Introduction
Glaucoma is the second most common cause of
blindness worldwide, and ~47% of the individuals with glaucoma are
of Asian origin (1). Notably,
primary open angle glaucoma (POAG) is one of the most prevalent
types of glaucoma, and its main clinical manifestations are high
intraocular pressure (IOP) and visual function damage (2). Although the mechanism of POAG
development is unclear, the main pathological change is considered
to be the reduced outflow of aqueous humor through the trabecular
meshwork (TM), the major site of IOP regulation (3). A previous study has demonstrated
that an increased amount of sheath-derived plaque material in the
TM is associated with an increased severity of optic nerve damage
in POAG (4). In addition, the TM
from glaucomatous ocular tissue has been found to be stiffer than
that from normal ocular tissue (5), indicating the significance of the
morphological and biophysical changes of the TM in glaucoma.
Therefore, primary cultured human trabecular meshwork (HTM) cells
have been studied in vitro in order to investigate the
pathogenic mechanism of POAG. Corticosteroids have long been
recognized as a cause of glaucoma (6,7).
Thus, a commonly used in vitro model for glaucoma is the
treatment of HTM cells with dexamethasone (DEX), a synthetic
glucocorticoid (8–11).
The Hippo signaling pathway, first discovered in
Drosophila, is a signaling pathway that controls organ size
by promoting cell death and cell differentiation (12,13) and inhibiting cell proliferation
(14–17). Yes-associated protein (YAP) and
transcriptional coactivator with PDZ-binding motif (TAZ) are two
Yorkie homologs in the Hippo pathway that have been identified in
mammals and are highly conserved (18). It has been reported that YAP and
TAZ exist at all levels of the TM (19), and are influenced by the stiffness
of the extracellular matrix (ECM) (20). Studies conducted by Raghunathan
et al (19) demonstrated
the expression of YAP and TAZ in HTM cells. In these studies,
polyacrylamide hydrogels mimicking the normal (5 kPa) and
glaucomatous (75 kPa) meshworks were used in the culture of HTM
cells, and the researchers observed that YAP and TAZ mRNA
expression levels were upregulated on the 75-kPa hydrogels in
comparison with the 5-kPa hydrogels. However, to the best of our
knowledge, no previous studies concerning the involvement of YAP
and TAZ in corticosteroid-induced glaucoma have been reported.
Therefore, the present study aimed to elucidate the roles of YAP
and TAZ in DEX-induced glaucoma. The knockdown and overexpression
of YAP and TAZ genes was conducted in order to investigate their
roles in the regulation of DEX-induced glaucoma. In addition, the
regulatory roles of YAP and TAZ in the cell proliferation and
cytoskeletal structure of HTM cells were determined.
Materials and methods
Chemicals
DEX, fluorescein isothiocyanate (FITC)-labeled
phalloidin (P5282), Triton X-100 (T9284), bovine serum albumin
(BSA; V900933), Bradford reagent (B6916) and paraformaldehyde (PFA;
158127) were all purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Small interfering RNAs (siRNAs) against human
YAP (sc-38637) and human TAZ (sc-38568), and scrambled siRNA
(sc-37007) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Click-iT 5-ethynyl-2′-deoxyuridine (EdU) Alexa
Fluor 488 Imaging kit (C10337), SYBR-Green PCR Master mix, NuPAGE
10% Bis-Tris protein gel (NP0315BOX), NuPAGE transfer buffer
(NP0006) and 4′,6-diamidino-2-phenylindole (DAPI; D1306) were
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Polyvinylidene fluoride membranes were purchased from Pall
Corporation (Port Washington, NY, USA).
Constitutively activated YAP (GST-YAP, Addgene
plasmid 38105), TAZ (HA-TAZ, Addgene plasmid 32839) and empty
vector (pcDNA3.1, Addgene plasmid 62803) were all purchased from
Addgene, Inc. (Cambridge, MA, USA).
Primary antibodies to the following proteins were
purchased from Santa Cruz Biotechnology, Inc.: YAP (sc-15407), TAZ
(sc-48805), fibronectin (sc-53285), laminin α1 (sc-6016), β-catenin
(sc-65480) and β-actin (sc-47778). Primary antibodies to collagen I
(ab34710) and collagen IV (ab19808) were both purchased from Abcam
(Cambridge, MA, USA). The secondary antibodies horseradish
peroxidase (HRP)-conjugated goat anti rabbit IgG (cat. no.
11-035-003) and goat anti-mouse IgG (cat. no. 11-035-003) were both
purchased from Jackson ImmunoResearch Laboratories, Inc. (West
Grove, PA, USA).
Cell culture
HTM cells were purchased from Bolise Co., Ltd.
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM)/F-12 containing 10% fetal bovine serum (FBS),
100 mg/ml streptomycin and 100 U/ml penicillin (all from Thermo
Fisher Scientific, Inc.), and maintained at 37°C with 5%
CO2 in a humidified atmosphere. All studies were
conducted using cells prior to the eighth passage.
Cell treatment, proliferation assay and
morphological analysis
HTMC cells were treated with PBS or DEX
(1×10−4, 1×10−5, 1×10−6 and
1×10−7 M) at 37°C for 48 h. Cell proliferation was
analyzed using a Click-iT EdU cell proliferation assay kit
according to the manufacturer's instructions. In brief,
3×105 HTM cells were plated on coverslips and incubated
with 10 μM EdU solution at 37°C for 16 h. The cells were
then fixed with 4% buffered PFA and incubated with 0.5 ml Click-iT
reaction mixture for 30 min at room temperature. The coverslides
were examined using a Nikon Eclipse 80i microscope system (Nikon,
Tokyo, Japan). DAPI was used as a nuclear counterstain (blue) at
room temperature for 5 min. EdU-positive cells (green) in 15–20
randomly selected fields were manually counted and the proportion
of all cells that were EdU-positive was determined.
For the immunofluorescence analysis of cell
morphology, 3×105 HTM cells plated on coverslips were
fixed with 4% PFA and permeabilized with Triton X-100. Following
the removal of the remaining Triton X-100, the cells were incubated
with 5 μg/ml FITC-labeled phalloidin for 1 h. Nuclear
staining of the cells was conducted by staining with DAPI at room
temperature for 5 min. The coverslides were examined using the
Nikon Eclipse 80i microscope system.
Transfection and gene silencing
HTM cells were plated in 60-mm dishes with DMEM/F-12
containing 10% FBS and 1% penicillin-streptomycin at a density of
1×106 cells/dish. The plated HTM cells were continuously
cultured in DMEM/F-12 without 10% FBS and without 1%
penicillin-streptomycin for a further 2 h. The YAP, TAZ and empty
plasmids were mixed with 25X diluent Lipofectamine LTX with PLUS
reagent, and incubated for 20 min at room temperature to allow
plasmid-Lipofectamine LTX complexation. Transfections were then
conducted in OptiMEM reduced-serum medium (cat. no. 31985070;
Thermo Fisher Scientific, Inc.) containing 1 mg/ml plasmid DNA,
0.1% PLUS reagent and 0.3% Lipofectamine LTX transfection reagent,
where HTM cells were cultured in the medium with plasmid in 60-mm
dishes for 48 h (21). Finally,
the transfection efficiency was detected using western blot
analysis. For the knockdown of YAP and TAZ genes, HTM cells were
transfected with YAP siRNA, TAZ siRNA or scrambled siRNA at 33 nM
using DharmaFect transfection reagent with SMARTpool ON-TARGET
siRNA (GE Healthcare Dharmacon, Inc., Lafayette, CO, USA) according
to the manufacturer's instructions (22). The transfected cells were used for
subsequent experiments 24 h later.
Western blot analysis
The cells were lysed using RIPA lysis buffer
(Beyotime Institute of Biotechnology, Suzhou, China) and the
protein lysates were centrifuged at 13,400 × g for 30 min. The
supernatants were collected and quantified using the Bradford
protein assay method with BSA as the standard. The protein was then
denatured by heating to 95°C for 5 min. Approximately 20 μg
protein was loaded into each well of the precast NuPAGE 10%
Bis-Tris protein gel. Following electrophoresis, the proteins were
transferred onto a polyvinylidene fluoride membrane using the
NuPAGE transfer buffer. The membranes were blocked with 5% BSA in
TBST at room temperature for 1 h, and then incubated overnight at
4°C with primary antibodies at the dilutions recommended by the
manufacturer (1:500). The blots were then incubated with
HRP-conjugated IgG secondary antibodies (1:5,000 dilutions) for 1 h
at 37°C. Bands were detected using Immobilon Western
Chemiluminescent HRP Substrate (WBKLS0500; EMD Millipore,
Billerica, MA, USA) and imaged using an ImageQuant 350 system (GE
Healthcare Life Sciences, Piscataway, NJ, USA) (23).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from the HTM cells using an RNeasy
Isolation kit according to the manufacturer's instructions (Qiagen,
Inc., Valencia, CA, USA), and then reverse transcribed. The
reaction mixture comprised 1 μg total RNA, 1 μl
random primer (50 μmol/l, cat. no. 48190011; Thermo Fisher
Scientific, Inc.), 1X reverse transcription buffer and 10 units
reverse transcriptase (A5003; Promega, Madison, WI, USA) in a total
volume of 20 μl. The RNA and primer were heated to 72°C and
slowly cooled prior to reverse transcription at 42°C for 1 h
(24). When cooled to room
temperature, the reaction was diluted to 100 μl with
RNase-free water. qPCR, was carried out with SYBR-Green PCR Master
mix in a total reaction volume of 20 μl using the following
amplification steps: Initial denaturation at 95°C for 10 min;
followed by 40 cycles of denaturation at 95°C for 15 sec; and then
elongation at 55°C for 30 sec. The expression levels were
normalized to those of the internal standard 18S rRNA. The
following primer sequences were used: YAP sense,
5′-ACCCACAGCTCAGCATCTTCG-3′ and anti-sense,
5′-TGGCTTGTTCCCATCCATCAG-3′; TAZ sense,
5′-GTCACCAACAGTAGCTCAGATC-3′ and antisense,
5′-AGTGATTACAGCCAGGTTAGAAAG-3′; 18S rRNA sense,
5′-GGGCATTCGTATTTCATAGTCAGAG-3′ and antisense,
5′-CGGTTCTTGATTAATGAAAACATCCT-3′. The experiment was performed in
triplicate. The YAP and TAZ mRNA expression levels of the
DEX-treated groups (treated with 10 μM dexamethasone at 37°C
for 48 h) were normalized relative to those of the DMSO (DEX-free)
group, which was assigned a value of 1.0. In order to control for
slight variations in the amount of RNA loaded for PCR, the
difference in cycle threshold (ΔCq) between the gene of interest
and the average cycle threshold (Cq) of the house keeping gene 18S
(also loaded in triplicate wells) were calculated. By calculating
the difference in ΔCq between the DEX-treated group and the control
group, the experimental data was normalized to the control data
using the 2−ΔΔCq method (25).
Permeability assay
The in vitro permeability assay was performed
as previously reported (26).
Briefly, HTM cells were plated onto the cell culture inserts of
Transwell plates (cat. no. 353104, BD Falcon; BD Biosciences,
Franklin Lakes, NJ, USA). When the cells reached subconfluence,
they were transfected with the aforementioned siRNA or plasmids. At
3 days after transfection, the cells were subjected to an in
vitro permeability assay. On the day of the assay, the medium
in the upper and lower chambers was replaced with fresh DMEM, and
150 μl FITC-Dextran (1:30 dilution, D1845; Thermo Fisher
Scientific, Inc.) was added to the medium in the upper chamber of
each insert. After 5 min at room temperature, 100 μl
solution from the lower chamber was transferred to a 96-well plate.
The plate was read using an Envision 2103 Multilabel Plate Reader
(PerkinElmer, Inc., Waltham, MA, USA) at an excitation wavelength
of 480 nm and emission wavelength of 530 nm, with a bandwidth of
10.
Statistical analysis
All data are presented as mean ± standard error of
the mean from at least three independent experiments. Statistical
significance was determined by one-way analysis of variance
followed by Bonferroni correction when three or more groups were
compared, and by Student's t-test when two groups were compared.
P<0.05 was considered to indicate a statistically significant
difference.
Results
DEX induces elevated YAP and TAZ
expression in HTM cells
A previous study has demonstrated a marked stiffness
of the HTM in glaucomatous patients (5). In addition, the HTM substratum
stiffness closely correlates with the expression and activity of
the YAP and TAZ proteins (27).
Thus, it was hypothesized that DEX-induced substratum stiffness is
accompanied by elevated expression levels of YAP and TAZ. Staining
of the HTM cells with the F-actin probe FITC-phalloidin, as shown
in Fig. 1A–E, indicates that DEX
altered the F-actin architecture in a concentration-dependent
manner, and promoted the formation of a cross-linked actin network
(CLAN) in the HTM cells (10). To
determine whether YAP and TAZ transcription was associated with the
DEX induced-CLAN formation, RT-qPCR was performed. It was observed
that 1×10−5 M DEX induced a significant increase in the
transcription level of the YAP gene (Fig. 1F). In addition, the transcription
level of the TAZ gene was also significantly elevated in the
presence of 1×10−5 M DEX (Fig. 1G). These results suggest an
association of the DEX-induced cytoskeletal changes in the cultured
HTM cells with the increased transcription levels of YAP and TAZ.
To further validate the correlation of the expression levels of YAP
and TAZ with the DEX-induced cytoskeletal reorganization, western
blotting was performed. As shown in Fig. 1H, the expression levels of the YAP
and TAZ proteins in the HTM cells were observed to increase as the
concentration of DEX increased. These data indicate that DEX has a
dose-dependent association with the transcription and expression
levels of YAP and TAZ.
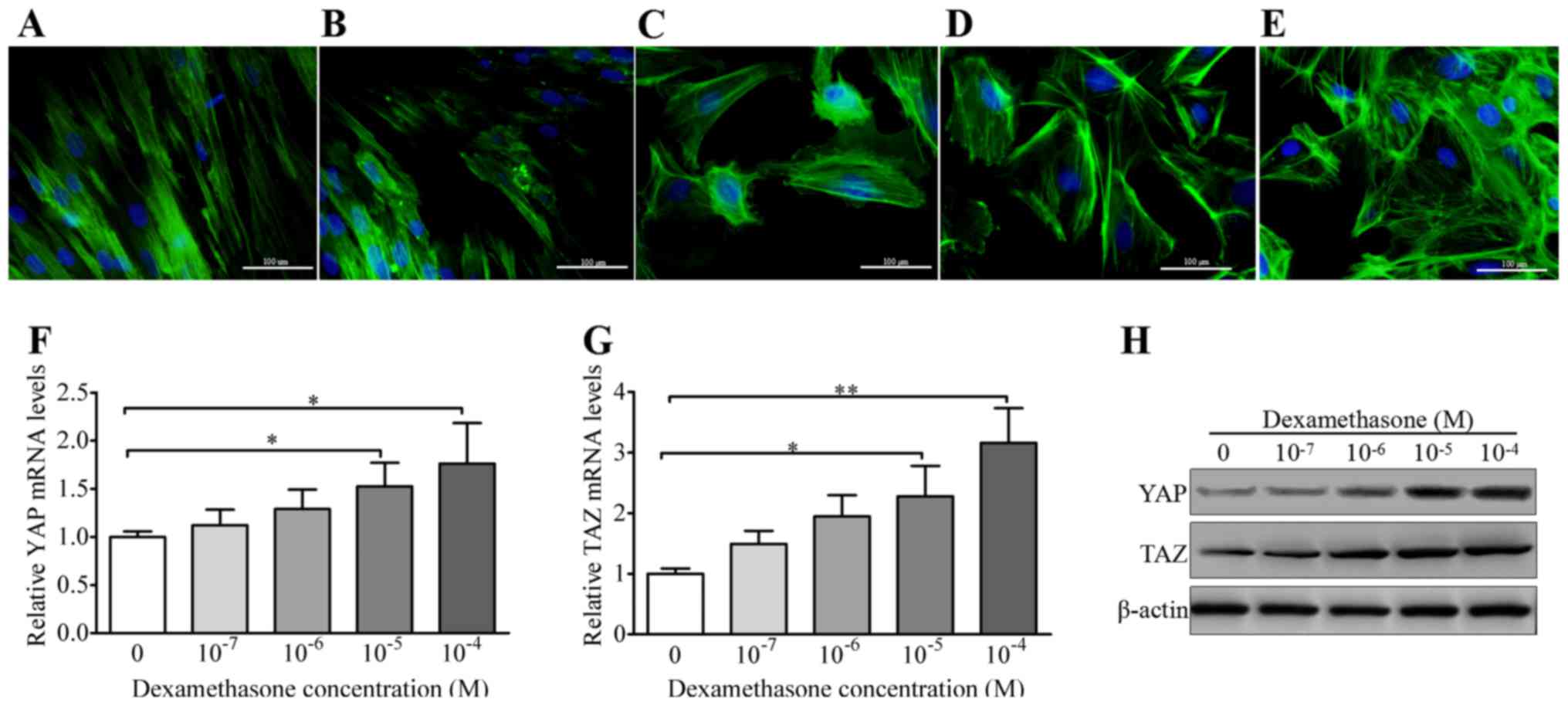 | Figure 1DEX increases the expression of YAP
and TAZ. (A–E) Observation of DEX-induced cross-linked actin
network formation by HTM cells. The cells were treated with (A)
DMSO, (B) 1×10−7 DEX, (C) 1×10−6 DEX, (D)
1×10−5 DEX and (E) 1×10−4 DEX. Green, F-actin
stain; blue, nuclear stain (DAPI). Scale bar, 100 μm. (F)
YAP mRNA levels and (G) TAZ mRNA levels in HTM cells treated with
different concentrations of DEX. (H) Western blot analysis of YAP
and TAZ proteins in the DEX-treated HTM cells. Protein bands shown
are representative of three independent experiments with similar
results. Data are presented as the mean ± standard error of the
mean from three independent experiments. *P<0.05 and
**P<0.01. DEX, dexamethasone; YAP, Yes-associated
protein; TAZ, transcriptional coactivator with PDZ-binding motif;
HTM, human trabecular meshwork. |
YAP and TAZ regulate actin-associated
proteins
Previous studies have demonstrated that in response
to DEX treatment, several actins and actin-associated proteins are
involved in the development of a CLAN (28). Furthermore, among those proteins,
fibronectin (29) and laminin
(30) have been shown to be
increased in DEX-treated HTM cells. Therefore, in order to further
elucidate the effects of YAP and TAZ on actin-associated proteins,
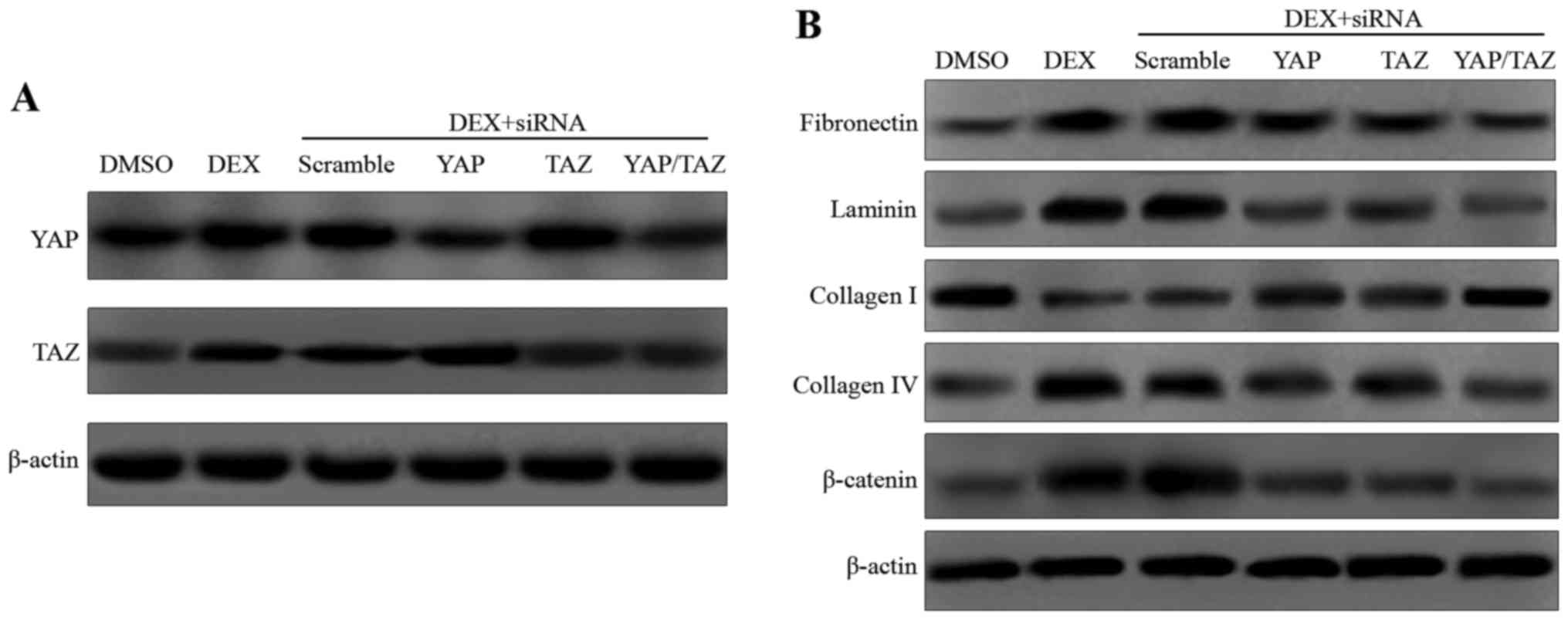
YAP and TAZ genes were knocked down using siRNA (Fig. 2A), and the expression levels of
fibronectin and laminin were measured. As shown by the western
blotting results in Fig. 2B, DEX
markedly increased the expression levels of fibronectin and
laminin. However, the elevated expression levels were attenuated by
the addition of YAP and TAZ siRNA. The expression of ECM-associated
molecules, including collagen types I and IV, is known to be
associated with DEX-induced CLAN formation (31,32). Thus, the expression levels of
types I and IV collagen were determined in the present study.
Increased expression levels of type I collagen and decreased
expression levels of type IV collagen were observed in the HTM
cells treated with DEX plus YAP and/or TAZ siRNA concurrently
compared with those treated with DEX alone (Fig. 2B). In addition, a previous study
by the present research team demonstrated that the expression of
β-catenin was induced by DEX in cultured HTM cells (33). The present study confirmed this,
and revealed that β-catenin expression was decreased in the
DEX-treated cells transfected with YAP and/or TAZ siRNA compared
with that in the DEX control.
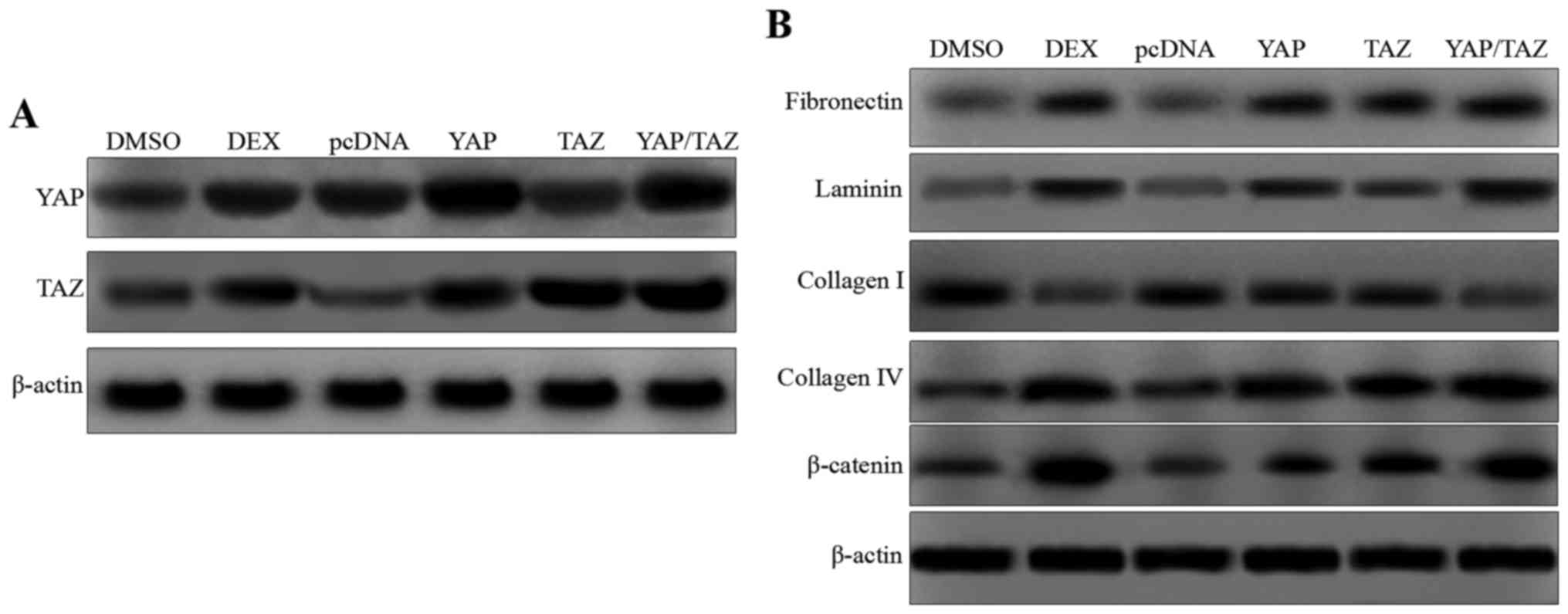
To further substantiate the association of YAP and
TAZ with the proteins shown in Fig.
2B, the HTM cells were cotransfected with YAP and/or TAZ
expression plasmids (Fig. 3A).
The western blotting results presented in Fig. 3B indicate that the expression
levels of fibronectin and laminin were increased in the presence of
YAP and TAZ overexpression, and the expression was comparable to
that in the DEX-treated cells. In addition, the expression of type
I collagen was inhibited in the YAP and TAZ overexpression groups,
whereas the expression of type IV collagen was clearly increased.
Increased expression of β-catenin was also observed in the YAP and
TAZ overexpressing cells. These data demonstrate that YAP and TAZ
regulate actin-associated proteins and the expression of
β-catenin.
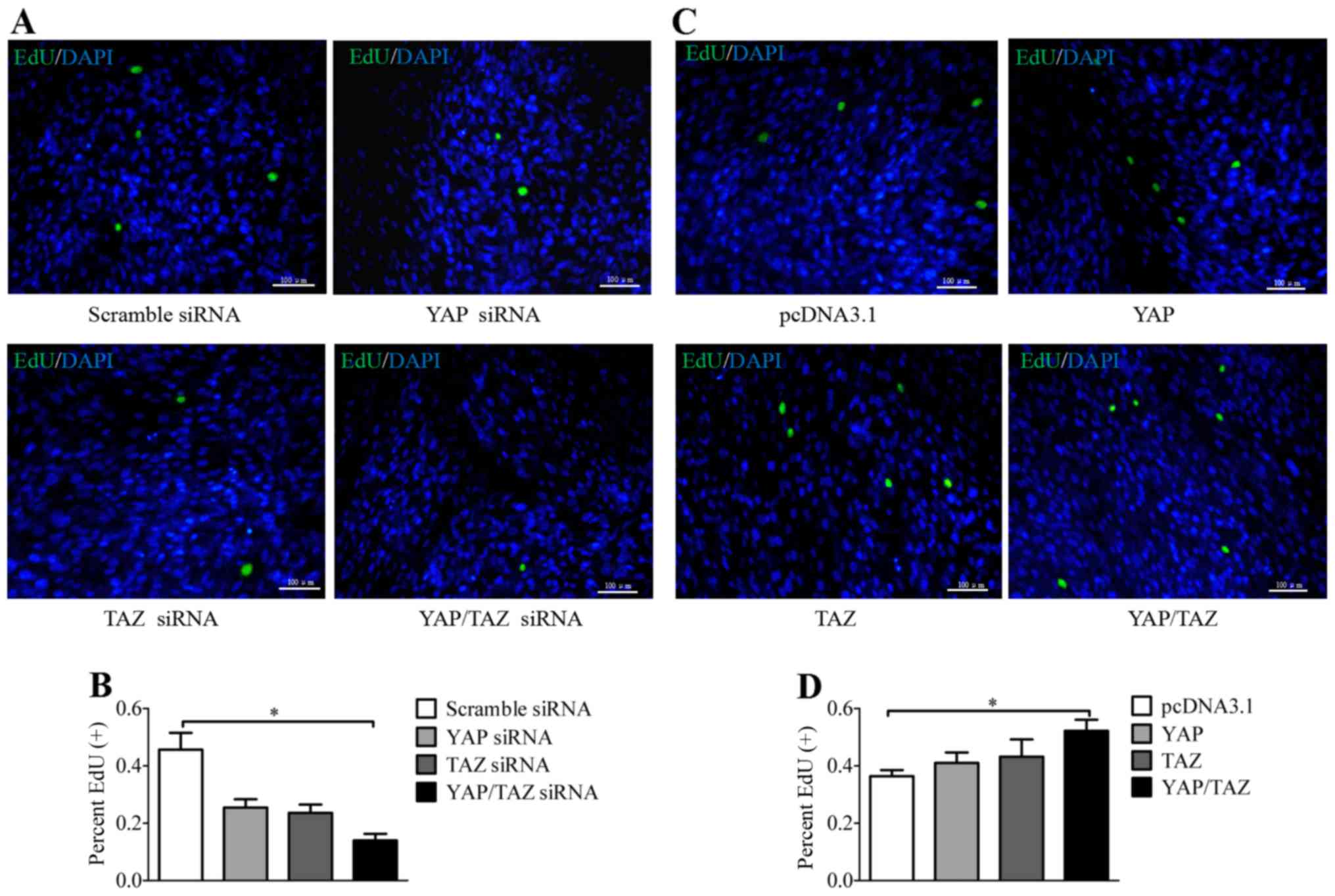
YAP and TAZ induce the proliferation of
HTM cells
The Hippo/Yap pathway is a well-conserved signaling
cascade that regulates cell proliferation and differentiation, and
controls organ size (34,35). In the present study, the
proliferation of HTM cells was investigated by labeling the cells
with EdU to fluorescently stain the replicating cells. Notably, the
fluorescence images indicate that the cells in which the YAP and
TAZ genes were both knocked down possessed significantly reduced
proliferation capability (Fig. 4A and
B), indicating that YAP and TAZ may have a pivotal role in the
regulation of HTM cell proliferation. By contrast, as depicted in
Fig. 4C and D, when expression
vectors for YAP and TAZ were employed, the HTM cells cotransfected
with YAP and TAZ plasmids exhibited significantly increased
proliferation ability. These results indicate the involvement of
YAP and TAZ in the regulation of the proliferation of HTM
cells.
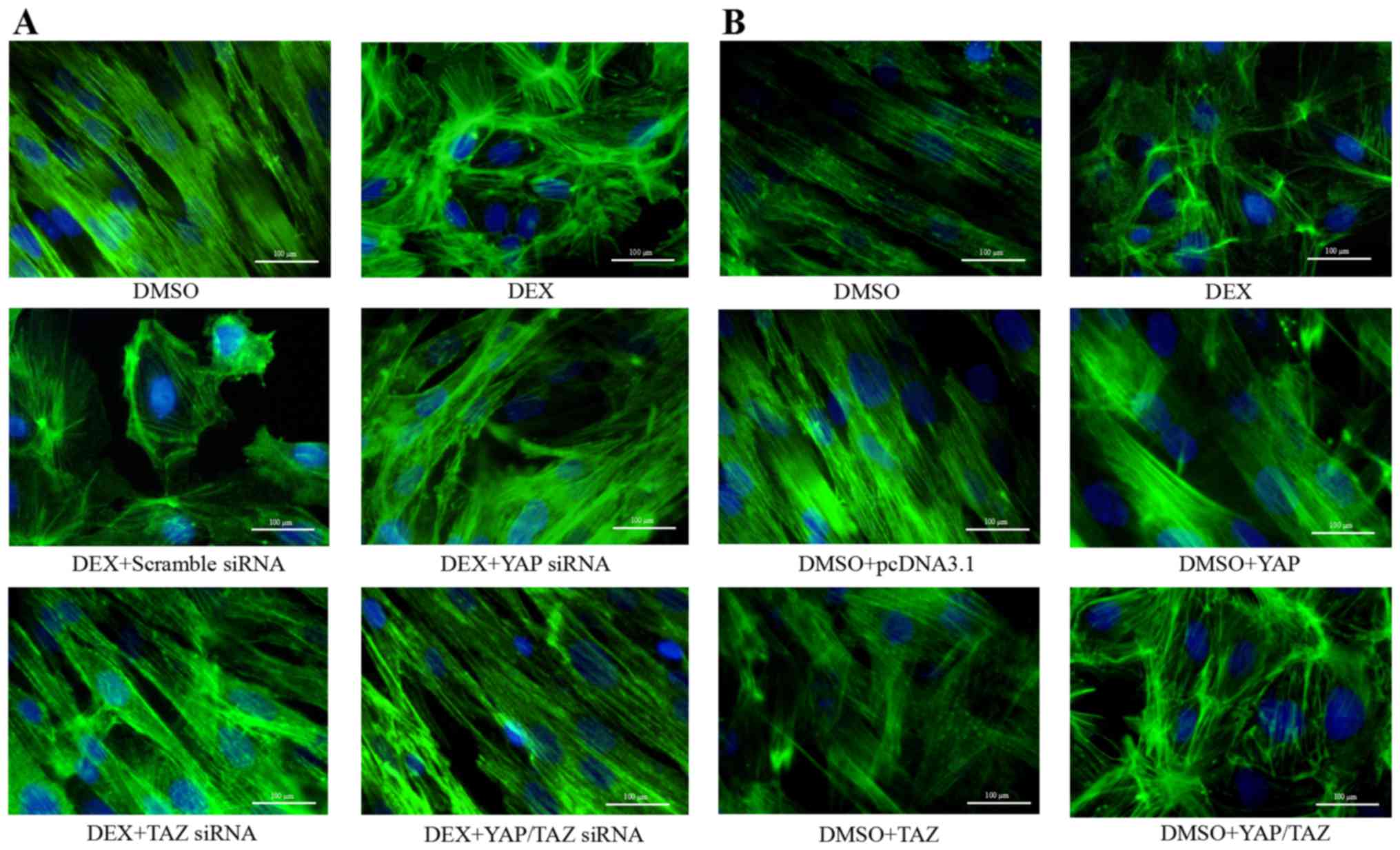
YAP and TAZ regulate CLAN formation in
HTM cells
Since CLAN formation in glaucomatous and DEX-treated
HTM cells has been indicated to contribute to reduced outflow
facility (36), CLAN formation in
the presence of DEX, YAP siRNA and/or TAZ siRNA was investigated in
the present study. From the images presented in Fig. 5A, it is clear that DEX-treated HTM
cells formed unusual geodesic dome-like cross-linked actin
networks. The majority of these cells exhibited a CLAN, which was
composed of a series of interconnected F-actin bundles that
radiated outward from central vertices in a geodesic network.
However, YAP siRNA and TAZ siRNA appeared to markedly alter overall
cell spreading, and induced reorganization of the actin skeleton.
Thus, is appears that CLAN formation in the HTM cells cotransfected
with YAP siRNA and TAZ siRNA was attenuated. The actin cytoskeleton
and actin microfilament patterns were comparable to those of the
DMSO-treated control cells. To further evaluate whether YAP and TAZ
contribute to rearrangement of the cytoskeleton in HTM cells, YAP
and/or TAZ overexpression plasmids were transfected into the HTM
cells. As shown in Fig. 5B, the
overexpression of YAP and TAZ induced CLAN formation with polygonal
actin arrangements in the HTM cells. The actin filaments were
dispersed around the cell, with a few microfilaments in the center,
and were similar in appearance to those of the DEX-treated control
cells. These observations suggest that YAP and TAZ play a key role
in CLAN formation in HTM cells.
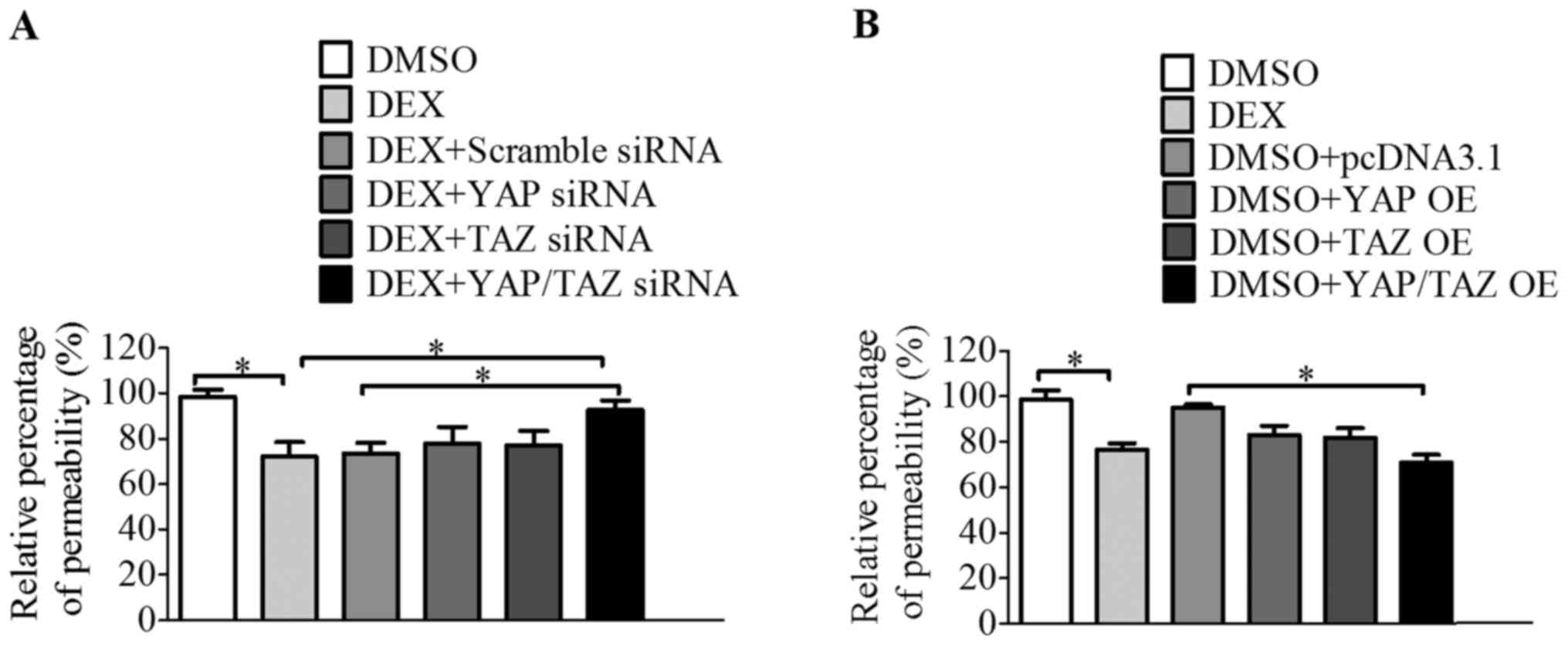
YAP and TAZ modulate the aqueous humor
outflow of HTM cells
It has been suggested that DEX-induced CLAN
formation in HTM cells decreases the overall contractility of the
tissue by causing the cells to become more rigid and unable to
change shape (10). Therefore,
the permeability of DEX-treated monolayer HTM cells may be markedly
impaired. In order to further investigate the hypothesis that YAP
and TAZ regulate the function of HTM cells, a permeability assay
was performed. The DEX-treated monolayer HTM cells exhibited
impaired permeability, which is consistent with previous studies
(26,37). However, when YAP and TAZ
expression was downregulated with a combination of YAP and TAZ
siRNA, the permeability was increased compared with that in the
cells treated with DEX alone and those treated with DEX plus
scramble siRNA (Fig. 6A). In
addition, cells transfected with a combination of YAP and TAZ
expression plasmids exhibited significantly decreased permeability
compared with the cells treated with DMSO and transfected with an
empty plasmid (Fig. 6B). This
observation indicates that YAP and TAZ regulate aqueous outflow
and, thereby alter the function of HTM cells.
Discussion
POAG is a common cause of blindness (1). Although the exact cause of POAG is
not yet clearly understood, the main abnormal changes associated
with this condition appear to affect the TM (3). TM cells are capable of the
phagocytosis, migration, synthesis and secretion of ECM components,
and the transduction of signals between ECMs. Dysfunction of the TM
and the lining cells of Schlemm's canal, as well as structural
abnormalities of the ECM are the main causes of resistance to the
outflow of aqueous humor. Changes in ECM components cause narrowing
of the outflow tract in adjacent areas, thereby increasing
resistance to the outflow of aqueous humor and elevating the IOP
(38). In the present study, the
signal transduction of YAP and TAZ in HTM cells, and its regulatory
role in the cytoskeletal arrangement of HTM cells were
investigated.
The symptoms of POAG include increased resistance to
the outflow of aqueous humor, increased TM hardness, hardening and
degeneration of the trabecular tissue, reduced mesh density,
irregularity or injury of the trabecular plate layer, increased
endothelial cell density, the degeneration of collagen or elastic
fibers, and the narrowing of spaces in the TM (5). These changes in TM properties are
indicative of a biophysical mechanism for the regulation of IOP and
glaucoma (39–43). However, the molecular mechanism by
which the biophysical properties affect cell behaviour is not yet
completely understood. YAP and TAZ have been identified as
mechanical signaling factors, which are affected by ECM hardness
(20). YAP and TAZ are the main
effectors of the classical Hippo pathway in the regulation of organ
size and tissue topology; however, they are considered to exert
actions beyond those of the classical pathway due to their dynamic
transduction effects as nuclear transcription factors in mechanical
signaling (44). As
mechanotransducers of the extracellular-microenvironment and
coactivators of transcription, YAP and TAZ have been shown to be
upregulated by the elastic hardness of the TM in glaucoma (19). In addition, previous studies have
demonstrated that YAP serves important roles in the regulation of
cell proliferation and apoptosis, the control of organ size, the
contact inhibition of cells and tumorigenesis (13–17,44).
Glucocorticoids have been shown to cause progressive
changes in the organization of the microfilaments in TM cells
(31,45). Therefore, in the present study,
the effects of DEX on HTM cells were investigated. It was observed
that as the concentration of DEX increased, the expression levels
of YAP also significantly increased, and the cytoskeleton was
damaged. TAZ and YAP genes are both conjugated with 14-3-3σ
protein, and have the same transcription activation function
(46). The similarity of the TAZ
and YAP genes was reflected in the dose response data for DEX,
which demonstrated that the expression levels of TAZ were also
elevated as the concentration of DEX increased. It has been
reported that in HTM cells cultured under high pressure, the
expression levels of YAP and TAZ greatly exceed than those of HTM
cells cultured at normal pressure (19). Their study demonstrated that
numerous proteins are involved in the dynamic induction and
transduction process, and identified that YAP and TAZ are dynamic
transduction and transcription factors. YAP and TAZ have been
confirmed to exist in all layers of the TM, including adjacent
tissue (14), which is considered
to be important in the generation of resistance to the outflow of
aqueous humor. Therefore, the further exploration of YAP and TAZ
genes in glaucoma is merited.
The fluorescence microscopic analysis conducted in
the present study demonstrated that the knockdown of YAP and TAZ
rescued HTM cells from the morphological changes induced by DEX.
Furthermore, it revealed that the overexpression of YAP and TAZ
contributed to the reorganization of HTM cells to form
geodesic-dome-like polygonal lattices. Since reorganization of the
TM cytoskeleton alters cell function (8,47),
the normalization of HTM cell morphology by silencing the
expression of YAP and TAZ was observed to restore the functionality
of the HTM cells in the presence of DEX in the cell permeability
assay.
The glucocorticoid treatment of cultured TM cells
has been reported to inhibit TM cell migration and proliferation
(45). Therefore, the present
study investigated the effect of YAP and TAZ knockdown on the
proliferation of HTM cells. It has been reported that β-catenin
binds with E-cadherin to bridge between the cytoplasmic domain of
cadherin and the actin cytoskeleton, thus realizing a connection
between cells and affecting the adhesion and motility of cells
(48). The Wnt signaling pathway
is considered a new target for intervention in the treatment of
glaucoma, and the Wnt/β-catenin signaling pathway has been
demonstrated to be modulated by YAP and TAZ, with TAZ exhibiting
antagonistic effects (49,50).
In the present study, it was demonstrated that the knockdown of YAP
and TAZ decreased the expression levels of β-catenin, whereas YAP
and TAZ overexpression increased them. In addition, the present
study indicated that YAP and TAZ overexpression stimulated cell
proliferation.
The present study on the mechanisms underlying the
effects of YAP and TAZ in HTM cells indicates that YAP and TAZ
serve critical roles in glaucoma. The expression of YAP and TAZ in
the cultured TM cells of normal human eyes was demonstrated by
western blotting and RT-qPCR. Notably, when the HTM cells were
treated with DEX, the expression levels of YAP and TAZ were
elevated, indicating the involvement of YAP and TAZ in the
pathogenesis of glaucoma. The treatment of cultured TM cells with
DEX is a classical model with which to simulate the state of TM
cells in glucocorticoid-induced glaucoma. In the present study, the
treatment of TM cells with DEX was demonstrated to markedly
increase the expression levels of fibronectin, laminin and collagen
IV in the ECM, reduce the expression of collagen I, induce the
restructuring of actin microfilaments, promote the formation of
classical cross-linked actin networks, and increase the expression
of β-catenin. These changes are likely to result in increased TM
hardness, restructuring of the TM and dynamic changes in the
microenvironment.
In conclusion, in the present study, the effects of
the knockdown and overexpression of YAP and TAZ on the expression
levels of fibronectin, laminin, collagen I, collagen IV and
β-catenin were analyzed, and the effects of YAP and TAZ on the ECM
and the actin microfilaments and cytoskeletons of DEX-treated HTM
cells were observed. The findings improve our understanding of the
involvement of YAP and TAZ in glaucoma pathogenesis, and suggest
new mechanisms for the development and progression of glaucoma.
Acknowledgments
This study was supported by the National Science
Foundation of China (grant no. 81300768), the Sichuan Science
Foundation (grant no. 2015JY0183), the Sichuan Health and Family
Planning Commission (grant no. 16ZD025), and by funding from
Sichuan Provincial People's Hospital and the Sichuan Scientific
Research Foundation of Returned Overseas Chinese Scholars for Dr.
Yi Wang.
References
|
1
|
Quigley HA and Broman AT: The number of
people with glaucoma worldwide in 2010–2020. Br J Ophthalmol.
90:262–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gordon MO, Beiser JA, Brandt JD, Heuer DK,
Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd,
Wilson MR, et al: The Ocular Hypertension Treatment Study: baseline
factors that predict the onset of primary open-angle glaucoma. Arch
Ophthalmol. 120:714–720. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson M: 'What controls aqueous humour
outflow resistance?'. Exp Eye Res. 82:545–557. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gottanka J, Johnson DH, Martus P and
Lütjen-Drecoll E: Severity of optic nerve damage in eyes with POAG
is correlated with changes in the trabecular meshwork. J Glaucoma.
6:123–132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Last JA, Pan T, Ding Y, Reilly CM, Keller
K, Acott TS, Fautsch MP, Murphy CJ and Russell P: Elastic modulus
determination of normal and glaucomatous human trabecular meshwork.
Invest Ophthalmol Vis Sci. 52:2147–2152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kersey JP and Broadway DC:
Corticosteroid-induced glaucoma: a review of the literature. Eye
(Lond). 20:407–416. 2006. View Article : Google Scholar
|
|
7
|
Stokes J, Walker BR, Campbell JC, Seckl
JR, O'Brien C and Andrew R: Altered peripheral sensitivity to
glucocorticoids in primary open-angle glaucoma. Invest Ophthalmol
Vis Sci. 44:5163–5167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhuo YH, He Y, Leung KW, Hou F, Li YQ,
Chai F and Ge J: Dexamethasone disrupts intercellular junction
formation and cytoskeleton organization in human trabecular
meshwork cells. Mol Vis. 16:61–71. 2010.PubMed/NCBI
|
|
9
|
Knepper PA, Collins JA and Frederick R:
Effects of dexamethasone, progesterone, and testosterone on IOP and
GAGs in the rabbit eye. Invest Ophthalmol Vis Sci. 26:1093–1100.
1985.PubMed/NCBI
|
|
10
|
Clark AF, Brotchie D, Read AT, Hellberg P,
English-Wright S, Pang IH, Ethier CR and Grierson I: Dexamethasone
alters F-actin architecture and promotes cross-linked actin network
formation in human trabecular meshwork tissue. Cell Motil
Cytoskeleton. 60:83–95. 2005. View
Article : Google Scholar
|
|
11
|
Clark AF, Wilson K, McCartney MD, Miggans
ST, Kunkle M and Howe W: Glucocorticoid-induced formation of
cross-linked actin networks in cultured human trabecular meshwork
cells. Invest Ophthalmol Vis Sci. 35:281–294. 1994.PubMed/NCBI
|
|
12
|
Kango-Singh M and Singh A: Regulation of
organ size: insights from the Drosophila Hippo signaling pathway.
Dev Dyn. 238:1627–1637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saucedo LJ and Edgar BA: Filling out the
Hippo pathway. Nat Rev Mol Cell Biol. 8:613–621. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buttitta LA and Edgar BA: How size is
controlled: from Hippos to Yorkies. Nat Cell Biol. 9:1225–1227.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan D: Hippo signaling in organ size
control. Genes Dev. 21:886–897. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao B, Lei QY and Guan KL: The Hippo-YAP
pathway: new connections between regulation of organ size and
cancer. Curr Opin Cell Biol. 20:638–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu FX and Guan KL: The Hippo pathway:
regulators and regulations. Genes Dev. 27:355–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang K, Degerny C, Xu M and Yang XJ: YAP,
TAZ, and Yorkie: a conserved family of signal-responsive
transcriptional coregulators in animal development and human
disease. Biochem Cell Biol. 87:77–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raghunathan VK, Morgan JT, Dreier B,
Reilly CM, Thomasy SM, Wood JA, Ly I, Tuyen BC, Hughbanks M, Murphy
CJ, et al: Role of substratum stiffness in modulating genes
associated with extracellular matrix and mechanotransducers YAP and
TAZ. Invest Ophthalmol Vis Sci. 54:378–386. 2013. View Article : Google Scholar :
|
|
20
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li C, Chen J, Lu B, Shi Z, Wang H, Zhang
B, Zhao K, Qi W, Bao J and Wang Y: Molecular switch role of Akt in
Polygonatum odoratum lectin-induced apoptosis and autophagy in
human non-small cell lung cancer A549 cells. PLoS One.
9:e1015262014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang G, Lv J, Li T, Huai G, Li X, Xiang
S, Wang L, Qin Z, Pang J, Zou B, et al: Notoginsenoside R1
ameliorates podocyte injury in rats with diabetic nephropathy by
activating the I3K/Akt signaling pathway. Int J Mol Med.
38:1179–1189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Liu Y, Wang H, Li C, Qi P and Bao
J: Agaricus bisporus lectins mediates islet β-cell proliferation
through regulation of cell cycle proteins. Exp Biol Med (Maywood).
237:287–296. 2012. View Article : Google Scholar
|
|
24
|
Wang Y, Wang H, Liu Y, Li C, Qi P and Bao
J: Antihyperglycemic effect of ginsenoside Rh2 by inducing islet
β-cell regeneration in mice. Horm Metab Res. 44:33–40. 2012.
View Article : Google Scholar
|
|
25
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perkins TW, Alvarado JA, Polansky JR,
Stilwell L, Maglio M and Juster R: Trabecular meshwork cells grown
on filters. Conductivity and cytochalasin effects. Invest
Ophthalmol Vis Sci. 29:1836–1846. 1988.PubMed/NCBI
|
|
27
|
Thomasy SM, Morgan JT, Wood JA, Murphy CJ
and Russell P: Substratum stiffness and latrunculin B modulate the
gene expression of the mechanotransducers YAP and TAZ in human
trabecular meshwork cells. Exp Eye Res. 113:66–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rozsa FW, Reed DM, Scott KM, Pawar H,
Moroi SE, Kijek TG, Krafchak CM, Othman MI, Vollrath D, Elner VM,
et al: Gene expression profile of human trabecular meshwork cells
in response to long-term dexamethasone exposure. Mol Vis.
12:125–141. 2006.PubMed/NCBI
|
|
29
|
Steely HT, Browder SL, Julian MB, Miggans
ST, Wilson KL and Clark AF: The effects of dexamethasone on
fibronectin expression in cultured human trabecular meshwork cells.
Invest Ophthalmol Vis Sci. 33:2242–2250. 1992.PubMed/NCBI
|
|
30
|
Filla MS, Clark R and Peters DM: A
syndecan-4 binding peptide derived from laminin 5 uses a novel PKCε
pathway to induce cross-linked actin network (CLAN) formation in
human trabecular meshwork (HTM) cells. Exp Cell Res. 327:171–182.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Clark AF, Miggans ST, Wilson K, Browder S
and McCartney MD: Cytoskeletal changes in cultured human glaucoma
trabecular meshwork cells. J Glaucoma. 4:183–188. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou L, Li Y and Yue BY: Glucocorticoid
effects on extracellular matrix proteins and integrins in bovine
trabecular meshwork cells in relation to glaucoma. Int J Mol Med.
1:339–346. 1998.PubMed/NCBI
|
|
33
|
Peng J, Feng XY, Ye ZM, Luo Q, Cheng YL,
Wu ZZ, Lei CT and Gong B: Effects of dexamethasone and HA1077 on
actin cytoskeleton and β-catenin in cultured human trabecular
meshwork cells. Int J Ophthalmol. 9:1376–1380. 2016.
|
|
34
|
Lange AW, Sridharan A, Xu Y, Stripp BR,
Perl AK and Whitsett JA: Hippo/Yap signaling controls epithelial
progenitor cell proliferation and differentiation in the embryonic
and adult lung. J Mol Cell Biol. 7:35–47. 2015. View Article : Google Scholar :
|
|
35
|
Morgan JT, Murphy CJ and Russell P: What
do mechanotransduction, Hippo, Wnt, and TGFβ have in common? YAP
and TAZ as key orchestrating molecules in ocular health and
disease. Exp Eye Res. 115:1–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Filla MS, Schwinn MK, Sheibani N, Kaufman
PL and Peters DM: Regulation of cross-linked actin network (CLAN)
formation in human trabecular meshwork (HTM) cells by convergence
of distinct beta1 and beta3 integrin pathways. Invest Ophthalmol
Vis Sci. 50:5723–5731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rao PV, Deng PF, Kumar J and Epstein DL:
Modulation of aqueous humor outflow facility by the Rho
kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci.
42:1029–1037. 2001.PubMed/NCBI
|
|
38
|
Sit AJ and Liu JH: Pathophysiology of
glaucoma and continuous measurements of intraocular pressure. Mol
Cell Biomech. 6:57–69. 2009.PubMed/NCBI
|
|
39
|
McKee CT, Wood JA, Shah NM, Fischer ME,
Reilly CM, Murphy CJ and Russell P: The effect of biophysical
attributes of the ocular trabecular meshwork associated with
glaucoma on the cell response to therapeutic agents. Biomaterials.
32:2417–2423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wood JA, McKee CT, Thomasy SM, Fischer ME,
Shah NM, Murphy CJ and Russell P: Substratum compliance regulates
human trabecular meshwork cell behaviors and response to
latrunculin B. Invest Ophthalmol Vis Sci. 52:9298–9303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wood JA, Shah NM, McKee CT, Hughbanks ML,
Liliensiek SJ, Russell P and Murphy CJ: The role of substratum
compliance of hydrogels on vascular endothelial cell behavior.
Biomaterials. 32:5056–5064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chew SY and Low WC: Scaffold-based
approach to direct stem cell neural and cardiovascular
differentiation: an analysis of physical and biochemical effects. J
Biomed Mater Res A. 97:355–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han H, Wecker T, Grehn F and Schlunck G:
Elasticity-dependent modulation of TGF-β responses in human
trabecular meshwork cells. Invest Ophthalmol Vis Sci. 52:2889–2896.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao B, Li L, Lei Q and Guan KL: The
Hippo-YAP pathway in organ size control and tumorigenesis: an
updated version. Genes Dev. 24:862–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wordinger RJ and Clark AF: Effects of
glucocorticoids on the trabecular meshwork: towards a better
understanding of glaucoma. Prog Retin Eye Res. 18:629–667. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Morrison DK: The 14-3-3 proteins:
integrators of diverse signaling cues that impact cell fate and
cancer development. Trends Cell Biol. 19:16–23. 2009. View Article : Google Scholar
|
|
47
|
Junglas B, Kuespert S, Seleem AA, Struller
T, Ullmann S, Bösl M, Bosserhoff A, Köstler J, Wagner R, Tamm ER,
et al: Connective tissue growth factor causes glaucoma by modifying
the actin cytoskeleton of the trabecular meshwork. Am J Pathol.
180:2386–2403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tovar-Vidales T, Roque R, Clark AF and
Wordinger RJ: Tissue transglutaminase expression and activity in
normal and glaucomatous human trabecular meshwork cells and
tissues. Invest Ophthalmol Vis Sci. 49:622–628. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mao W, Millar JC, Wang WH, Silverman SM,
Liu Y, Wordinger RJ, Rubin JS, Pang IH and Clark AF: Existence of
the canonical Wnt signaling pathway in the human trabecular
meshwork. Invest Ophthalmol Vis Sci. 53:7043–7051. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Varelas X, Miller BW, Sopko R, Song S,
Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill
H, et al: The Hippo pathway regulates Wnt/beta-catenin signaling.
Dev Cell. 18:579–591. 2010. View Article : Google Scholar : PubMed/NCBI
|