Introduction
Bone deficiency is a socioeconomic challenge in
stomatology. It not only affects the appearance of patients, but
also increases the difficulty of restoring oral function (1). Currently, tissue engineering using
suitable seed cells is considered to be the most effective method
in the treatment of bone deficiencies. Mesenchymal stem cells
(MSCs) are capable of self-renewal and can differentiate into
multiple connective tissue cell types, including osteogenic,
chondrogenic and adipogenic lineages. Human bone-derived marrow
MSCs (HBMSCs) are associated with a high availability, low
immunogenic properties and an ease of genetic manipulation; thus,
they are regarded as a suitable source of cells in bone and
cartilage tissue engineering (2,3).
However, the molecular mechanisms of the differentiation processes
of these cells involve complex pathways which remain largely
unknown.
Recently, growing attention has been paid to a class
of non-coding RNAs (ncRNAs), which are those RNAs that do not
encode any proteins. Long non-coding RNAs (lncRNAs), which belong
to a novel heterogeneous class of ncRNAs, play crucial roles in
both cell fate determination and disease pathogenesis (4,5).
The length of lncRNAs does not exceed 200 nt, and they can fold
into complex structures to provide greater potential and
versatility for both protein and target recognition (6). lncRNAs have been proposed to play
crucial regulatory roles in a wide range of biological pathways and
cellular processes, including chromosome inactivation (7) and differentiation (4), the reprogramming of stem cell
pluripotency (8), and the
modulation of invasion and apoptosis (9). Previous studies have suggested that
the downregulation of the lncRNA, differentiation antagonizing
non-protein coding RNA (DANCR) promotes the osteogenic
differentiation of human periodontal ligament stem cells (10) and human fetal osteoblastic cells
(11). However, whether lncRNA
DANCR is also involved in the osteogenic differentiation of HBMSCs
remains unknown.
In the present study, we obtained differential
expression profiles of lncRNA DANCR in undifferentiated vs.
osteogenic-differentiated HBMSCs. We found that the lncRNA DANCR
expression level was significantly decreased in HBMSCs during
osteogenic differentiation. DANCR knockdown abnormally enhanced the
levels of alkaline phosphatase activity (ALP), the mRNA expression
of osteogenic marker genes and mineralized matrix deposition in
HBMSCs, whereas the overexpression of DANCR yielded opposite
results. Moreover, we demonstrated that DANCR regulates the
proliferation and osteogenic differentiation via the p38
mitogen-activated protein kinase (MAPK) pathway. The association of
DANCR with the MAPK pathway may provide new insight into the
mechanisms involved in the regulation of osteogenic
differentiation.
Materials and methods
Cell culture
The HBMSC cell line, PTA-1058, was obtained from
(The American Type Culture Collection, Manassas, VA, USA). The
cells were cultured in Dulbecco's modified Eagle's medium (HyClone,
Logan, UT, USA) supplemented with 100 U/l penicillin (Gibco Life
Technologies, Carlsbad, CA, USA), 100 mg/l streptomycin (Gibco Life
Technologies) and 10% FBS (Gibco Life Technologies) in a humidified
atmosphere of 5% CO2 at 37°C. The confluent cells were
transferred to the following passage using 0.25% trypsin for up to
3 passages and the culture medium was changed every 3 days.
Proliferation assay
Cell proliferation was measured on days 3, 5 and 7
by flow cytometry (FCM). The HBMSCs were treated with serum-free
medium for 24 h, and the medium was then replaced with culture
medium containing 10% FBS. The cells were harvested on days 3, 5
and 7 and then fixed with 75% ice-cold ethanol. Cell cycle
fractions (G0, G1, S, and G2 M phases) were determined by FCM and
the DNA content was measured using a FACScan flow cytometer (BD
Biosciences, San Diego, CA, USA).
Osteogenic differentiation of HBMSCs
The HBMSCs (at passage 3) were cultured in
osteogenic supplement (OS) medium containing 10 mM
β-glycerophosphate, 100 nM ascorbic acid and 100 nM dexamethasone
(all from Sigma-Aldrich, St. Louis, MO, USA). The cells were
subjected to ALP activity assay and Alizarin Red staining. ALP
activity was measured using the ALP assay kit (Jiancheng Technology
Co., Ltd., Nanjing, China) according to the manufacturer's
instructions. Alizarin Red staining was measured using 40 mM
Alizarin Red S (pH 4.4; Jiancheng Technology Co., Ltd.) for 15 min
at room temperature. Mineralized nodules were visualized using an
inverted microscope (Carl Zeiss AG, Oberkochen, Germany) after
rinsing with PBS and 10 images were captured for each group.
SB203580 (Cell Signaling Technology Inc., Danvers, MA, USA), a
highly selective inhibitor of p38 signaling, was prepared in DMSO
and used in the signaling inhibition assay at the concentration of
10 mM as previously described (12). The osteogenenic differentiation
was measured on days 7, 14 and 21.
Plasmid construction and cell
transfection
The ectopic expression of DANCR was induced by
transfection with a DANCR overexpression vector (pcDNA3.1-DANCR)
using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad,
CA, USA), with an empty pCDNA3.1 vector used as the control. Both
vectors were obtained from and created by RiboBio (Guangzhou,
China). The knockdown of DANCR was induced by transfection with
siRNA targeting DANCR (si-DANCR; obtained from RiboBio) using
Lipofectamine 2000 (Invitrogen Life Technologies), with an empty
si-NC vector used as the control (RiboBio). According to the
manufacturer's instructions, the fusion and transfection of the
HBMSCs were performed when the cells were cultivated 0n 6-well
plates using Lipofectamine 2000.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total cellular RNA was isolated from the HBMSCs
using TRIzol (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the instructions recommended by the manufacturer.
The RNA was reverse transcribed into cDNA using a PrimeScript RT
Master Mix kit (Jiancheng Technology Co., Ltd.). Quantitative PCR
was performed using a SYBR Green PCR kit (Toyobo Co., Ltd., Osaka,
Japan) and the ABI 7300 Real-Time PCR System (Applied Biosystems,
Foster City, CA, USA) according to the instructions provided by the
manufacturer. The primers used were as follows: human DANCR
forward, 5′-GCCACTATGTAGCGGGTTTC-3′and reverse,
5′-ACCTGCGCTAAGAACTGAGG-3′; human bone gamma-carboxyglutamate
protein (BGLAP) forward, 5′-CAG CGGTGCAGAGTCCAGCAAA-3′ and reverse,
5′-GATGT GGTCAGCCAACTCGTCA-3′; human collagen I (COL1) forward,
5′-GGACACAATGGATTGCAAGG-3′ and reverse, 5′-TAACCACTGCTCCACTCTGG-3′;
human Runt-related transcription factor 2 (RUNX2) forward,
5′-CCGCACAACC GCACCAT-3′ and reverse, 5′-CGCTCCGGCCCACAAATCTC-3′
and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward,
5′-GGGCTGCTTTTAACTCTGGT-3′ and reverse, 5′-GCAGGTTTTTCTAGACGG-3′.
For each sample, GAPDH expression was analyzed to normalize target
gene expression. Each sample was analyzed in triplicate. Relative
gene expression was calculated using the 2-ΔΔCq
method.
Western blot analysis
The HBMSCs were lysed in RIPA buffer containing 1 mM
phenylmethane sulfonyl-fluoride according to the manufacturer's
instructions. The total protein concentration was determined using
a bicinchoninic acid assay kit. Protein lysates (20 μg) were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and then transferred onto 0.22 μm
polyvinylidene difluoride membranes (Millipore Corp., Bedford, MA,
USA). After blocking, the membranes were incubated overnight at 4°C
with specific primary antibodies [anti-p-p38 (Thr180/Tyr182; D3F9;
cat. no. 4511), p-38 (D13E1; cat. no. 8690), p-c-Jun N-terminal
kinase (JNK; Thr183/Tyr185; 81E11; cat. no. 4668), JNK (cat. no.
9252), p-extracellular signal-regulated protein kinase (ERK)1/2,
ERK1/2 and β-actin (13E5; cat. no. 4970) antibodies; diluted,
1/1,000; Cell Signaling Technology Inc). Following 3 washes with
PBST (0.5% Tween-20 in PBS), the membranes were incubated with the
relevant secondary antibodies (L27A9; cat. no. 3678) 1/1,000
diluted (Cell Signaling Technology Inc.) for 1 h at 37°C, washed
and visualized with an ECL detection kit (Amersham Pharmacia
Biotech, Piscataway, NJ, USA). β-actin served as an internal
control.
Statistical analysis
All data are expressed as the mean ± SD and were
processed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA).
Data were analyzed using one-way analysis of variance. Three
independent experiments were performed. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
DANCR levels are downregulated in HBMSCs
during osteogenic differentiation
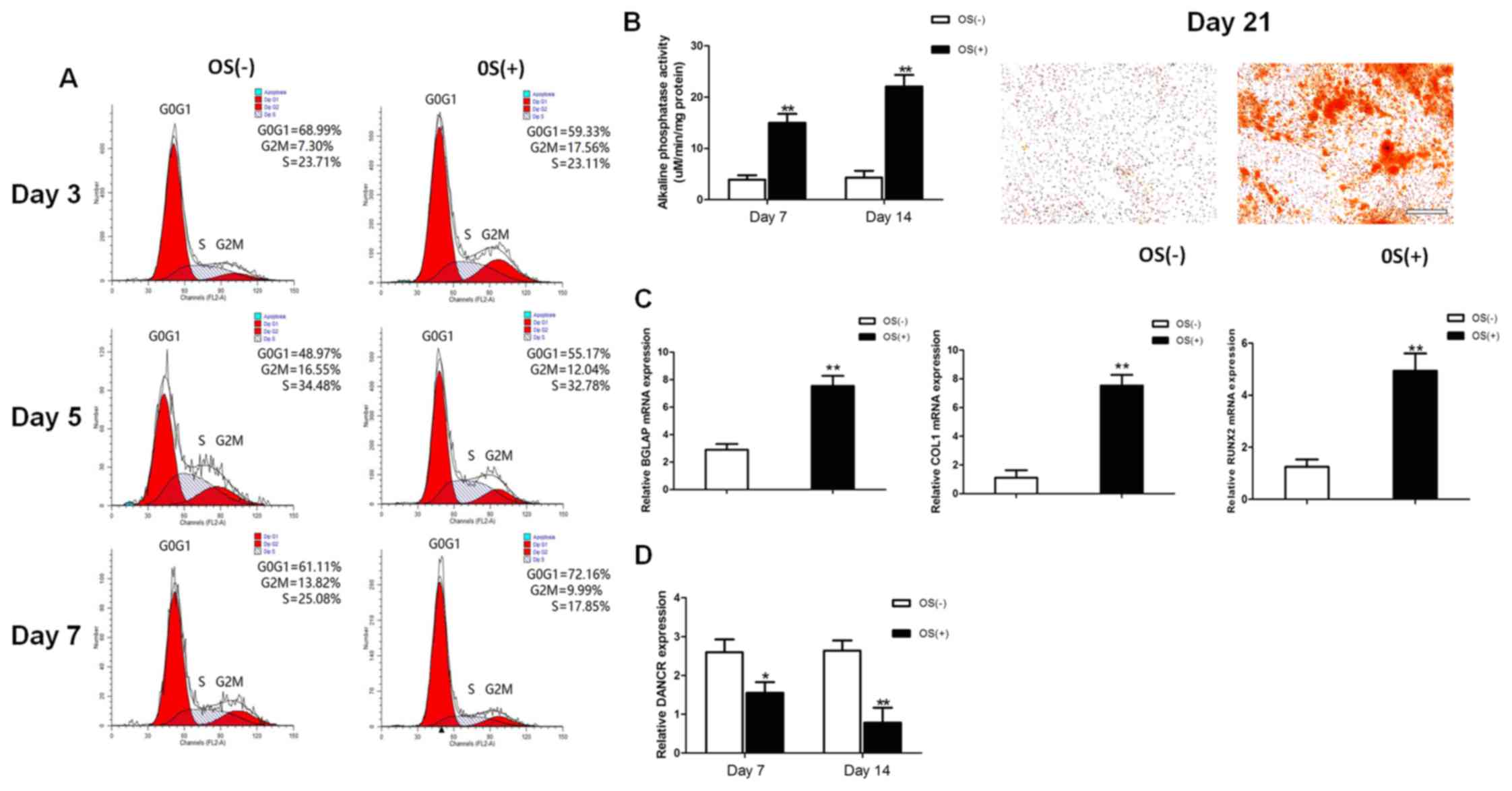
The osteogenic differentiation of HBMSCs was induced
by using OS medium. Cell cycle fractions (G0, G1, S, and G2M
phases) were determined by FCM on days 3, 5 and 7. The number of
cells in the S phase decreased significantly in the
osteogenic-differentiated HBMSCs compared to the undifferentiated
HBMSCs on day 7 (Fig. 1A), while
no significant differences were observed on days 3 and 5. Both ALP
activity assay and Alizarin Red staining revealed that the
micromass pellets had differentiated toward the osteogenic lineage
following induction with OS medium, as shown by increased ALP
activity and a greater amount of cells stained red (Fig. 1B). RT-qPCR detected a marked
increase in the expression of osteogenic marker genes on day 14,
including BGLAP, COL1 and RUNX2 (Fig.
1C). The lncRNA DANCR expression profiles during osteogenic
differentiation were investigated on days 7 and 14 after osteogenic
induction. The expression levels of lncRNA DANCR were significantly
decreased between the undifferentiated and differentiated cells
(Fig. 1D).
Expression of osteogenic marker genes is
enhanced by the knockdown of DANCR and is suppressed by
overexpression of DANCR
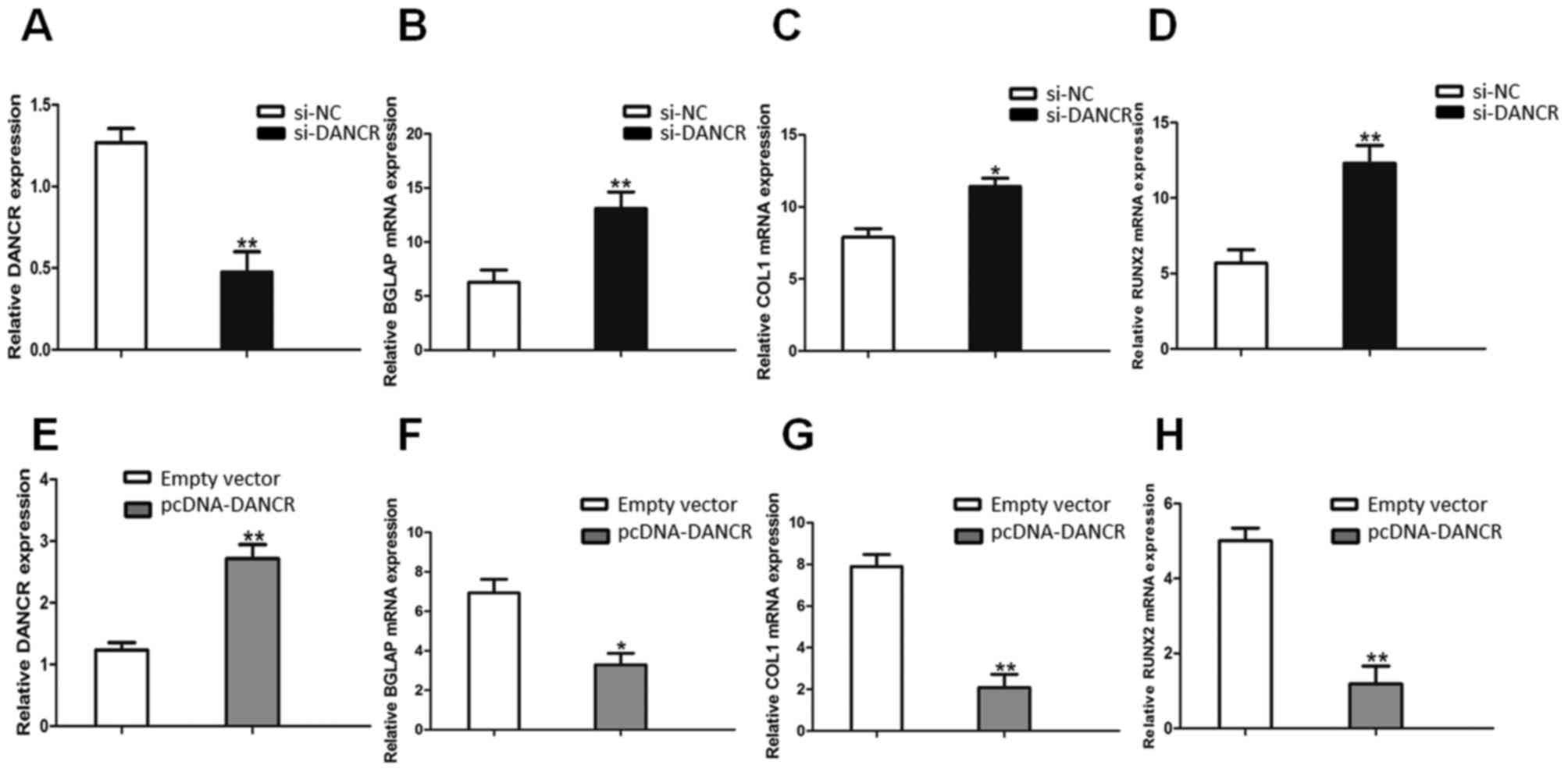
The knockdown of DANCR expression was successfully
performed using RNA interference (si-NC and si-DANCR) in HBMSCs.
After the HBMSCs were transfected with si-DANCR, the DANCR relative
levels were significantly downregulated (Fig. 2A), compared with the cells
transfected with si-NC. Following treatment with OS medium for 14
days, RT-qPCR assays revealed that the downregulation of DANCR
significantly promoted the expression of osteogenic marker genes,
including BGLAP, COL1 and RUNX2 (Fig.
2B, C and D). On the other hand, the DANCR relative levels in
the pcDNA-DANCR-transfected HBMSCs were significantly upregulated
(Fig. 2E). Compared with the
cells transfected with the empty vector, the expression levels of
osteogenic marker genes in the pcDNA-DANCR-transfected cells were
significantly descreased following treatment with OS medium for 14
days (Fig. 2F, G and H).
Proliferation and osteogenic
differentiation of HBMSCs is promoted by the knockdown of DANCR and
inhibited by the overexpression of DANCR
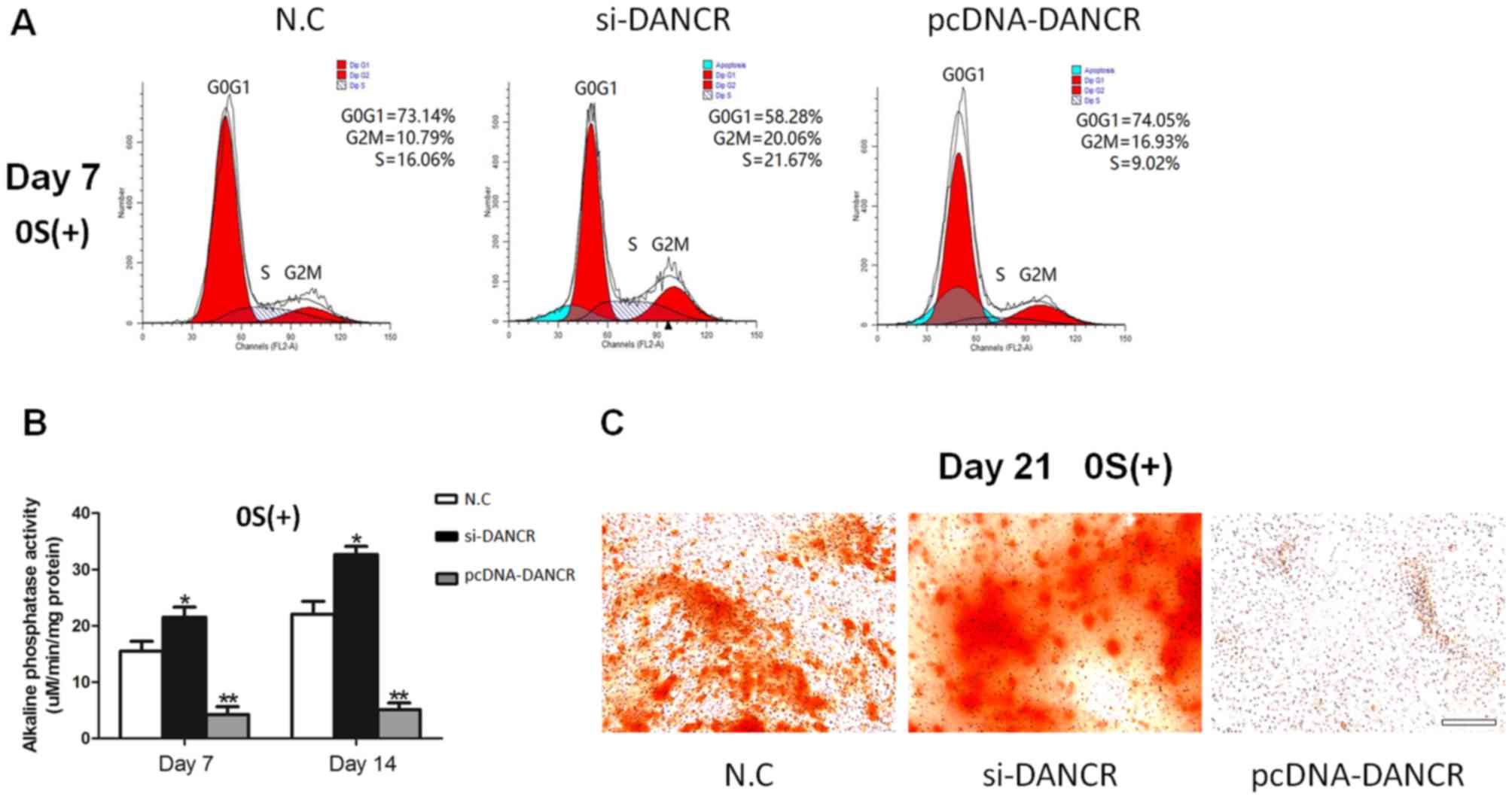
In order to determine the role of DANCR in
regulating the proliferation and osteogenic differentiation of
HBMSCs, the cells transfected with si-DANCR or pcDNA-DANCR were
subjected to cell cycle fractions, ALP activity assay and Alizarin
Red staining. We found that the knockdown of DANCR significantly
increased the number of cells in the S phase in the
osteogenic-differentiated HBMSCs on day 7, whereas the
overexpression of DANCR inhibited cell proliferation (Fig. 3A). Moreover, higher ALP activity
and a greater number of mineralized nodules were observed during
osteogenic differentiation in the si-DANCR-transfected HBMSCs, as
evidenced by increased ALP activity assay and Alizarin Red staining
(Fig. 3B and C). These results
suggested that the downregulation of DANCR promoted the
proliferation and osteogenic differentiation of HBMSCs.
DANCR regulates the proliferation and
osteogenic differentiation of HBMSCs via the p38 MAPK pathway
It is well known that the MAPK family regulates the
differentiation, mineralization and proliferation of HBMSCs
(13). Therefore, in this study,
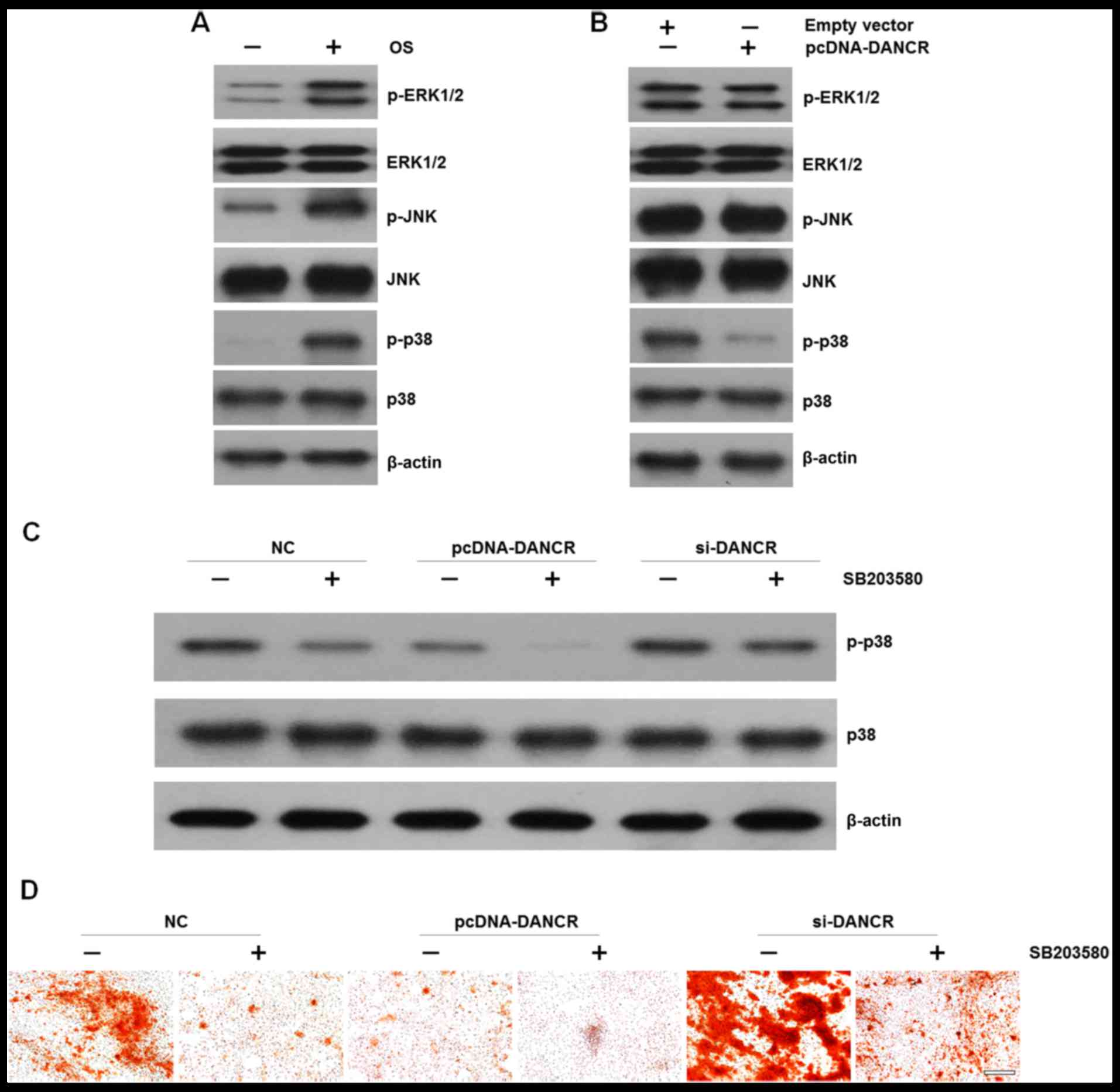
we examined the effects of DANCR on MAPK signal activation in
HBMSCs. We found that cultured in OS medium enhaced the
phosphorylation of ERK1/2, JNK and p38 (Fig. 4A). However, when the cells were
transfected with pcDNA-DANCR, the levels of p-p38 were markedly
decreased, while no significant difference was observed in the
levels of p-ERK1/2 or p-JNK (Fig.
4B). In order to elucidate the effects of p38 on DANCR-related
cell proliferation and osteogenic differentiation, HBMSCs that were
transfected with si-DANCR or pcDNA-DANCR were treated with the
specific p38 inhibitor, SB203580, which was followed by western
blot analysis and Alizarin Red staining. We found that the
upregulation of DANCR resulted in the inactivation of the p38 MAPK
pathway. More importantly, when the p38 MAPK pathway was
inactivated by the inhibitor, the inhibitory effects were reversed
by DANCR knockdown (Fig. 4C). In
accordance, mineralized matrix formation inhibited by the
suppression of the p38 MAPK pathway was also ameliorated by DANCR
knockdown (Fig. 4D)
Discussion
Bone deficiency is an emerging medical threat in
stomatology. Stomatological diseases can cause bone deficiency,
which in turn can increase the difficulty of restoring oral
function (14). Currently, tissue
engineering using suitable seed cells that mimics the natural
process has shown great potential in the treatment of bone
deficiencies (15–17). Among these seed cells, MSCs
present in a variety of mesenchymal tissues, have gained increasing
attention. MSCs have selfrenewable capacities and multi-directional
differentiation potentials (18,19). Given appropriate culture
conditions, they are capable of differentiating into chondrocytes
(2), adipocytes (20), endothelial cells (21), cardiomyocytes (22), osteocytes (23), as well as other cell lineages.
HBMSCs have the advantages of availability, culture expansion, low
immunogenicity (2) and
anti-inflammatory properties (24), and thus they are widely used in
tissue engineering and regenerative orthopaedics (25). The differentiation processes
involve complex pathways regulated at both transcriptional and
post-transcriptional levels. However, data on the precise molecular
mechanisms of osteogenic differentiation remain
incomprehensive.
lncRNAs are evolutionarily conserved ncRNAs that are
abundantly encoded in mammalian genomes, numbering in the tens of
thousands. The ratio of lncRNAs in total ncRNAs is beyond 80% but
is the least well understood (26). Although initially thought to be
transcriptional noise, recent evidence suggests that lncRNAs play
crucial roles in gene regulatory processes, such as cellular
metabolism (27), drug resistance
(28) and apoptosis (29). Previous studies have suggested
that some lncRNAs modulate pluripotency in embryonic stem cells
(30) and play a key role in
controlling the cell state (4).
lncRNA DANCR, highly expressed in the epidermis and downregulated
during the procedure of stem cell differentiation, is required to
maintain the undifferentiated state of epidermal stem cells or
osteoblasts (31). Recent studies
have demonstrated that the downregulation of ANCR promotes the
osteogenic differentiation of human periodontal ligament stem cells
(10) and human fetal
osteoblastic cells (11).
Therefore, we speculated the potential role of lncRNA DANCR in the
osteogenic differentiation of HBMSCs.
In the present study, we investigated the
differential expression of lncRNA DANCR in undifferentiated vs.
osteogenic-differentiated HBMSCs. We found that the lncRNA DANCR
expression level was significantly decreased during the osteogenic
differentiation of HBMSCs. By downregulating DANCR, the number of
cells in the S phase, the levels of ALP activity, the mRNA
expression of osteogenic marker genes and mineralized matrix
deposition in the HBMSCs was abnormally increased. On the other
hand, the upregulation of DANCR yielded opposite results. Thus, we
confirmed that the downregulation of DANCR promoted the
proliferation and osteogenic differentiation of HBMSCs.
The core purpose of the present study was to
investigate the potential molecular signaling pathways engaged by
DANCR. The MAPK signaling pathways, including the JNK, ERK1/2 and
p38 pathways have emerged as major regulators of cellular
physiology and the activation of MAPK is a crucial trigger of
osteogenic differentiation (32–35). Our data indicated that the
DANCR-regulated osteogenic differentiation was neither
ERK1/2-dependent nor JNK-dependent, but p38-dependent, and the
inhibitory effects of DANCR overexpression were more evident when
introduced in combination with p38 inactivation induced by the
specific inhibitor, SB203580. Moreover, we found that these
inhibitory effects were reversed by DANCR knockdown.
In conclusion, the present study highlights the
potential role of lncRNA DANCR in regulating HBMSC proliferation
and osteogenic differentiation. We further found that the p38 MAPK
pathway was involved in the precise molecular mechanisms. These
findings suggested that DANCR may be both a novel potential target
and regulatory factor of bone-destructive diseases. Further
investigations are warranted in order to fully elucidate the
complete molecular mechanisms involved in this process.
Acknowledgments
The present study was supported by the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (PAPD; 2014–37).
References
|
1
|
Maire P: Calibrated autologous bone
grafts–their use in oral implantology. Widening - crest
augmentation. Personal technic Rev Stomatol Chir Maxillofac.
98(Suppl 1): 27–30. 1997.In French.
|
|
2
|
Rastegar F, Shenaq D, Huang J, Zhang W,
Zhang BQ, He BC, Chen L, Zuo GW, Luo Q, Shi Q, et al: Mesenchymal
stem cells: Molecular characteristics and clinical applications.
World J Stem Cells. 2:67–80. 2010. View Article : Google Scholar
|
|
3
|
Macchiarini P, Jungebluth P, Go T, Asnaghi
MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto
PP, et al: Clinical transplantation of a tissue-engineered airway.
Lancet. 372:2023–2030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guttman M, Donaghey J, Carey BW, Garber M,
Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, et al:
lincRNAs act in the circuitry controlling pluripotency and
differentiation. Nature. 477:295–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheetham SW, Gruhl F, Mattick JS and
Dinger ME: Long noncoding RNAs and the genetics of cancer. Br J
Cancer. 108:2419–2425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guttman M and Rinn JL: Modular regulatory
principles of large non-coding RNAs. Nature. 482:339–346. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JT and Bartolomei MS: X-inactivation,
imprinting, and long noncoding RNAs in health and disease. Cell.
152:1308–1323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ng SY and Stanton LW: Long non-coding RNAs
in stem cell pluripotency. Wiley Interdiscip Rev RNA. 4:121–128.
2013. View Article : Google Scholar
|
|
9
|
Ørom UA and Shiekhattar R: Long noncoding
RNAs usher in a new era in the biology of enhancers. Cell.
154:1190–1193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia Q, Jiang W and Ni L: Down-regulated
non-coding RNA (lncRNA-ANCR) promotes osteogenic differentiation of
periodontal ligament stem cells. Arch Oral Biol. 60:234–241. 2015.
View Article : Google Scholar
|
|
11
|
Zhu L and Xu PC: Downregulated LncRNA-ANCR
promotes osteoblast differentiation by targeting EZH2 and
regulating Runx2 expression. Biochem Biophys Res Commun.
432:612–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Y, Chan E, Wang SX and Li B: Activation
of p38 mitogen-activated protein kinase is required for osteoblast
differentiation. Endocrinology. 144:2068–2074. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Q, Chen B, Cao M, Sun J, Wu H, Zhao
P, Xing J, Yang Y, Zhang X, Ji M and Gu N: Response of MAPK pathway
to iron oxide nanoparticles in vitro treatment promotes osteogenic
differentiation of hBMSCs. Biomaterials. 86:11–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hertz J: Problems of maxillofacial and
oral surgery. J Int Coll Surg. 26:63–79. 1956.PubMed/NCBI
|
|
15
|
Kim SH, Kim KH, Seo BM, Koo KT, Kim TI,
Seol YJ, Ku Y, Rhyu IC, Chung CP and Lee YM: Alveolar bone
regeneration by transplantation of periodontal ligament stem cells
and bone marrow stem cells in a canine peri-implant defect model: A
pilot study. J Periodontol. 80:1815–1823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Zhang Z, Zhao J, Zhang X, Sun X,
Xia L, Chang Q, Ye D and Jiang X: Vertical alveolar ridge
augmentation with beta-tricalcium phosphate and autologous
osteoblasts in canine mandible. Biomaterials. 30:2489–2498. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao J, Zhang Z, Wang S, Sun X, Zhang X,
Chen J, Kaplan DL and Jiang X: Apatite-coated silk fibroin
scaffolds to healing mandibular border defects in canines. Bone.
45:517–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bianco P and Robey PG: Stem cells in
tissue engineering. Nature. 414:118–121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jones E and McGonagle D: Human bone marrow
mesenchymal stem cells in vivo. Rheumatology (Oxford). 47. pp.
126–131. 2008, View Article : Google Scholar
|
|
20
|
Karagianni M, Brinkmann I, Kinzebach S,
Grassl M, Weiss C, Bugert P and Bieback K: A comparative analysis
of the adipogenic potential in human mesenchymal stromal cells from
cord blood and other sources. Cytotherapy. 15:76–88. 2013.
View Article : Google Scholar
|
|
21
|
Crisan M: Transition of mesenchymal
stem/stromal cells to endothelial cells. Stem Cell Res Ther.
4:952013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao JW, Zhang MR, Ji QY, Xing FJ, Meng LJ
and Wang Y: The role of slingshot-1L (SSH1L) in the differentiation
of human bone marrow mesenchymal stem cells into cardiomyocyte-like
cells. Molecules. 17:14975–14994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heino TJ and Hentunen TA: Differentiation
of osteoblasts and osteocytes from mesenchymal stem cells. Curr
Stem Cell Res Ther. 3:131–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu P, Deng Z, Han S, Liu T, Wen N, Lu W,
Geng X, Huang S and Jin Y: Tissue-engineered skin containing
mesenchymal stem cells improves burn wounds. Artif Organs.
32:925–931. 2008. View Article : Google Scholar
|
|
25
|
Pećina M and Vukičević S: Tissue
engineering and regenerative orthopaedics (TERO). Int Orthop.
38:1757–1760. 2014. View Article : Google Scholar
|
|
26
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ellis BC, Graham LD and Molloy PL: CRNDE,
a long non-coding RNA responsive to insulin/IGF signaling,
regulates genes involved in central metabolism. Biochim Biophys
Acta. 1843:372–386. 2014. View Article : Google Scholar
|
|
28
|
Jiang M, Huang O, Xie Z, Wu S, Zhang X,
Shen A, Liu H, Chen X, Wu J, Lou Y, et al: A novel long non-coding
RNA-ARA: Adriamycin resistance-associated. Biochem Pharmacol.
87:254–283. 2014. View Article : Google Scholar
|
|
29
|
Wu W, Zhang S, Li X, Xue M, Cao S and Chen
W: Ets-2 regulates cell apoptosis via the Akt pathway, through the
regulation of urothelial cancer associated 1, a long non-coding
RNA, in bladder cancer cells. PLoS One. 8:e739202013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sheik Mohamed J, Gaughwin PM, Lim B,
Robson P and Lipovich L: Conserved long noncoding RNAs
transcriptionally regulated by Oct4 and Nanog modulate pluripotency
in mouse embryonic stem cells. RNA. 16:324–337. 2010. View Article : Google Scholar :
|
|
31
|
Kretz M, Webster DE, Flockhart RJ, Lee CS,
Zehnder A, Lopez-Pajares V, Qu K, Zheng GX, Chow J, Kim GE, et al:
Suppression of progenitor differentiation requires the long
noncoding RNA ANCR. Genes Dev. 26:338–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu N, Feng C, Jiang Y, Miao Q and Liu H:
Regulative Effect of Mir-205 on Osteogenic Differentiation of Bone
Mesenchymal Stem Cells (BMSCs): Possible Role of SATB2/Runx2 and
ERK/MAPK Pathway. Int J Mol Sci. 16:10491–10506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Herbert BA, Valerio MS, Gaestel M and
Kirkwood KL: Sexual Dimorphism in MAPK-Activated Protein Kinase-2
(MK2) Regulation of RANKL-Induced Osteoclastogenesis in Osteoclast
Progenitor Subpopulations. PLoS One. 10:e01253872015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qin L, Tang B, Deng B, Mohan C, Wu T and
Peng A: Extracellular regulated protein kinases play a key role via
bone morphogenetic protein 4 in high phosphate-induced endothelial
cell apoptosis. Life Sci. 131:37–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ki YW, Park JH, Lee JE, Shin IC and Koh
HC: JNK and p38 MAPK regulate oxidative stress and the inflammatory
response in chlorpyrifos-induced apoptosis. Toxicol Lett.
218:235–245. 2013. View Article : Google Scholar : PubMed/NCBI
|