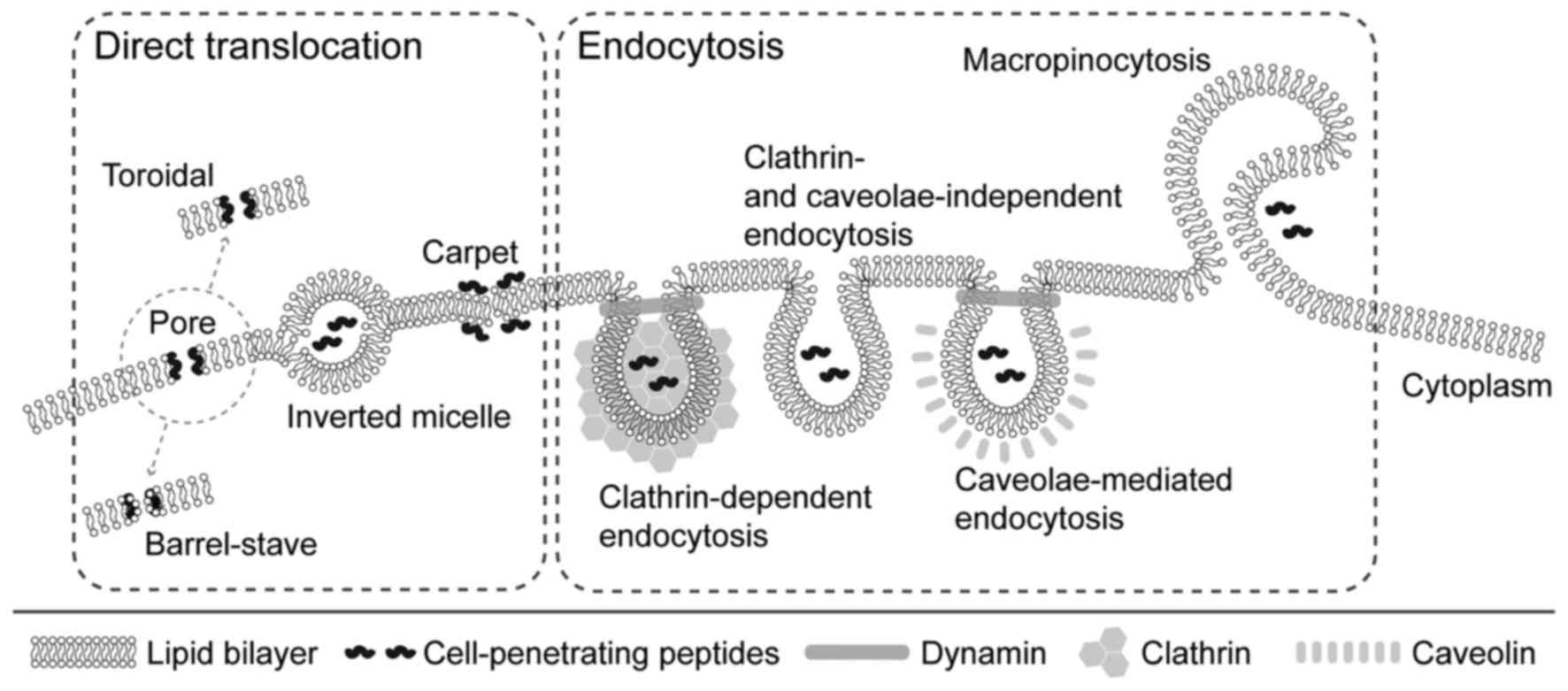
|
1
|
Zorko M and Langel U: Cell-penetrating
peptides: Mechanism and kinetics of cargo delivery. Adv Drug Deliv
Rev. 57:529–545. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karro K, Männik T, Männik A and Ustav M:
DNA transfer into animal cells using stearylated CPP based
transfection reagent. Methods Mol Biol. 1324:435–445. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramakrishna S, Kwaku Dad A-BB, Beloor J,
Gopalappa R, Lee SK and Kim H: Gene disruption by cell-penetrating
peptide-mediated delivery of Cas9 protein and guide RNA. Genome
Res. 24:1020–1027. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zatsepin TS, Turner JJ, Oretskaya TS and
Gait MJ: Conjugates of oligonucleotides and analogues with cell
penetrating peptides as gene silencing agents. Curr Pharm Des.
11:3639–3654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Segovia N, Dosta P, Cascante A, Ramos V
and Borrós S: Oligopeptide-terminated poly(β-amino ester)s for
highly efficient gene delivery and intracellular localization. Acta
Biomater. 10:2147–2158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu C, Luo Q, Tu Y, Wang G, Liu Y and Xie
Y: Drug-carrier interaction analysis in the cell penetrating
peptide-modified liposomes for doxorubicin loading. J
Microencapsul. 32:745–754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Wen G, Wang D, Zhang X, Lu Y, Wang
J, Zhong L, Cai H, Zhang X and Wang Y: A complementary strategy for
enhancement of nanoparticle intracellular uptake. Pharm Res.
31:2054–2064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jo J, Hong S, Choi WY and Lee DR:
Cell-penetrating peptide (CPP)-conjugated proteins is an efficient
tool for manipulation of human mesenchymal stromal cells. Sci Rep.
4:43782014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khafagy S, Morishita M, Isowa K, Imai J
and Takayama K: Effect of cell-penetrating peptides on the nasal
absorption of insulin. J Control Release. 133:103–108. 2009.
View Article : Google Scholar
|
|
10
|
Skotland T, Iversen TG, Torgersen ML and
Sandvig K: Cell-penetrating peptides: Possibilities and challenges
for drug delivery in vitro and in vivo. Molecules. 20:13313–13323.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frankel AD and Pabo CO: Cellular uptake of
the tat protein from human immunodeficiency virus. Cell.
55:1189–1193. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Green M and Loewenstein PM: Autonomous
functional domains of chemically synthesized human immunodeficiency
virus tat trans-activator protein. Cell. 55:1179–1188. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruben S, Perkins A, Purcell R, Joung K,
Sia R, Burghoff R, Haseltine WA and Rosen CA: Structural and
functional characterization of human immunodeficiency virus tat
protein. J Virol. 63:1–8. 1989.PubMed/NCBI
|
|
14
|
Derossi D, Joliot AH, Chassaing G and
Prochiantz A: The third helix of the Antennapedia homeodomain
translocates through biological membranes. J Biol Chem.
269:10444–10450. 1994.PubMed/NCBI
|
|
15
|
Milletti F: Cell-penetrating peptides:
Classes, origin, and current landscape. Drug Discov Today.
17:850–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Derossi D, Chassaing G and Prochiantz A:
Trojan peptides: The penetratin system for intracellular delivery.
Trends Cell Biol. 8:84–87. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pooga M, Hällbrink M, Zorko M and Langel
U: Cell penetration by transportan. FASEB J. 12:67–77.
1998.PubMed/NCBI
|
|
18
|
Kwon SJ, Han K, Jung S, Lee JE, Park S,
Cheon YP and Lim HJ: Transduction of the MPG-tagged fusion protein
into mammalian cells and oocytes depends on amiloride-sensitive
endocytic pathway. BMC Biotechnol. 9:732009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mo RH, Zaro JL and Shen WC: Comparison of
cationic and amphipathic cell penetrating peptides for siRNA
delivery and efficacy. Mol Pharm. 9:299–309. 2012. View Article : Google Scholar :
|
|
20
|
Bechara C, Pallerla M, Burlina F, Illien
F, Cribier S and Sagan S: Massive glycosaminoglycan-dependent entry
of Trp-containing cell-penetrating peptides induced by exogenous
sphingomyelinase or cholesterol depletion. Cell Mol Life Sci.
72:809–820. 2015. View Article : Google Scholar
|
|
21
|
Melikov K, Hara A, Yamoah K, Zaitseva E,
Zaitsev E and Chernomordik LV: Efficient entry of cell-penetrating
peptide nona-arginine into adherent cells involves a transient
increase in intracellular calcium. Biochem J. 471:221–230. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zavaglia D, Favrot MC, Eymin B, Tenaud C
and Coll JL: Intercellular trafficking and enhanced in vivo
antitumour activity of a non-virally delivered P27-VP22 fusion
protein. Gene Ther. 10:314–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin YZ, Yao SY, Veach RA, Torgerson TR and
Hawiger J: Inhibition of nuclear translocation of transcription
factor NF-kappa B by a synthetic peptide containing a cell
membrane-permeable motif and nuclear localization sequence. J Biol
Chem. 270:14255–14258. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oehlke J, Krause E, Wiesner B, Beyermann M
and Bienert M: Extensive cellular uptake into endothelial cells of
an amphipathic beta-sheet forming peptide. FEBS Lett. 415:196–199.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rousselle C, Clair P, Temsamani J and
Scherrmann JM: Improved brain delivery of benzylpenicillin with a
peptide-vector-mediated strategy. J Drug Target. 10:309–315. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Järver P and Langel U: Cell-penetrating
peptides - a brief introduction. Biochim Biophys Acta.
1758:260–263. 2006. View Article : Google Scholar
|
|
27
|
Meade BR and Dowdy SF: Exogenous siRNA
delivery using peptide transduction domains/cell penetrating
peptides. Adv Drug Deliv Rev. 59:134–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simeoni F, Morris MC, Heitz F and Divita
G: Insight into the mechanism of the peptide-based gene delivery
system MPG: Implications for delivery of siRNA into mammalian
cells. Nucleic Acids Res. 31:2717–2724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muñoz-Morris MA, Heitz F, Divita G and
Morris MC: The peptide carrier Pep-1 forms biologically efficient
nanoparticle complexes. Biochem Biophys Res Commun. 355:877–882.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gros E, Deshayes S, Morris MC,
Aldrian-Herrada G, Depollier J, Heitz F and Divita G: A
non-covalent peptide-based strategy for protein and peptide nucleic
acid transduction. Biochim Biophys Acta. 1758:384–393. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deshayes S, Morris M, Heitz F and Divita
G: Delivery of proteins and nucleic acids using a non-covalent
peptide-based strategy. Adv Drug Deliv Rev. 60:537–547. 2008.
View Article : Google Scholar
|
|
32
|
Guo Z, Peng H, Kang J and Sun D:
Cell-penetrating peptides: Possible transduction mechanisms and
therapeutic applications. Biomed Rep. 4:528–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mäger I, Langel K, Lehto T, Eiríksdóttir E
and Langel U: The role of endocytosis on the uptake kinetics of
luciferin-conjugated cell-penetrating peptides. Biochim Biophys
Acta. 1818:502–511. 2012. View Article : Google Scholar
|
|
34
|
Dutta D and Donaldson JG: Search for
inhibitors of endocytosis: Intended specificity and unintended
consequences. Cell Logist. 2:203–208. 2012. View Article : Google Scholar
|
|
35
|
Tomoda H, Kishimoto Y and Lee YC:
Temperature effect on endocytosis and exocytosis by rabbit alveolar
macrophages. J Biol Chem. 264:15445–15450. 1989.PubMed/NCBI
|
|
36
|
Bode SA1, Thévenin M, Bechara C, Sagan S,
Bregant S, Lavielle S, Chassaing G and Burlina F: Self-assembling
mini cell-penetrating peptides enter by both direct translocation
and glycosaminoglycan-dependent endocytosis. Chem Commun (Camb).
48:7179–7181. 2012. View Article : Google Scholar
|
|
37
|
Cleal K, He L, Watson PD and Jones AT:
Endocytosis, intracellular traffic and fate of cell penetrating
peptide based conjugates and nanoparticles. Curr Pharm Des.
19:2878–2894. 2013. View Article : Google Scholar
|
|
38
|
Derossi D, Calvet S, Trembleau A,
Brunissen A, Chassaing G and Prochiantz A: Cell internalization of
the third helix of the Antennapedia homeodomain is
receptor-independent. J Biol Chem. 271:18188–18193. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuzaki K, Sugishita K and Miyajima K:
Interactions of an antimicrobial peptide, magainin 2, with
lipopolysaccharide-containing liposomes as a model for outer
membranes of gram-negative bacteria. FEBS Lett. 449:221–224. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deshayes S, Plénat T, Aldrian-Herrada G,
Divita G, Le Grimellec C and Heitz F: Primary amphipathic
cell-penetrating peptides: Structural requirements and interactions
with model membranes. Biochemistry. 43:7698–7706. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Regberg J, Eriksson JN and Langel U:
Cell-penetrating peptides: From cell cultures to in vivo
applications. Front Biosci (Elite Ed). 5:509–516. 2013. View Article : Google Scholar
|
|
42
|
Safety and efficacy study of AVI-5126 when
used on vein grafts before use in heart by-pass graft surgery
(CABG). https://clinicaltrials.gov/ct2/show/NCT00451256.
2009
|
|
43
|
Safety and efficacy study of KAI-1678 to
treat pain in subjects with postherpetic neuralgia. https://clinicaltrials.gov/ct2/show/NCT01106716.
2010
|
|
44
|
Safety and efficacy study of KAI-1678 to
treat pain in subjects with spinal cord injury. https://clinicaltrials.gov/ct2/show/NCT01135108.
2010
|
|
45
|
Safety and efficacy study of KAI-1678 to
treat subjects with postoperative pain. https://clinicaltrials.gov/ct2/show/NCT01015235.
2011
|
|
46
|
Efficacy of AM-111 in patients with acute
sensorineural hearing loss. https://clinicaltrials.gov/ct2/show/NCT00802425.
2014
|
|
47
|
Safety and efficacy study of RT001 to
treat moderate to severe lateral canthal lines. https://clinicaltrials.gov/ct2/show/NCT00888914.
2013
|
|
48
|
Safety, tolerability and PK of a single iv
infusion of 10, 40, and 80 µg/kg XG-102 administered to healthy
volunteers. https://clinicaltrials.gov/ct2/show/NCT01570205.
2012
|
|
49
|
Efficacy and safety of XG-102 in reduction
of post-cataract surgery intraocular inflammation. https://clinicaltrials.gov/ct2/show/NCT02235272.
2015
|
|
50
|
AM-111 in the treatment of acute inner ear
hearing loss (HEALOS). https://clinicaltrials.gov/ct2/show/NCT02561091.
2014
|
|
51
|
Efficacy and safety of AM-111 as acute
sudden sensorineural hearing loss treatment (ASSENT). https://clinicaltrials.gov/ct2/show/NCT02809118.
2017
|
|
52
|
Bakhtiyari S, Haghani K, Basati G and
Karimfar MH: siRNA therapeutics in the treatment of diseases. Ther
Deliv. 4:45–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nakase I, Tanaka G and Futaki S:
Cell-penetrating peptides (CPPs) as a vector for the delivery of
siRNAs into cells. Mol Biosyst. 9:855–861. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wierzbicki PM, Kogut-Wierzbicka M,
Ruczynski J, Siedlecka-Kroplewska K, Kaszubowska L, Rybarczyk A,
Alenowicz M, Rekowski P and Kmiec Z: Protein and siRNA delivery by
transportan and transportan 10 into colorectal cancer cell lines.
Folia Histochem Cytobiol. 52:270–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Moschos SA, Jones SW, Perry MM, Williams
AE, Erjefalt JS, Turner JJ, Barnes PJ, Sproat BS, Gait MJ and
Lindsay MA: Lung delivery studies using siRNA conjugated to
TAT(48–60) and penetratin reveal peptide induced reduction in gene
expression and induction of innate immunity. Bioconjug Chem.
18:1450–1459. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Turner JJ, Jones S, Fabani MM, Ivanova G,
Arzumanov AA and Gait MJ: RNA targeting with peptide conjugates of
oligonucleotides, siRNA and PNA. Blood Cells Mol Dis. 38:1–7. 2007.
View Article : Google Scholar
|
|
57
|
Muratovska A and Eccles MR: Conjugate for
efficient delivery of short interfering RNA (siRNA) into mammalian
cells. FEBS Lett. 558:63–68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Davidson TJ, Harel S, Arboleda VA, Prunell
GF, Shelanski ML, Greene LA and Troy CM: Highly efficient small
interfering RNA delivery to primary mammalian neurons induces
MicroRNA-like effects before mRNA degradation. J Neurosci.
24:10040–10046. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chiu YL, Ali A, Chu CY, Cao H and Rana TM:
Visualizing a correlation between siRNA localization, cellular
uptake, and RNAi in living cells. Chem Biol. 11:1165–1175. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Turner JJ, Williams D, Owen D and Gait MJ:
Disulfide conjugation of peptides to oligonucleotides and their
analogs. Current protocols in nucleic acid chemistry. Chapter 4:
Unit 4.28. 2006. View Article : Google Scholar
|
|
61
|
Huang YW, Lee HJ, Tolliver LM and Aronstam
RS: Delivery of nucleic acids and nanomaterials by cell-penetrating
peptides: Opportunities and challenges. Biomed Res Int.
2015:8340792015.PubMed/NCBI
|
|
62
|
Crowet JM, Lins L, Deshayes S, Divita G,
Morris M, Brasseur R and Thomas A: Modeling of non-covalent
complexes of the cell-penetrating peptide CADY and its siRNA cargo.
Biochim Biophys Acta. 1828:499–509. 2013. View Article : Google Scholar
|
|
63
|
Crombez L, Morris MC, Dufort S,
Aldrian-Herrada G, Nguyen Q, Mc Master G, Coll JL, Heitz F and
Divita G: Targeting cyclin B1 through peptide-based delivery of
siRNA prevents tumour growth. Nucleic Acids Res. 37:4559–4569.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kadkhodayan S, Jafarzade BS, Sadat SM,
Motevalli F, Agi E and Bolhassani A: Combination of cell
penetrating peptides and heterologous DNA prime/protein boost
strategy enhances immune responses against HIV-1 Nef antigen in
BALB/c mouse model. Immunol Lett. 188:38–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bivalkar-Mehla S, Mehla R and Chauhan A:
Chimeric peptide-mediated siRNA transduction to inhibit HIV-1
infection. J Drug Target. 25:307–319. 2017. View Article : Google Scholar
|
|
66
|
Kato T, Yamashita H, Misawa T, Nishida K,
Kurihara M, Tanaka M, Demizu Y and Oba M: Plasmid DNA delivery by
arginine-rich cell-penetrating peptides containing unnatural amino
acids. Bioorg Med Chem. 24:2681–2687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Rudolph C, Plank C, Lausier J, Schillinger
U, Müller RH and Rosenecker J: Oligomers of the arginine-rich motif
of the HIV-1 TAT protein are capable of transferring plasmid DNA
into cells. J Biol Chem. 278:11411–11418. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rádis-Baptista G, Campelo IS, Morlighem
JR, Melo LM and Freitas VJ: Cell-penetrating peptides (CPPs): From
delivery of nucleic acids and antigens to transduction of
engineered nucleases for application in transgenesis. J Biotechnol.
252:15–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nagy A: Cre recombinase: The universal
reagent for genome tailoring. Genesis. 26:99–109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jo D, Nashabi A, Doxsee C, Lin Q, Unutmaz
D, Chen J and Ruley HE: Epigenetic regulation of gene structure and
function with a cell-permeable Cre recombinase. Nat Biotechnol.
19:929–933. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wadia JS, Stan RV and Dowdy SF:
Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion
proteins after lipid raft macropinocytosis. Nat Med. 10:310–315.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hashimoto M, Taniguchi M, Yoshino S, Arai
S and Sato K: S Phase-preferential Cre-recombination in mammalian
cells revealed by HIV-TAT-PTD-mediated protein transduction. J
Biochem. 143:87–95. 2008. View Article : Google Scholar
|
|
73
|
Xu Y, Liu S, Yu G, Chen J, Chen J, Xu X,
Wu Y, Zhang A, Dowdy SF and Cheng G: Excision of selectable genes
from transgenic goat cells by a protein transducible TAT-Cre
recombinase. Gene. 419:70–74. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
De Coupade C, Fittipaldi A, Chagnas V,
Michel M, Carlier S, Tasciotti E, Darmon A, Ravel D, Kearsey J,
Giacca M, et al: Novel human-derived cell-penetrating peptides for
specific subcellular delivery of therapeutic biomolecules. Biochem
J. 390:407–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gitton Y, Tibaldi L, Dupont E, Levi G and
Joliot A: Efficient CPP-mediated Cre protein delivery to developing
and adult CNS tissues. BMC Biotechnol. 9:402009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sonsteng KM, Prigge JR, Talago EA, June RK
and Schmidt EE: Hydrodynamic delivery of Cre protein to
lineage-mark or time-stamp mouse hepatocytes in situ. PLoS One.
9:e912192014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gaj T, Guo J, Kato Y, Sirk SJ and Barbas
CF III: Targeted gene knockout by direct delivery of zinc-finger
nuclease proteins. Nat Methods. 9:805–807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen Z, Jaafar L, Agyekum DG, Xiao H, Wade
MF, Kumaran RI, Spector DL, Bao G, Porteus MH, Dynan WS, et al:
Receptor-mediated delivery of engineered nucleases for genome
modification. Nucleic Acids Res. 41:e1822013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cornu TI, Thibodeau-Beganny S, Guhl E,
Alwin S, Eichtinger M, Joung JK and Cathomen T: DNA-binding
specificity is a major determinant of the activity and toxicity of
zinc-finger nucleases. Mol Ther. 16:352–358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pruett-Miller SM, Reading DW, Porter SN
and Porteus MH: Attenuation of zinc finger nuclease toxicity by
small-molecule regulation of protein levels. PLoS Genet.
5:e10003762009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Puria R, Sahi S and Nain V:
HER2+ breast cancer therapy: By CPP-ZFN mediated
targeting of mTOR? Technol Cancer Res Treat. 11:175–180. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nain V, Sahi S and Verma A: CPP-ZFN: A
potential DNA-targeting anti-malarial drug. Malar J. 9:2582010.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu J, Gaj T, Patterson JT, Sirk SJ and
Barbas CF III: Cell-penetrating peptide-mediated delivery of TALEN
proteins via bioconjugation for genome engineering. PLoS One.
9:e857552014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Horvath P and Barrangou R: CRISPR/Cas, the
immune system of bacteria and archaea. Science. 327:167–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Selle K and Barrangou R: Harnessing
CRISPR-Cas systems for bacterial genome editing. Trends Microbiol.
23:225–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Harms DW, Quadros RM, Seruggia D, Ohtsuka
M, Takahashi G, Montoliu L and Gurumurthy CB: Mouse genome editing
using the CRISPR/Cas system. Curr Protoc Hum Genet.
83:15.7.1–15.7.27. 2014. View Article : Google Scholar
|
|
87
|
Bortesi L and Fischer R: The CRISPR/Cas9
system for plant genome editing and beyond. Biotechnol Adv.
33:41–52. 2015. View Article : Google Scholar
|
|
88
|
Park A, Hong P, Won ST, Thibault PA,
Vigant F, Oguntuyo KY, Taft JD and Lee B: Sendai virus, an RNA
virus with no risk of genomic integration, delivers CRISPR/Cas9 for
efficient gene editing. Mol Ther Methods Clin Dev. 3:160572016.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Suresh B, Ramakrishna S and Kim H:
Cell-penetrating peptide-mediated delivery of Cas9 protein and
guide RNA for genome editing. Methods Mol Biol. 1507:81–94. 2017.
View Article : Google Scholar
|
|
90
|
Langel U: Handbook of Cell-Penetrating
Peptides. 2nd edition. Taylor and Francis Group; 2006, View Article : Google Scholar
|
|
91
|
Saar K, Lindgren M, Hansen M, Eiríksdóttir
E, Jiang Y, Rosenthal-Aizman K, Sassian M and Langel U:
Cell-penetrating peptides: A comparative membrane toxicity study.
Anal Biochem. 345:55–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Suhorutsenko J, Oskolkov N, Arukuusk P,
Kurrikoff K, Eriste E, Copolovici DM and Langel U: Cell-penetrating
peptides, PepFects, show no evidence of toxicity and immunogenicity
in vitro and in vivo. Bioconjug Chem. 22:2255–2262. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Amantana A, Moulton HM, Cate ML, Reddy MT,
Whitehead T, Hassinger JN, Youngblood DS and Iversen PL:
Pharmacokinetics, biodistribution, stability and toxicity of a
cell-penetrating peptide-morpholino oligomer conjugate. Bioconjug
Chem. 18:1325–1331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Maiolo JR, Ferrer M and Ottinger EA:
Effects of cargo molecules on the cellular uptake of arginine-rich
cell-penetrating peptides. Biochim Biophys Acta. 1712:161–172.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cox DB, Platt RJ and Zhang F: Therapeutic
genome editing: Prospects and challenges. Nat Med. 21:121–131.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y,
Wu Q, Park A, Yang J, Suresh S, Bizhanova A, et al: Therapeutic
genome editing by combined viral and non-viral delivery of CRISPR
system components in vivo. Nat Biotechnol. 34:328–333. 2016.
View Article : Google Scholar : PubMed/NCBI
|