Introduction
Aging is defined as a time-dependent degenerative
process caused by accumulated damage that leads to cellular
dysfunction, tissue damage and ultimately death (1,2).
Aging of the kidney is associated with structural changes and
functional decline (3), which
render the elderly more vulnerable to stress factors, including
heart failure, dehydration and hypertension, eventually leading to
the development of chronic kidney disease (4,5).
The kidney has an essential role in the aging process, and renal
function has been suggested to be an important predictor of
longevity (4,6). Furthermore, with aging, several
structural and functional changes reportedly occur in renal
glomeruli and in proximal tubular epithelial cells (7).
Acute kidney injury (AKI) occurs when there is a
rapid decline in kidney function (8). In general, AKI affects proximal
tubular epithelial cells. In addition, activated immune cells
produce a number of inflammatory mediators and reactive oxygen
species (ROS) that may cause tubular cell damage (8,9).
AKI may be triggered by multiple stimuli, including ischemia,
nephrotoxins, radiocontrast media and bacterial endotoxins
(8,10,11). Nuclear factor erythroid 2-related
factor 2 (Nrf2) and nuclear factor-κB (NF-κB) are representative
transcription factors that function in transcriptional adaption to
chemical stress (10,12).
Cisplatin is one of the most widely used and
effective drugs for the treatment of various types of solid tumor
(13,14). Cisplatin-induced renal injury may
be pathophysiologically classified into the following four types:
Tubular toxicity, vascular damage, glomerular injury and
interstitial injury (14,15). The stepwise and complex processes
that result in renal damage involve accumulation of potentially
toxic compounds in the tubular fluid, which then diffuse into the
highly permeable tubular cells (13). Numerous studies have demonstrated
that several mechanisms, including oxidative stress, DNA damage and
inflammatory responses, mediate cisplatin-induced nephrotoxicity
(13,16). The key pathological occurrences in
cisplatin-induced nephrotoxicity are renal tubular cell injury and
death (14,17).
Brown algae may represent a natural renewable source
of novel therapeutic agents, as they are rich in functional
bioactive substances, including sulfated polysaccharides,
carotenoids, dietary fiber and proteins (18–28). However, despite the increased rate
of discovery of biologically active substances from marine
resources, little is known regarding the potential effects of
seaweeds in terms of changes in renal structure and function during
cisplatin treatment (29–31).
Previous studies by our group reported on the
cellular and molecular effects of peptide and hydrophilic protein
extracts of the brown seaweed Porphyra yezoensis (P.
yezoensis) based on analyses of cytotoxicity (20,32), inflammation (33–37) and cell proliferation (38–41). However, the biological effects of
P. yezoensis protein (PYP) on cisplatin-induced renal injury
have remained elusive. In the present study, PYP, a specific active
factor, was obtained from P. yezoensis through water-based
extraction followed by sequential ammonium sulfate precipitation.
The therapeutic potential of PYP in reducing cisplatin-induced AKI
during chemotherapy was assessed using the HK2 human proximal
tubular epithelial cell line as well as model mice.
Materials and methods
Preparation of PYP
PYP was prepared according to a previous protocol
(37), with slight modifications.
P. yezoensis was desalted by washing and then lyophilized. A
crude aqueous extract was prepared by mixing the P.
yezoensis powder with distilled water (DW) and incubating the
resulting mixture at room temperature for 4 h. The aqueous extract
was clarified by centrifugation and filtered to remove any
insoluble material. The crude extract was subjected to 80% ammonium
sulfate saturation and then centrifuged at 5,000 × g for 30 min at
4°C. The precipitate was suspended in the minimum volume of DW and
dialyzed for 48 h at 4°C against 2,000 ml of DW. The dialyzed
sample was lyophilized for 3 days and the residue was stored at
−70°C until further use. The purity and molecular weight of PYP
were assessed by sodium dodecyl sulfate-poly-acrylamide gel
electrophoresis (SDS-PAGE).
Cell culture and treatment with PYP
The HK2 immortalized human proximal tubular
epithelial cell line (no. CRL-2190) was obtained from the American
Type Culture Collection (Manassas, VA, USA). Cells were cultured in
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (GenDepot, Inc., Barker, TX, USA) and antibiotics (50
µg/ml penicillin, 25 µg/ml amphotericin B and 50
µg/ml streptomycin). Prior to incubation with cisplatin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), cells were
pretreated with various concentrations of PYP for the indicated
durations.
Cell viability assay
Cell viability was assessed using a CytoX cell
viability assay kit (CYT3000; LPS solution, Daejeon, Korea). Cells
were seeded in 96-well plates at 4×104 cells/well and
allowed to attach for 24 h. Attached cells were treated with
cisplatin (0–40 µM) or PYP (0–100 µg/ml) in
serum-free medium (SFM) for 24 h. The CytoX solution was added to
the cells, followed by incubation for 1 h, and the absorbance of
each well was then measured at 450 nm using a microplate reader
(Gen 5; Epoch BioTek Instrument, Inc., Winooski, VT, USA). In
addition, cell morphological changes were observed under a light
microscope (magnification, ×200) using a Nikon inverted Eclipse
TS100-F microscope system (Epi-Fluorescence attachment; Nikon
Corp., Tokyo, Japan).
Protein extraction and western blot
analysis
HK2 cells were grown to 80% confluence in 100 mm
dish plates. Attached cells were treated with PYP (0–100
µg/ml) in SFM for 24 h and cisplatin (20 µM) for 8 h.
After treatment, the cells were washed twice with
phosphate-buffered saline (PBS), harvested and lysed in
radioimmunoprecipitation assay buffer (50 mM Tris, pH 7.4, 1 mM
EDTA, 150 mM sodium chloride, 1% nonidet-40 and 0.25% sodium
deoxycholate) containing a protease inhibitor cocktail (Geno
Technology Inc., St. Louis, MO, USA). The lysate was centrifuged at
9,750 × g for 10 min at 4°C and the supernatants were used for
western blot analysis.
In addition, cytoplasmic and nuclear lysates were
separated using an NE-PER® extraction reagents kit
(78833; Pierce; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. To isolate mitochondria, cells were lysed
using a Dounce homogenizer in buffer containing 20 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.5, 250 mM
sucrose, 20 mM potassium chloride, 1.5 mM magnesium chloride, 1 mM
phenylmethylsulfonyl fluoride, 10 µg/ml aprotinin and 10
µg/ml leupeptin at 4°C. The cell lysates were centrifuged at
6,000 × g for 10 min to remove unbroken cells and nuclei. The
supernatants were then centrifuged at 7,000 × g for 10 min at 4°C
and the resulting pellets were collected as the mitochondrial
fraction.
Protein concentrations were determined using a BCA
protein assay kit (23225; Thermo Fisher Scientific, Inc.). Equal
amounts of protein (30 µg) were boiled for 10 min and
resolved by 7.5–12% SDS-PAGE. The resolved proteins were then
transferred to polyvinylidene difluoride membranes (Millipore
Corp., Billerica, MA, USA). The membranes were blocked by
incubation with 1% bovine serum albumin (BSA; GenDepot, Inc.) in a
buffer of 10 mM Tris-HCl (pH 7.5) containing 150 mM NaCl and 0.1%
Tween-20 (TBS-T) at room temperature for 1 h, and then incubated
with specific primary antibodies (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 3 h. The membranes were washed three times
with TBS-T and incubated for 2 h with the appropriate horseradish
peroxidase (HRP)-conjugated goat anti-rabbit, goat anti-mouse or
rabbit anti-goat secondary antibody (Santa Cruz Biotechnology,
Inc.) diluted at 1:10,000 in TBS-T and 1% BSA. The respective
proteins were detected using the chemiluminescent substrate
(K-12045; Advansta, Menlo Park, CA, USA) and visualized on a
GeneSys imaging system (SynGene Synoptics, Ltd., London, UK). The
following primary antibodies were used: Anti-B-cell lymphoma-2
[Bcl-2; cat. no. sc-492; anti-rabbit immunoglobulin (Ig)G],
anti-Bcl-2-associated X protein (Bax; cat. no. sc-493; anti-rabbit
IgG), anti-caspase-3 (cat. no. sc-7148; anti-rabbit IgG),
anti-cytochrome c (cat. no. sc-7159; anti-rabbit IgG),
anti-NF-κB (cat. no. sc-7151; anti-rabbit IgG), anti-Nrf2 (cat. no.
sc-722; anti-rabbit IgG), anti-phospho (p)-p38 (cat. no. sc-7973,
anti-mouse IgG), anti-p38 (cat. no. sc-7149; anti-rabbit IgG),
anti-p-mitogen-activated protein kinase (p-MAPK; cat. no. sc-7383;
anti-mouse IgG), anti-MAPK (cat. no. sc-94; anti-rabbit IgG),
anti-p-c-Jun N-terminal kinase (JNK; cat. no. sc-6254; anti-mouse
IgG), anti-JNK (cat. no. sc-7345; anti-mouse IgG),
anti-nitrotyrosine (cat. no. sc-32757; anti-mouse IgG),
anti-histone H3 (cat. no. sc-374669; anti-mouse IgG), anti-heat
shock protein 60 (cat. no. sc-59567; anti-mouse IgG) and
anti-β-actin (cat. no. sc-4778; anti-mouse IgG). The secondary
antibodies used were HRP-conjugated anti-mouse IgG (cat. no.
sc-2031; Santa Cruz Biotechnology, Inc.), anti-rabbit IgG (cat. no.
A-0545; Sigma-Aldrich Co., St. Louis, MO, USA) and anti-goat IgG
(cat. no. A50-101P; Bethyl Laboratories, Inc., Montgomery, TX,
USA).
Reactive oxygen species (ROS) content
assay
The relative levels of ROS were determined using a
2′,7′-dichlo-rofluorescin diacetate (DCF-DA) fluorescence assay.
Cells were pretreated with PYP and incubated in Hank's Balanced
Salt Solution (HBSS; pH 7.4) containing 20 µM DCF-DA
(Sigma-Aldrich; Merck KGaA) at 37°C for 1 h. Cells were then washed
with HBSS and treated with 20 µM cisplatin in HBSS for 8 h.
DCF-DA fluorescence intensity was determined using a
SpectraMax® M2 Multimode Microplate Reader (Molecular
Devices, Sunnyvale, CA, USA) with an excitation wavelength of 485
nm and an emission wavelength of 524 nm.
Animal care and PYP treatments
A total of 21 male C57BL/6 mice (22±2 g) were
purchased from Samtako Bio Korea Co. (Gyeonggi-do, Korea). They
were fed a standard commercial diet and housed at an ambient
temperature of 20–22°C with a relative humidity of 50±5% under a
12-h light/dark cycle in a specific pathogen-free facility. Food
and water were provided ad libitum. Mice (age, 8 weeks) were
divided into three treatment groups (n=7/group): Control group,
cisplatin group and PYP + cisplatin group. The mice in the
cisplatin group received cisplatin (20 mg/kg body weight) in 5%
dimethyl sulfoxide (v/v) as a single intraperitoneal injection. The
mice of the PYP + cisplatin group were orally administered PYP (100
mg/kg body weight) for 7 days. At 72 h after cisplatin injection,
mice were sacrificed and the kidney tissues and blood samples were
collected for the subsequent experiments.
The animal protocol of present study was approved by
the Institutional Animal Care and Use Committee of Pukyong National
University (Busan, Korea; approval no. 2016-14).
Renal function monitoring
For the renal function analysis, serum was separated
from blood and stored at -80°C until use. Serum creatinine and
blood urea nitrogen (BUN) levels were measured using an assay kit
(BioVision, Milpitas, CA, USA) according to the manufacturer's
instructions.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA from each sample was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total
RNA (1 µg) was subjected to first-strand complementary
(c)DNA synthesis using a Reverse Transcriptase Premix kit (IP25084;
Intron Biotechnology, Inc., Gyeonggi-do, Korea). PCR amplification
of the cDNA products was performed with 2X TOPsimple™ DyeMIX
(aliquot)-nTag (P561T; Enzynomics, Daejeon, Korea) and primer
pairs. The PCR primer sequences for the tested genes were as
follows: Tumor necrosis factor-α (TNF-α) forward, 5′-TGC ACC ACA
GTT TAA ACC CA-3′ and reverse, 5′-GAC TCCT TCA GGT GCT CAG G-3′
(42,43); interleukin-6 (IL-6) forward,
5′-AGG AGA CTT GCC TGG TGA AA-3′ and reverse, 5′-CAG GGG T GG TTA
TTG CAT CT-3′ (43); IL-1β
forward, 5′-CTG TCC TGC GTG TTG AAA GA-3′ and reverse, 5′-TTC TGC
TTG AGA GGT GCT GA-3′ (43);
monocyte chemoattractant protein-1 (MCP-1) forward, 5′-ACT GAA GCT
CGC ACT CTC-3′ and reverse, 5′-GAG TGA GGT GTT GGG TTC-3′;
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward, 5′-CAG
CCG AGC CAC ATC G-3′ and reverse, 5′-TGA GGC TGT TGT CAT ACT TCT
C-3′. Reaction mixtures were incubated for and initial denaturation
at 95°C for 3 min followed by 30 cycles of 95°C for 30 sec, 60°C
for 45 sec and 72°C for 60 sec. The amplified products were
normalized using GAPDH as an internal control, and separated using
1% agarose gel electrophoresis. The densitometry was determined
using GeneTools software, version 4.03 (Syngene, Cambridge,
UK).
Statistical analysis
Values are expressed as the mean ± standard
deviation (SD) of three independent experiments and seven animals.
Significant differences among multiple mean values were assessed by
one-way analysis of variance followed by the Bonferrori multiple
comparison test using GraphPad Prim 6 (GraphPad Software Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Preparation of PYP
PYP was isolated from the aqueous extract of P.
yezoensis by simple fractionation based on ammonium sulfate
precipitation. After ultrafiltration and lyophilization of the
pooled desalted PYP, a total of 120 mg of PYP was obtained from 5 g
of dried P. yezoensis. SDS-PAGE analysis revealed six major
bands and proteins of 25–37 kDa in size accounted for 50% of the
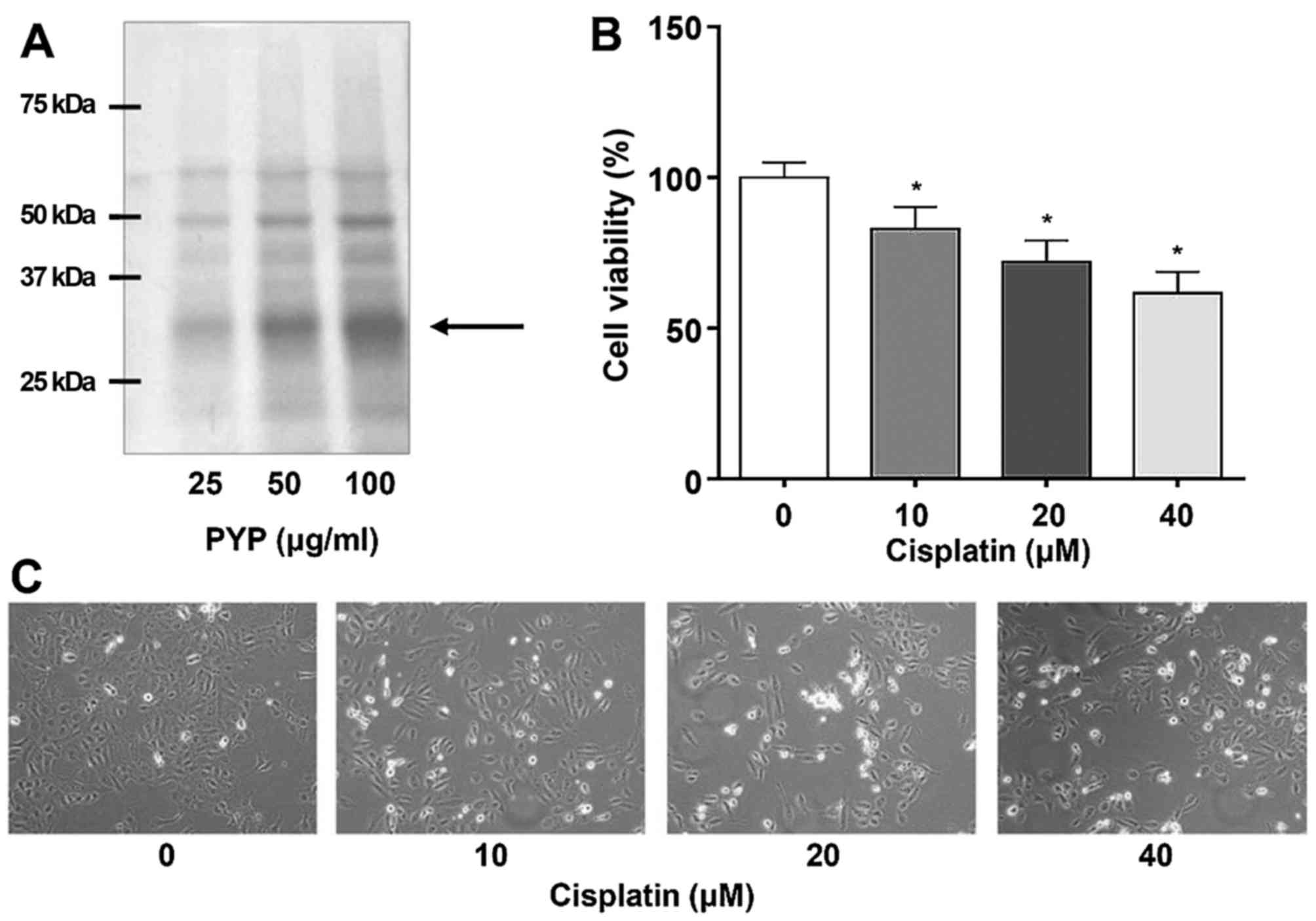
total protein (Fig. 1A).
Cisplatin decreases the viability of the
HK2 renal cell line
Renal proximal tubular epithelial cells are the
major targets of toxic drugs such as cisplatin (8). To assess the effects of cisplatin on
cell viability, HK2 cells were incubated with various
concentrations (0–40 µM) of cisplatin. The cisplatin
treatment significantly decreased the viability of HK2 cells in a
concentration-dependent manner (Fig.
1B). After incubation with 0, 10, 20 and 40 µM cisplatin
for 24 h, cell viability was significantly reduced to 100.0±5.00,
83.0±7.21, 72.0±7.00 and 61.7±7.02%, respectively. In addition, to
examine the effects of cisplatin at the microscopic level, HK2
cells were treated with various concentrations of cisplatin and
assessed under a microscope, indicating that the cell viability was
decreased in a cisplatin concentration-dependent manner (Fig. 1C).
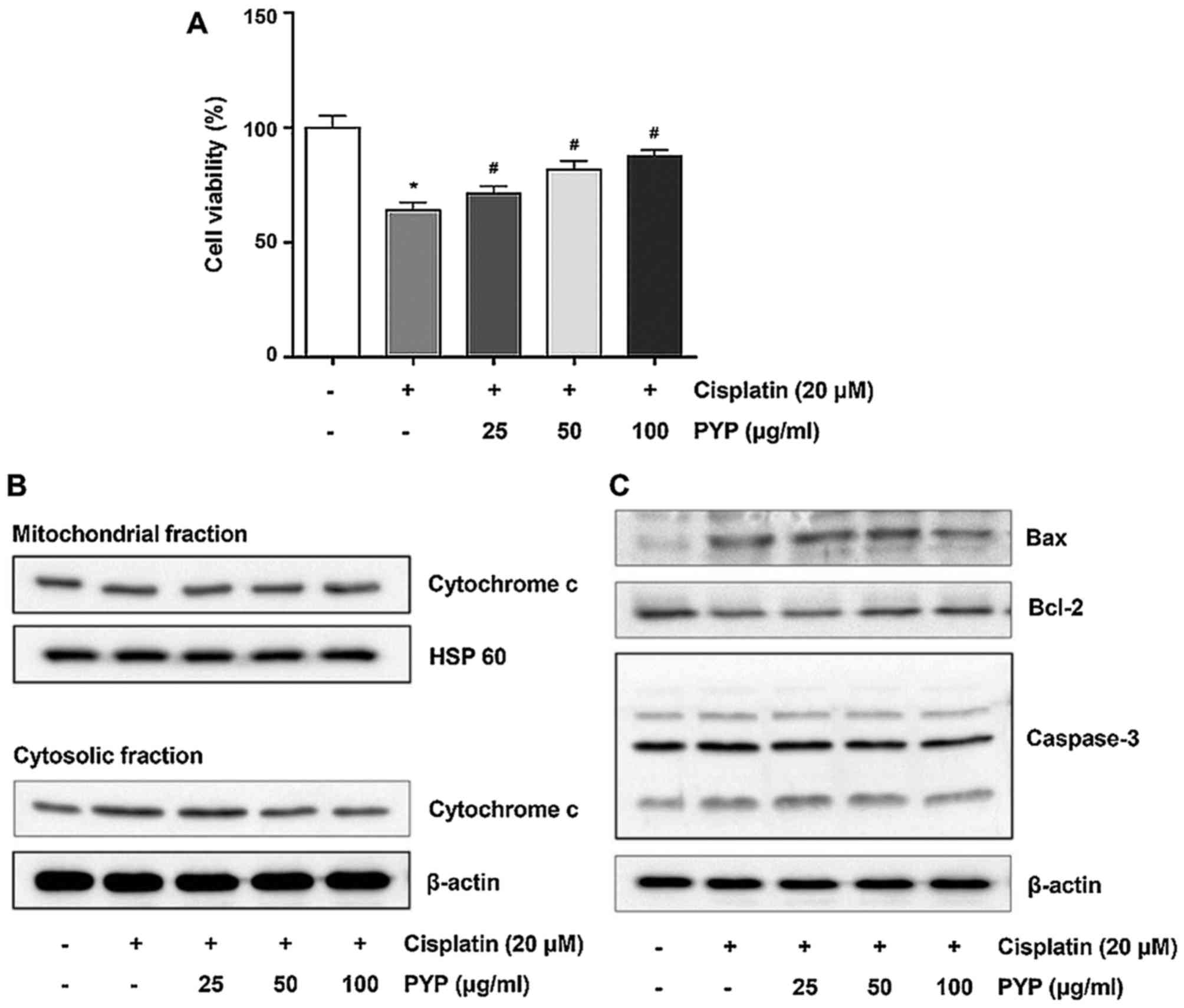
PYP protects HK2 cells against
cisplatin-induced cytotoxicity
The effects of PYP on cisplatin-induced decreases in
cell viability were then assessed. HK2 cells were incubated with 20
µM cisplatin for 8 h following pretreatment with various
concentrations of PYP for 24 h. As presented in Fig. 2A, PYP treatment increased the
viability of cisplatin-induced HK2 cells in a
concentration-dependent manner (Fig.
2A). The cell viability in the group treated with 20 µM
cisplatin was 64.0±3.61% of that in the control group. However,
treatment with 25, 50 and 100 µg/ml of PYP significantly
increased the cell viability in a concentration-dependent manner to
71.3±3.21, 81.7±3.79 and 87.7±2.52%, respectively, of that in the
control group.
Next, the effects of PYP treatment on the
translocation of cytochrome c in cisplatin-treated HK2 cells
were examined. As presented in Fig.
2B, treatment with cisplatin alone increased the release of
cytochrome c from the mitochondria into the cytosolic
fraction. However, treatment with PYP decreased the
cisplatin-induced release of cytochrome c from the
mitochondria to the cytosol. Furthermore, treatment with cisplatin
alone increased Bax and caspase-3 expression, and decreased Bcl-2
expression (Fig. 2C), which was
inhibited by PYP in a concentration-dependent manner.
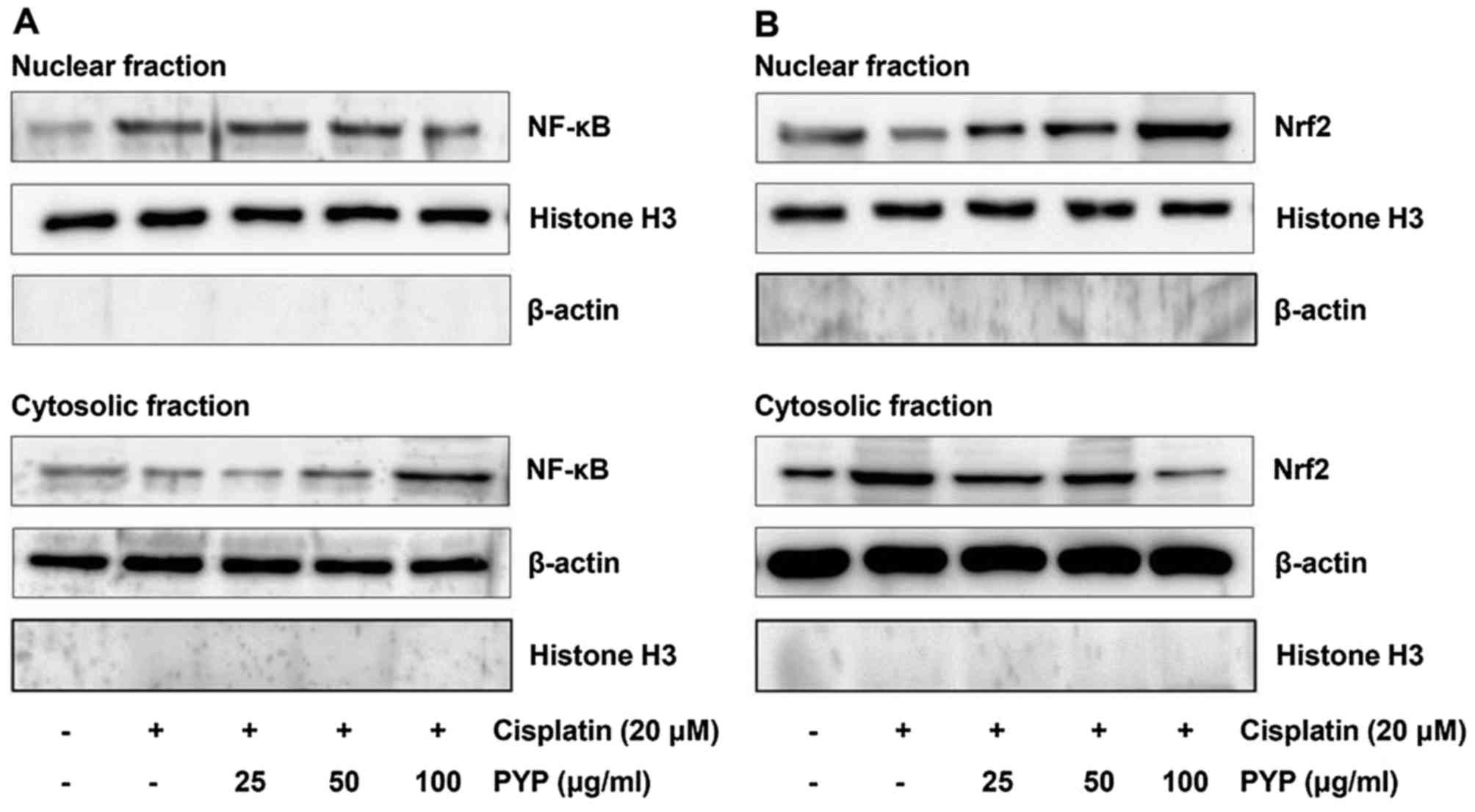
PYP inhibits NF-κB activation and
restores Nrf2 inactivation in cisplatin-treated HK2 cells
Drugs such as cisplatin may cause AKI, which may be
associated with increases of ROS or inflammation. AKI is also
associated with transcription factors such as NF-κB and Nrf2. NF-κB
is a key transcription factor in renal inflammation and the
oxidative stress response. NF-κB regulates the inflammatory
response by regulating anti- or pro-cytokine expression. Therefore,
the effects of PYP treatment on NF-κB translocation in
cisplatin-treated HK2 cells were examined by western blot analysis.
As presented in Fig. 3A,
cisplatin increased the levels of nuclear NF-κB p65 and decreased
the levels of cytosolic NF-κB p65, which was inhibited by PYP. Nrf2
inhibits cell damage by reducing intracellular ROS production. As
displayed in Fig. 3B, cisplatin
decreased the content of Nrf2 in the nuclear fraction and increased
that in the cytosolic fraction, which was inhibited by PYP
treatment.
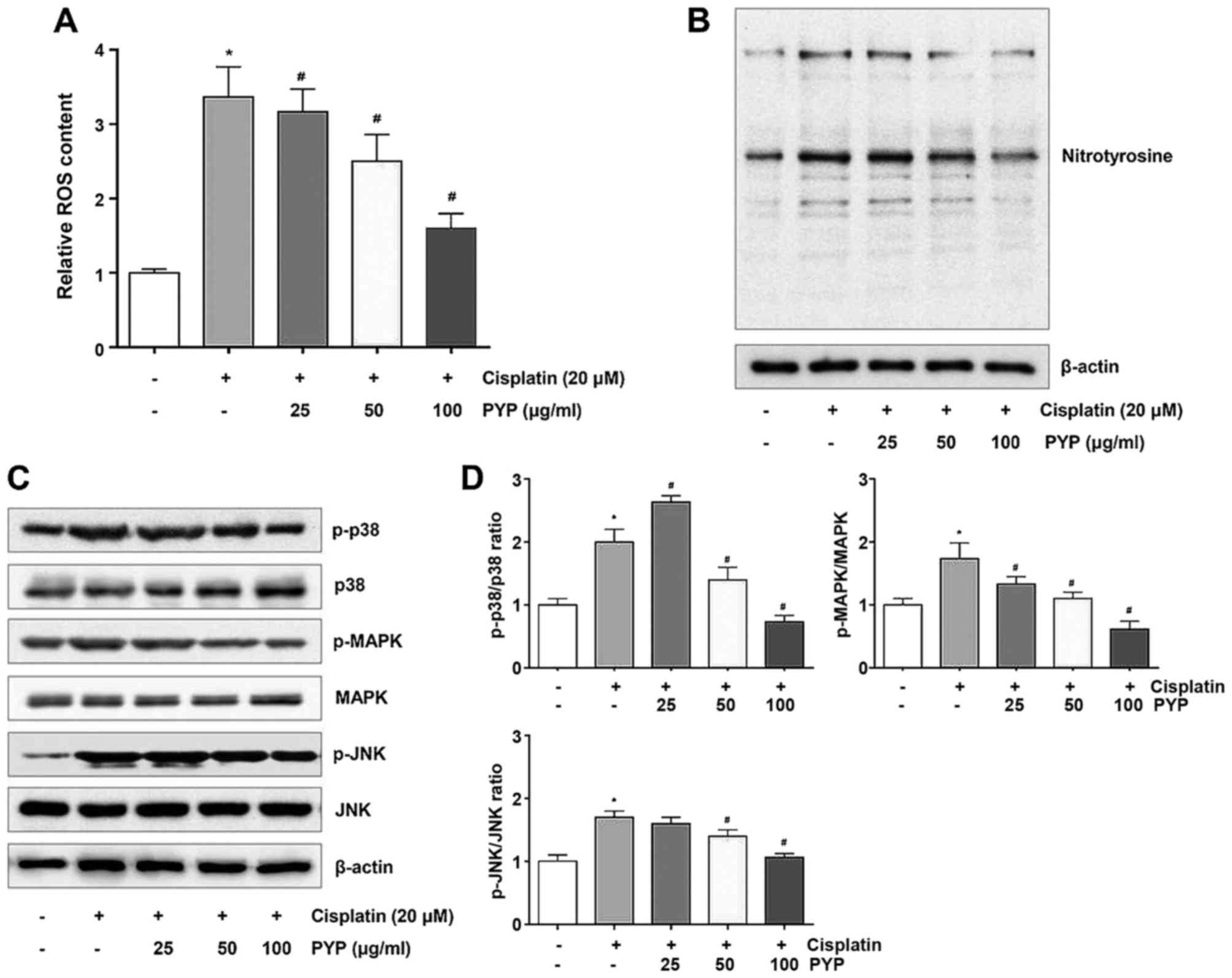
PYP decreases cisplatin-induced oxidative
stress by downregulating the p38/MAPK/JNK pathway
To determine the role of ROS production in the
cytoprotective effects of PYP, its effects on ROS production and
nitrotyrosine expression in cisplatin-treated HK2 cells were
examined. HK2 cells were incubated with 20 µM cisplatin for
8 h after pretreatment with PYP, and intracellular ROS production
and nitrotyrosine expression levels were then determined by
measuring the fluorescence intensity of DCF-DA and western blot
analysis, respectively. While ROS were significantly induced in the
cisplatin treatment group, they were significantly and
concentration-dependently reduced in the groups pretreated with PYP
(Fig. 4A). Nitrotyrosine
expression was also significantly reduced in the presence of PYP in
a concentration-dependent manner (Fig. 4B). The phosphorylation levels of
MAPK proteins including p38, ERK and JNK are correlated with
oxidative stress. In the present study, it was determined that the
phosphorylation levels of p38, MAPK and JNK were increased in the
cisplatin treatment group, which was inhibited in the presence of
PYP in a concentration-dependent manner (Fig. 4C and D).
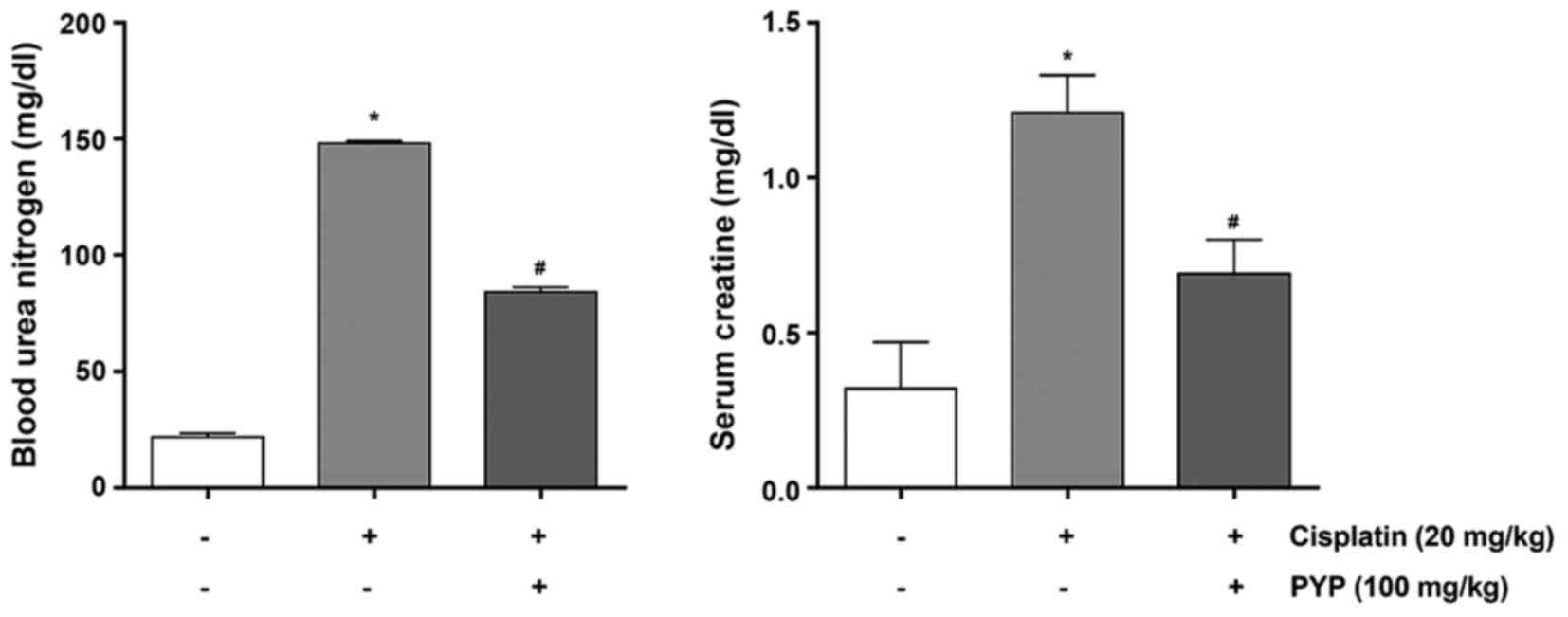
PYP treatment improves cisplatin-induced
renal dysfunction in vivo
The renoprotective role of PYP against
cisplatin-induced AKI was then examined in vivo. BUN and
creatinine levels of cisplatin/PYP-treated mice were assessed to
evaluate kidney function. As presented in Fig. 5, the levels of BUN were
significantly higher in the cisplatin group (148.1±1.01 mg/dl)
compared with those in the control group (21.6±1.74 mg/dl).
However, in the PYP + cisplatin group, the cisplatin-associated
increase in BUN was significantly inhibited by PYP (84.2±2.07
mg/dl). In addition, as presented in Fig. 5, the levels of serum creatinine in
the cisplatin group (1.20±0.12 mg/dl) were higher than those the
control group (0.32±0.15 mg/dl). PYP significantly prevented the
change in serum creatinine induced by cisplatin, resulting in serum
creatinine levels of 0.69±0.11 mg/dl.
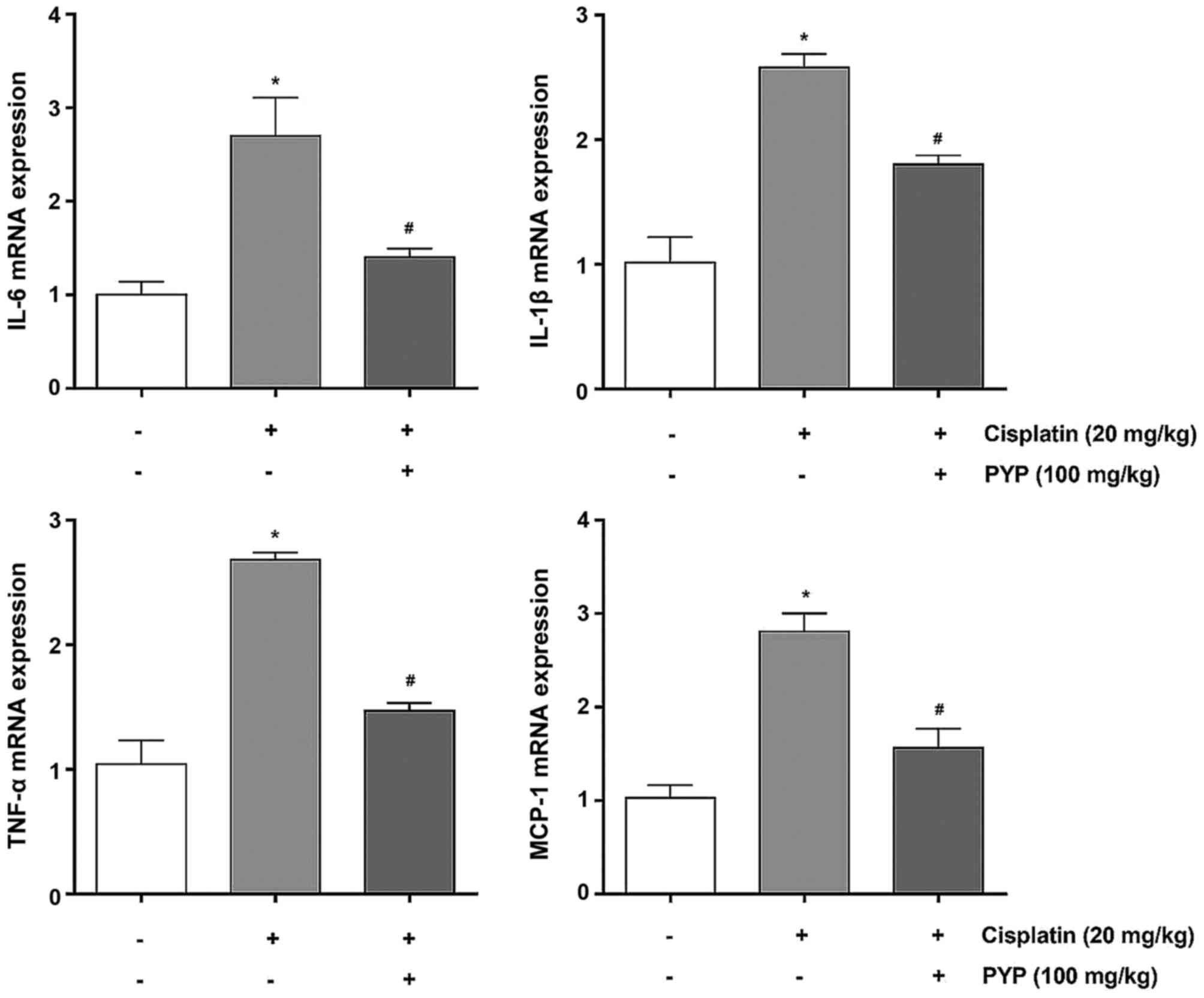
PYP treatment reduces inflammatory gene
expression in cisplatin-induced AKI
Next, the mRNA expression levels of various
cytokines associated with the inflammatory process were assessed in
the kidney tissues of the experimental animals. The mRNA levels of
IL-6, IL-1β, TNF-α and MCP-1 in the cisplatin group were
significantly higher than those in the control group (Fig. 6). By contrast, the mRNA expression
levels of IL-6, IL-1β, TNF-α and MCP-1 in the PYP + cisplatin group
were significantly decreased compared with those in the cisplatin
group (1.4±0.09 vs. 2.7±0.41-fold, 1.80±0.07 vs. 2.6±0.11-fold,
1.5±0.06 vs. 2.7±0.06-fold and 1.6±0.21 vs. 2.8±0.19-fold of those
in the control group, respectively).
Discussion
AKI is defined as a rapid loss of renal function due
to kidney damage, which results in retention of the nitrogenous and
non-nitrogenous waste products normally excreted by the kidney
(44,45). AKI is common among hospitalized
patients; indeed, its incidence has increased over the past few
decades, possibly due to the aging population and an increased
frequency of comorbidities (46).
AKI is associated with increased mortality and a greater risk of
chronic kidney disease, as well as end-stage renal disease
(47). Recognizing and treating
AKI as early as possible may prevent kidney damage. Therefore,
understanding the key regulatory factors in drug-induced kidney
dysfunction is critical.
Cisplatin is one of the most widely used
chemotherapeutic drugs for the treatment of various malignancies
such as bladder cancer, lung cancer, breast cancer and malignant
melanoma (48–50). Cisplatin is a front-line
chemotherapeutic agent for solid tumors, and AKI is an important
side effect, as is a chronic decline in kidney function. Renal
tubular cells are particularly sensitive to cisplatin treatment.
Cisplatin-induced renal cell death involves the activation of
multiple pathways, including the intrinsic and extrinsic apoptotic
pathways, oxidative stress and inflammatory responses (15). Unfortunately, numerous of these
pathways are also involved in the desirable cytotoxic effects of
cisplatin on tumor cells (51,52). Therefore, development of an
effective method of preventing cisplatin-induced AKI is of critical
importance. In the present study, a novel investigation of the
protective effects of PYP, extracted from P. yezoensis, on
cisplatin-induced cell death in human renal proximal tubular
epithelial cells and an animal model was pursued. The results
indicated that PYP inhibited the cisplatin-induced death of HK2
cells, and reduced AKI-associated clinical parameters (Nrf2 and
NF-κB) and inflammatory biomarkers (IL-6, IL-1β, TNF-α and MCP-1)
in vivo.
In the present study, the effects of PYP on the
cisplatin-induced reduction in HK2 cell viability were assessed.
PYP treatment resulted in a recovery of cell viability in a
concentration-dependent manner (Fig.
2A). The cisplatin-induced cell death was ameliorated by PYP
treatment, which was also in parallel with the recovery of changes
in the expression levels of various apoptotic proteins. The
cisplatin-treated cells exhibited increased cytochrome c
release from the mitochondria to the cytosol, as well as increased
Bax and caspase-3. However, compared with the cisplatin-treated
group, pretreatment with PYP resulted in decreased cytochrome
c release from the mitochondria to the cytosol, decreased
Bax and caspase-3 expression. In addition, western blot analysis
revealed that cisplatin treatment significantly reduced Bcl-2
expression: The protein levels of Bcl-2 were decreased by ~40%
following treatment with 20 µM cisplatin, which was
inhibited by pretreatment with PYP. These results suggested that
PYP protects HK2 cells against cisplatin-induced cell death.
The NF-κB pathway involves an important family of
transcription factors, which control the expression of cytokines,
cell adhesion molecules, growth factors and also apoptosis,
proliferation, differentiation and survival (53–55). Of note, NF-κB activation has been
observed in renal tubular cells treated with cisplatin (56,57), and the activation of NF-κB in
kidneys has been demonstrated in cisplatin-induced AKI (58,59). PYP ameliorated cisplatin-induced
nephrotoxicity in HK2 cells by reducing NF-κB activation and
inducing Nrf2 activation to inhibit cisplatin-induced cell death.
In addition, an inverse correlation in expression was detected
between Nrf2 and NF-κB. This suggested that PYP blocked NF-κB
activation and restored Nrf2 inactivation in cisplatin-treated HK2
cells.
Oxidative stress also has a critical role in the
pathogenesis of cisplatin-induced nephrotoxicity (17). ROS-induced cell death has been
reported in renal proximal tubular epithelial cells, and ROS
promoted cisplatin-induced renal failure (15,17). The effects of various functional
substances derived from seaweeds on oxidative stress and Nrf2/Heme
oxygenase-1 activation have been evaluated (17,43). In the present study, PYP treatment
suppressed cisplatin-induced p38/MAPK/JNK activation. This was
mediated by a reduction in ROS content. These results indicate that
PYP decreases cisplatin-induced oxidative stress by downregulating
the p38/MAPK/JNK pathway. These results also indicate that PYP may
suppress the cisplatin-induced death of HK2 cells by ameliorating
ROS-mediated oxidative stress.
PYP may protect renal tissue from cisplatin-induced
damage without reducing the tumoricidal activity of the drug, which
involves enzymatic activation and subsequent regulation of the
levels of intracellular metabolites and signaling molecules. Thus,
strategies that attenuate cisplatin-induced renal injury may have
the unintended consequence of reducing its antitumor activity.
Preventive strategies must therefore be designed in such a way that
this deleterious consequence is avoided.
Although the molecular mechanisms underlying
cisplatin-induced nephrotoxicity are complex and remain to be fully
elucidated, induction of oxidative damage, renal tubular cell death
and activation of inflammatory-related pathways have been
demonstrated in the kidneys of cisplatin-treated animals. Cisplatin
application enhances oxidative stress in kidney tissue,
demonstrated by increased levels of free radicals, structural
modifications of proteins and lipid peroxidation. Cisplatin
generates reactive free radicals, which interact with and modify
cellular components, leading to cell death. Cisplatin-induced ROS
generation in renal tubular cells activates the expression of NF-κB
and pro-inflammatory cytokines and chemokines. In the gentamicin
animal model, increased MAPK and NF-κB activation as well as
increased plasma creatinine levels were observed in the renal
cortex of gentamicin-injected rats compared with the control group
(60). These results indicated
that inhibiting NF-κB activation attenuates gentamicin-induced
kidney dysfunction.
Oxidative stress in kidneys is considered to be a
major cause of renal injury and inflammation, giving rise to a
variety of pathological disorders. Several studies have
demonstrated that excessive ROS production in AKI tissues triggers
cell death, and antioxidant agents have been used to prevent
associated increases in lipid peroxidation, resulting in reduced
kidney injury (61,62).
In the present study, cellular ROS production was
attenuated by PYP treatment, that cisplatin-induced Nrf2 activation
through its antioxidant effects. These results suggested that the
renoprotective effect of PYP may be attributable to its free
radical-scavenging and antioxidant activities. In addition, the
present results revealed that PYP treatment decreased nitrotyrosine
expression and suppressed NF-κB activation in the presence of
cisplatin. The predominant mechanism underlying kidney dysfunction
in response to cisplatin exposure is downregulation of the
NF-κB/MAPK pathway. These results suggested a novel mechanism of
cisplatin-induced nephro-toxicity through metabolic regulation by
PYP. Therefore, administration of PYP or dietary supplementation of
seaweed may represent a promising strategy to reduce this side
effect of cisplatin treatment.
Based on these results, experimental mice were
divided into three groups (control, cisplatin, PYP + cisplatin) and
the effects of PYP were evaluated in this mouse model of
cisplatin-induced AKI. Renal function was assessed by measuring the
concentration of BUN and creatinine. The results indicated that BUN
and serum creatinine were lower in the PYP + cisplatin group than
in the cisplatin group. In addition, the mRNA expression levels of
inflammatory factors in kidney tissues were examined. The mRNA
expression levels of IL-6, IL-1β, TNF-α and MCP-1 in the PYP +
cisplatin group were lower than those in the cisplatin group. The
levels of inflammatory factors in kidney tissues were examined, and
were confirmed to be consistent with the results obtained in HK2
cells. For instance, the expression of p38 and JNK was lower in the
PYP + cisplatin group than that in the cisplatin group (data not
shown). Therefore, PYP appears to prevent cisplatin-induced
nephrotoxicity by regulating the expression of inflammatory factors
in vivo.
The present results demonstrated, for the first
time, to the best of our knowledge, the potential therapeutic
effect of the seaweed P. yezoensis against cisplatin-induced
nephrotoxicity in vitro and in vivo, which was
mediated by downregulation of the MAPK and NF-κB pathways. The
present results suggested that P. yezoensis is a valuable
source of potential anti-nephrotoxic proteins that act by
inhibiting cell death and kidney dysfunction induced by cisplatin.
Therefore, further research to isolate and identify bioactive
functional proteins from P. yezoensis is warranted.
In conclusion, the present study demonstrated that
PYP exerted protective effects against cisplatin-induced AKI,
possibly by regulating the MAPK/NF-κB pathways. These effects
substantially contributed to the elimination of excessive oxidative
stress and to protected against cell death.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
2012R1A6A1028677).
References
|
1
|
Kowald A and Kirkwood TB: Can aging be
programmed? A critical literature review. Aging Cell. 15:986–998.
2016. View Article : Google Scholar
|
|
2
|
Vaiserman AM, Lushchak OV and Koliada AK:
Anti-aging pharmacology: Promises and pitfalls. Ageing Res Rev.
31:9–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen J, Zeng M, Shu Y, Guo D, Sun Y, Guo Z,
Wang Y, Liu Z, Zhou H and Zhang W: Aging increases the
susceptibility of cisplatin-induced nephrotoxicity. Age (Dordr).
37:1122015. View Article : Google Scholar
|
|
4
|
Wang X, Bonventre JV and Parrish AR: The
aging kidney: Increased susceptibility to nephrotoxicity. Int J Mol
Sci. 15:15358–15376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anand S, Johansen KL and Kurella Tamura M:
Aging and chronic kidney disease: The impact on physical function
and cognition. J Gerontol A Biol Sci Med Sci. 69:315–322. 2014.
View Article : Google Scholar
|
|
6
|
He YH, Pu SY, Xiao FH, Chen XQ, Yan DJ,
Liu YW, Lin R, Liao XP, Yu Q, Yang LQ, et al: Improved lipids,
diastolic pressure and kidney function are potential contributors
to familial longevity: A study on 60 Chinese centenarian families.
Sci Rep. 6:219622016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calvo-Rubio M, Burón MI, López-Lluch G,
Navas P, de Cabo R, Ramsey JJ, Villalba JM and González-Reyes JA:
Dietary fat composition influences glomerular and proximal
convoluted tubule cell structure and autophagic processes in
kidneys from calorie-restricted mice. Aging Cell. 15:477–487. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chevalier RL: The proximal tubule is the
primary target of injury and progression of kidney disease: Role of
the glomerulotubular junction. Am J Physiol Renal Physiol.
311:F145–F161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Sheng Y, Liu C, Li K, Huang X, Huang
J and Xu K: Nox4 has a crucial role in uric acid induced oxidative
stress and apoptosis in renal tubular cells. Mol Med Rep.
13:4343–4348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rogers NM, Stephenson MD, Kitching AR,
Horowitz JD and Coates PT: Amelioration of renal
ischaemia-reperfusion injury by liposomal delivery of curcumin to
renal tubular epithelial and antigen-presenting cells. Br J
Pharmacol. 166:194–209. 2012. View Article : Google Scholar :
|
|
11
|
Li J, Gui Y, Ren J, Liu X, Feng Y, Zeng Z,
He W, Yang J and Dai C: Metformin protects against
cisplatin-induced tubular cell apoptosis and acute kidney injury
via AMPKα-regulated autophagy induction. Sci Rep. 6:239752016.
View Article : Google Scholar
|
|
12
|
Markó L, Vigolo E, Hinze C, Park JK, Roël
G, Balogh A, Choi M, Wübken A, Cording J, Blasig IE, et al: Tubular
epithelial NF-κB activity regulates ischemic AKI. J Am Soc Nephrol.
27:2658–2669. 2016.
|
|
13
|
Stathopoulos GP: Cisplatin: Process and
future. J BUON. 18:564–569. 2013.PubMed/NCBI
|
|
14
|
Dugbartey GJ, Bouma HR, Lobb I and Sener
A: Hydrogen sulfide: A novel nephroprotectant against
cisplatin-induced renal toxicity. Nitric Oxide. 57:15–20. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herrera-Pérez Z, Gretz N and Dweep H: A
comprehensive review on the genetic regulation of cisplatin-induced
nephrotoxicity. Curr Genomics. 17:279–293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El-Naga RN and Mahran YF:
Indole-3-carbinol protects against cisplatin-induced acute
nephrotoxicity: Role of calcitonin gene-related peptide and
insulin-like growth factor-1. Sci Rep. 6:298572016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thongnuanjan P and Soodvilai S,
Chatsudthipong V and Soodvilai S: Fenofibrate reduces
cisplatin-induced apoptosis of renal proximal tubular cells via
inhibition of JNK and p38 pathways. J Toxicol Sci. 41:339–349.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cunha L and Grenha A: Sulfated seaweed
polysaccharides as multifunctional materials in drug delivery
applications. Mar Drugs. 14:e422016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grasa-López A, Miliar-García Á,
Quevedo-Corona L, Paniagua-Castro N, Escalona-Cardoso G,
Reyes-Maldonado E and Jaramillo-Flores ME: Undaria pinnatifida and
fucoxanthin ameliorate lipogenesis and markers of both inflammation
and cardiovascular dysfunction in an animal model of diet-induced
obesity. Mar Drugs. 14:e1482016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi JW, Kim IH, Kim YM, Lee MK and Nam
TJ: Pyropia yezoensis glycoprotein regulates antioxidant status and
prevents hepatotoxicity in a rat model of
D-galactosamine/lipopolysaccharide-induced acute liver failure. Mol
Med Rep. 13:3110–3114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cian RE, Drago SR, de Medina FS and
Martínez-Augustin O: Proteins and carbohydrates from red seaweeds:
Evidence for beneficial effects on gut function and microbiota. Mar
Drugs. 13:5358–5383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park HK, Kim IH, Kim J and Nam TJ:
Induction of apoptosis and the regulation of ErbB signaling by
laminarin in HT-29 human colon cancer cells. Int J Mol Med.
32:291–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park HK, Kim IH, Kim J and Nam TJ:
Induction of apoptosis by laminarin, regulating the insulin-like
growth factor-IR signaling pathways in HT-29 human colon cells. Int
J Mol Med. 30:734–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Min EY, Kim IH, Lee J, Kim EY, Choi YH and
Nam TJ: The effects of fucodian on senescence are controlled by the
p16INK4a-pRb and p14Arf-p53 pathways in hepatocellular carcinoma
and hepatic cell lines. Int J Oncol. 45:47–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park HY, Han MH, Park C, Jin CY, Kim GY,
Choi IW, Kim ND, Nam TJ, Kwon TK and Choi YH: Anti-inflammatory
effects of fucoidan through inhibition of NF-κB, MAPK and Akt
activation in lipopolysaccharide-induced BV2 microglia cells. Food
Chem Toxicol. 49:1745–1752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Go H, Hwang HJ and Nam TJ: A glycoprotein
from Laminaria japonica induces apoptosis in HT-29 colon cancer
cells. Toxicol In Vitro. 24:1546–1553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koyanagi S, Tanigawa N, Nakagawa H, Soeda
S and Shimeno H: Oversulfation of fucoidan enhances its
anti-angiogenic and antitumor activities. Biochem Pharmacol.
65:173–179. 2003. View Article : Google Scholar
|
|
28
|
Heo SJ, Park EJ, Lee KW and Jeon YJ:
Antioxidant activities of enzymatic extracts from brown seaweeds.
Bioresour Technol. 96:1613–1623. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mhadhebi L, Mhadhebi A, Robert J and
Bouraoui A: Antioxidant, anti-inflammatory and antiproliferative
effects of aqueous extracts of three mediterranean brown seaweeds
of the genus Cystoseira. Iran J Pharm Res. 13:207–220.
2014.PubMed/NCBI
|
|
30
|
Song MY, Ku SK, Kim HJ and Han JS: Low
molecular weight fucoidan ameliorating the chronic
cisplatin-induced delayed gastrointestinal motility in rats. Food
Chem Toxicol. 50:4468–4478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Easton C, Turner A and Sewell G: An
evaluation of the toxicity and bioaccumulation of cisplatin in the
marine environment using the macroalga, Ulva lactuca. Environ
Pollut. 159:3504–3508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi JW, Kim YM, Park SJ, Kim IH and Nam
TJ: Protective effect of Porphyra yezoensis glycoprotein on
D-galactosamine induced cytotoxicity in Hepa 1c1c7 cells. Mol Med
Rep. 11:3914–3919. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee HA, Kim IH and Nam TJ: Bioactive
peptide from Pyropia yezoensis and its anti-inflammatory
activities. Int J Mol Med. 36:1701–1706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi JW, Kim IH, Kim YM, Lee MK, Choi YH
and Nam TJ: Protective effect of Pyropia yezoensis glycoprotein on
chronic ethanol consumption-induced hepatotoxicity in rats. Mol Med
Rep. 14:4881–4886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi JW, Kwon MJ, Kim IH, Kim YM, Lee MK
and Nam TJ: Pyropia yezoensis glycoprotein promotes the M1 to M2
macrophage phenotypic switch via the STAT3 and STAT6 transcription
factors. Int J Mol Med. 38:666–674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ryu J, Park SJ, Kim IH, Choi YH and Nam
TJ: Protective effect of porphyra-334 on UVA-induced photoaging in
human skin fibroblasts. Int J Mol Med. 34:796–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shin ES, Hwang HJ, Kim IH and Nam TJ: A
glycoprotein from Porphyra yezoensis produces anti-inflammatory
effects in liposaccharide-stimulated macrophages via the TLR4
signaling pathway. Int J Mol Med. 28:809–815. 2011.PubMed/NCBI
|
|
38
|
Lee MK, Kim IH, Choi YH and Nam TJ: A
peptide from Porphyra yezoensis stimulates the proliferation of
IEC-6 cells by activating the insulin-like growth factor I receptor
signaling pathway. Int J Mol Med. 35:533–538. 2015. View Article : Google Scholar :
|
|
39
|
Lee MK, Kim IH, Choi YH, Choi JW, Kim YM
and Nam TJ: The proliferative effects of Pyropia yezoensis peptide
on IEC-6 cells are mediated through the epidermal growth factor
receptor signaling pathway. Int J Mol Med. 35:909–914. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park SJ, Ryu J, Kim IH, Choi YH and Nam
TJ: Activation of the mTOR signaling pathway in breast cancer MCF 7
cells by a peptide derived from Porphyra yezoensis. Oncol Rep.
33:19–24. 2015. View Article : Google Scholar
|
|
41
|
Park SJ, Ryu J, Kim IH, Choi YH and Nam
TJ: Induction of apoptosis by a peptide from Porphyra yezoensis:
Regulation of the insulin-like growth factor I receptor signaling
pathway in MCF-7 cells. Int J Oncol. 45:1011–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kang BY, Kim S, Lee KH, Lee YS, Hong I,
Lee MO, Min D, Chang I, Hwang JS, Park JS, et al: Transcriptional
profiling in human HaCaT keratinocytes in response to kaempferol
and identification of potential transcription factors for
regulating differential gene expression. Exp Mol Med. 40:208–219.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ryu J, Kwon MJ and Nam TJ: Nrf2 and NF-κB
signaling pathways contribute to porphyra-334-mediated inhibition
of UVA-induced inflammation in skin fibroblasts. Mar Drugs.
13:4721–4732. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aiello S and Noris M: Klotho in acute
kidney injury: Biomarker, therapy, or a bit of both? Kidney Int.
78:1208–1210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Basile DP, Anderson MD and Sutton TA:
Pathophysiology of acute kidney injury. Compr Physiol. 2:1303–1353.
2012.PubMed/NCBI
|
|
46
|
Simmons EM, Himmelfarb J, Sezer MT,
Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K,
Spratt D, et al PICARD Study Group: Plasma cytokine levels predict
mortality in patients with acute renal failure. Kidney Int.
65:1357–1365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rewa O and Bagshaw SM: Acute kidney
injury-epidemiology, outcomes and economics. Nat Rev Nephrol.
10:193–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Maruyama T, Yamamoto S, Qiu J, Ueda Y,
Suzuki T, Nojima M and Shima H: Apoptosis of bladder cancer by
sodium butyrate and cisplatin. J Infect Chemother. 18:288–295.
2012. View Article : Google Scholar
|
|
49
|
Wang M, Liu ZM, Li XC, Yao YT and Yin ZX:
Activation of ERK1/2 and Akt is associated with cisplatin
resistance in human lung cancer cells. J Chemother. 25:162–169.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Brechbuhl HM, Kachadourian R, Min E, Chan
D and Day BJ: Chrysin enhances doxorubicin-induced cytotoxicity in
human lung epithelial cancer cell lines: The role of glutathione.
Toxicol Appl Pharmacol. 258:1–9. 2012. View Article : Google Scholar :
|
|
51
|
Wang R, MoYung KC, Zhao YJ and Poon K: A
Mechanism for the temporal potentiation of genipin to the
ctotoxicity of cisplatin in colon cancer cells. Int J Med Sci.
13:507–516. 2016. View Article : Google Scholar :
|
|
52
|
Yan D, An G and Kuo MT: C-Jun N-terminal
kinase signalling pathway in response to cisplatin. J Cell Mol Med.
20:2013–2019. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View Article : Google Scholar
|
|
54
|
Basak S and Hoffmann A: Crosstalk via the
NF-kappaB signaling system. Cytokine Growth Factor Rev. 19:187–197.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kim J, Long KE, Tang K and Padanilam BJ:
Poly(ADP-ribose) polymerase 1 activation is required for cisplatin
nephrotoxicity. Kidney Int. 82:193–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee S, Kim W, Moon SO, Sung MJ, Kim DH,
Kang KP, Jang YB, Lee JE, Jang KY and Park SK: Rosiglitazone
ameliorates cisplatin-induced renal injury in mice. Nephrol Dial
Transplant. 21:2096–2105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Al-Lamki RS, Lu W, Finlay S, Twohig JP,
Wang EC, Tolkovsky AM and Bradley JR: DR3 signaling protects
against cisplatin nephrotoxicity mediated by tumor necrosis factor.
Am J Pathol. 180:1454–1464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Benedetti G, Fokkelman M, Yan K,
Fredriksson L, Herpers B, Meerman J, van de Water B and de Graauw
M: The nuclear factor κB family member RelB facilitates apoptosis
of renal epithelial cells caused by cisplatin/tumor necrosis factor
α synergy by suppressing an epithelial to mesenchymal
transition-like phenotypic switch. Mol Pharmacol. 84:128–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sahu BD, Tatireddy S, Koneru M, Borkar RM,
Kumar JM, Kuncha M, Srinivas R, Shyam Sunder R and Sistla R:
Naringin ameliorates gentamicin-induced nephrotoxicity and
associated mitochondrial dysfunction, apoptosis and inflammation in
rats: Possible mechanism of nephroprotection. Toxicol Appl
Pharmacol. 277:8–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Taye A and Ibrahim BM: Activation of renal
haeme oxygenase-1 alleviates gentamicin-induced acute
nephrotoxicity in rats. J Pharm Pharmacol. 65:995–1004. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sepand MR, Ghahremani MH,
Razavi-Azarkhiavi K, Aghsami M, Rajabi J, Keshavarz-Bahaghighat H
and Soodi M: Ellagic acid confers protection against
gentamicin-induced oxidative damage, mitochondrial dysfunction and
apoptosis-related nephrotoxicity. J Pharm Pharmacol. 68:1222–1232.
2016. View Article : Google Scholar : PubMed/NCBI
|