Introduction
Daunorubicin (DNR), a anthracycline chemotherapeutic
drug, is widely used in the treatment of various types of cancer,
including acute leukemia, breast cancer, soft tissue sarcoma and
aggressive lymphoma (1). However,
the clinical application of DNR is always restricted due to its
cumulative and dose- dependent cardiotoxicity (2). Increasing evidence indicates that
cardio myocyte apoptosis may contribute to the progression of
anthracycline-based cardiotoxicity (3). The mechanisms involved in
DNR-induced cardiotoxicity include reactive oxygen species (ROS)
production, caspase activation and cell cycle arrest, which
ultimately result in cardiomyocyte apoptosis (4).
Cell apoptosis is an essential process which
maintains the dynamic equilibrium under normal conditions (5). Calcium (Ca2+) is a
pivotal regulator of cell survival, and the sustained elevation of
intracellular Ca2+ concentrations can activate various
Ca2+ signals connected to apoptosis (6). A recent study reported that BN52021,
a platelet activating factor antagonist, protected H9c2 cells
against DNR-induced death by decreasing Ca2+ influx and
attenuating the activation of p38 mitogen-activated protein kinase
(MAPK) signaling (7). In
addition, as previously demonstrated, the overexpression of
calreticulin suppresses phosphatidylinositol 3-kinase (PI3K)/Akt
signaling and causes the increased apoptosis of H9c2 cells via
altered Ca2+ homeostasis (8). Thus, it is suggested that the
disruption of Ca2+ homeostasis can lead to caspase
activation and subsequent cellular apoptosis (9).
The traditional Chinese medicine, puerarin, known as
Ge-gen in Chinese, is isolated from Radix puerariae as an
active component and possesses a series of pharmacological
activities, including antioxidant, anti-osteoporotic,
anti-inflammatory, anti-apoptotic, neuroprotective and
cardioprotective properties (10). Puerarin can protect against
apoptosis in a variety of cell types, including osteoblasts,
microglia, neurons and cardiomyocytes (11–14). However, its role in the
DNR-induced apoptosis of H9c2 cells and the underlying mechanisms
remain unclear.
In this study, we demonstrate that puerarin
attenuates H9c2 cell apoptosis induced by DNR by promoting the
activation of the PI3K/Akt signaling pathway via the inhibition of
Ca2+ influx. These findings provide new insight into the
the detailed mechanisms underlying the cardioprotective effects of
puerarin. Puerarin may thus have potential for use as an
alternative drug in the treatment of cardiomyopathy indcued by
DNR.
Materials and methods
Materials
Rat H9c2 cardiomyocytes were purchased from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). DNR was
obtained from Pfizer, Inc. (New York, NY, USA). Specific
inhibitors, including PD98059 (MAPK inhibitor), LY294002 (PI3K
inhibitor), SB203580 (p38 MAPK inhibitor) and SP600125 (JNK
inhibitor) were purchased from Sigma-Aldrich, (St. Louis, MO,
USA).
H9c2 cell culture
H9c2 cells were cultured in Dulbecco's modified
eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) and antibiotics (100 U/ml streptomycin and 100 U/ml
penicillin) (all from Gibco Life Technologies, Carlsbad, CA, USA)
at 37°C in a humified incubator with 5% CO2.
Cell treatments
The H9c2 cells were treated with various
concentrations of puerarin (1–100 µg/ml; Sigma-Aldrich) for
24 h. The cells were then cultured for an additional 24 h in the
presence of 1 µM DNR to induce cell apoptosis. The cells
were then collected for use in the follow-up experiments.
Treatment of cells with signaling pathway
inhibitors
The H9c2 cells were treated with various signaling
pathway inhibitors, including ERK1/2 inhibitor (PD98059, 10
µM), PI3K inhibitor (LY294002, 10 µM), p38 MAPK
inhibitor (SB203580, 10 µM) and JNK inhibitor (SP600125, 10
µM), for 30 min prior to the addition of puerarin. Following
24 h of incubation, the cells were treated with 1 µM DNR for
24 h. The suppressive effects of puerarin on DNR-induced apoptosis
were then evaluated.
MTT assay
The H9c2 cell suspensions (5×104
cells/ml) were seeded in 96-well plates at approximately 200
µl per well, and 1×104 cells per well for MTT
assay. At the end of the drug incubation,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(Sigma-Aldrich) working solution (0.5 mg/ml) was added, and the
plates were incubated for an additional 4 h at 37°C. Following
centrifugation, the medium was replaced with dimethyl sulfoxide
(DMSO) (Sigma-Aldrich). The absorbance of each well at 570 nm was
measured using a plate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Cell viability was calculated as follows:
inhibitory rate of H9c2 cell viability (%) = (OD value of control
group - OD value of test group) ×100%/OD value of control
group.
Flow cytometric detection of
apoptosis
The apoptotic cells were detected using a FITC
Apoptosis Detection kit (BD Pharmingen, San Diego, CA, USA). The
H9c2 cells were collected, washed and resuspended in buffer
containing 5 µl of Annexin V-FITC and 5 µl of
propidium iodide (PI). Following incubation for 15 min in the dark
at room temperature, the fluorescence of the cells was analyzed
using BD FACSCanto II with Diva software (BD Biosciences, San
Diego, CA, USA). The apoptotic cells contained the early apoptotic
cells (Annexin V+ and PI−) and the late
apoptotic or necrotic cells (both Annexin V+ and
PI+).
Enzymatic assay for caspase-3
Caspase-3 activity was detected using a Caspase-3
Activity Assay kit (BestBio, Shanghai, China). The cells were
harvested and extracted on ice in lysis buffer for 15 min. The
lysates were centrifuged and the supernatants were assessed for
protein content. Subsequently, supernatant samples containing 50
µg proteins were incubated with 90 µl reaction buffer
and 10 µl caspase-3 substrate
(N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide) at 37°C for 2 h in the
dark. The optical density (OD) values were measured at 405 nm using
a microtiter plate reader (Thermo Fisher Scientific, Inc., Waltham,
MA, USA).
Western blot analysis
The H9c2 cells were collected, washed with ice-cold
sterile phosphate-buffered saline (PBS), and lysed with RIPA buffer
(50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 0.2%
deoxycholic acid and 1 mM PMSF, 1:100 protease inhibitor cocktail).
The lysates were centrifuged and supernatants were analyzed for
protein concentration using a BCA Protein Assay kit (Thermo Fisher
Scientific, Inc). An equal amount of the proteins (30 µg)
was separated by 10% SDS-PAGE and transferred onto a PVDF membrane.
The blotted membrane was then blocked with 5% non-fat dry milk for
1 h at room temperature and incubated overnight at 4°C with primary
antibodies [1:1,000, rabbit antibodies to cleaved caspase-3 (9661),
caspase-3 (9665), phosphorylated Akt (p-Akt; 4058), Akt (4685) and
mouse antibodies to β-actin (3700)] (Cell Signaling Technology,
Inc., Danvers, MA, USA). The immunoreactive bands were further
incubated with peroxidase-conjugated anti-mouse (7076)/rabbit
(7074) secondary antibodies for 1 h at room temperature, were then
detected by the enhanced chemiluminescence (ECL) method and
captured using a scanner (HP Scanjet 7400C; Hewlett-Packard, Palo
Alto, CA, USA). The protein intensities were quantified using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Cytosolic Ca2+
measurement
Ratiometric imaging of intracellular Ca2+
using cells loaded with Fura-2 was carried out as previously
described (15). The H9c2 cells
were grown in round coverslips (30 mm) under normal culture
conditions, unless otherwise indicated. Coverslips with cells were
placed in a cation-safe solution composed of 107 mM NaCl, 7.2 mM
KCl, 1.2 mM MgCl2, 11.5 g lucose, 20 mM HEPES/NaOH (pH
7.3) and loaded with Fura-2/AM (2 µM final concentration)
for 30 min at 37°C. The cells were washed, and de-esterification
was allowed for a minimum of 15 min. Ca2+ measurements
were made using a Leica DMI 6000B fluorescence microscope
controlled by the slidebook software (Intelligent Imaging
Innovations, Inc., Denver, CO, USA). Fluorescence emission at 505
nm was monitored, while alternating excitation wavelengths between
340 and 380 nm at a frequency of 0.5 Hz; intracellular
Ca2+ measurements are shown as 340/380 nm ratio obtained
from groups (15) of single
cells. External solutions contained: 135 mM NaCl, 5.4 mM KCl, 10 mM
HEPES, 0.02 mM NaH2PO4, 2 mM Mg2+,
10 g glucose (pH 7.4).
Statistical analysis
The results were analyzed with statistical analysis
software SPSS 16.0 (SPSS, Inc., Chicago, IL, USA), and the values
are represented as the means ± SD. Differences between 2 groups
were evaluated using the Student's t-test. One-way analysis of
variance (ANOVA) was used for multi-group comparisons with Tukey's
post hoc test. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
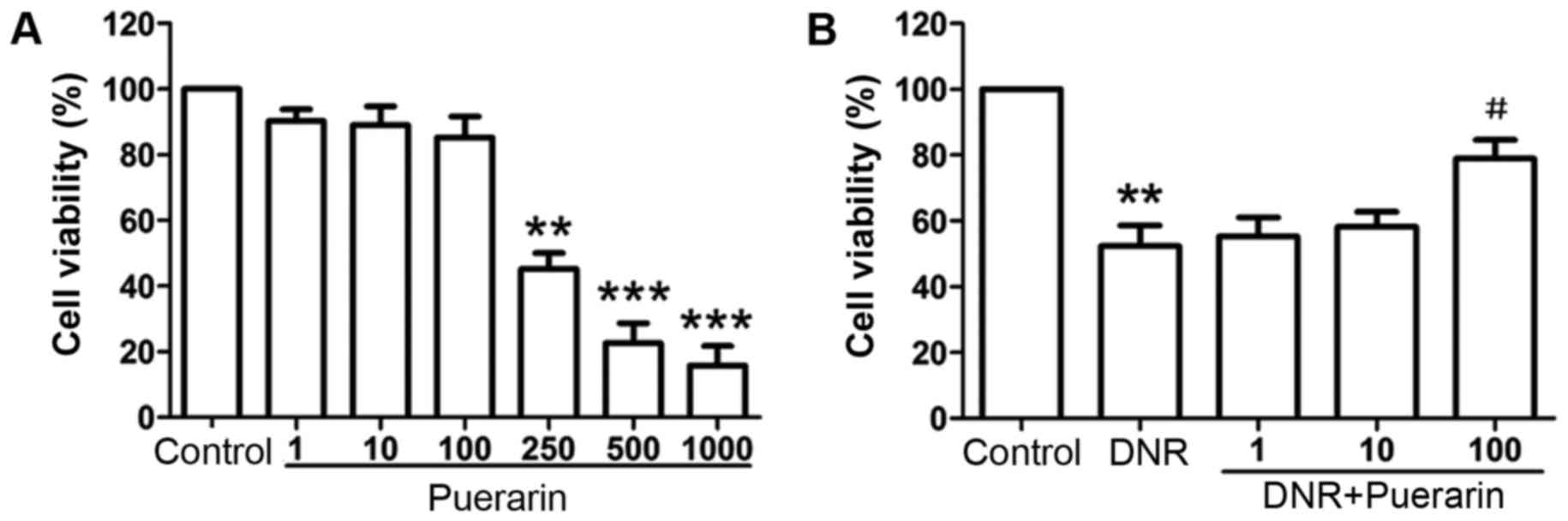
Puerarin suppresses DNR-induced
cytotoxicity in H9c2 cells
When the H9c2 cells were treated with low
concentrations of puerarin (1, 10 and 100 µg/ml), no
significant changes in cell viability were observed apart from a
slight decrease compared with the controls. However, the viability
of the H9c2 cells was inhibited in a dose-dependent manner
following treatment with higher concentrations of puerarin
(250–1,000 µg/ml) (Fig.
1A), suggesting strong cytotoxicity to H9c2 cells treated with
high concentrations of puerarin.
According to the inhibitory effects of puerarin at
various concentrations on H9c2 cell viability
(IC50=168.5 µg/ml) (Table I), a concentration range of
puerarin (1, 10 and 100 µg/ml) was selected for use in this
study. As shown in Fig. 1B,
treatment with DNR significantly suppressed the viability of the
H9c2 cells compared with the controls (P<0.01). Importantly,
puerarin at 100 µg/ml markedly suppressed DNR-induced
cytotoxicity (DNR vs. puerarin, 52.38±6.22% vs. 78.98±5.65%;
P<0.05).
 | Table IEffects of puerarin on the viability
of H9c2 cells. |
Table I
Effects of puerarin on the viability
of H9c2 cells.
| Puerarin
(µg/ml) | OD 570 (mean ±
SD) | Inhibition rate
(%) |
|---|
| 0 | 0.992±0.006 | 0 |
| 1 | 0.895±0.014 | 9.716±0.563 |
| 10 | 0.883±0.018 | 10.038±0.862 |
| 100 | 0.845±0.021 | 13.718±1.056 |
| 250 | 0.447±0.016 | 53.582±1.256 |
| 500 | 0.224±0.013 | 76.202±1.124 |
| 1,000 | 0.156±0.011 | 84.535±0.682 |
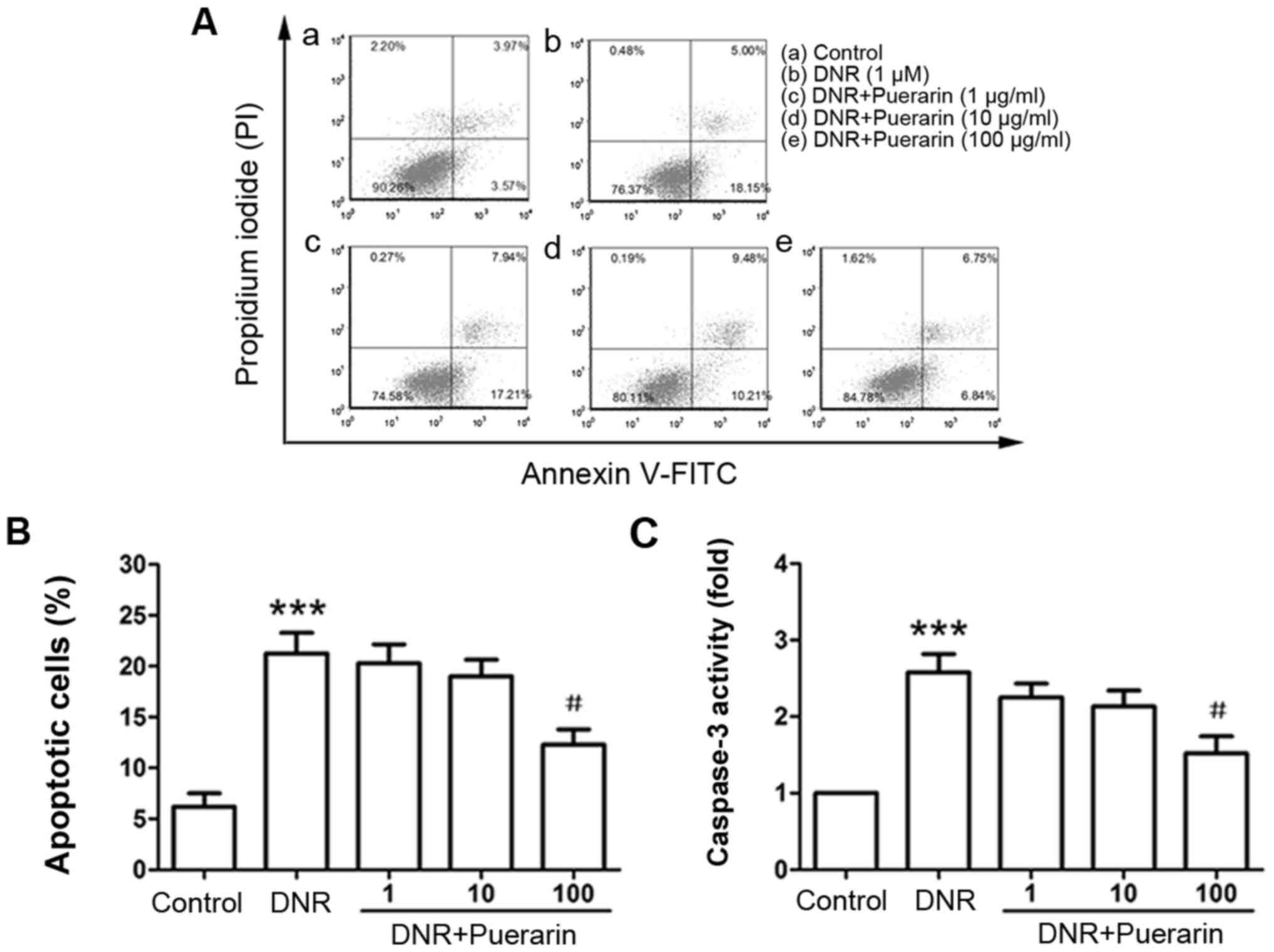
Puerarin inhibits the DNR-induced
apoptosis of H9c2 cells
By flow cytometric analysis, we observed that DNR
markedly induced the apoptosis of H9c2 cells (control vs. DNR,
6.21±1.30% vs. 21.25±2.05%, P<0.001). Pre-treatment with
puerarin at 100 µg/ml significantly inhibited DNR-induced
apoptosis (DNR vs. puerarin, 21.25±2.05% vs. 12.28±1.52%;
P<0.05) (Fig. 2A and B). In
line with the Annexin V/PI staining results, the enzymatic activity
of caspase-3 was also markedly decreased following treatment with
puerarin prior to exposure to DNR (P<0.05) (Fig. 2C).
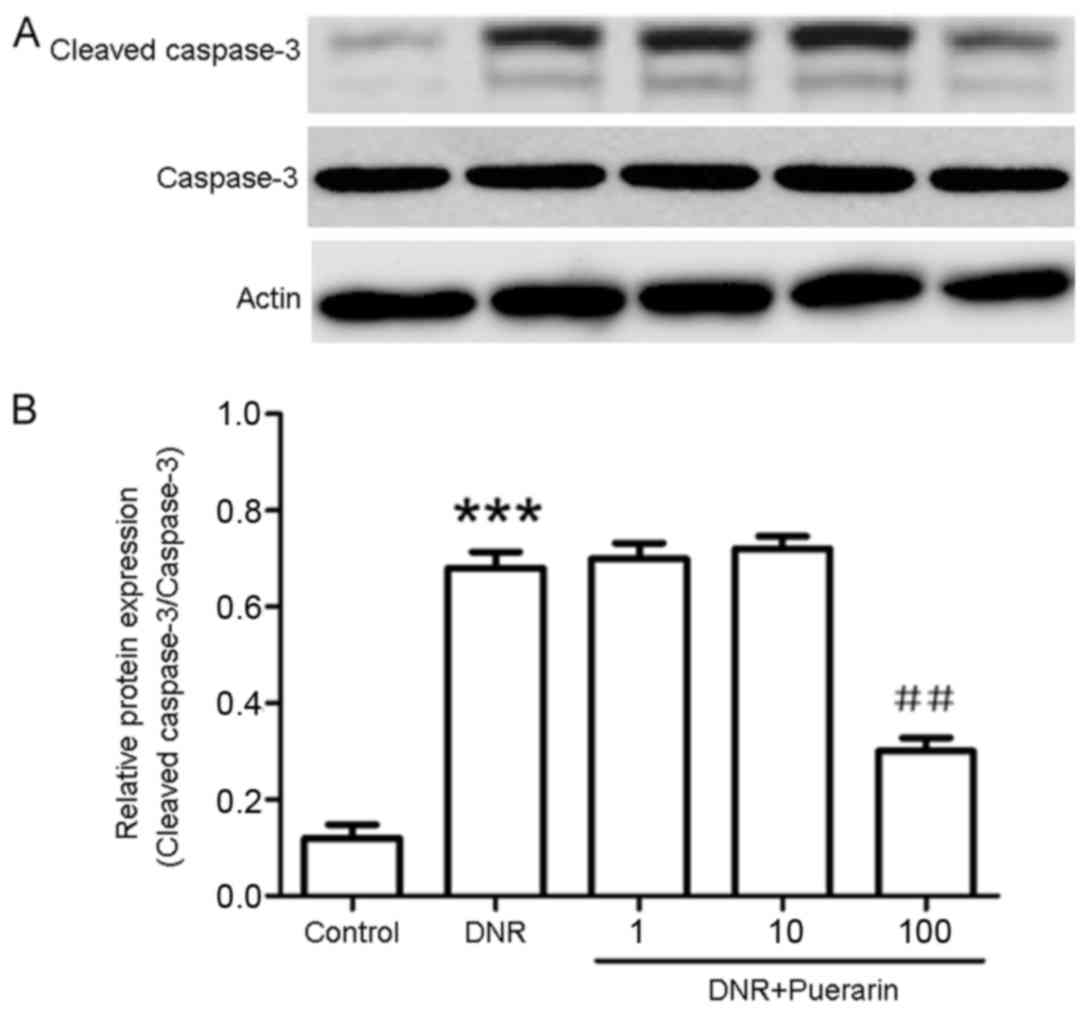
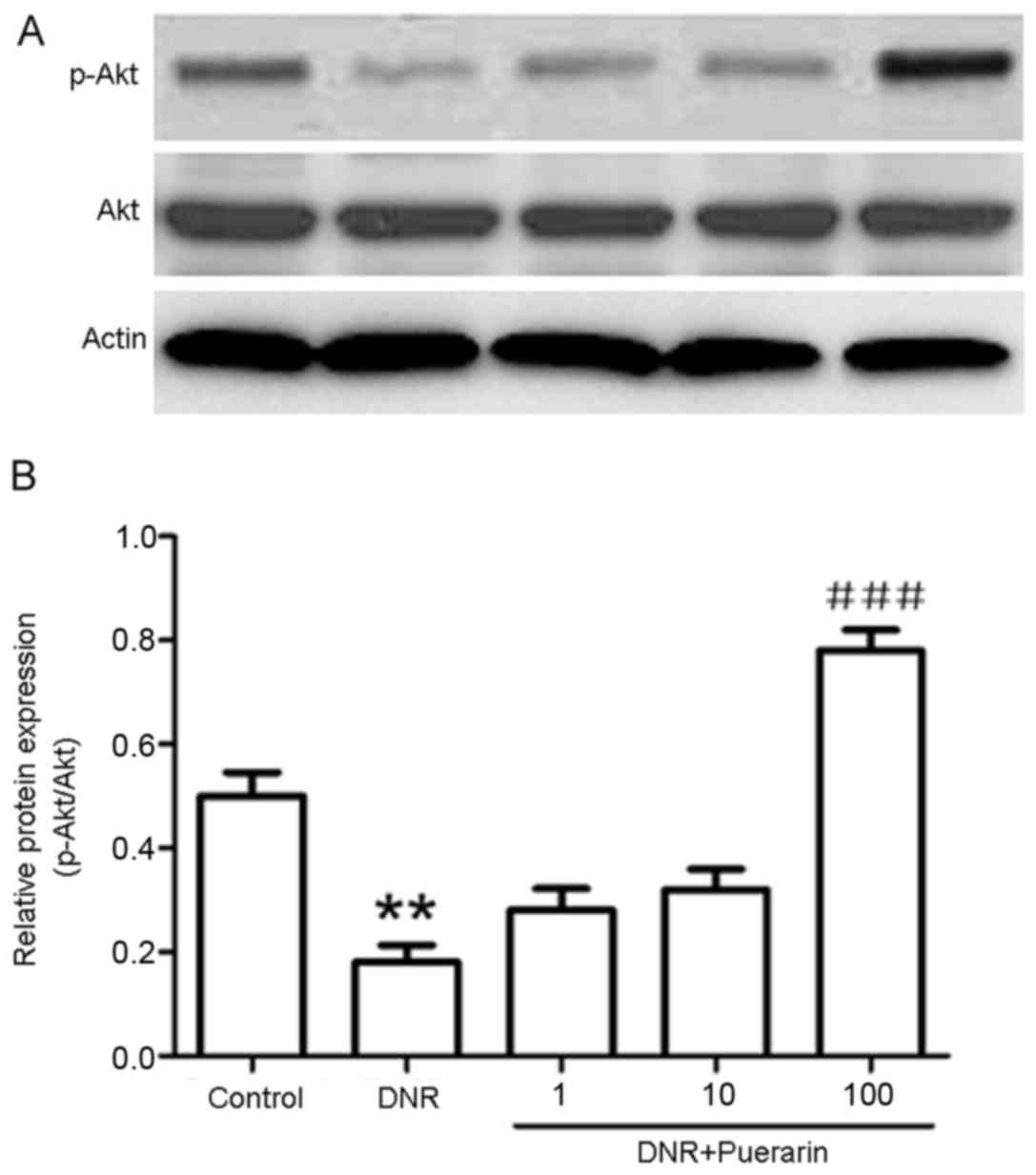
Puerarin promotes p-Akt (Ser473)
activation which is suppressed by DNR
As shown in Fig.
3, puerarin markedly decreased the DNR-induced protein
expression of cleaved caspase-3 (P<0.01). In addition, treatment
with DNR markedly suppressed the phosphorylation of Akt (Ser473)
compared with the control (P<0.01). Conversely, treatment with
puerarin prior to exposure to DNR evidently promoted p-Akt (Ser473)
activation (P<0.001) (Fig.
4).
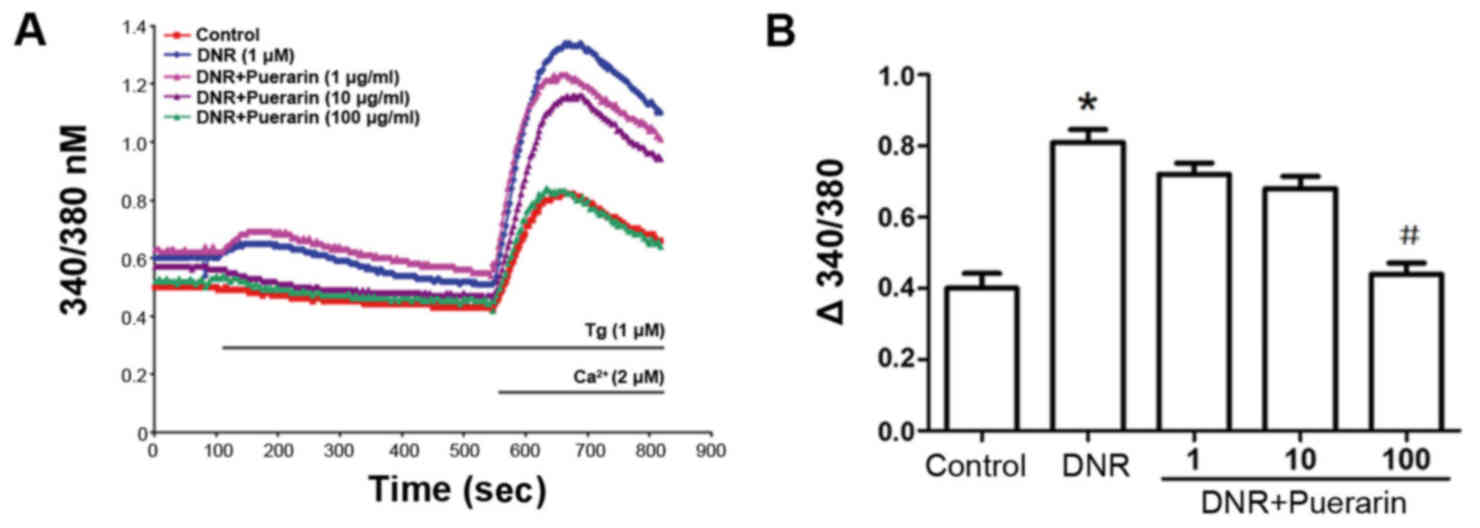
Puerarin restricts Ca2+ influx
in H9c2 cells treated with DNR
As shown in Fig.
5, DNR induced an increment in Ca2+ influx in a
time-dependent manner. Of note, puerarin clearly restricted the
DNR-induced Ca2+ influx (P<0.05), suggesting that the
inhibition of Ca2+ influx was involved in the
cardioprotective effects of puerarin.
Puerarin attenuates DNR-induced apoptosis
via the PI3K/Akt signaling pathway
We treated the H9c2 cells with various inhibitors,
including PD98059, LY294002, SB203580 and SP600125 prior to
treatment with puerarin. As shown in Fig. 6D, pre-treatment with the PI3K
inhibitor, LY294002, markedly reversed the inhibition of
Ca2+ influx by puerarin (P<0.05); however, the ERK
inhibitor (PD98059), p38 MAPK inhibitor (SB203580) and JNK
inhibitor (SP600125) (Fig. 6) did
not exert any significant effects.
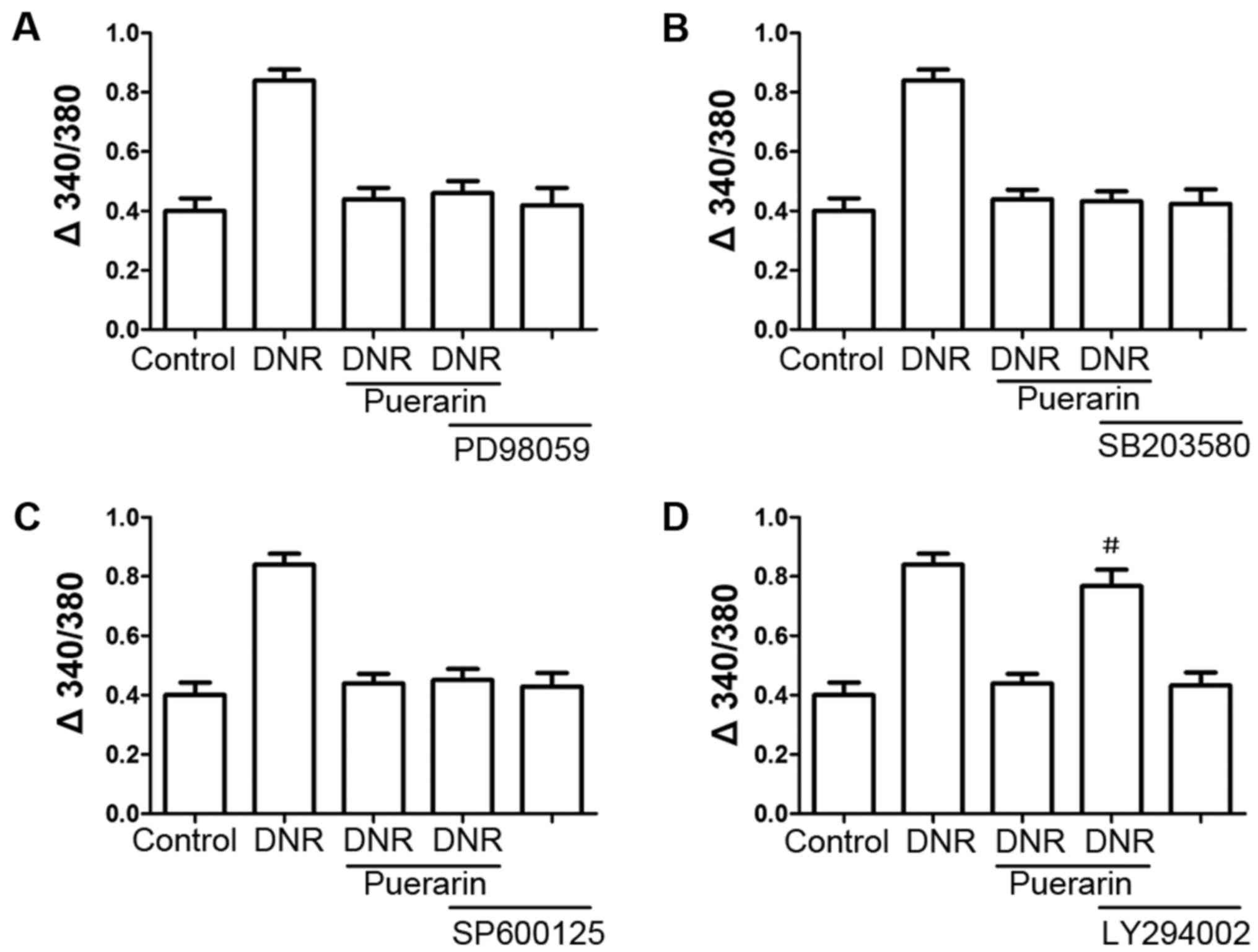 | Figure 6(A–D) The phosphatidylinositol
3-kinase (PI3K) inhibitor, LY294002, attenuates the
cardioprotective effects of puerarin. H9c2 cells were treated with
inhibitors, including PD98059, LY294002, SB203580 and SP600125 for
30 min prior to the addition of 100 µg/ml puerarin.
Following 24 h of incubation, the cells were treated with 1
µM daunorubicin (DNR) for 24 h. The suppressive effects of
puerarin on DNR-induced apoptosis were antagonized by the PI3K
inhibitor (LY294002, 10 µM), but not by the ERK1/2 inhibitor
(PD98059, 10 µM), p38 MAPK inhibitor (SB203580, 10
µM), or JNK inhibitor (SP600125, 10 μM).
#P<0.05 vs. DNR with puerarin, n=3. |
Discussion
At present, the avoidance of the cardiotoxic
side-effects of DNR without compromising its antitumor efficacy in
clinical treatment is a major challenge. In this study, we found
that puerarin exerted protective effects against DNR-induced
cytotoxicity in H9c2 cells by suppressing apoptosis, promoting the
activation of the PI3K/Akt signaling pathway and the inhibition of
caspase-3 expression. Simultaneously, the decreased Ca2+
influx was involved in the cardioprotective effects of puerarin;
these effects were abolished by the PI3K inhibitor, LY294002. To
our knowledge, this study is the first to report the direct effects
of puerarin on DNR-mediated cardiotoxicity.
Accumulating evidence indicates that puerarin plays
differential roles in cell apoptosis related to distinct cell
types. As shown in a previous study, in human mantle Z138 cells,
puerarin induced dose-dependent cytotoxicity, and exerted
anti-proliferative and pro-apoptotic effects by inhibiting the
PI3K/Akt/nuclear factor-κB (NF-κB) pathway (16). A recent study indicated that
puerarin inhibited the proliferation and induced the apoptosis of
U251 and U87 human glioblastoma cells. Treatment with puerarin
suppressed the expression of p-Akt and Bcl-2 and promoted the
expression of Bax and cleaved caspase-3 in cells (17). In line with the above-mentioned
studies, treatment with puerarin has also been shown to result in
the dose-dependent inhibition of the growth of various cancer cell
lines, including HT-29 colon cancer, SMMC-7721 hepatocellular
carcinoma, and HS578T, MDA-MB-231 and MCF-7 cells (18–20). Based on these findings, we
speculated that puerarin exerted its antitumor effects by
inhibiting proliferation and promoting apoptosis, although its
pro-apoptotic characteristics are mainly observed in cancer cells.
In the present study, low concentrations of puerarin (1, 10 and 100
µg/ml) exerted little cytotoxicity; however, higher
concentrations of puerarin (250–1,000 µg/ml) decreased the
viability of the H9c2 cells (Fig.
1A). In contrast to previous findings (18–20), puerarin at 100 µg/ml
significantly attenuated DNR-induced cell apoptosis and cleaved
caspase-3 expression (Figs. 2 and
3). Thus, various concentrations
of puerarin thus contribute to distinct cell responses as regards
apoptosis. Therefore, further investigations are required for more
in depth clarifications.
It is possible that different molecular mechanisms
are involved in the anti-apoptotic effects of puerarin. A previous
study reported that puerarin attenuated amyloid-β (Aβ)-induced
microglial apoptosis by activating the PI3K-dependent pathway in
association with Akt phosphorylation, which inhibited the
activation of caspase-3 apoptotic cascade (21). The PI3K/Akt signaling pathway was
proposed as an essential pathway in the prevention of cell
apoptosis and plays a critical role in cell survival. Puerarin may
have an ability to attenuate the progression of cardiac hypertrophy
induced by pressure overload by targeting PI3K/Akt signaling
(14). Puerarin pre-treatment may
attenuate myocardial hypoxia/reoxygenation injury by inhibiting
autophagy via the Akt signaling pathway (22). A recent study also indicated that
puerarin prevented the TNF-α-induced apoptosis of PC12 cells by
activating the PI3K/Akt signaling pathway (23). These studies suggested that the
PI3K/Akt pathway may be involved in the biological effects of
puerarin. Our results also revealed that puerarin on inhibited the
DNR-induced apoptosis of H9c2 cells and promoted p-Akt activation
(Fig. 4), which was associated
with its cardioprotective effects.
It has been demonstrated that puerarin exerts
protective effects on the cardiovascular system and nervous system,
and against osteoporosis, liver injury, diabetes and inflammation
in vitro and in vivo and (24 and refs therein). The
cardioprotective effects of puerarin are partly attributed to its
effects on Ca2+ in cardiomyocytes (25). Puerarin has been shown to block
L-type Ca2+ channels in ventricular myocytes from
Langendorff rat heart preparations, and to inhibit the amplitude of
peak Ca2+ and decrease the level of Ca2+ flux
(26). Accompanying mechanical
strain, intracellular Ca2+ plays a role in regulating
signaling through the PI3K/Akt pathway in osteoblasts (27). Cytoplasmic Ca2+
overload results in cytotoxicity, and an increased cytosolic
Ca2+ concentration induces cellular apoptosis (28). In this study, we illustrated that
the inhibition of Ca2+ influx participated in the
suppressive effects of puerarin on the DNR-induced apoptosis of
H9c2 cells (Fig. 5), which was
abolished by the PI3K inhibitor, LY294002, suggesting that the
inhibition of the Ca2+ influx was related to the
activation of PI3K/Akt signaling (Fig. 6). Further studies are required in
order to elucidate the precise mechanisms underlying the
cardioprotective effects of puerarin.
In conclusion, the present study demonstrated that
puerarin attenuated the DNR-induced apoptosis of H9c2 cells by
activating the PI3K/Akt signaling pathway via the inhibition of
Ca2+ influx, which contributed to decreased caspase-3
activation. These findings partly clarify the detailed mechanisms
underlying the cardioprotective effects of puerarin, suggesting
that puerarin may have potential for use as a cardioprotective drug
in the treatment of cardiotoxicity triggered by DNR.
Acknowledgments
This study was supported by a grant from the Natural
Science Foundation of Hubei Province of China (no. 2015CFA096).
References
|
1
|
Rosa GM, Gigli L, Tagliasacchi MI, Di
Iorio C, Carbone F, Nencioni A, Montecucco F and Brunelli C: Update
on cardiotoxicity of anti-cancer treatments. Eur J Clin Invest.
46:264–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feijen EA, Leisenring WM, Stratton KL,
Ness KK, van der Pal HJ, Caron HN, Armstrong GT, Green DM, Hudson
MM, Oeffinger KC, et al: Equivalence ratio for daunorubicin to
doxorubicin in relation to late heart failure in survivors of
childhood cancer. J Clin Oncol. 33:3774–3780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi J, Abdelwahid E and Wei L: Apoptosis
in anthracycline cardiomyopathy. Curr Pediatr Rev. 7:329–336. 2011.
View Article : Google Scholar :
|
|
4
|
Zhang YW, Shi J, Li YJ and Wei L:
Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch
Immunol Ther Exp (Warsz). 57:435–445. 2009. View Article : Google Scholar
|
|
5
|
Meier P, Finch A and Evan G: Apoptosis in
development. Nature. 407:796–801. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orrenius S, Zhivotovsky B and Nicotera P:
Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol
Cell Biol. 4:552–565. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan W, Xuan C, Xuan L, Xu R and Wang J:
BN52021 protects rat cardiomyocyte from doxorubicin induced
cardiotoxicity. Int J Clin Exp Pathol. 8:1719–1724. 2015.PubMed/NCBI
|
|
8
|
Kageyama K, Ihara Y, Goto S, Urata Y, Toda
G, Yano K and Kondo T: Overexpression of calreticulin modulates
protein kinase B/Akt signaling to promote apoptosis during cardiac
differentiation of cardiomyoblast H9c2 cells. J Biol Chem.
277:19255–19264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu MJ, Wang Z, Ju Y, Wong RN and Wu QY:
Diosgenin induces cell cycle arrest and apoptosis in human leukemia
K562 cells with the disruption of Ca2+ homeostasis.
Cancer Chemother Pharmacol. 55:79–90. 2005. View Article : Google Scholar
|
|
10
|
Zhou YX, Zhang H and Peng C: Puerarin: a
review of pharmacological effects. Phytother Res. 28:961–975. 2014.
View Article : Google Scholar
|
|
11
|
Liu LJ, Liu LQ, Bo T, Li SJ, Zhu Z, Cui RR
and Mao DA: Puerarin suppress apoptosis of human osteoblasts via
ERK signaling pathway. Int J endocrinol. 2013:7865742013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang C, Xie N, Zhang H, Li Y and Wang Y:
Puerarin protects against β-amyloid-induced microglia apoptosis via
a PI3K-dependent signaling pathway. Neurochem Res. 39:2189–2196.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie N, Wang C, Lian Y, Wu C, Zhang H and
Zhang Q: Puerarin protects hippocampal neurons against cell death
in pilocarpine-induced seizures through antioxidant and
anti-apoptotic mechanisms. Cell Mol Neurobiol. 34:1175–1182. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan Y, Zong J, Zhou H, Bian ZY, Deng W,
Dai J, Gan HW, Yang Z, Li H and Tang QZ: Puerarin attenuates
pressure overload-induced cardiac hypertrophy. J Cardiol. 63:73–81.
2014. View Article : Google Scholar
|
|
15
|
Yin N, Hong X, Han Y, Duan Y, Zhang Y and
Chen Z: Cortex Mori Radicis Extract induces neurite outgrowth in
PC12 cells activating ERK signaling pathway via inhibiting Ca(2+)
influx. Int J Clin Exp Med. 8:5022–5032. 2015.PubMed/NCBI
|
|
16
|
Gan M and Yin X: Puerarin induced in
mantle cell lymphoma apoptosis and its possible mechanisms
involving multi-signaling pathway. Cell Biochem Biophys.
71:367–373. 2015. View Article : Google Scholar
|
|
17
|
Yang JA, Li JQ, Shao LM, Yang Q, Liu BH,
Wu TF, Wu P, Yi W and Chen QX: Puerarin inhibits proliferation and
induces apoptosis in human glioblastoma cell lines. Int J Clin Exp
Med. 8:10132–10142. 2015.PubMed/NCBI
|
|
18
|
Yu Z and Li W: Induction of apoptosis by
puerarin in colon cancer HT-29 cells. Cancer Lett. 238:53–60. 2006.
View Article : Google Scholar
|
|
19
|
Zhang WG, Liu XF, Meng KW and Hu SY:
Puerarin inhibits growth and induces apoptosis in SMMC-7721
hepatocellular carcinoma cells. Mol Med Rep. 10:2752–2758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin YJ, Hou YC, Lin CH, Hsu YA, Sheu JJ,
Lai CH, Chen BH, Lee Chao PD, Wan L and Tsai FJ: Puerariae radix
isoflavones and their metabolites inhibit growth and induce
apoptosis in breast cancer cells. Biochem Biophys Res Commun.
378:683–688. 2009. View Article : Google Scholar
|
|
21
|
Xing G, Dong M, Li X, Zou Y, Fan L, Wang
X, Cai D, Li C, Zhou L, Liu J, et al: Neuroprotective effects of
puerarin against beta-amyloid-induced neurotoxicity in PC12 cells
via a PI3K-dependent signaling pathway. Brain Res Bull. 85:212–218.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang H, Song X, Ling Y, Wang X, Yang P,
Luo T and Chen A: Puerarin attenuates myocardial
hypoxia/reoxygenation injury by inhibiting autophagy via the Akt
signaling pathway. Mol Med Rep. 15:3747–3754. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang F and Xie S: Puerarin prevents tumor
necrosis factor-α- induced apoptosis of PC12 cells via activation
of the PI3K/Akt signaling pathway. Exp Ther Med. 14:813–818. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei SY, Chen Y and Xu XY: Progress on the
pharmacological research of puerarin: a review. Chin J Nat Med.
12:407–414. 2014.PubMed/NCBI
|
|
25
|
Gao Q, Pan HY, Qiu S, Lu Y, Bruce IC, Luo
JH and Xia Q: Atractyloside and 5-hydroxydecanoate block the
protective effect of puerarin in isolated rat heart. Life Sci.
79:217–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao Q, Yang B, Ye ZG, Wang J, Bruce IC and
Xia Q: Opening the calcium-activated potassium channel participates
in the cardio-protective effect of puerarin. Eur J Pharmacol.
574:179–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Danciu TE, Adam RM, Naruse K, Freeman MR
and Hauschka PV: Calcium regulates the PI3K-Akt pathway in
stretched osteoblasts. FEBS Lett. 536:193–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mekahli D, Parys JB, Bultynck G, Missiaen
L and De Smedt H: Polycystins and cellular Ca2+
signaling. Cell Mol Life Sci. 70:2697–2712. 2013. View Article : Google Scholar
|