Introduction
Fibromyalgia (FM) is characterized by generalized
tenderness in ≥11 of 18 tender points (1) and chronic widespread pain that lasts
>3 months (2). Mechanical
hyperalgesia is a common symptom of FM (3), which is a painful syndrome of
largely unknown etiology and pathology that is often accompanied by
various phenomena. In addition, emerging evidence has indicated
that pain amplification within the central nervous system serves an
important role in the pathology of FM-associated pain (4), which is associated with numerous
other symptoms (5). The symptoms
of this painful syndrome include fatigue (6), anxiety (7), sleep disturbance (8), temporomandibular disease and
depression (9). An animal model
of FM must include widespread pain and associated symptoms of
fatigue and psychological disturbance (3).
There are numerous animal models of FM pain, which
are induced by either intramuscular injection of acidic saline
(10), vagotomy (11), sound stress (12) or depletion of biogenic amines
(13). A previous study described
a novel generalized chronic pain or FM-like mouse model as part of
a pharmacological study. This FM model was induced by intermittent
cold stress (ICS), which is useful for inducing abnormal pain
(14,15). Xu et al previously revealed
that complex interactions exist between pain and depression
(16). Previous studies have
suggested that FM is associated with emotional disorders, including
depression (17); ≥30% of
patients with chronic pain have major depression, and 30% are
diagnosed with panic and diffuse anxiety disorder (18).
Brain-derived neurotrophic factor (BDNF) is an
upstream activator and a downstream target of cAMP response
element-binding protein (CREB)-mediated signaling (19) CREB activity is tightly regulated
by its phosphorylation at serine 133 (20); therefore, it would be useful to
analyze BDNF and phosphorylated (p)-CREB levels following identical
treatments (21). Furthermore,
the BDNF/tropomyosin receptor kinase B-mediated signaling pathway
within the spinal cord may be involved in the induction of
neuropathic pain. In a previous study, treatment with a selective
N-methyl-D-aspartate (NMDA)-2B receptor antagonist completely
blocked BDNF-induced mechanical allodynia in animals (22). BDNF may play a protective role in
FM, including pain modulation and mental disorders, such as
depression. In addition, it has been suggested that the expression
of BDNF is a downstream target of various antidepressants (23) and BDNF is an important candidate
gene in antidepressant medication (24). In addition, the principal
treatment for depression consists of pharmacotherapy with selective
serotonin reuptake inhibitors (25), including Cymbalta (duloxetine),
which is a Food and Drug Administration-approved drug for the
treatment of FM (26).
Valeriana fauriei Briq. (VF) is a perennial
herb found in all of North America, most parts of Europe, and
Northern Asia (27). The genus
Valeriana contains >250 species with many subspecies
containing medicinal plants (28). VF has been used for many years in
China and Korea (29), and
contains 150–200 chemical constituents of biologically active
components that can be divided into volatile oils, epoxy iridoid
esters and alkaloids (30). These
components have been reported to inhibit γ-aminobutyric acid (GABA)
re-uptake (31) The genus
Valeriana has been widely used in popular medicine for
centuries to treat sleep disorders, anxiety and epilepsy. It can
also modulate insomnia by interacting with various neurotransmitter
systems (32). Previous studies
have focused on the novel pharmacological effects of VF species in
various human diseases. Some studies have attempted to explain the
pharmacological functions of VF, particularly with regards to its
neuroprotective effects in neurodegenerative diseases (33–35), and its ability to reduce pain,
cyclic cramps and stress (36,37).
These previous findings have resulted in the
hypothesis that VF may exert beneficial effects on FM with
depression. Therefore, in the present study, the behavior of mice
and the protein levels in the medial prefrontal cortex and
hippocampus of a mouse model of FM were examined, in order to
determine whether treatment with VF reverses any changes.
Materials and methods
Animals
Male adult C57BL/6J mice were obtained from Daehan
Biolink, Co., Ltd. (Eumseong, South Korea). All groups contained
between 12 and 14 mice with similar numbers of males: the 'control'
(n=12 males) group; the drug non-treated model group 'FMS' (n=14
males); and the VF administration model group (n=14 males). Mice
were housed in clear cages with access to free food and water, and
were maintained in an environment with the temperature-controlled
to 23±2°C, humidity-controlled to 50–60% and under a 12-h
light-dark cycle (lights were turned on at 6:30 a.m.). All animal
procedures were performed in accordance with the Guidelines for the
Care and Use of Laboratory Animals of the US National Institutes of
Health (38). The present study
was approved by the Animal Care and Use Committee of Soonchunhyang
University (Cheonan, South Korea; SCH16-0062).
Drugs and treatments
VF extract was purchased from Yunpung (Eumsung,
Chungbuk, South Korea), and the specimens were identified
taxonomically by an Oriental medicine physician at the National
Institute of Horticultural and Herbal Science, Rural Development
Administration (Eumsung, South Korea). The voucher specimen
(HPR-207) was deposited in the herbarium of the Herbal Crop
Research Institute (Eumsung, South Korea). VF was dissolved in tap
water to a concentration of 100 mg/kg/day. The animals were orally
administered VF solution during stress for 24 days (39).
Experimental model of FM
An animal model of FM was constructed as previously
described, with slight modification (40,41). For the chronic restraint stress
(CRS) and intermittent cold stress (ICS) paradigm, the mice were
restrained for 6 h (between 12 a.m. and 6 p.m.) daily in
well-ventilated 50-ml conical tubes and were deprived of food and
water. Control mice remained undisturbed in their cages. The
protocol was scheduled for 21 days. For the ICS paradigm, mice were
placed on a stainless steel mesh plate in a cold room at 4°C
overnight (between 4:30 p.m. and 10:00 a.m.), followed by ICS with
experimental temperatures alternating between 24 and 4°C every 30
min, between 10:00 a.m. and 4:30 p.m. These procedures were
repeated for 2 days. On day 3, the mice were adapted to 24°C for 1
h prior to behavioral analysis. Control mice were maintained at
24°C for the 3 days (from 4:30 p.m. on day 1 to 10:00 a.m. on day
3) (15).
Behavioral analysis
Behavioral assessments were conducted 1 day after
the final day of ICS (n=10 mice/group). Mice were allowed to
acclimate to the testing room for ≥1 h prior to the assessments.
Tests for nociception and depression were performed. A tail flick
test (TFT), a plantar test (PTL), and the von Frey test paw
withdrawal threshold (PWT) test were used to assess nociception.
Subsequently, a tail suspension test (TST) was used to assess
depression. For these thermal and mechanical tests, thresholds were
determined from three repeated challenges at 15 min intervals, and
the averages were used for statistical analysis.
TFT
A TFT is used as a test of pain response, which can
be used to assess the antinociceptive effects of drugs by measuring
the latency time from the onset of radiant heat exposure to
withdrawal of the tail (42,43). The thermal pain threshold of the
tail was measured using tail flick apparatus (IITC Life Science
Inc., Woodland Hills, CA, USA). Each animal was gently restrained
under a 50-ml conical tube with light manual pressure so as to
minimize stress. Radiant heat was applied to the tail (2 cm distal
part) and latency (seconds) was determined as the time taken for
the tail to flick away from the radiant heat source. A cutoff time
of 20 sec of radiant heat application was applied to avoid tissue
damage to the tail. Each mouse was tested three times at each
time-point, with an interval of 15 min between replicates. The
average of three replicates was calculated to obtain tail
withdrawal latency.
PTL
In the thermal paw withdrawal test, nociceptive
threshold is assessed by determining the latency of paw withdrawal
upon thermal stimulus (44,45). Mice were placed in individual
clear plastic chambers on top of a glass sheet and were acclimated
for 1 h. Radiant heat (IITC Life Science Inc.) was positioned under
the glass sheet, and the focus of the projection bulb was aimed
exactly at the plantar surface of the hind paw of the animal. Paw
withdrawal latency (PWL), defined as the first occurrence of
licking the hind paw, was scored. Each mouse was tested three times
at each time-point, with an interval of 15 min between replicates.
The average of three replicates was calculated to obtain PWL.
Von Frey PWT test
The pressure required to induce a flexor response
was defined as the pain threshold. The Von Frey PWT test was
conducted using digital von Frey apparatus (Aesthesiometer; IITC
life Sciences Inc.), as previously reported (46,47). Mice were placed in a plastic
chamber on a wire mesh grid floor and were allowed to acclimate for
1 h. In this experiment, the threshold of a given pressure with a
rigid tip used to induce paw withdrawal behavior was evaluated. To
prevent tissue damage, the interval time was set at at least 5 min
for each paw.
TST
The TST is a widely used model for the assessment of
depression-like behaviors in mice. In this test, mice are subjected
to short-term, inescapable stress by being suspended by the tail
resulting in the development of immobility. In the present study,
the total duration of tail suspension-induced immobility was
measured according to Steru et al (44). Mice were suspended 50 cm above the
floor using adhesive tape placed ~1 cm from the tip of the tail and
the total duration of immobility during a 6-min period was
measured.
Measurement of corticosterone
Following completion of the behavioral analyses, the
mice were placed in 50-ml conical tubes for 60 min (n=6
mice/group). The animals for analysis were sacrificed by
decapitation using a guillotine using a decapicone (48). Trunk blood was immediately
collected in plastic tubes and was centrifuged at 15,814 × g at
room temperature; the serum was placed in a fresh tube. Serum
corticosterone levels were determined by immunoassay using the
Cortisol ELISA kit (cat no. 500360; Cayman Chemical, Ann Arbor, MI,
USA) Assays were conducted according to the manufacturer's
protocol.
Western blot analysis
mPFC and hippocampal tissues were lysed in mixture
solution as radioimmunoprecipitation assay buffer [RIPA and
EBA-1149 (Elpis Biotech, Inc., Daejeon, South Korea) and leupeptin
and sodium fluoride (cat. nos. 103476-89-7 and 7681-49-4,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany], and were
centrifuged at 18,341 × g for 10 min at 4°C (n=6–7 mice/group).
Protein concentration was calculated using a standard Bradford
protein assay. These samples (100 μg) were separated by 15%
SDS-PAGE and were transferred to a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). After blocking with
5% skim milk for 1h at room temperature, the membranes were probed
with the following antibodies overnight at 4°C: Anti-p-CREB
(1:1,000; #9198), anti-CREB (1:1,000; #9197) (both from Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-BDNF (1:3,000;
ab108319; Abcam, Cambridge, UK) and anti-β-tubulin (1:3,000;
MA5-16308; Thermo Fisher Scientific, Inc., Rockford, IL, USA).
Subsequently, the membrane was incubated with peroxidase-conjugated
anti-mouse secondary antibody (1:10,000; A9044; Sigma-Aldrich;
Merck KGaA) and goat anti-rabbit IgG-HRP antibody (1:5,000;
LF-SA8002; Abfrontier, Seoul, Korea) for 1 h at room temperature.
Immunoreactive bands were detected using an enhanced
chemiluminescence kit (Elpis Biotech, Inc.). Semi-quantitative
analysis of p-CREB, CREB, BDNF and β-tubulin protein expression was
conducted using ImageJ software version 1.51k (National Institutes
of Health, Bethesda, MD, USA).
Immunohistochemistry
The mice for immunohistochemical analysis had been
anaesthetized with diethyl ether and were perfused through the left
cardiac ventricle with 4% paraformaldehyde (n=4–5 mice/group). The
fixed brains were removed, frozen and cut into 30-μm
sections (n=4 mice/group). Frozen sections from the hippocampus
were treated with 0.3% hydrogen peroxide for 5 min, blocked with
normal horse serum (S-2000; Vector Laboratories, Inc., Burlingame,
CA, USA), and were incubated with anti-p-CREB (1:800; #9198),
anti-CREB (1:800; #9197) (both Cell Signaling Technology, Inc.).
Subsequently, sections were incubated with Cy3-conjugated
anti-rabbit (111-165-003) and anti-mouse (715-545-151) secondary
antibodies (1:500; Jackson ImmunoResearch Laboratories, Inc., west
Grove, PA, USA). After washing in PBS, sections were stained with
DAPI to identify nuclei. Fluorescent images were captured using a
confocal laser-scanning microscope (FV10-ASW; Olympus Corporation,
Tokyo, Japan), and images were semi-quantified with ImageJ software
version 1.51k using a protocol described previously with slight
modifications (49).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean, and assessed using one-way analysis of variance (ANOVA)
with subsequent Tukey's tests. All statistical analyses were
performed using IBM SPSS Statistics 19 software (IBM Corp., Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
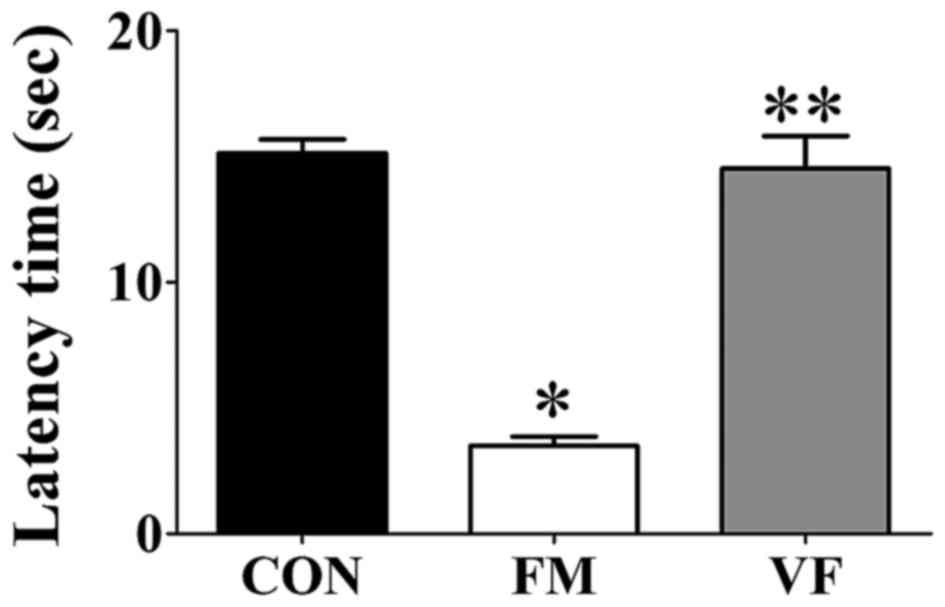
Tail-flick latency
Tail-flick latency was significantly different among
the control, FM and VF-administered FM groups (Fig. 1). The FM group exhibited decreased
tail-flick latency compared with the control group (Fig. 1). Conversely, the FM-induced
decrease in tail-flick latency was attenuated following VF
administration (Fig. 1).
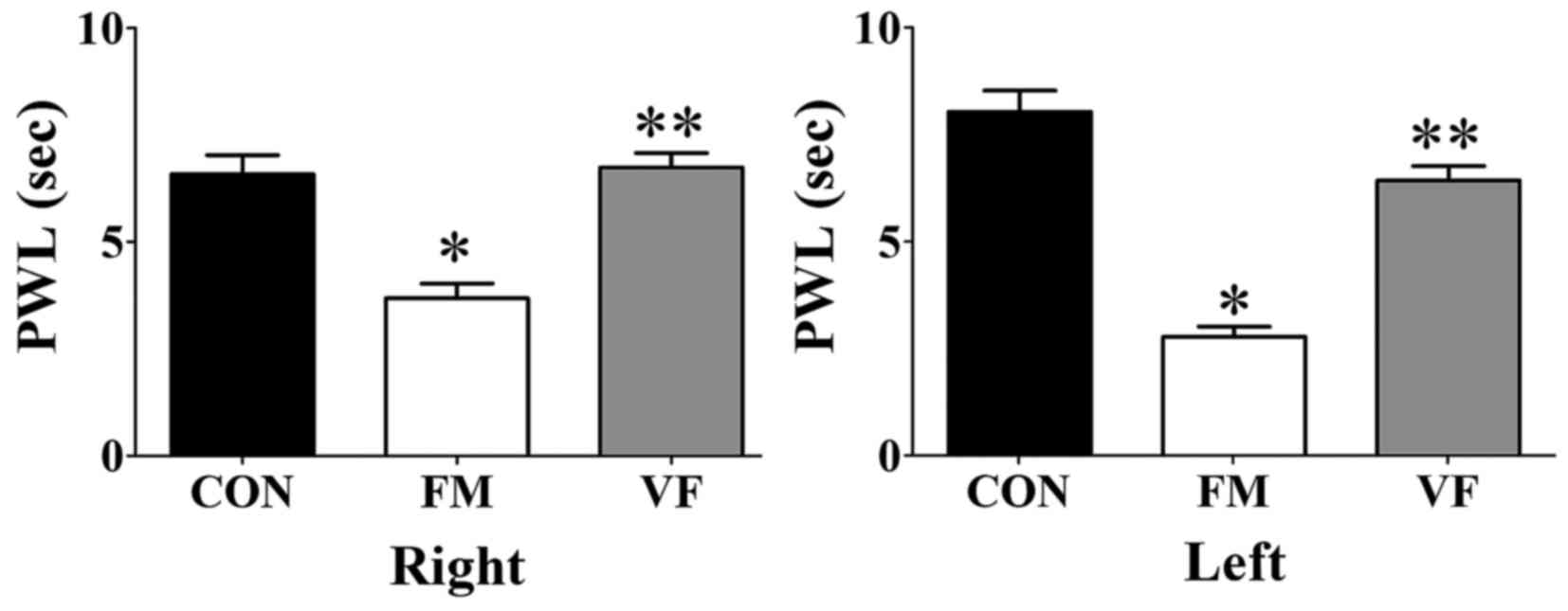
PWL
Significant differences among the control, FM and
VF-administered FM groups were detected with regards to the PWL of
both paws (Fig. 2). The FM group
exhibited decreased PWL compared with the control group (Fig. 2). Conversely, this FM-induced
decrease in PWL in the plantar test was attenuated following VF
administration (Fig. 2).
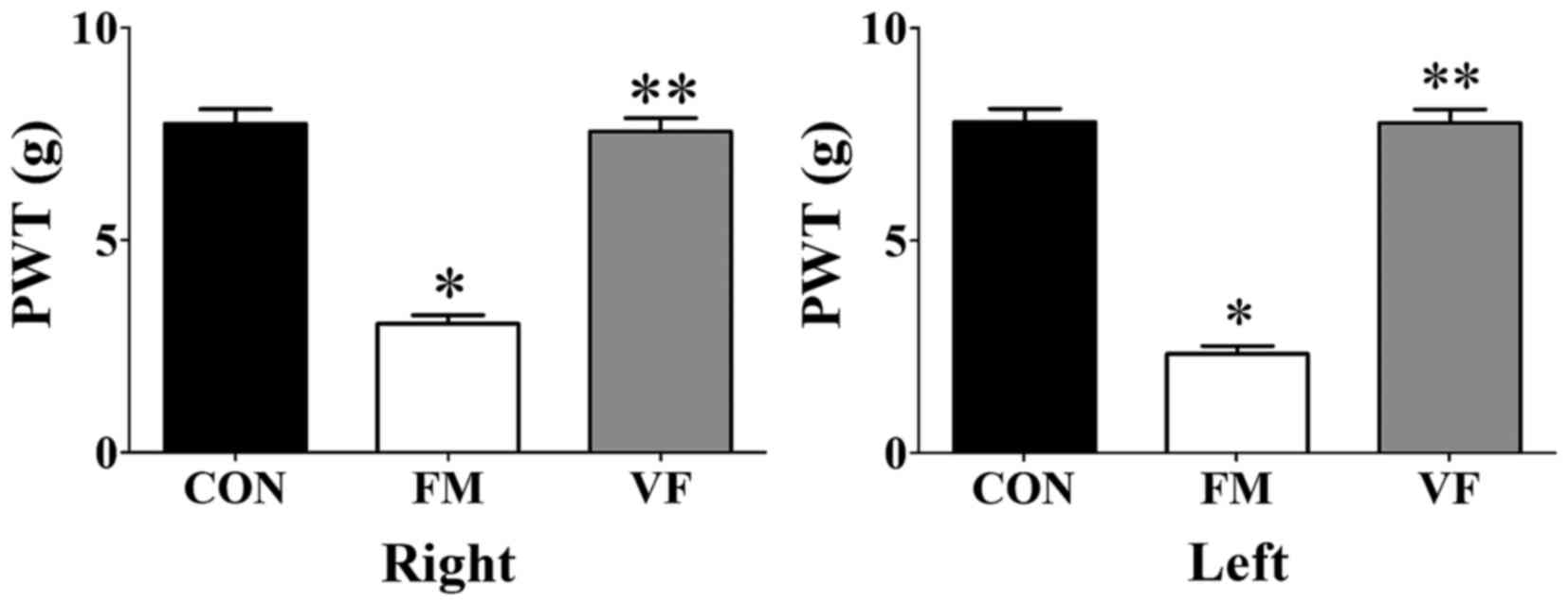
PWT
VF exerted beneficial effects on the PWT of both
paws in the VF-administered FM group (Fig. 3). The FM group exhibited a
decreased PWT compared with the control group (Fig. 3). Conversely, this FM-induced
decrease in PWT was attenuated following VF administration
(P<0.01; Fig. 3).
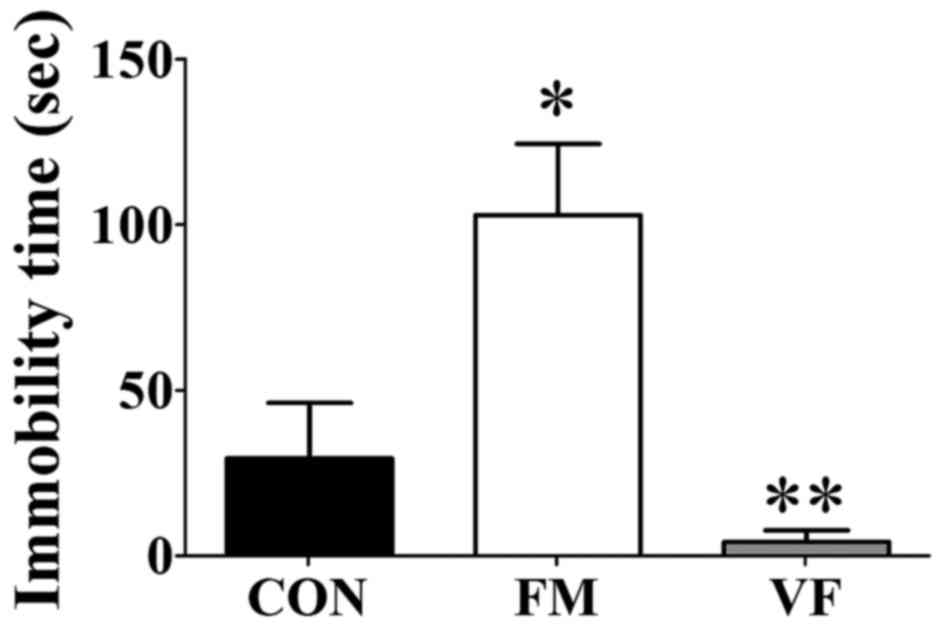
Tail suspension
The duration of immobility was measured in the TST,
in order to evaluate stress-associated depression in mice. The
duration of immobility in the FM group was significantly increased
compared with the control group (P<0.05; Fig. 4). Following VF administration, the
duration of immobility was significantly decreased compared with
the FM group (Fig. 4), thus
suggesting that VF may reverse depression in the FM group.
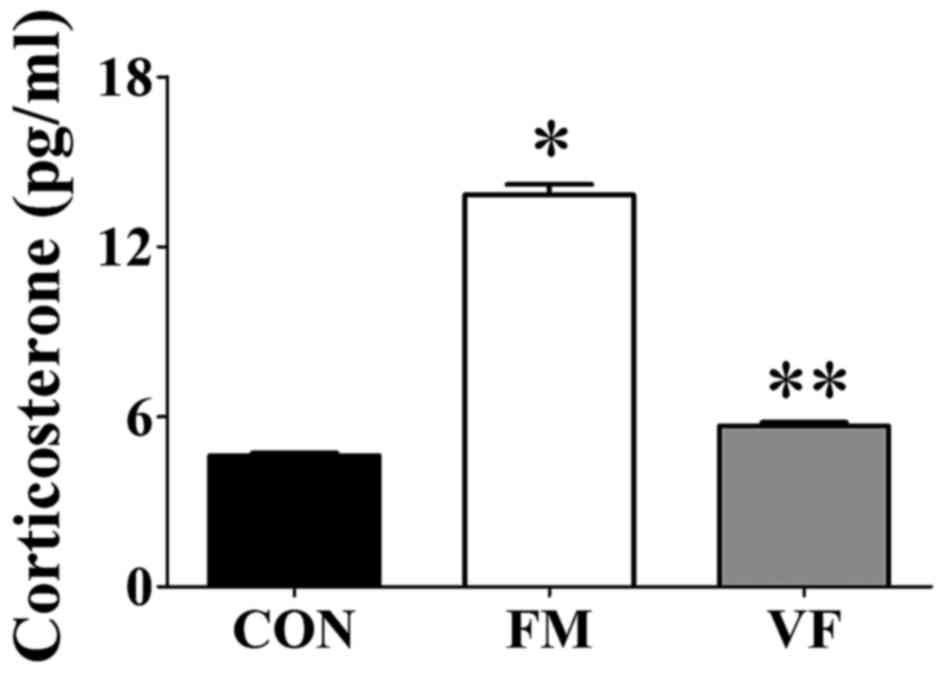
Corticosterone levels
Corticosterone levels were increased in the FM group
(Fig. 5) compared with those in
the control group. However, the corticosterone levels in the
VF-administered FM group returned to the control levels
(P<0.001; Fig. 5).
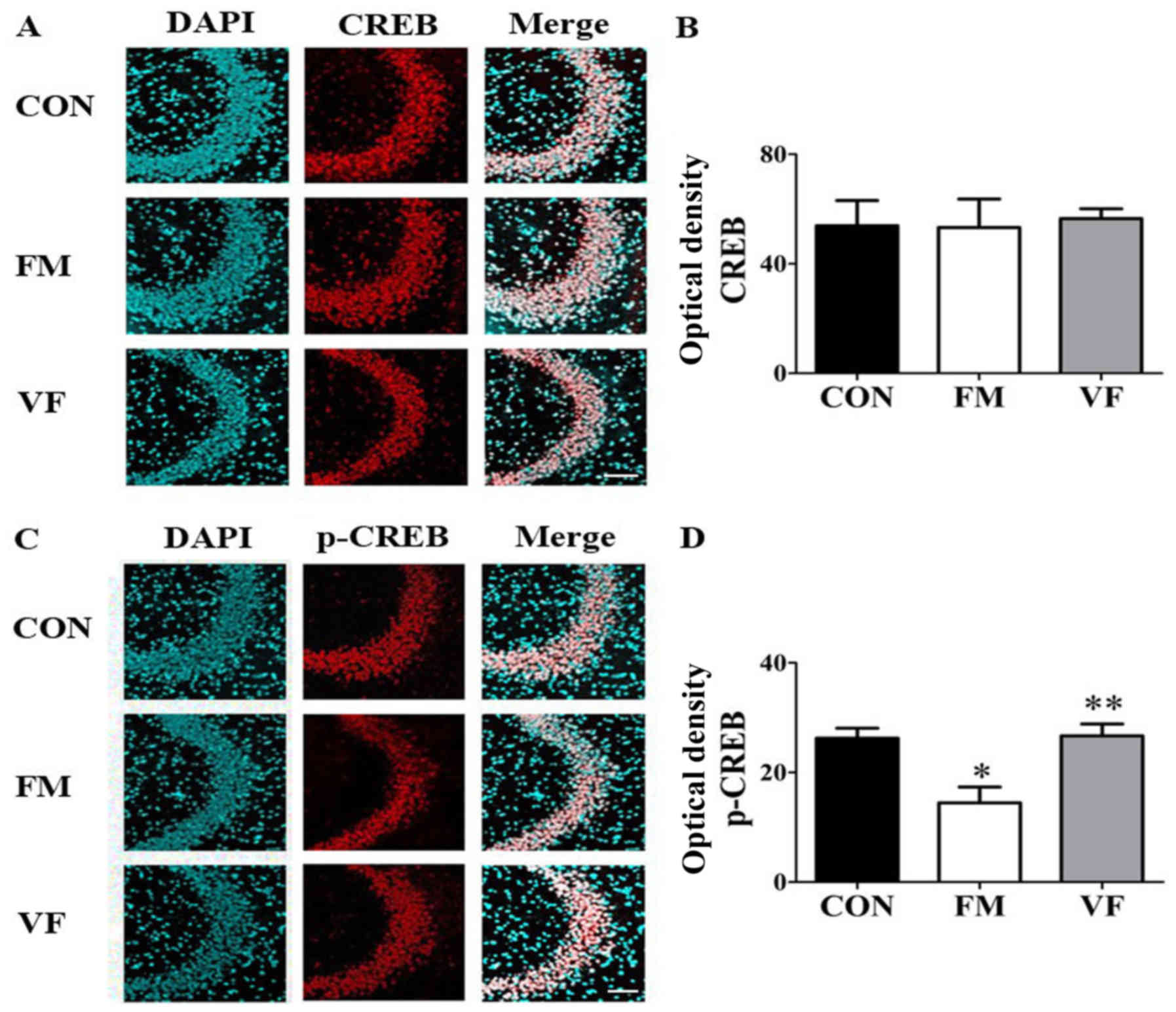
Western blot analysis and
immunohistochemistry
In order to investigate whether the BDNF-CREB
pathway, which is known to be associated with depression and pain,
is involved in behavioral abnormalities in the FM group, western
blotting (Figs. 6 and 7) was performed on samples from the
medial prefrontal cortex and hippocampus. In addition,
immunohistochemical analyses (Fig.
8) were performed on samples from the hippocampus of the
control, FM and VF-administered FM groups. Western blot analysis
revealed that the protein expression levels of BDNF and p-CREB in
the medial prefrontal cortex and hippocampus were significantly
reduced in the FM group compared with in the control group
(P<0.05). However, these alterations were reversed by VF
administration (P<0.05; Figs.
6 and 7). In addition, p-CREB
was differentially expressed in the immunofluorescent-stained brain
images of the control, FM and VF-administered FM groups (P<0.05;
Fig. 8).
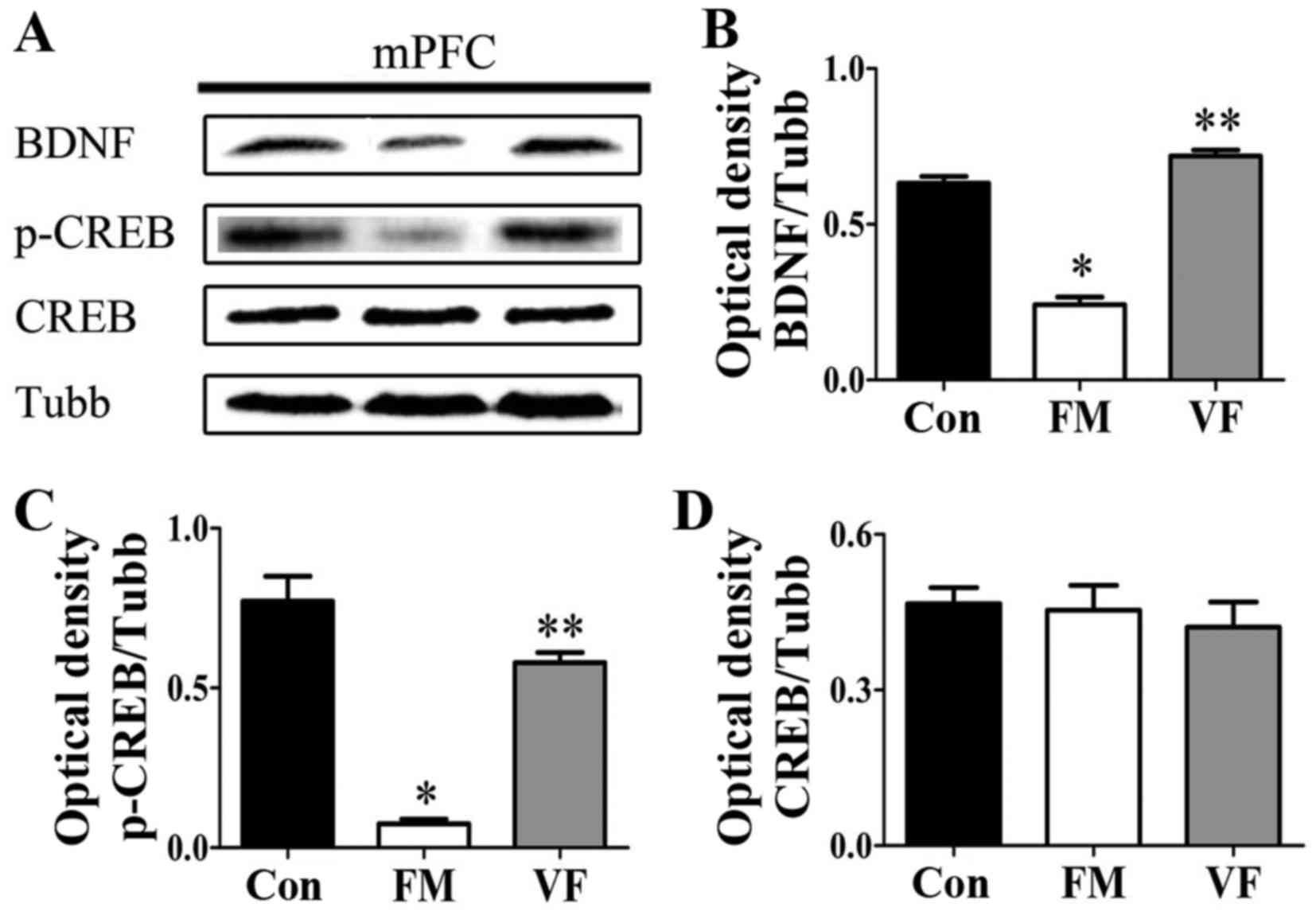 | Figure 6Effects of VF treatment on the
protein expression levels of BDNF and p-CREB in FM mice (n=5
mice/group). (A) BDNF, p-CREB and CREB levels in the mPFC were
determined by western blot analysis. (B-D) Semi-quantitative
analysis of western blotting data for BDNF, p-CREB, CREB expression
was conducted using ImageJ. There was a significant difference in
BDNF and p-CREB expression between the FM and VF-administered FM
groups. *P<0.05 vs. Con group; **P<0.05
vs. FM group. BDNF, brain-derived neurotrophic factor; Con, control
mice; CREB, cAMP response element-binding protein; FM, fibromyalgia
mouse model; mPFC, medial prefrontal cortex; p-CREB,
phosphorylated-CREB; Tubb, β-tubulin; VF, Valeriana fauriei
extract-administered FM mouse model. |
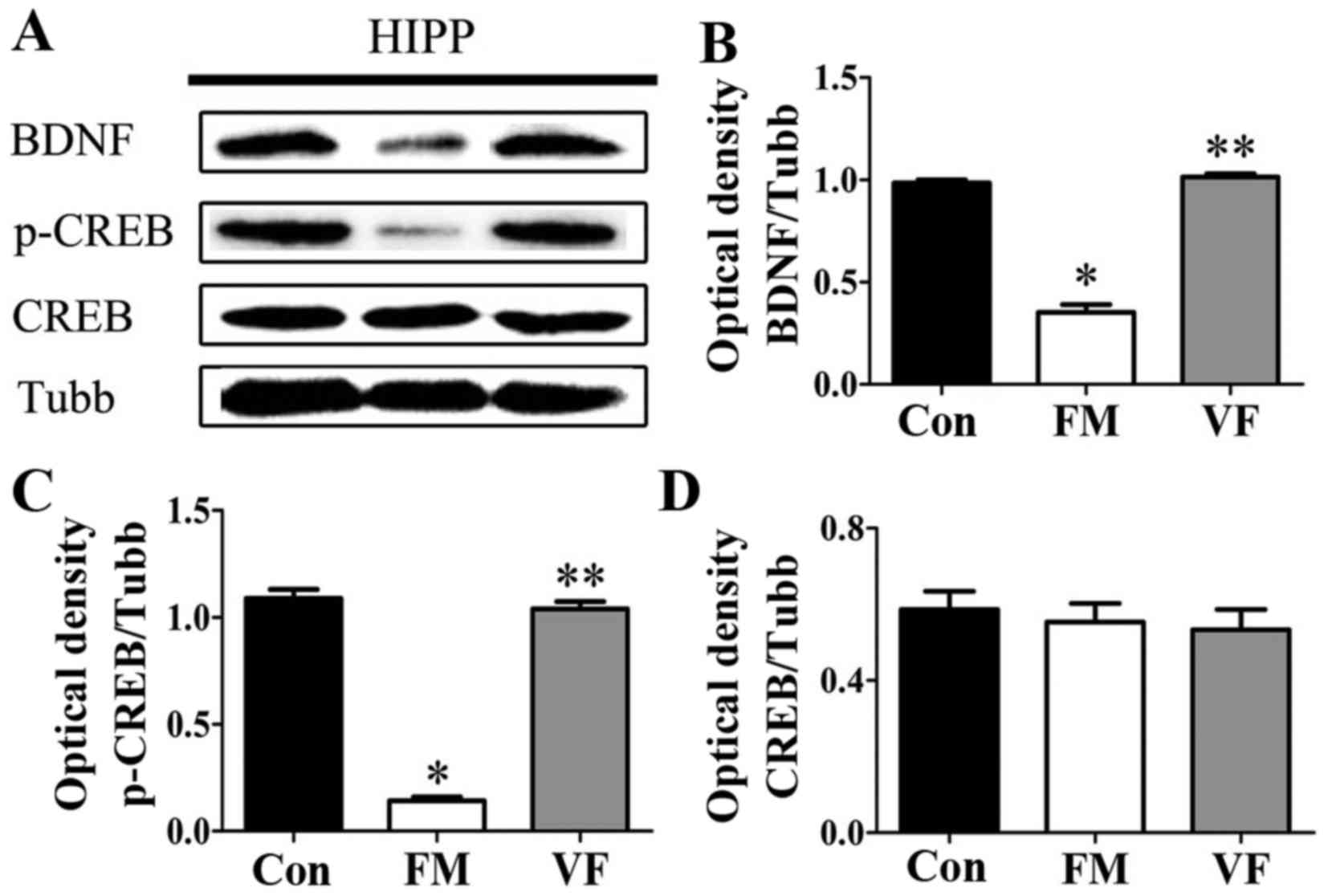 | Figure 7Effects of VF treatment on the
protein expression levels of BDNF and p-CREB in FM mice (n=5
mice/group). (A) p-CREB, CREB and BDNF levels in the HIPP were
determined by western blot analysis. (B-D) Semi-quantitative
analysis of western blotting data for BDNF, p-CREB, CREB expression
was conducted using ImageJ. There was a significant difference in
BDNF and p-CREB expression between the FM and VF-administered FM
groups. *P<0.05 vs. Con group; **P<0.05
vs. FM group. BDNF, brain-derived neurotrophic factor; Con, control
mice; CREB, cAMP response element-binding protein; FM, fibromyalgia
mouse model; HIPP hippocampus; p-CREB, phosphorylated-CREB; Tubb,
β-tubulin; VF, Valeriana fauriei extract-administered FM
mouse model. |
Discussion
In order to evaluate the extent to which treatment
with VF alters behavioral activity and protein expression that may
be associated with the pathophysiology of FM, the present study
determined the effects of VF treatment on behavioral phenotypes
using the TST, TFT, PWL and PWT. The present study demonstrated
that BDNF and p-CREB expression were significantly decreased in the
medial prefrontal cortex and hippocampus of mice in the FM group.
The protein expression levels of BDNF and p-CREB were increased in
the VF-administered FM model, as determined by western blotting and
immunohistochemistry. In addition, mice in the FM model group
exhibited a transient increase in serum corticosterone levels.
These results provide evidence of a possible association between
the BDNF-CREB pathway and FM susceptibility and
pathophysiology.
Previous interest in herbal medicine has focused on
the mechanisms underlying neuroendocrinological abnormalities,
including the hypothalamic-pituitary-adrenal axis, cortisol
production and BDNF, as well as impaired endogenous opioid
function, alterations in GABAergic and/or glutamatergic
transmission, cytokine or steroidal alterations, and abnormal
circadian rhythm (50,51). It has been previously reported
that BDNF, a key protein in the BDNF pathway, is a member of the
neurotrophin family, which includes nerve growth factor,
neurotrophin (NT)-3, NT-4 and BDNF (52). BDNF is involved in the development
and survival of neurons and in modulating the activity of
neurotransmitter systems (53),
particularly serotonin and dopamine (52), which are abnormal in FM (54). Furthermore, increased BDNF-related
hyperalgesia is dependent on an NMDA receptor-mediated mechanism
(55). This study has reported
that BDNF produces an acute, dose-dependent thermal hyperalgesic
response in normal mice while antisense directed against either
BDNF or its receptor, prevent inflammation-induced hyperalgesia.
Furthermore, our FM model showed a thermal hyperalgesic response
with decreased BDNF expression. There is growing evidence
indicating that BDNF also serves a role in major depressive
disorders and that antidepressant treatments increase serum BDNF
levels (56,57). In animal and human studies,
antidepressant treatments may increase central, as well as
peripheral, BDNF levels (23,24). Liu et al reviewed studies
that had been performed to identify additional modes of action of
various herbal medicines on several mood disorders and
antidepressants (57). The
results suggested that Fuzi total alkaloid increased the
phosphorylation levels of CREB and the expression of BDNF in the
frontal cortex and hippocampus of an animal model. In addition, in
murine models it was reported that the Fuzi total alkaloid-induced
generation of antidepressant-like effects may involve the CREB-BDNF
pathway (58). Furthermore,
hydrophilic constituents of Morinda citiforlia are well
known in folk medicine for a wide range of health purposes,
including anti-inflammatory, antioxidative, detoxifying and
cell-rejuvenating properties (59). In the present study, it was
suggested that the BDNF-CREB pathway may be associated with
FM-related pain. In addition, VF was revealed to exert
anti-depressive and anti-hyperalgesic effects via the BDNF-CREB
pathway.
Several studies have indicated that herbal hypnotics
and sedatives, including Valeriana spp. (valerian) and
Humulus lupulus (hops), usually work via modulation of
adenosine receptors and via melatoninergic effects (60–62). In a previous study, valerian and
its primary active component, valerenic acid, produced anxiolytic
and sedative effects mainly via GABA-ergic mechanisms, similar to
benzodiazepine drugs (62,63).
Numerous studies have reported that the phenomenons of anxiety,
psychological distress and depression are associated with the
chronicity of pain (64,65), rather than tissue damage (66). The relationship between physical
disease, psychiatric disorders and chronic pain is likely caused by
a complex interaction. Ammer and Melnizky assessed the effects of
pine oil and valerian on pain, sleep and tender point count in FM.
This previous study indicated that valerian baths were associated
with improved sleep, pine oil baths with increased sensitivity to
pain in certain body areas, and plain water baths appeared to
reduce pain intensity (67).
In the present study, the decrease in the protein
expression levels of BDNF and p-CREB following ICS and CRS
procedures was revealed to be reversed by treatment with VF. In
addition, the results confirmed that the FM animal model was able
to induce FM-like pain via abnormalities in BDNF signaling. The
present study demonstrated the beneficial effects of VF on
FM-associated symptoms, including depression and hyperalgesia.
However, further research using cellular and animal model systems
or human patients is required to fully characterize the
pharmacological functions of VF on FM.
Acknowledgments
The present study was carried out with the support
of the Cooperative Research Program for Agriculture Science and
Technology Development (grant no. PJ01158203), Rural Development
Administration, Republic of Korea.
References
|
1
|
Lindell L, Bergman S, Petersson IF,
Jacobsson LT and Herrström P: Prevalence of fibromyalgia and
chronic widespread pain. Scand J Prim Health Care. 18:149–153.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolfe F, Smythe HA, Yunus MB, Bennett RM,
Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M,
Clark P, et al: The American College of Rheumatology 1990 criteria
for the classification of fibromyalgia. Report of the Multicenter
Criteria Committee. Arthritis Rheum. 33:160–172. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Montserrat-de la Paz S, García-Giménez MD,
Ángel-Martín M and Fernández-Arche A: Validation and additional
support for an experimental animal model of fibromyalgia. Mod
Rheumatol. 25:116–122. 2015. View Article : Google Scholar
|
|
4
|
Schmidt-Wilcke T and Clauw DJ:
Pharmacotherapy in fibromyalgia (FM) - implications for the
underlying pathophysiology. Pharmacol Ther. 127:283–294. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Green PG, Alvarez P, Gear RW, Mendoza D
and Levine JD: Further validation of a model of fibromyalgia
syndrome in the rat. J Pain. 12:811–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schur EA, Afari N, Furberg H, Olarte M,
Goldberg J, Sullivan PF and Buchwald D: Feeling bad in more ways
than one: Comorbidity patterns of medically unexplained and
psychiatric conditions. J Gen Intern Med. 22:818–821. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilson HD, Starz TW, Robinson JP and Turk
DC: Heterogeneity within the fibromyalgia population: Theoretical
implications of variable tender point severity ratings. J
Rheumatol. 36:2795–2801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anderson RJ, McCrae CS, Staud R, Berry RB
and Robinson ME: Predictors of clinical pain in fibromyalgia:
Examining the role of sleep. J Pain. 13:350–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balasubramaniam R, de Leeuw R, Zhu H,
Nickerson RB, Okeson JP and Carlson CR: Prevalence of
temporomandibular disorders in fibromyalgia and failed back
syndrome patients: A blinded prospective comparison study. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod. 104:204–216. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sluka KA, Kalra A and Moore SA: Unilateral
intramuscular injections of acidic saline produce a bilateral,
long-lasting hyperalgesia. Muscle Nerve. 24:37–46. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khasar SG, Miao JP, Jänig W and Levine JD:
Modulation of bradykinin-induced mechanical hyperalgesia in the rat
by activity in abdominal vagal afferents. Eur J Neurosci.
10:435–444. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khasar SG, Dina OA, Green PG and Levine
JD: Sound stress-induced long-term enhancement of mechanical
hyperalgesia in rats is maintained by sympathoadrenal
catecholamines. J Pain. 10:1073–1077. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagakura Y, Oe T, Aoki T and Matsuoka N:
Biogenic amine depletion causes chronic muscular pain and tactile
allodynia accompanied by depression: A putative animal model of
fibromyalgia. Pain. 146:26–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishiyori M and Ueda H: Prolonged
gabapentin analgesia in an experimental mouse model of
fibromyalgia. Mol Pain. 4:522008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishiyori M, Uchida H, Nagai J, Araki K,
Mukae T, Kishioka S and Ueda H: Permanent relief from intermittent
cold stress-induced fibromyalgia-like abnormal pain by repeated
intrathecal administration of antidepressants. Mol Pain. 7:692011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Lin D, Yu X, Xie X, Wang L, Lian L,
Fei N, Chen J, Zhu N, Wang G, et al: The antinociceptive effects of
ferulic acid on neuropathic pain: Involvement of descending
monoaminergic system and opioid receptors. Oncotarget.
7:20455–20468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uçar M, Sarp Ü, Karaaslan Ö, Gül AI, Tanik
N and Arik HO: Health anxiety and depression in patients with
fibromyalgia syndrome. J Int Med Res. 43:679–685. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alok R, Das SK, Agarwal GG, Salwahan L and
Srivastava R: Relationship of severity of depression, anxiety and
stress with severity of fibromyalgia. Clin Exp Rheumatol. 29(Suppl
69): S70–S72. 2011.
|
|
19
|
Marco EM, Granstrem O, Moreno E, Llorente
R, Adriani W, Laviola G and Viveros MP: Subchronic nicotine
exposure in adolescence induces long-term effects on hippocampal
and striatal cannabinoid-CB1 and mu-opioid receptors in rats. Eur J
Pharmacol. 557:37–43. 2007. View Article : Google Scholar
|
|
20
|
Kivinummi T, Kaste K, Rantamäki T, Castrén
E and Ahtee L: Alterations in BDNF and phospho-CREB levels
following chronic oral nicotine treatment and its withdrawal in
dopaminergic brain areas of mice. Neurosci Lett. 491:108–112. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geng SJ, Liao FF, Dang WH, Ding X, Liu XD,
Cai J, Han JS, Wan Y and Xing GG: Contribution of the spinal cord
BDNF to the development of neuropathic pain by activation of the
NR2B-containing NMDA receptors in rats with spinal nerve ligation.
Exp Neurol. 222:256–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Masaki E, Mizuta K, Ohtani N and Kido K:
Early postoperative nociceptive threshold and production of
brain-derived neurotrophic factor induced by plantar incision are
not influenced with minocycline in a rat: Role of spinal microglia.
Neurosignals. 24:15–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuluğ B, Ozan E, Gönül AS and Kilic E:
Brain-derived neurotrophic factor, stress and depression: a
minireview. Brain Res Bull. 78:267–269. 2009. View Article : Google Scholar
|
|
24
|
Rybakowski JK: BDNF gene: Functional
Val66Met polymorphism in mood disorders and schizophrenia.
Pharmacogenomics. 9:1589–1593. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Licinio J, Dong C and wong ML: Novel
sequence variations in the brain-derived neurotrophic factor gene
and association with major depression and antidepressant treatment
response. Arch Gen Psychiatry. 66:488–497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Russell IJ, Mease PJ, Smith TR, Kajdasz
DK, Wohlreich MM, Detke MJ, Walker DJ, Chappell AS and Arnold LM:
Efficacy and safety of duloxetine for treatment of fibromyalgia in
patients with or without major depressive disorder: Results from a
6-month, randomized, double-blind, placebo-controlled, fixed-dose
trial. Pain. 136:432–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hobbs C: Valerian: A literature review.
HerbalGram. 21:19–34. 1989.
|
|
28
|
Liu XG, Gao PY, Wang GS, Song SJ, Li LZ,
Li X, Yao XS and Zhang ZX: In vivo antidepressant activity of
sesquiterpenes from the roots of Valeriana fauriei Briq.
Fitoterapia. 83:599–603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang BS, Kang SS, Kang SK, Kang CK, Ko WC
and Ko HG: Dictionary of traditional Chinese medicines. Jungdam
Publ Korea. 10:5171–5174. 1985.
|
|
30
|
Yao M, Ritchie HE and Brown-Woodman PD: A
developmental toxicity-screening test of valerian. J
Ethnopharmacol. 113:204–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ortiz JG, Nieves-Natal J and Chavez P:
Effects of Valeriana officinalis extracts on [3H]flunitrazepam
binding, synaptosomal [3H]GABA uptake, and hippocampal [3H]GABA
release. Neurochem Res. 24:1373–1378. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sudati JH, Vieira FA, Pavin SS, Dias GR,
Seeger RL, Golombieski R, Athayde ML, Soares FA, Rocha JB and
Barbosa NV: Valeriana officinalis attenuates the rotenone-induced
toxicity in Drosophila melanogaster. Neurotoxicology. 37:118–126.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Oliveria DM, Barreto G, De Andrade DV,
Saraceno E, Aon-Bertolino L, Capani F, Dos Santos El Bachá R and
Giraldez LD: Cytoprotective effect of Valeriana officinalis extract
on an in vitro experimental model of Parkinson disease. Neurochem
Res. 34:215–220. 2009. View Article : Google Scholar
|
|
34
|
Malva JO, Santos S and Macedo T:
Neuroprotective properties of Valeriana officinalis extracts.
Neurotox Res. 6:131–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pereira RP, Fachinetto R, de Souza Prestes
A, Wagner C, Sudati JH, Boligon AA, Athayde ML, Morsch VM and Rocha
JB: Valeriana officinalis ameliorates vacuous chewing movements
induced by reserpine in rats. J Neural Transm Vienna.
118:1547–1557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barnes J, Anderson LA and Phillipson JD:
Herbal Medicines. A Guide for Heathcare Professionals. 2nd edition.
Pharmaceutical Press; London: 2002
|
|
37
|
Pizzorno JE and Murray MT: Textbook of
Natural Medicine. 2nd edition. Churchill Livingstone; London:
1999
|
|
38
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. The National Academies Press;
washington, DC: 1996
|
|
39
|
Lee H, Won H, Im J, Kim YO, Lee S, Cho IH,
Kim HK, Kwon JT and Kim HJ: Effect of Valeriana fauriei extract on
the offspring of adult rats exposed to prenatal stress. Int J Mol
Med. 38:251–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ejchel-Cohen TF, Wood GE, Wang JF, Barlow
K, Nobrega JN, S McEwen B and Trevor Young L: Chronic restraint
stress decreases the expression of glutathione S-transferase pi2 in
the mouse hippocampus. Brain Res. 1090:156–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Magariños AM, Li CJ, Gal Toth J, Bath KG,
Jing D, Lee FS and McEwen BS: Effect of brain-derived neurotrophic
factor haploinsufficiency on stress-induced remodeling of
hippocampal neurons. Hippocampus. 21:253–264. 2011. View Article : Google Scholar
|
|
42
|
Keyhanfar F, Shamsi Meymandi M, Sepehri G,
Rastegaryanzadeh R and Heravi G: Evaluation of antinociceptive
effect of pregabalin in mice and its combination with tramadol
using Tail Flick test. Iran J Pharm Res. 12:483–493.
2013.PubMed/NCBI
|
|
43
|
Meymandi MS, Sepehri G and Mobasher M:
Gabapentin enhances the analgesic response to morphine in acute
model of pain in male rats. Pharmacol Biochem Behav. 85:185–189.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Steru L, Chermat R, Thierry B and Simon P:
The tail suspension test: A new method for screening
antidepressants in mice. Psychopharmacology (Berl). 85:367–370.
1985. View Article : Google Scholar
|
|
45
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Inoue M, Rashid MH, Fujita R, Contos JJ,
Chun J and Ueda H: Initiation of neuropathic pain requires
lysophosphatidic acid receptor signaling. Nat Med. 10:712–718.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rashid MH, Inoue M, Toda K and Ueda H:
Loss of peripheral morphine analgesia contributes to the reduced
effectiveness of systemic morphine in neuropathic pain. J Pharmacol
Exp Ther. 309:380–387. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Morley-Fletcher S, Darnaudery M, Koehl M,
Casolini P, Van Reeth O and Maccari S: Prenatal stress in rats
predicts immobility behavior in the forced swim test. Effects of a
chronic treatment with tianeptine. Brain Res. 989:246–251. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Joo J, Lee S, Nah SS, Kim YO, Kim DS, Shim
SH, Hwangbo Y, Kim HK, Kwon JT, Kim JW, et al: Lasp1 is
down-regulated in NMDA receptor antagonist-treated mice and
implicated in human schizophrenia susceptibility. J Psychiatr Res.
47:105–112. 2013. View Article : Google Scholar
|
|
50
|
Hindmarch I: Expanding the horizons of
depression: Beyond the monoamine hypothesis. Hum Psychopharmacol.
16:203–218. 2001. View Article : Google Scholar
|
|
51
|
Raison CL, Capuron L and Miller AH:
Cytokines sing the blues: Inflammation and the pathogenesis of
depression. Trends Immunol. 27:24–31. 2006. View Article : Google Scholar
|
|
52
|
Sebastião AM, Assaife-Lopes N, Diógenes
MJ, Vaz SH and Ribeiro JA: Modulation of brain-derived neurotrophic
factor (BDNF) actions in the nervous system by adenosine A(2A)
receptors and the role of lipid rafts. Biochim Biophys Acta.
1808:1340–1349. 2011. View Article : Google Scholar
|
|
53
|
Pillai A: Brain-derived neurotropic
factor/TrkB signaling in the pathogenesis and novel pharmacotherapy
of schizophrenia. Neurosignals. 16:183–193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Laske C, Stransky E, Eschweiler GW, Klein
R, Wittorf A, Leyhe T, Richartz E, Köhler N, Bartels M, Buchkremer
G, et al: Increased BDNF serum concentration in fibromyalgia with
or without depression or antidepressants. J Psychiatr Res.
41:600–605. 2007. View Article : Google Scholar
|
|
55
|
Groth R and Aanonsen L: Spinal
brain-derived neurotrophic factor (BDNF) produces hyperalgesia in
normal mice while antisense directed against either BDNF or trkB,
prevent inflammation-induced hyperalgesia. Pain. 100:171–181. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xu Y, Ku B, Tie L, Yao H, Jiang W, Ma X
and Li X: Curcumin reverses the effects of chronic stress on
behavior, the HPA axis, BDNF expression and phosphorylation of
CREB. Brain Res. 1122:56–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu L, Liu C, Wang Y, Wang P, Li Y and Li
B: Herbal medicine for anxiety, depression and insomnia. Curr
Neuropharmacol. 13:481–493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu L, Li B, Zhou Y, Wang L, Tang F, Shao
D, Jiang X, Zhao H, Cui R and Li Y: Antidepressant-like effect of
Fuzi total alkaloid on ovariectomized mice. J Pharmacol Sci.
120:280–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Deng S, West BJ, Palu AK, Zhou BN and
Jensen CJ: Noni as an anxiolytic and sedative: A mechanism
involving its gamma-aminobutyric acidergic effects. Phytomedicine.
14:517–522. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sarris J: Herbal medicines in the
treatment of psychiatric disorders: A systematic review. Phytother
Res. 21:703–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sarris J and Byrne GJ: A systematic review
of insomnia and complementary medicine. Sleep Med Rev. 15:99–106.
2011. View Article : Google Scholar
|
|
62
|
Murphy K, Kubin ZJ, Shepherd JN and
Ettinger RH: Valeriana officinalis root extracts have potent
anxiolytic effects in laboratory rats. Phytomedicine. 17:674–678.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dietz BM, Mahady GB, Pauli GF and
Farnsworth NR: Valerian extract and valerenic acid are partial
agonists of the 5-HT5a receptor in vitro. Brain Res Mol Brain Res.
138:191–197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
No authors listed. Classification of
chronic pain. Descriptions of chronic pain syndromes and
definitions of pain terms Prepared by the International Association
for the study of pain, subcommittee on taxonomy. Pain Suppl.
3:S1–S226. 1986.
|
|
65
|
Livingston G, Watkin V, Milne B, Manela MV
and Katona C: Who becomes depressed? The Islington community study
of older people. J Affect Disord. 58:125–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
McBeth J and Silman AJ: Role Psychiatr
Disord Fibromyalgia. 3:157–166. 2001.
|
|
67
|
Ammer K and Melnizky P: Medicinal baths
for treatment of generalized fibromyalgia. Forsch Komplementarmed.
6:80–85. 1999.In German. PubMed/NCBI
|