Introduction
Coronary artery disease (CAD) and its major
complication, acute coronary syndrome (ACS), remain the most common
causes of morbidity and mortality worldwide (1). ACS comprises two main entities:
Unstable angina (UA) and acute myocardial infarction (AMI), which
is usually triggered by the rupture of unstable atherosclerotic
plaques (2). Vulnerable plaques,
also known as unstable plaques, are typically characterized by a
thin fibrous cap, large lipid core (>40% of the plaque),
inflammatory infiltration, angiogenesis and intraplaque hemorrhage
(3). The risk factors of CAD
include hypertension, diabetes mellitus, elevated low-density
lipoprotein cholesterol levels and cigarette smoke, all of which
may lead to endothelial injury. Impaired vascular endothelium has
an essential role in the initiation of atherosclerosis and in
ultimate formation of unstable plaques (4). Dysfunctional endothelial cells (ECs)
exhibit increased proliferation and migration, eventually resulting
in re-endothelization and angiogenesis of the atherosclerotic
plaque (5); the latter is a major
step in promoting plaque destabilization, rupture and thrombus
formation (6).
MicroRNAs (miRNAs/miRs) are small non-coding RNA
molecules that negatively regulate gene expression, predominantly
at the post-transcriptional level. Altered miRNA expression has
been identified in the circulation of patients with ACS (7). Furthermore, it is now recognized
that miRNAs are involved in almost all steps of atherogenesis,
including endothelial damage and dysfunction, monocyte invasion and
activation, lipoprotein formation and deposition, and vascular
smooth muscle cell and platelet dysfunction, where they exert
either beneficial or harmful effects (8). miR-126 is the most abundant miRNA in
ECs, which may prompt EC repair by inhibiting inflammation
(9). In addition, miR-181b and
miR-146a are able to alleviate EC inflammation by regulating the
nuclear factor-κB signaling pathway (10–12). Our previous study revealed that
miR-19b may function as an antithrombotic protective miRNA in UA by
targeting tissue factor in ECs (13). However, it remains unclear as to
whether there are other protective roles for miR-19b in UA. It has
previously been suggested that miR-19b may be closely associated
with atherosclerosis. miR-19b is downregulated in the aortic walls
of apolipoprotein E (apoE) knockout mice, and suppressor of
cytokine signaling 3 is a potential target of this miRNA (14). Signal transducer and activator of
transcription 3 (STAT3) is an important nuclear transcription
factor. The promoters of numerous proliferation, migration and
angiogenesis-associated genes, including vascular endothelial
growth factor, placental growth factor and SRY-box 18, contain
STAT3 consensus sequences (15–17). Therefore, STAT3 activation may
induce the expression of these genes and regulate cell
proliferation, migration and angiogenesis.
The aim of the study was to evaluate the potential
role and mechanism of miR-19b in plaque stability. The present
study demonstrated that miR-19b was markedly upregulated in the
plasma of patients with UA, and was predicted to be involved in the
regulation of cell proliferation, migration and angiogenesis by
bioinformatics analysis. Finally, the present study indicated that
miR-19b may inhibit EC proliferation, migration and tube formation
in vitro by suppressing STAT3 transcriptional activity.
Materials and methods
Study population
The present study was performed in accordance with
the Helsinki declaration and was approved by the ethics review
board of Peking University People's Hospital (Beijing, China). All
individuals recruited to the present study provided written
informed consent. Patients who were suspected of CAD with negative
angiography were enrolled in the control group (n=36). Patients
with typical UA that were angiographically documented as having CAD
were enrolled in the UA group (n=46). All individuals, recruited to
the present study between April 2012 to November 2013, provided
written informed consent. The criteria for the diagnosis of UA were
based on the American College of Cardiology Foundation/American
Heart Association 2010 guidelines for the management of patients
with UA/non-ST-segment-elevation MI (18). Patients presenting elevated
troponin I (≥0.04 ng/ml) and/or creatine kinase-MB (≥5 ng/ml)
levels, myocarditis, cardiogenic shock, a history of severe hepatic
or renal dysfunction, leukemia, leukopenia and ongoing inflammatory
malignant disease were excluded from the present study.
Blood collection and RNA extraction
Blood was collected from patients via arterial
puncture into tubes containing EDTA (BD Biosciences, Franklin
Lakes, NJ, USA) prior to coronary angiography, and was processed
for isolation of plasma within 4 h. Blood was centrifuged at 1,300
× g for 10 min at 4°C. miRNeasy mini kit (Qiagen, Inc., Valencia,
CA, USA) was used for RNA extraction according to the
manufacturer's protocol.
miRNA TaqMan low density array
(TLDA)
TLDA was used to detect differentially expressed
miRNAs in the plasma of patients with UA (n=12) and controls
(n=12). Total RNA (~15 ng) was reverse transcribed using the TaqMan
miRNA reverse transcription (RT) kit and TaqMan miRNA Multiplex RT
assays (human pool) (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The RT products were preamplified with
TaqMan PreAmp kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.), after which preamplification reaction products were
amplified using Human MicroRNA TLDA card A+B version 3.0 (Applied
Biosystems; Thermo Fisher Scientific, Inc.), which could detect 754
miRNAs simultaneously.
All steps were performed using a 7900HT Fast
real-time polymerase chain reaction (PCR) system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Results were expressed
as Cq. Raw data were analyzed using Data Assist software for TaqMan
gene expression assays version 3.0 (Applied Biosystems; Thermo
Fisher Scientific, Inc.). miRNA expression levels were normalized
to RNU6B. Significance analysis of microarrays (SAM) was used to
identify differentially expressed miRNAs between the two groups.
The miRNAs that exhibited ≥2-fold change, q-value <0.0001% and
false discovery rate (FDR) <0.05 were considered differentially
expressed.
RT-quantitative (q)PCR
TaqMan miRNA RT kit (cat. no. 4366596),
miRNA-specific stem-loop primers (miR-19b, cat. no. 000396;
cel-miR-39, cat. no. 000200; RNU6B, cat. no. 001093) and Universal
PCR Master Mix (cat. no. 4324018) (Applied Biosystems; Thermo
Fisher Scientific, Inc.) were used for miRNAs quantification
according to the manufacturer's protocol. Real-time PCR reactions
were performed on an Applied Biosystem ViiA™ 7 Real-Time PCR system
with the following program: 10 min pre-incubation at 95°C, 40
cycles of 15 sec denaturation at 95°C and 60 sec of elongation at
60°C. Values were expressed as 2−ΔΔCq (19). miR-19b levels in the plasma were
normalized to a spiked-in control, synthetic Caenorhabditis
elegans miR-39 (10 fmol/sample; Qiagen, Inc.).
Cell culture and transfection
Cell culture and transfection were performed as
previously described (11).
Briefly, EA.hy926 cells (fusion cell line derived from human
umbilical vein ECs and lung carcinoma cells), obtained from
Shanghai Institutes for Biological Sciences (Chinese Academy of
Sciences, Shanghai China), were cultured in Dulbecco's modified
Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (both
from Gibco, Grand Island, NY, USA) at 37°C in a humidified
atmosphere containing 5% CO2. Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used for
transfection at a final concentration of 3 µg/ml according
to the manufacturer's protocol. miR-19b mimic (cat. no. MC10629) or
negative control (NC) mimic (cat. no. 4464058) (Applied Biosystems;
Thermo Fisher Scientific, Inc.) were transfected into EA.hy926
cells (70–80% confluence) at a final concentration of 30 pmol/ml
for 24 or 48 h.
Western blot analysis
Western blot analysis was performed as previously
described (20). The following
antibodies were used in the present study: Anti-STAT3 (cat. no.
9132S; 1:1,000 diluted) and anti-phosphorylated-STAT3 (Tyr 705)
(cat. no. 9131S; 1:1,000 diluted; both from Cell Signaling
Technology, Inc., Beverly, MA, USA). GAPDH (cat. no. SC-32233;
1:3,000 diluted; Santa Cruz Biotechnology, Santa Cruz, CA, USA) as
a loading control. HRP-conjugated goat-anti-rabbit (cat. no.
sc-2004)/mouse (cat. no. sc-2005) IgG secondary antibodies (both
from Santa Cruz Biotechnology).
Luciferase reporter assay
EA.hy926 cells plated in a 24-well plate at 70–80%
confluence were cotransfected with NC or miR-19b mimic (final
concentration, 60 pmol/ml) and STAT3-driven promoter (2×APRE)
firefly luciferase reporter plasmid (final concentration, 300
ng/ml), as well as the internal control Renilla luciferase
reporter plasmid (final concentration, 10 ng/ml; phRL-TK; Promega
Corporation, Madison, WI, USA) using Lipofectamine 2000 (final
concentration, 4 µg/ml). After 24 h, cell extracts were
prepared and assessed according to the manufacturer's protocol
(Dual Luciferase assay system; Promega Corporation). Firefly
luciferase activity was normalized to Renilla luciferase
activity in the same well. To construct the STAT3-driven promoter,
the 2×APRE sequence was cloned into the multiple cloning site of
pGL3-TATA plasmid, which encodes the firefly luciferase gene
containing an upstream TATA element (21).
Cell proliferation assay
Cell proliferation activity was measured using Cell
Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). EA.hy926 cells in 96-well plates were transfected
with miR-19b or NC mimic for 24 or 48 h. Subsequently, 20 µl
CCK-8 solution was added to each well with 200 µl culture
medium and the plates were incubated for 1 h at 37°C. The
absorbance was measured at 450 nm using a microplate reader.
Wound healing migration assay
EA.hy926 cells in 24-well plates were transfected
with miR-19b or NC mimic for 24 h, and were then serum-starved in
DMEM containing 1% FBS overnight. Subsequently, the cell monolayer
was scratched with a sterile 10-µl pipette tip. The cells
were gently washed with DMEM to remove detached cells and were
incubated with DMEM containing 1% FBS for 24 h at 37°C. Images of
four injured fields in each well were captured (Olympus IX70;
Olympus Corporation, Tokyo, Japan). Cell migration was
semi-quantified by measuring the recovered area using ImageJ
software (version 1.38; National Institutes of Health, Bethesda,
MD, USA) (22).
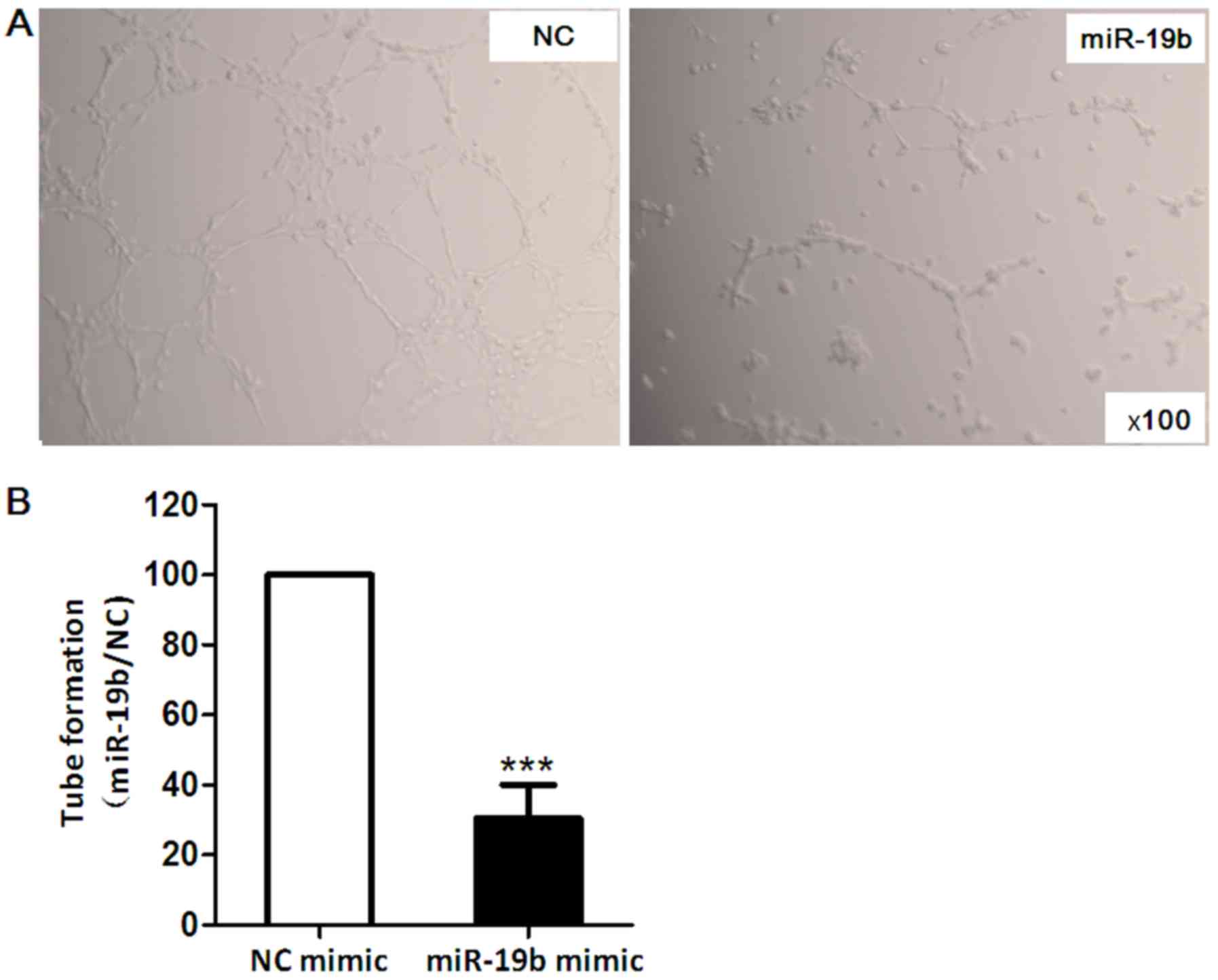
Tube formation assay
Melted Matrigel (150 µl; BD Biosciences) was
applied to 48-well plates and was incubated at 37°C for 30 min.
EA.hy926 cells were transfected with miR-19b mimic or NC mimic for
24 h. Transfected cells (4×104/well) were seeded in the
gel and incubated at 37°C for 16 h to allow tube formation. The
effects of miR-19b on EA.hy926 cell differentiation stimulated by
EC growth supplement (contained within Matrigel) at 37°C were
observed under a microscope (Olympus IX70) and images of four
representative fields were captured. Average total tube numbers in
four representative fields were determined.
Functional enrichment analysis
The target genes of miR-19b were predicted using
DIANA-microT (http://www.microrna.gr/microT-CDS), TargetScan
(http://www.targetscan.org/) and miRanda
(http://34.236.212.39/microrna/getDownloads.do)
databases. Target genes simultaneously predicted by all three
databases were selected to obtain the vascular endothelial target
genes from CGAP SAGE via Database for Annotation, Visualization and
Integrated Discovery (DAVID) platform (23). The target genes of miR-19b in the
endothelium were classified into functional groups using DAVID
according to the biological process (BP) classification of Gene
Ontology (GO) (24). As input,
the GenBank ID of each gene target was used. As output, significant
processes (GOTERM) with P<0.05 were generated. All the details
can be freely found at http://david.abcc.ncifcrf.gov/.
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation or standard error of the mean. For continuous
variables, statistical significance was calculated using one-way
analysis of variance followed by the Tukey multiple comparisons
test. For comparisons between two groups, Student's t-test was
conducted. For categorical variables, statistical significance was
calculated using the χ2 test for the comparison of two
groups. SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used for all
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-19b levels were increased in plasma
samples from patients with UA
The present study initially detected the miRNA
expression profiles in plasma samples from patients with UA (n=12)
and controls (n=12) by TLDA. The clinical characteristics of the 24
subjects are summarized in Table
I. Analysis of array data using SAM algorithm identified 28
miRNAs that were significantly upregulated in patients with UA
compared with in the controls, among which miR-19b exhibited the
most marked upregulation (Table
II).
 | Table IClinical characteristics of study
populations. |
Table I
Clinical characteristics of study
populations.
| Characteristic | Profiles
| Validation
|
|---|
| Controls
(n=12) | UA (n=12) | Controls
(n=24) | UA (n=34) |
|---|
| General data | | | | |
| Age (years) | 56±9 | 61±8 | 61±12 | 63±9 |
| Gender
(males/females) | 4/8 | 6/6 | 11/13 | 14/20 |
| BMI
(kg/m2) | 26±4 | 25±3 | 26±3 | 25±3 |
| SBP (mmHg) | 126±12 | 131±12 | 128±13 | 131±18 |
| DBP (mmHg) | 76±6 | 79±11 | 79±11 | 78±10 |
| HR (bpm) | 68±6 | 71±10 | 70±8 | 69±9 |
| LVEF (%) | 70±6 | 68±7 | 68±10 | 69±6 |
| Medical
history | | | | |
| Hypertension
(%) | 50 | 83 | 58 | 53 |
| Diabetes (%) | 17 | 33 | 21 | 24 |
| Hyperlipidemia
(%) | 50 | 58 | 42 | 35 |
| Laboratory
test | | | | |
| WBC
(109/l) | 6.00±1.17 | 6.10±1.65 | 6.68±1.67 | 5.93±1.66 |
| LDL-C
(mmol/l) | 2.11±0.42 | 2.24±0.51 | 2.52±0.58 | 2.19±0.56 |
| HDL-C
(mmol/l) | 1.02±0.23 | 0.99±0.30 | 1.12±0.32 | 0.96±0.27 |
| TC (mmol/l) | 3.71±0.49 | 3.94±0.63 | 4.54±0.73 | 3.73±0.69 |
| Creatinine
(µmol/l) | 54.63±12.24 | 70.50±17.13 | 67.43±13.61 | 67.91±15.46 |
| Medication (%) | | | | |
| Aspirin | 42 | 58 | 63 | 74 |
| Clopidogrel | 25 | 50 | 46 | 50 |
| Statin | 58 | 58 | 67 | 68 |
| Calcium
antagonist | 25 | 42 | 33 | 27 |
| ACEI | 17 | 25 | 13 | 15 |
| ARB | 17 | 28 | 13 | 27 |
| β-Blocker | 47 | 75 | 67 | 47 |
 | Table IImiRNA expression profiles in the
plasma of patients with UA compared with controls. |
Table II
miRNA expression profiles in the
plasma of patients with UA compared with controls.
| No. | Gene ID | Score (d) | Fold change
(UA/controls) | q-value (%) |
|---|
| 1 | hsa-miR-19b | 3.37 | 17.03 | <0.0001 |
| 2 | hsa-miR-106a | 3.09 | 11.04 | <0.0001 |
| 3 | hsa-miR-20a | 3.04 | 21.22 | <0.0001 |
| 4 | hsa-miR-16 | 3.00 | 9.18 | <0.0001 |
| 5 | hsa-miR-17 | 2.97 | 9.07 | <0.0001 |
| 6 | hsa-miR-451 | 2.78 | 28.65 | <0.0001 |
| 7 | hsa-miR-24 | 2.72 | 7.67 | <0.0001 |
| 8 | hsa-miR-223 | 2.58 | 10.13 | <0.0001 |
| 9 | hsa-miR-92a | 2.53 | 15.46 | <0.0001 |
| 10 | hsa-miR-146a | 2.23 | 9.01 | <0.0001 |
| 11 | hsa-miR-320 | 2.05 | 7.01 | <0.0001 |
| 12 | hsa-miR-19a | 1.99 | 4.84 | <0.0001 |
| 13 | hsa-miR-30a-5p | 1.98 | 37.19 | <0.0001 |
| 14 | hsa-miR-126 | 1.97 | 7.16 | <0.0001 |
| 15 | hsa-miR-126* | 1.95 | 18.13 | <0.0001 |
| 16 | hsa-miR-720 | 1.94 | 6.82 | <0.0001 |
| 17 | hsa-miR-26a | 1.91 | 22.25 | <0.0001 |
| 18 | hsa-miR-26b | 1.88 | 20.72 | <0.0001 |
| 19 | hsa-miR-191 | 1.80 | 4.58 | <0.0001 |
| 20 | hsa-miR-195 | 1.79 | 11.81 | <0.0001 |
| 21 | hsa-miR-20b | 1.78 | 23.13 | <0.0001 |
| 22 | hsa-miR-30c | 1.77 | 23.08 | <0.0001 |
| 23 | hsa-miR-1274b | 1.76 | 16.24 | <0.0001 |
| 24 | hsa-miR-30b | 1.71 | 28.93 | <0.0001 |
| 25 | hsa-miR-93 | 1.54 | 35.92 | <0.0001 |
| 26 | hsa-miR-484 | 1.53 | 4.89 | <0.0001 |
| 27 | hsa-miR-197 | 1.52 | 2.52 | <0.0001 |
| 28 | hsa-miR-222 | 1.39 | 3.52 | <0.0001 |
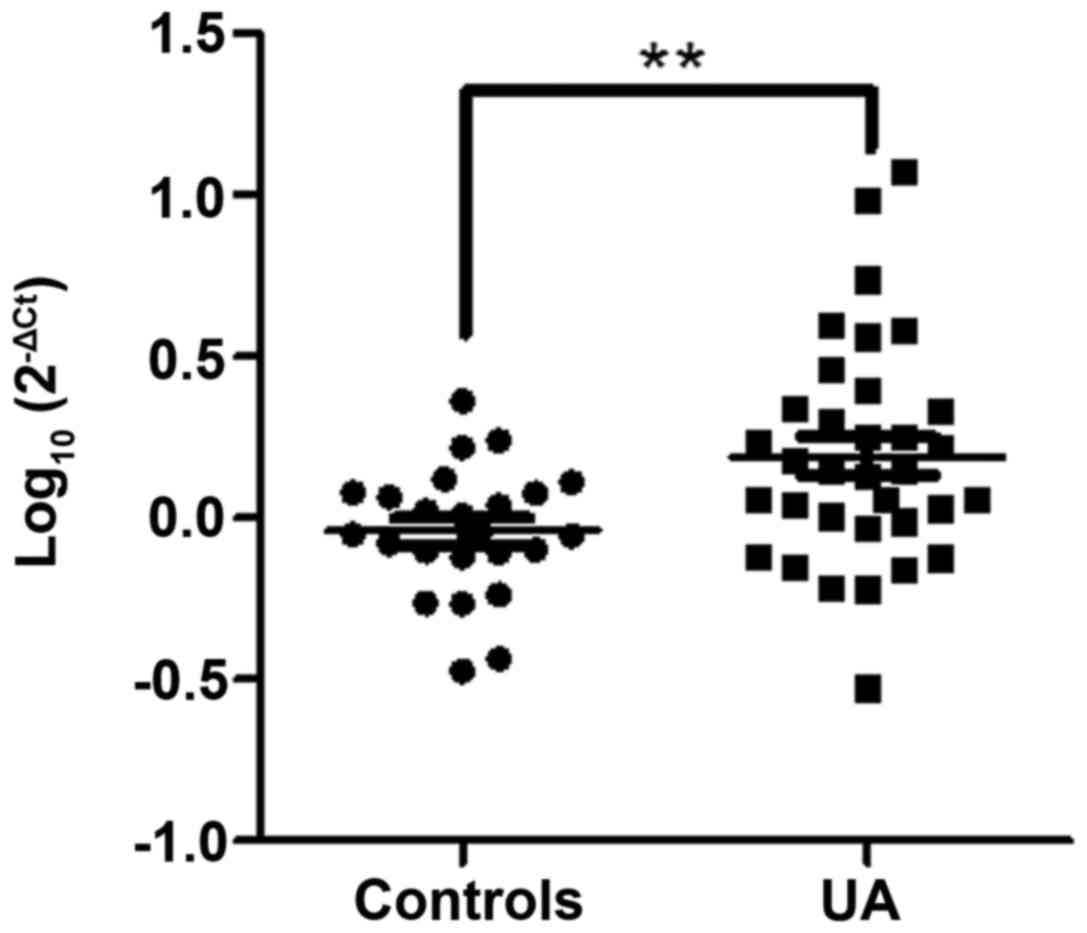
The present study further validated circulating
miR-19b levels in another independent cohort (Table I), which comprised 34 patients
with UA and 24 controls, by RT-qPCR. The results indicated that the
expression levels of miR-19b were increased in the UA group
compared with in the control group (P<0.01; Fig. 1); this finding was consistent with
the miRNA array data.
Functional enrichment analysis of
miR-19b
Dysfunctional endothelium is an important
pathological basis of unstable plaque formation, which triggers the
clinical symptoms of UA. Therefore, the present study performed a
functional enrichment analysis for miR-19b in ECs, in order to
reveal the potential role of miR-19b in patients with UA. GO
annotations of 276 predicted target genes in ECs demonstrated that
miR-19b may be involved in regulating cell proliferation, migration
and angiogenesis, which are closely associated with the formation
of unstable plaques (Table
III).
 | Table IIITop ten enriched GO terms of
microRNA-19b potential target genes in vascular endothelial
cells. |
Table III
Top ten enriched GO terms of
microRNA-19b potential target genes in vascular endothelial
cells.
| ID | Definition | Gene count | P-value |
|---|
| GO:0042127 | Regulation of cell
proliferation | 33 |
1.60×10−6 |
| GO:0001944 | Vasculature
development | 16 |
4.36×10−5 |
| GO:0048514 | Blood vessel
morphogenesis | 14 |
4.36×10−5 |
| GO:0019220 | Regulation of
phosphate metabolic process | 22 |
4.65×10−5 |
| GO:0042981 | Regulation of
apoptosis | 30 |
4.90×10−5 |
| GO:0051270 | Regulation of cell
motion | 13 |
7.87×10−5 |
| GO:0042325 | Regulation of
phosphorylation | 21 |
7.94×10−5 |
| GO:0030334 | Regulation of cell
migration | 12 |
1.04×10−4 |
| GO:0001525 | Angiogenesis | 11 |
1.58×10−4 |
| GO:0048545 | Response to steroid
hormone stimulus | 12 |
3.18×10−4 |
Effects of miR-19b on EC proliferation,
migration and angiogenesis
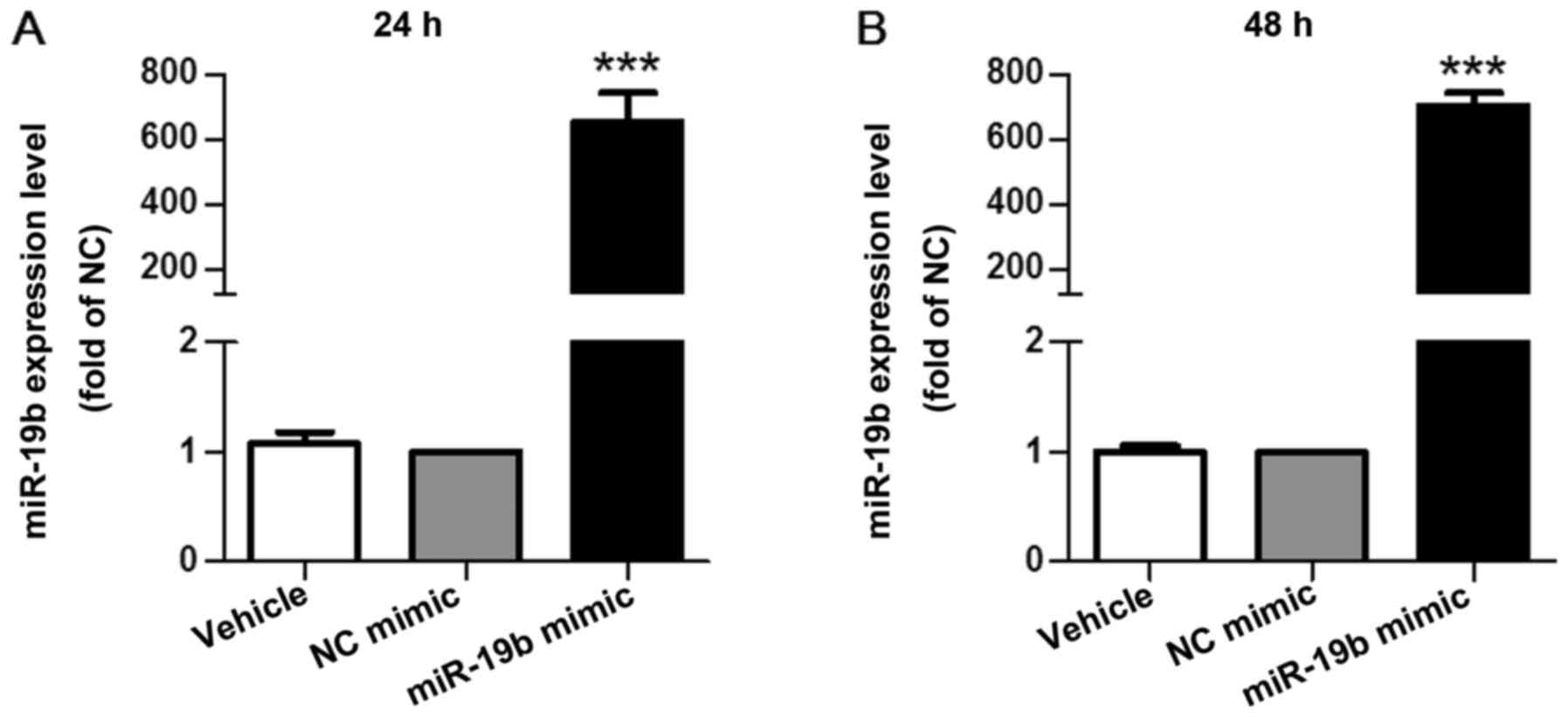
To determine the effects of miR-19b on EC
proliferation, miR-19b mimic was transfected into EA.hy926 cells
for 24 or 48 h. The degree of miR-19b overexpression was detected
24 h (fold change, 653±90; P<0.001; Fig. 2A) and 48 h (fold change, 704±40;
P<0.001; Fig. 2B)
post-transfection compared with the NC mimic. Furthermore, the
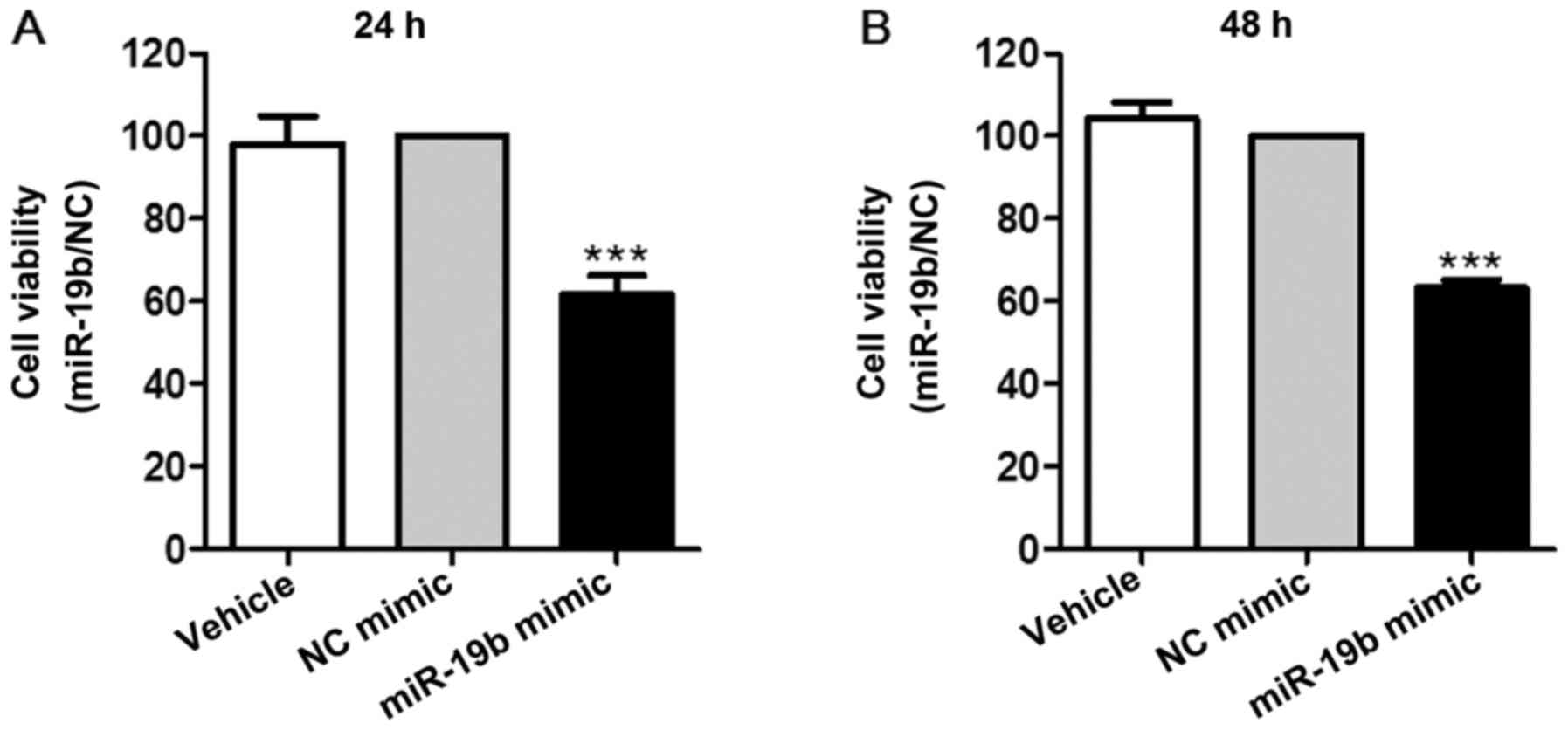
results of a CCK-8 assay demonstrated that miR-19b significantly
inhibited cell proliferation at the two time points (P<0.001;
Fig. 3).
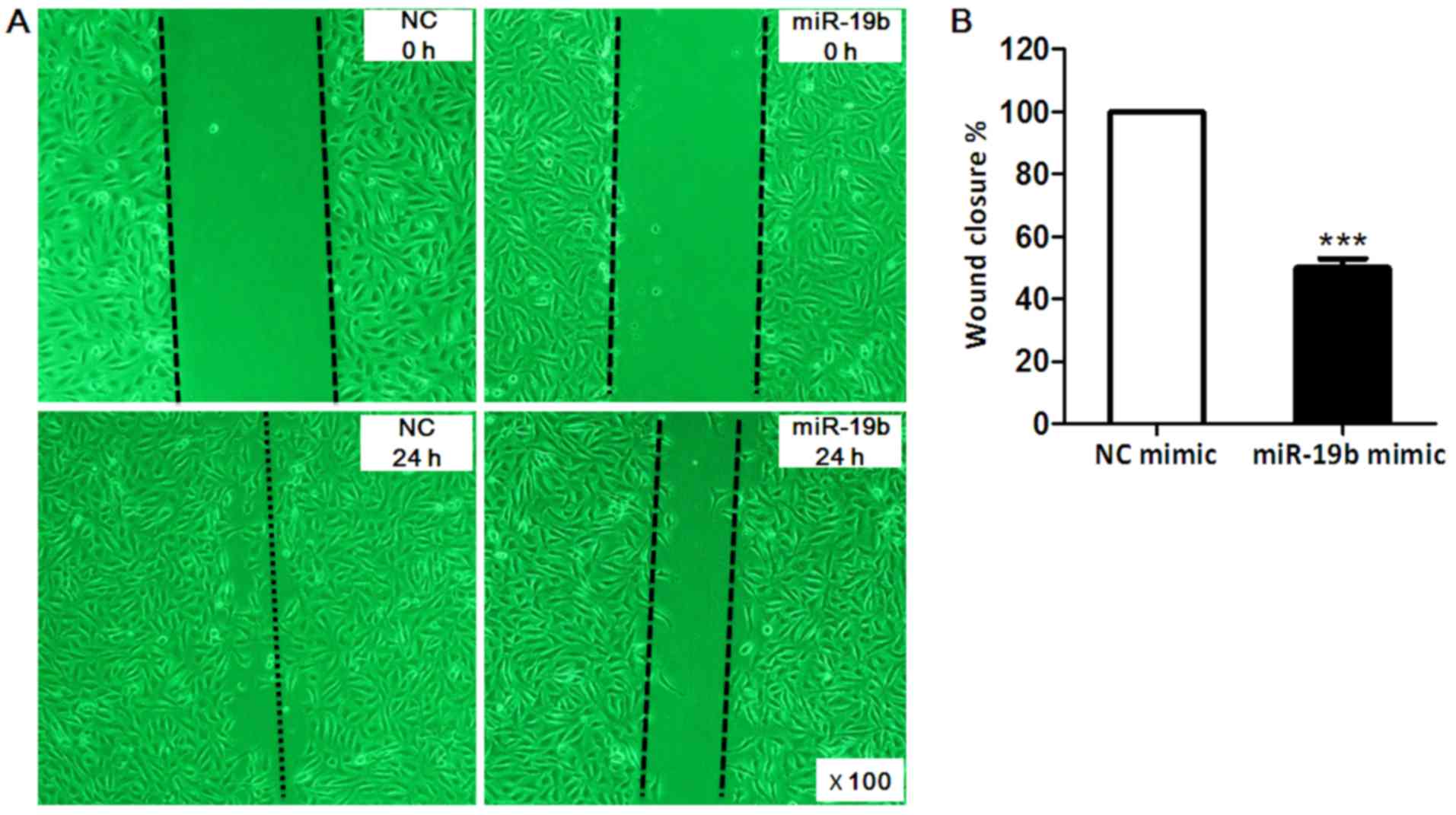
Wound healing assay was conducted to investigate the
effects of miR-19b on EA.hy926 cell migration. As presented in
Fig. 4, EA.hy926 cell migration
was markedly inhibited by 50% post- transfection with miR-19b mimic
for 24 h compared with in the NC group (P<0.001).
Tube formation assay was performed to determine the
role of miR-19b in angiogenesis. The results demonstrated that
compared with in the NC group, EA.hy926 cells tube formation was
decreased by ~70% in the miR-19b mimic group (P<0.001; Fig. 5).
All together, these findings indicated that miR-19b
may act as an inhibitor of unstable plaque formation by negatively
regulating EC proliferation, migration and angiogenesis.
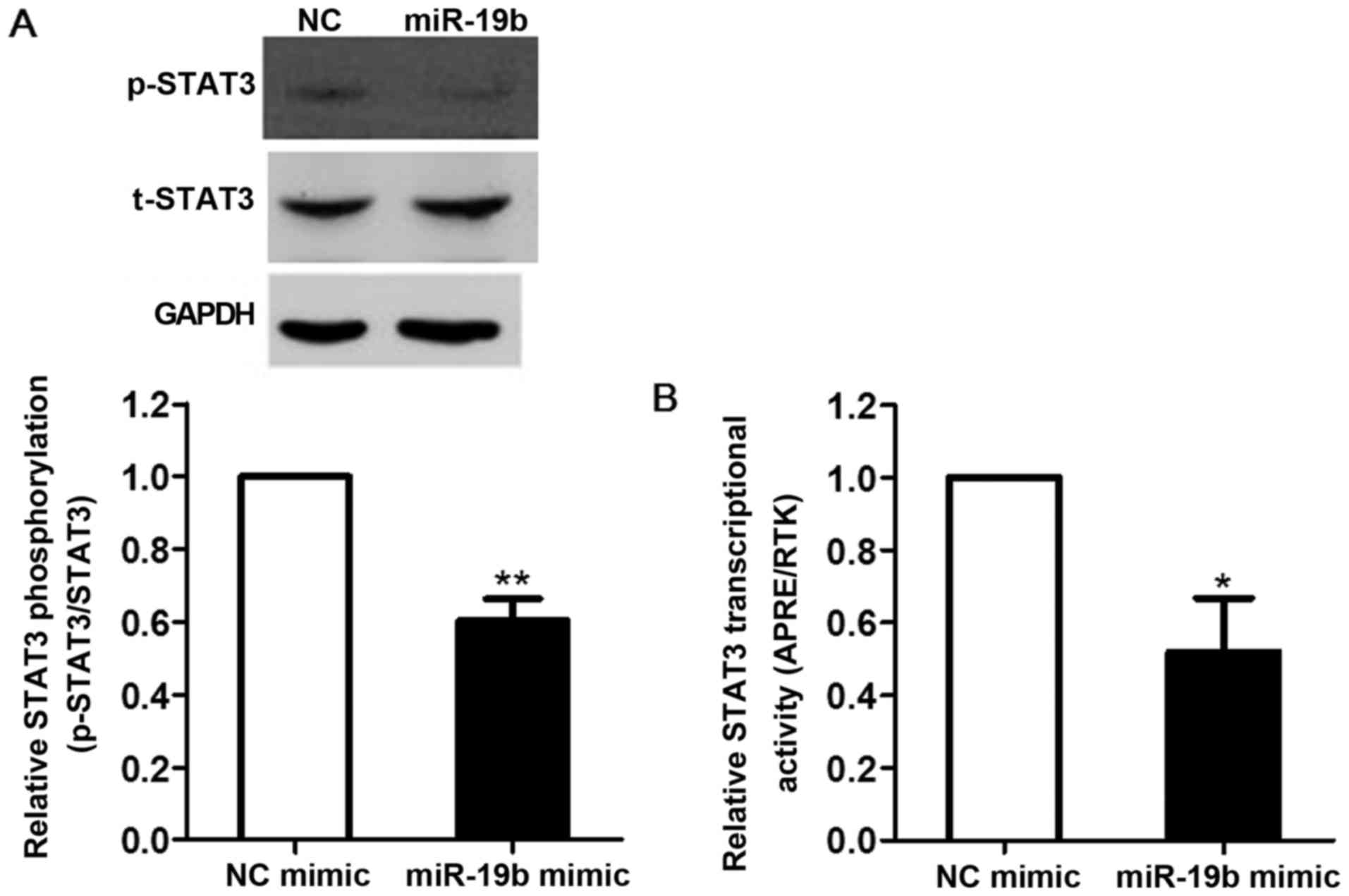
Effects of miR-19b on STAT3
transcriptional activity
STAT3 is an important nuclear transcription factor
and a major regulator of cell proliferation, migration and
angiogenesis. In order to investigate whether the inhibitory role
of miR-19b in ECs was mediated by STAT3, EA.hy926 cells were
transfected with miR-19b mimic for 24 h and STAT3 tyrosine
phosphorylation was detected by western blot analysis. As shown in
Fig. 6A, STAT3 phosphorylation
was markedly decreased by ~40% in the miR-19b mimic group compared
with in the NC group (P<0.01).
STAT3 transcriptional activity was also examined by
transfecting STAT3-driven promoter luciferase plasmid into EA.hy926
cells. Consistent with the results of STAT3 tyrosine
phosphorylation analysis, miR-19b significantly inhibited the
transcriptional activity of STAT3 by ~48% compared with in the NC
group (P<0.05; Fig. 6B).
These results suggested that the negative regulation
of miR-19b in vulnerable plaque formation may be mediated by
inhibiting STAT3 signaling.
Discussion
Angiogenesis serves a critical role in the
development and ultimate rupture of unstable plaques, which is
considered to be responsible for the majority of acute coronary
artery events (25). Previous
studies have suggested that miRNAs have an important role in
regulating atherosclerotic diseases (26), and some miRNAs have been reported
to regulate the angiogenic response to various pathological stimuli
(27). The present study
demonstrated that miR-19b may serve as a regulator of unstable
plaque formation in patients with UA by inhibiting EC
proliferation, migration and angiogenesis.
There are specific circulating miRNA profiles
associated with various diseases. The present study revealed that
circulating miR-19b was significantly upregulated in patients with
UA compared with in controls (Fig.
1); this finding was consistent with our previous study
(13). Zeller et al also
reported that miR-19b levels were increased in plasma samples from
patients with UA (28).
Furthermore, a recent study observed that the levels of plasma
miR-19b were significantly increased in early stage AMI (29). Our previous study indicated that
miR-19b acted as a potential antithrombotic miRNA in patients with
UA by targeting tissue factor in ECs (13). A single miRNA may serve various
biological roles by targeting numerous genes; therefore, the
present study aimed to explore the possible effects of miR-19b
upregulation on patients with UA. By bioinformatics analysis, the
present study demonstrated that miR-19b may be involved in EC
proliferation, migration and angiogenesis (Table III), which is closely associated
with unstable atherosclerotic plaque formation. Subsequently,
numerous assays were conducted to determine the effects of miR-19b
on ECs. Consistent with the bioinformatics prediction, the
experimental results indicated that miR-19b could inhibit EC
proliferation, migration and tube formation in vitro
(Figs. 3Figure 4–5). In addition, Han et al
reported that miR-19b was downregulated in aortic tissues of apoE
gene knockout mice compared with in healthy C57BL/6 (B6) mice
(14). Tang et al
suggested that miR-19b alleviated EC apoptosis, which was also
predicted in the functional enrichment analysis of the present
study (Table III) (30). These results suggested that
atherosclerosis may be delayed by supplementing miR-19b mimic.
However, miR-19b has also been reported to prompt macrophage
cholesterol accumulation and aortic atherosclerosis by targeting
ATP-binding cassette transporter A1, whereas diosgenin could
inhibit atherosclerosis via suppressing the effects of miR-19b
(31,33). Therefore, it is unfeasible to
prevent the development of atherosclerosis by delivering a miR-19b
mimic in a systemic manner. Previous studies have demonstrated
success at penetrating the vascular endothelium of the vessel wall
and peripheral blood mononuclear cells (10,11). Delivery of a miRNA mimic (or
inhibitor) in a targeted cell- or tissue-specific manner may
represent a novel strategy to suppress the progression of
atherosclerosis.
STAT3 inhibitor could suppress STAT3-mediated human
EC proliferation, migration and tube formation (17). However, it remains unclear as to
whether the inhibitory effect of miR-19b on EC proliferation,
migration and tube formation is mediated by STAT3. In the present
study, overexpression of miR-19b in ECs markedly decreased STAT3
tyrosine phosphorylation (Fig.
6A). The inhibitory effects of miR-19b on STAT3 transcriptional
activity were further confirmed by STAT3-driven promoter luciferase
assay (Fig. 6B). These results
suggested that the role of miR-19b in regulating EC proliferation,
migration and tube formation may be mediated by the STAT3 signaling
pathway. Furthermore, miR-19b has also been reported to inhibit the
migration and angiogenesis of human umbilical vein ECs by targeting
the proangiogenic protein, fibroblast growth factor receptor 2, and
by suppressing the expression of cyclin D1. However, this previous
study failed to observe the inhibitory role of miR-19b on cell
proliferation (33), which may be
due to differences in cell culture.
In patients with UA, differentially expressed
circulating miRNAs may serve opposite effects in regulating
angiogenesis. miR-19b belongs to the miRNA-17–92 cluster, which
comprises miR-17–5p, miR-18a, miR-19a, miR-20a, miR-19b and
miR-92a. This miRNA cluster is highly expressed in human ECs and is
upregulated by ischemia (34). In
the present study, miR-17–5p, miR-19a, miR-20a, miR-19b and miR-92a
were all upregulated in patients with UA compared with in controls.
It has also been reported that members of the miRNA-17–92 cluster
exhibit an antiangiogenic effect in ECs (34). Upregulated miR-106a, miR-222,
miR-320 and miR-451 were also observed to inhibit angiogenic
activity in ECs (35–42). Conversely, miR-30a/b/c, miR-93,
miR-126, miR-146a and miR-24 may promote proangiogenic activity
(43–47). These circulating miRNAs may
influence vulnerable plaque formation by forming complex regulatory
networks in patients with UA. Our future studies aim to determine
the key miRNAs in the network using systems biology, in order to
identify effective therapeutic targets.
In conclusion, the present findings suggested that
miR-19b may exert protective effects on plaque stability by
modulating the STAT3-mediated signaling pathway. These findings may
provide information regarding vulnerable plaque formation
intervention in patients with UA.
Acknowledgments
The present study was supported by the Beijing
Science and Technology Major Project (grant no. D141100003014002)
and the National Natural Science Foundation of China (grant nos.
81270274, 81470473, 81400265, 81400264 and 81600340). The present
study was presented at the ESC Congress 2017, August 26–30, 2017,
in Barcelona, Spain and published as abstract no. P675 in
Atherosclerosis 38 (Suppl 1): 2017.
References
|
1
|
GBD 2013 Mortality and Causes of Death
Collaborators: Global, regional, and national age-sex specific
all-cause and cause-specific mortality for 240 causes of death,
1990–2013: A systematic analysis for the Global Burden of Disease
Study 2013. Lancet. 385:117–171. 2015. View Article : Google Scholar
|
|
2
|
Fuster V, Stein B, Ambrose JA, Badimon L,
Badimon JJ and Chesebro JH: Atherosclerotic plaque rupture and
thrombosis. Evolving concepts. Circulation. 82(Suppl 3): II47–II59.
1990.PubMed/NCBI
|
|
3
|
Hellings WE, Peeters W, Moll FL and
Pasterkamp G: From vulnerable plaque to vulnerable patient: The
search for biomarkers of plaque destabilization. Trends Cardiovasc
Med. 17:162–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hopkins PN: Molecular biology of
atherosclerosis. Physiol Rev. 93:1317–1542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi L, Fisslthaler B, Zippel N, Frömel T,
Hu J, Elgheznawy A, Heide H, Popp R and Fleming I: MicroRNA-223
antagonizes angiogenesis by targeting β1 integrin and preventing
growth factor signaling in endothelial cells. Circ Res.
113:1320–1330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haver VG, Slart RH, Zeebregts CJ,
Peppelenbosch MP and Tio RA: Rupture of vulnerable atherosclerotic
plaques: microRNAs conducting the orchestra. Trends Cardiovasc Med.
20:65–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okamura K, Ishizuka A, Siomi H and Siomi
MC: Distinct roles for Argonaute proteins in small RNA-directed RNA
cleavage pathways. Genes Dev. 18:1655–1666. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feinberg MW and Moore KJ: MicroRNA
Regulation of Atherosclerosis. Circ Res. 118:703–720. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harris TA, Yamakuchi M, Ferlito M, Mendell
JT and Lowenstein CJ: MicroRNA-126 regulates endothelial expression
of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA.
105:1516–1521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun X, Icli B, Wara AK, Belkin N, He S,
Kobzik L, Hunninghake GM, Vera MP, Blackwell TS, Baron RM, et al:
MICU Registry: MicroRNA-181b regulates NF-κB-mediated vascular
inflammation. J Clin Invest. 122:1973–1990. 2012.PubMed/NCBI
|
|
11
|
Sun X, He S, Wara AK, Icli B, Shvartz E,
Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, et al:
Systemic delivery of microRNA-181b inhibits nuclear factor-κB
activation, vascular inflammation, and atherosclerosis in
apolipoprotein E-deficient mice. Circ Res. 114:32–40. 2014.
View Article : Google Scholar
|
|
12
|
Cheng HS, Sivachandran N, Lau A, Boudreau
E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI and Fish JE:
MicroRNA-146 represses endothelial activation by inhibiting
pro-inflammatory pathways. EMBO Mol Med. 5:1017–1034. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li S, Ren J, Xu N, Zhang J, Geng Q, Cao C,
Lee C, Song J, Li J and Chen H: MicroRNA-19b functions as potential
anti-thrombotic protector in patients with unstable angina by
targeting tissue factor. J Mol Cell Cardiol. 75:49–57. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han H, Wang YH, Qu GJ, Sun TT, Li FQ,
Jiang W and Luo SS: Differentiated miRNA expression and validation
of signaling pathways in apoE gene knockout mice by
cross-verification microarray platform. Exp Mol Med. 45:e132013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wegrzyn J, Potla R, Chwae YJ, Sepuri NB,
Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, et
al: Function of mitochondrial Stat3 in cellular respiration.
Science. 323:793–797. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Folkman J: Fundamental concepts of the
angiogenic process. Curr Mol Med. 3:643–651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mehta JL, Mercanti F, Stone A, Wang X,
Ding Z, Romeo F and Khaidakov M: Gene and microRNA transcriptional
signatures of angiotensin II in endothelial cells. J Cardiovasc
Pharmacol. 65:123–129. 2015.
|
|
18
|
Anderson JL, Adams CD, Antman EM, Bridges
CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS,
Levin TN, et al: 2011 WRITING GROUP MEMBERS; ACCF/AHA TASK FORCE
MEMBERS: 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA
2007 Guidelines for the Management of Patients With Unstable
Angina/Non-ST-Elevation Myocardial Infarction: A report of the
American College of Cardiology Foundation/American Heart
Association Task Force on Practice Guidelines. Circulation. 123.
pp. e426–e579. 2011, View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Li S, Chen H, Ren J, Geng Q, Song J, Lee
C, Cao C, Zhang J and Xu N: MicroRNA-223 inhibits tissue factor
expression in vascular endothelial cells. Atherosclerosis.
237:514–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Feng W, Liao W, Ma X, Han Q and
Zhang Y: The gp130/STAT3 signaling pathway mediates beta-adrenergic
receptor-induced atrial natriuretic factor expression in
cardio-myocytes. FEBS J. 275:3590–3597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu Z, Maiti D, Kisiel W and Duh EJ: Tissue
factor pathway inhibitor-2 is upregulated by vascular endothelial
growth factor and suppresses growth factor-induced proliferation of
endothelial cells. Arterioscler Thromb Vasc Biol. 26:2819–2825.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boon K, Osorio EC, Greenhut SF, Schaefer
CF, Shoemaker J, Polyak K, Morin PJ, Buetow KH, Strausberg RL, De
Souza SJ and Riggins GJ: An anatomy of normal and malignant gene
expression. Proc Natl Acad Sci USA. 99:11287–11292. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar
|
|
25
|
O'Brien ER, Garvin MR, Dev R, Stewart DK,
Hinohara T, Simpson JB and Schwartz SM: Angiogenesis in human
coronary atherosclerotic plaques. Am J Pathol. 145:883–894.
1994.PubMed/NCBI
|
|
26
|
Menghini R, Stöhr R and Federici M:
MicroRNAs in vascular aging and atherosclerosis. Ageing Res Rev.
17:68–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Welten SM, Goossens EA, Quax PH and
Nossent AY: The multifactorial nature of microRNAs in vascular
remodelling. Cardiovasc Res. 110:6–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeller T, Keller T, Ojeda F, Reichlin T,
Twerenbold R, Tzikas S, Wild PS, Reiter M, Czyz E, Lackner KJ, et
al: Assessment of microRNAs in patients with unstable angina
pectoris. Eur Heart J. 35:2106–2114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang KJ, Zhao X, Liu YZ, Zeng QT, Mao XB,
Li SN, Zhang M, Jiang C, Zhou Y, Qian C, et al: Circulating
MiR-19b-3p, MiR-134-5p and MiR-186-5p are Promising novel
biomarkers for early diagnosis of acute myocardial infarction. Cell
Physiol Biochem. 38:1015–1029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang Y, Zhang YC, Chen Y, Xiang Y, Shen CX
and Li YG: The role of miR-19b in the inhibition of endothelial
cell apoptosis and its relationship with coronary artery disease.
Sci Rep. 5:151322015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lv YC, Tang YY, Peng J, Zhao GJ, Yang J,
Yao F, Ouyang XP, He PP, Xie W, Tan YL, et al: MicroRNA-19b
promotes macrophage cholesterol accumulation and aortic
atherosclerosis by targeting ATP-binding cassette transporter A1.
Atherosclerosis. 236:215–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lv YC, Yang J, Yao F, Xie W, Tang YY,
Ouyang XP, He PP, Tan YL, Li L, Zhang M, et al: Diosgenin inhibits
atherosclerosis via suppressing the MiR-19b-induced downregulation
of ATP-binding cassette transporter A1. Atherosclerosis. 240:80–89.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin R, Bao W, Xing Y, Xi T and Gou S:
MiR-19b-1 inhibits angiogenesis by blocking cell cycle progression
of endothelial cells. Biochem Biophys Res Commun. 417:771–776.
2012. View Article : Google Scholar
|
|
34
|
Doebele C, Bonauer A, Fischer A, Scholz A,
Reiss Y, Urbich C, Hofmann WK, Zeiher AM and Dimmeler S: Members of
the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic
function in endothelial cells. Blood. 115:4944–4950. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun CY, She XM, Qin Y, Chu ZB, Chen L, Ai
LS, Zhang L and Hu Y: miR-15a and miR-16 affect the angiogenesis of
multiple myeloma by targeting VEGF. Carcinogenesis. 34:426–435.
2013. View Article : Google Scholar
|
|
36
|
Yin R, Wang R, Guo L, Zhang W and Lu Y:
MiR-17-3p inhibits angiogenesis by downregulating flk-1 in the cell
growth signal pathway. J Vasc Res. 50:157–166. 2013. View Article : Google Scholar
|
|
37
|
He QY, Wang GC, Zhang H, Tong DK, Ding C,
Liu K, Ji F, Zhu X and Yang S: miR-106a-5p suppresses the
proliferation, migration, and invasion of osteosarcoma cells by
targeting HMGA2. DNA Cell Biol. 35:506–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pin AL, Houle F, Guillonneau M, Paquet ER,
Simard MJ and Huot J: miR-20a represses endothelial cell migration
by targeting MKK3 and inhibiting p38 MAP kinase activation in
response to VEGF. Angiogenesis. 15:593–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khella HW, Butz H, Ding Q, Rotondo F,
Evans KR, Kupchak P, Dharsee M, Latif A, Pasic MD, Lianidou E, et
al: miR-221/222 are involved in response to sunitinib treatment in
metastatic renal cell carcinoma. Mol Ther. 23:1748–1758. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bonauer A, Carmona G, Iwasaki M, Mione M,
Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et
al: MicroRNA-92a controls angiogenesis and functional recovery of
ischemic tissues in mice. Science. 324:1710–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu YY, Chen YL, Jao YC, Hsieh IS, Chang KC
and Hong TM: miR-320 regulates tumor angiogenesis driven by
vascular endothelial cells in oral cancer by silencing neuropilin
1. Angiogenesis. 17:247–260. 2014. View Article : Google Scholar
|
|
42
|
Liu X, Zhang A, Xiang J, Lv Y and Zhang X:
miR-451 acts as a suppressor of angiogenesis in hepatocellular
carcinoma by targeting the IL-6R-STAT3 pathway. Oncol Rep.
36:1385–1392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang Q, Lagos-Quintana M, Liu D, Shi Y,
Helker C, Herzog W and le Noble F: miR-30a regulates endothelial
tip cell formation and arteriolar branching. Hypertension.
62:592–598. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fang L, Du WW, Yang W, Rutnam ZJ, Peng C,
Li H, O'Malley YQ, Askeland RW, Sugg S, Liu M, et al: MiR-93
enhances angiogenesis and metastasis by targeting LATS2. Cell
Cycle. 11:4352–4365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang S, Aurora AB, Johnson BA, Qi X,
McAnally J, Hill JA, Richardson JA, Bassel-Duby R and Olson EN: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu HY, Bai WD, Liu JQ, Zheng Z, Guan H,
Zhou Q, Su LL, Xie ST, Wang YC, Li J, et al: Upregulation of FGFBP1
signaling contributes to miR-146a-induced angiogenesis in human
umbilical vein endothelial cells. Sci Rep. 6:252722016. View Article : Google Scholar
|
|
47
|
Zhou Q, Gallagher R, Ufret-Vincenty R, Li
X, Olson EN and Wang S: Regulation of angiogenesis and choroidal
neovascularization by members of microRNA-23~27~24 clusters. Proc
Natl Acad Sci USA. 108:8287–8292. 2011. View Article : Google Scholar : PubMed/NCBI
|