Introduction
Eco1/Eso1 protein plays an important role in
chromosome segregation, DNA repair and gene regulation (1–3).
Eco1/Eso1 protein mediates the formation of the cohesion complex
during DNA replication, chromosome separation and cell mitosis from
the metaphase to the anaphase transition of the cell cycle. The
cohesion complex is critical for the faithful chromosome
segregation between two sister chromatids at the anaphase (4), consists of 4 highly conserved
proteins (Smc3, Smc1, Scc3 and Scc1) (5,6),
and functions to entrap the chromatids by forming a topological
ring (7) during the cell cycle.
Eco1/Eso1 protein is an acetyltransferase and is able to acetylate
the two conserved lysine sites in the Smc3 protein, enabling Smc3
to form the cohesion complex (8–11).
A previous study revealed that mutations of ESCO2, the human
ortholog of Eco1, were associated with a human developmental
disorder known as Roberts syndrome (12). The role of Eco1/Eso1 in the
contribution to chromosome structure and organization was then
proposed (13). For example, our
previous genetics screening data also demonstrated an intimate
association between cohesion and chromatin separation (14). Recently, rRNA transcription and
protein translation defects occurred in budding yeast that carry
Roberts syndrome-related Eco1 mutations and in cells from
patients with Roberts syndrome (15,16). These data indicate that Eco1/Eso1
protein may play an important role in Schizosaccharomyces pombe
(S. pombe) viability. However, the molecular mechanisms through
which Eco1 protein functions in S. pombe, remain to be
defined. For example, determining how and which protein Eco1/Eso1
protein binds to or interacts with, and any additional functions of
Eco1/Eso1 protein may shed light into this matter.
In the present study, we first purified Eso1 protein
using a tandem affinity purification (TAP) tag antibody and
immunoprecipitated whole cell extracts from the wild-type S.
pombe strain to identify Eso1 binding partners. We then focused
on one of its interacting proteins, polymerase 5 (Pol5), as the
Pol5 protein provides a conserved function in rDNA transcription in
yeast (17,18). In addition, Myb-binding protein 1a
(Mybbp1a), a human homologue of Pol5, has been shown to function in
rDNA transcription and processing, and to be associated with early
embryonic development and carcinogenesis (19,20). Subsequently, we assessed how Eso1
interacts with Pol5 and and examined alterations in Pol5 protein
acetylation, and the effects of these on S. pombe viability.
This study aimed to provide insightful information regarding the
interaction of Eso1 with Pol5 and the acetylation of Pol5 protein,
and the effects of these on S. pombe viability. Future
studies are also required in order to investigate whether and how
Eso1 acetylates the Pol5 K47 site for S. pombe survival.
Materials and methods
Strains and culture media
The S. pombe strains used in this study are
listed in Table I and were
cultured in standard conditions, as previously described (21). For S. pombe transformation,
the lithium acetate protocol was utilized, as described in a
previous study (22). pDblet
(acquired from Baumann's Lab, Stowers Institute, Kansas City, MO,
USA) was used as a plasmid vector for the expression of Pol5 with
or without K47 mutations, and 0.1%(w/v) 5-fluoroorotic acid (5FOA)
was used to select against ura + S. pombe cells, which is
supposed to contain transformed pDblet plasmids.
 | Table IYeast strains used in this study. |
Table I
Yeast strains used in this study.
| Name | Genotype | Source |
|---|
| ZCA002 | H− leu1-32 ura4-D18
his3-D1 ade6-M216 | Our stock |
| ZCA005 | h− leu1-32 ura4-D18
his3-D1 ade6-M216 ESO1-TAP::KAN | Our stock |
| ZCA006 | h− leu1-32 ura4-D18
his3-D1 ade6-M216 ESO1-FLAG::KAN | Our stock |
| ZCB001 | h− leu1-32 ura4-D18
his3-D1 ade6-M216 POL5-HA::NAT | This study |
| ZCB002 | h− leu1-32 ura4-D18
his3-D1 ade6-M216 ESO1-FLAG::KAN POL5-HA::NAT | This study |
| ZCB056 | h− leu1-32 ura4-D18
his3-D1 ade6-M216 POL5-TAP::KAN | This study |
| ZCB057 | h+/h− leu1-32
ura4-D18 his3-D1 ade6-M216/M210 POL5/POL5K47N::NAT | This study |
| ZCB058 | h+/h− leu1-32
ura4-D18 his3-D1 ade6-M216/M210 POL5/POL5K47R::NAT | This study |
| ZCB060 | h? leu1-32 ura4-D18
his3-D1 ade6-M216/M210 POL5K47R::NAT p-pdblet-POL5::URA | This study |
Mutagenesis and protein tagging
DNA sequences corresponding to Pol5 cDNA were
amplified using standard PCR from the S. pombe genomic DNA.
The primers used are listed in Table
II. The resulting PCR product was then cloned into a pCloneNat1
vector [kind gift from Gregan's Lab, Max F. Perutz Laboratories,
Vienna Biocenter (VBC), Vienna, Austria]. To create the Pol5 K47N
and Pol5 K47R mutant strains, a site directed mutagenesis kit
(QuikChange II; Agilent Technologies, Santa Clara, CA, USA) was
employed at the corresponding residues using the primers listed in
Table II. The resulting
plasmids, pCloneNat1-pol5 K47N and pCloneNat1-pol5 K47R, were
linearized with the restriction enzyme, BstBI, by digesting
nucleotides at the start codon, and then transferred into the
diploid S. pombe strains. The tetrad analysis of the
expected mutation strains was then confirmed by DNA sequencing and
used in this study. These wild-type and mutated Pol5 with TAP, HA
or FLAG tag were then tagged according to a previously study
(23) and online protocol
(http://mendel.imp.ac.at/Pombe_tagging/).
 | Table IIPrimers used in this study. |
Table II
Primers used in this study.
| Primers | Nucleotide
sequences |
|---|
| Wild-type Pol5 |
5′-atatctcgagttgagaacgttcccatctac-3′ |
|
5′-atatggatccatccttgggcttggt-3′ |
| Pol5 K47N
mutated |
5′-cgttaaaccgtttgaccaatggtctttctagtggtcg-3′ |
|
5′-cgaccactagaaagaccattggtcaaacggtttaacg-3′ |
| Pol5 K47R
mutated |
5′-ttcgttaaaccgtttgacccgaggtctttctagtggtcgc-3′ |
|
5′-gcgaccactagaaagacctcgggtcaaacggtttaacgaa-3′ |
| Pol5 mutated |
5′-tacaacctcgattgtgag-3′ |
Cloning and expression of GST-Eso1
protein
The full-length Eso1 cDNA and its different
truncated forms, including Rad30 homologue fragment (1-519 or
M519*), Rad30 fragment plus the additional second
zinc-finger domain (1-568 or R568*) and Eco1 homologue
fragment (520–871) were amplified from the genomic DNA of S.
pombe using PCR primers (Table
II). The PCR product containing the BamHI and
XmaI restriction sites were cloned into the pGEX-4T-1 vector
(kind gift from Gerton's Lab, Stowers Institute) in a manner that
produces an N-terminally GST tagged Eso1 protein and its different
truncated forms, respectively. The resulting plasmids were
transferred into E. coli Bl21 (DE3) cells (# 200131; Agilent
Technologies), and were amplified and DNA sequence-confirmed. To
express the N-terminally GST tagged Eso1 protein and its different
truncated forms, plasmids were transferred into E. coli Bl21
(DE3) cells and 0.3 mM of IPTG was added to induce the expression
of Eso1 protein in DE3 cells at 25°C for 3 h. The glutathione resin
was then utilized to pull-down and purify these GST fused proteins,
as previously described (24).
TAP purification and liquid
chromatography-mass spectrometry (LC-MS/MS) analysis
Trichloroacetic acid (TCA)-precipitated proteins
from cultured S. pombe strains were resuspended in 30
µl of buffer containing 100 mM of Tris-HCl pH 8.5, 8 M of
urea and 5 mM of Tris(2-carboxylethyl)-phosphine hydrochloride
(Pierce, Rockford, IL, USA), and alkylated with 10 mM of
iodoacetamide (Sigma, St. Louis, MO, USA). Subsequently, a two-step
digestion procedure was applied. In brief, 0.5 µg of
endoproteinase Lys-C (Roche Applied Science, Indianapolis, IN, USA)
was added to each sample and incubated for at least 6 h at 37°C.
The samples were then diluted with 2 M of urea in 100 mM of
Tris-HCl pH 8.5 and 2 mM of calcium chloride. Subsequently, the
samples were digested with 0.5 µg of trypsin (Promega,
Madison, WI, USA) at 37°C overnight with shaking. The following,
the reaction was quenched by the addition of 5% formic acid, and
the peptide mixtures were then loaded onto a 100-µm fused
silica microcapillary column packed with 8 cm of reverse-phase
material (Aqua; Phenomenex, Torrance, CA, USA) for LC-MS/MS
analysis in a Deca-XP ion trap mass spectrometer equipped with a
nano-LC electrospray ionization source (Thermo Fisher Scientific,
Waltham, MA, USA). The full MS spectra were recorded on the
peptides over a 400–1,600 m/z range. Mass spectrometer scan
functions and HPLC solvent gradients were controlled by the
XCalibur data system (Thermo Fisher Scientific). RAW files were
extracted into ms2 file format using RAW Xtract v.1.0. The protein
spectra were searched against the Swiss-Prot database using the
Mascot software program (http://www.matrixscience.com/).
Immunoprecipitation and western blot
analysis
S. pombe cells from a 100-ml culture grown
into a 0.8 OD-595 were pelleted and immediately frozen in liquid
nitrogen. The S. pombe cell pellets were then suspended in 1
ml of lysis buffer containing 50 mM of Tris pH 7.5, 150 mM of NaCl,
0.1% NP-40, 1 mM of DTT, 10% glycerol and a protease inhibitor
tablet. The cells were lysed by adding glass beads followed by
bead-beating for 60 sec 5 times with 1 min intervals on ice. The
supernatant was then separated by centrifugation at 14,000 rpm at
4°C for 20 min, and subjected to co-immunoprecipitation assay with
an anti-FLAG antibody for Eso1-FLAG protein and an anti-HA antibody
for Pol5-HA protein, respectively. Specifically, the supernatant
was added with 30 µl of anti-FLAG or anti-HA affinity gel
(#E6779; EZview™ Red Anti-HA Affinity Gel; Sigma-Aldrich, Shanghai,
China) and incubated at 4°C overnight. The following day, the
mixtures were washed 5 times with wash buffer containing 50 mM of
Tris pH 7.5, 150 mM of NaCl and 1% Triton X-100; they were then
eluted in 2X SDS buffer containing 10 mM of Tris pH 7.5, 1 mM of
EDTA and 1% SDS. The elutes were loaded onto a 4–12% Bis-Tris gel
for sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE), and the proteins were transferred onto a nitrocellulose
membrane (Whatman, Piscataway, NJ, USA). For western blot anaysis,
the membrane was blocked with 5% skimmed milk for 1 h at room
temperature and blotted with primary antibodies [anti-FLAG antibody
(1:3,000; #F3165; Sigma), GST antibody (1:3,000; Abmart, Berkeley
Heights, NJ, USA), a-HA antibody (1:10,000; #sc-7392; Santa Cruz
Biotechnology, Santa Cruz, CA, USA)] against HA and FLAG tags
(Sigma-Aldrich) respectively, as previously described (24). The glutathione resin bound with
variant forms of Eso1 was incubated with whole cell extracts of
S. pombe expressing C-terminally HA-tagged Pol5 at 4°C
overnight, and the resulting samples were subjected to western blot
analysis with the anti-HA tag antibody (Sigma-Aldrich).
In vitro acetylation assay
Purified GST-tagged Eco1p and HA affinity gel bound
with Pol5-HA was added with a HAT buffer containing 50 mM of Tris
pH 8.0, 5% glycerol, 0.1 mM of EDTA, 50 mM of KCl, 1 mM of DTT, 1
mM of PMSF and 10 µM of Acetyl-CoA. This was followed by
incubation at 30°C for 60 min, as previously described (24). The samples were then subjected to
western blot analysis with anti-acetyl-lysine antibody for
acetylation levels and HA antibody as a loading control.
Results
Eso1 protein interaction with Pol5
protein in S. pombe
To identify proteins that can physically interact
with Eso1 protein, Eso1 cDNA was tagged with a TAP tag at the
C-terminus and this fusion protein was expressed under its
endogenous promoter (25) using a
protocol we have previously described (25). The proteins binding to Eso1-TAP
protein were then purified using immunoprecipitation and mass
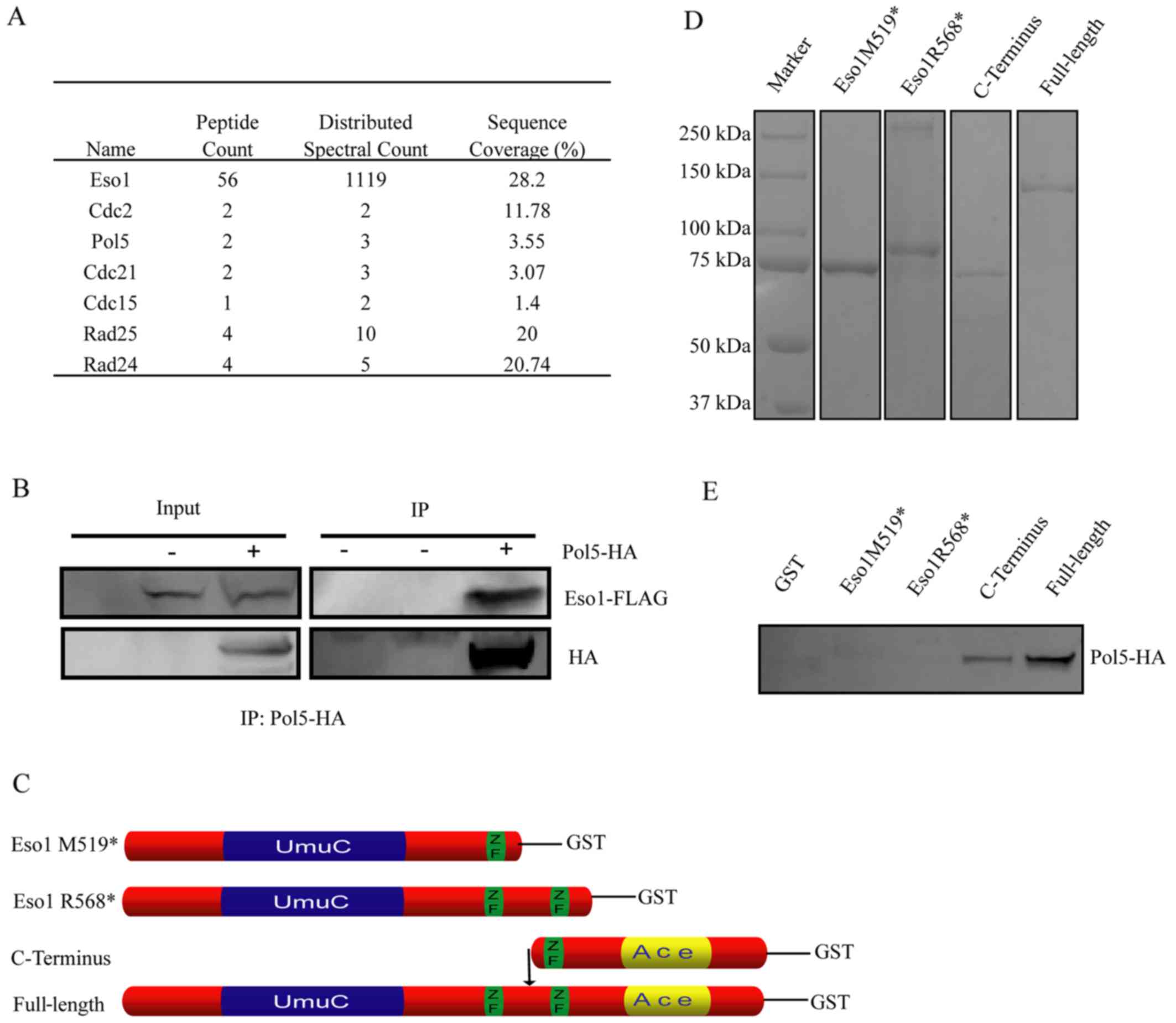
spectrometry followed by TAP. Our data revealed that Eso1 protein
was able to bind to Cdc2, Pol5 and Cdc21, as well as other proteins
(Fig. 1A). The following
experiments focused on Pol5 protein due to its function in rRNA
synthesis and cell proliferation. The specificity of their binding
was confirmed by immunoprecipitating the whole cell extracts of
S. pombe from the wild-type strain, the strain that only
expressed C-terminally FLAG-tagged Eso1, and the strain that
expressed both C-terminally HA-tagged Pol5 and FLAG-tagged Eso1
with anti-HA or FLAG antibodies. Following western blot analysis
using anti-FLAG or α-HA antibody, their binding and interaction
(i.e., the proper expression of Eso1-FLAG and Pol5-HA) were
directly confirmed from the indicated strains (Fig. 1B). Specifically, Pol5-HA protein
could only be detected from whole cell extracts of strains that
express both HA-tagged Pol5 and FLAG-tagged Eso1, indicating the
specificity of their interaction.
However, given the characteristics of the fusion
Eso1 protein in S. pombe, which expresses both budding yeast
Eco1 and Rad30 homologue (26),
our present data was unable to distinguish the binding of Pol5
protein to Eco1 protein from the binding of Pol5 to the Rad30
homologue of Eso1 protein. Thus, we amplified DNA sequences of
Rad30 homologue fragment (1-568 or R568*), Rad30
fragment plus the additional second zinc-finger domain (1-519 or
M519*), Eco1 homologue fragment (520–871) and the full
length Eso1 cDNA using genomic DNA (Fig. 1C). We then inserted these
amplicants into pGEX-4T-1 to produce the expected form of Eso1
protein with the N-terminal GST-tag (Fig. 1D). Following GST pull-down assays
and western blot analysis with anti-GST antibody, the correct
expression of Eso1 proteins were confirmed (Fig. 1D). These proteins were then
utilized to pull-down Pol5 in the whole cell lysis of S.
pombe expressing C-terminally HA-tagged Pol5 for western blot
analysis with anti-α-HA antibody. We found that the results were
consistent with our first set of data, which showed that Pol5
protein binds to Eso1 protein from either Eso1-FLAG and Pol5-HA
co-immunoprecipitation or mass spectrometry analysis following the
purification of Eso1 by TAP (Fig.
1); Pol5-HA was able to pull-down the full-length recombinant
GST-Eso1 protein. Strikingly, Pol5-HA could have also pulled-down
the Eco1 homology part of Eso1, but not the Rad30 part or Rad30
part plus additional zinc finger domain of Eco1 protein (Fig. 1E), suggesting that the Eco1
homology part of Eso1 mediated binding to the Pol5 protein.
Mass spectrometry identification of Pol5
protein acetylation sites
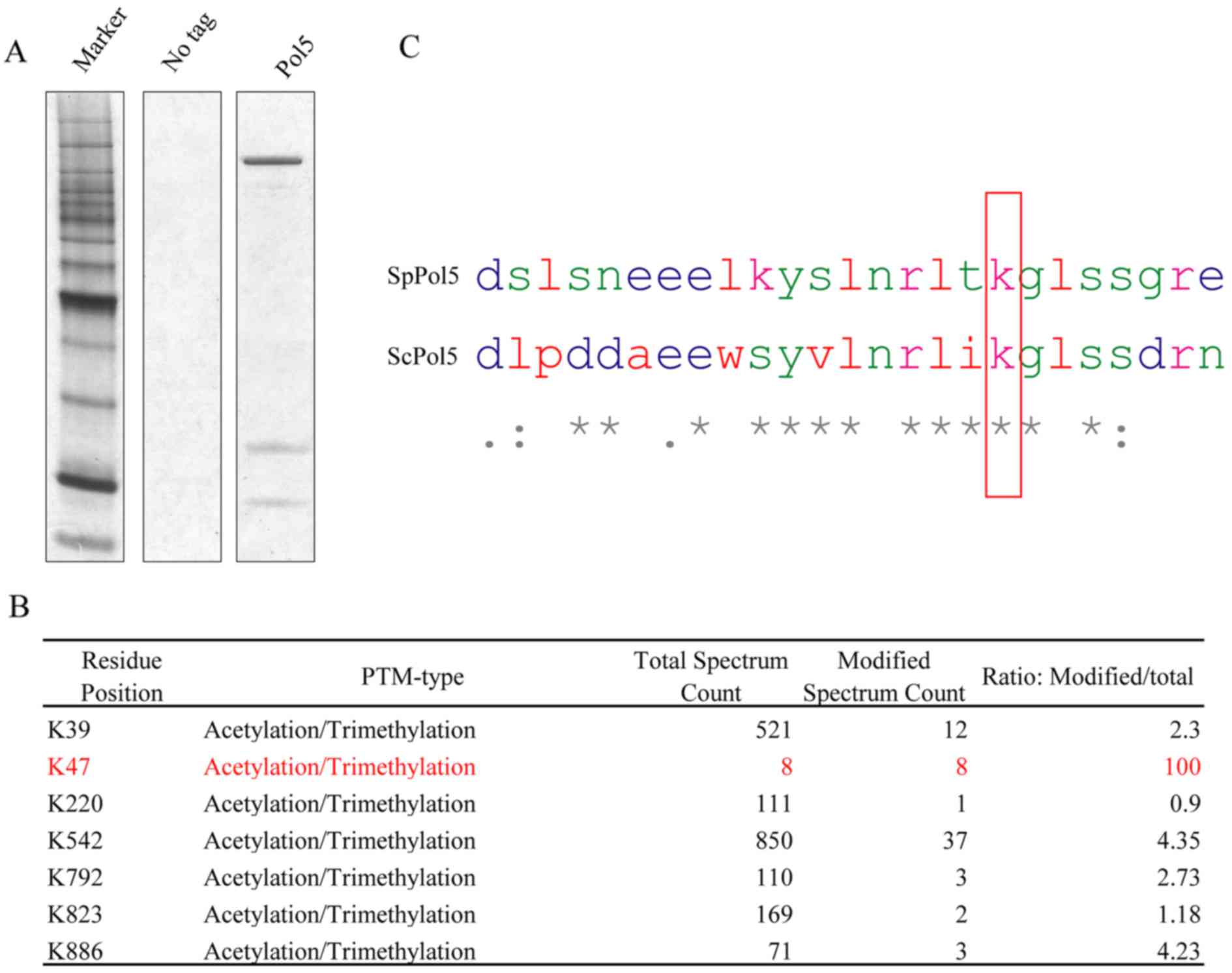
C-terminal TAP tagging and Pol5 protein expression
in vitro was established according to a protocol described
in a previous study (23).
Proteins were purified using an anti-TAP tag antibody and separated
using SDS-PAGE. A prominent protein band of approximately 120 kDa
was found after silver staining (Fig.
2A), which was in agreement with the predicted molecular weight
of Pol5 protein plus TAP tag. Possible post-translational
modifications of this purified Pol5 protein were then analyzed
using mass spectrometry, and several acetylation or trimethylation
modification sites in the lysine residues of Pol5 protein were
identified (Fig. 2B). The Pol5
K47 residue attracted our attention, because there was a 100%
modification ratio (although the number of the total spectrum count
was low); the DNA sequence alignment of S. pombe and S.
cerevisiae indicate that K47 residue is well conserved in
budding yeast (Fig. 2C).
Acetylation of Pol5 protein K47 residue
mediated S. pombe viability
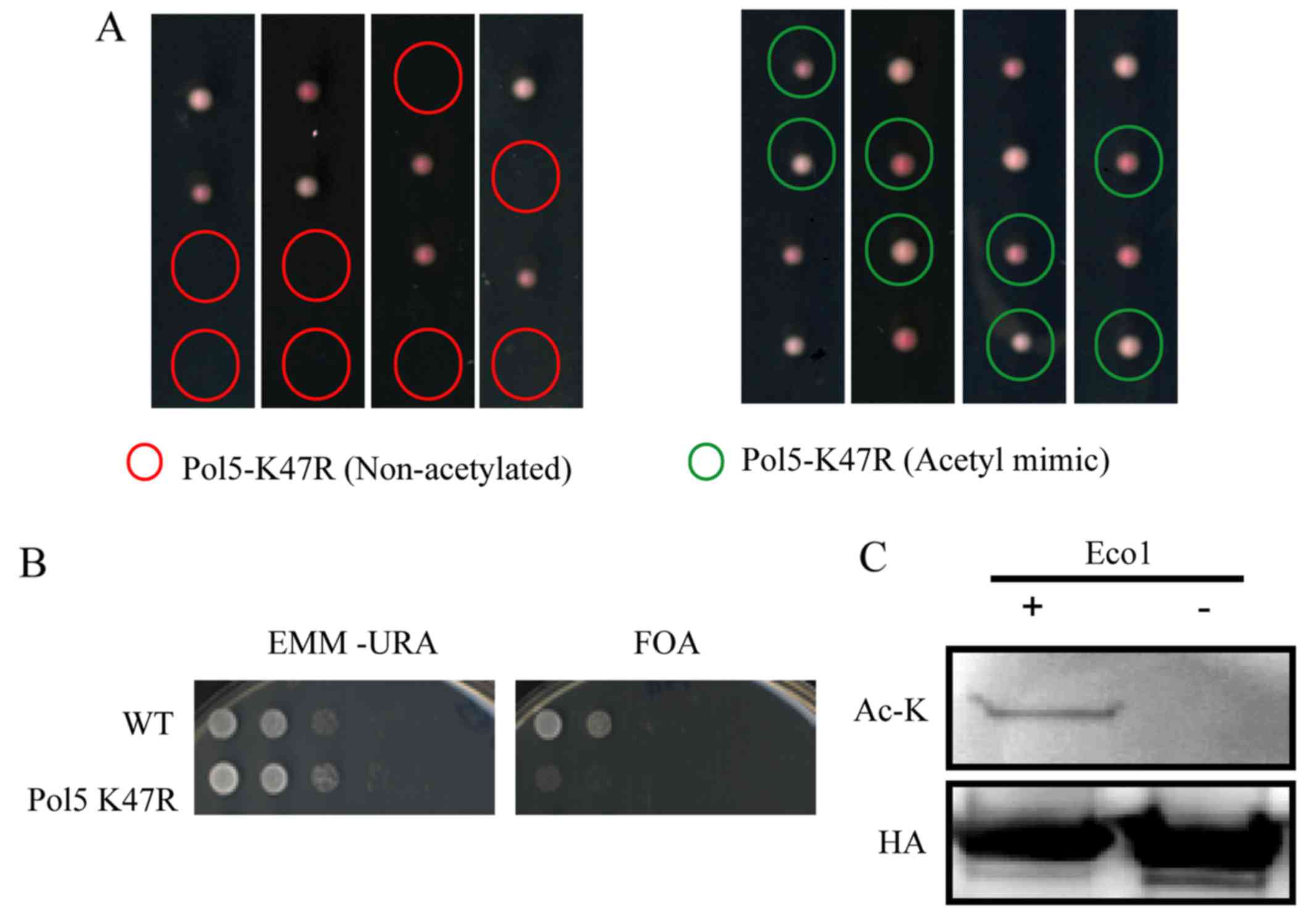
The effects of Pol5 K47 acetylation on S.
pombe cells were assessed, and
pol5-K47R/pol5+ heterozygous diploids were
prepared. Following sporulation, only two spores in each diploid
grew (Fig. 3A). DNA sequencing
analysis was then performed. The results revealed that all viable
spores contained a wild-type (non-mutated) allele of the
Pol5 gene, indicating that S. pombe expressing mutant
Pol5 K47R was unable to survive. Moreover, since arginine (R) is
structurally similar to lysine (K), which does not undergo
acetylation change, we found that arginine substitution of lysine
led to the absence of the acetylation of Pol5 K47; this resulted in
the lethality to S. pombe. In addition, our data also
revealed that Pol5 K47N acetyl-mimetic mutant S. pombe cells
grew normally (Fig. 3A).
Furthermore, we constructed the shuffle plasmid by
integrating the Pol5 coding region cDNA plus the 200-bp upstream
and downstream sequences into the pDblet vector. The transformation
of this plasmid into S. pombe fully rescued the growth of
Pol5 K47R mutants (Fig. 3).
Subsequently, 5FOA was added into S. pombe to select against
the expression of Ura4+ in pDblet-Pol5 plasmid-transferred S.
pombe. Consistent with the data on Pol5 K47R mutation without
pDblet-Pol5 plasmid, our data revealed that S. pombe growth
was limited and lethal (Fig.
3B).
Eso1 acetylation of Pol5 protein K47
residue mediates S. pombe viability
We explored whether Eso1 protein is able to
acetylate Pol5 using acetylation assay following a protocol
described in a previous study (27). We found that Pol5-HA protein was
immunoprecipitated with EZview™ Red Anti-HA Affinity Gel from S.
pombe. This was then incubated with the recombinant Eco1
protein purified from E. coli via the GST tag. Pol5 protein
acetylation was detected by western blot analysis with an
anti-acetyl-lysine antibody following the addition of Eco1 protein
(Fig. 3C).
Discussion
In the present study, we first performed
immunoprecipitation and mass spectrometry assays to identify
several candidate proteins that bind to Eso1 protein (i.e., Cdc2,
Cdc21, Cdc15, Rad24, Rad25 and Pol5). Functionally, Cdc2, as a
serine/threonine kinase, is a highly conserved protein and a key
player in the regulation of cell cycle progression (22,28). Cdc21 is a member of the MCM family
of nuclear proteins, which can regulate DNA replication and cell
cycle progression (29). Cdc15 is
also involved in the formation of the cytokinetic contractile ring
(28). Moreover, Rad24 and Rad25
are c ell cycle checkpoint proteins that belong to the highly
conserved 14-3-3 protein family (29–31), which regulates cell cycle arrest
and DNA damage repair in response to UV light-induced DNA damage
(24–27). Pol5 protein functions in yeast
rDNA transcription (17,18) and the human homologue of Pol5,
Mybbp1a, demonstrating functions in rDNA transcription, early
embryonic development and tumorigenesis (19,20). Thus, given the important functions
of Eso1 protein in cells, including the sister chromatin cohesion
complex and mediation of DNA repair (2), it seems reasonable to hypothesize
that the binding of Eso1 to these proteins illustrates the
functions of Eso1 protein.
Furthermore, we focused on the interaction of Pol5
protein with Eso1 protein to assess their regulation of S.
pombe viability. We performed a co-immunoprecipitation assay to
further confirm the binding of Eco1 to Pol5 protein and found that
Pol5 protein only bound to Eso1 protein, but not to the Rad30 part
or Rad30 part, plus the additional zinc finger domain of Eco1
protein, indicating that their binding was specific. Upon their
binding, Eso1 protein was able to acetylate Pol5 protein. Our
confirmative evidence indicated that there were several acetylation
sites in Pol5 protein lysine residues (Fig. 2B) and that arginine substitution
of lysine led to the lack of Pol5 K47 acetylation, which resulted
in the lethality of S. pombe; whereas for Pol5 K47N, the
acetyl-mimetic mutant had normal S. pombe proliferation;
thus, the Eso1 acetylation site in Pol5 protein could be at the
Pol5 K47. However, at present, there are no Pol5 K47 acetylation
antibodies available; thus, a definitive proof would require the
involvement of a mass-spec analysis of the acetylation on Pol5
before and after incubation with Eso1 protein. Future studies are
required to further verify the Eso1 acetylating Pol5 K47 site,
although our present data suggest that Pol5 protein acetylation was
essential in the regulation of S. pombe viability.
In conclusion, previous studies have revealed the
essential role of Eco1/Eso1 protein in the formation of the
cohesion complex between the two sister chromatids, and that the
acetyl-mimetic mutation, Smc3 K47N, made Eco1 protein dispensable.
In the present study, we found that the S. pombe carrying
non-acetylatable mutations of Pol5 protein (i.e., Pol5 K47R) was
unable to survive, indicating the essential role of Pol5
acetylation in S. pombe. However, this study was not able to
rule out whether other acetyltransferases, apart from Eso1,
acetylate Pol5 protein. Future studies are warranted to verify
Eso1, particularly the acetylate Pol5 K47 site; although the
present study suggested so.
Acknowledgments
The authors would like to thank Dr Juraj Gregan of
Max F. Perutz Laboratories, Department of Chromosome Biology,
University of Vienna, Vienna, Austria; and the Stowers Proteomics
and Molecular Biology groups, MO, USA. This study was supported by
a grant from the National Natural Science Foundation of China
(#81672122), and Jilin Provincial Science and Technology Agency
(#20160414048GH).
References
|
1
|
Skibbens RV, Corson LB, Koshland D and
Hieter P: Ctf7p is essential for sister chromatid cohesion and
links mitotic chromosome structure to the DNA replication
machinery. Genes Dev. 13:307–319. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanaka K, Yonekawa T, Kawasaki Y, Kai M,
Furuya K, Iwasaki M, Murakami H, Yanagida M and Okayama H: Fission
yeast Eso1p is required for establishing sister chromatid cohesion
during S phase. Mol Cell Biol. 20:3459–3469. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Unal E, Heidinger-Pauli JM and Koshland D:
DNA double-strand breaks trigger genome-wide sister-chromatid
cohesion through Eco1 (Ctf7). Science. 317:245–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nasmyth K and Schleiffer A: From a single
double helix to paired double helices and back. Philos Trans R Soc
Lond B Biol Sci. 359:99–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirano T: Chromosome cohesion,
condensation, and separation. Annu Rev Biochem. 69:115–144. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nasmyth K: Disseminating the genome:
Joining, resolving, and separating sister chromatids during mitosis
and meiosis. Annu Rev Genet. 35:673–745. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koshland DE and Guacci V: Sister chromatid
cohesion: The beginning of a long and beautiful relationship. Curr
Opin Cell Biol. 12:297–301. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Shi X, Li Y, Kim BJ, Jia J, Huang
Z, Yang T, Fu X, Jung SY, Wang Y, et al: Acetylation of Smc3 by
Eco1 is required for S phase sister chromatid cohesion in both
human and yeast. Mol Cell. 31:143–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Unal E, Heidinger-Pauli JM, Kim W, Guacci
V, Onn I, Gygi SP and Koshland DE: A molecular determinant for the
establishment of sister chromatid cohesion. Science. 321:566–569.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rolef Ben-Shahar T, Heeger S, Lehane C,
East P, Flynn H, Skehel M and Uhlmann F: Eco1-dependent cohesin
acetylation during establishment of sister chromatid cohesion.
Science. 321:563–566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feytout A, Vaur S, Genier S, Vazquez S and
Javerzat JP: Psm3 acetylation on conserved lysine residues is
dispensable for viability in fission yeast but contributes to
Eso1-mediated sister chromatid cohesion by antagonizing Wpl1. Mol
Cell Biol. 31:1771–1786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vega H, Waisfisz Q, Gordillo M, Sakai N,
Yanagihara I, Yamada M, van Gosliga D, Kayserili H, Xu C, Ozono K,
et al: Roberts syndrome is caused by mutations in ESCO2, a human
homolog of yeast ECO1 that is essential for the establishment of
sister chromatid cohesion. Nat Genet. 37:468–470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dorsett D: Cohesin: Genomic insights into
controlling gene transcription and development. Curr Opin Genet
Dev. 21:199–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Z, McCrosky S, Guo W, Li H and Gerton
JL: A genetic screen to discover pathways affecting cohesin
function in Schizosaccharomyces pombe identifies chromatin
effectors. G3 (Bethesda). 2:1161–1168. 2012. View Article : Google Scholar
|
|
15
|
Bose T, Lee KK, Lu S, Xu B, Harris B,
Slaughter B, Unruh J, Garrett A, McDowell W, Box A, et al: Cohesin
proteins promote ribosomal RNA production and protein translation
in yeast and human cells. PLoS Genet. 8:e10027492012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu B, Lee KK, Zhang L and Gerton JL:
Stimulation of mTORC1 with L-leucine rescues defects associated
with Roberts syndrome. PLoS Genet. 9:e10038572013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang W, Rogozin IB and Koonin EV: Yeast
POL5 is an evolutionarily conserved regulator of rDNA transcription
unrelated to any known DNA polymerases. Cell Cycle. 2:120–122.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimizu K, Kawasaki Y, Hiraga S,
Tawaramoto M, Nakashima N and Sugino A: The fifth essential DNA
polymerase phi in Saccharomyces cerevisiae is localized to the
nucleolus and plays an important role in synthesis of rRNA. Proc
Natl Acad Sci USA. 99:9133–9138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mori S, Bernardi R, Laurent A, Resnati M,
Crippa A, Gabrieli A, Keough R, Gonda TJ and Blasi F: Myb-binding
protein 1A (MYBBP1A) is essential for early embryonic development,
controls cell cycle and mitosis, and acts as a tumor suppressor.
PLoS One. 7:e397232012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hochstatter J, Hölzel M, Rohrmoser M,
Schermelleh L, Leonhardt H, Keough R, Gonda TJ, Imhof A, Eick D,
Längst G, et al: Myb-binding protein 1a (Mybbp1a) regulates levels
and processing of pre-ribosomal RNA. J Biol Chem. 287:24365–24377.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sabatinos SA and Forsburg SL: Molecular
genetics of Schizosaccharomyces pombe. Methods Enzymol.
470:759–795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gregan J, Rabitsch PK, Rumpf C,
Novatchkova M, Schleiffer A and Nasmyth K: High-throughput knockout
screen in fission yeast. Nat Protoc. 1:2457–2464. 2006. View Article : Google Scholar
|
|
23
|
Cipak L, Spirek M, Novatchkova M, Chen Z,
Rumpf C, Lugmayr W, Mechtler K, Ammerer G, Csaszar E and Gregan J:
An improved strategy for tandem affinity purification-tagging of
Schizosaccharomyces pombe genes. Proteomics. 9:4825–4828. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong B, Lu S and Gerton JL: Hos1 is a
lysine deacetylase for the Smc3 subunit of cohesin. Curr Biol.
20:1660–1665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Z, Cao H, Guo W and Lu Y:
Identification of two forms of the Eso1 protein in
Schizosaccharomyces pombe. Cell Biol Int. 38:682–688. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Madril AC, Johnson RE, Washington MT,
Prakash L and Prakash S: Fidelity and damage bypass ability of
Schizosaccharomyces pombe Eso1 protein, comprised of DNA polymerase
eta and sister chromatid cohesion protein Ctf7. J Biol Chem.
276:42857–42862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu S, Goering M, Gard S, Xiong B, McNairn
AJ, Jaspersen SL and Gerton JL: Eco1 is important for DNA damage
repair in S. cerevisiae. Cell Cycle. 9:3315–3327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fankhauser C, Reymond A, Cerutti L, Utzig
S, Hofmann K and Simanis V: The S. pombe cdc15 gene is a key
element in the reorganization of F-actin at mitosis. Cell.
82:435–444. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ford JC, al-Khodairy F, Fotou E, Sheldrick
KS, Griffiths DJ and Carr AM: 14-3-3 protein homologs required for
the DNA damage checkpoint in fission yeast. Science. 265:533–535.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ozoe F, Kurokawa R, Kobayashi Y, Jeong HT,
Tanaka K, Sen K, Nakagawa T, Matsuda H and Kawamukai M: The 14-3-3
proteins Rad24 and Rad25 negatively regulate Byr2 by affecting its
localization in Schizosaccharomyces pombe. Mol Cell Biol.
22:7105–7119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Heusden GP and Steensma HY: Yeast
14-3-3 proteins. Yeast. 23:159–171. 2006. View Article : Google Scholar : PubMed/NCBI
|