Introduction
Low-grade inflammation is involved in the
pathogenesis of cardiovascular diseases. The activation of the
immune system contributes to the development of hypertension
(1). Studies have demonstrated
that T cells are required for the development of hypertension and
the infiltration of target tissues (2,3).
Previous studies have implicated changes in T cells in the immune
status of experimental animals, such as angiotensin (Ang) II and
DOCA-salt hypertension (2,4,5).
Therefore, T cells are critical for the development of essential
hypertension and targeting organ damage. However, exactly which
type of T cells may contribute to inflammatory responses and renal
end-organ damage during hypertension, as well as the mechanisms
responsible for hypertensive inflammation remain speculative and
unclear.
T cells can be divided into several subtypes, and
each subtype plays differential roles in infection and immune
homeostasis. T cells consist of T helper (Th) cells
(CD4+) and cytotoxic T cells (CD8+). Th cells
(CD4+) are classified into Th1, Th2, Th17 and regulatory
T (Treg) cells. CD4+ T cells remove pathogens by
activating innate cells and B cells of the adaptive immune system.
CD8+ T cells kill pathogens by releasing cytotoxic
molecules, such as perforin and granzyme B. The surface markers of
Treg cells, which have an immunosuppressive T cell lineage, contain
CD4+CD25+ to prevent the elevation of blood
pressure and the damage to target organs induced by Ang II
(6,7). The abnormity of T cell subsets leads
to hypertension and end-organ damage by releasing cytokines
(1). Th1 cells are known to
secrete adaptive cytokines, namely, interleukin-2 (IL)-2,
interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), whereas
Th2 cells secrete IL-4, IL-6 and IL-13. It has previously been
reported elevated Th1 activity in Ang-dependent hypertensive mice,
as shown by the increased expression of lymphoid Th1-specific
cytokines, namely, IFN-γ and TNF-α (2). Central signals activate macrophages
and T cells, which target the kidneys and vasculature, and release
cytokines, including IL-6 and IL-17, thereby inducing renal and
vascular dysfunction and elevating blood pressure (8,9).
It is known that gap junctions (GJs) between Tregs
and T cells function in a novel signaling pathway to suppress T
cell proliferation by mediating the transfer of Cyclic adenosine
monophosphate (cAMP) (10). Gap
junctional intercellular communication (GJIC) relies on the
intercellular channels that span the lipid bilayers of contiguous
cells, thereby allowing the direct bidirectional passage of 1–1.5
kDa molecules (11). A GJ is
composed of two end-to-end connected hemichannels (also called
connexons), each hemichannel is composed of six connexin (Cx)
molecules (12). Cx43 is a major
GJ protein of immune cells (13-15). Cx43-mediated GJs provide direct
intercellular channels to link attached lymphocytes. Cx43 also
plays an important role in the proliferation and activation of, as
well as cytokine production in T lymphocytes (16,17). However, the involvement of GJs
between lymphocytes in triggering inflammation during hypertension
remains unknown.
In this study, we hypothesized that Cx43 in T cells
can modulate the inflammatory response in hypertension by
regulating lymphocyte proliferation and cytokine production.
Therefore, the present study was carried out to examine whether
Cx43 in splenic T cell subsets is associated with
hypertension-induced inflammation.
Materials and methods
Source of reagents
The fluorochrome-conjugated monoclonal antibodies
(mAbs) to rat antigens for flow cytometric analysis, including
anti-CD45 FITC (Cat. no. 202205), anti-CD3 FITC (Cat. no. 201403),
anti-CD4 APC (Cat. no. 201509), anti-CD8a PE (Cat. no. 200608),
anti-CD25 PE (Cat. no. 202105), mouse FITC-IgG1, κ Isotype Ctrl
(Cat. no. 400107), mouse FITC-IgM, κ Isotype Ctrl (Cat. no.
401606), mouse APC-IgG1, κ Isotype Ctrl (Cat. no. 400122), mouse
PE-IgG2a, κ Isotype Ctrl (Cat. no. 400212) and mouse PE-IgG1, κ
Isotype Ctrl (Cat. no. 981804) were obtained from BioLegend (San
Diego, CA, USA). Anti-Cx43 antibody (Cat. no. ab79010) was obtained
from Abcam (Cambridge, MA, USA). The goat anti-mouse IgG/FITC
secondary antibody (Cat. no. 405305) was acquired from BioLegend,
and 10X erythrocyte lysis buffer was supplied by Mindray (Shenzhen,
China). Rat IL-2 and rat IL-6 enzyme-linked immunosorbent assay
(ELISA) kits were provided by MultiSciences Biotech Co., Ltd.
(Hangzhou, China). RPMI-1640 medium was obtained from Gibco (Grand
Island, NY, USA) and fetal bovine serum (FBS) was acquired from
HyClone (Logan, UT, USA). Penicillin, streptomycin, concanavalin A
(ConA) and TRI reagent were obtained from Sigma-Aldrich (St. Louis,
MO, USA). Gap27 was supplied by Apexbio (Boston, MA, USA), and the
cell counting kit-8 (CCK-8) kit was provided by Zomanbio (Beijing,
China). The PrimeScript™ RT reagent kit was acquired from Takara
(Shiga, Japan), and the QuantiNova SYBR-Green PCR kit was obtained
from Qiagen (Hilden, Germany). Primers were synthesized by Sangon
Biotech (Shanghai, China).
Experimental animals
Age-matched 16- to 17-week-old male spontaneously
hypertensive rats (SHRs) and normotensive Wistar-Kyoto (WKY) rats
(n=15 in each group) were used in this study. The experimental
animals were purchased from vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The protocol of this study
was approved by the Institutional Animal Care and Use Committees
(IACUC) of the Medical College of Shihezi University, Shihezi,
China. The study was performed in accordance with the guidelines
for the Care and Use of Laboratory Animals published by the US
National Institutes of Health (Public Health Service Policy on
Humane Care and Use of Animals, DHEW Publication no. 96-01, PHS
Policy revised in 2002).
Blood pressure monitoring
The systolic blood pressure (SBP) was measured
non-invasively using a tail-cuff apparatus (Chengdu Thai Union Co.,
Chengdu, China) prior to the experiment. The rats were placed for
10 min in a chamber heated to 37°C. A cuff equipped with a
pneumatic pulse sensor was wrapped around the tail of the rat and
was placed in an individual plastic restrainer. Blood pressure was
accurately recorded for each rat and averaged from at least 3
consecutive readings.
Histological analysis
The SHRs and WKY rats were euthanized under general
anesthesia by an intraperitoneal injection of pentobarbital sodium
(50 mg/kg). The kidneys and spleens were harvested, weighed and
fixed in formalin. After 72 h, the samples were dehydrated,
embedded in paraffin, and sliced into 5-μm-thick sections.
These sections were mounted on slides and stained with hematoxylin
and eosin (H&E). The slides were photographed under a light
microscope (Olympus, Tokyo, Japan). The damaged tubules were
identified based on the presence and severity of the renal tubular
epithelial cell degeneration, necrosis, loss of brush border cells,
intraluminal casts and inflammatory cell infiltration, as assessed
by two professional pathologists at 10 high-power fields
(magnification, ×400) per section. The percentages of histological
changes in the kidney tissues were scored with a semi-quantitative
scale, which was designed to evaluate the degree of tubular damage
as previously described (18): 0,
normal kidney; 1, minimal damage (5% involvement); 2, mild damage
(5–25% involvement); 3, moderate damage (25–50% involvement); 4,
severe damage (50–75% involvement); and 5, most severe damage
(>75% involvement).
Detection of serum cytokine levels
The SHR and WKY rats were euthanized by 30 mg/l
sodium pentobarbital anesthesia (50 mg/kg, i.p.). Peripheral blood
was collected with heparin-coated capillary glass tubes from
abdominal aorta and allowed to clot at ambient temperature for 15
min. The blood samples obtained were centrifuged at 800 × g for 10
min at 4°C to obtain serum. ELISA was performed to quantify and
compare the cytokine levels (IL-2 and IL-6) in serum according to
the manufacturer's instructions with rat platinum ELISA kits. The
reaction was measured at 450 nm with a microplate reader (Dynatech,
Denkendorf, Germany). A standard curve was prepared from IL-2 and
IL-6 standard dilutions before the IL-2 and IL-6 concentrations
were determined in the rat samples. The concentration of each
cytokine was expressed in pg/ml.
Analysis of splenic lymphocyte
subsets
The spleens were harvested from the same rats as
those above into a tissue culture dish and used to prepare a single
cell suspension by gentle pressing with the plunger of a syringe.
The isolated cells were passed through a 200 μM cell
strainer (BD Biosciences, San Jose, CA, USA) and collected. The
cell suspensions were centrifuged at 200 × g for 10 min at room
temperature. The red blood cells were isolated, treated with the
erythrocyte lysis buffer, and washed twice with phosphate-buffered
saline (PBS). After cell counting with a counting chamber and
viability detection by trypan blue exclusion (>95%), they were
resuspended in an appropriate volume of PBS to a final
concentration of approximately 1×106 cells/ml. The cells
were incubated at 4°C for 15 min in the dark with
fluorochrome-labelled mAbs for surface antigen markers, including T
lymphocytes (CD3+), T-helper cells
(CD3+CD4+), cytotoxic T cells
(CD3+CD8+) and regulatory T cells
(CD4+CD25+). The cells were resuspended in
500 μl of PBS. Subsequently, approximately 20,000 cells were
analyzed using a flow cytometer (Becton-Dickinson, Franklin Lakes,
NJ, USA), with an initial gate set by forward/side scatter
(FSC/SSC) dot plots. Isotype-matched antibodies were used as
negative control samples to confirm the specificity of antibody
binding and eliminate nonspecific receptor binding or other
cellular protein interactions. The proper fluorescence compensation
was set to ensure that negative and positive cells were identical
and used to gate the population. For the splenic cell suspension,
CD45 staining was used to identify leukocytes, in the
CD45+ gate, T cells were identified by the anti-CD3,
-CD4, -CD8 and -CD25 antibodies.
Analysis of Cx43 in splenic lymphocyte
subsets
The spleens were harvested from the same SHRs and
WKY rats as those above into a tissue culture dish and used to
prepare a single cell suspension via the method above. Cell
suspension was resuspended in an appropriate volume of PBS to a
final concentration of approximately 1×106 cells/ml and
fixed with 4% paraformaldehyde for 10 min and permeabilized with
0.1% Tween-20 for 20 min at room temperature. Following incubation
in blocking buffer containing 10% goat serum, the cells were
incubated with the anti-rat Cx43 antibodies at 4°C for 30 min prior
to incubation with the FITC-labeled goat anti-mouse IgG secondary
antibody in the dark for 30 min at 4°C with. Negative control
samples were incubated with isotype-matched antibodies. The cells
were washed twice with PBS and incubated with CD4 or CD8 antibodies
for flow cytometry analysis as described above.
Lymphocyte proliferation assay
Splenic cells from the SHRs and WKY rats were
collected in respective centrifuge tube containing the lymphocyte
separation medium. After density gradient centrifugation (400 × g
for 30 min), the splenic mono-nuclear cells were isolated. The red
blood cells were lysed by the addition of the erythrocyte lysis
solution for 5 sec before washing the pellet twice with PBS. The
pellet was resuspended in 1 ml of RPMI-1640 medium containing 10%
FBS, 100 units penicillin and 100 μg/ml streptomycin.
Following 3 h of incubation at 37°C in 5% CO2,
non-adherent cells were collected and adjusted to 1×106
cells/ml in the culture medium. The mitogen ConA was used to
stimulate the lymphocytes. Furthermore, the Gap27 peptide was used
to evaluate the effects on lymphocytes activation and proliferation
(Gap27 dissolved in Hanks balanced salt solution was used as gap
junctional communication inhibitor). The cells were dispensed into
96-well round-bottomed plates as 100 μl triplicates. The
wells were divided into the following 4 treatment groups: the
control, ConA, Gap27 and Gap27 + ConA groups. The control group
contained cells without stimulation. In the ConA group, the
lymphocytes were stimulated with ConA (5 μg/ml) for 24 h. In
the Gap27 group, the lymphocytes were stimulated with Gap27 (500
μM) for 48 h. In the Gap27 + ConA group, Gap27 (500
μM) was used to incubate the lymphocytes for 48 h prior to
stimulation with ConA (5 μg/ml) for 24 h. The plates were
incubated at 37°C under 5% CO2 in a humidified
incubator. After 72 h, the splenic lymphocytes from the SHRs and
WKY rats were harvested to determine the cell proliferation with
CCK-8 kits according to the manufacturer's instructions. The
optical density was measured using a microplate reader (Dynatech),
with a test wavelength of 450 nm.
Analysis of cultured splenic lymphocyte
subsets in vitro
To calculate the frequency of different lymphocyte
subpopulations in response to ConA stimulation and treatment with
the GJ inhibitor, Gap27, each group of cultured cell suspensions
from the WKY rats and SHRs was incubated and stained for 15 min at
4°C in the dark for flow cytometic analysis with the following
antibodies: FITC-anti-CD3, APC-anti-CD4, PE-anti-CD8 and
PE-anti-CD25. The samples were analyzed with the experimental
procedure for flow cytometry as mentioned above. The cell
proliferation rate was caclulated as follows: cell proliferation
rate = (ConA group-control group)/ConA group ×100%. The cell
inhibition rate was caclulated as follows: cell inhibition rate =
[ConA group−(Gap27 + ConA) group]/ConA group ×100%
Analysis of IL-2 and IL-6 mRNA expression
by reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from the cultured lymphocytes from the
SHRs and WKY rats was extracted using TRI reagent. The RNA
concentration and purity were determined by measuring
spectrophotometric absorbance at 260 nm (A260) and the
A260/280 ratio with a Nanovue™ spectrophotometer (Thermo
Fisher Scientific, Waltham, MA, USA). A 1 μl aliquot of RNA
was used to generate cDNA in 10 μl reaction volumes with the
PrimeScript™ RT reagent kit. Reverse transcriptase reactions were
operated in a thermocycler (Takara, Mountain view, CA, USA) for 15
min at 37°C, followed by incubation at 85°C for 5 sec. For each
diluted cDNA sample (performed in triplicate for each gene), 2
μl of cDNA was amplified in 10 μl reactions with 5
μl of 2X SYBR-Green PCR master mix as gene-specific primers:
IL-2 forward, 5′-GCA CCT GTA AGT CCA GCA AC-3′ and reverse primer,
3′-ACG CTT GTC CTC CTT GTC A-5′; IL-6 forward, 5′-TTG GGA CTG ATG
TTG TTG AC-3′ and reverse primer, 3′-TGT GGG TGG TAT CCT CTG T-5′;
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward,
5′-CTC TCT GCT CCT CCC TGT TC-3′, and reverse primer, 3′-GCC AAA
TCC GTT CAC ACC G-5′. The real-time PCR system (Bio-Rad, Hercules,
CA, USA) was operated with the following reaction program: 20 sec
at 37°C and 2 min at 95°C, followed by 40 cycles of 5 sec at 95°C
and 10 sec at 60°C. All assays were done in triplicate and the ΔCq
value was computed. A relative quantification assay was performed
to evaluate the ratio of each cytokine normalized to the GAPDH
expression in each sample.
Statistical analysis
All results were presented as the means ± SEM for
each assessment. All statistical analyses were performed with
GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA). One-way ANOVA, followed by Tukey's multiple comparison test,
was performed to compare more than 3 groups. An unpaired two-tailed
t-test was used to establish 2 group comparisons. Differences with
p<0.05 were considered statistically significant.
Results
Systematic analysis of body weight and
systolic blood pressure between SHRs and WKY rats
The basal SBP of the WKY rats and SHRs was measured
prior to the start of the experiments. At 16-17 weeks of age, the
SBP was significantly higher in the SHRs (188.9±3.7 mmHg) than in
the age-matched WKY rats (109.3±2.6 mmHg; p<0.01). A tendency
toward an increased spleen weight (SW) was observed in the SHRs,
although the difference was not significant as compared with the
WKY rats. No significant difference was observed in the body weight
(BW) or the ratio of the spleen weight to the body weight (SW/BW)
ratio between the SHRs and WKY rats (p>0.05) (Table I).
 | Table IGeneral information of SHRs and WKY
rats. |
Table I
General information of SHRs and WKY
rats.
| Rats | BW
(g) | SBP
(mmHg) | SW
(mg) | SW/BW
(mg/g) |
|---|
| WKY (n=15) | 282.0±3.2 | 109.3±2.6 | 629.0±15.6 | 2.2±0.2 |
| SHR (n=15) | 290.8±2.9 | 188.9±3.7a | 652.5±11.2 | 2.2±0.1 |
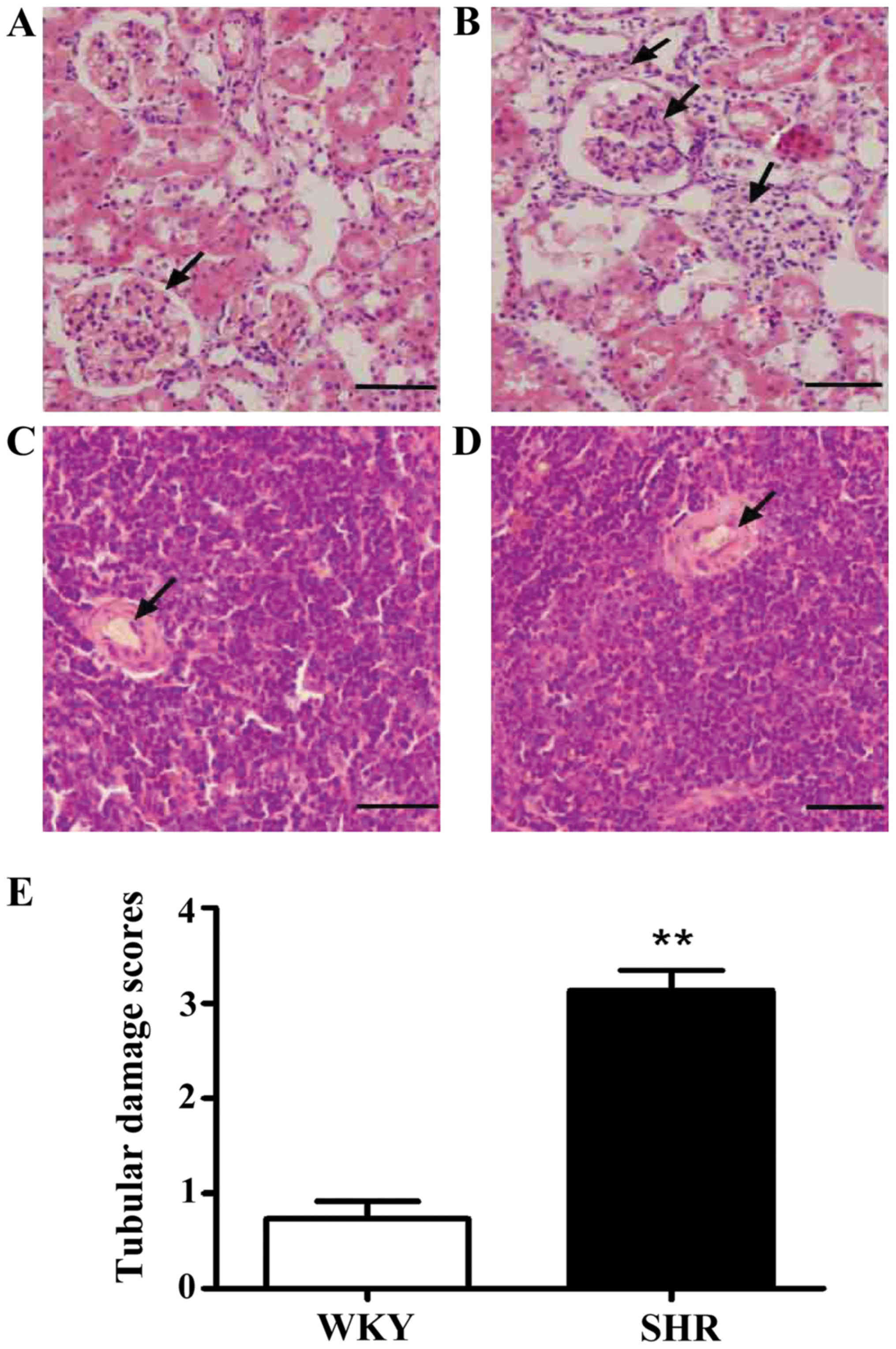
Target organ damage caused by
hypertensive inflammation
To observe the pathological changes in the target
organs resulting from hypertension, we determined the pathological
changes in the kidneys and spleens of the SHRs by H&E staining.
H&E staining revealed a significant thickening of the vascular
wall, inflammatory cell infiltration into part of the renal
interstitium, and glomerular atrophy in the kidneys of the SHRs as
compared with those of the WKY rats. After blinded
semi-quantitative scoring, the tubular damage score in the SHRs
(3.1±0.2) was greater than that in the WKY rats (0.7±0.2;
p<0.01) (Fig. 1). The SHRs
exhibited stenosis in the wall of the central artery in the spleen
(Fig. 1).
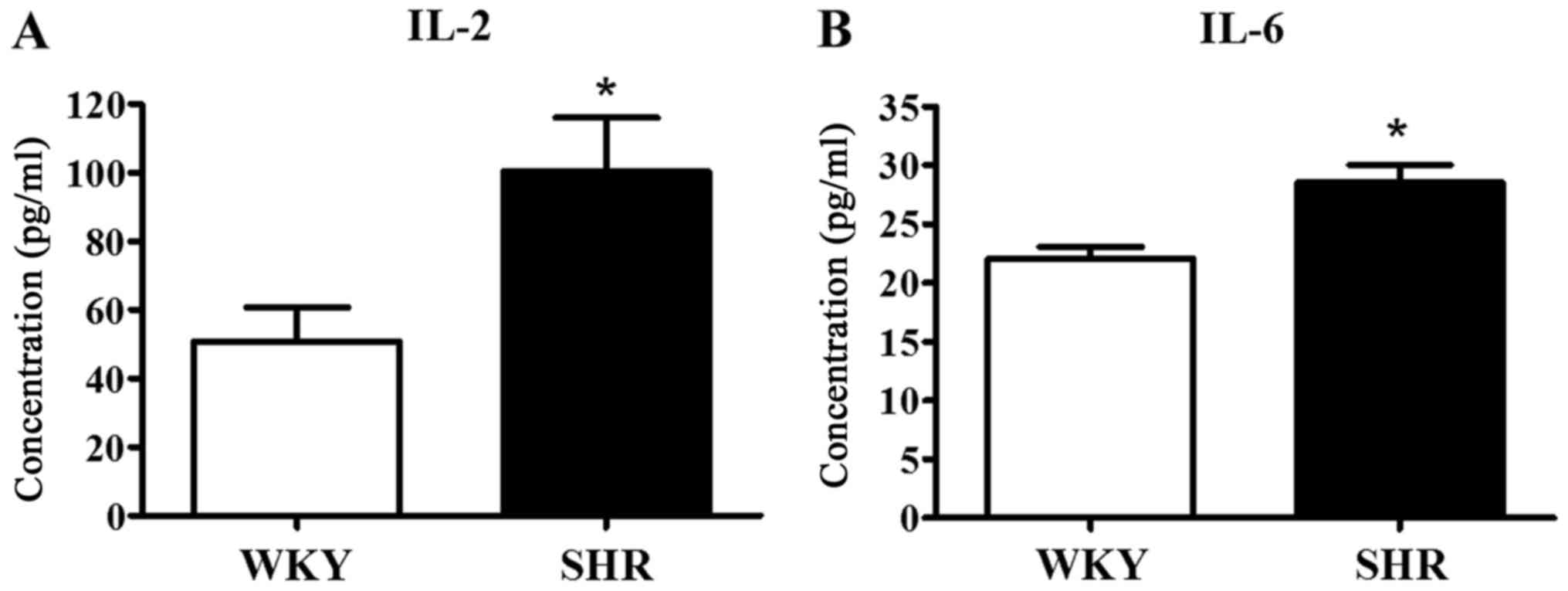
We evaluated the hypertensive inflammatory response
by measuring the concentration of typical Th1 (IL-2) and Th2 (IL-6)
cytokines in the serum of the WKY rats and SHRs, as these cytokines
are generally regarded as signs of the pro-inflammatory response.
The serum concentrations of IL-2 (100.5±15.5 pg/ml) and IL-6
(28.5±1.5 pg/ml) in the SHRs were significantly higher than the
IL-2 (50.8±10.0 pg/ml) and IL-6 (22.1±1.0 pg/ml; p<0.05) levels
in the WKY rats (Fig. 2). These
results suggested that the development of hypertension was
accompanied by an inflammatory response and the impairment of
kidney and spleen function was caused by inflammation.
Simultaneously, the elevated blood pressure may promote lymphocyte
activation and the secretion of pro-inflammatory cytokines.
Hypertension-induced reduction of the
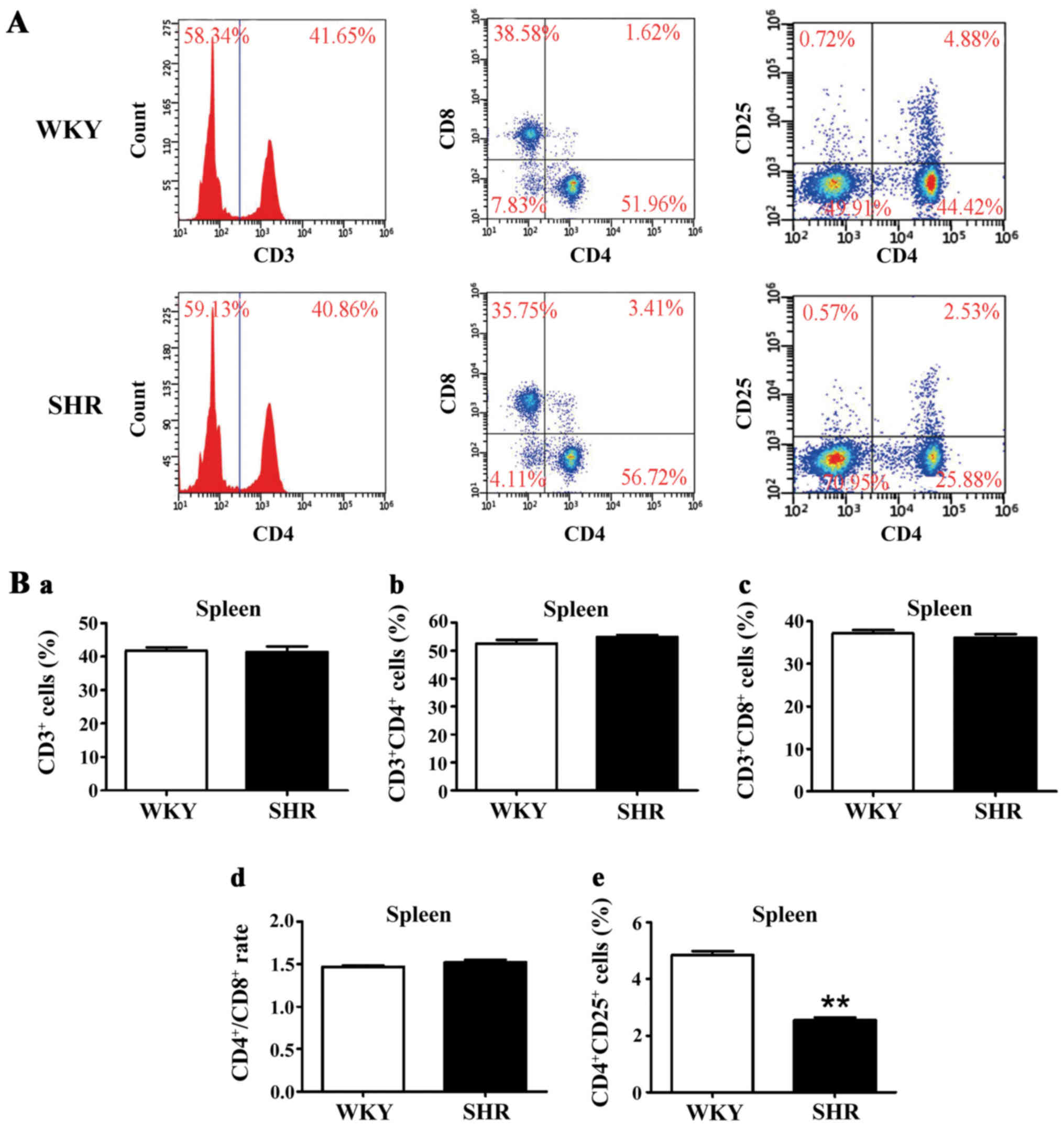
percentage of Tregs in spleen
We investigated whether pro-inflammatory lymphocytes
can be detected in the spleens of normotensive and hypertensive
rats. Flow cytometric analysis (Fig.
3) was used to determine the percentages of CD3+ T
cells (WKY, 41.8±1.0%; SHR, 41.5±1.6%; p>0.05),
CD3+CD4+ T cells (WKY, 52.5±1.4%; SHR,
54.9±0.7%; p>0.05), CD3+CD8+ T cells (WKY,
37.2±0.7%; SHR, 36.2±0.9%; p>0.05) and
CD4+CD25+ T cells (WKY, 4.9±0.1%; SHR,
2.6±0.1%; p<0.01), as well as the ratio of
CD4+/CD8+ (WKY, 1.47±0.02; SHR, 1.52±0.03;
p>0.05) cells. The frequencies of
CD4+CD25+ T cells were significantly lower in
the spleens of SHRs as compared with those of the WKY rats. The
percentage of other T cell subtypes, including the CD3+,
CD3+CD4+, and CD3+CD8+
T cells in the spleen and the CD4+/CD8+ ratio
did not differ significantly between the SHRs and WKY rats. These
results demonstrated that a population of Tregs may be required for
hypertension.
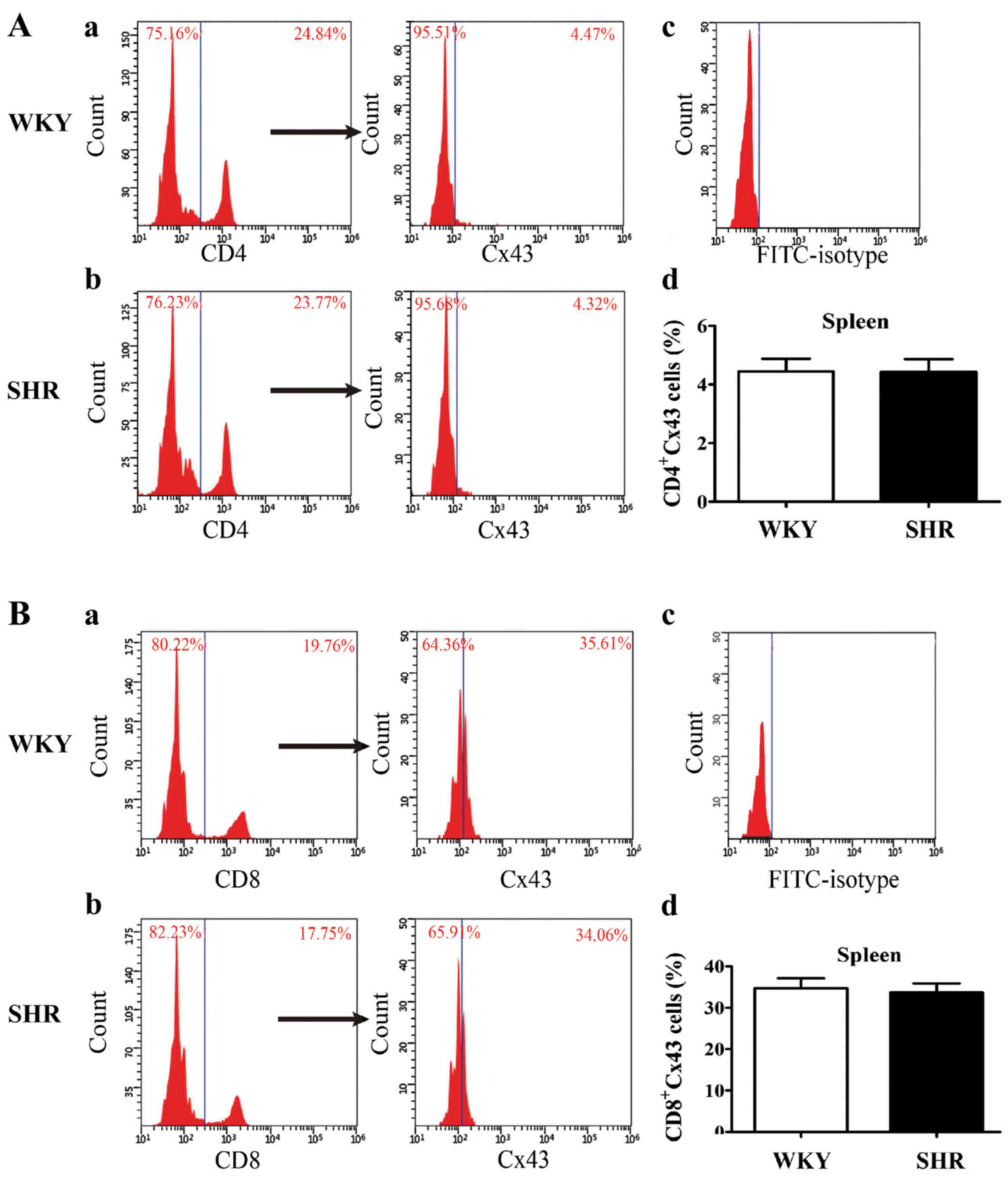
After analyzing the phenotypic characterization of
lymphocyte subsets, we compared Cx43 surface expression in splenic
lymphocyte subsets between the SHRs and WKY rats. A representative
scatter plot in Fig. 4 shows the
percentage of CD4+Cx43 and CD8+Cx43 cells in
the spleens from the SHRs and WKY rats. Flow cytometric analysis
revealed the absence of significant statistical differences in the
percentage of CD4+Cx43 or CD8+Cx43 in the
spleens between the SHRs and WKY rats. However, Cx43 surface
expression could not be detected in the SHRs and WKY rats due to
the low percentage of CD4+CD25+ T cells
(Fig. 4).
Role of Gap27 in lymphocyte proliferation
and activation between the SHRs and WKY rats
To confirm the involvement of GJs in hypertensive
inflammation responses, cultured splenic lymphocytes from the SHRs
and WKY rats were treated with ConA (5 μg/ml) alone or ConA
for 24 h following treatment with the Cx43 mimetic peptide, Gap27
(500 μM) for 48 h. The activation of lymphocytes in
vitro was determined by CCK-8 assay. Statistical analysis of
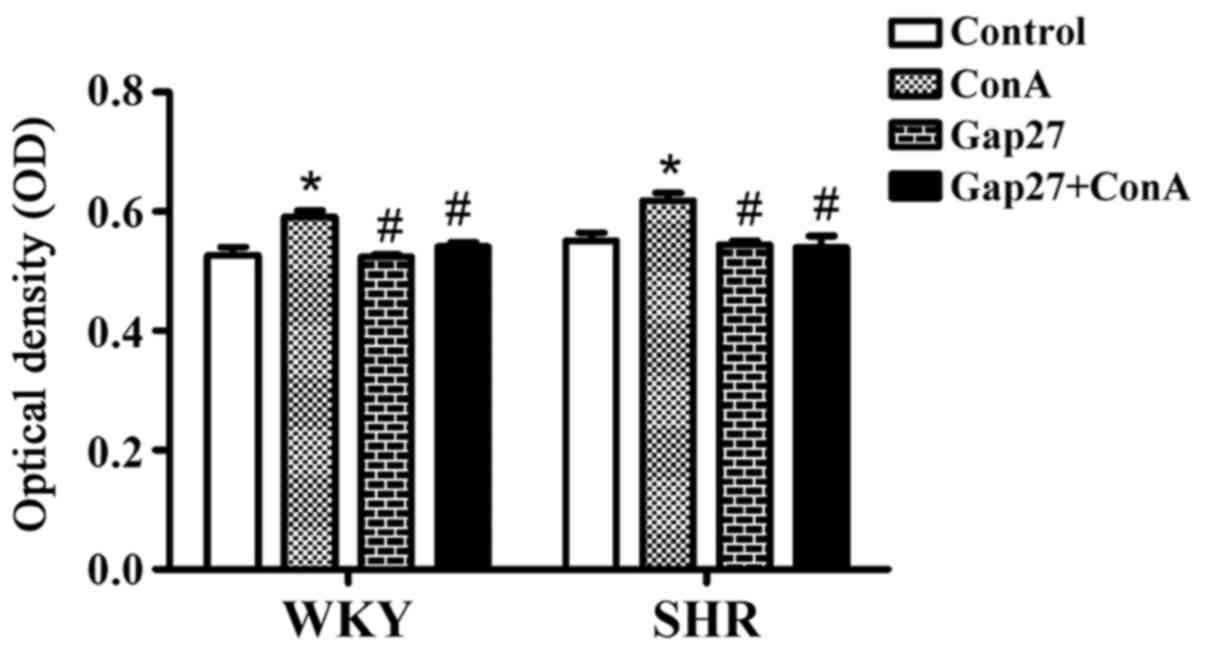
the results is shown in Fig. 5.
In the lymphocytes from the WKY rats, compared with the control
group (0.53±0.01), significant lymphocyte proliferation was
observed in the ConA group (0.59±0.01; p<0.05). Compared to the
ConA group, lymphocyte proliferation was significantly decreased in
the Gap27 group (0.52±0.01) and Gap27 + ConA group (0.54±0.01;
p<0.05). No statistically significant differences were noted
between the Gap27 + ConA and control groups. In the lymphocytes
from the SHRs, significant lymphocyte proliferation was observed in
the ConA group (0.62±0.01) as compared with the control group
(0.55±0.01; p<0.05). The lymphocytes in the Gap27 group
(0.54±0.01) and Gap27 + ConA group (0.54±0.02) exhibited a
significantly decreased proliferation as compared with the ConA
group (p<0.05). By contrast, lymphocyte proliferation following
Gap27 intervention was similar to that of the control group even
after ConA stimulation (p>0.05) (Fig. 5). These data demonstrated that the
proliferation of T lymphocytes may be directly regulated by GJs
during essential hypertension.
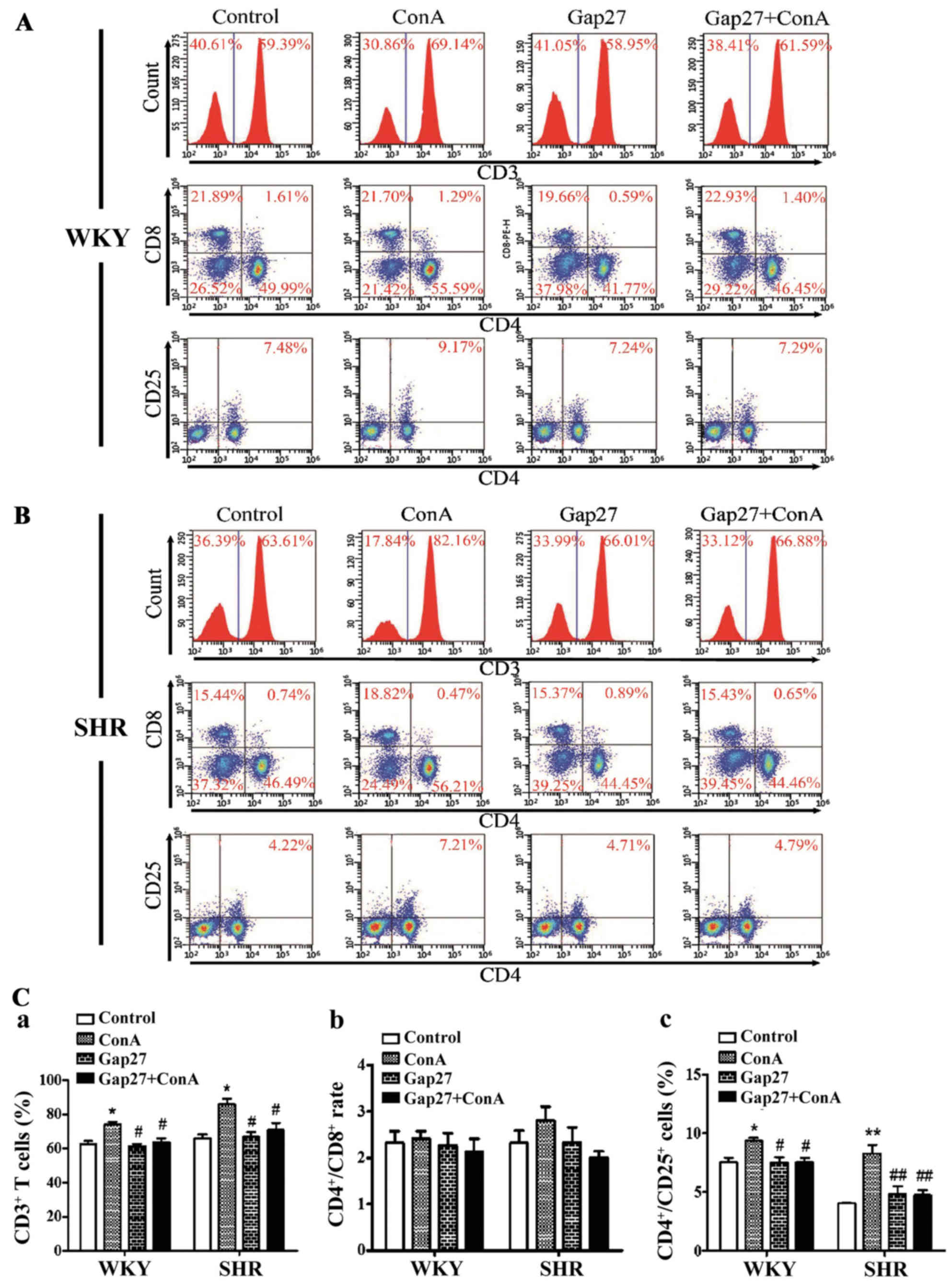
The percentage of T cell subsets was determined for
the SHRs and WKY rats treated with Gap27. In the lymphocytes from
the WKY rats, the percentages of total T cells (CD3+)
and CD4+CD25+ T cells differed significantly
among the 4 groups. The percentages of CD3+ and
CD4+CD25+ T cells were higher in the
ConA-treated lymphocytes than in the control group lymphocytes
(p<0.05) (Fig. 6). Under the
effects of Gap27, the percentages of CD3+ and
CD4+CD25+ T cells in the Gap27 and Gap27 +
ConA groups were significantly decreased compared to those of the
ConA group (p<0.05). However, the percentages of CD3+
and CD4+CD25+ T cells did not differ
significantly between the Gap27 + ConA and control groups. More
specifically, the CD4+/CD8+ ratio was
statistically similar in all comparisons (Fig. 6). In the lymphocytes from the
SHRs, the trends for the percentages of CD3+ and
CD4+CD25+ T cells, as well as the
CD4+/CD8+ ratio, among the 4 groups were
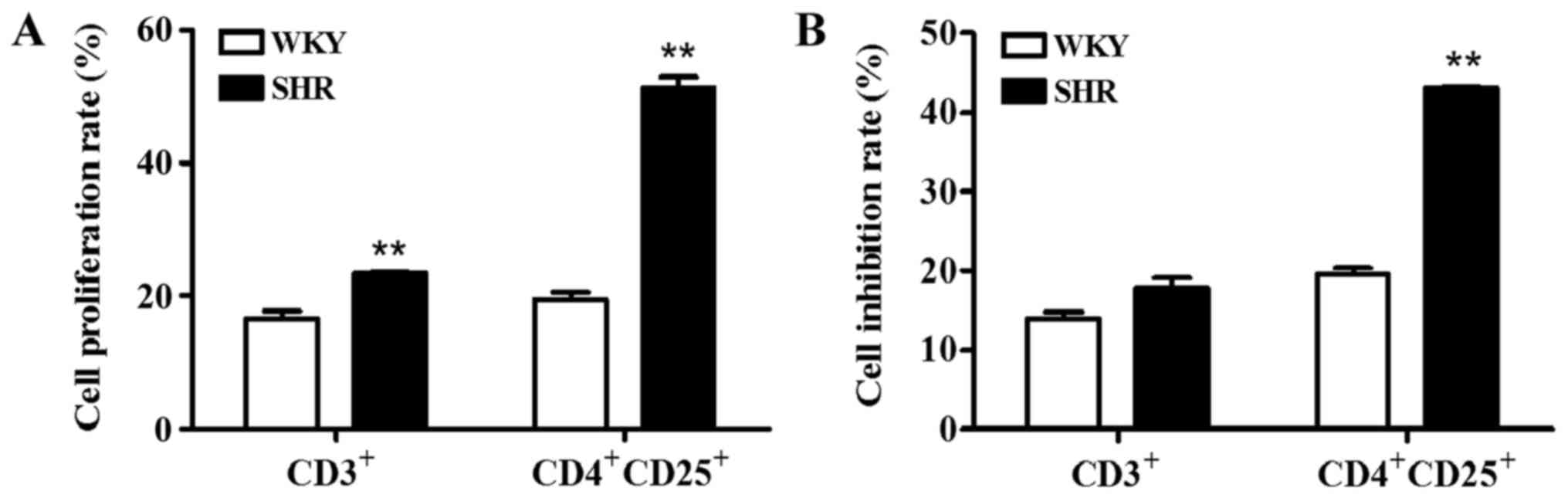
consistent with those of the WKY rats (Fig. 6). In addition, the proliferation
rates of the CD3+ and CD4+CD25+ T
cells, as well as the inhibition rate of the
CD4+CD25+ T cells in the lymphocytes from the
SHRs were higher than those of the WKY rats (p<0.01) (Fig. 7).
The blocking of direct intercellular communications
among adjacent lymphocytes suppresses the synthesis and release of
pro-inflammatory cytokines, such as IFN-γ and IL-2 (17,19). Thus, to further investigate
involvement of Cx43-mediated GJIC in the synthesis of
pro-inflammatory cytokines in changed T cells subsets during
hypertension, the IL-2 and IL-6 cytokine levels were detected
following intervention with Gap27 by RT-qPCR. The results obtained
fro the 4 groups are presented in Table II. In the SHRs and WKY rats, the
IL-2 and IL-6 mRNA expression levels were significantly increased
following ConA stimulation (p<0.05). However, the IL-2 and IL-6
mRNA expression levels decreased following treatment with Gap27 in
the Gap27 and ConA + Gap27 groups (p<0.05). Notably, the IL-2
and IL-6 mRNA expression levels did not differ significantly
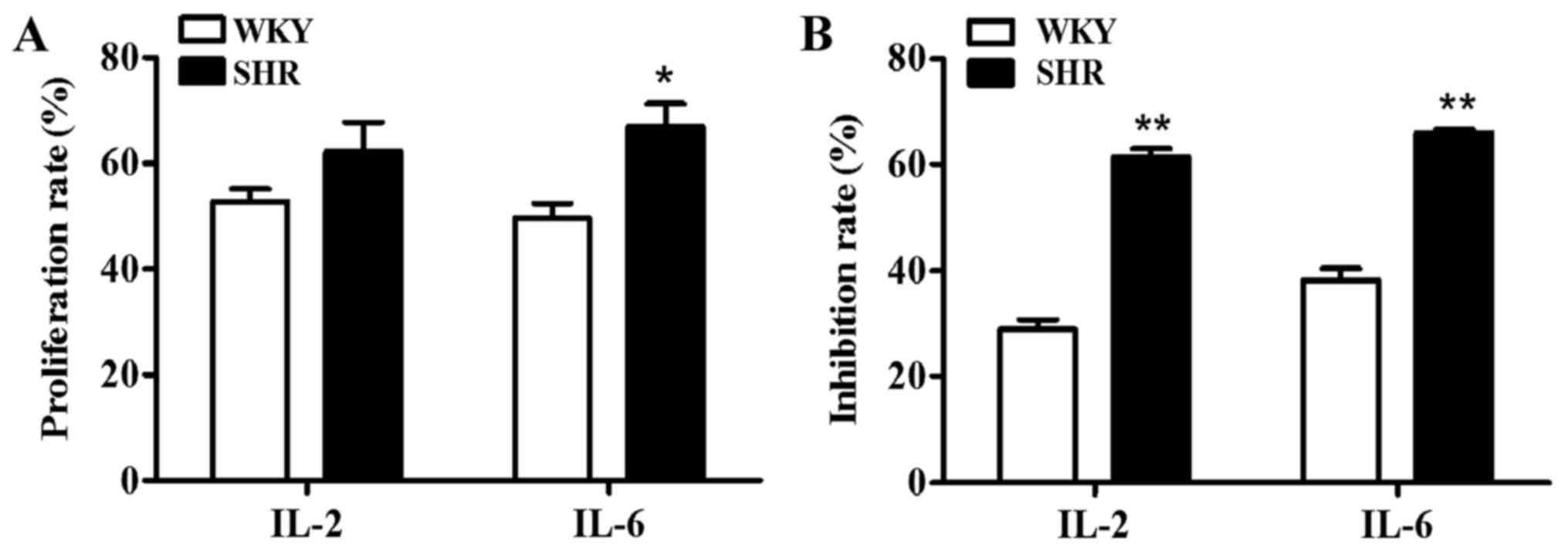
between the Gap27 + ConA and control groups (Table II). In addition, the
proliferation rate of the lymphocytes from the SHRs expressing IL-2
and IL-6 was higher than that of those from the WKY rats
(p<0.05) (Fig. 8). Compared
with the lymphocytes from the WKY rats, the Gap27 + ConA group
lymphocytes from the SHRs exhibited a significant decrease in IL-2
and IL-6 mRNA expression compared with the ConA group (Table II), and thus the inhibition rate
of lymphocytes expressing IL-2 and IL-6 was significantly higher in
the lymphocytes from the SHRs than in those from the WKY rats
(p<0.01) (Fig. 8). This trend
may be explained by the obvious responsiveness of these cytokines
to ConA, such that cytokine secretion was immediately inhibited by
Gap27. The effects of Gap27 also differed in the lymphocytes
obtained from the hypertensive and normotensive rats.
 | Table IIThe expression of IL-2, IL-6 mRNA in
cultured lymphocytes. |
Table II
The expression of IL-2, IL-6 mRNA in
cultured lymphocytes.
| Rats | mRNA | Control | ConA | Gap27 | Gap27+ConA |
|---|
| WKY | IL-2 | 1 | 2.13±0.18a | 0.94±0.10d | 1.52±0.20c |
| IL-6 | 1 | 2.00±0.19a | 1.25±0.15c | 1.24±0.19c |
| SHR | IL-2 | 1 | 2.76±0.69b | 0.99±0.17d | 1.05±0.20c |
| IL-6 | 1 | 3.13±0.69a | 1.08±0.27d | 1.07±0.27c |
Discussion
The findings of the present study demonstrate that
hypertension causes renal and splenic injury by downregulating the
percentage of CD4+CD25+ T cells in secondary
lymphoid organ (i.e., the spleen), and upregulating serum
inflammatory cytokine (IL-2 and IL-6) expression. The kidney plays
an important role in the regulation of water and electrolyte
balance, with various endocrine functions that are closely related
to hypertension. Wang et al reported that elevated systemic
blood pressure accelerated the progression of kidney injury in rats
(20). Gestational hypertension,
particularly in preeclampsia, also causes significant kidney damage
(21). In accordance with
previous studies (3,22,23), significant thickening of the
vascular wall, inflammatory cell infiltration into part of the
blood vessels and glomerular atrophy were observed in the kidneys
of hypertensive rats in this study (Fig. 1). A number of different types of
infiltrating immune cells, such as macrophages, T lymphocytes and B
lymphocytes have been identified in the kidneys of hypertensive
rats (24,25). However, the mechanisms leading to
the infiltration of these inflammatory cells into the kidneys
during hypertension remain to be determined. We speculate that the
infiltration of immune cells in the kidneys of hypertensive rats is
a secondary effect, which may be mediated by a primary increase in
arterial pressure.
As a secondary lymphoid organ and a source of
vasoactive factors, the spleen controls the amount of peripheral
neuroendocrine and immune mediators in the blood, and maintains a
close interaction with the central system via sympathetic
innervation in response to stress (26,27). Spleen removal can induce
hypertension and lead to tissue injury. Spleen re-implantation
reverses the elevation of blood pressure and reducestissue injury
induced by Ang II (28). In the
present study, the spleens from SHRs exhibited central artery wall
thickening and stenosis (Fig. 1).
The mammalian spleen is conventionally considered to be the main
filter for blood-borne pathogens and antigens, and this organ is
also important for maintaining the lymphocyte populations and
immune homeostasis (29). T cells
are involved in the pathophysiology of chronic hypertension and
target organ damage (30–32). However, the association between
hypertensive inflammation and splenic lymphocytes and the
mechanisms implicating immune response in hypertension remain
elusive. In this study, we compared the different lymphocyte
subsets, including T cells (CD3+), T-helper cells
(CD3+CD4+), cytotoxic T cells
(CD3+CD8+) and Treg
(CD4+CD25+) in the spleens between SHRs and
WKY rats. The percentages of CD3+,
CD3+CD4+ and CD3+CD8+ T
cells, as well as the CD4+/CD8+ ratio
exhibited similar trends in the spleens of the SHRs and WKY rats
(Fig. 3). Similar findings have
been reported by Pascual et al in the spleens of SHRs
younger than 12 weeks (33). Treg
populations are decreased; likewise, the adoptive transfer of Treg
cells and their anti-inflammatory effects are prevented in Ang
II-induced hypertension (7,34).
Consistent with the literature, we observed the significantly
decreased number of CD4+CD25+ T cells in the
spleens of SHRs compared with those of WKY rats (Fig. 3). This result suggests that
splenic Treg cells are involved in the pathogenesis of hypertensive
inflammation and are associated with vascular diseases. Our
findings imply that the prevention of regulatory T cell reduction
may be a potential therapeutic technique with which to attenuate
inflammation in hypertension.
Cx43 is ubiquitously expressed in different types of
immune cells (35). The transfer
of information by GJs plays an important role in various immune
response processes. However, the effects of Cx43 in splenic
lymphocytes on inflammation in hypertension are largely unknown. In
our experiments, the level of CD4+Cx43 and
CD8+Cx43 in the spleen did not differ significantly
between the SHRs and WKY rats (Fig.
4). Of note, the expression of Cx43 in CD8+ T cells
was higher than that in CD4+ T cells, thereby indicating
of the differential expression of Cx43 in different lymphocyte
subsets. Of course, we also detected the expression of Cx43 in
CD4+CD25+ T cells; however, the surface
expression of Cx43 was not detected due to the low percentage of
CD4+CD25+ cells. The function of Cx43 in
CD4+CD25+ cells and their relationship
requires further investigation.
A vital role of Cx43 is to mediate the GJs,
regulating key inflammation processes (36). The transmission of signals
mediated by Cx43 depends on hemichannels-independent and
channel-independent mechanisms through GJs (37). It has also been shown that the
upregulation of Cx43 expression can promote the activation of
CD4+ T lymphocytes (10,16,38). Given that arterial hypertension
activates T lymphocytes (1), ConA
is used as a T cell activator that induces various cytokine
secretions (39,40). In addition, to confirm the
involvement of GJs in lymphocyte proliferation after activation,
lymphocytes were treated with a Cx extracellular loop mimetic
peptide (Gap27), which can restrictintercellular communication
across GJs and reduce or block Cx hemichannel function in
CD4+ T cells and other cell systems (i.e., non-diabetic
cells) (16,41). The results revealed that ConA
significantly increased the proliferation of lymphocytes from WKY
rats and SHRs compared with the controls. Gap27 significantly
inhibited the ConA-stimulated proliferative response of
lymphocytes, whereas no significant differences were noted between
the Gap27 + ConA and Gap27 groups (Fig. 5). Second, the proliferative
response of CD3+ and CD4+CD25+
lymphocytes from the WKY rats and SHRs were significantly inhibited
by Gap27 as compared with the ConA group. No significant
differences were observed for the CD4+/CD8+
ratio in all comparisons (Fig.
6). The proliferation rates of CD3+ and
CD4+CD25+ T cells, as well as the inhibition
rate of CD4+CD25+ T cells, were higher in the
lymphocytes from the SHRs than those from the WKY rats (Fig. 7). We mainly focused on the
proportion of the different cell subsets in these in vitro
stimulation experiments, and the absolute cell numbers were not
considered. Therefore, variations in the percentages of cell
subsets cannot exclude the possible loss or increased cell survival
during the cell culture. The results indicated that the
Cx43-induced mediation of GJ channels or hemichannels may be
involved in the proliferation of lymphocyte subsets, particularly
in CD4+CD25+ T cells. The precise mechanisms
by which Cx43 regulate the proliferative response of lymphocyte
subsets remain unknown.
IL-2, is a Th1 cytokine which participates in the
inflammatory responses, antitumor activity and anti-graft
rejection. Elevated IL-6 levels also contribute to hypertension in
pregnant rats with chronic reductions in uterine perfusion
(42). As previously
demonstrated, Ang II-mediated hypertension was attenuated in
IL-6-knockout mice (43–45). Knocking down IL-6 by RNA
interference has been shown to block cold-induced hypertension
(46). Luther et al
demmonstrated that Ang II induced IL-6 expression via a
mineralocorticoid receptor-dependent mechanism (47). These findings strongly support the
essential role of IL-2 and IL-6 in the development of hypertension.
Kumral et al reported that renovascular hypertension induced
the elevation of IL-2 and IL-6 in serum (48). In the present study, the
concentration of IL-2 and IL-6 significantly increased in the serum
from SHRs compared with that from WKY rats (Fig. 2), thereby verifying that
hypertension is chronic low-grade inflammation. The interruption of
GJIC between lymphocytes or blocking of Cx43 hemichannel causes the
reduced synthesis and release of cytokines, such as IFN-γ, IL-2 and
IL-10 (49). Mendoza-Naranjo
et al demonstrated that Cx43 regulated IFN-γ secretion, but
the blockade of the Cx43-GJ function obviously decreased IFN-γ
secretion by the primed T cells (15). Cytokines also positively regulate
Cx expression in immune cells (14,50), suggesting the bidirectional
regulation between GJs and cytokines. In our study, a marked
decrease in IL-2 and IL-6 mRNA expression after Gap27 treatment was
observed (Table II), which
suggests the inhibitory effect of Gap27 on pro-inflammatory
cytokine production. These data are consistent with those of recent
study by Oviedo-Orta et al (16). The study by Oviedo-Orta
et al indicated that Cx43 is a vital functional component of
the immunological synapse between DCs and T lymphocytes (16). However, there were differences in
hypertensive and normotensive rats with the action of ConA and
Gap27 (Fig. 8). These results
clearly demonstrate that Cx mimetic peptide Gap27 inhibits the
ConA-induced lymphocyte activation and proliferative response in
vitro. The proliferation and activation of T lymphocytes and
the synthesis and secretion of pro-inflammatory cytokines are
regulated by intracellular Ca2+ levels (51). The increase in intracellular
Ca2+ is a key step in the cascade of signals that
results in T cell proliferation (52). More recently, Mendoza-Naranjo
et al reported that the impaired Ca2+ signals
result from GJs blocking in DC-T cell reduced lymphocyte activation
and IFN-γ secretion. This finding suggests that Ca2+
signals, which are regulated by GJs, may be some of the important
mechanisms that control T cell activation and proinflammatory
cytokines levels (17).
Therefore, it is conceivable that Gap27 is likely to block CxHc and
thus blocks ATP release and transfer of second messengers such as
Ca2+ entry (49,53). Further studies are required to
demonstrate this hypothesis. Above all, these findings indicate
that GJIC may regulate the production of inflammatory cytokines
after lymphocyte activation.
In conclusion, our results suggest that splenic
Tregs may be involved in the suppression of inflammation in
hypertension. Cx43 may serve as an important functional component
in the modulation of the pro-inflammatory. The action of ConA and
Gap27 differed in hypertensive and normotensive rats. The
mechanisms by which Gap27 is involved in the control of lymphocyte
activation have not yet been established and elucidated. The
functional interplays between GJs, cytokines and signaling pathways
in Treg-mediated immunosuppressive properties are of important
interest. Further studies are warranted to further investigate the
interplay between the immune response and the expression of Cx43;
some non-specific inhibitor such as niflumic acid (NFA) and
18β-glycyrrhetinic acid affecting Cx43 expression can be used to
analyze whether the alteration of Cx43 expression will affect
hypertensive inflammatory response in vivo or in
vitro. Further studies using Cx43 tissue-specific gene knockout
mutants may also provide useful tools with which to discern the
contribution of Cx to T cell activation. In addition, further
investigations into the role of Cxs and the electrophysiological
characteristics of GJs in Treg-mediated immune response suppression
and effector T cell activation may lead to the discovery of novel
immune therapeutic targets for the treatment of hypertension.
Acknowledgments
This study was supported by grants from the
National Natural Science Foundation of China (nos. 81660271,
31260247 and 31460264 to Ke-Tao Ma; no. 81460098 to Xin-Zhi Li; no.
81560081 to Jun-Qiang Si and no. 81260159 to Li Li). The authors
would like to thank the cooperation of Dr Jin-Yao Li and Mr. Yong
Ji (DingJu Biotechnology Co., Ltd., Xinjiang, China) for their
guidance in the experimental techniques.
References
|
1
|
Harrison DG, Vinh A, Lob H and Madhur MS:
Role of the adaptive immune system in hypertension. Curr Opin
Pharmacol. 10:203–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guzik TJ, Hoch NE, Brown KA, McCann LA,
Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG: Role of
the T cell in the genesis of angiotensin II induced hypertension
and vascular dysfunction. J Exp Med. 204:2449–2460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crowley SD, Song YS, Lin EE, Griffiths R,
Kim HS and Ruiz P: Lymphocyte responses exacerbate angiotensin
II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol.
298:R1089–R1097. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muller DN, Kvakan H and Luft FC:
Immune-related effects in hypertension and target-organ damage.
Curr Opin Nephrol Hypertens. 20:113–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rudemiller N, Lund H, Jacob HJ, Geurts AM,
Mattson DL and PhysGen Knockout Program: CD247 modulates blood
pressure by altering T-lymphocyte infiltration in the kidney.
Hypertension. 63:559–564. 2014. View Article : Google Scholar
|
|
6
|
Kvakan H, Kleinewietfeld M, Qadri F, Park
JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, et
al: Regulatory T cells ameliorate angiotensin II-induced cardiac
damage. Circulation. 119:2904–2912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barhoumi T, Kasal DA, Li MW, Shbat L,
Laurant P, Neves MF, Paradis P and Schiffrin EL: T regulatory
lymphocytes prevent angiotensin II-induced hypertension and
vascular injury. Hypertension. 57:469–476. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Madhur MS, Lob HE, McCann LA, Iwakura Y,
Blinder Y, Guzik TJ and Harrison DG: Interleukin 17 promotes
angiotensin II-induced hypertension and vascular dysfunction.
Hypertension. 55:500–507. 2010. View Article : Google Scholar :
|
|
9
|
Zhang W, Wang W, Yu H, Zhang Y, Dai Y,
Ning C, Tao L, Sun H, Kellems RE, Blackburn MR, et al: Interleukin
6 underlies angiotensin II-induced hypertension and chronic renal
damage. Hypertension. 59:136–144. 2012. View Article : Google Scholar
|
|
10
|
Bopp T, Becker C, Klein M, Klein-Hessling
S, Palmetshofer A, Serfling E, Heib v, Becker M, Kubach J, Schmitt
S, et al: Cyclic adenosine monophosphate is a key component of
regulatory T cell-mediated suppression. J Exp Med. 204:1303–1310.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hervé JC, Bourmeyster N, Sarrouilhe D and
Duffy HS: Gap junctional complexes: From partners to functions.
Prog Biophys Mol Biol. 94:29–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Evans WH and Martin PE: Gap junctions:
Structure and function (Review). Mol Membr Biol. 19:121–136. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oviedo-Orta E and Howard Evans W: Gap
junctions and connexin-mediated communication in the immune system.
Biochim Biophys Acta. 1662:102–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsue H, Yao J, Matsue K, Nagasaka A,
Sugiyama H, Aoki R, Kitamura M and Shimada S: Gap junction-mediated
intercellular communication between dendritic cells (DCs) is
required for effective activation of DCs. J Immunol. 176:181–190.
2006. View Article : Google Scholar
|
|
15
|
Mendoza-Naranjo A, Saéz PJ, Johansson CC,
Ramírez M, Mandakovic D, Pereda C, López MN, Kiessling R, Sáez JC
and Salazar-Onfray F: Functional gap junctions facilitate melanoma
antigen transfer and cross-presentation between human dendritic
cells. J Immunol. 178:6949–6957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oviedo-Orta E, Perreau M, Evans WH and
Potolicchio I: Control of the proliferation of activated
CD4+ T cells by connexins. J Leukoc Biol. 88:79–86.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mendoza-Naranjo A, Bouma G, Pereda C,
Ramírez M, Webb KF, Tittarelli A, López MN, Kalergis AM, Thrasher
AJ, Becker DL, et al: Functional gap junctions accumulate at the
immunological synapse and contribute to T cell activation. J
Immunol. 187:3121–3132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rodríguez-Iturbe B, Quiroz Y, Nava M,
Bonet L, Chávez M, Herrera-Acosta J, Johnson RJ and Pons HA:
Reduction of renal immune cell infiltration results in blood
pressure control in genetically hypertensive rats. Am J Physiol
Renal Physiol. 282:F191–F201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bermudez-Fajardo A, Ylihärsilä M, Evans
WH, Newby AC and Oviedo-Orta E: CD4+ T lymphocyte subsets express
connexin 43 and establish gap junction channel communication with
macrophages in vitro. J Leukoc Biol. 82:608–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Johnson AC, Sasser JM, Williams
JM, Solberg Woods LC and Garrett MR: Spontaneous one-kidney rats
are more susceptible to develop hypertension by DOCA-NaCl and
subsequent kidney injury compared with uninephrectomized rats. Am J
Physiol Renal Physiol. 310:F1054–F1064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Craici IM, Wagner SJ, Weissgerber TL,
Grande JP and Garovic VD: Advances in the pathophysiology of
pre-eclampsia and related podocyte injury. Kidney Int. 86:275–285.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodríguez-Iturbe B, Pons H, Quiroz Y and
Johnson RJ: The immunological basis of hypertension. Am J
Hypertens. 27:1327–1337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McMaster WG, Kirabo A, Madhur MS and
Harrison DG: Inflammation, immunity, and hypertensive end-organ
damage. Circ Res. 116:1022–1033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alvarez V, Quiroz Y, Nava M, Pons H and
Rodríguez-Iturbe B: Overload proteinuria is followed by
salt-sensitive hypertension caused by renal infiltration of immune
cells. Am J Physiol Renal Physiol. 283:F1132–F1141. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bravo Y, Quiroz Y, Ferrebuz A, vaziri ND
and Rodríguez-Iturbe B: Mycophenolate mofetil administration
reduces renal inflammation, oxidative stress, and arterial pressure
in rats with lead-induced hypertension. Am J Physiol Renal Physiol.
293:F616–F623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamza SM and Kaufman S: Role of spleen in
integrated control of splanchnic vascular tone: Physiology and
pathophysiology. Can J Physiol Pharmacol. 87:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liezmann C, Stock D and Peters EM: Stress
induced neuroendocrine-immune plasticity: A role for the spleen in
peripheral inflammatory disease and inflammaging.
Dermatoendocrinol. 4:271–279. 2012. View Article : Google Scholar
|
|
28
|
Carnevale D, Pallante F, Fardella V,
Fardella S, Iacobucci R, Federici M, Cifelli G, De Lucia M and
Lembo G: The angiogenic factor PlGF mediates a neuroimmune
interaction in the spleen to allow the onset of hypertension.
Immunity. 41:737–752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mebius RE and Kraal G: Structure and
function of the spleen. Nat Rev Immunol. 5:606–616. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harrison DG, Guzik TJ, Lob HE, Madhur MS,
Marvar PJ, Thabet SR, Vinh A and Weyand CM: Inflammation, immunity,
and hypertension. Hypertension. 57:132–140. 2011. View Article : Google Scholar
|
|
31
|
Quiroz Y, Johnson RJ and Rodríguez-Iturbe
B: The role of T cells in the pathogenesis of primary hypertension.
Nephrol Dial Transplant. 27(Suppl 4): iv2–iv5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schiffrin EL: Immune mechanisms in
hypertension and vascular injury. Clin Sci (Lond). 126:267–274.
2014. View Article : Google Scholar
|
|
33
|
Pascual VH, Oparil S, Eldridge JH, Jin H,
Bost KL and Pascual DW: Spontaneously hypertensive rat: lymphoid
depression is age dependent and mediated via a mononuclear cell
subpopulation. Am J Physiol. 262:R1–R7. 992PubMed/NCBI
|
|
34
|
Matrougui K, Abd Elmageed Z, Kassan M,
Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P,
Belmadani S and Partyka M: Natural regulatory T cells control
coronary arteriolar endothelial dysfunction in hypertensive mice.
Am J Pathol. 178:434–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Neijssen J, Pang B and Neefjes J: Gap
junction-mediated inter-cellular communication in the immune
system. Prog Biophys Mol Biol. 94:207–218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Montecino-Rodriguez E, Leathers H and
Dorshkind K: Expression of connexin 43 (Cx43) is critical for
normal hematopoiesis. Blood. 96:917–924. 2000.PubMed/NCBI
|
|
37
|
Kuczma M, Lee JR and Kraj P: Connexin 43
signaling enhances the generation of Foxp3+ regulatory T cells. J
Immunol. 187:248–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oviedo-Orta E, Hoy T and Evans WH:
Intercellular communication in the immune system: Differential
expression of connexin40 and 43, and perturbation of gap junction
channel functions in peripheral blood and tonsil human lymphocyte
subpopulations. Immunology. 99:578–590. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lombardi VR, Martínez E, Chacón R,
Etcheverría I and Cacabelos R: Effects of FR-91 on immune cells
from healthy individuals and from patients with non-Hodgkin
lymphoma. J Biomed Biotechnol. 187015:2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin CM, Jeng CR, Hsiao SH, Lee Y, Tsai YC,
Chia MY and Pang VF: Monocyte-derived dendritic cells enhance cell
proliferation and porcine circovirus type 2 replication in
concanavalin A-stimulated swine peripheral blood lymphocytes in
vitro. Vet Immunol Immunopathol. 145:368–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pollok S, Pfeiffer AC, Lobmann R, Wright
CS, Moll I, Martin PE and Brandner JM: Connexin 43 mimetic peptide
Gap27 reveals potential differences in the role of Cx43 in wound
repair between diabetic and non-diabetic cells. J Cell Mol Med.
15:861–873. 2011. View Article : Google Scholar
|
|
42
|
Gadonski G, LaMarca BB, Sullivan E,
Bennett W, Chandler D and Granger JP: Hypertension produced by
reductions in uterine perfusion in the pregnant rat: Role of
interleukin 6. Hypertension. 48:711–716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee DL, Sturgis LC, Labazi H, Osborne JB
Jr, Fleming C, Pollock JS, Manhiani M, Imig JD and Brands MW:
Angiotensin II hypertension is attenuated in interleukin-6 knockout
mice. Am J Physiol Heart Circ Physiol. 290:H935–H940. 2006.
View Article : Google Scholar
|
|
44
|
Coles B, Fielding CA, Rose-John S,
Scheller J, Jones SA and O'Donnell VB: Classic interleukin-6
receptor signaling and interleukin-6 trans-signaling differentially
control angiotensin II-dependent hypertension, cardiac signal
transducer and activator of transcription-3 activation, and
vascular hypertrophy in vivo. Am J Pathol. 171:315–325. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brands MW, Banes-Berceli AK, Inscho EW,
Al-Azawi H, Allen AJ and Labazi H: Interleukin 6 knockout prevents
angiotensin II hypertension: Role of renal vasoconstriction and
janus kinase 2/signal transducer and activator of transcription 3
activation. Hypertension. 56:879–884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Crosswhite P and Sun Z: Ribonucleic acid
interference knockdown of interleukin 6 attenuates cold-induced
hypertension. Hypertension. 55:1484–1491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Luther JM, Gainer JV, Murphey LJ, Yu C,
Vaughan DE, Morrow JD and Brown NJ: Angiotensin II induces
interleukin-6 in humans through a mineralocorticoid
receptor-dependent mechanism. Hypertension. 48:1050–1057. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kumral ZN, Sener G, Ozgur S, Koc M,
Suleymanoglu S, Hurdag C and Yegen BC: Regular exercise alleviates
renovascular hypertension-induced cardiac/endothelial dysfunction
and oxidative injury in rats. J Physiol Pharmacol. 67:45–55.
2016.PubMed/NCBI
|
|
49
|
Oviedo-Orta E, Gasque P and Evans WH:
Immunoglobulin and cytokine expression in mixed lymphocyte cultures
is reduced by disruption of gap junction intercellular
communication. FASEB J. 15:768–774. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Eugenín EA, Brañes MC, Berman JW and Sáez
JC: TNF-alpha plus IFN-gamma induce connexin43 expression and
formation of gap junctions between human monocytes/macrophages that
enhance physiological responses. J Immunol. 170:1320–1328. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vogel SZ, Schlickeiser S, Jürchott K,
Akyuez L, Schumann J, Appelt C, Vogt K, Schröder M, Vaeth M,
Berberich-Siebelt F, et al: TCAIM decreases T cell priming capacity
of dendritic cells by inhibiting TLR-induced Ca2+ influx and IL-2
production. J Immunol. 194:3136–3146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Litvinov IS and Mersiyanova IV: The role
of extracellular calcium ions reduction in T cell activation in
human peripheral blood. Bioorg Khim. 41:432–442. 2015.PubMed/NCBI
|
|
53
|
Ring S, Karakhanova S, Johnson T, Enk AH
and Mahnke K: Gap junctions between regulatory T cells and
dendritic cells prevent sensitization of CD8(+) T cells. J Allergy
Clin Immunol. 125:237–246. e1–e7. 2010. View Article : Google Scholar : PubMed/NCBI
|