Introduction
The flavonoids comprise a large group of unique
compounds that are widely distributed throughout the plant Kingdom.
Flavonoids are widely consumed in foodstuffs, including fruits,
vegetables and tea (1).
Flavonoids can be divided into various classes on the basis of
their molecular structure. Flavonols are a subgroup of dietary
flavonoids, consisting predominantly of myricetin, quercetin,
kaempferol and galangin, contai ning 3, 2, 1, and 0 hydroxy groups
on the B-ring structure, respectively (2). Flavonols are the strongest
antioxidants among flavonoids. 3,3′,4′,5,7-Pentahydroxyflavone
(quercetin) (Fig. 1A), with two
hydroxy groups on the B-ring, located in herbs, fruits and
vegetables, has long been used as a component in certain
traditional Chinese medicines as an anti-inflammatory and
anticancer agent (3,4). 3,5,7-Trihy droxyflavone (galangin)
(Fig. 1A), with no hydroxy group
on the B-ring, exists in high concentrations in propolis and
Alpinia officinarum, a plant that has been used as a spice
and as a herbal medicine for various ailments in Asia (5).
Inflammation is a natural host defense response to
invading pathogens and tissue injury involving phagocytic cells,
such as macrophages, mast cells, dendritic cells and innate
lymphocytes, ultimately leading to the restoration of normal cell
structure and function (6).
Nevertheless, the instability of immune homeostasis and a prolonged
inflammatory response may result in the development of various
chronic diseases, such as cancer and inflammation (7,8).
Atopic dermatitis (AD) is an inflammatory skin
disease associated with severe pruritic, long-term swelling and
redness of the skin. It is characterized by the infiltration of
inflammatory cells, such as eosinophils, mast cells and
macrophages, into lesioned skin (9). The causes of AD have yet to be
completely elucidated, but complex inflammatory immune
dysregulation and the response to allergens are considered to be
involved (10).
Macrophages, which are a differentiated tissue cell
type originating as blood monocytes, serve a critical role in the
initiation and propagation of inflammatory responses by releasing
proinflammatory mediators, including nitric oxide (NO),
interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and
prostaglandins by inducible cyclooxygenase (COX-2) (11).
Lipopolysaccharide (LPS) is a major component of the
outer membrane of Gram-negative bacteria, and one of the most
potent initiators of inflammation. LPS activates monocytes and
macrophages to produce proinflammatory cytokines (12). The mitogen-activated protein
kinase (MAPK) family consists of extracellular signal-regulated
kinase (ERK), p38 and c-Jun N-terminal kinase (JNK) (13). MAPKs are serine-threonine kinases
that mediate intracellular signaling associated with various
cellular activities, including cell proliferation, differentiation,
cell death and inflammation (14).
Nuclear factor-κB (NF-κB) regulates the
transcription of numerous genes involved in immunity, inflammation
and protection from programmed cell death (apoptosis). The
activation of NF-κB is mediated by various upstream protein
kinases, including MAPKs (15).
p50/p65 NF-κB is bound to inhibitory inhibitor of κB (IκB) proteins
in the cytoplasm (16). The
cytoplasmic NF-κB/IκB complex is activated by phosphorylation; in
the case of IκB-α, this modification occurs at serines 32 and 36 by
the IκB kinase (IKK) complex (17). A free p50/p65 NF-κB complex
translocates from the cytosol to the nucleus, and ultimately binds
to the promoter region of target genes encoding various
proinflammatory factors (18).
Several pharmacological studies have shown that
quercetin and galangin exhibit anti-inflammatory efficacy in
vitro and in vivo (19–21). However, those studies had
limitations, and it is unknown whether quercetin or galangin
exhibits higher activity. Therefore, the aim of the present study
was to analyze the efficacy of flavonols as anti-inflammatory
compounds in RAW264.7 macrophages by evaluating the generation of
NO, prostaglandin E2 (PGE2), inducible NO
synthase (iNOS), COX-2, TNF-α, and IL-6, and also to investigate
the activation of NF-κB and MAPK signaling. Subsequently, the
anti-inflammatory effects of quercetin, galangin, and
co-administration of the two flavonols were measured by assessing
ear thickness, immunoglobulin E (IgE) production, inflammation and
mast cell infiltration in 2,4-dinitrochlorobenzene (DNCB)-induced
AD models.
Materials and methods
Chemicals, drugs and antibodies
Quercetin and galangin (purity >95%) were both
purchased from Merck KGaA (Darmstadt, Germany), dissolved in
dimethyl sulfoxide (DMSO), and stored at −20°C. Dulbecco's modified
Eagle's medium (DMEM), penicillin-streptomycin and fetal bovine
serum (FBS) were all purchased from Welgene (Gyeongsan, Korea).
LPS, 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) and DMSO were purchased from Merck KGaA. The nitrate/nitrite
colorimetric assay kit was purchased from Cayman Chemical Company
(Ann Arbor, MI, USA); the mouse TNF ELISA and mouse IL-6 ELISA kits
were both purchased from BD Biosciences (San Jose, CA, USA).
β-actin (#4967), iNOS (#2982), COX-2 (#4842), p-NF-κB/p65 (#3033),
IκB-α (#9242), ERK1/2 (#9102), phospho-ERK1/2 (#4376), p38 MAPK
(#9212), phospho-p38 MAPK (#9211), JNK (#9252), phospho-JNK (#4668)
and anti-rabbit horseradish peroxidase (HRP; #7074) antibodies, as
well as Alexa Fluor 594-conjugated anti-rabbit immunoglobulin G
(IgG) (#8889), were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA).
Cell culture and stimulation
The RAW264.7 macrophage line was obtained from the
Korean Cell Line Bank (Seoul, Korea), and maintained in DMEM
supplemented with 5% FBS/1% penicillin-streptomycin at 37°C in a 5%
CO2-humidified air environment. The cells were incubated
for 24 h in medium supplemented with 10% FBS. Subsequently, the
cells were pre-incubated with or without the indicated
concentrations of quercetin and galangin for 2 h in serum-free
media, prior to the addition of LPS (1 μg/ml).
Cell viability assay
RAW264.7 cells were seeded in a 96-well plate at a
density of 1×105/ml and a volume of 200 μl/well.
After incubation for 24 h at 37°C, the cells were treated with
quercetin and galangin at the indicated concentrations for 24 h,
followed by the addition of 5 mg/ml MTT solution to each well, and
the plates were further incubated for 2 h at 37°C. The supernatant
was removed, and 200 μl DMSO was added to each well to
solubilize the water-insoluble purple formazan crystals. The
absorbance at a wavelength of 595 nm was measured using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The percentage of viable cells compared with that in untreated
control cells was estimated.
Measurement of NO
RAW264.7 cells were seeded (1×105/ml) and
cultured in 96-well plates. After incubation for 24 h at 37°C, the
cells were treated with quercetin and galangin at the indicated
concentrations for 2 h in serum-free medium, prior to the addition
of LPS (1 μg/ml). After 24 h, the supernatants were measured
for NO production using the nitrate/nitrite assay kit (Cayman
Chemical Company). NO was measured as the accumulation of nitrite
and nitrate reductase, which were determined spectrophotometrically
using Griess reagent at an optical density of 540 nm.
Determination of TNF-α and IL-6
production
Production of the proinfla mmatory cytokines, TNF-α
and IL-6, in the culture medium was determined using commercially
available enzyme-linked immunosorbent assay (ELISA) kits (BD
Biosciences), according to the manufacturer's protocol.
Western blot analysis
The cells were preincubated with or without the
indicated concentrations of quercetin and galangin for 2 h prior to
exposure to LPS (1 μg/ml), and the cells were subsequently
harvested at various time points. The cells were washed with
phosphate-buffered saline (PBS) and treated with trypsin-EDTA. The
cell pellets were obtained by centrifugation at 260 × g for 5 min
at 4°C, lysed in lysis buffer (Invitrogen Life Technologies; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and were centrifuged at
27,237 × g for 5 min at 4°C to obtain whole-cell lysates. The
protein concentrations were determined by the Bradford protein
assay (Bio-Rad Laboratories, Inc.). The proteins were resolved
using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred electrophoretically to nitrocellulose
membranes (Bio-Rad Laboratories, Inc.). Transferred membranes were
blocked with Tris-buffered saline (TBS) containing 5% non-fat dried
milk and 0.1% Tween®-20 at 4°C for 2 h. After blocking,
the membranes were incubated with primary antibodies (1:1,000)
overnight at 4°C with gentle shaking. After incubation with the
primary antibodies, the membranes were incubated with
HRP-conjugated anti-rabbit IgG secondary antibodies (1:1,000) for 2
h at room temperature with gentle shaking. After washing the
membranes three times for 10 min in TBS containing 0.1%
Tween®-20, bands were detected using enhanced
chemiluminescence western blotting detection reagents (Pierce;
Thermo Fisher Scientific, Inc.) accor ding to the manufacturer's
protocol. β-actin was used as a loading control. Band density was
measured using ImageJ software (National Institutes of Health,
Bethesda, MD, USA).
Immunofluorescence assay
RAW264.7 cells were cultured directly on glass
coverslips in 6-well plates at a density of 1×105/ ml
for 24 h to detect NF-κB/p65 localization by fluorescence
microscopy. After stimulation with LPS for 15 min in the presence
or absence of quercetin and galangin, the cells were fixed with 4%
paraformaldehyde for 10 min at room temperature and permeabilized
with 100% methanol for 10 min at 20°C. After several washings with
PBS, the cells were blocked in PBS containing 3% BSA (Cell
Signaling Technology, Inc.). The cells were subsequently incubated
with the primary antibody, diluted 1:200, at 4°C overnight. The
cells were then washed with PBS, and Alexa Fluor 594-conjugated
anti-rabbit IgG, diluted 1:200, was applied for 1 h to the cells.
This process was performed in the dark at room temperature. After
washing with PBS, the nuclei were stained with DAPI, and
fluorescence was visualized using a fluorescence microscope (BX41;
Olympus, Tokyo, Japan) at a magnification of ×400.
Animals
BALB/c female mice (4 weeks old) were purchased from
the animal production company of Orient Bio, Inc. (Gyeonggi-do,
Korea) and maintained at 23±5°C at 40±10% relative humidity with
artificial lighting from 8:00 a.m. to 8:00 p.m. in facilities
approved by the Companion and Laboratory Animal Science Department
of Kongju National University (Chungnam, Korea). The animals were
housed in cages and allowed access to sterilized water and
commercial rodent chow (Biopia, Seoul, Korea) ad libitum.
All the animal experiments were performed with the approval of the
Institutional Animal Care and Use Committee, following the
guidelines of Kongju National University. All experiments with mice
were performed in accordance with the national guidelines, and
approved by Kongju National University (approval no. KNU_2015–10;
Yesan, Korea).
Induction of AD
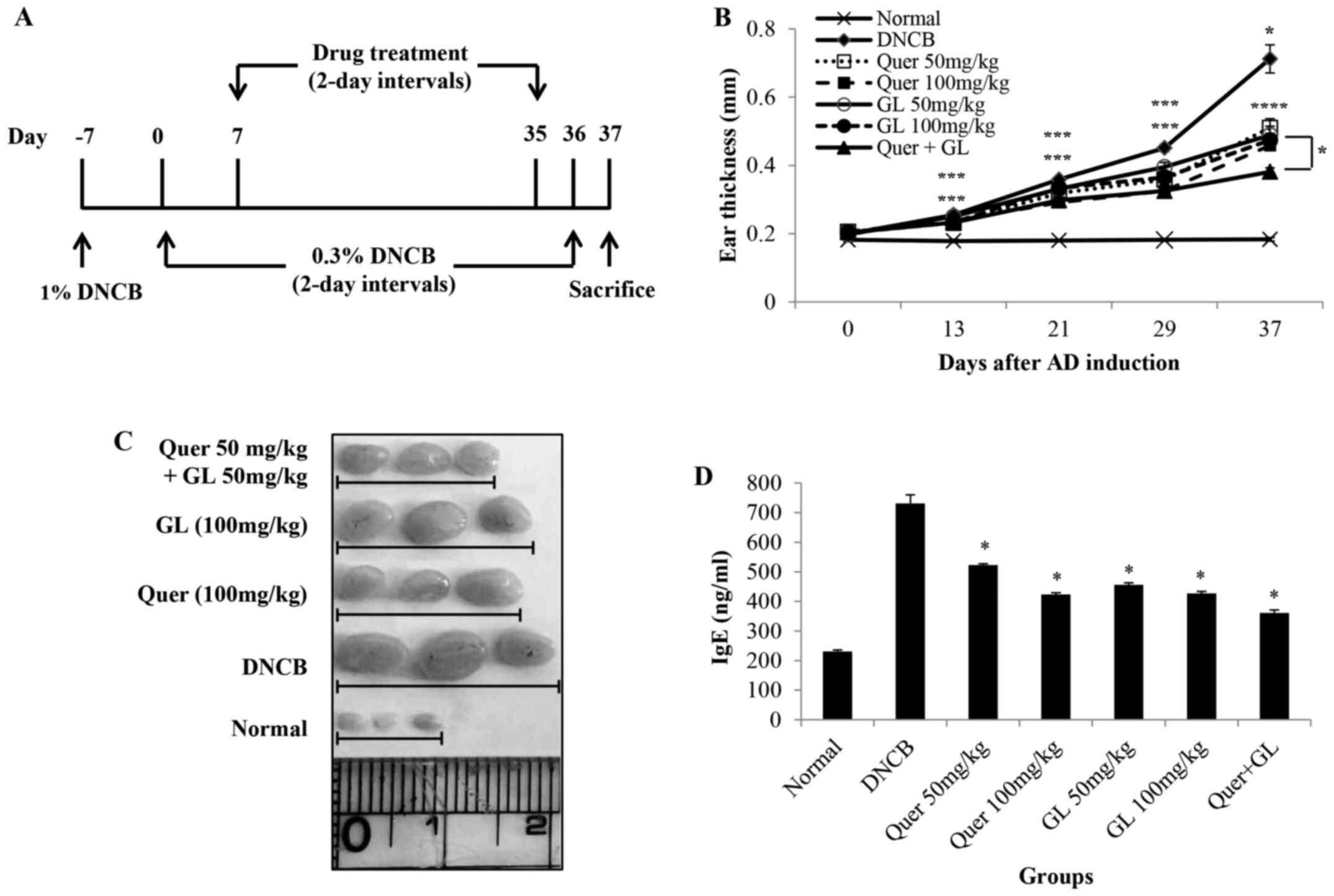
AD was induced in BALB/c mice according to a
published method, but with minor modifications (22). The schematic experimental
procedure is described in Fig.
6A. Briefly, BALB/c mice were divided into seven groups
(n=3/group). Group I (normal control) and group II (DNCB) were
provided with vehicle orally, while group III (50 mg/kg quercetin),
group IV (100 mg/kg quercetin), group V (50 mg/kg galangin), group
VI (100 mg/kg galangin), and group VII (50 mg/kg quercetin + 50
mg/kg galangin) received quercetin, galangin or their combination,
respectively. For the induction of AD, the surfaces of both ears of
the mice were stripped with surgical tape. After stripping, 1% DNCB
dissolved in acetone/olive oil solution (acetone:olive oil, 3:1)
was painted on each ear. On day 0, the mice were challenged again
by applying 0.3% DNCB to the ears every other day for up to 36
days. From day 7 until the completion of the experiment, the mice
were treated with quercetin, galangin and quercetin + galangin via
gavage every other day. The mice were sacrificed on day 37. A
vernier caliper (Mitutoyo, Kawasaki, Japan) was used to measure ear
thickness 24 h after the application of DNCB. On day 37, blood
samples were collected from abdominal aorta, and the plasma was
stored at -70̊C until further analysis. After blood collection, the
ears were excised and subjected to histopathological analysis.
Measurement of immunoglobulin levels
Blood samples were obtained from each treatment
group 37 days following AD induction. Total IgE levels in serum
were measured using an ELISA kit, following the manufacturer's
protocol.
Histological observation
The ears were immediately fixed in 10% formaldehyde,
and embedded in paraffin. Blocks were then cut into
5-μm-thick slices. For the measurement of thickening of the
epidermis, hematoxylin and eosin (H&E) staining was performed.
For the measurement of mast cells, the skin sections were stained
with toluidine blue.
Statistical analysis
The results are expressed as the mean ± standard
deviation (SD). Differences between the mean values for the
individual groups were assessed by one-way analysis of variance
(ANOVA), with Dunnett's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of quercetin and galangin on the
viability of RAW264.7 macrophages
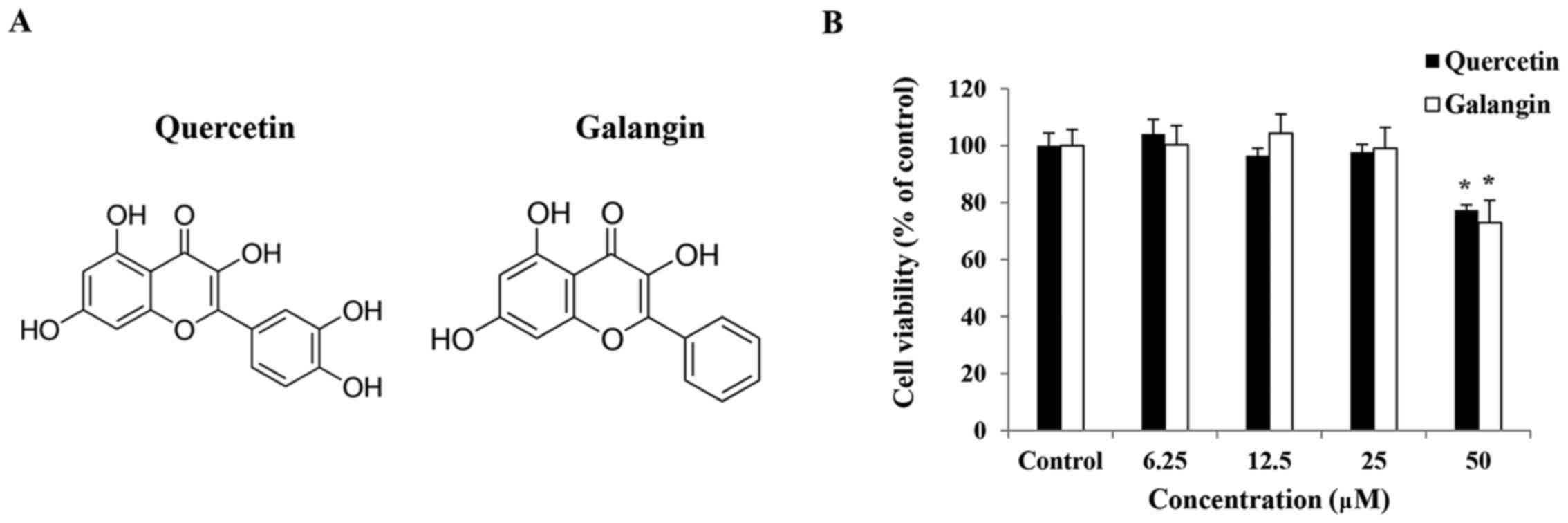
The cytotoxic effects of quercetin and galangin were
evaluated after treatment with various concentrations of quercetin
and galangin for 24 h using an MTT assay. This analysis revealed
that quercetin and galangin did not affect the viability of
RAW264.7 macrophages at concentrations of 6.25, 12.5 and 25
μM (Fig. 1B). Hence, these
concentrations of quercetin and galangin were considered suitable
for further assays.
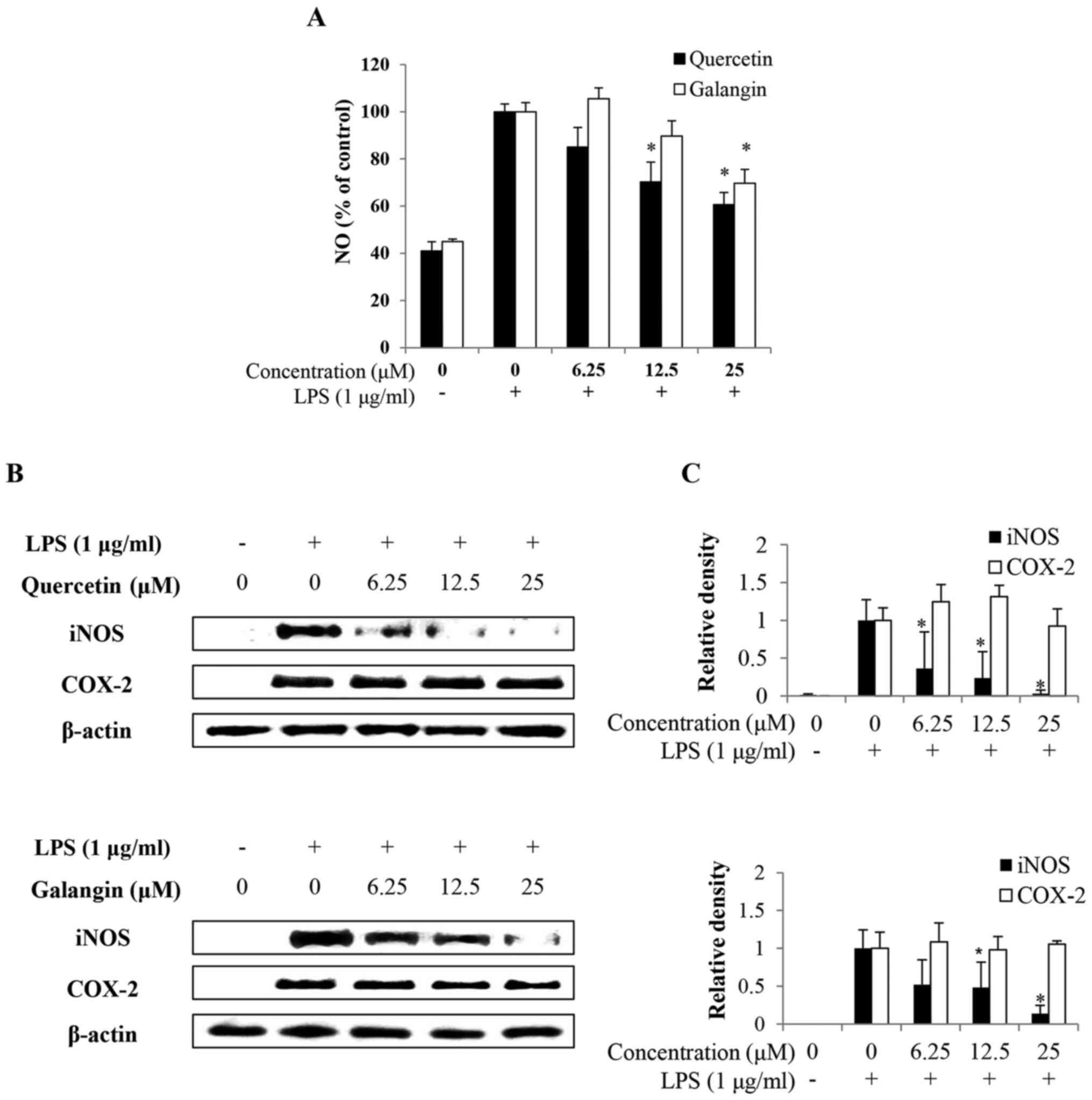
Effects of quercetin and galangin on
LPS-induced NO production
To investigate the effects of quercetin and galangin
on NO production in macrophages, RAW264.7 cells were pretreated
with or without quercetin and galangin (0, 6.25, 12.5 and 25
μM) for 2 h prior to stimulation with 1 μg/ml LPS for
24 h. Consequently, LPS alone markedly induced NO production
compared with the untreated control. However, pretreatment with
12.5–25 μM quercetin and 25 μM galangin significantly
reduced NO production in LPS-stimulated RAW264.7 cells in a
dose-dependent manner (Fig.
2A).
Effects of quercetin and galangin on
LPS-induced iNOS and COX-2 protein
To confirm whether the effects of quercetin and
galangin involved modulation of the expression of iNOS and COX-2,
which are associated with NO and PGE2 production, the
expression levels of iNOS and COX-2 were examined by western blot
analysis. After treatment with 1 μg/ml LPS, the expression
levels of iNOS and COX-2 were significantly increased, whereas
pretreatment with 6.25–25 μM quercetin or with 12.5–25
μM galangin markedly decreased iNOS expression, but had no
effect on the expression of COX-2 (Fig. 2B and C). These results indicated
that the reduced expression of iNOS at the protein level
contributed to the inhibitory effect of quercetin and galangin on
LPS-induced NO production.
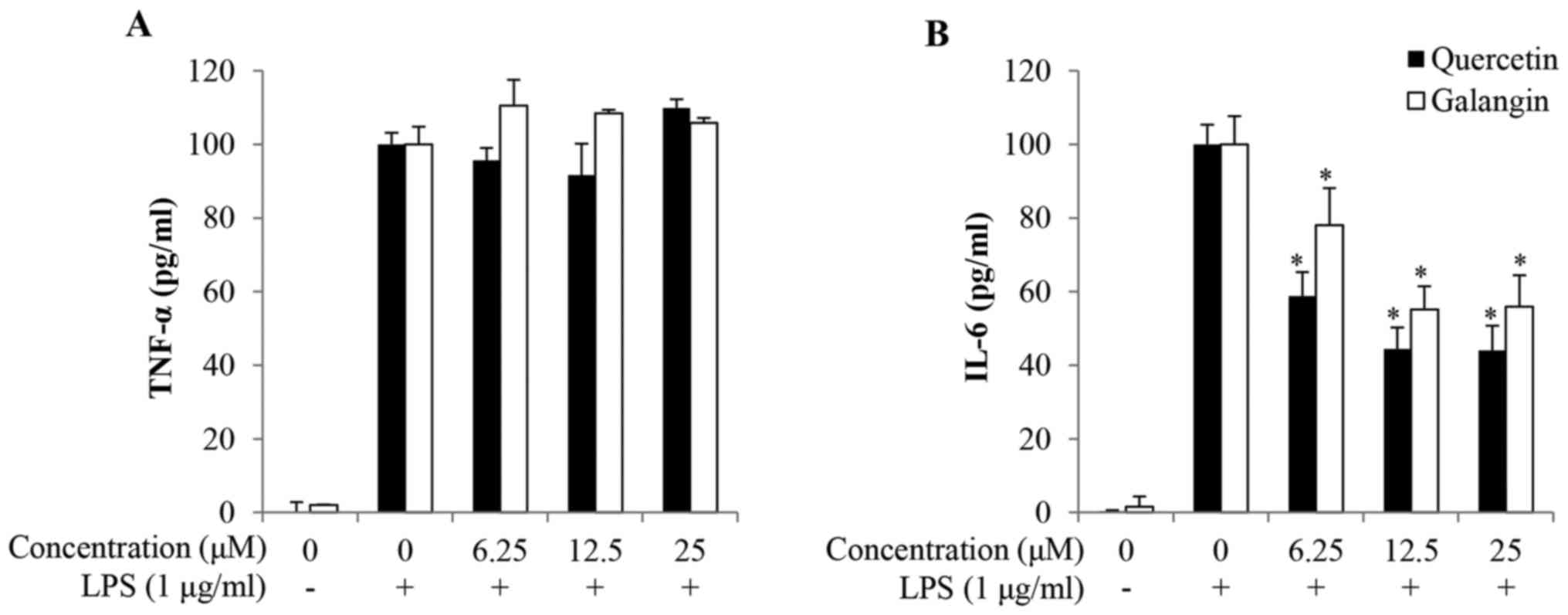
Effects of quercetin and galangin on
LPS-induced proinflammatory cytokine production
To investigate the anti-inflammatory effects of
quercetin and galangin, the production of TNF-α and IL-6 in the
culture supernatants of RAW264.7 cells was quantified. As shown by
ELISA, the expression levels of both TNF-α and IL-6 were relatively
low in untreated control cells, but were markedly increased upon
exposure to LPS alone. Pretreatment with quercetin and galangin
significantly reduced IL-6 production in LPS-stimulated RAW264.7
cells in a dose-dependent manner from 6.25 to 25 μM
quercetin and galangin. However, quercetin and galangin had no
effect on the production of TNF-α following 24 h of incubation
(Fig. 3A and B).
Effects of quercetin and galangin on the
activation and nuclear translocation of NF-κB in LPS-stimulated
RAW264.7 macrophages
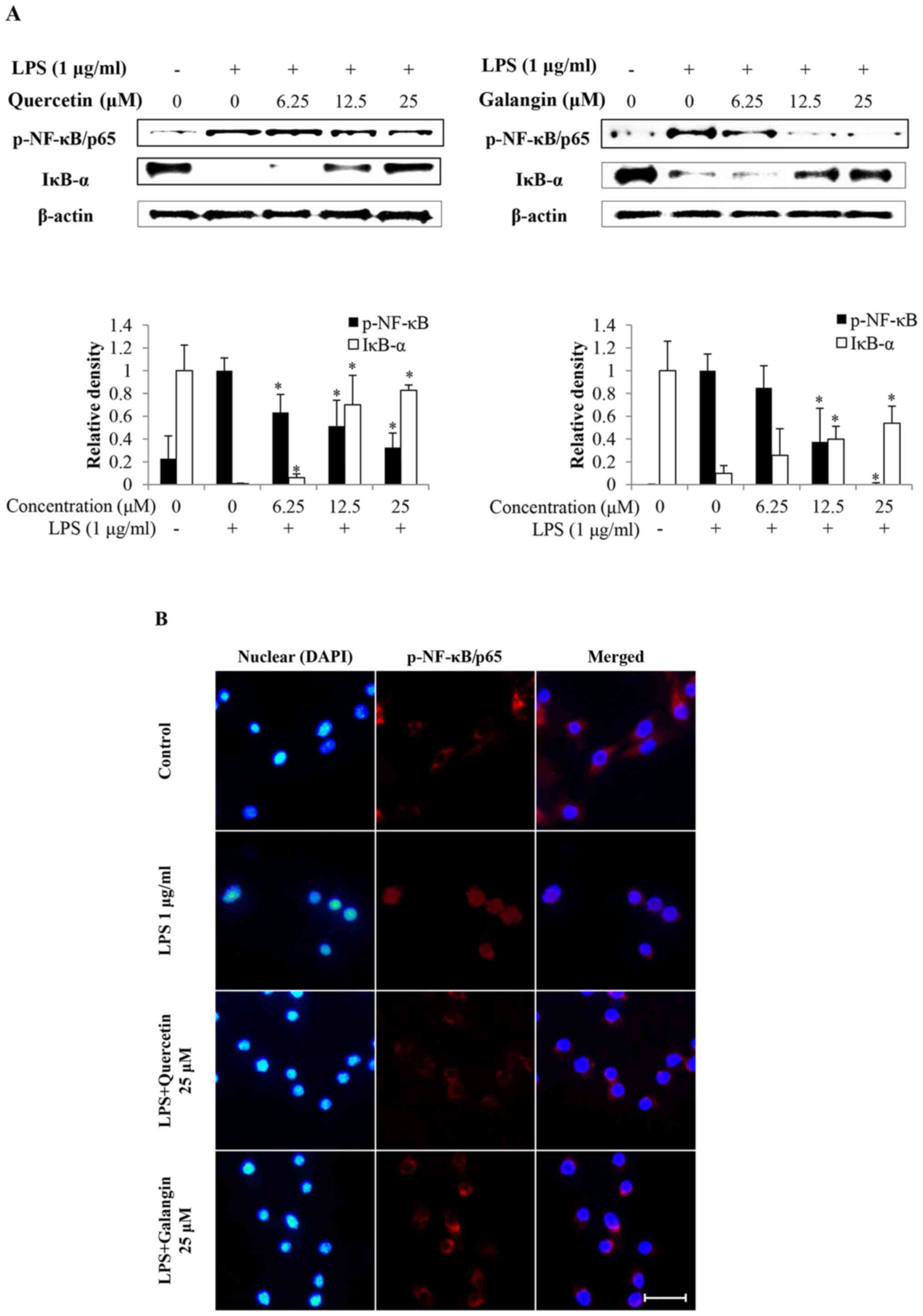
Subsequently, whether quercetin and galangin
suppressed the degradation of IκB-α, and translocation of NF-κB
into the nucleus, was investigated. As shown in Fig. 4A, LPS stimulation induced the
degradation of IκB-α and activation of NF-κB in RAW264.7 cells.
However, quercetin and galangin pretreatment significantly
attenuated IκB-α degradation and NF-κB activation.
Immunofluorescence staining of NF-κB revealed that NF-κB was
exclusively distributed in the cytoplasmic compartment prior to LPS
stimulation. Treatment with LPS resulted in the enrichment of NF-κB
in the nucleus. The nuclear translocation of NF-κB was markedly
attenuated by quercetin and galangin treatment (Fig. 4B). These results indicate the
potential role of NF-κB in the suppression of inflammatory mediator
production by quercetin and galangin.
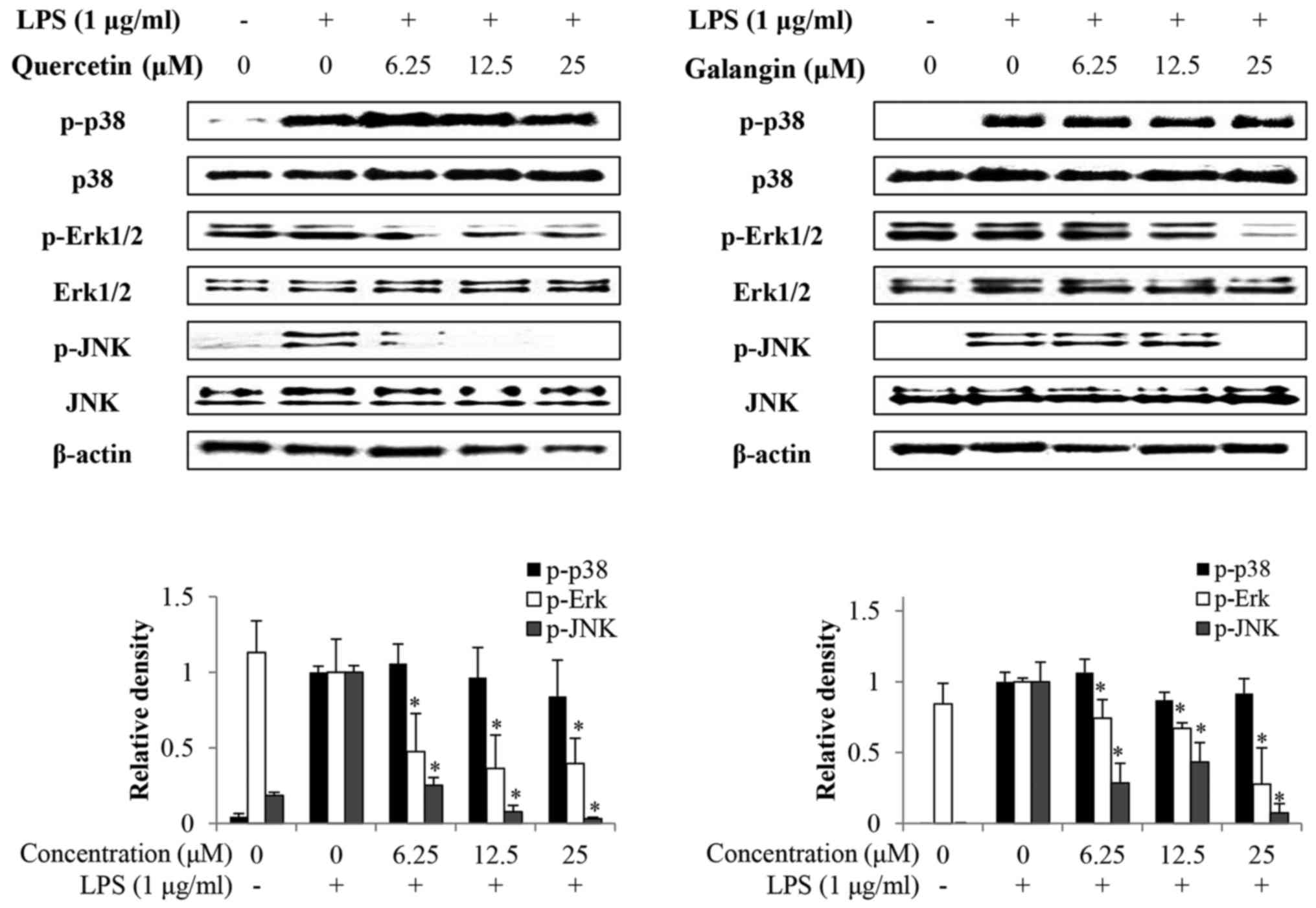
Effects of quercetin and galangin on
LPS-induced MAPKs phosphorylation
Whether quercetin and galangin suppressed MAPK
signaling pathways in LPS-stimulated RAW264.7 cells was
subsequently evaluated. It was identified that the phosphorylation
of p38 by LPS stimulation in RAW264.7 cells was not affected by
quercetin or galangin. However, phosphorylation of Erk1/2 and JNK
was markedly reduced by quercetin and galangin treatment in a
concentration-dependent manner, without a change in total protein
expression (Fig. 5). These
results suggest that suppression of phosphorylation of Erk1/2 and
JNK may be involved in the inhibitory effect of quercetin and
galangin on LPS-stimulated NF-κB activation in RAW264.7 cells.
Effect of orally administered quercetin,
galangin and their combination on DNCB-induced AD-like skin lesions
in BALB/c mice
Treatment of mice with DNCB (see the scheme in
Fig. 7A) resulted in severe
discernible inflammation, with a significant increase in ear
thickness compared with the normal group. The oral administration
of quercetin and galangin in AD mice led to a noticeable reduction
in ear thickness and AD symptoms, which were significant on day 21
and thereafter. Additionally, the data revealed that the
combination of quercetin and galangin was more effective in
suppressing ear thickness compared with each flavonol alone
(Fig. 6B). Subsequently, the
morphological changes in the lymph nodes of AD mice were measured.
The lymph nodes from mice in the DNCB-only group were very swollen,
whereas those from quercetin and galangin mice were smaller and
weighed less (Fig. 6C). The IgE
levels in AD mice were also measured. As expected, AD mice
receiving quercetin and galangin produced significantly less IgE
than did DNCB-only mice. Furthermore, the combination of quercetin
and galangin was more effective in reducing IgE levels compared
with each flavonol alone (Fig.
6D). These data indicated that quercetin and galangin
ameliorated AD symptoms in mice, thereby suggesting that quercetin
and galangin may be effective modulators of immune responses in
AD.
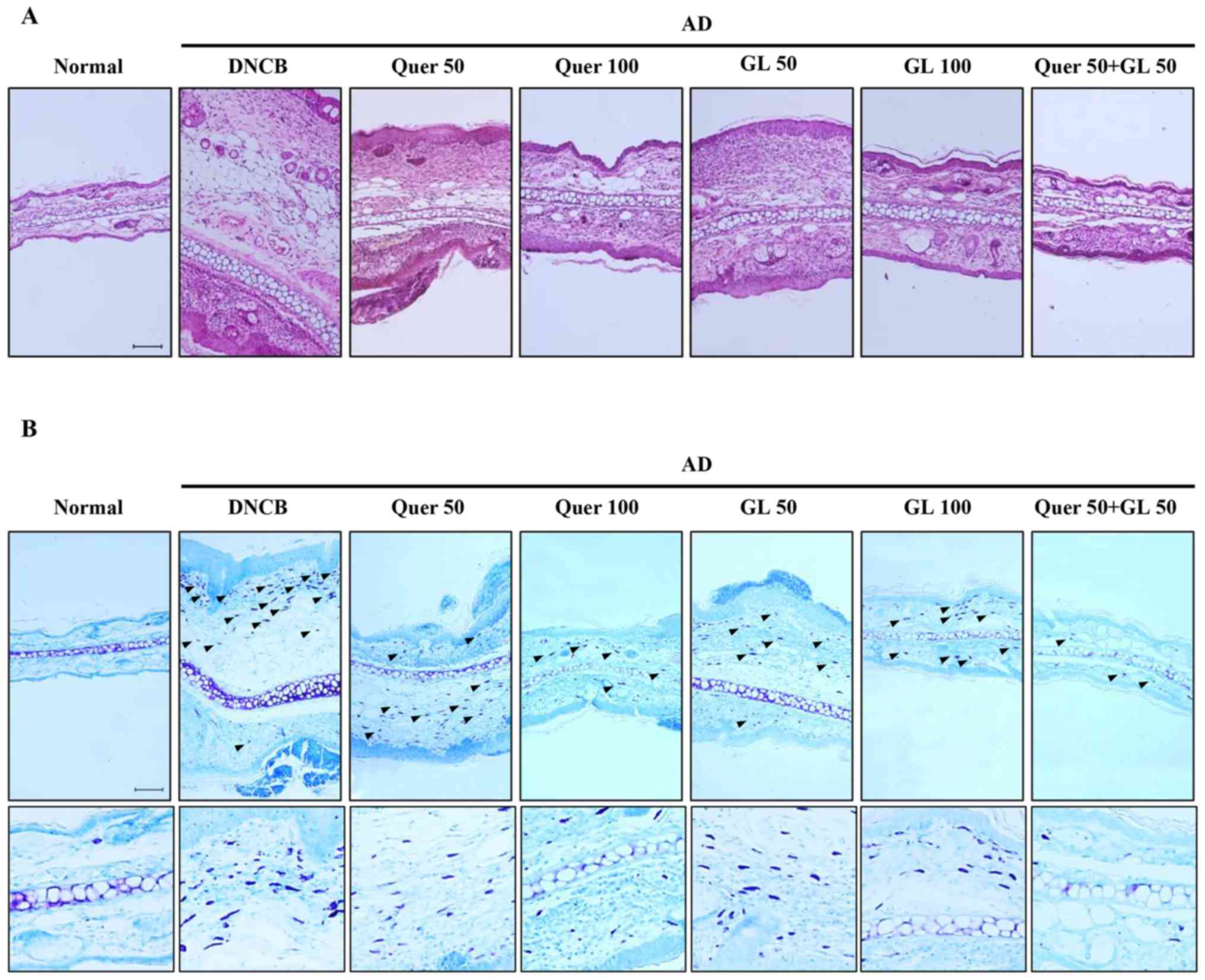 | Figure 7Effects of orally administered Quer
(at concentrations of 50 or 100 mg/kg), GL (also at concentrations
of 50 or 100 mg/kg), and their combination (both compounds at 50
mg/kg) on tissue inflammation and infiltration of immune cell in AD
mice. (A) Hematoxylin and eosin (red) and (B) toluidine blue (blue)
stainingwas performed, and the cells were examined under a light
microscope (magnification, ×200). The arrows indicate mast cells.
AD, atopic dermatitis; Quer, quercetin; GL, galangin; AD, atopic
dermatitis; DCNB, 2,4-dinitrochlorobenzene. |
Effect of quercetin and galangin on
DNCB-induced immune cell infiltration in BALB/c mice
Following induction of AD in mice, the ears were
excised, and the thickness of ear tissues and the number of mast
cells in the skin lesions were examined. First, H&E staining
revealed extensive epidermal and dermal changes in the ears of AD
mice, whereas these changes were attenuated in AD mice that were
administered quercetin and galangin. The epidermal and dermal
tissues in AD mice were significantly thinner following the
administration of quercetin and galangin (Fig. 7A). Secondly, toluidine blue
staining of ear tissue sections revealed mast cell infiltration on
AD mice. This was abrogated by administration of quercetin and
galangin (Fig. 7B). Taken
together, these results suggested that quercetin and galangin
reduce inflammation and mast cell infiltration in the skin, thereby
attenuating AD.
Discussion
Flavonoids, also known as natural substances,
possess various biological activities, including antioxidant,
anti-inflammatory and anticancer properties (23). However, analyses of the
anti-inflammatory properties of quercetin and galangin are limited
to in vitro studies, and whether quercetin or galangin
exhibits higher activity has yet to be elucidated. Thus, in the
present study, the anti-inflammatory effects of quercetin and
galangin in LPS-stimulated RAW264.7 cells in vitro, and the
DNCB-induced AD model in vivo, were evaluated. Furthermore,
whether the number of hydroxy groups on the B-ring of two flavonols
would influence their anti-inflammatory effects was also
investigated. The difference between the chemical structures of the
two flavonols is associated with the number of hydroxy groups on
the B-ring. This is considered to be involved in the biological
activities of flavonols (24).
Therefore, the present study aimed to compare the anti-inflammatory
effects of the two flavonols.
Macrophages are crucial for the host's defense
against infections and in inflammatory processes through the
release of molecules, including NO, TNF-α and IL-6. The
overproduction of these mediators has been implicated in several
inflammatory diseases and cancer (25). Thus, the murine macrophage
RAW264.7 cell line was used in the present study.
In the MTT assay results, quercetin and galangin
exhibited no cytotoxicity towards RAW264.7 macrophage cells up to
concentrations of 25 μM, and therefore concentrations above
this were not considered for further in vitro experiments
(Fig. 1B).
NO is produced from L-arginine by three NOS enzymes:
Endothelial NOS (eNOS), neuronal NOS (nNOS), and iNOS. Low
physiological levels of NO are produced by constitutively expressed
eNOS and nNOS, whereas iNOS is responsible for prolonged production
of larger amounts of NO (26).
iNOS is induced by bacterial products and inflammatory cytokines in
macrophages and several other cells (27). In the present study, it has been
demonstrated that quercetin and galangin markedly attenuated the
LPS-induced expression of NO from RAW264.7 cells (Fig. 2A). Furthermore, these results
indicated that quercetin, which has a higher number of hydroxy
groups, was more effective compared with galangin.
Another important enzyme, COX-2, is an inducible
enzyme that catalyzes the conversion of arachidonic acid into
prostaglandin. Numerous studies have suggested that increased
levels of prostaglandin and COX activity promote inflammatory pain
(28). Thus, modulation of iNOS
and COX-2 expression is considered to be a putative strategy for
alleviating inflammatory disorders. The present study showed that
quercetin and galangin inhibited the production of NO through
downregulation of iNOS expression in LPS-stimulated RAW264.7 cells.
However, quercetin and galangin had no effect on the production of
COX-2 (Fig. 2B). In a previous
study, the expression of iNOS and COX-2 by drug treatment yielded
different results, i.e. that iNOS expression was decreased, but
COX-2 expression was not affected (29). The differences noted in these
results may be due to the degree of dependence of iNOS and COX-2
promoters on the various transcription factors.
TNF-α serves a major role in the cascade of
proinflammatory cytokines, and subsequent inflammatory processes
(30). IL-6 production is rapidly
increased in acute inflammatory responses associated with
infection, injury, trauma, and other stresses. As such, a
dysregulated, high-level production of IL-6 may induce an
undesirable inflammatory state (31). In the present study, IL-6
production was also inhibited by quercetin and galangin (Fig. 3B), but no effect was observed with
respect to TNF-α production (Fig.
3A). These results provide the possibility that potentially
separate mechanisms may exist in the production of TNF-α and IL-6
in LPS-stimulated macrophages. Therefore, the effects of quercetin
and galangin on the expression of the NF-κB signaling pathway
associated with IL-6 were examined in the following
experiments.
NF-κB is a transcription factor that promotes the
expression of inflammatory cytokines such as IL-6, and its
downstream targets, including iNOS. Under normal conditions, NF-κB
is present in an inactivated form in complex with IκB in the
cytoplasm (32). However, LPS
stimulation leads to the phosphorylation and degradation of IκB. As
a result, NF-κB is released from the inhibition mediated by IκB,
and activated NF-κB is subsequently translocated into the nucleus
(33). The present study
indicated that quercetin and galangin inhibited the nuclear
translocation of NF-κB/p65 by suppressing degradation of the IκB-α
protein levels (Fig. 4A).
Subsequently, nuclear translocation of NF-κB/p65 was observed under
a fluorescence microscope. Quercetin (25 μM) and galangin
(25 μM) markedly inhibited the LPS-induced nuclear
trans-location of NF-κB/p65 (Fig.
4B). These data suggested that the inhibitory effect of
quercetin and galangin on the production of various inflammatory
factors, including NO, iNOS and IL-6, occurs through blocking the
activation and nuclear translocation of NF-κB/p65.
A previous study has reported that MAPKs (p38,
Erk1/2 and JNK) are associated with TLR4-mediated signaling events
that lead to activation of the transcription factors, NF-κB and
activator protein-1 (AP-1), in LPS-stimulated macrophages (34). The effects of quercetin and
galangin on MAPK phosphorylation in RAW264.7 cells were also
investigated. In the present study, it was shown that quercetin and
galangin inhibited the LPS-induced phosphorylation of Erk1/2 and
JNK, but had no effect on the phosphorylation of p38 (Fig. 5), suggesting that the Erk1/2 and
JNK pathway may be involved in the quercetin- and galangin-mediated
inhibition of LPS-induced inflammation. Taken together, these
results suggested that quercetin and galangin may suppress the
LPS-induced expression of iNOS and IL-6 by inhibiting
phosphorylation of Erk1/2 and JNK in RAW264.7 cells.
In accordance with the in vitro results,
quercetin and galangin significantly inhibited the LPS-induced
inflammatory response. A noteworthy feature is that quercetin,
which has two hydroxy groups on the B-ring, exhibited markedly more
pronounced anti-inflammatory effects compared with galangin, which
possesses no hydroxy groups on the B-ring. According to a previous
study, the presence of hydroxy groups on the B-ring was shown to
increase the pro-apoptotic activity of the flavonoids (35). Also, the antioxidant effect of
various flavonoids was increased, depending on the number of
hydroxy groups on the B-ring (36). Taken together, the number of
hydroxy groups on the B-ring in quercetin and galangin is
considered to exert an influence on the inflammatory response of
LPS-induced RAW264.7 macrophages.
The number of published studies on the bioactive
effects of various flavonoids has been continually increasing,
although the majority of these studies has been focused on the
efficacy of a single compound. However, synergistic effects have
been reported for several flavonoids (37–39). Based on these results, the
anti-inflammatory effects of quercetin, galangin and their
combination in the DNCB-induced AD-like skin lesions of BALB/c
mouse models were investigated.
DNCB is a representative irritant that induces
contact dermatitis. Numerous studies have indicated the importance
of the method used to deliver a compound to target tissues and
cells in studying diseases in animal models. Topical or oral
administration is widely used in AD models. Topical administration
has relatively rapid effects on the administration site, although
it is difficult to control the dosage. Oral administration is more
convenient for AD animal studies, and it is much easier to control
the dosage compared with topical administration (40). Since the symptoms of AD are
systemic, rather than being limited to the skin, oral
administration was selected for the present study.
Skin inflammatory diseases, such as contact
dermatitis and AD, have features in common, including the
infiltration of immune cells, epidermal hyperplasia, elevated serum
IgE levels and increased inflammatory cytokines (41). To investigate the
anti-inflammatory effect, ear thickness of the mice was measured.
It was revealed that the increase in DNCB-induced ear thickness was
markedly suppressed by quercetin, galangin and their combination
compared with the DNCB control in the following order: Quercetin +
galangin > quercetin 100 > galangin 100 > galangin 50 >
quercetin 50 > DNCB-control (see the Materials and methods
section for a fuller description of these groups). Among them, the
most effective group was the flavonol combination group (Fig. 6B).
Lymph nodes exert a critical role in the
cell-mediated immune response. Therefore, morphological changes in
AD-induced mice were investigated. The lymph nodes from mice in the
DNCB-only group were significantly larger compared with the
quercetin- and galangin-treatment groups (Fig. 6C).
IgE is an important therapeutic target for AD, as it
is a major activator of mast cells, which release histamine. IgE
expression causes acute- and chronic-phase skin inflammation
(42). Consequently, the levels
of serum IgE in DNCB-induced AD mouse models were also
investigated. As a result, the quercetin, galangin and flavonol
combination groups exhibited significantly reduced levels of IgE
compared with the control group. Among them, the most effective
group was the flavonol combination group (Fig. 6D).
Histological examination of the skin lesions stained
by H&E and toluidine blue revealed thickening of the epidermis
and dermis due to leukocyte infiltration, hyperplasia, dermis
ulcers, and infiltration of immunocytes on the skin,
including T-cells, mast cells and eosinophils (43). Mast cells are key effector cells
in IgE-mediated allergic disorders, and are activated by
cross-linking of the high-affinity IgE receptor. In addition, it
has been reported that numerous mast cells may be identified in AD
skin lesions (44). In the
quercetin, galangin and flavonol combination groups, the
thicknesses of the epidermis and dermis were reduced, and the
expression of mast cells was significantly decreased, compared with
the DNCB-only group (Fig. 7A and
B). These experimental results indicated that the
anti-inflammatory effect of quercetin combined with galangin is
more potent compared with that of either flavonol alone.
For the in vitro experiments, marked
anti-inflammatory effects were revealed, depending on the number of
hydroxy groups on the B-ring, whereas the in vivo
experiments showed no significant effects according to the
difference in the number of hydroxy groups on the B-ring. However,
these findings cannot be completely correlated, due to the
differences that result from performing experiments in vitro
and in vivo. Thus, it may be necessary to perform further
experiments to gain an improved understanding of the differences
between these findings.
In conclusion, the present study has demonstrated
that pretreatment with quercetin and galangin ameliorated the
LPS-induced inflammatory response in RAW264.7 macrophages through
the inhibition of NF-κB, Erk1/2 and JNK signaling. Furthermore, the
presence of hydroxy groups in flavonols has been confirmed to lead
to significant anti-inflammatory effects in vitro.
Additionally, to the best of our knowledge for the first time, it
has been reported that the combination of quercetin and galangin
works much more effectively in DNCB-induced AD-like skin lesions
compared with either compound alone. Compared with the in
vitro results, no significant differences attributable to the
hydroxy groups were identified for quercetin and galangin treatment
in the in vivo experiments. However, quercetin and galangin
both exerted anti-inflammatory effects on the DNCB-induced AD
animal model. Therefore, it may be hypothesized that quercetin and
galangin treatment may be further developed as a medicine for AD
once further experimental work has helped to delineate the
molecular mechanisms of how quercetin and galangin act.
Acknowledgments
This study was supported by a research grant of the
Kongju National University in 2016.
References
|
1
|
Aherne SA and O'Brien NM: Dietary
flavonols: chemistry, food content, and metabolism. Nutrition.
18:75–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao J, Suzuki M, Jiang X, Chen X,
Yamamoto K, Ren F and Xu M: Influence of B-ring hydroxylation on
interactions of flavonols with bovine serum albumin. J Agric Food
Chem. 56:2350–2356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rogerio AP, Kanashiro A, Fontanari C, da
Silva EV, Lucisano-Valim YM, Soares EG and Faccioli LH:
Anti-inflammatory activity of quercetin and isoquercitrin in
experimental murine allergic asthma. Inflamm Res. 56:402–408. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamson DW and Brignall MS: Antioxidants
and cancer, part 3: quercetin. Altern Med Rev. 5:196–208.
2000.PubMed/NCBI
|
|
5
|
Heo MY, Sohn SJ and Au WW:
Anti-genotoxicity of galangin as a cancer chemopreventive agent
candidate. Mutat Res. 488:135–150. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galli SJ, Grimbaldeston M and Tsai M:
Immunomodulatory mast cells: negative, as well as positive,
regulators of immunity. Nat Rev Immunol. 8:478–486. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hofseth LJ and Ying L: Identifying and
defusing weapons of mass inflammation in carcinogenesis. Biochim
Biophys Acta. 1765:74–84. 2006.
|
|
8
|
Leung DY: Atopic dermatitis: the skin as a
window into the pathogenesis of chronic allergic diseases. J
Allergy Clin Immunol. 96:302–319. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bieber T and Simon HU: Allergen-specific
immunotherapy: current concepts and future directions. Allergy.
66:709–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akdis CA, Akdis M, Trautmann A and Blaser
K: Immune regulation in atopic dermatitis. Curr Opin Immunol.
12:641–646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanno S, Shouji A, Tomizawa A, Hiura T,
Osanai Y, Ujibe M, Obara Y, Nakahata N and Ishikawa M: Inhibitory
effect of naringin on lipopolysaccharide (LPS)-induced endotoxin
shock in mice and nitric oxide production in RAW 264.7 macrophages.
Life Sci. 78:673–681. 2006. View Article : Google Scholar
|
|
12
|
Fujihara M, Muroi M, Tanamoto K, Suzuki T,
Azuma H and Ikeda H: Molecular mechanisms of macrophage activation
and deactivation by lipopolysaccharide: roles of the receptor
complex. Pharmacol Ther. 100:171–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schaeffer HJ and Weber MJ:
Mitogen-activated protein kinases: specific messages from
ubiquitous messengers. Mol Cell Biol. 19:2435–2444. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Longpré F, Garneau P, Christen Y and
Ramassamy C: Protection by EGb 761 against β-amyloid-induced
neurotoxicity: involvement of NF-kappaB, SIRT1, and MAPKs pathways
and inhibition of amyloid fibril formation. Free Radic Biol Med.
41:1781–1794. 2006. View Article : Google Scholar
|
|
16
|
Reber L, Vermeulen L, Haegeman G and
Frossard N: Ser276 phosphorylation of NF-kB p65 by MSK1 controls
SCF expression in inflammation. PLoS One. 4:e43932009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hiscott J, Kwon H and Génin P: Hostile
takeovers: viral appropriation of the NF-kappaB pathway. J Clin
Invest. 107:143–151. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gloire G, Legrand-Poels S and Piette J:
NF-kappaB activation by reactive oxygen species: fifteen years
later. Biochem Pharmacol. 72:1493–1505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kleemann R, Verschuren L, Morrison M,
Zadelaar S, van Erk MJ, Wielinga PY and Kooistra T:
Anti-inflammatory, anti-proliferative and anti-atherosclerotic
effects of quercetin in human in vitro and in vivo models.
Atherosclerosis. 218:44–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung YC, Kim ME, Yoon JH, Park PR, Youn
HY, Lee HW and Lee JS: Anti-inflammatory effects of galangin on
lipo-polysaccharide-activated macrophages via ERK and NF-κB pathway
regulation. Immunopharmacol Immunotoxicol. 36:426–432. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YN, Zha WJ, Ma Y, Chen FF, Zhu W, Ge
A, Zeng XN and Huang M: Galangin attenuates airway remodelling by
inhibiting TGF-β1-mediated ROS generation and MAPK/Akt
phosphorylation in asthma. Sci Rep. 5:117582015. View Article : Google Scholar
|
|
22
|
Kang NJ, Han SC, Kang GJ, Koo DH, Koh YS,
Hyun JW, Lee NH, Ko MH, Kang HK and Yoo ES:
Diphlorethohydroxycarmalol inhibits interleukin-6 production by
regulating NF-κB, STAT5 and SOCS1 in lipopolysaccharide-stimulated
RAW264.7 cells. Mar Drugs. 13:2141–2157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
García-Lafuente A, Guillamón E, Villares
A, Rostagno MA and Martínez JA: Flavonoids as anti-inflammatory
agents: implications in cancer and cardiovascular disease. Inflamm
Res. 58:537–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karawajczyk A, Drgan V, Medic N, Oboh G,
Passamonti S and Novič M: Properties of flavonoids influencing the
binding to bilitranslocase investigated by neural network
modelling. Biochem Pharmacol. 73:308–320. 2007. View Article : Google Scholar
|
|
25
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alderton WK, Cooper CE and Knowles RG:
Nitric oxide synthases: structure, function and inhibition. Biochem
J. 357:593–615. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bogdan C: Nitric oxide and the immune
response. Nat Immunol. 2:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giuliano F and Warner TD: Origins of
prostaglandin E2: involvements of cyclooxygenase (COX)-1
and COX-2 in human and rat systems. J Pharmacol Exp Ther.
303:1001–1006. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JA, Song HY, Ju SM, Lee SJ, Kwon HJ,
Eum WS, Jang SH, Choi SY and Park JS: Differential regulation of
inducible nitric oxide synthase and cyclooxygenase-2 expression by
superoxide dismutase in lipopolysaccharide stimulated RAW 264.7
cells. Exp Mol Med. 41:629–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Möller B and Villiger PM: Inhibition of
IL-1, IL-6, and TNF-α in immune-mediated inflammatory diseases.
Springer Semin Immunopathol. 27:391–408. 2006. View Article : Google Scholar
|
|
31
|
Kwon HS, Park JH, Kim DH, Kim YH, Park
JHY, Shin HK and Kim JK: Licochalcone A isolated from licorice
suppresses lipopolysaccharide-stimulated inflammatory reactions in
RAW264.7 cells and endotoxin shock in mice. J Mol Med (Berl).
86:1287–1295. 2008. View Article : Google Scholar
|
|
32
|
Tak PP and Firestein GS: NF-kappaB: a key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi Y, Tu Z, Tang D, Zhang H, Liu M, Wang
K, Calderwood SK and Xiao X: The inhibition of LPS-induced
production of inflammatory cytokines by HSP70 involves inactivation
of the NF-kappaB pathway but not the MAPK pathways. Shock.
26:277–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oeckinghaus A, Hayden MS and Ghosh S:
Crosstalk in NF-κB signaling pathways. Nat Immunol. 12:695–708.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Monasterio A, Urdaci MC, Pinchuk IV,
López-Moratalla N and Martínez-Irujo JJ: Flavonoids induce
apoptosis in human leukemia U937 cells through caspase- and
caspase-calpain-dependent pathways. Nutr Cancer. 50:90–100. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Husain SR, Cillard J and Cillard P:
Hydroxyl radical scavenging activity of flavonoids. Phytochemistry.
26:2489–2491. 1987. View Article : Google Scholar
|
|
37
|
Heeba GH, Mahmoud ME and El Hanafy AA:
Anti-inflammatory potential of curcumin and quercetin in rats: role
of oxidative stress, heme oxygenase-1 and TNF-α. Toxicol Ind
Health. 30:551–560. 2014. View Article : Google Scholar
|
|
38
|
Campbell JK, King JL, Harmston M, Lila MA
and Erdman JW: Synergistic effects of flavonoids on cell
proliferation in Hepa-1c1c7 and LNCaP cancer cell lines. J Food
Sci. 71:S358–S363. 2006. View Article : Google Scholar
|
|
39
|
Harasstani OA, Moin S, Tham CL, Liew CY,
Ismail N, Rajajendram R, Harith HH, Zakaria ZA, Mohamad AS,
Sulaiman MR, et al: Flavonoid combinations cause synergistic
inhibition of proinflammatory mediator secretion from
lipopolysaccharide-induced RAW 264.7 cells. Inflamm Res.
59:711–721. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Turner PV, Brabb T, Pekow C and Vasbinder
MA: Administration of substances to laboratory animals: routes of
administration and factors to consider. J Am Assoc Lab Anim Sci.
50:600–613. 2011.
|
|
41
|
Leung DY: Atopic dermatitis: new insights
and opportunities for therapeutic intervention. J Allergy Clin
Immunol. 105:860–876. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arshad SH and Holgate S: The role of IgE
in allergen-induced inflammation and the potential for intervention
with a humanized monoclonal anti-IgE antibody. Clin Exp Allergy.
31:1344–1351. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Karki R, Jung MA, Kim KJ and Kim DW:
Inhibitory effect of Nelumbo nucifera (Gaertn.) on the development
of atopic dermatitis-like skin lesions in NC/Nga mice. Evid Based
Complement Alternat Med. 153568:2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012. View Article : Google Scholar : PubMed/NCBI
|