Introduction
High-density lipoprotein (HDL) particles are
particles with a density of 1.063–1.210 g/ml that are composed of
proteins and lipids (1). Compared
with other lipoproteins, HDL contains a high level of protein. The
protein/lipid ratio differs among HDL subpopulations. In the large
and light HDL subfraction HDL2, the ratio is 1:2, and in small
dense pre-HDL it is 10:1 (2).
Apolipoprotein A-I (apoA-I) is the most abundant protein component
(3) in HDL particles. ApoA-I
constitutes ~70% of the protein content of HDL, followed by
apoA-II, which constitutes 15–20% (4). The remaining proteins include
apolipoprotein C (apoC-I, apoC-II and apoC-III), apolipoprotein E
(apoE), apolipoprotein D (apoD), apolipoprotein M (apoM),
apolipoprotein A-IV (apoA-IV) and other proteins that are involved
in lipid metabolism, including lecithin:cholesterol acyl
transferase and cholesteryl ester transfer protein. Proteomic
studies (5–8) have identified ≥75 different proteins
that are contained in HDL obtained by ultracentrifugation.
Regarding disease states, HDL proteomic studies have
observed substantial changes in HDL in individuals with
cardiovascular disease (8–11),
diabetes mellitus (DM) (12),
chronic kidney disease (13–15) and rheumatoid arthritis (16) in comparison with healthy
individuals, while the plasma HDL-cholesterol (HDL-C) did not
change markedly (8,9). Disorders such as atherosclerosis and
type 2 DM cause a prominent chronic inflammatory state affecting
endothelial cells, which induces a proteomic change of HDL with the
subsequent impairment of their antiatherogenic, antioxidant and
anti-inflammatory functions (17). HDL subpopulations are associated
with stroke subtype; a previous study identified that smaller HDL-C
particles were associated with a reduced risk of lacunar infarction
(LACI) (18).
The biological mechanism underlying the beneficial
role of small HDL particles in LACI is not well understood. It is
considered that the anti-inflammatory effects of smaller HDL
particles (19,20) inhibit angionecrosis or
microatheroma formation in cerebral vessels, and thereby reduce the
risk of LACI (21,22). Proteomics may be helpful in
identifying the molecules associated with HDL that intervene in
their inverse association with cerebrovascular disease, as the
quantitative measurement of HDL-C level does not explain this
satisfactorily (23). Proteomic
analysis may drive a shift in the classical classification of HDL
subfractions, from the previous physicochemical model towards a
novel pattern based on their physiological function and
pathophysiological roles (24,25). In the present study, the HDL
proteomes in patients with LACI and controls were investigated.
Materials and methods
Ethics statement
The study was approved by the Ethics Committee of
Peking University First Hospital (Beijing, China). Written informed
consent was provided by all patients and controls.
Subjects and biochemical analysis
The study included 12 patients with LACI (7 males
and 5 females, aged 45–69 years old) without any evident large
vessel occlusions and 12 control subjects (7 males and 5 females,
aged 48–62 years old). The patients and controls were recruited in
the Neurology Ward of Peking University First Hospital from January
1, 2015 to March 31, 2015. Exclusion criteria were having
infectious, inflammatory or autoimmune disorders, advanced kidney
or liver failure, neoplastic disease, and a history of major
surgery or trauma within the previous month. Patients with LACI
were defined as having lacunar syndrome and signs, and brain
neuroimaging evidence of an infarct of size ≤1.5 cm at a typical
location (26,27). The patient and control groups were
equivalent with regard to sex proportion and age range. Blood
samples were drawn into EDTA-coated tubes following an 8-h fast.
Fasting blood glucose and creatinine were determined using a
Beckman CX5 Automated Analyzer. DM was defined either as records of
fasting blood glucose >7.0 mmol/l, post-prandial blood glucose
>11.1 mmol/l or used to on anti-diabetic treatments.
Hyperlipidemia was defined as abnormally elevated levels of any or
all lipids or lipoproteins in the blood and needed treatment with
lipid-lowering therapy. The plasma was obtained by centrifugation
at 200 × g for 10 min at 15°C, and was reserved at −80°C. All
plasma samples underwent 2 freeze/thaw cycles. Measurements of the
total cholesterol, HDL-C and triglycerides in the plasma were
conducted by biochemical assays using a Beckman CX5 Automated
Analyzer (Beckman Coulter, Inc., Brea, CA, USA). The quantification
of apoA-II, serum amyloid A (SAA) and apoC-III in the individual
HDL samples was conducted by an immunoblotting assay. A sample
containing 10 µg total proteins was separated by 10%
SDS-PAGE and blotted onto a nitrocellulose membrane. Anti-apoA-II
(ab109897), anti-SAA (ab190802) and anti-apoC-III (ab76305)
anti-bodies (all from Abcam, Cambridge, UK) were used as the
primary antibodies. The primary antibodies were incubated at 4°C
overnight. Horseradish peroxidase-conjugated goat anti-rabbit
monoclonal antibody (cat. no. sc-2004; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA; 1:1,000) was used as the secondary antibody.
The secondary antibody was incubated at 25°C for 1 h. Antibody
binding was detected using a Super Signal West Pico kit (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol.
HDL isolation from plasma
HDL particles (density, 1.063–1.21 g/ml) in the
plasma were separated by sequential density ultracentrifugation
using potassium bromide (KBr) as described previously (28). Briefly, the plasma density was
adjusted to 1.3 g/ml with KBr, and normal saline (1.006 g/ml) was
layered over the adjusted plasma to form a discontinuous NaCl/KBr
density gradient. The tubes loaded with sample and gradient were
placed in the P40ST rotor of an ultracentrifuge (model CP70MX;
Hitachi, Ltd., Tokyo, Japan) and were centrifuged at 350,000 × g
for 3.5 h at 4°C. The HDL layer was collected. The protein
concentration was measured in triplicate using a Micro BCA kit
(Pierce; Thermo Fisher Scientific, Inc.). The purity of the HDL was
evaluated by 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and western blot analysis using goat
anti-apoA-I polyclonal antibody (ab64308; Abcam) and quantified
through the measurement of apoA-I content by nephelometry
(Dimension XPand; Siemens Healthineers, Erlangen, Germany).
Mass spectrometry (MS)
The specific gel band was excised and destained with
25 mM NH4HCO3 in 50% acetonitrile. Proteins
were reduced by 10 mM dithiothreitol at 56°C for 30 min and
alkylated using 50 mM iodoacetamide at 25°C for 30 min. After
drying in 100% acetonitrile, the gel band was digested using
sequencing grade trypsin (Promega Corporation, Madison, WI, USA) at
37°C overnight. The extracted peptides were suspended in 0.1%
formic acid and subjected to nano liquid chromatography-MS/MS
analysis. Peptides were eluted with a linear gradient from 5 to 40%
of 100% acetonitrile and 0.1% formic acid at a flow rate of 300
nl/min using a self-made 100 µm × 10 cm reversed-phase C18
fused silica emitter. The data-dependent mass spectra were acquired
with an LTQ Orbitrap Elite mass spectrometer equipped with a
nano-electrospray ion source (Thermo Fisher Scientific, Inc.). Raw
mass spectra files were processed with Proteome Discoverer 1.4
(Thermo Fisher Scientific, Inc.) and searched using the SEQUEST
search engine (29) against the
human uniprot database (version 2014_02; http://www.uniprot.org/). The precursor ion mass
tolerance was set to 10 ppm, and the MS/MS tolerance was 0.02 Da.
Relative quantification was based on the ratio of the areas under
the reporter peaks. Protein-protein interaction networks of
differentially expressed proteins were constructed using STRING 9.1
(http://string-db.org/). The name of each
individual protein was given as a query to the STRING database and
the corresponding PPI information was retrieved by enabling
different prediction methods. The networks were made with a
confidence cutoff of 0.4.
THP-1 cell adhesion assay and
determination of adhesion molecules by western blot analysis
The method used was as described previously
(30). THP-1 monocytic cells were
labeled with 3 µg/ml
2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein,
acetoxymethyl ester at 37°C for 30 min. The labeled cells were
washed three times with phosphate-buffered saline (PBS), and were
then resuspended in RPMI-1640 (R1640; Gibco, Paisley, UK)
containing 0.1% bovine serum albumin (Gibco). The cell suspensions
were overlaid (1.5×106 cells/ml, 500 µl/well) on
confluent monolayers of human umbilical vein endothelial cells
(HUVECs; CRL-1730; ATCC, Manassas, VA, USA) that had been grown in
12-well plates and treated with various types of HCL: HDL from the
control group (HDLn), HDL from LACI patients
(HDLLI) and HDL with an elevated level of apoC-III
[HDL/apoC-III; obtained after incubation of HDL with apoC-III (100
µg/ml; TP306566; Origene, Beijing, China) for 2 h at 37°C].
PBS treatment was used as a negative control. Following incubation
for 15 min at 37°C, nonadherent THP-1 monocytic cells were removed
by washing five times with prewarmed RPMI-1640 containing 0.1%
bovine serum albumin. The number of THP-1 monocytic cells on the
HUVECs was counted in four views using fluorescence microscopy at
×100 magnification to determine the number of adhering cells.
Western blot analysis was conducted to investigate
the expression of vascular cell adhesion molecule-1 (VCAM-1) by the
endothelial cells. The cells were washed twice with cold PBS, and
the cytoplasmic proteins were collected using Nucleoprotein
Extraction kit (BSP009; Shenggong, Shanghai, China) following the
manuals. The proteins were quantified using Protein Quantitative
kit (DQ111-01; Transgen Biotech, Beijing, China) following the
manuals. A sample containing 30 µg cytoplasmic proteins was
separated by 10 or 15% SDS-PAGE and blotted onto a nitrocellulose
membrane. Rabbit monoclonal antibody to VCAM-1 (ab134047; 1:1,000;
Abcam) was used as the primary antibody and was incubated at 4°C
overnight. Horseradish peroxidase-conjugated goat anti-rabbit
monoclonal antibody (1:1,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) was used as the secondary antibody and was
incubated at 25°C for 1 h. Antibody binding was detected using a
Super Signal West Pico kit (Pierce; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± standard deviation
or number (%). Continuous data were analyzed using t-tests, while
discrete data were analyzed using Chi-square tests. One-way ANOVA
method was used for comparison of multiple groups. P<0.05 was
considered to indicate a statistically significant difference. The
SPSS 16.0 software package (SPSS, Inc., Chicago, IL, USA) was used
for data analysis. Gene Ontology (GO) analysis was conducted using
DAVID bioinformatics resources (http://david.abcc.ncifcrf.gov) as described previously
(31,32).
Results
Characteristics of the study
population
The characteristics of the patients and controls are
summarized in Table I. No
difference in the proportions of sex, DM and hyperlipidemia was
observed between the two groups. In addition, there was no
difference in the plasma total cholesterol, triglyceride, HDL-C,
fasting blood glucose and serum creatinine levels between the LACI
and control subjects.
 | Table IClinical characteristics of study
subjects. |
Table I
Clinical characteristics of study
subjects.
| Features | LACI
patients
(n=12) | control
(n=12) |
|---|
| Age (years) | 57±6 | 55±4 |
| Male, n (%) | 7 (58.33) | 7 (58.33) |
| DM, n (%) | 3 (25) | 3 (25) |
| Hyperlipidemia, n
(%) | 6 (50) | 4 (33.33) |
| Total cholesterol
(mg/dl) | 4.16±1.09 | 4.30±0.63 |
| Triglyceride
(mg/dl) | 1.65±0.69 | 1.70±0.55 |
| HDL cholesterol
(mmol/l) | 1.04±0.24 | 1.05±0.37 |
| Fasting blood
glucose (mg/dl) | 5.59±1.41 | 6.08±1.71 |
| Creatinine
(mg/dl) | 93.90±14.32 | 90.02±15.17 |
HDL proteomics
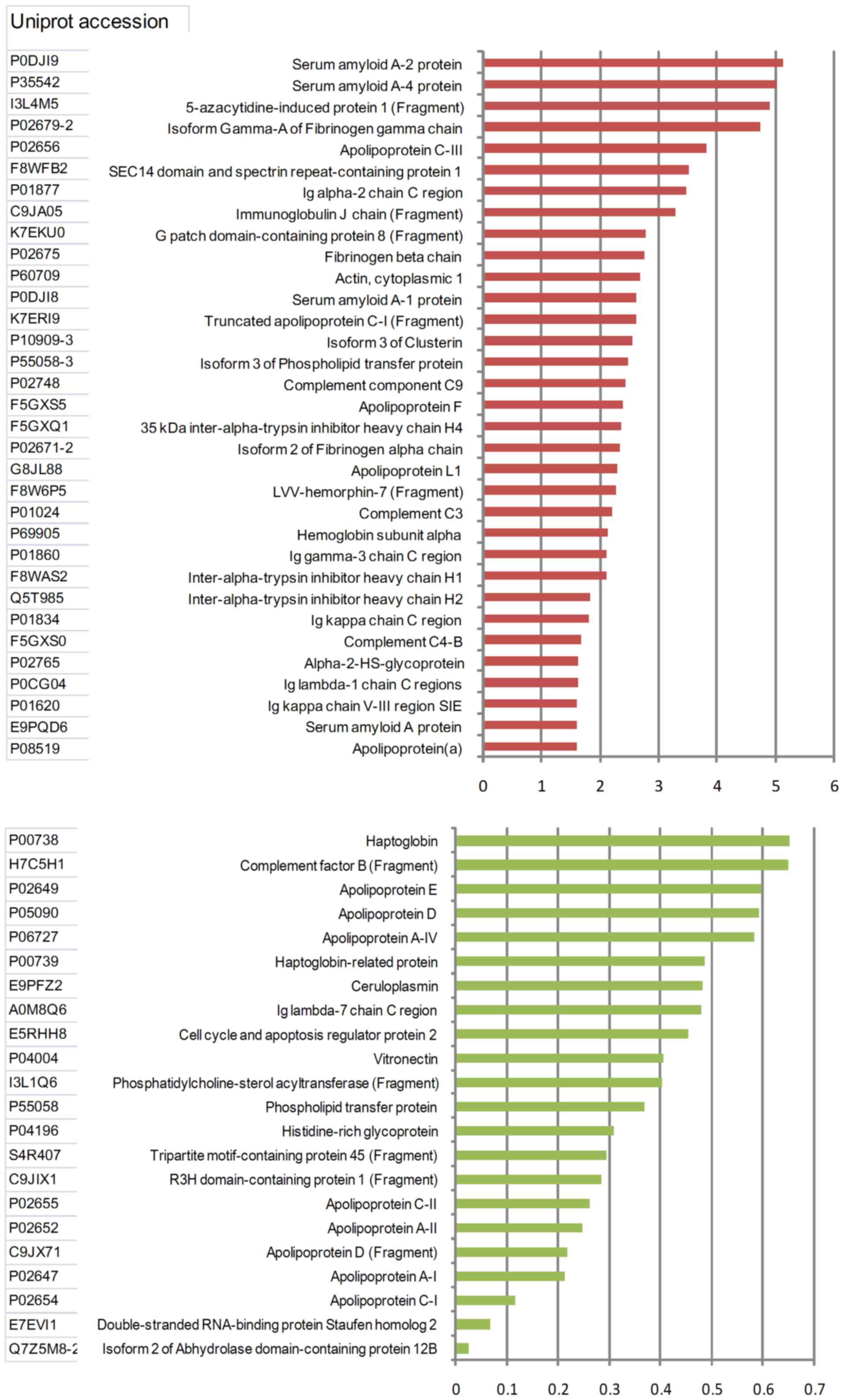
Proteins that had a differential expression of
≥1.5-fold or ≤0.67-fold relative in the LACI samples compared with
the control samples were considered as differentially expressed. In
total, 55 proteins were identified to be differentially expressed
in the LACI and control groups (Fig.
1). Among these 55 proteins, 33 were upregulated and 22 were
downregulated in the patients with LACI compared with the control
subjects. The level of LACI's haptoglobin was less compared to 1/5
of the control subjects.
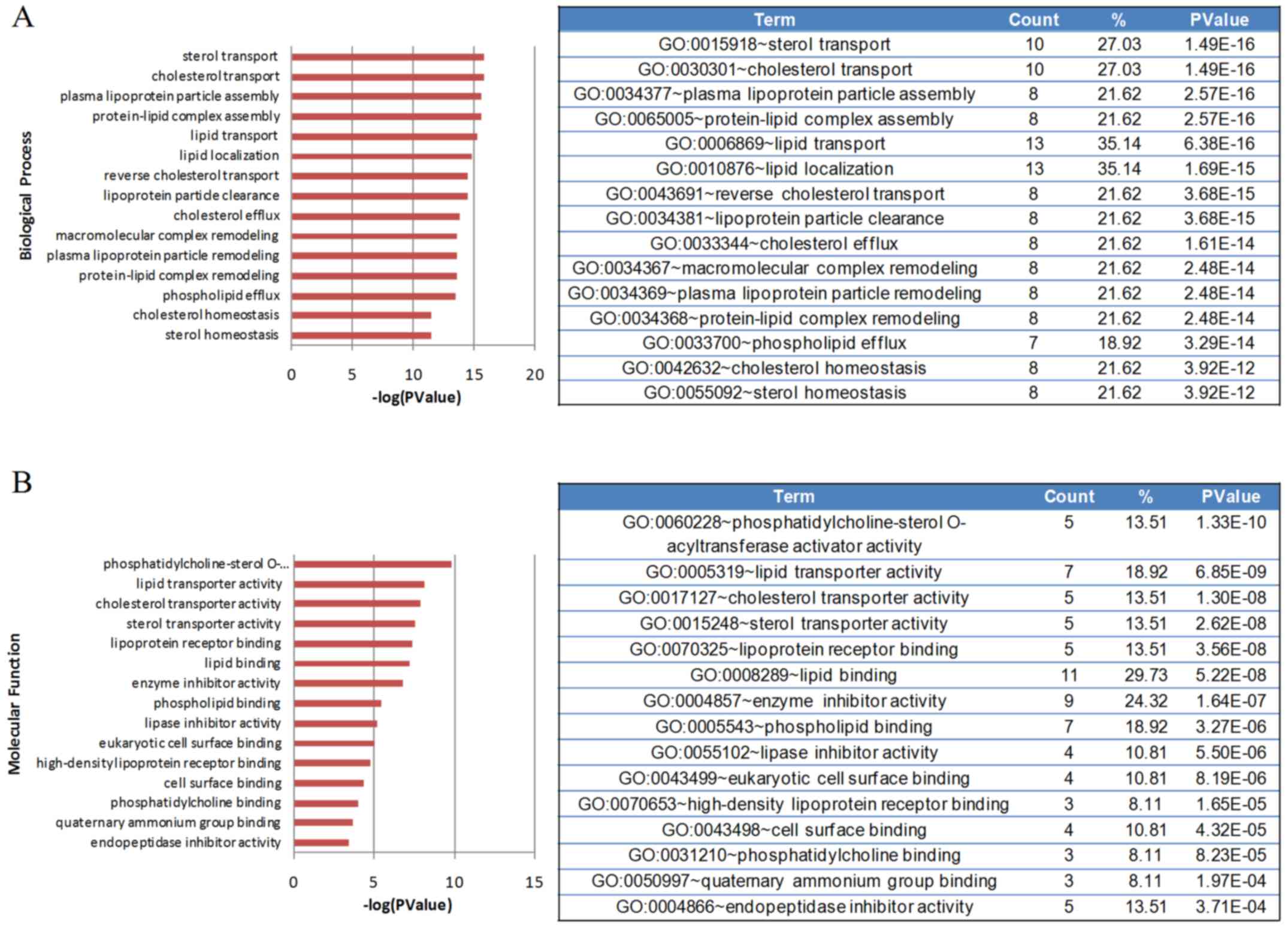
The GO classification system was used to classify
the identified proteins into different clusters according to
biological processes and molecular functions. Fig. 2 shows the GO categories of the
identified proteins. The differentially identified proteins were
associated with numerous molecular functions, including lipid and
cholesterol metabolism, inflammatory response, the complement and
coagulation pathway, hemostasis, metal ion metabolism and
endopeptidase inhibitory activity. Proteins associated with
lipid/cholesterol transport and metabolism exhibited the most
significant changes.
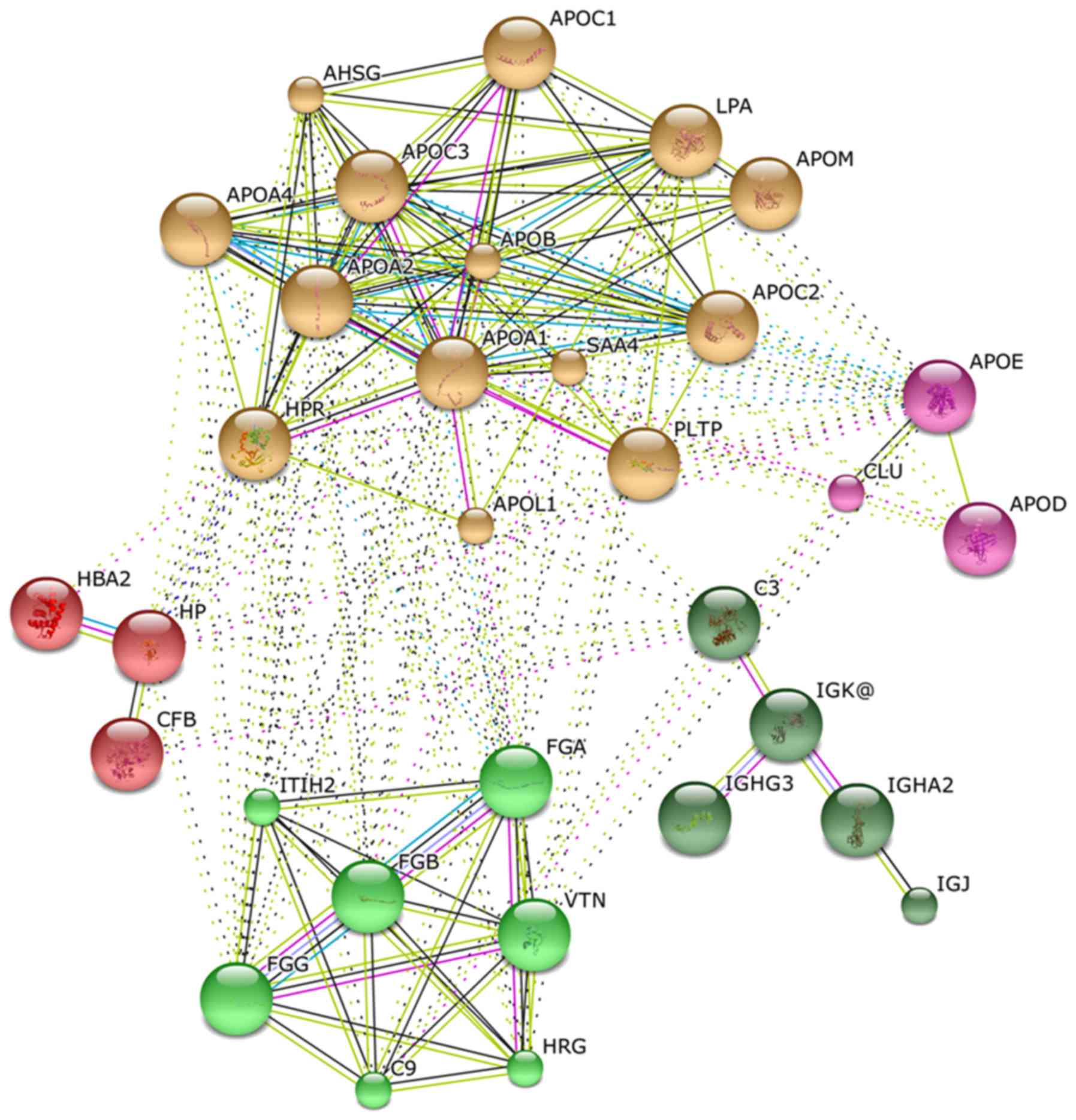
Fig. 3 shows an
organic network that graphically depicts statistically significant
correlations between identified proteins (nodes) as connecting
lines (edges). Long/thin lines indicate weak correlations.
Short/thick lines indicate strong correlations; tightly correlated
proteins appear in the same color in the network. The
differentially expressed proteins formed five different functional
clusters, indicating an involvement in lipid metabolism,
hemostasis, metal binding, hemoglobin metabolism and inflammatory
response.
Biochemical confirmation of the
differentially expressed proteins
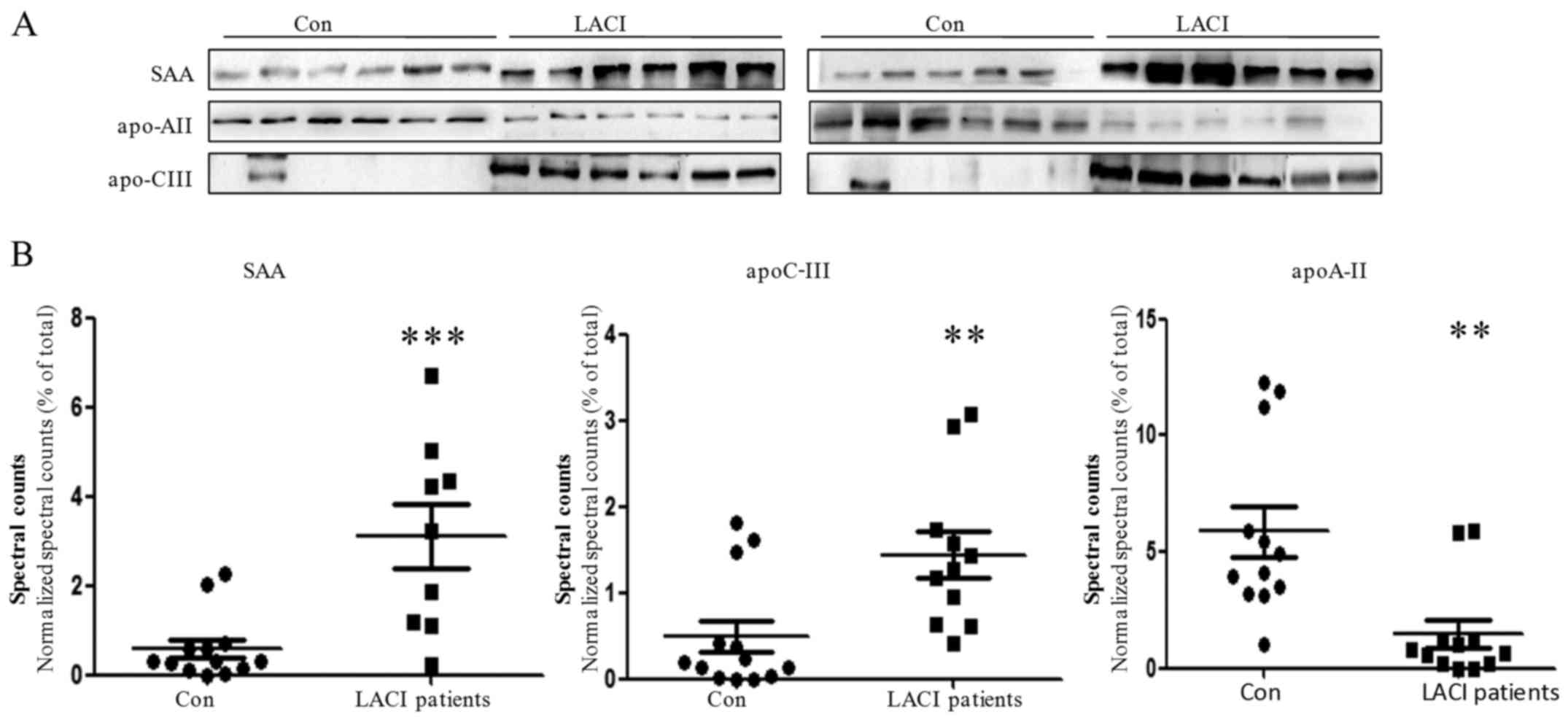
The expression levels of three proteins identified
by MS analysis in the majority of the 12 LACI patients were
evaluated by western blot assay to validate the difference
observed. SAA (P<0.001) and apoC-III (P= 0.007) were
significantly upregulated whereas apoA-II (P=0.002) was
significantly downregulated in the LACI patient group compared with
the controls in the proteomic analysis. The results of the western
blot analysis were in agreement with the MS findings, with the
majority of the patients having a decreased level of apoA-II and
increased levels of apoC-III and SAA (Fig. 4).
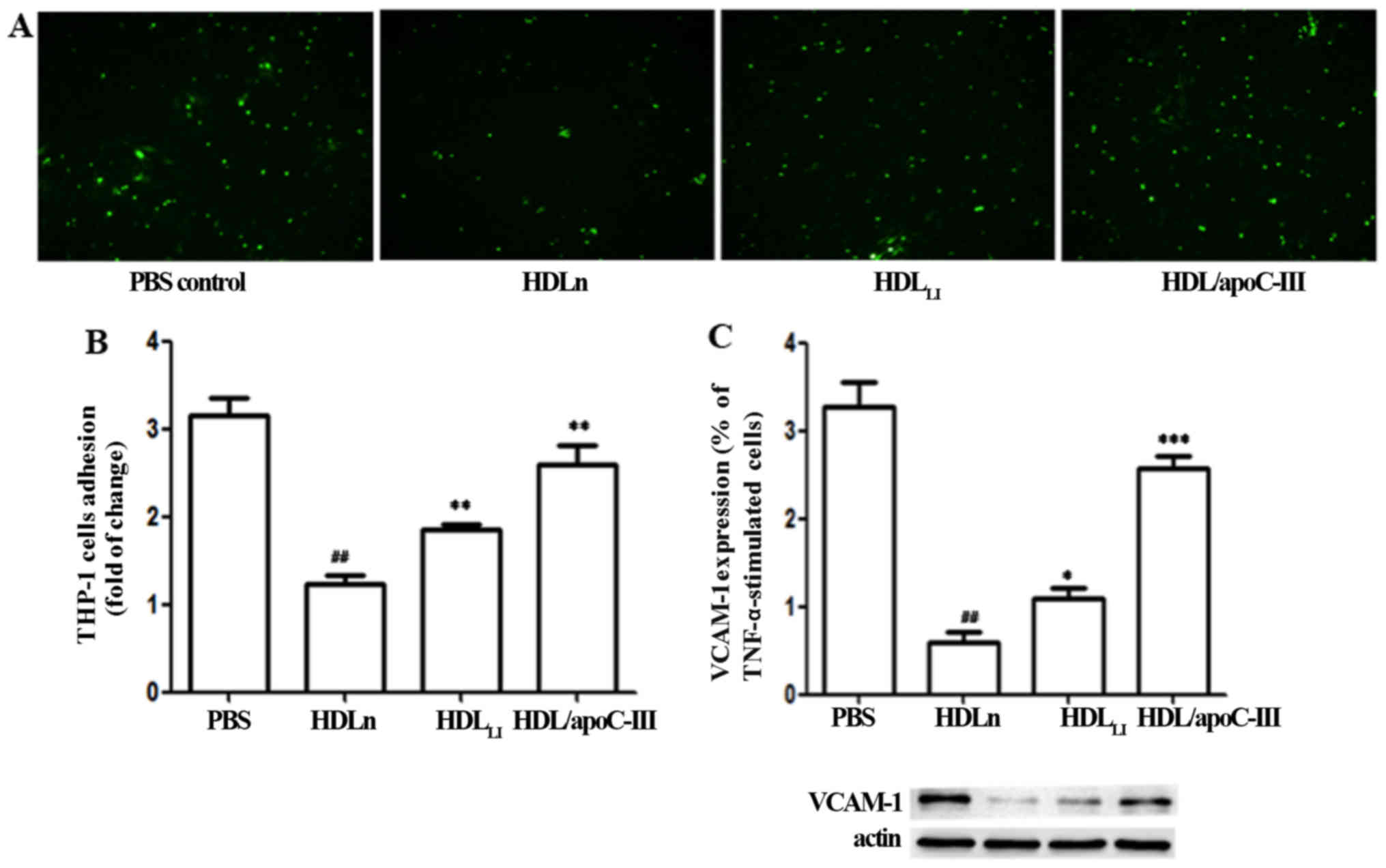
HDL of LACI patients has an increased
apoC-III content that impairs its anti-inflammatory function
The treatment of HUVECs with HDLn reduced
the binding of THP-1 cells to the HUVECs compared with the
PBS-treated controls (Fig. 5).
However, HDLLI had an impaired ability to inhibit the
binding of THP-1 cells to HUVECs compared with HDLn (P<0.01).
HDL/apoC-III also exhibited a significantly reduced ability to
inhibit the binding of THP-1 cells to HUVECs compared with HDLn
(P<0.01). The expression of VCAM-1 protein exhibited a response
to the HDL treatments that paralleled the response of the binding
ability. Treatment with HDL/apoC-III induced a significantly higher
expression of VCAM-1 compared with HDLn (P<0.001).
Treatment with HDLLI induced a significantly higher expression of
VCAM-1 compared with HDLn (P<0.05).
Discussion
There are ~50 proteins associated with the HDL
fraction that have been identified by previous studies using MS
methods (5,7,8,13,25,33). In the present study, 55
differentially expressed proteins were identified, which included
the majority of the previously identified proteins (19,25–28,30,31). The proteins identified as being
associated with HDL are involved in numerous functions, including
lipid metabolism, inflammatory response, the complement and
coagulation pathway, and endopeptidase inhibitory activity. Several
apolipoproteins and enzymes associated with lipid metabolism were
detected, including apoA-I, apoA-II, apoC-III, apoA-IV, apoC-I,
apoC-II, apoD, apoJ, apoE, apoF, apoL-I, apoM, phospholipid
transfer protein and phosphatidylcholine-sterol acyltransferase.
Other proteins that are involved in the inflammation response and
oxidative pathways were also identified, including serum amyloid A
proteins and certain complement components.
There were 55 proteins that were identified to be
differentially expressed between the patients and controls in the
present study. Among these proteins, three proteins that were
differentially expressed in the majority of the 12 LACI patients
were further validated using a biochemical method. The results of
western blot analysis validated the increase of apoC-III and SAA,
as well as the reduction of apoA-II in the HDL fraction of LACI
patients compared with the controls.
Another notable finding in the present study was
that an increased level of apoC-III in the HDL of LACI patients was
associated with an impaired anti-inflammatory function. ApoC-III is
a small apolipoprotein that is synthesized mainly in the liver and
circulates in the plasma in association with apoB-containing
lipoproteins and HDL (35). The
main physiological processes that apoC-III is involved in are
inhibition of lipoprotein lipase and hepatic lipase, and inhibition
of the hepatic uptake of triglyceride-rich particles (34). Furthermore, apoC-III activates
β-integrin, protein kinase C β and downstream VCAM-1 in monocytes,
which increases the adhesion of monocytes to vascular endothelial
cells (35). In the present
study, the HDL of LACI patients, with its upregulation of apoC-III,
had a reduced ability to inhibit leukocyte binding to endothelial
cells. Furthermore, ApoC-III-rich HDL did not inhibit monocyte
adhesion to endothelial cells, while the HDL of control subjects
decreased the adhesion, suggesting that apoC-III in HDL reduces the
anti-inflammatory property of HDL. This phenomenon clearly implies
that the apoC-III metabolism is changed in cerebrovacular
disease.
In addition to a reverse cholesterol transport
function, HDL has other vasculoprotective effects, including
antioxidative and anti-inflammatory properties (4,36,37). For instance, HDL attenuates
low-density lipoprotein (LDL) oxidation, a critical process in the
onset and aggravation of atherosclerotic plaques (38). Metal ions, such as iron or copper,
may promote lipid peroxidation in the process of LDL oxidation
(39). The present study
identified a number of proteins associated with metal ion
metabolism. The level of haptoglobin was decreased in the HDL
fraction of LACI patients compared with that of the control
subjects. Haptoglobin is an acute phase protein. It exclusively
binds to hemoglobin and releases it into the plasma during
physiological and pathological hemolysis, thereby preventing iron-
and heme-mediated oxidative side effects (40,41).
In conclusion, the present study revealed the
proteomic changes of HDL in patients with LACI. There were 55
proteins that were identified to be quantitatively different
between the patients with LACI and the control group, which were
mainly associated with the processes of lipid metabolism, the
inflammatory response, metal ion homeostasis, and the complement
pathway. The expression levels of apoA-II, apoC-III and SAA were
validated biochemically, and the results were consistent with the
MS data. The ApoC-III enrichment of the HDL in patients with LACI
may reduce the adhesion of THP-1 to endothelial cells, and thereby
decrease the anti-inflammatory effect of HDL. Future studies with a
larger number of subjects are required to determine whether the
identified proteins are suitable and relevant risk biomarkers.
Furthermore, the underlying mechanism of the apoC-III mediated HDL
dysfunction requires elucidation.
References
|
1
|
Havel RJ, Eder HA and Bragdon JH: The
distribution and chemical composition of ultracentrifugally
separated lipoproteins in human serum. J Clin Invest. 34:1345–1353.
1955. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kontush A and Chapman MJ: Antiatherogenic
small, dense HDL–guardian angel of the arterial wall. Nat Clin
Pract Cardiovasc Med. 3:144–153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davidson WS and Thompson TB: The structure
of apolipoprotein A-I in high density lipoproteins. J Biol Chem.
282:22249–22253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kontush A and Chapman MJ: Functionally
defective high-density lipoprotein: A new therapeutic target at the
crossroads of dyslipidemia, inflammation, and atherosclerosis.
Pharmacol Rev. 58:342–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karlsson H, Leanderson P, Tagesson C and
Lindahl M: Lipoproteomics II: Mapping of proteins in high-density
lipoprotein using two-dimensional gel electrophoresis and mass
spectrometry. Proteomics. 5:1431–1445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heller M, Stalder D, Schlappritzi E, Hayn
G, Matter U and Haeberli A: Mass spectrometry-based analytical
tools for the molecular protein characterization of human plasma
lipoproteins. Proteomics. 5:2619–2630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rezaee F, Casetta B, Levels JH, Speijer D
and Meijers JC: Proteomic analysis of high-density lipoprotein.
Proteomics. 6:721–730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vaisar T, Pennathur S, Green PS, Gharib
SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P,
et al: Shotgun proteomics implicates protease inhibition and
complement activation in the antiinflammatory properties of HDL. J
Clin Invest. 117:746–756. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan Y, Liu TR, Hu SW, Tian D, Li C, Zhong
JK, Sun HG, Luo TT, Lai WY and Guo ZG: Acute coronary syndrome
remodels the protein cargo and functions of high-density
lipoprotein subfractions. PLoS One. 9:e942642014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan LR, Wang DX, Liu H, Zhang XX, Zhao H,
Hua L, Xu P and Li YS: A pro-atherogenic HDL profile in coronary
heart disease patients: An iTRAQ labelling-based proteomic
approach. PLoS One. 9:e983682014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lepedda AJ, Nieddu G, Zinellu E, De Muro
P, Piredda F, Guarino A, Spirito R, Carta F, Turrini F and Formato
M: Proteomic analysis of plasma-purified VLDL, LDL, and HDL
fractions from atherosclerotic patients undergoing carotid
endarterectomy: Identification of serum amyloid A as a potential
marker. Oxid Med Cell Longev. 385214:2013. View Article : Google Scholar
|
|
12
|
Ståhlman M, Fagerberg B, Adiels M, Ekroos
K, Chapman JM, Kontush A and Borén J: Dyslipidemia, but not
hyperglycemia and insulin resistance, is associated with marked
alterations in the HDL lipidome in type 2 diabetic subjects in the
DIWA cohort: Impact on small HDL particles. Biochim Biophys Acta.
1831:1609–1617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holzer M, Birner-Gruenberger R, Stojakovic
T, El-Gamal D, Binder V, Wadsack C, Heinemann A and Marsche G:
Uremia alters HDL composition and function. J Am Soc Nephrol.
22:1631–1641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mangé A, Goux A, Badiou S, Patrier L,
Canaud B, Maudelonde T, Cristol JP and Solassol J: HDL proteome in
hemodialysis patients: A quantitative nanoflow liquid
chromatography-tandem mass spectrometry approach. PLoS One.
7:e341072012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kopecky C, Genser B, Drechsler C, Krane V,
Kaltenecker CC, Hengstschläger M, März W, Wanner C, Säemann MD and
Weichhart T: Quantification of HDL proteins, cardiac events, and
mortality in patients with type 2 diabetes on hemodialysis. Clin J
Am Soc Nephrol. 10:224–231. 2015. View Article : Google Scholar :
|
|
16
|
Watanabe J, Charles-Schoeman C, Miao Y,
Elashoff D, Lee YY, Katselis G, Lee TD and Reddy ST: Proteomic
profiling following immunoaffinity capture of high-density
lipoprotein: Association of acute-phase proteins and complement
factors with proinflammatory high-density lipoprotein in rheumatoid
arthritis. Arthritis Rheum. 64:1828–1837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chait A, Han CY, Oram JF and Heinecke JW:
Thematic review series: The immune system and atherogenesis.
Lipoprotein-associated inflammatory proteins: Markers or mediators
of cardiovascular disease. J Lipid Res. 46:389–403. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chei CL, Yamagishi K, Kitamura A, Kiyama
M, Imano H, Ohira T, Cui R, Tanigawa T, Sankai T, Ishikawa Y, et
al: CIRCS Investigators: High-density lipoprotein subclasses and
risk of stroke and its subtypes in Japanese population: The
Circulatory Risk in Communities Study. Stroke. 44:327–333. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashby DT, Rye KA, Clay MA, Vadas MA,
Gamble JR and Barter PJ: Factors influencing the ability of HDL to
inhibit expression of vascular cell adhesion molecule-1 in
endothelial cells. Arterioscler Thromb Vasc Biol. 18:1450–1455.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barter PJ, Nicholls S, Rye KA,
Anantharamaiah GM, Navab M and Fogelman AM: Antiinflammatory
properties of HDL. Circ Res. 95:764–772. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fisher CM: The arterial lesions underlying
lacunes. Acta Neuropathol. 12:1–15. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogata J, Yamanishi H and Ishibashi-Ueda H:
Review: Role of cerebral vessels in ischaemic injury of the brain.
Neuropathol Appl Neurobiol. 37:40–55. 2011. View Article : Google Scholar
|
|
23
|
Sorci-Thomas MG and Thomas MJ: Why
targeting HDL should work as a therapeutic tool, but has not. J
Cardiovasc Pharmacol. 62:239–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsieh JY, Chang CT, Huang MT, Chang CM,
Chen CY, Shen MY, Liao HY, Wang GJ, Chen CH, Chen CJ, et al:
Biochemical and functional characterization of charge-defined
subfractions of high-density lipoprotein from normal adults. Anal
Chem. 85:11440–11448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davidson WS, Silva RA, Chantepie S, Lagor
WR, Chapman MJ and Kontush A: Proteomic analysis of defined HDL
subpopulations reveals particle-specific protein clusters:
Relevance to antioxidative function. Arterioscler Thromb Vasc Biol.
29:870–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patel B and Markus HS: Magnetic resonance
imaging in cerebral small vessel disease and its use as a surrogate
disease marker. Int J Stroke. 6:47–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Turin TC, Kita Y, Rumana N, Nakamura Y,
Takashima N, Ichikawa M, Sugihara H, Morita Y, Hirose K, Okayama A,
et al: Ischemic stroke subtypes in a Japanese population: Takashima
Stroke Registry, 1988–2004. Stroke. 41:1871–1876. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu D, Ji L, Tong X, Pan B, Han JY, Huang
Y, Chen YE, Pennathur S, Zhang Y and Zheng L: Human apolipoprotein
A-I induces cyclooxygenase-2 expression and prostaglandin I-2
release in endothelial cells through ATP-binding cassette
transporter A1. Am J Physiol Cell Physiol. 301:C739–C748. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eng JK, McCormack AL and Yates JR: An
approach to correlate tandem mass spectral data of peptides with
amino acid sequences in a protein database. J Am Soc Mass Spectrom.
5:976–989. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kimura T, Tomura H, Mogi C, Kuwabara A,
Damirin A, Ishizuka T, Sekiguchi A, Ishiwara M, Im DS, Sato K, et
al: Role of scavenger receptor class B type I and sphingosine
1-phosphate receptors in high density lipoprotein-induced
inhibition of adhesion molecule expression in endothelial cells. J
Biol Chem. 281:37457–37467. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar :
|
|
32
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering
C, et al: STRING v9.1: Protein-protein interaction networks, with
increased coverage and integration. Nucleic Acids Res.
41:D808–D815. 2013. View Article : Google Scholar :
|
|
33
|
Gordon SM, Deng J, Lu LJ and Davidson WS:
Proteomic characterization of human plasma high density lipoprotein
fractionated by gel filtration chromatography. J Proteome Res.
9:5239–5249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bernelot Moens SJ, van Capelleveen JC and
Stroes ESG: Inhibition of ApoCIII: The next CSK9? Curr Opin
Lipidol. 25:418–422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kawakami A and Yoshida M: Apolipoprotein
CIII links dyslipidemia with atherosclerosis. J Atheroscler Thromb.
16:6–11. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feig JE, Shamir R and Fisher EA:
Atheroprotective effects of HDL: Beyond reverse cholesterol
transport. Curr Drug Targets. 9:196–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ansell BJ, Fonarow GC and Fogelman AM: The
paradox of dysfunctional high-density lipoprotein. Curr Opin
Lipidol. 18:427–434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Watson AD, Berliner JA, Hama SY, La Du BN,
Faull KF, Fogelman AM and Navab M: Protective effect of high
density lipoprotein associated paraoxonase. Inhibition of the
biological activity of minimally oxidized low density lipoprotein.
J Clin Invest. 96:2882–2891. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Blokhina O, Virolainen E and Fagerstedt
KV: Antioxidants, oxidative damage and oxygen deprivation stress: A
review. Ann Bot (Lond). 91:179–194. 2003. View Article : Google Scholar
|
|
40
|
Katoh N and Nakagawa H: Detection of
haptoglobin in the high-density lipoprotein and the very
high-density lipoprotein fractions from sera of calves with
experimental pneumonia and cows with naturally occurring fatty
liver. J Vet Med Sci. 61:119–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nielsen MJ, Petersen SV, Jacobsen C, Oxvig
C, Rees D, Møller HJ and Moestrup SK: Haptoglobin-related protein
is a high-affinity hemoglobin-binding plasma protein. Blood.
108:2846–2849. 2006. View Article : Google Scholar : PubMed/NCBI
|