Introduction
Recent industrial advancements have resulted in
rapid social changes, including changes in everyday lifestyle and
worsening of environmental pollution. In particular, various mental
and physical pathologies have accelerated the rate of chronic
metabolic diseases, including obesity, cerebrovascular disease,
heart disease and hypertension (1). The major causes of chronic
degenerative diseases are reactive oxygen species (ROS), comprising
superoxide (O2-), singlet oxygen
(1O2) and hydrogen peroxide
(H2O2), which are produced during metabolic
processes. ROS generated in the body are the cause of oxidative
stress (2,3). They are not only involved in
processes of energy metabolism, immune reaction and neuronal signal
transduction, but when they are produced excessively, ROS also have
a role in various dysfunctions, including protein and DNA
modification, aging of biological membranes, metabolic diseases and
cancer (4,5).
Obesity arises from the accumulation of
triglycerides in the body, resulting from an imbalance in the use
and intake of energy from food. A typical disease caused by obesity
is insulin resistance and diabetic dyslipidemia (6,7).
The cause of the lipid accumulation is the differentiation of
preadipocytes to adipocytes. This differentiation leads to
hypertrophy and hyperplasia of adipocytes (8,9).
In addition, when the preadipocytes differentiate to adipocytes,
the expression of oxidant enzymes, such as NADPH oxidase, is
increased. Therefore, adipocyte differentiation generates ROS and
leads to oxidative stress (10).
For the maintenance of the anti-oxidative system, a
healthy functional diet is required so that individuals with a
modern lifestyle, who are exposed to a high frequency of stress,
obesity-associated factors, chemicals and environmental hormones
may compensate for the risk these factors represent for their
health (11,12). A healthy functional diet contains
radical-quenching substances that exert a protective mechanism to
remove toxic ROS (13). These
foods are required to contain high quantities of ascorbic acid,
α-tocopherol, glutathione, phenolics and flavonoids that protect
against oxidative damage by removing ROS (14).
Of note, due to the increasing demand for more
effective and safer in anti-obesity and antioxidant products, it is
important to identify novel natural substances that serve this
purpose. Therefore, the present study was designed to investigate
the antioxidant and anti-obesity effects of Alnus firma,
which is common in South Korea, to observe its inhibitory effects
on lipid accumulation and ROS generation.
Materials and methods
Materials
The leaves of Alnus firma (A. firma) 70%
ethanolic extract (AFE) were prepared as follows: A. firma
was collected in Goryeong-gun (Gyeongsangbuk-do, Korea) in July
2014 and identified by the National Institute of Biological
Resources (Incheon, Korea). A sample was also deposited in a
library of the National Institute of Biological Resources. Coarse,
grounded, dried samples (100 g) were extracted with 1,000 ml 70%
ethanol at room temperature for 24 h. The extraction process was
repeated three times. The extracted materials were filtered (no. 3;
Whatman; GE Healthcare, Little Chalfont, UK) and concentrated with
a rotary evaporator (N-3000; Eyela, Tokyo, Japan). Subsequently,
the extracted materials were dried using a freeze dryer (Biotron,
Bucheon, Korea). The extracts obtained after freeze drying weighed
12.7 g, with a yield of 12.70%. The samples were dissolved in
dimethyl sulfoxide (DMSO) to prepare a stock solution used in the
experiments. Oil Red O, 3-isobutyl-1-methylxanthine (IBMX),
dexamethasone (DEX), isopropanol, rutin, ascorbic acid, gallic
acid, 1,1-diphenyl-2-picryl hydrazyl radical (DPPH),
N-acetyl-L-cysteine (NAC),
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium
salt (ABTS), Folin-Ciocalteu phenol reagent, potassium
ferricyanide, sodium carbonate, potassium persulfate,
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox),
insulin, acetic acid, 2,2-azobis(2-amidino propane) dihydrochloride
(AAPH), fluorescein sodium salt and sodium nitrite were from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Dulbecco's modified
Eagle's medium (DMEM), bovine serum (BS), fetal bovine serum (FBS),
penicillin-streptomycin (P/S), phosphate-buffered saline (PBS) and
trypsin-EDTA were from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Total phenolic, flavonoid and
proanthocyanidin contents
The total phenolic content of AFE was determined by
the method of Gutfinger (15)
with certain modifications. The sample dissolved in 100% DMSO
solution (1 ml) was placed in a test tube with (2%) sodium
carbonate solution (1 ml) and 10% Folin-Ciocalteu reagent (1 ml).
After the mixture was incubated at 25°C for 40 min, the absorbance
was measured at 750 nm and the calibration curve was prepared using
gallic acid. The results are expressed in milligram of gallic acid
equivalents (mg GAE)/g.
The total flavonoid content of AFE was determined by
the method of Moreno et al (16) with certain modifications. The
sample dissolved in 100% DMSO solution (0.5 ml) was mixed with 0.5
ml aluminum chloride (2% w/v). After incubation at room temperature
for 60 min, the absorbance was determined at 420 nm, and a
calibration curve was prepared using quercetin. The results are
expressed in milligram of quercetin equivalents (mg QE)/g.
The total proanthocyanidin content of AFE was
determined by the method of Mitsunaga et al (17) with certain modifications. The
sample (0.5 mg) was diluted with 5 ml methanol. The sample solution
was mixed with 3 ml vanillin (4% w/v) and 1.5 ml concentrated
hydrochloric acid. After the mixture was incubated at room
temperature for 15 min, the absorbance was determined at 429 nm,
and a calibration curve was prepared using catechin. The results
are expressed in milligram of catechin equivalents (mg CE)/g.
DPPH radical scavenging assay
The DPPH assay was performed according to the method
of Chu et al (18) with
certain modifications. The sample solution (0.1 ml) was mixed with
0.4 mM DPPH solution (0.1 ml) in anhydrous ethanol and the mixture
was incubated in the dark for 30 min. The absorbance of the
solution was adjusted to 1.0±0.1 at 515 nm. Subsequently, 0.2 ml of
the sample (or a control) was mixed with 0.8 ml DPPH solution and
incubated for 10 min in the dark at room temperature. The decrease
in absorbance was measured at 515 nm, and the radical scavenging
activity was calculated and expressed as a percentage using the
following formula: DPPH radical scavenging activity (%) = [(Ac −
As)/Ac] ×100 (i), with Ac being the absorbance of the control and
As the absorbance of the sample.
ABTS radical scavenging activity
The ABTS assay was based on the method of Re et
al (19) with certain
modifications. ABTS (7 mM) dissolved in water was mixed with 2.45
mM potassium persulfate. The mixture was incubated in the dark at
20°C for 16 h. The ABTS solution was diluted with ethanol until an
absorbance of 0.70±0.02 was achieved at 734 nm. Subsequently, 150
μl ABTS solution was added to 50 μl of the sample
solutions. After 20 min, the absorbance of the mixture was measured
at 734 nm. The ABTS radical scavenging activity was calculated and
expressed as a percentage using formula (i).
Reducing power
The reducing power assay was performed according to
the method of Oyaizu (20) with
certain alterations. Various concentrations of sample solutions
were mixed with 0.5 ml sodium phosphate buffer (0.2 M) and 0.5 ml
potassium ferricyanide (1% v/v). The mixtures were incubated at
50°C for 20 min. After the addition of 0.5 ml trichloroacetic acid
(10% w/v), the mixture was centrifuged at 1,790 × g for 10 min. A
2.5-ml aliquot of the supernatant was mixed with 2.5 ml distilled
water and 0.2 ml Iron(III) chloride (0.1% w/v), and the absorbance
was measured at 700 nm.
Nitrate scavenging ability assay
The nitric oxide (NO) assay was performed according
to the method of Kato et al (21) with certain modifications. Sample
solution (0.5 ml) was mixed with 0.5 ml sodium nitrite solution (1
mM). The mixture was calibrated at pH 1.2 with HCl (0.1 N) and
filled up to a volume of 5 ml with distilled water. The mixture was
then incubated at 37°C for 60 min. Subsequently, 1 ml of the
solution was mixed with 5 ml acetic acid (2% v/v) and 0.4 ml Griess
reagent (0.5% sulfanilic acid and 0.5% naphthylamine in 30% acetic
acid). The mixture was incubated at room temperature for 60 min and
the absorbance was measured at 734 nm. The NO radical scavenging
activity was calculated and expressed as a percentage using formula
(i).
Oxygen radical absorbance capacity (ORAC)
assay
The ORAC assay was based on the method of Ou et
al (22) with certain
modifications. AFE was dissolved in 75 mM phosphate buffer (pH 7.4)
at 37°C. Aliquots of 25 μl of this solution, 150 μl
fluorescein (40 nM) and 25-μl AAPH (18 mM) were filled into
each well of black 96-well plates and incubated at 37°C for 15 min.
The plate was then immediately placed in the fluorescence
microplate reader (Spectramax Gemini EM; Molecular Devices,
Sunnyvale, CA, USA). The reader was programmed to record the
fluorescence of fluorescein every 3 for 90 min at emission and
excitation wavelengths of 520 and 485 nm, respectively. Trolox was
used as a standard, and a blank was measured that contained
phosphate buffer in place of the sample. The ORAC values were
calculated using a Trolox calibration curve and the area under the
fluorescence decay curve. ORAC values were expressed as TE in
μmol/ml and the area under the curve (AUC) was determined as
follows: AUC = 1+f1/f0+f2/f0+f3/f0+f4/f0+...f/31/f0 (ii), with f(0)
being the initial fluorescence values and f(n) the fluorescence
value measured every 3 min.
Cell culture
3T3-L1 preadipocytes obtained from the American Type
Culture Collection (no. CL-173; Manassas, VA, USA) were cultured,
maintained and differentiated as described by Lee et al
(23). In brief, the cells were
plated in a 5% CO2 atmosphere at 37°C and grown in DMEM
with 3.7 g/l sodium bicarbonate, 1% P/S and 10% BS. Adipocyte
differentiation was induced by treating post-confluent cells with
10% FBS and a hormonal cocktail consisting of 0.5 mM IBMX, 1.0
μM DEX, and 1.0 μM insulin (MDI) for 2 days. The
culture medium was then replaced with DMEM supplemented with 1.67
μM insulin and 10% FBS only. 3T3-L1 cells were grown in
100-mm tissue culture dishes in DMEM supplemented with 10% BS, as
previously described. When the cells were in the log phase, they
were divided into suitable dishes for the study. This medium was
then replenished every 2 days. For the treatments, 2-days
post-confluent cells were differentiated with MDI in the presence
of AFE (25, 50 or 100 μg/ml) or NAC (5 mM). AFE was prepared
at 25, 50 and 100 mg/ml in DMSO and then diluted to 0.1% with
media.
Cell proliferation assay
Cell proliferation was assessed using the
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
inner salt (XTT) assay (WelGene, Seoul, Korea). When the cells were
cultured to be in the log phase, they were seeded on a 96-well
plate (1×105 cells/well) and incubated for 24 h. The
cells were divided into a control group and the treatment groups,
which were treated at the concentrations indicated. The absorbance
(A) was determined with an enzyme calibrator at 450 nm. The cell
viability was determined as follows: Viability = (A study group/A
of control group) ×100% (iii).
Determination of ROS by flow
cytometry
The levels of ROS were analyzed using
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA;
Invitrogen; Thermo Fisher Scientific, Inc.). In brief, the 3T3-L1
cells were differentiated with AFE for 6 days. Differentiated cells
were incubated for at 37°C with H2DCFDA (10 μM)
for 30 min. The cell pellet was analysed using a FACSCalibur flow
cytometer (BD Biosciences, San Jose, CA, USA), with an excitation
wavelength of 488 nm and an emission wavelength of 545 nm. Data
analysis was based on 10,000 detected events using Cell Quest
software 6.0 (BD Biosciences).
Nitroblue tetrazolium (NBT) assay
3T3-L1 preadipocytes were grown to confluence and
induced to differentiate into adipocytes as previously described
(24). ROS production was
detected by an NBT assay. NBT is reduced by ROS to a dark blue,
insoluble form of NBT called formazan. At 8 days after induction,
the cells were incubated in PBS containing 0.2% NBT for 90 min. The
formazan was dissolved in 50% acetic acid and the absorbance was
determined at 570 nm. NBT assay was calculated and expressed as a
percentage using formula (iii).
Oil Red O staining
The extent of differentiation was determined by the
amount of lipid accumulation after 8 days following induction of
differentiation by Oil Red O staining. In brief, the cells were
fixed in 10% formaldehyde in distilled water for 1 h, washed with
distilled water and completely dried. The cells were stained with
0.5% Oil Red O dissolved in isopropanol (60% v/v) for 30 min at
room temperature, washed four times with water and dried.
Differentiation was also monitored under a microscope and
quantified via elution with isopropanol. The optical density at 490
nm (OD490) was then measured (25). Oil Red O staining was calculated
and expressed as a percentage using formula (iii).
RNA extraction and semi-quantitative
reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from mature 3T3-L1 adipocyte
cells with TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) in accordance with the manufacturer's recommended protocols.
RNA samples with OD260/OD280 ratios >2.0
were employed for semi-quantitative RT-PCR. One microgram of total
RNA was employed for the production of complementary DNA using a
Maxime RT premix kit (Intron Biotechnology, Inc., Seongnam, Korea)
at 45°C for 60 min and an RT-PCR system (c1000 thermal cycler;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). PCR amplification
was performed with primers, cDNA and Taq MasterMix (MG Taq
MasterMix; Doctor Protein; MGMED, Inc., Seoul, Korea), and it was
performed at 95°C for 5 min, followed by 30 cycles of 95°C for 30
sec, annealing for 30 sec at 54°C and an extension step of 1 min at
72°C, and a final extension step of 5 min at 72°C. All primers used
in this study are listed in Table
I. The PCR products were then run on 1.5% (v/v) agarose gels
and stained with ethidium bromide, and images were captured with a
gel documentation and analysis system (care stream MISE; Carestream
Health, Inc., Rochester, NY, USA). The expression levels were
quantified using ImageJ software 1.48 (National Institutes of
Health, Bethesda, MD, USA).
 | Table IGene specific primer used for
RT-PCR. |
Table I
Gene specific primer used for
RT-PCR.
| Gene | Primer sequences
(5′-3′) |
|---|
| PPAR-γ | F: CCA GAG TCT GCT
GAT CTG CG |
| R: GCC ACC TCT TTG
CTC TGA TC |
| C/EBP-α | F: GCA GTG TGC ACG
TCT ATG CT |
| R: AGC CCA CTT CAT
TTC ATT GG |
| aP2 | F: GAC CTG GAA ACT
CGT CTC CA |
| R: CAT GAC ACA TTC
CAC CAC CA |
| β-actin | F: ATG GAT GAC GAT
ATC GCT GC |
| R: GCT GGA AGG TGG
ACA GTG AG |
Western blot analysis
For western blot analysis, cells were lysed using a
protein lysis buffer containing 50 mM Tris-HCL at pH 7.4, 150 mM
NaCl, 1% NP-40, 0.25% sodium deoxycholate, 0.1% SDS, 1 mM PMSF and
1 mM pepstatin A. The lysates were centrifuged at 12,000 × g for 20
min at 4°C, and the protein concentrations were determined using a
Bradford protein assay kit (Bio-Rad Laboratories, Inc.). Equal
volumes of supernatant (30 μg) were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc.). Nonspecific binding was blocked using
Tris-buffered saline containing Tween-20 (TBS-T) and 5% skimmed
milk for 1 h and the membranes were incubated with primary
antibodies at 4°C overnight. Finally, the membranes were treated
with secondary antibodies for 1 h at room temperature and washed
with TBS-T. Each protein was detected using Super Signal West Pico
Chemiluminescence detection reagents (Pierce; Thermo Fisher
Scientific, Inc.), and images were evaluated using Chemi Doc image
software 5.2.1 (Bio-Rad Laboratories, Inc.). The primary antibodies
specific for β-actin (1:1,000; cat. no. 4967), peroxisome
proliferator-activated receptor-γ (PPAR-γ; 1:1,000; cat. no. 2443),
CCAAT/enhancer-binding protein-α (C/EBP-α; 1:1,000; cat. no. 2295),
adipocyte protein 2 (aP2; 1:1,000; cat. no. 3544) and the secondary
antibodies (1:3,000; cat. no. 7076) were obtained from Cell
Signalling Technology (Danvers, MA, USA).
Statistical analysis
All measurements were repeated three times. Values
are expressed as the mean ± standard deviation. The results were
statistically analyzed by analysis of variance and Duncan's
multiple-range tests. P<0.05 was considered to indicate a
statistically significant difference. SAS software 9.4 (1998; SAS
Institute, Inc., Cary, NC, USA) was used for all statistical
comparisons.
Results
Total phenolic, flavonoid and
proanthocyanidin contents
Phenols are aromatic compounds bearing hydroxyl
groups (–OH). These phenolic compounds have antioxidant capacities
and protect DNA, cell proteins and enzymes when exposed to ROS,
thereby exerting anticancer and other protective effects. Due to
their antioxidant effects, the increased total phenolic content
achieved by A. firma supplementation increases the
physiological antioxidant activity (26,27). Flavonoids and proanthocyanidins,
which are polyphenol compounds contained in the roots, stems,
leaves, flowers and fruits of numerous plants, have also been
reported to have antioxidant, anti-bacterial, anti-allergic,
anticancer and anti-inflammatory effects (28,29). The present study examined the
total phenolic, flavonoid and proanthocyanidin contents of AFE
(Table II). It had a high total
phenolic content (436.26±3.30 mg GAE/g), total flavonoid content
(73.82±0.54 mg QE/g) and total anthocyanin content (149.25±6.06 mg
CE/g). These results are in good agreement with another study by
Stević et al (30),
according to which the total phenolic content of other species of
the Alnus genus, methanolic extract of A. incana and
A. viridis, were 316.2±6.9 and 238.6±6.6 mg GAE/g,
respectively. Futhermore, Acero and Muñoz-Mingarro (31) reported that the total flavonoid
content of methanolic extract of A. glutinosa, another
species of the Alnus genus, was 34.55±0.19 mg QE/g.
Therefore, these results indicated that AFE, compared with the
extracts of other Alnus species, contained high levels of
flavonoids and phenolic compounds.
 | Table IIQuantification of antioxidant
components in 70% ethanolic extract of Alnus firma. |
Table II
Quantification of antioxidant
components in 70% ethanolic extract of Alnus firma.
| Compound class | Amount |
|---|
| Total phenols | 436.26±3.30 mg
GAE/g |
| Total
flavonoids | 73.82±0.54 mg
QE/g |
| Total
proanthocyanidins | 149.25±6.06 mg
CE/g |
Effect of AFE on antioxidant
activity
In order to measure the antioxidant activity of AFE,
in vitro assays assessing parameters including the DPPH
radical scavenging activity, ABTS radical cation scavenging
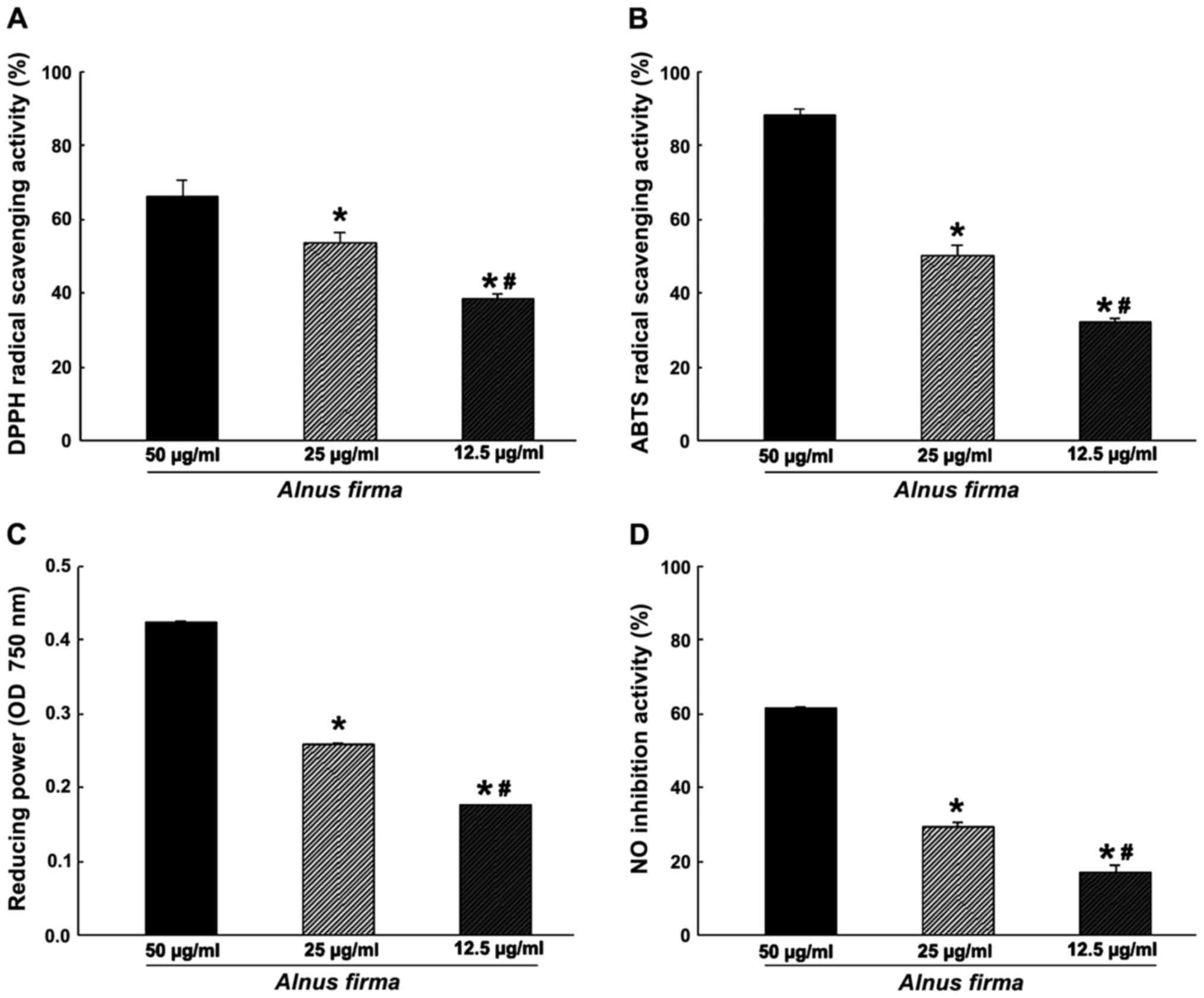
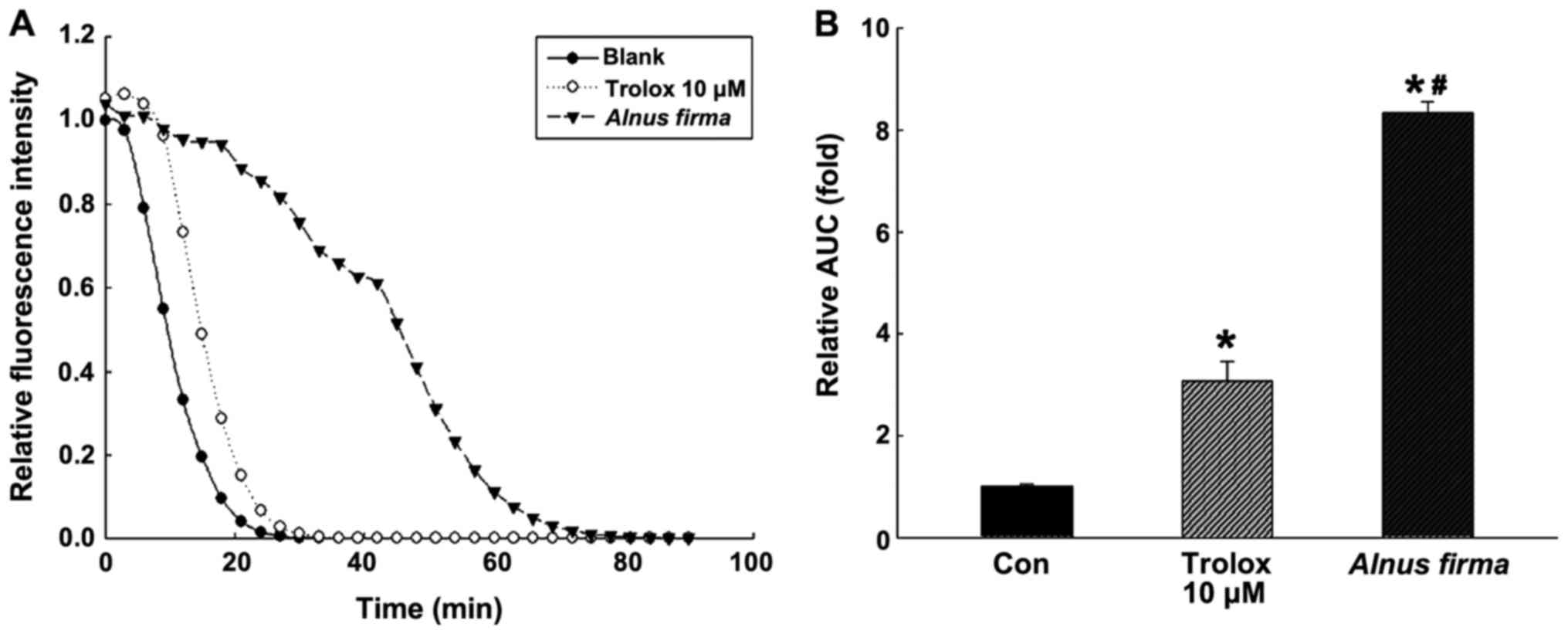
activity, reducing power and nitrite scavenging ability (Fig. 1), as well as the ORAC (Fig. 2) were used. These assays measure
the antioxidant and radical quenching capacity of AFE through
different mechanisms. The DPPH radical scavenging activity of AFE
was dose-dependent, and scavenging of 38.57±1.04, 53.63±2.68 and
66.16±4.54% of DPPH was obtained with 12.5, 25 and 50 μg/ml
AFE, respectively (Fig. 1A). ABTS
radical scavenging proceeds via a similar mechanism to that of DPPH
radical scavenging, and was also dose-dependent with 32.09±1.11,
50.29±2.74 and 88.19±1.47% ABTS scavenging obteined with 12.5, 25
and 50 μg/ml AFE, respectively (Fig. 1B). However, as the DPPH assay
cancels out the free radicals whereas the ABTS assay cancels out
cation radicals, a discrepancy in radical scavenging ability
between the DPPH assay and the ABTS assay was observed, which may
indicate a combination of reactant and substrate at different
degrees (32). The nitrite
scavenging ability and reducing power assays, which demonstrate the
antioxidant effect of AFE, are based on different mechanisms. One
of the radicals of nitrite reacts with the Griess reagent to form a
purple Azo-dye. The nitrite scavenging activities were 17.19±1.89,
29.23±1.43 and 61.30±0.34% for 12.5, 25 and 50 μg/ml AFE,
respectively (Fig. 1D). The
reducing power assay is a method to measure the degree of reduction
from oxidation of the analyte substance. AFE (12.5 to 25 to 50
μg/ml) was determined to have dose-dependent
nitrite-scavenging and reducing capacities (Fig. 1C and D). The ORAC assay is a
measure of the reduction of fluorescence by the peroxyl radical
(Fig. 2). The ORAC of AFE was
792.01±13.10 μM Trolox equivalents (TE)/g when it was
created by Trolox standard curve (data not shown). Kim et al
(33) reported that the ORAC
value of methanolic extract of A. firma was 198.0±9.1
μM TE/g, which differed from the result of the present
study.
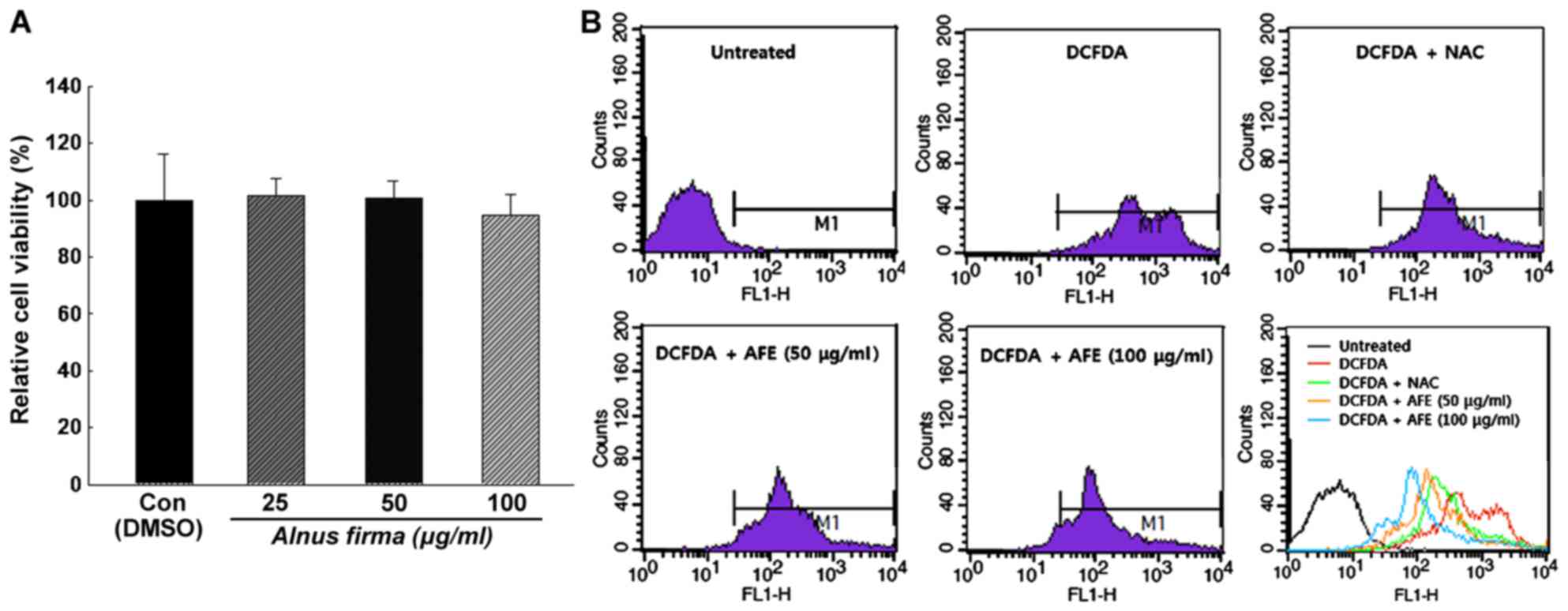
Effect of AFE on cell viability
In order to determine the cytotoxicity and the
concentration range of AFE suitable for treating 3T3-L1
preadipocytes, the XTT assay was performed. The cytotoxicity of AFE
at various concentrations was analyzed by calculating the degree of
change in cell growth as a percentage of the mock control group. As
presented in Fig. 3A, AFE did not
have any cytotoxic effects at 25, 50 and 100 μg/ml
concentration and the absorbance values were 101±6.3, 100±6.1 and
94±7.6% of the control values, respectively, after 24 h of
incubation. Also, no changes in cell morphology were observed under
the microscope (data not shown). Thus, the concentrations of 25, 50
and 100 μg/ml were used in the experiments described
below.
Determination of ROS by flow
cytometry
The inhibition of ROS production during the
differentiation of 3T3-L1 cells by AFE was analyzed using
H2DCFDA. Treatment with AFE (50 or 100 μg/ml) or
NAC during the adipogenic stage (days 0-6 post-induction) resulted
in a inhibition of ROS generation compared with that in a control
group, which was only treated with MDI for induction. As presented
in Fig. 3B, the production of ROS
was increased during adipocyte differentiation, which was inhibited
in the presence of NAC or AFE. It was also revealed that the
production of ROS was decreased by AFE in a dose-dependent manner,
although statistical analysis was not conducted on this data.
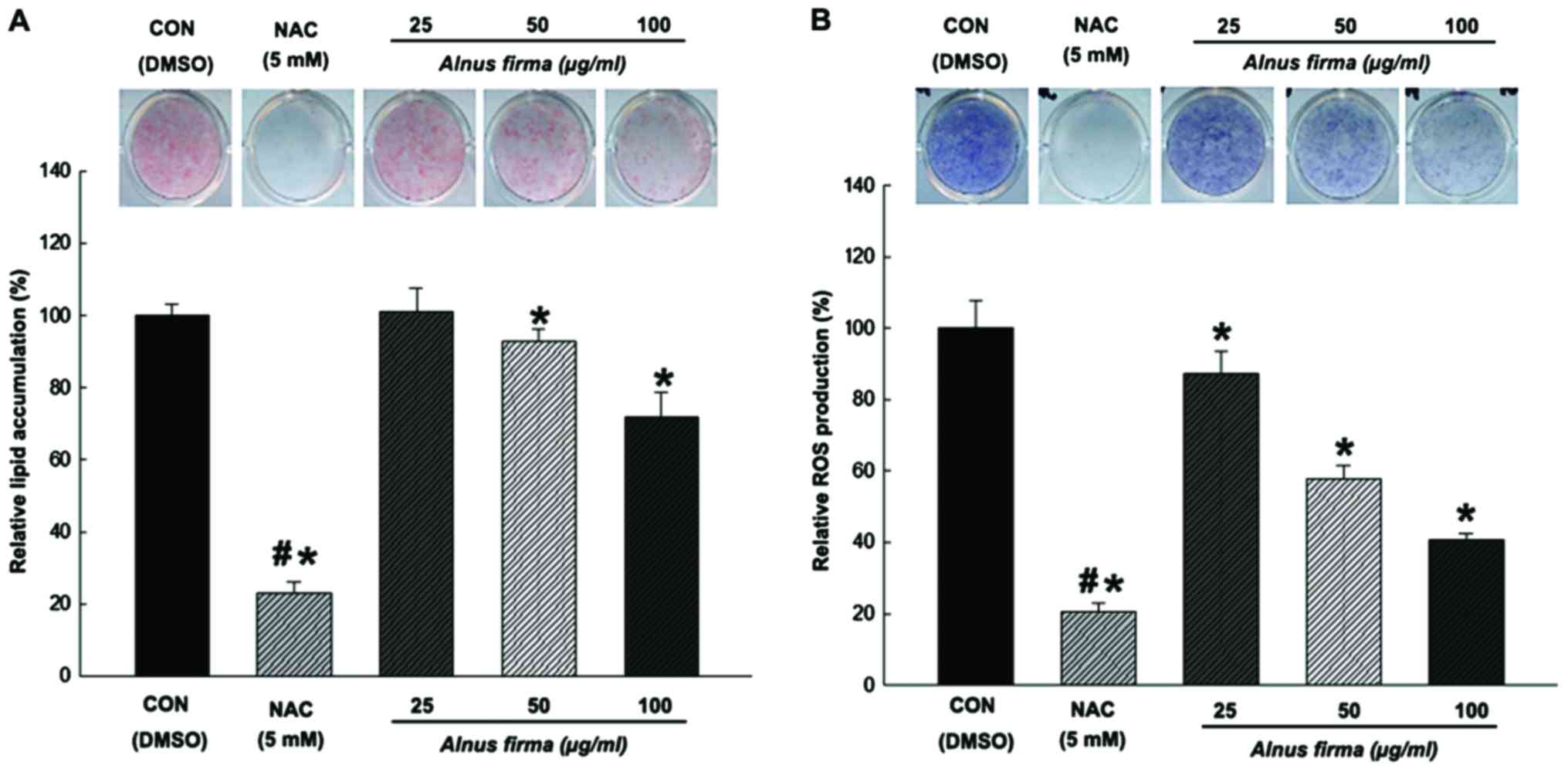
Effects of AFE on lipid accumulation and
ROS production during adipocyte differentiation
The present study examined the capacity of AFE to
inhibit adipogenic differentiation of preadipocytes. Treatments
with AFE (25, 50 and 100 μg/ml) and NAC during adipogenic
differentiation (days 0–8 post-induction) resulted in a significant
inhibition of lipid accumulation compared with that in the control
group. The 3T3-L1 cells treated with NAC also displayed
significantly reduced lipid accumulation (23±3.1% compared with
that in the control) (Fig. 4A).
While AFE at 25 μg/ml did not affect the lipid accumulation
(101±6.6% of control), 50 and 100 μg/ml of AFE significantly
reduced lipid accumulation to 92±3.4 and 71±6.8%, respectively,
compared with the mock control. Next, the effect of AFE on ROS
production in 3T3-L1 during adipogenic differentiation was examined
using an NBT assay. Treatment with AFE (25, 50 or 100 μg/ml)
or NAC during adipogenic differentiation (days 0–8 post-induction)
resulted in a significant inhibition of ROS accumulation.
NAC-treated cells displayed significantly reduced ROS levels
(20±2.5% compared with the control) (Fig. 4B). Cells treated with AFE at all
concentrations (25, 50 and 100 μg/ml) exhibited drastically
reduced ROS production (87±6.2, 57±3.8 and 40±1.6%, respectively,
compared to mock control). These results indicated that lipids
accumulate in parallel with ROS production during adipogenesis,
which is dose-dependently inhibited by AFE.
Effect of AFE on the expression of
factors associated with lipid accumulation during
differentiation
In order to determine whether AFE inhibitis
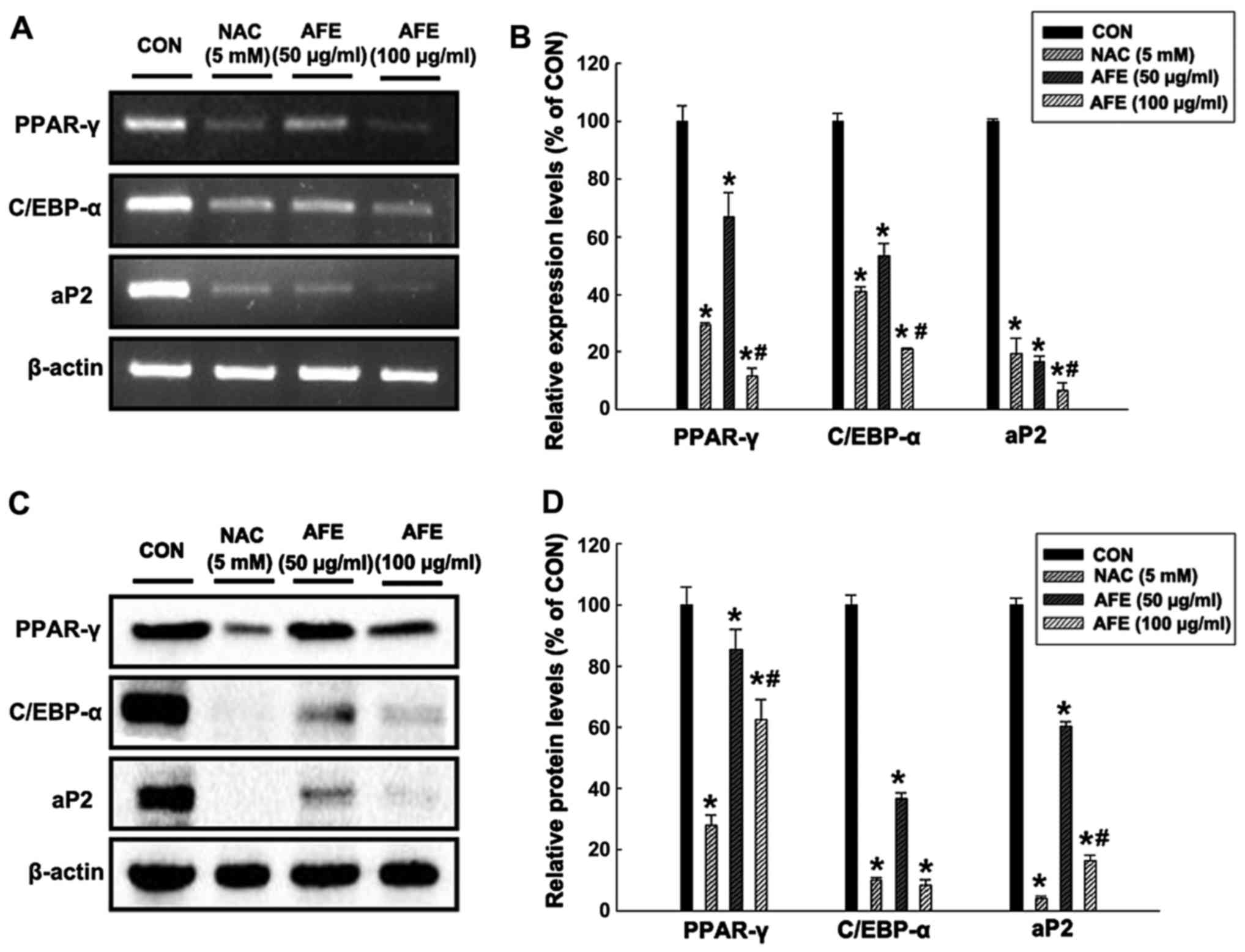
adipogenic mechanisms, the mRNA (Fig.
5A and B) and protein (Fig. 5C
and D) expression levels of the adipogenic transcription
factors PPAR-γ, C/EBP-α and aP2 in 3T3-L1 cells treated with 50 and
100 μg/ml of AFE or NAC during differentiation were assessed
by RT-PCR and western blot analysis, respectively. The results in
Fig. 5 demonstrate that in 3T3-L1
cells treated with 100 μg/ml AFE during differentiation,
PPAR-γ, C/EBP-α and aP2 levels were decreased to 12, 21 and 6% of
those in the control group, respectively. In addition, the protein
expression levels of PPAR-γ, C/EBP-α and aP2 in 3T3-L1 cells
treated with 100 μg/ml AFE during differentiation were
significantly decreased to 62, 8 and 16% of those in the control
group, respectively. These results were similar to those in the
positive control group treated with NAC.
Discussion
The members of the botanical genus Alder have
been reviewed for their biological activities, including certain
common species with importance in traditional medicine. Plant
species of the genus Alder have been used for treating
hemorrhage, burn injuries, fever, diarrhea and alcoholism in
traditional Korean medicine. The stems of these plants are
available on oriental herb market in the form of a health tea for
treating alcoholism in Korea. Evidence from pharmacological studies
has suggested that these species have anti-inflammatory, antitumor,
anti-obesity and anti-oxidative effects (34,35). The present study demonstrated the
antioxidant effects, and the inhibition of lipid accumulation and
ROS production by AFE during the adipogenesis of 3T3-L1 cells.
Plants contain numerous phytochemicals, including
proanthocyanidins, phenolics and flavonoids, which provide a number
of benefits to the human body (36). Of these phytochemicals, the genus
Alder contains flavonoids and phenols (34). Numerous studies suggested that
these compounds are important antioxidant substances that act as
reducing agents, singlet oxygen quenchers or electron donors with
chelating properties (37,38).
Therefore, the total anthocyanin, phenolic and flavonoid content of
AFE were investigated and the results indicated their presence at
high levels. Comparison of the levels of these components among
other species of the genus Alder reported in the literature
indicated that their levels were not low at all. A. firma
was determined to be of medicinal value due to the high levels of
phenolic compounds.
The DPPH radical has the capacity to compromise the
stability of molecules. It accepts an electron or hydrogen radical
from ascorbic acid, tocopherol, polyhydroxy aromatic compounds and
aromatic amines. In the DPPH assay, this reaction triggers a color
change from purple to yellow measured at OD517, and
which may be interfered with by radical-quenching substrates
(39). This principle has been
widely used to explore the antioxidant activity of a variety of
natural materials, as this method is quick, reliable and
reproducible (40). The ABTS
radical scavenging activity assay was also applied. The ABTS
radical generated by reaction with potassium persulfate is quenched
by the antioxidant substrate at different concentrations in the
sample, which leads to changes in color that are detected at 734 nm
(41). Another antioxidant
activity assay, the reducing power is usually associated with the
presence of reductones, which exert their antioxidant effects by
breaking the free radical chain via donating a hydrogen atom
(42). Ferric-ferricyanide
(Fe3+) stabilizes the free radical by sharing a hydrogen
atom, and is thereby reduced to a ferrous ion (Fe2+) and
the reducing power is quantified by measuring the OD700
(20). The nitrite scavenging
ability is also associated with the antioxidant capacity. Nitrite
radicals react with Griess reagent to form a purple Azo dye. In
this method, the nitrite scavenging activity is measured by
colorimetric determination with the color change being proportional
to the concentration of nitrite (21). Finally, the ORAC assay is a method
of measuring the antioxidant activity of a sample via the reduction
of fluorescein by peroxyl radicals. When the peroxyl radical is
generated by 2,2′-azobis(2-methylpropionamidine) dihychloride, the
antioxidants in the sample maintain the fluorescence by removing
the peroxyl radicals, which would oxidize the fluorescein, until
the antioxidants in the sample are depleted. The antioxidant
capacity of the sample over time is represented by a decay curve
and quantified by comparison with a standard, which is
water-soluble vitamin E (Trolox) (22). In general, these analytical
methods assessing various radical-quenching and antioxidant effects
should be employed for the evaluation of the antioxidant activity
of a compound, as ROS are formed by a number of different
mechanisms and may be detected by various techniques. AFE were
evaluated using these methods, revealing that it had a high
radical-quenching and antioxidant capacity, and all antioxidant
values increased in a dose-dependent manner. Due to these effects,
A. firma is considered to have a wide applicability as a
functional food supplement.
The effects of the AFE on lipid accumulation and ROS
production during the differentiation of 3T3-L1 cells were
determined by Oil Red O staining and the NBT assay. Two-days
post-confluent 3T3-L1 preadipocytes (day 0) were treated with AFE
(25, 50 or 100 μg/ml) every other day for 8 days. When the
preadipocytes were differentiated into adipocytes via the
application of the MDI cocktail, morphological changes were
observed due to the accumulation of lipid droplets in the
cytoplasm. During the differentiation of 3T3-L1 cells from
preadipocytes to adipocytes, the neutral lipids produced during
this process were detected by Oil Red O staining, with red droplets
displaying in the cells. A greater amount of reddish 3T3-L1 cells
indicated more lipid accumulation and represented the extent of
adipogenesis. The absorbance of cells treated with AFE or NAC
during adipogenesis was compared with that of the control that was
only induced with MDI. NAC is a well-known inhibitor used as a
positive control during adipogenensis and causes a significant
reduction in lipid accumulation in 3T3-L1 cells (43). The results of the present study
indicated significantly reduced lipid accumulation in the cells
treated with AFE during differentiation. This result means that
A. firma was effective in reducing lipid accumulation in
preadipocytes during adipogenesis. Numerous studies have emphasized
the link between oxidative stress and obesity (24). It is expected that the reduction
of ROS production and the reduction in lipid accumulation by AFE
has a positive impact to interfere with obesity-associated
processes. In this context, the NBT assay and flow cytometry were
used to measure ROS production during adipocyte differentiation.
The NBT assay is a well-established technique that is used to
quantify the cellular oxidative metabolism (44). In this assay, the NBT reagent
reacts with ROS accumulating in the 3T3-L1 cells to produce
dark-blue formazan crystals, which are then densitometrically
quantified after being dissolved to determine the amount of ROS
production. The H2DCFDA assay is one of the most widely
used methods for directly measuring the reductive and oxidative
states of cells. In particular, it is sensitive to changes in the
redox state of cells and may be used to monitor changes in the
quantity of ROS over time (45).
The results of the present study demonstrated significantly reduced
ROS accumulation in the presence of different concentrations of AFE
during adipogenesis. This reduction of ROS accumulation by AFE was
in parallel with inhibition of lipid accumulation, further
indicating a link between the two processes.
The anti-obesity effects of extracts from natural
resources may be screened through in vitro inhibition of
adipocyte differentiation and of lipid accumulation in 3T3-L1 cells
(46). The differentiation of
confluent preadipocytes into adipocytes requires a variety of
effectors that activate a cascade of transcription factors,
including C/EBP-α and PPAR-γ. PPAR-γ induces most
adipocyte-specific genes, including aP2 (47), which is a marker gene for
adipogenesis and has a critical role in the regulation of gene
expression during early development (48,49). In the present study, AFE
downregulated the mRNA and protein expression levels of PPAR-γ,
C/EBP-α and aP2. It was therefore suggested that A. firma
has beneficial anti-adipogenic effects.
In conclusion, the present study evaluated the
antioxidant capacity of AFE, as well as its effects on lipid
accumulation and ROS production during adipogenesis of 3T3-L1
cells. The total phenolic and flavonoid content of AFE were
assessed. Various measurement methods were applied for assessing
the antioxidant activity, including the DPPH and the ABTS radical
scavenging, reducing power and ORAC assays, revealing that AFE
exhibited significant antioxidant activity. Furthermore, AFE
decreased lipid accumulation and ROS production in 3T3-L1 cells. In
addition, AFE downregulated the mRNA and protein expression levels
of PPAR-γ, C/EBP-α and aP2. Based on these results, A. firma
was revealed to possess antioxidant and anti-adipocyte activities
and to be potentially beneficial in preventing obesity.
Acknowledgments
This study was supported by a grant from the
National Institute of Biological Resources (NIBR), funded by the
Ministry of Environment of the Republic of Korea (grant no.
NIBR201628101).
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
ORAC
|
oxygen radical absorbance capacity
|
|
ORO
|
Oil Red O
|
|
PPAR-γ
|
peroxisome proliferator-activated
receptor γ
|
|
C/EBP-α
|
CCAAT/enhancer-binding protein α
|
|
aP2
|
adipocyte protein 2
|
References
|
1
|
Dröge W and Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Halliwell B, Aeschbach R, Löliger J and
Aruoma OI: The characterization of antioxidants. Food Chem Toxicol.
33:601–617. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar
|
|
4
|
Yamashina K, Miller BE and Heppner GH:
Macrophage-mediated induction of drug-resistant variants in a mouse
mammary tumor cell line. Cancer Res. 46:2396–2401. 1986.PubMed/NCBI
|
|
5
|
Fridovich I: Superoxide dismutases. An
adaptation to a paramagnetic gas. J Biol Chem. 264:7761–7764.
1989.PubMed/NCBI
|
|
6
|
Spiegelman BM and Flier JS: Obesity and
the regulation of energy balance. Cell. 104:531–543. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marcelin G and Chua S Jr: Contributions of
adipocyte lipid metabolism to body fat content and implications for
the treatment of obesity. Curr Opin Pharmacol. 10:588–593. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holst D and Grimaldi PA: New factors in
the regulation of adipose differentiation and metabolism. Curr Opin
Lipidol. 13:241–245. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Ferranti S and Mozaffarian D: The
perfect storm: Obesity, adipocyte dysfunction, and metabolic
consequences. Clin Chem. 54:945–955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee OH, Kwon YI, Hong HD, Park CS, Lee BY
and Kim YC: Production of reactive oxygen species and changes in
antioxidant enzyme activites during differentiation of 3T3-L1
adipocyte. J Korean Soc Appl Biol Chem. 52:70–75. 2009. View Article : Google Scholar
|
|
11
|
Sureda A, Tauler P, Aguiló A, Cases N,
Fuentespina E, Córdova A, Tur JA and Pons A: Relation between
oxidative stress markers and antioxidant endogenous defences during
exhaustive exercise. Free Radic Res. 39:1317–1324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HK, Kwon YJ, Kim YE and Nahmgang B:
Changes of total polyphenol content and antioxidant activity of
aster scaber thunb extracts with different microwave assisted
extraction conditions. Korean J Food Preserv. 11:88–95. 2004.
|
|
13
|
Larson RA: The antioxidants of higher
plants. Phytochemistry. 27:969–978. 1988. View Article : Google Scholar
|
|
14
|
Schöner S and Heinrich Krause G:
Protective systems against active oxygen species in spinach:
Response to cold acclimation in excess light. Planta. 180:383–389.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gutfinger T: Polyphenols in olive oils. J
Am Oil Chem Soc. 58:966–968. 1981. View Article : Google Scholar
|
|
16
|
Moreno MI, Isla MI, Sampietro AR and
Vattuone MA: Comparison of the free radical-scavenging activity of
propolis from several regions of Argentina. J Ethnopharmacol.
71:109–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitsunaga T, Doi T, Kondo Y and Abe I:
Color development of proanthocyanidins in vanillin-hydrochloric
acid reaction. J Wood Sci. 44:125–130. 1998. View Article : Google Scholar
|
|
18
|
Chu YH, Chang CL and Hsu HF: Flavonoid
content of several vegetables and their antioxidant activity. J Sci
Food Agric. 80:561–566. 2000. View Article : Google Scholar
|
|
19
|
Re R, Pellegrini N, Proteggente A, Pannala
A, Yang M and Rice-Evans C: Antioxidant activity applying an
improved ABTS radical cation decolorization assay. Free Radic Biol
Med. 26:1231–1237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oyaizu M: Antioxidative activities of
browning products of glucosamine fractionated by organic solvent
and thin-layer chromatography. J Jpn Soc Food Sci. 35:771–775.
1988. View Article : Google Scholar
|
|
21
|
Kato H, Lee IE, Chuyen V, Kim SB and
Hayase F: Inhibition of nitrosamine formation by nondialyzable
melanoidins. Agric Biol Chem. 51:1333–1388. 1987.
|
|
22
|
Ou B, Hampsch-Woodill M and Prior RL:
Development and validation of an improved oxygen radical absorbance
capacity assay using fluorescein as the fluorescent probe. J Agric
Food Chem. 49:4619–4626. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee OH, Kwon YI, Apostolidis E, Shetty K
and Kim YC: Rhodiola-induced inhibition of adipogenesis involves
antioxidant enzyme response associated with pentose phosphate
pathway. Phytother Res. 25:106–115. 2011. View Article : Google Scholar
|
|
24
|
Furukawa S, Fujita T, Shimabukuro M, Iwaki
M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M and
Shimomura I: Increased oxidative stress in obesity and its impact
on metabolic syndrome. J Clin Invest. 114:1752–1761. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blumberg JM, Tzameli I, Astapova I, Lam
FS, Flier JS and Hollenberg AN: Complex role of the vitamin D
receptor and its ligand in adipogenesis in 3T3-L1 cells. J Biol
Chem. 281:11205–11213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaur C and Kapoor HC: Antioxidant activity
and total phenolic content of some Asian vegetables. Int J Food Sci
Technol. 37:153–161. 2002. View Article : Google Scholar
|
|
27
|
Machowetz A, Poulsen HE, Gruendel S,
Weimann A, Fitó M, Marrugat J, de la Torre R, Salonen JT, Nyyssönen
K, Mursu J, et al: Effect of olive oils on biomarkers of oxidative
DNA stress in Northern and Southern Europeans. FASEB J. 21:45–52.
2007. View Article : Google Scholar
|
|
28
|
Heim KE, Tagliaferro AR and Bobilya DJ:
Flavonoid antioxidants: Chemistry, metabolism and
structure-activity relationships. J Nutr Biochem. 13:572–584. 2002.
View Article : Google Scholar
|
|
29
|
Fine AM: Oligomeric proanthocyanidin
complexes: History, structure, and phytopharmaceutical
applications. Altern Med Rev. 5:144–151. 2000.PubMed/NCBI
|
|
30
|
Stević T, Savikin K, Zdunić G, Stanojković
T, Juranić Z, Janković T and Menković N: Antioxidant, cytotoxic,
and antimicrobial activity of Alnus incana (L.) ssp. incana Moench
and A. viridis (Chaix) DC ssp. viridis extracts. J Med Food.
13:700–704. 2010. View Article : Google Scholar
|
|
31
|
Acero N and Muñoz-Mingarro D: Effect on
tumor necrosis factor-α production and antioxidant ability of black
alder, as factors related to its anti-inflammatory properties. J
Med Food. 15:542–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li H, Choi YM, Lee JS, Park JS, Yeon KS
and Han CS: Drying and antioxidant characteristics of the shiitake
(Lentinus edodes) mushroom in a conveyer type far-infrared dryer. J
Korean Soc Food Sci Nutr. 36:250–254. 2007. View Article : Google Scholar
|
|
33
|
Kim MB, Hyun SH, Park JS, Kang M, Ko YH
and Lim SB: Integral antioxidative capacity of extracts by
pressurized organic solvent from natural plants in Jeju. J Korean
Soc Food Sci Nutr. 37:1491–1496. 2008. View Article : Google Scholar
|
|
34
|
Sati SC, Sati N and Sati OP: Bioactive
constituents and medicinal importance of genus Alnus. Pharmacogn
Rev. 5:174–183. 2011. View Article : Google Scholar
|
|
35
|
Lee M, Song JY, Chin YW and Sung SH:
Anti-adipogenic diarylheptanoids from Alnus hirsuta f. sibirica on
3T3-L1 cells. Bioorg Med Chem Lett. 23:2069–2073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hanasaki Y, Ogawa S and Fukui S: The
correlation between active oxygens scavenging and antioxidative
effects of flavonoids. Free Radic Biol Med. 16:845–850. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rice-Evans CA, Miller NJ and Paganga G:
Structure-antioxidant activity relationships of flavonoids and
phenolic acids. Free Radic Biol Med. 20:933–956. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cao G, Sofic E and Prior RL: Antioxidant
and prooxidant behavior of flavonoids: Structure-activity
relationships. Free Radic Biol Med. 22:749–760. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
MacDonald-Wicks LK, Wood LG and Garg ML:
Methodology for the determination of biological antioxidant
capacity in vitro: A review. J Sci Food Agric. 86:2046–2056. 2006.
View Article : Google Scholar
|
|
40
|
Que F, Mao L, Zhu C and Xie G: Antioxidant
properties of Chinese yellow wine, its concentrate and volatiles.
LWT Food Sic Technol. 39:111–117. 2006. View Article : Google Scholar
|
|
41
|
Tachakittirungrod S, Okonogi S and
Chowwanapoonpohn S: Study on antioxidant activity of certain plants
in Thailand: Mechanism of antioxidant action of guava leaf extract.
Food Chem. 103:381–388. 2007. View Article : Google Scholar
|
|
42
|
Duh PD: Antioxidant activity of burdock
(Arctium lappa Linné): Its scavenging effect on free-radical and
active oxygen. J Am Oil Chem Soc. 75:455–461. 1998. View Article : Google Scholar
|
|
43
|
Calzadilla P, Sapochnik D, Cosentino S,
Diz V, Dicelio L, Calvo JC and Guerra LN: N-acetylcysteine reduces
markers of differentiation in 3T3-L1 adipocytes. Int J Mol Sci.
12:6936–6951. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Baehner RL, Boxer LA and Davis J: The
biochemical basis of nitroblue tetrazolium reduction in normal
human and chronic granulomatous disease polymorphonuclear
leukocytes. Blood. 48:309–313. 1976.PubMed/NCBI
|
|
45
|
Eruslanov E and Kusmartsev S:
Identification of ROS using oxidized DCFDA and flow-cytometry.
Methods Mol Biol. 594:57–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Morrison RF and Farmer SR: Insights into
the transcriptional control of adipocyte differentiation. J Cell
Biochem. 33(Suppl 32): 59–67. 1999. View Article : Google Scholar
|
|
47
|
Tontonoz P, Hu E, Graves RA, Budavari AI
and Spiegelman BM: mPPAR gamma 2: Tissue-specific regulator of an
adipocyte enhancer. Genes Dev. 8:1224–1234. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hilger-Eversheim K, Moser M, Schorle H and
Buettner R: Regulatory roles of AP-2 transcription factors in
vertebrate development, apoptosis and cell-cycle control. Gene.
260:1–12. 2000. View Article : Google Scholar
|
|
49
|
Makowski L and Hotamisligil GS: Fatty acid
binding proteins - the evolutionary crossroads of inflammatory and
metabolic responses. J Nutr. 134:2464S–2468S. 2004.
|