Introduction
Influenza, which is caused by influenza A virus
(IAV) infection, is one of the most common infectious diseases in
humans (1). The clinical symptoms
of influenza vary according to the virulence of the IAV strain and
the exposure history, age and immune status of the host. Seasonal
influenza induces serious illness in 3–5 million people each year,
with 2–500,000 deaths worldwide (2) and ~30,000 deaths in the United
States (3). In addition to annual
epidemics, IAV is also the cause of infrequent pandemics, which may
affect >50% of the population in a single year and often leads
to more severe disease than epidemic influenza. The 1918 pandemic
was accountable for the death of an estimate of 50–100 million
individuals (4,5). The 2009 H1N1 outbreak in Mexico and
the United States quickly spread to numerous countries in the
Americas, Europe and Asia, to become a new pandemic that seriously
threatened global public health (6).
Vaccination is the most effective method to protect
against IAV infection. Protection against influenza is primarily
mediated by neutralizing antibodies against the major viral antigen
hemagglutinin (HA) (7,8). However, seasonal as well as pandemic
influenza occur due to a lack of preformed immunity against HA, as
it is susceptible to mutation. Epidemic strains with new
epidemiological features will emerge along with the evolution of
HA, which results in new viruses and a lagging immune protection
(8).
The IAV vaccine provides protection to recipients
depending on whether the vaccine matches circulating IAV strains. A
mismatched IAV vaccine will fail to induce protective immune
responses. However, outside their protective or non-protective
effects, current IAV vaccines have side effects, which may include
shock, renal function damage and the neurologic disorder
Guillain-Barre syndrome (GBS) (9). The symptoms associated with IAV
infection and the side effects of IAV vaccination have been issues
for scientists, the vaccine industry and governments year after
year. The mechanisms of influenza vaccination-associated GBS and
numerous other serious side effects remain to be fully elucidated
and continue to pose risks. For instance, numerous efforts have
been made to determine the association between GBS and
anti-ganglioside antibodies (anti-GM-1), while the involved
mechanisms have remained elusive (10–16). The 1976 swine flu vaccines were
known for resulted in a higher incidence of GBS compared with
unvaccinated people (17,18). Preserved samples of monovalent and
bivalent 1976 vaccines were demonstrated to induce anti-GM-1
antibodies in mice, as did vaccines from 1991-1992 and 2004-2005
(10). These studies ruled out
the initial suspicion that the vaccines were contaminated by
moieties including Campylobacter jejuni antigens, which
mimic human gangliosides to elicit an anti-GM-1 antibody response
in susceptible recipients (10);
however, questions remain regarding the other vaccine
components.
Cross-protective vaccines (19–22), antibodies (23–26) and T cells (27–30) against IAV infections have been
well studied and reported. However, the cross-reactive immune
response associated with the pathology of influenza has remained to
be elucidated. By preparing monoclonal antibodies (McAbs) against
HA, the present study successfully obtained a series of McAbs with
a spectrum of cross-reactivities. In addition, two antigenic
epitopes on HA were identified that may induce antibodies with a
wide range of cross-reactivity, including that in nervous tissues,
hemoglobin (Hb) and numerous other crucial types of organs and
tissues. This implies that the symptoms of IAV infection and the
side effects of IAV vaccination may result from immunopathological
reactions induced by their HA cross-reactive epitopes.
Materials and methods
Ethical approval
This study was also performed in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The
protocol was approved by the Committee on the Ethics of Animal
Experiments of Shaanxi Provincial People's Hospital (Xi'an, China;
permit no. 01–0420). All surgeries on animals were performed under
sodium pentobarbital anesthesia and every effort was made to
minimize suffering.
McAb preparation
Immunogens
HA protein vaccine purified from 2009 H1N1 lysate is
a State Food and Drug Administration-approved influenza vaccine
(cat. no. S20090015; Hualan Vaccine Ltd., Xinxiang, China). The
seasonal influenza A1, A3 and B vaccines were obtained from Dalian
Yalifeng Biotechnology, Ltd. (Dalian, China). The 2009 H1N1 HA
epitope polypeptides were synthesized by Meilian Biotechnology,
Ltd. (Xi'an, China). Carboxy-terminal polyglutamic acid was added
to facilitate coating onto the ELISA plate and two amino acids
after each epitope were included to reduce the effect of negative
charges of polyglutamic acid. The following epitopes were used:
P11, WGIHH-PS-EEEE corresponding to the 2009 H1N1 HA amino acids
194–198; P14, WYGYHH-QN-EEEE corresponding to the 2009 H1N1 HA
amino acids 365–370; P11F, FGIHH-PS-EEEE, modified P11 by
substitution of phenylalanine with tryptophan to disrupt epitope
P11; P14R, WYGYRH-QN-EEEE, modified P14 by substitution of the
first histidine with arginine to disrupt epitope P14.
Antibodies
Five McAbs (H1-13, A1-6, H1-15, A1-10 and H1-17)
against the HA protein of H1N1 influenza virus were previously
prepared in our laboratory (31).
BALB/c mice
A total of 40 female BALB/c mice (age, 8 weeks;
weight, 18±20 g) were obtained from the Laboratory Animal Center of
the Fourth Military Medical University (Xi'an, China) and
maintained at 23°C, with a humidity of 40–70% in a 12 h light/dark
cycle. Mice were provided with free access to water and food.
Immunization protocol
For the H1N1 vaccine, priming was performed by
subcutaneous injection of 2 μg vaccine in 0.1 ml
phosphate-buffered saline (PBS). Boosting was performed four weeks
later by intraperitoneal injection of 2 μg vaccine in 0.1 ml
PBS and an intraperitoneal immunization with 2 μg vaccine in
0.1 ml PBS vaccine was performed three days prior to fusion (6
weeks following first boost). For epitope polypeptides, 2 μg
polypeptide was applied onto a nitrocellulose membrane, followed by
air drying in the hood and inguinal subcutaneous embedding for
priming. After four weeks, boosting was performed twice with 2
μg/0.1 ml peptide through intraperitoneal immunizations with
a biweekly interval. A pulse immunization was performed by
intraperitoneal injection of 2 μg/0.1 ml peptide three days
prior to fusion.
McAb preparation
The feeder layer cells, immune spleen cells and
myeloma Sp2/0 cells were routinely prepared, and the hybridoma were
prepared using polyethylene glycol-mediated chemical fusion
(31).
Biological characterization of the
McAbs
A McAb subtype identification kit from Southern
Biotech (Birmingham, AL, USA; cat. no. 5300-05) was used to
identify the antibody class and subclass according to the
manufacturer's instructions. The titration of the antibodies was
performed as follows: Serial dilutions of the McAb containing
ascetic fluid were prepared and the antibody reactivity was
detected with the indirect ELISA established in-house (32). The McAb titer was considered to be
the highest dilution determined to display binding with the cut-off
value of antibody optical density at 450 nm
(OD450)/control OD450≥2.1 (33). Similar to the indirect ELISA with
HA antigen, the specificities of the McAbs were determined with
HA-coated plates (National Vaccine and Serum Institute, Beijing,
China) and cross-reactivity was evaluated with heme-protoporphyrin,
chlorophyll and vitamin B12 and Hg-coated plates (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). The coating concentration for the
above antigens was 2.5 μg/well.
Western blot analysis
The specificity of the McAbs was analyzed using
western blot developed with diaminobenzidine (DAB) substrate. A
total of 20 μg of each HA sample (1.392 mg/ml obtained from
H1N1 vaccine lysate) and each human leukocyte antigen (HLA)-B27E
and each HLA-B27E protein with a His tag was mixed with an SDS
electrophoresis sample buffer, resolved by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto a nitrocellulose membrane (GE Healthcare Life
Sciences, Little Chalfont, UK). After blocking with 5% skimmed milk
for 30 min at room temperature, the membranes were treated with
H1N1 McAb supernatants of the cultivated hybridoma at 37°C for 1 h.
Membranes were probed with horseradish peroxidase (HRP)-linked
anti-mouse polyclonal secondary antibody (cat. no. CW102s; 1:2,000)
at 37°C for 1 h, and bands were visualized via chemiluminescence
with a DAB substrate (both from CW Biotech, Beijing, China).
Immunohistochemical staining of the
tissue microarray (TMA)
A normal human TMA was purchased from Shaanxi
Chaoying Biotech, Ltd. (Xi'an, China; parent company: Cybrdi,
Frederick, MD, USA), which included 33 human tissue specimens in
duplicates for a total of 66 specimens. The slide was previously
treated with aminopropyltriethoxysilane. Immunohistochemical
staining was performed as previously described (34). In brief, paraffin sections of
tissues were deparaffinized, hydrated, endogenous peroxidase was
blocked with 3% H2O2 at room temperature for
20 min, followed by blocking of unspecific binding with buffer
containing goat serum (cat. no. CW2134; CW Biotech) for 30 min. The
samples were stained with primary antibody (McAb supernatants of
cultured hybridoma against HA) at 4°C overnight, stored at room
temperature for 60 min, washed 3 times with PBS, and incubated with
1:500 HRP-labeled sheep anti-mouse secondary antibody (cat. no.
CW0102S; CW Biotech) at 37°C for 40 min. After 3 washes with PBS,
DAB and hematoxylin at room temperature for 3 min were added for
color development and color enhancement, respectively, according to
the manufacturer's instructions.
Immunogold electron microscopic
analysis of normal rat brain tissue
Pre-embedding immunogold-silver cytochemistry was
performed as previously described (35). In brief, 2 healthy female Sprague
Dawley rats (180-220 g) were purchased from the laboratory animal
center of Fourth Military Medical University and kept under
specific pathogen conditions at 23°C with a humidity of 40–70% and
a 12 h light/dark cycle. Rats were provided with free access to
food and water. Rats were intraperitoneally anesthetized with 100
mg/kg sodium pentobarbital and perfused with 4% paraformaldehyde.
The skull was rapidly opened, and the cerebral cortex tissue was
taken and immersed in 3% glutaraldehyde fixative solution for 3 h.
The snap-frozen tissue was sectioned into 60–70 μm slices
using a vibratome (VT1000S; Leica Microsystems, Wetzlar, Germany).
The slices were transferred to PBS containing 25% sucrose and 10%
glycerol and were soaked for 1 h. After a repeated freezing and
thawing process, the tissue slices were placed in a blocking
solution containing 5% bovine serum albumin (Takara Biotechnology
Co., Ltd., Dalian, China) at 37°C for 4 h. The primary antibody
(A1-10 or H1-13; 1:100) was added, and the tissue slices were
incubated overnight at 4°C; after washing the tissue slices with
PBS, the secondary antibody biotinylated goat anti-mouse
immunoglobulin (Ig) G containing gold particles (cat. no.
2002-06D232; 1:200; Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA) was added, and the slices were incubated overnight at 4°C.
After washing, the sections were fixed with PBS fixation buffer
containing 0.5% osmium tetroxide for 1 h and dehydration was
performed with a gradient series of ethanol. After being immersed
in propylene oxide, the slices were embedded in Epon-812 resin.
After 50 nm ultrathin sectioning, the slices were counterstained
with uranyl acetate and lead citrate. The sections were observed
under a transmission electron microscope (JEOL Ltd., Tokyo,
Japan).
Immunoblocking ELISA
After identifying the three cross-reactive McAbs
A1-10, H1-13 and H1-15, their specificity was further determined
with an in-house immunoblocking ELISA. The 96-well plates were
coated with H1N1 influenza vaccine (2.5 μg/ml). After
washing three times with Tris-buffered saline (TBS), antibodies
diluted at 1:100 and pre-reacted with (200 μg/ml) heme, Hg,
polypeptide P11 or polypeptide P14 for 1 h at 37°C were added (100
μl/well), followed by incubation for l h at 37°C. After
washing three times with TBS, HRP-labeled sheep anti-mouse
secondary antibody (100 μl/well; cat. no. CW0102S; 1:1,000;
CW Biotech) was added, and then the plates were incubated for 1 h
at 37°C. After another three washes, O-phenylenediamine
dihydrochloride (0.4 mg/ml) in 50 mM sodium citrate (pH 5.5)
containing 0.03% H2O2 was added to each well,
followed by incubation at 37°C for 30 min. A 0.9 M
H2SO4 solution was added to terminate the
reaction; the OD450 nm was then detected using an ELISA
reader.
The relative inhibitor index (RI) was calculated as
follows: RI=(OD450 McAb-OD450 antigen
pre-reacted McAb)/OD450 McAb. RI≤0.4 indicates that the
antibody does not react with the blocking antigen; RI 0.4–0.8
indicates that the antibody is reactive with the blocking antigen;
RI≥0.8 indicates that the antibody consistently reacted with the
blocking antigen.
Statistical analysis
All values are expressed as the mean ± standard
deviation. The SPSS 10.0 statistical package (SPSS Inc., Chicago,
IL, USA) was used for all statistical analyses. Comparisons of
group means were performed by analysis of variance with
Newman-Keuls post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
McAbs produced by immunization of mice
with IAV HA antigen
Sixty-seven hybridoma cell lines reactive to McAb
against IAV HA antigen were obtained from 3 experiments; among
these, 47 produced IgG class and 20 produced IgM class antibodies;
25 were H1 subtype-specific, and the remaining ones cross-reacted
with the other HA subtypes, including 3 (H1-13, H1-15 and A1-10)
that reacted not only with IAV HA but also with a wide variety of
antigens as previously reported (31,32). The present study focused on these
3 cross-reactive McAbs.
Characteristics of the cross-reactive
McAbs
The titers of the McAbs against IAV HA vary in a
considerable range (as measured by indirect ELISA) from
10−2 to 10−7 (Table I). While a limited number of
experiments was performed in the present study, McAbs with a higher
titer usually have a higher specificity and that McAbs with a lower
titer usually have a lower specificity; however, this is not a
universal rule. A group of 3 lower-titer McAbs (H1-13, H1-15 and
A1-10) reacted not only with IAV HA but also with recombinant
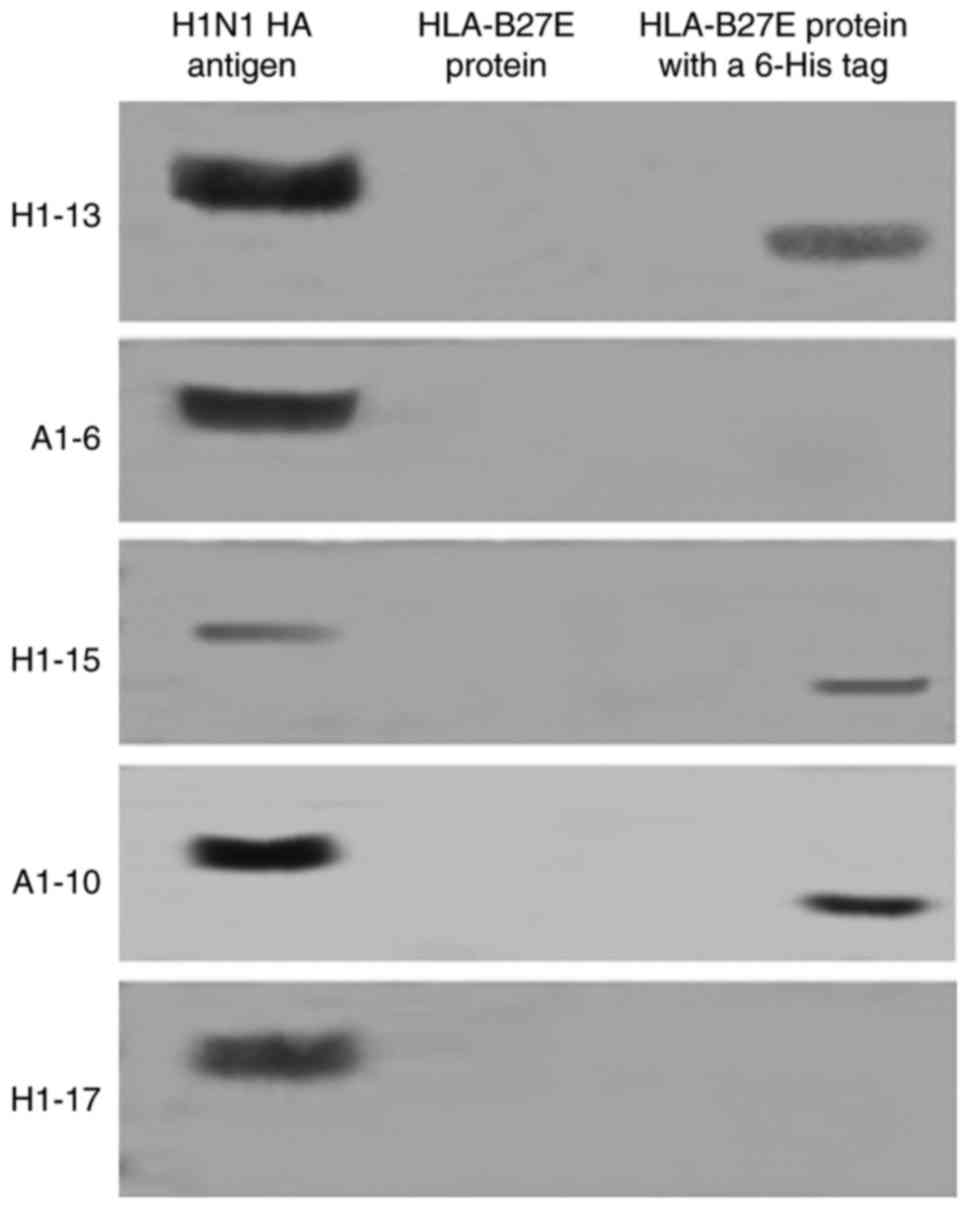
proteins with His-tag that were not associated with HA (Table I and Fig. 1). Two IVA H1 subtype-specific
McAbs (A1-6 and H1-17) were included in this study as controls. The
western blot analysis included the following recombinant proteins
with a His-tag: HLA-B27E. The recombinant proteins without His-tag
testing included HLA-B27E. The 3 McAbs with the lower ELISA titers
reacted with proteins with a His-tag but not with the recombinant
proteins without a His-tag, with the exception of IAV HA. Fig. 1 displays the results of only
HLA-B27E with and without His-tag as the representation.
 | Table ICharacterization of the
cross-reactivity of McAbs. |
Table I
Characterization of the
cross-reactivity of McAbs.
| McAb | Ig subtype | Ascitic fluid | HA | Hg | Heme | PP | Cp | VitB12 |
|---|
| H1-13 | IgG |
10−2 | 14.10±0.26 | 2.90±0.26a | 9.43±1.03a | 2.53±0.06a | 2.17±0.06a | 1.93±0.15a |
| H1-15 | IgM |
10−2 | 3.00±0.26b | 6.80±0.35b | 9.27±0.31b | 3.47±0.64 | 2.23±0.23b | 2.90±0.10b |
| A1-10 | IgM |
10−3 | 14.33±0.78 | 4.97±0.51b | 12.20±0.10b | 2.43±0.15b | 3.77±0.06b | 2.43±0.58b |
| A1-6 | IgG |
10−7 | 12.23±1.36 | 1.17±0.21 | 1.13±0.15 | 1.43±0.21 | 1.07±0.23 | 0.93±0.15 |
| H-17 | IgM |
10−6 | 13.37±1.21 | 1.00±0.10 | 1.03±0.06 | 1.13±0.06 | 1.07±0.12 | 1.50±0.17 |
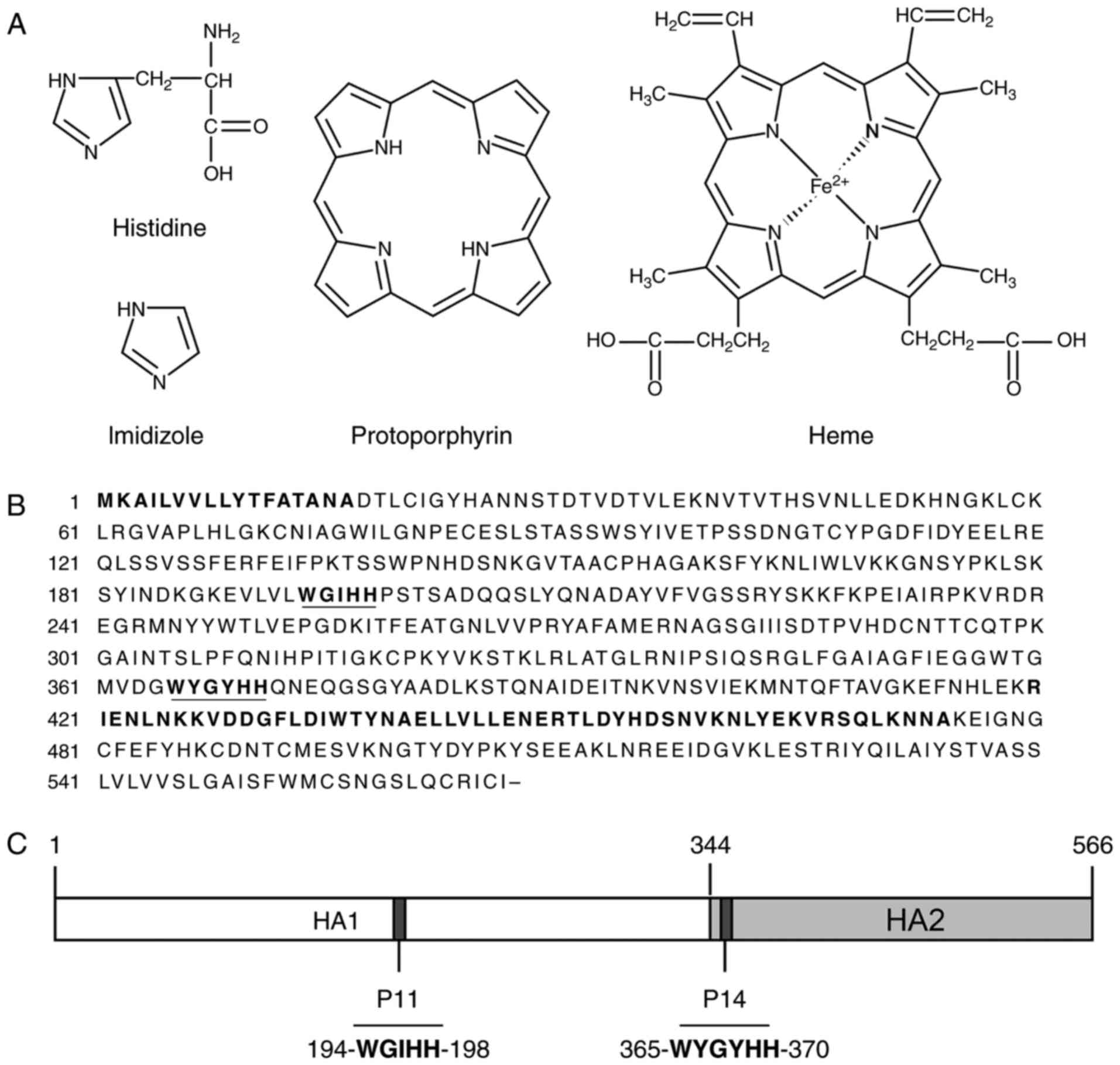
The imidazole ring is an important structural
feature of histidine and a the His-tag is composed of a chain of
histidines, rendering it structurally similar to the porphyrin ring
containing four imidazole moieties. Along this line of reasoning,
the cross-reactive McAbs were assessed using indirect ELISA with
porphyrin ring-containing molecules (including Hg, protoporphyrin,
chlorophyll and vitamin B12) in parallel with IAV HA-coated
microwell plates with positive results from all. This experiment
expanded the knowledge on the cross-reactivity of the McAbs from
the His-tag to a spectrum of important biomolecules (Table I).
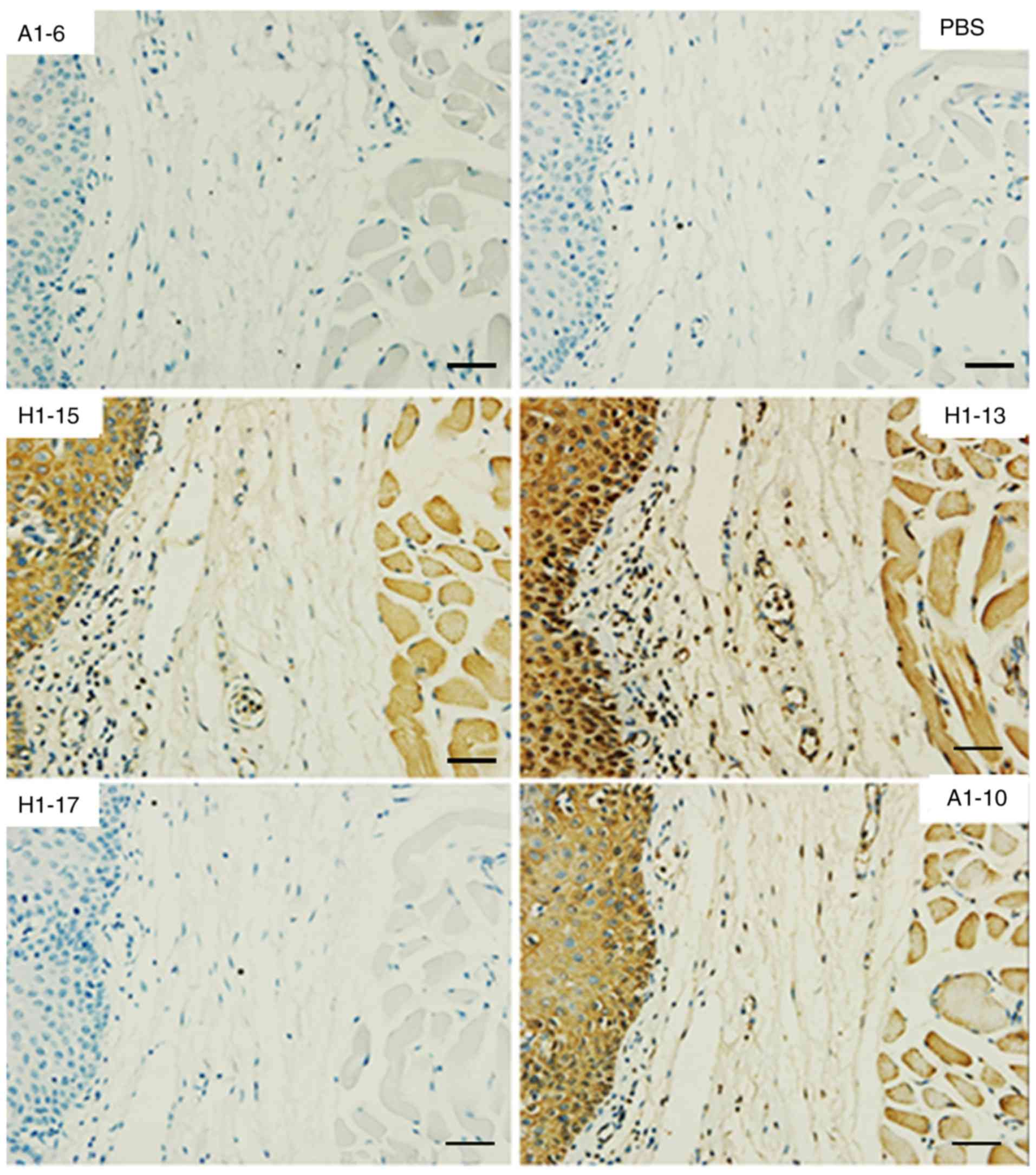
Reactivity of the anti-HA McAbs to human
normal tissues
To accurately determine the reactivity of the
cross-reactive anti-HA McAbs with normal human tissues, they were
tested using a normal human TMA. The results are summarized in
Table II. Fig. 2 displays the reactivity to normal
human brain tissue as an example. All three cross-reactive anti-HA
McAbs consistently reacted with red blood cells, neuronal cells and
certain neurogliocytes. No significant binding was observed with
the other organs assessed. The HA-specific McAbs A1-6 (IgG) and
H1-17 (IgM) were used as an isotype control; they were not reactive
to the TMA.
 | Table IIReactivity of the monoclonal
antibodies to a normal human tissue array. |
Table II
Reactivity of the monoclonal
antibodies to a normal human tissue array.
| Organs and
tissues | A1-6 | H1-17 | A1-10 | H1-15 | H1-13 |
|---|
| Brain | − | − | +/− | +/− | + Neuron cells |
| Cerebellum
tissue | − | − | − | − | +/− |
| Peripheral nerve
tissue | − | − | − | − | +/− |
| Adrenal cortical
tissue | − | − | + | + | − |
| Thyroid tissue | − | − | − | − | − |
| Spleen | − | − | − | − | − |
| Thymic tissue | − | − | − | − | − |
| Myeloid tissue | +/− | − | ++ | ++ | +/− |
| Lymph node | − | − | − | − | − |
| Tonsil | − | − | − | ++ Squamous
epithelium | − |
| Liver | − | − | +/− | ++ Cytoplasm | +/− |
| Esophageal
tissue | − | − | +/− Squamous
epithelium | ++ Squamous cell
cytoplasm | − |
| Gastric tissue | − | − | +/− | +/− | − |
| Small
intestine | − | − | +/− | + Gland
cytoplasm | − |
| Smooth muscle | − | − | +/− | + Gland
cytoplasm | − |
| Lung tissue | − | − | +/− | − | − |
| Normal salivary
gland | − | − | + Glandular
tube | + Glandular
tube | − |
| Nasopharyngeal
epithelial tissue | − | − | ++ Squamous
epithelium and muscle tissues | +++ Squamous
epithelium and muscle tissues | ++ Squamous
epithelium and muscle tissues |
| Kidney tissue | − | − | ++ Kidney
tubules | ++ Proximal
convoluted tubule | +/− Proximal
convoluted tubule |
| Bladder tissue | − | − | +/− | + Glands | + Transitional
epithelial cells |
| Testis | − | − | + Interstitial
cells | ++ Interstitial
cells | − |
| Prostate
glands | − | − | − | +/− | + Gland cell
nuclei |
| Penis tissue | − | − | − | − | − |
| Ovarian tissue | − | − | − | +/− | + |
| Fibrovascular
tissue | − | − | +/− | + | − |
| Mammary tissue | − | − | − | − | − |
| Endometrial
tissue | − | − | − | + | + |
| Cervical
tissue | − | − | +/− Gland
cytoplasm | − | + |
| Cardiac muscular
tissue | − | − | ++ | +++ | + |
| Skeletal muscle
tissue | − | − | ++ | + | + |
| Diaphragmatic
muscle mesothelial tissue | − | +/− | ++ | ++ | + |
| Skin | − | − | +/− | − | − |
Anti-HA McAb binds to normal rat brain
tissue
In the immune colloidal gold-silver electron
microscopy study, normal rat brain tissue was stained with the
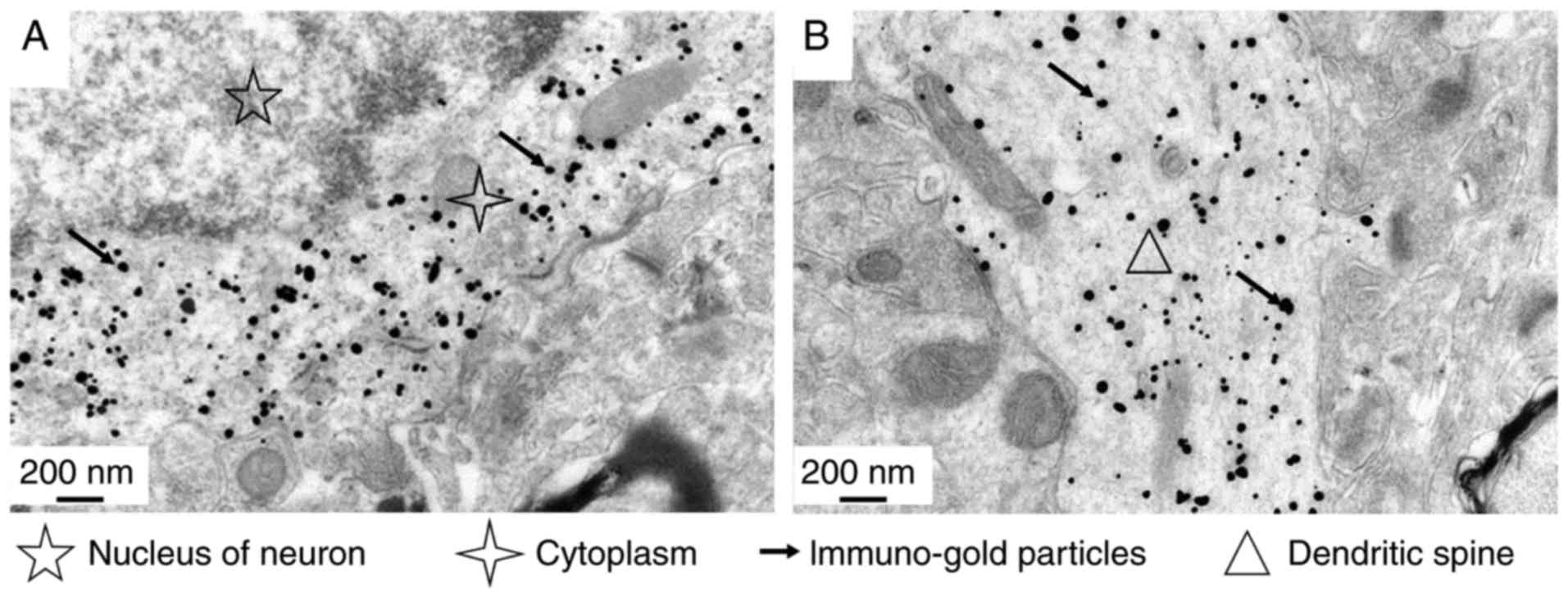
cross-reactive McAb A1-10. The results indicated that the antibody
specifically binds to neuronal cells of rat brain cortex tissue
(Fig. 3). The binding sites were
identified to be located in the neuronal cell cytoplasm (Fig. 3A) and dendrites (Fig. 3B). However, there was no
indication of binding to any specific organelles, and there was no
specific reaction with the neuronal nucleus or surrounding
cells.
Prediction and characterization of the
cross-reactive epitopes on IAV HA
Two imidazole-rich clusters were identified by
visually scanning the HA amino acid sequence (Fig. 4B). They are the P11
(194-WGIHH-198) and P14 (365-WYGHH-370) epitopes (Fig. 4C). To prove the importance of the
imidazole ring cluster for these two cross-reactive epitopes,
peptide P11F (FGIHH) were designed by substituting one
imidazole-containing amino acid (tryptophan) with another aromatic
amino acid (phenylalanine). In the same manner, peptide P14R
(WYGYRH) was designed by substituting the first histidine with
another basic amino acid (arginine).
To confirm the selected HA cross-reactive epitopes,
two McAbs were prepared from hybridoma made by the fusion of Sp2/0
cells with spleen cells of mice immunized with polypeptide P11 and
P14, respectively. These are P11-A1 and P11-A2, as well as P14-A1
and P14-A2; all of which are IgM class. All reacted with the two
native epitopes but not with the modified peptides P11F or P14R in
the indirect ELISA. This indicated that a minimum number of
imidazole rings is required and that there were cross-reactions
between the two epitopes. Of note, all of the epitopes reacted with
HA and heme more efficiently than with their own peptides (Table III). This also indicates that
the number of imidazole rings in the antigen made a difference; the
more imidazol rings in the molecule, the stronger its reaction. In
addition, the McAb Hb-A18 generated by immunizing mice with Hb
reacted with heme and the unassociated HA in the indirect ELISA;
this further suggests the possibility of sharing an epitope between
the imidazole ring-rich molecules including Hg and HA (Table III). The HA-specific McAb H1-17
was included in the ELISA as a negative control and system
control.
 | Table IIIEpitope specificity of the McAbs. |
Table III
Epitope specificity of the McAbs.
| McAbs | Immunization
antigens | Detection antigens
|
|---|
| HA | Heme | Peptide P11 | Peptide P14 | Peptide P11F | Peptide P14R |
|---|
| P11-A1 | 11P | 4.03±0.67 | 6.93±0.06 | 2.73±0.65 | 2.97±0.25 | 1.17±0.21b | 1.20±0.10c |
| P11-A2 | 11P | 5.00±0.98 | 4.50±0.44 | 3.36±0.32 | 2.50±0.35 | 1.23±0.06b | 1.00±0.27c |
| P14-A1 | 14P | 4.70±0.62 | 6.83±0.51 | 3.43±0.76 | 3.27±0.12 | 1.50±0.17b | 1.17±0.23c |
| P14-A2 | 14P | 5.63±0.15 | 9.67±0.42a | 2.57±0.57 | 4.03±0.55 | 1.20±0.35b | 1.37±0.21c |
| Hb-A18 | Hb | 7.23±0.55 | 15.63±1.65a | ND | ND | ND | ND |
| H1-17 | HA | 24.25±1.06 | 1.03±0.06a | 1.07±0.12 | 1.03±0.15 | 1.30±0.10 | 1.00±0.17c |
Further confirmation of the broad
cross-reactive epitopes of HA
P11 and P14 are liner epitopes. In the above
experiments, they reacted with HA and other antigens coated on the
plate or attached to the NC membrane after SDS-PAGE, which means
that those antigens may already be denatured. To confirm these
epitopes, they were first reacted with a series of soluble native
antigens to block the McAbs from binding to HA coated on the
microwell plate of the indirect ELISA in parallel with the McAbs
without pre-reaction. As presented in Fig. 5, protoporphyrin, heme, P11 and P14
efficiently blocked all 3 cross-reactive McAbs from binding to HA
on the ELISA plate. However, neither the modified polypeptide P11F
nor P14R were able to block any of the McAbs. The HA-specific McAbs
A1-16 and H1-17 were included as the negative control and system
control. This result confirmed that all 3 cross-reactive McAbs
react with either of the two epitopes, which also indicated that
the 2 cross-reactive HA epitopes P11 and P14 are cross-reactive
with each other; all 3 cross-reactive McAbs reacted with
protoporphyrin, heme, P11 and P14, not only on the nitrocellulose
membrane, but also in solution.
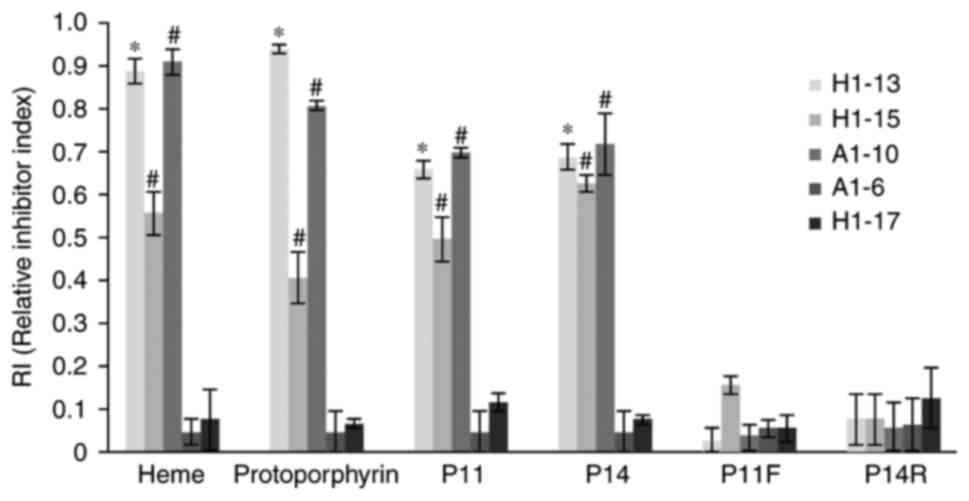 | Figure 5Cross-reactivity inhibitory assay of
the anti-HA McAbs. The antigens (including heme, protoporphyrin,
and P11, P14, P11F and P14R polypeptides; displayed on the x-axis)
were pre-reacted with individual McAb prior to addition to the
HA-coated microtiter plate. The y-axis displays the RI value
calculated as RI=(OD450 of McAb-OD450 McAb
pre-reacted with antigen)/OD450 of McAb. RI≤0.4
indicates that the antibody does not react with the blocking
antigen; RI 0.4–0.8 indicates that the antibody is reactive with
the blocking antigen; RI≥0.8 indicates that the antibody
consistently reacted with the blocking antigen.
*P<0.05, RI of H1-13 antibody in each group compared
with that of A1-6 antibody (all are IgG subtypes).
#P<0.05, RI of A1-10 and H1-15 antibody in each group
are compared with that of H1-17 antibody (all are IgM subtypes).
P11F, imidazole-rich P11 epitope from HA (FGIHH) with substitution
of one imidazole-containing amino acid (tryptophan) with
phenylalanine; P14R, imidazole-rich P14 epitope from HA (WYGYRH)
with substitution of the first histidine with arginine; RI,
relative inhibitor index; Ig, immunoglobulin; McAbs, monoclonal
antibodies; HA, hemagglutinin; OD450, optical density at
450 nm. |
Discussion
HA is an important glycoprotein of IAV and a major
antigenic component of the IAV vaccine that stimulates the body to
produce protective antibodies. IAV vaccination may produce side
effects or even lead to death. Identification of impurities in the
vaccine has been performed to assess the cause of these serious
side effects (10); however, few
efforts were made to investigate the antigen itself. In the present
study, 67 McAbs were prepared with the 2009 H1N1 HA as the antigen;
among these, H1-13, H1-15 and A1-10 exhibited broad
cross-activities. Antibodies against these HA epitopes react with
imidazol ring-containing molecules, including His-tag and the
porphyrin derivatives Hb, protoporphyrin, chlorophyll and vitamin
B12. In addition, the porphyrin ring is the basic structure for Hg
in erythrocytes, myoglobin in muscle cells and the cytochrome
c respiratory chain in the mitochondrial membrane; thus, the
present study speculated and confirmed that these cross-reactive
antibodies may react with various cells in the body, including
heme-rich red blood cells, myoglobin-rich muscle cells and
metabolically active tissues including bone marrow, the liver and
certain glandular tissues. In addition, the McAb A1-10 was
demonstrated to react with brain cell cytoplasm and dendrites.
The HA protein shares a similar structure with
imidazol ring-rich molecules, including His-tag, which reacted with
all 3 cross-reactive McAbs. The antibody binds to the porphyrin
ring-like structure, and the N atom in the imidazolyl group of
histidine and the indolyl group of tryptophan form a coordination
bond through binding with a transition metal ion to form a
porphyrin ring-like structure; thus, it was assumed that a
cross-reactive epitope on HA should contain a high abundance of
histidine or tryptophan. By analyzing the amino acid sequence of
the 2009 H1N1 HA protein, the amino acid sequences 194-WGIHH-198
and 365-WYGYHH-370 were identified to have the characteristics
required to form a porphyrin ring-like structure. The present
results indicated that substitution of one of these amino acids
abrogated the cross-reactivity of the McAbs, probably by disrupting
the porphyrin ring-like structure. These 2 cross-reactive epitopes
have been recognized as novel HA epitopes (36).
In addition, the antibodies of the HA cross-reactive
epitopes induced were identified to have similar reactivity to
those of cross-reactive antibodies generated through HA
immunization; more interestingly, these antibodies have a higher
reactivity with HA and heme than with the epitopes themselves, as
HA and heme have more antigenic determinants.
Since the identification of broadly cross-reactive
epitopes on HA, their clinical importance has been studied,
including their roles in influenza symptoms and side effects of IAV
vaccines. Only few studies on the anti-HA antibody-associated
pathological effects of the antibody-dependent enhancement of
infection, and its possible role in the pathogenesis of influenza
have been reported (37);
however, other speculations have been made regarding the
associations between antibody and flu pathology. Lung samples of 75
young and middle-aged patients from the 2009 IAV pandemic
demonstrated abnormally elevated C4d levels. C4d usually binds with
an immune complex; this suggested that severe influenza may result
from pathological damage caused by immune complexes (38).
Cross-reactivity of HA-induced antibodies with heme
may result in serious problems, as hemeproteins have diverse
biological functions, including oxygen transport (Hg, myoglobin,
neuroglobin, cytoglobin, legHg), catalysis (cytochrome P450s,
cytochrome c oxidase, ligninases, peroxidases), electron
transfer/transport (cytochrome a-c), sensory
disambiguation (oxygen sensor FixL, soluble guanylyl cyclase, CO
sensor CooA) and defense (catalase). The HA cross-reactive
epitope-induced antibodies strongly bind with numerous important
tissue types and organs; thus, HA-associated auto-immunopathology
should be addressed.
Any hemeprotein malfunction may cause specific
diseases. In addition, HA cross-reactive epitope-induced
antibodies, which bind with hemoproteins, may induce malfunction.
For instance, studies regarding Hb release in IAV infection are
lacking; however, this is likely to occur, as HA interacts with
erythrocytes, and the release of Hb occurs in certain infectious
and autoimmune diseases (39). Hb
also occurs outside of red blood cells and their progenitor lines.
Other cell types that contain Hb include mesangial cells in the
kidney, macrophages, alveolar cells and the A9 dopaminergic neurons
in the substantia nigra. In these tissues, Hg has a
non-oxygen-carrying function as a regulator of iron metabolism and
an antioxidant (40). The
autoantibody to human Hb was identified in the sera of patients
with malaria, leishmania and systemic lupus erythematosus. Serum Hb
antibody levels in lupus-prone mice also exhibited an age-dependent
increase, with progressive organ sequestration. A suggestive link
between anti-Hb and anti-Smith antibody responses was also observed
(39). Myoglobin was identified
in vertebrate muscle cells, including myocardial cells. Myocarditis
is a common and severe complication of influenza (41); however, the underlying mechanism
have remained elusive.
In conclusion, the present study identified 2 broad
cross-reactive epitopes on HA, P11 (194-WGIHH-198) and P14
(365-WYGYHH-370), and demonstrated that antibodies against these
epitopes react with Hb and numerous important normal tissue/organ
types. Further study into whether such antibodies are involved in
reducing anti-IAV immunity (42,43) and immunopathological damage, as
well as whether cross-reactive epitopes are associated with severe
symptoms of influenza infection and serious adverse reactions to
influenza vaccination may provide critical insight for influenza
research and management.
Acknowledgments
The authors would like to thank Dr Mingjie Zhang
from the Laboratory of Molecular Virology of the Center for
Biologics Evaluation and Research (Food and Drug Administration,
Silver Spring, MD, USA) for his assistance with the design and
editing of the manuscript. The present study was supported by the
National Key Research and Development Program of China (grant no.
2016YFD0500701-5), the Key Research and Development Plan of Shaanxi
Province (grant no. 2017SF-091), The Health and family planning
commission research fund project of Shaanxi Province (grant no.
2016D035) and the Natural Science Foundation of China (grant no.
81202373).
References
|
1
|
Cox NJ and Subbarao K: Influenza. Lancet.
354:1277–1282. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen GL and Subbarao K: Live attenuated
vaccines for pandemic influenza. Curr Top Microbiol Immunol.
333:109–132. 2009.PubMed/NCBI
|
|
3
|
Thompson WW, Shay DK, Weintraub E, Brammer
L, Cox N, Anderson LJ and Fukuda K: Mortality associated with
influenza and respiratory syncytial virus in the United States.
Jama. 289:179–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson NP and Mueller J: Updating the
accounts: Global mortality of the 1918–1920 'Spanish' influenza
pandemic. Bull Hist Med. 76:105–115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tumpey TM, Basler CF, Aguilar PV, Zeng H,
Solórzano A, Swayne DE, Cox NJ, Katz JM, Taubenberger JK, Palese P,
et al: Characterization of the reconstructed 1918 Spanish influenza
pandemic virus. Science. 310:77–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santos-Preciado J, Franco-Paredes C,
Hernandez-Flores I, Tellez I, Del Rio C and Tapia-Conyer R: What
have we learned from the novel influenza A (H1N1) pandemic in 2009
for strengthening pandemic influenza preparedness? Arch Med Res.
40:673–676. 2009. View Article : Google Scholar
|
|
7
|
Wiley DC and Skehel JJ: The structure and
function of the hemagglutinin membrane glycoprotein of influenza
virus. Annu Rev Biochem. 56:365–394. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Drescher J and Aron R: Influence of the
amino acid differences between the hemagglutinin HA1 domains of
influenza virus H1N1 strains on their reaction with antibody. J Med
Virol. 57:397–404. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Evans D, Cauchemez S and Hayden FG:
'Prepandemic' immunization for novel influenza viruses, 'swine flu'
vaccine, Guillain-Barré syndrome, and the detection of rare severe
adversee vents. J Infect Dis. 200:321–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nachamkin I, Shadomy SV, Moran AP, Cox N,
Fitzgerald C, Ung H, Corcoran AT, Iskander JK, Schonberger LB and
Chen RT: Anti-ganglioside antibody induction by swine
(A/NJ/1976/H1N1) and other influenza vaccines: Insights into
vaccine-associated Guillain-Barré syndrome. J Infect Dis.
198:226–233. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Israeli E, Agmon-Levin N, Blank M, Chapman
J and Shoenfeld Y: Guillain-Barré syndrome-a classical autoimmune
disease triggered by infection or vaccination. Clin Rev Allergy
Immunol. 42:121–130. 2012. View Article : Google Scholar
|
|
12
|
Simpson BS and Rajabally YA: Sensori-motor
Guillain-Barré syndrome with anti-GD1b antibodies following
influenza A infection. Eur J Neurol. 16:e812009. View Article : Google Scholar
|
|
13
|
Nakamura N, Nokura K, Zettsu T, Koga H,
Tachi M, Terada M, Katoh H, Itoh Y, Osawa H, Ozeki T and Yamamoto
H: Neurologic complications associated with influenza vaccination:
Two adult cases. Intern Med. 42:191–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang DJ, Boltz DA, McElhaney J, McCullers
JA, Webby RJ and Webster RG: No evidence of a link between
influenza vaccines and Guillain-Barre syndrome-associated
antiganglioside antibodies. Influenza Other Respir Viruses.
6:159–166. 2012. View Article : Google Scholar
|
|
15
|
Lei T, Siu KL, Kok KH, Chan KH, Chan EY,
Hung IF, To KK, Li PC, Zhou J, Zheng BJ, et al: Anti-ganglioside
antibodies were not detected in human subjects infected with or
vaccinated against 2009 pandemic influenza A (H1N1) virus. Vaccine.
30:2605–2610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuki N, Takahashi Y, Ihara T, Ito S,
Nakajima T, Funakoshi K, Furukawa K, Kobayashi K and Odaka M: Lack
of antibody response to Guillain-Barré syndrome-related
gangliosides in mice and men after novel flu vaccination. J Neurol
Neurosurg Psychiatry. 83:116–117. 2012. View Article : Google Scholar
|
|
17
|
Sencer DJ and Millar JD: Reflections on
the 1976 swine flu vaccination program. Emerg Infect Dis. 12:29–33.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Souayah N, Nasar A, Suri MF and Qureshi
AI: Guillain-Barre syndrome after vaccination in United States a
report from the CDC/FDA vaccine adverse event reporting system.
Vaccine. 25:5253–5255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manicassamy B, Medina RA, Hai R, Tsibane
T, Stertz S, Nistal-Villán E, Palese P, Basler CF and García-Sastre
A: Protection of mice against lethal challenge with 2009 H1N1
influenza A virus by 1918-like and classical swine H1N1 based
vaccines. PLoS Pathog. 6:e10007452010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanson BJ, Boon AC, Lim AP, Webb A, Ooi EE
and Webby RJ: Passive immunoprophylaxis and therapy with humanized
monoclonal antibody specific for influenza A H5 hemagglutinin in
mice. Respir Res. 7:1262006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lipatov AS, Gitelman AK and Smirnov YuA:
Prevention and treatment of lethal influenza A virus
bronchopneumonia in mice by monoclonal antibody against
haemagglutinin stem region. Acta Virol. 41:337–340. 1997.
|
|
22
|
Stephenson I, Bugarini R, Nicholson KG,
Podda A, Wood JM, Zambon MC and Katz JM: Cross-reactivity to highly
pathogenic avian influenza H5N1 viruses after vaccination with
nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97
(H5N3) vaccine: A potential priming strategy. J Infect Dis.
191:1210–1215. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hancock K, Veguilla V, Lu X, Zhong W,
Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, et al:
Cross-reactive antibody responses to the 2009 pandemic H1N1
influenza virus. N Engl J Med. 361:1945–1952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krause JC, Tsibane T, Tumpey TM, Huffman
CJ, Basler CF and Crowe JE Jr: A broadly neutralizing human
monoclonal antibody that recognizes a conserved, novel epitope on
the globular head of the influenza H1N1 virus hemagglutinin. J
Virol. 85:10905–10908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simmons CP, Bernasconi NL, Suguitan AL,
Mills K, Ward JM, Chau NV, Hien TT, Sallusto F, Ha do Q, Farrar J,
et al: Prophylactic and therapeutic efficacy of human monoclonal
antibodies against H5N1 influenza. PLoS Med. 4:e1782007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan GS, Krammer F, Eggink D, Kongchanagul
A, Moran TM and Palese P: A pan-H1 anti-hemagglutinin monoclonal
antibody with potent broad-spectrum efficacy in vivo. J Virol.
86:6179–6188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alam S and Sant AJ: Infection with
seasonal influenza virus elicits CD4 T cells specific for
genetically conserved epitopes that can be rapidly mobilized for
protective immunity to pandemic H1N1 influenza virus. J Virol.
85:13310–13321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clute SC, Watkin LB, Cornberg M, Naumov
YN, Sullivan JL, Luzuriaga K, Welsh RM and Selin LK: Cross-reactive
influenza virus-specific CD8+ T cells contribute to
lymphoproliferation in Epstein-Barr virus-associated infectious
mononucleosis. J Clin Invest. 115:3602–3612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weinfurter JT, Brunner K, Capuano SV III,
Li C, Broman KW, Kawaoka Y and Friedrich TC: Cross-reactive T cells
are involved in rapid clearance of 2009 pandemic H1N1 influenza
virus in nonhuman primates. PLoS Pathog. 7:e10023812011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu CI, Gallegos M, Marches F, Zurawski G,
Ramilo O, Garcia-Sastre A, Banchereau J and Palucka AK: Broad
influenza-specific CD8+ T-cell responses in humanized
mice vaccinated with influenza virus vaccines. Blood.
112:3671–3678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo C, Xie X, Li H, Zhao P, Zhao X, Sun J,
Wang H, Liu Y and Li Y, Hu Q, Hu J and Li Y: Prediction of common
epitopes on hemagglutinin of the influenza A virus (H1 subtype).
Exp Mol Pathol. 98:79–84. 2015. View Article : Google Scholar
|
|
32
|
Guo CY, Li HJ, Liu Y, Zhao XR, Wang X,
Feng Q, Li Y and Hu J: Preparation and partial characterization of
monoclonal antibodies against HA protein of H1 subtype influenza
virus. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 28:177–180. 2012.In
Chinese. PubMed/NCBI
|
|
33
|
Zhang MJ, Wang MX, Jiang SZ, Xiu ZZ and Ma
WY: Preparation and characterization of the monoclonal antibodies
against Japanese encephalitis virus. Acta Virol. 36:533–540.
1992.PubMed/NCBI
|
|
34
|
Thomson TA, Zhou C, Chu C and Knight B:
Tissue microarray for routine analysis of breast biomarkers in the
clinical laboratory. Am J Clin Pathol. 132:899–905. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yi H, Leunissen J, Shi G, Gutekunst C and
Hersch S: A novel procedure for pre-embedding double
immunogold-silver labeling at the ultrastructural level. J
Histochem Cytochem. 49:279–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu W: Highly conserved domains in
hemagglutinin of influenza viruses characterizing dual receptor
binding. Natural Science. 2:1005–1014. 2010. View Article : Google Scholar
|
|
37
|
Dutry I, Yen Hl, Lee H, Peiris M and Jaume
M: Antibody-dependent enhancement (ADE) of infection and its
possible role in the pathogenesis of influenza. BMC Proc. 5(Suppl
1): S622011. View Article : Google Scholar
|
|
38
|
Monsalvo AC, Batalle JP, Lopez MF, Krause
JC, Klemenc J, Hernandez JZ, Maskin B, Bugna J, Rubinstein C,
Aguilar L, et al: Severe pandemic 2009 H1N1 influenza disease due
to pathogenic immune complexes. Nat Med. 17:195–199. 2011.
View Article : Google Scholar :
|
|
39
|
Bhatnagar H, Kala S, Sharma L, Jain S, Kim
KS and Pal R: Serum and organ-associated anti-hemoglobin humoral
autoreactivity: Association with anti-Sm responses and
inflammation. Eur J Immunol. 41:537–548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Biagioli M, Pinto M, Cesselli D, Zaninello
M, Lazarevic D, Roncaglia P, Simone R, Vlachouli C, Plessy C,
Bertin N, et al: Unexpected expression of alpha- and beta-globin in
mesencephalic dopaminergic neurons and glial cells. Proc Natl Acad
Sci USA. 106:15454–15459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ukimura A, Satomi H, Ooi Y and Kanzaki Y:
Myocarditis associated with influenza A H1N1pdm2009. Influenza Res
Treat. 2012:3519792012.
|
|
42
|
Chen AT, Cornberg M, Gras S, Guillonneau
C, Rossjohn J, Trees A, Emonet S, de la Torre JC, Welsh RM and
Selin LK: Loss of anti-viral immunity by infection with a virus
encoding a cross-reactive pathogenic epitope. PLoS Pathog.
8:e10026332012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marazzi I, Ho JS, Kim J, Manicassamy B,
Dewell S, Albrecht RA, Seibert CW, Schaefer U, Jeffrey KL, Prinjha
RK, et al: Suppression of the antiviral response by an influenza
histone mimic. Nature. 483:428–433. 2012. View Article : Google Scholar : PubMed/NCBI
|