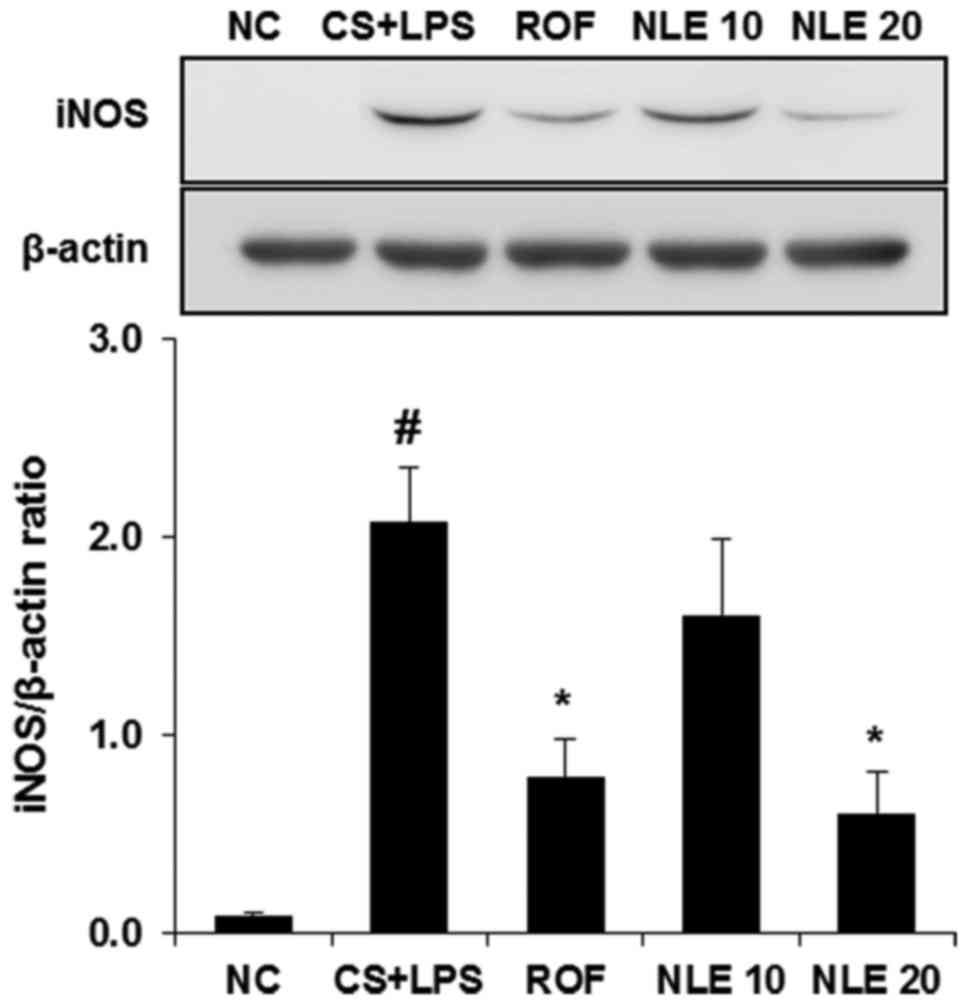
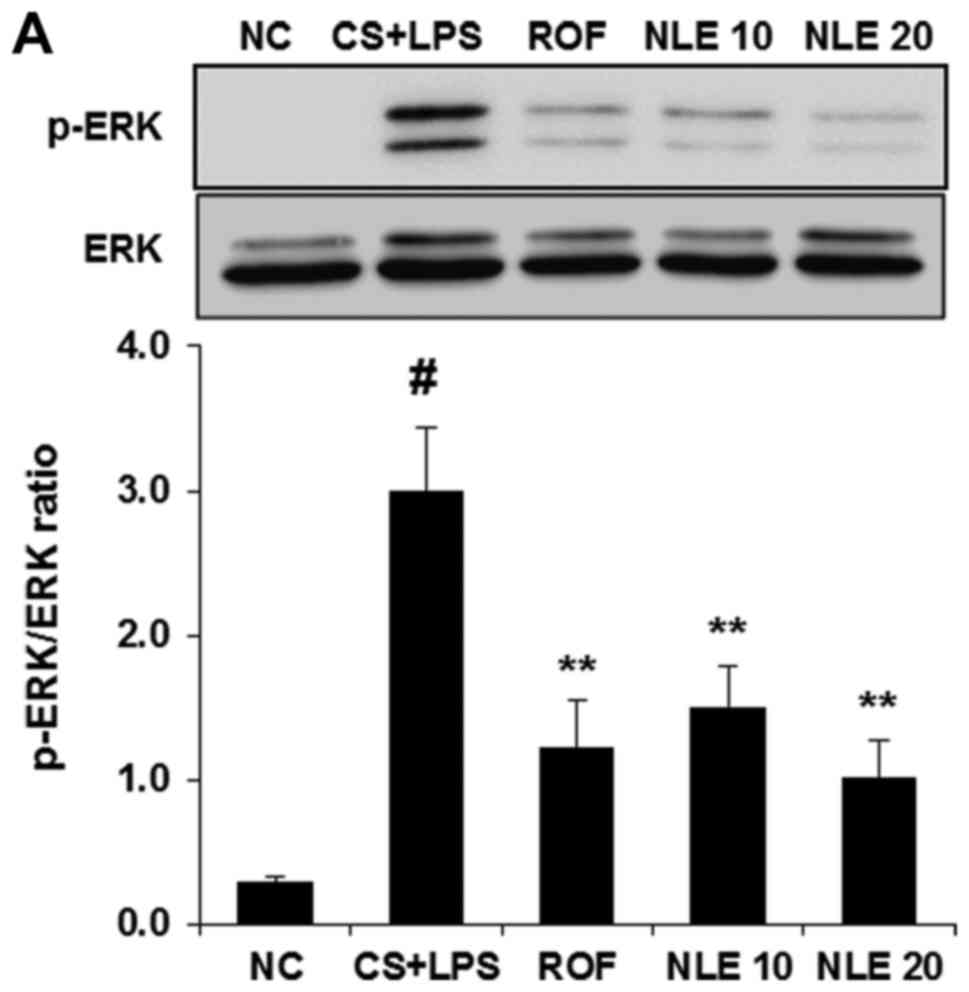
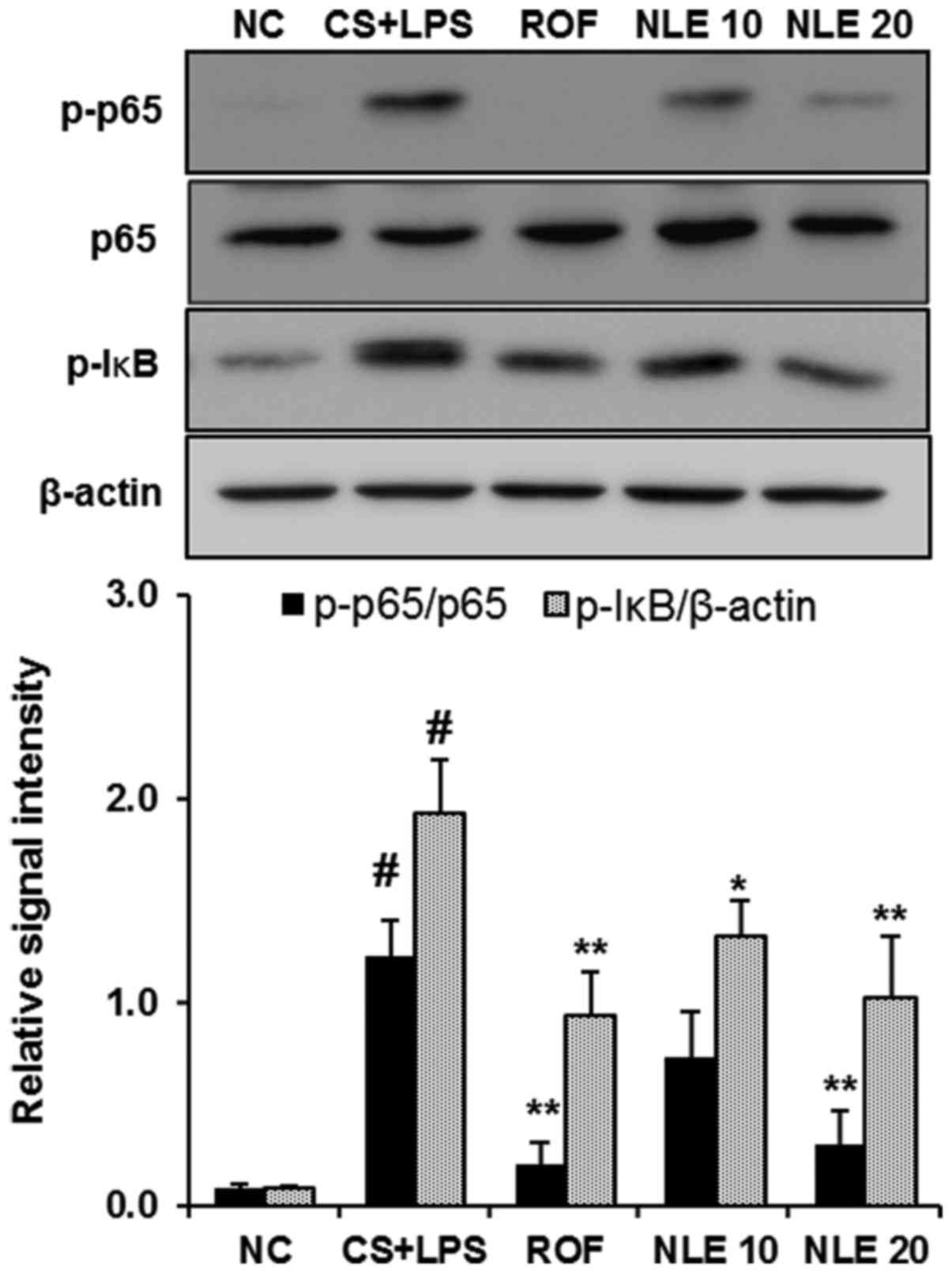
|
1
|
Lee H, Jung KH, Lee H, Park S, Choi W and
Bae H: Casticin, an active compound isolated from Vitex Fructus,
ameliorates the cigarette smoke-induced acute lung inflammatory
response in a murine model. Int Immunopharmacol. 28:1097–1101.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnes PJ: Chronic obstructive pulmonary
disease * 12: new treatments for COPD. Thorax. 58:803–808. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park YC, Jin M, Kim SH, Kim MH, Namgung U
and Yeo Y: Effects of inhalable microparticle of flower of Lonicera
japonica in a mouse model of COPD. J Ethnopharmacol. 151:123–130.
2014. View Article : Google Scholar
|
|
4
|
Bak JH, Lee SM and Lim HB: Safety
assessment of mainstream smoke of herbal cigarette. Toxicol Res.
31:41–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shin IS, Shin NR, Park JW, Jeon CM, Hong
JM, Kwon OK, Kim JS, Lee IC, Kim JC, Oh SR, et al: Melatonin
attenuates neutrophil inflammation and mucus secretion in cigarette
smoke-induced chronic obstructive pulmonary diseases via the
suppression of Erk-Sp1 signaling. J Pineal Res. 58:50–60. 2015.
View Article : Google Scholar
|
|
6
|
Lee JW, Shin NR, Park JW, Park SY, Kwon
OK, Lee HS, Hee Kim J, Lee HJ, Lee J, Zhang ZY, et al: Callicarpa
japonica Thunb. attenuates cigarette smoke-induced neutrophil
inflammation and mucus secretion. J Ethnopharmacol. 175:1–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kruger P, Saffarzadeh M, Weber AN, Rieber
N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J and Hartl
D: Neutrophils: between host defence, immune modulation, and tissue
injury. PLoS Pathog. 11:e10046512015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin IS, Ahn KS, Shin NR, Lee HJ, Ryu HW,
Kim JW, Sohn KY, Kim HJ, Han YH and Oh SR: Protective effect of
EC-18, a synthetic monoacetyldiglyceride on lung inflammation in a
murine model induced by cigarette smoke and lipopolysaccharide. Int
Immunopharmacol. 30:62–68. 2016. View Article : Google Scholar
|
|
9
|
Song HH, Shin IS, Woo SY, Lee SU, Sung MH,
Ryu HW, Kim DY, Ahn KS, Lee HK, Lee D, et al: Piscroside C, a novel
iridoid glycoside isolated from Pseudolysimachion rotundum var.
subinegrum suppresses airway inflammation induced by cigarette
smoke. J Ethnopharmacol. 170:20–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rahman I and Adcock IM: Oxidative stress
and redox regulation of lung inflammation in COPD. Eur Respir J.
28:219–242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shapiro SD, Goldstein NM, Houghton AM,
Kobayashi DK, Kelley D and Belaaouaj A: Neutrophil elastase
contributes to cigarette smoke-induced emphysema in mice. Am J
Pathol. 163:2329–2335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukhopadhyay S, Hoidal JR and Mukherjee
TK: Role of TNFalpha in pulmonary pathophysiology. Respir Res.
7:1252006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lundblad LK, Thompson-Figueroa J, Leclair
T, Sullivan MJ, Poynter ME, Irvin CG and Bates JH: Tumor necrosis
factor-alpha overexpression in lung disease: a single cause behind
a complex phenotype. Am J Respir Crit Care Med. 171:1363–1370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rincon M and Irvin CG: Role of IL-6 in
asthma and other inflammatory pulmonary diseases. Int J Biol Sci.
8:1281–1290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan MX, Wang Y, Liu Q, Schramm R and
Thorlacius H: CC chemokines induce P-selectin-dependent neutrophil
rolling and recruitment in vivo: intermediary role of mast cells.
Br J Pharmacol. 138:698–706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): an overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Traves SL, Culpitt SV, Russell RE, Barnes
PJ and Donnelly LE: Increased levels of the chemokines GROalpha and
MCP-1 in sputum samples from patients with COPD. Thorax.
57:590–595. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McNeill E, Crabtree MJ, Sahgal N, Patel J,
Chuaiphichai S, Iqbal AJ, Hale AB, Greaves DR and Channon KM:
Regulation of iNOS function and cellular redox state by macrophage
Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2
activation. Free Radic Biol Med. 79:206–216. 2015. View Article : Google Scholar :
|
|
19
|
Webb JL, Polak JM and Evans TJ: Effect of
adhesion on inducible nitric oxide synthase (iNOS) production in
purified human neutrophils. Clin Exp Immunol. 123:42–48. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JW, Bae CJ, Choi YJ, Kim SI, Kwon YS,
Lee HJ, Kim SS and Chun W: 3,4,5-Trihydroxycinnamic acid inhibits
lipopolysaccharide (LPS)-induced inflammation by Nrf2 activation in
vitro and improves survival of mice in LPS-induced endotoxemia
model in vivo. Mol Cell Biochem. 390:143–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marinovic MP, Morandi AC and Otton R:
Green tea catechins alone or in combination alter functional
parameters of human neutrophils via suppressing the activation of
TLR-4/NFκB p65 signal pathway. Toxicol In Vitro. 29:1766–1778.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roh GS, Yi CO, Cho YJ, Jeon BT,
Nizamudtinova IT, Kim HJ, Kim JH, Oh YM, Huh JW, Lee JH, et al:
Anti-inflammatory effects of celecoxib in rat lungs with
smoke-induced emphysema. Am J Physiol Lung Cell Mol Physiol.
299:L184–L191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang WT, Liu XS, Xu YJ, Ni W and Chen SX:
Expression of nitric oxide synthase isoenzyme in lung tissue of
smokers with and without chronic obstructive pulmonary disease.
Chin Med J (Engl). 128:1584–1589. 2015. View Article : Google Scholar
|
|
24
|
Singh D: P38 inhibition in COPD; cautious
optimism. Thorax. 68:705–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh YC, Jeong YH, Ha JH, Cho WK and Ma JY:
Oryeongsan inhibits LPS-induced production of inflammatory
mediators via blockade of the NF-kappaB, MAPK pathways and leads to
HO-1 induction in macrophage cells. BMC Complement Altern Med.
14:2422014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang Z, Xie W, Wu R, Geng H, Zhao L, Xie
C, Li X, Zhu M, Zhu W, Zhu J, et al: Inhibition of tobacco
smoke-induced bladder MAPK activation and epithelial-mesenchymal
transition in mice by curcumin. Int J Clin Exp Pathol. 8:4503–4513.
2015.PubMed/NCBI
|
|
27
|
Li L, Sun J, Xu C, Zhang H, Wu J, Liu B
and Dong J: Icariin ameliorates cigarette smoke induced
inflammatory responses via suppression of NF-κB and modulation of
GR in vivo and in vitro. PLoS One. 9:e1023452014. View Article : Google Scholar
|
|
28
|
Mahfuzul Hoque MD, Bari ML, Inatsu Y,
Juneja VK and Kawamoto S: Antibacterial activity of guava (Psidium
guajava L.) and Neem (Azadirachta indica A. Juss.) extracts against
foodborne pathogens and spoilage bacteria. Foodborne Pathog Dis.
4:481–488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okpanyi SN and Ezeukwu GC:
Anti-inflammatory and anti-pyretic activities of Azadirachta
indica. Planta Med. 41:34–39. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rao AD, Devi KN and Thyagaraju K:
Isolation of antioxidant principle from Azadirachta seed kernels:
determination of its role on plant lipoxygenases. J Enzyme Inhib.
14:85–96. 1998. View Article : Google Scholar
|
|
31
|
Yanpallewar SU, Sen S, Tapas S, Kumar M,
Raju SS and Acharya SB: Effect of Azadirachta indica on
paracetamol-induced hepatic damage in albino rats. Phytomedicine.
10:391–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Almas K: The antimicrobial effects of
extracts of Azadi-rachta indica (Neem) and Salvadora persica (Arak)
chewing sticks. Indian J Dent Res. 10:23–26. 1999.
|
|
33
|
Badam L, Joshi SP and Bedekar SS: 'In
vitro' antiviral activity of neem (Azadirachta indica. A Juss) leaf
extract against group B coxsackieviruses. J Commun Dis. 31:79–90.
1999.
|
|
34
|
Siddiqui BS, Afshan F, Gulzar T and Hanif
M: Tetracyclic triterpenoids from the leaves of Azadirachta indica.
Phytochemistry. 65:2363–2367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang YC, Tsai MH, Sheu WH, Hsieh SC and
Chiang AN: The therapeutic potential and mechanisms of action of
quercetin in relation to lipopolysaccharide-induced sepsis in vitro
and in vivo. PLoS One. 8:e807442013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Loizou S, Lekakis I, Chrousos GP and
Moutsatsou P: Beta-sitosterol exhibits anti-inflammatory activity
in human aortic endothelial cells. Mol Nutr Food Res. 54:551–558.
2010. View Article : Google Scholar
|
|
37
|
Pillai NR and Santhakumari G:
Anti-arthritic and anti-inflammatory actions of nimbidin. Planta
Med. 43:59–63. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim WH, Song HO, Jin CM, Hur JM, Lee HS,
Jin HY, Kim SY and Park H: The methanol extract of Azadirachta
indica A. Juss leaf protects mice against lethal endotoxemia and
sepsis. Biomol Ther (Seoul). 20:96–103. 2012. View Article : Google Scholar
|
|
39
|
Sakuma T, Takahashi K, Ohya N, Usuda K,
Handa M and Abe T: ONO-5046 is a potent inhibitor of neutrophil
elastase in human pleural effusion after lobectomy. Eur J
Pharmacol. 353:273–279. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rytilä P, Rehn T, Ilumets H, Rouhos A,
Sovijärvi A, Myllärniemi M and Kinnula VL: Increased oxidative
stress in asymptomatic current chronic smokers and GOLD stage 0
COPD. Respir Res. 7:692006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee JW, Kwon JH, Lim MS, Lee HJ, Kim SS,
Lim SY and Chun W: 3,4,5-Trihydroxycinnamic acid increases
heme-oxygenase-1 (HO-1) and decreases macrophage infiltration in
LPS-induced septic kidney. Mol Cell Biochem. 397:109–116. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barnes PJ: Chronic obstructive pulmonary
disease: a growing but neglected global epidemic. PLoS Med.
4:e1122007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vestbo J, Hurd SS, Agustí AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar
|
|
44
|
Yang J, Yu HM, Zhou XD, Huang HP, Han Zh,
Kolosov VP and Perelman JM: Cigarette smoke induces mucin
hypersecretion and inflammatory response through the p66shc adaptor
protein-mediated mechanism in human bronchial epithelial cells. Mol
Immunol. 69:86–98. 2016. View Article : Google Scholar
|
|
45
|
Shao MX, Nakanaga T and Nadel JA:
Cigarette smoke induces MUC5AC mucin overproduction via tumor
necrosis factor-alpha-converting enzyme in human airway epithelial
(NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol.
287:L420–L427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chung KF: Inflammatory mediators in
chronic obstructive pulmonary disease. Curr Drug Targets Inflamm
Allergy. 4:619–625. 2005. View Article : Google Scholar
|
|
47
|
Thorley AJ and Tetley TD: Pulmonary
epithelium, cigarette smoke, and chronic obstructive pulmonary
disease. Int J Chron Obstruct Pulmon Dis. 2:409–428. 2007.
|
|
48
|
Kwak HG and Lim HB: Inhibitory effects of
Cnidium monnieri fruit extract on pulmonary inflammation in mice
induced by cigarette smoke condensate and lipopolysaccharide. Chin
J Nat Med. 12:641–647. 2014.PubMed/NCBI
|
|
49
|
Baraldo S, Turato G, Badin C, Bazzan E,
Beghé B, Zuin R, Calabrese F, Casoni G, Maestrelli P, Papi A, et
al: Neutrophilic infiltration within the airway smooth muscle in
patients with COPD. Thorax. 59:308–312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li H, Yang T, Ning Q, Li F, Chen T, Yao Y
and Sun Z: Cigarette smoke extract-treated mast cells promote
alveolar macrophage infiltration and polarization in experimental
chronic obstructive pulmonary disease. Inhal Toxicol. 27:822–831.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Neofytou E, Tzortzaki EG, Chatziantoniou A
and Siafakas NM: DNA damage due to oxidative stress in chronic
obstructive pulmonary disease (COPD). Int J Mol Sci.
13:16853–16864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chan KH, Chan SC, Yeung SC, Man RY, Ip MS
and Mak JC: Inhibitory effect of Chinese green tea on cigarette
smoke-induced up-regulation of airway neutrophil elastase and
matrix metal-loproteinase-12 via antioxidant activity. Free Radic
Res. 46:1123–1129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Iizuka T, Ishii Y, Itoh K, Kiwamoto T,
Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, et
al: Nrf2-deficient mice are highly susceptible to cigarette
smoke-induced emphysema. Genes Cells. 10:1113–1125. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bhowmik A, Chahal K, Austin G and
Chakravorty I: Improving mucociliary clearance in chronic
obstructive pulmonary disease. Respir Med. 103:496–502. 2009.
View Article : Google Scholar
|
|
55
|
van Eeden SF and Sin DD: Oxidative stress
in chronic obstructive pulmonary disease: a lung and systemic
process. Can Respir J. 20:27–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hwang JH, Lee BJ, Jung HJ, Kim KI, Choi
JY, Joo M and Jung SK: Effects of Chung-pae inhalation therapy on a
mouse model of chronic obstructive pulmonary disease. Evid Based
Complement Alternat Med. 2015:4612952015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee E, Yun N, Jang YP and Kim J: Lilium
lancifolium Thunb. extract attenuates pulmonary inflammation and
air space enlargement in a cigarette smoke-exposed mouse model. J
Ethnopharmacol. 149:148–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pedroza M, Schneider DJ, Karmouty-Quintana
H, Coote J, Shaw S, Corrigan R, Molina JG, Alcorn JL, Galas D,
Gelinas R, et al: Interleukin-6 contributes to inflammation and
remodeling in a model of adenosine mediated lung injury. PLoS One.
6:e226672011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fernandez-Real JM, Broch M, Vendrell J and
Ricart W: Smoking, fat mass and activation of the tumor necrosis
factor-alpha pathway. Int J Obes Relat Metab Disord. 27:1552–1556.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu GH, Shen J, Sun P, Yang ML, Zhao PW,
Niu Y, Lu JK, Wang ZQ, Gao C, Han X, et al: Anti-inflammatory
effects of potato extract on a rat model of cigarette smoke-induced
chronic obstructive pulmonary disease. Food Nutr Res. 59:288792015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen X, Guan XJ, Peng XH, Cui ZL, Luan CY
and Guo XJ: Acetylation of lysine 9 on histone H3 is associated
with increased pro-inflammatory cytokine release in a cigarette
smoke-induced rat model through HDAC1 depression. Inflamm Res.
64:513–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhao Y, Cui A, Wang F, Wang XJ, Chen X,
Jin ML and Huang KW: Characteristics of pulmonary inflammation in
combined pulmonary fibrosis and emphysema. Chin Med J (Engl).
125:3015–3021. 2012.
|
|
63
|
Hesslinger C, Strub A, Boer R, Ulrich WR,
Lehner MD and Braun C: Inhibition of inducible nitric oxide
synthase in respiratory diseases. Biochem Soc Trans. 37:886–891.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li D, Xu D, Wang T, Shen Y, Guo S, Zhang
X, Guo L, Li X, Liu L and Wen F: Silymarin attenuates airway
inflammation induced by cigarette smoke in mice. Inflammation.
38:871–878. 2015. View Article : Google Scholar
|
|
65
|
Ng DS, Liao W, Tan WS, Chan TK, Loh XY and
Wong WS: Anti-malarial drug artesunate protects against cigarette
smoke-induced lung injury in mice. Phytomedicine. 21:1638–1644.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ma WJ, Sun YH, Jiang JX, Dong XW, Zhou JY
and Xie QM: Epoxyeicosatrienoic acids attenuate cigarette smoke
extract-induced interleukin-8 production in bronchial epithelial
cells. Prostaglandins Leukot Essent Fatty Acids. 94:13–19. 2015.
View Article : Google Scholar
|
|
67
|
Coskun M, Olsen J, Seidelin JB and Nielsen
OH: MAP kinases in inflammatory bowel disease. Clin Chim Acta.
412:513–520. 2011. View Article : Google Scholar
|
|
68
|
Hoshino S, Yoshida M, Inoue K, Yano Y,
Yanagita M, Mawatari H, Yamane H, Kijima T, Kumagai T, Osaki T, et
al: Cigarette smoke extract induces endothelial cell injury via JNK
pathway. Biochem Biophys Res Commun. 329:58–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xu X, Balsiger R, Tyrrell J, Boyaka PN,
Tarran R and Cormet-Boyaka E: Cigarette smoke exposure reveals a
novel role for the MEK/ERK1/2 MAPK pathway in regulation of CFTR.
Biochim Biophys Acta. 1850:1224–1232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Marumo S, Hoshino Y, Kiyokawa H, Tanabe N,
Sato A, Ogawa E, Muro S, Hirai T and Mishima M: p38
mitogen-activated protein kinase determines the susceptibility to
cigarette smoke-induced emphysema in mice. BMC Pulm Med. 14:792014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shen N, Gong T, Wang JD, Meng FL, Qiao L,
Yang RL, Xue B, Pan FY, Zhou XJ, Chen HQ, et al: Cigarette
smoke-induced pulmonary inflammatory responses are mediated by
EGR-1/GGPPS/MAPK signaling. Am J Pathol. 178:110–118. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Metcalfe HJ, Lea S, Hughes D, Khalaf R,
Abbott-Banner K and Singh D: Effects of cigarette smoke on
toll-like receptor (TLR) activation of chronic obstructive
pulmonary disease (COPD) macrophages. Clin Exp Immunol.
176:461–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhao Y, Xu Y, Li Y, Xu W, Luo F, Wang B,
Pang Y, Xiang Q, Zhou J, Wang X, et al: NF-κB-mediated inflammation
leading to EMT via miR-200c is involved in cell transformation
induced by cigarette smoke extract. Toxicol Sci. 135:265–276. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Edwards MR, Bartlett NW, Clarke D, Birrell
M, Belvisi M and Johnston SL: Targeting the NF-kappaB pathway in
asthma and chronic obstructive pulmonary disease. Pharmacol Ther.
121:1–13. 2009. View Article : Google Scholar
|
|
75
|
Akihisa T, Noto T, Takahashi A, Fujita Y,
Banno N, Tokuda H, Koike K, Suzuki T, Yasukawa K and Kimura Y:
Melanogenesis inhibitory, anti-inflammatory, and chemopreventive
effects of limonoids from the seeds of Azadirachta indicia A. Juss.
(neem). J Oleo Sci. 58:581–594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Faccin-Galhardi LC, Yamamoto KA, Ray S,
Ray B, Carvalho Linhares RE and Nozawa C: The in vitro antiviral
property of Azadirachta indica polysaccharides for poliovirus. J
Ethnopharmacol. 142:86–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Akihisa T, Takahashi A, Kikuchi T, Takagi
M, Watanabe K, Fukatsu M, Fujita Y, Banno N, Tokuda H and Yasukawa
K: The melanogenesis-inhibitory, anti-inflammatory, and
chemopreventive effects of limonoids in n-hexane extract of
Azadirachta indica A. Juss. (neem) seeds. J Oleo Sci. 60:53–59.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Agyare C, Spiegler V, Sarkodie H, Asase A,
Liebau E and Hensel A: An ethnopharmacological survey and in vitro
confirmation of the ethnopharmacological use of medicinal plants as
anthelmintic remedies in the Ashanti region, in the central part of
Ghana. J Ethnopharmacol. 158:255–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Quelemes PV, Perfeito ML, Guimarães MA,
dos Santos RC, Lima DF, Nascimento C, Silva MP, Soares MJ, Ropke
CD, Eaton P, et al: Effect of neem (Azadirachta indica A. Juss)
leaf extract on resistant Staphylococcus aureus biofilm formation
and Schistosoma mansoni worms. J Ethnopharmacol. 175:287–294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sharma J, Gairola S, Sharma YP and Gaur
RD: Ethnomedicinal plants used to treat skin diseases by Tharu
community of district Udham Singh Nagar, Uttarakhand, India. J
Ethnopharmacol. 158:140–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Othman F, Motalleb G, Lam Tsuey Peng S,
Rahmat A, Basri R and Pei Pei C: Effect of neem leaf extract
(Azadirachta indica) on c-Myc oncogene expression in 4T1 breast
cancer cells of BALB/c mice. Cell J. 14:53–60. 2012.
|
|
82
|
Manikandan P, Anandan R and Nagini S:
Evaluation of Azadirachta indica leaf fractions for in vitro
antioxidant potential and protective effects against
H2O2-induced oxidative damage to pBR322 DNA
and red blood cells. J Agric Food Chem. 57:6990–6996. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sithisarn P, Supabphol R and Gritsanapan
W: Comparison of free radical scavenging activity of Siamese neem
tree (Azadirachta indica A. Juss var. siamensis Valeton) leaf
extracts prepared by different methods of extraction. Med Princ
Pract. 15:219–222. 2006. View Article : Google Scholar : PubMed/NCBI
|