Introduction
Ulcerative colitis (UC) and Crohn’s disease,
collectively termed inflammatory bowel disease (IBD), are two
separate clinical entities characterized by intestinal inflammation
with a chronic relapsing course and, in recent years, the incidence
of IBD is increasing worldwide (1–3).
Though the pathogenesis of IBD is not totally clear, it is known
that IBD occurs due to the role of complex interactions between
genetic, environmental and immunological factors (4–6).
Exogenous and endogenous triggers and multiple mediators are
involved in the pathogenetic mechanisms of IBD, and their
interactions lead to abnormalities of multiple immunological
signaling pathways in genetically susceptible individuals.
Solute carrier family 26, member 3 (Slc26a3) ion
transporter, also termed downregulated-in-adenoma (DRA), is a
Cl−/HCO3− exchanger. Slc26a3
absorbs Cl− and exports HCO3−,
contributing to intestinal fluid absorption and enterocyte
acid/base balance (7). DRA
predominantly locates at the mid-distal colon and its mutation may
lead to congenital chloride diarrhea, an autosomal recessive
disorder, which occurs worldwide (8). In addition, the risk of acute and
chronic intestinal inflammation was significantly increased,
presenting as clinical manifestations of IBD (9). Our previous study indicated that
Slc26a3 deficiency is associated with the absence of a firmly
adherent mucus layer and HCO3−/mucus barrier
impairment in mice; thus, Slc26a3−/− mice are
susceptible to dextran sulfate sodium (DSS)-induced colitis
(10). Farkas et al
(11) demonstrated a robust
reduction in Cl− absorption with a corresponding
decrease of ~50% in DRA mRNA expression levels in the surface cells
of the colonic crypts in UC patients. Furthermore, the modulation
of DRA in the inflammation environment remains unknown. Tumor
necrosis factor (TNF)-α, which is predominantly synthesized by
lymphocytes and activated macrophages, promotes the activation and
recruitment of immune cells, induces apoptosis of intestinal
epithelial cells and abnormal expression levels of tight junction
molecules, thus finally leading to disruption of the intestinal
mucosal barrier and increased permeability (12–14). TNF-α is a central mediator of
intestinal inflammation in IBD, and the clinical efficacy of
anti-TNF-α drugs indicates that TNF-α performs an important role in
the pathogenesis of IBD. A recent study indicated that loss of DRA
is associated with decreased mucosal HCO3−
secretion and may occur due to the effect of proinflammatory
cytokines on epithelial ion transporters (15). In addition, a study by Juric et
al (16) demonstrated that
DRA mRNA expression levels are significantly higher in the distal
colon, and the expression levels differ significantly between
TNF+/+ and TNF+/ΔARE mice. Based on these
data, the present study hypothesizes that TNF-α may act
reciprocally with DRA leading to the development of gut
inflammation in IBD.
In the present study, the expression levels of DRA
and TNF-α were examined in UC patients and DSS-induced colitis
mice. Subsequently, the expression level of DRA was evaluated
following treatment with TNF-α or silencing of TNF-α in colonic
epithelial Caco2BBE cells. In addition, DRA was overexpressed or
depleted to observe the expression level of TNF-α in Caco2BBE cells
and supernatant. The aim of the present study was to reveal the
association between DRA and TNF-α, and establish a novel
therapeutic target for IBD.
Materials and methods
Ethics statement
All animal experiments were performed in accordance
with the relevant national guidelines. The protocols were approved
by the Committee on the Ethics of Animal Experiments of Tongji
Medical School, Huazhong University of Science and Technology
(Wuhan, China).
Patients and specimens
Endoscopic biopsy specimens from the sigmoid colon
of active UC patients and healthy control patients were obtained
from the endoscopy ward of the Department of Gastroenterology,
Tongji Hospital, Huazhong University of Science and Technology. The
specimens were obtained during routine diagnostic sample collection
and patient data were made anonymous. The collection of samples was
approved by the local ethical committee and the institutional
review board of Huazhong University of Science and Technology and
each patient provided written informed consent.
DSS-induced colitis model and evaluation
of the severity of clinical colitis
Male C57BL/6 mice (age, 6–8 weeks; weight, 19–23 g)
were purchased from the Department of Experimental Animals of
Tongji Medical School (Wuhan, China). The mice were maintained on a
standard mouse diet with free access to tap water and food, under a
12-h light-dark cycle at room temperature (22±2°C) in relative
humidity (55–60%). All C57BL/6 mice were housed together for 2
weeks before commencing studies. The mice were divided randomly
into control and experimental groups, and each group contained 8
mice. Mice in the treatment group were administered with 3% DSS (MP
Biomedicals, Solon, OH, USA) drinking solution ad libitum
for 7 days, followed by regular water for another 2 days. The
animals were weighed daily and monitored for signs of distress, as
well as rectal bleeding, weight loss, stool consistency, water
content and rectal bleeding. To evaluate the colitis severity, the
disease activity index (DAI) was, measured as described previously
(17). Briefly, stool samples
from individual mice were evaluated using a test for occult blood,
whereas the macroscopic examination was performed by two
independent researchers under blinded conditions: 0=Normal, no
occult blood; 1=loose stool, no occult blood; 2=loose stool,
positive for occult blood; 3=diarrhea, positive for occult blood;
and 4=bleeding. Following oral DSS for 7 days and regular water for
2 days, the blood was collected from the angular vein and the mice
were sacrificed. Colons were collected for haematoxylin and eosin
(H&E) staining, immunohistochemistry (IHC), immunofluorescence,
quantitative polymerase chain reaction (qPCR) and western blot
analysis.
IHC and immunofluorescence staining
In order to detect the location and expression level
of DRA in colitis mice and healthy mice, histological analysis was
performed on colon tissue samples that were fixed with 4%
phosphate-buffered paraformaldehyde at room temperature for 24 h,
embedded in paraffin and sectioned at a thickness of 4 μm.
Following hydration in a decreasing ethanol gradient, all sections
were deparaffinized and stain in Harris hematoxylin solution for 5
min at 37°C. For IHC, the sections were routinely dewaxed and
rehydrated followed by endogenous peroxidase blocking. Antigen
retrieval was performed by pressurized steam cooking. A DRA
antibody (1:50, sc-34942; Santa Cruz Biotechnology, Heidelberg,
Germany) served as the primary antibody and was used at 4°C
overnight. Following incubation with horseradish
peroxidase-conjugated polyclonal goat anti-rabbit secondary
antibody (Dako REAL™ EnVision™ kit; cat. no. K5007; Dako, Agilent
Technologies GmbH, Waldbronn, Germany) for 30 min at 37°C, the
expression of the proper antigen in cells was detected with Dako
3,3′-diaminobenzidine (Agilent Technologies GmbH).
For immunofluorescent analysis, frozen sections of
the distal colon were washed in phosphate-buffered saline (PBS; pH
7.2) and stained to detect the expression level of DRA. Sections
were blocked with 5% horse serum for 30 min at room temperature and
incubated at 4°C overnight with a primary antibody against DRA,
followed by incubation with Cy3-labeled donkey anti-goat secondary
antibody (1:200, #705-165-003; Jackson ImmunoResearch Laboratories,
Inc., West Grove, PA, USA) for 1 h at room temperature. After
washing with PBS for three times, sections were stained with
4′,6-diamidino-2-phenylindole at room temperature for 10 min
(1:1,000, C1002) for nuclear staining and mounted with antifade
medium (P0126) (both from Beyotime Institute of Biotechnology,
Jiangsu, China). Images were acquired under an Olympus 1 X71
fluorescence microscope.
Cell culture and lentiviral-mediated DRA
overexpressed in Caco2BBE cells
Caco2BBE cells were provided by Professor Seidler U
(Hannover Medical School, Germany) and all the experiments were
performed within 25 passages. The cells were cultured in Gibco
Dulbecco’s modified Eagle’s medium (DMEM) medium supplemented with
10% fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 1% penicillin and streptomycin, 1% non-essential amino
acid and 1% L-glutamine, and incubated in a 5% CO2
incubator at 37°C as previously described (18). Recombinant lentivirus
overexpressing DRA was constructed by Lenti-Easy Packaging System
(Shanghai GeneChem Co., Ltd., Shanghai, China). Infection cells
were obtained by selection in puromycin conditioned culture. Thus,
Caco2BBE cell proliferation occurred following transduction with
recombinant lentivirus, and infected cells were used for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
RNA interference
For RNA interference, synthesis of DRA siRNA (sense,
GAACCAACATCTATAAGAA) and TNF-α siRNA (sense, GCGTGGAGCTGAGAGATAA)
was performed by Guangzhou RiboBio Co., Ltd. (Guangzhou, China) and
transfected into cells using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) as previously described (19). Cells were transfected at a density
of 80–90% and used for experiments 24 h after transfection. When
necessary, the DMEM medium of cell culture was collected.
RNA extraction and RT-qPCR
Total RNA was extracted using TRIzol reagent (Life
Technologies; Thermo Fisher Scientific, Inc.). Reverse-transcribed
complementary DNA was synthesized using the PrimeScript RT reagent
kit (RR036A; Takara Bio, Inc., Otsu, Japan). qPCR was performed for
40 cycles of 95°C for 30 sec and 60°C for 30 sec using SYBR Premix
ExTaq (Takara Bio, Inc.) on an ABI StepOne Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The value of
2−ΔΔCq (20) was used
to determine the fold difference between samples. The primer
sequences used for PCR are presented in Table I.
 | Table IPrimer sequences used for polymerase
chain reaction. |
Table I
Primer sequences used for polymerase
chain reaction.
| Name | Sequence 5′-3′
|
|---|
| Sense | Antisense |
|---|
| Mouse GAPDH |
TGACCTCAACTACATGGTCTACA |
CTTCCCATTCTCGGCCTTG |
| Human GAPDH |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG |
| Mouse Slc26A3 |
GCCGTGGTTGGGAACATGA |
GCAAATCCTTTGAATGCTCCAG |
| Human Slc26A3 |
GTTGCAGAAGCTCCTCACAGA |
GGCTGTGGCAAGTTGAAGAC |
| Mouse TNF-α |
CATCTTCTCAAAATTCGAGTGACAA |
TGGGAGTAGACAAGGTACAACCC |
| Human TNF-α |
CCTCTCTCTAATCAGCCCTCTG G |
AGGACCTGGGAGTAGATGAG |
Western blot analysis
For western blot analysis, colon tissue samples from
mice were added to RIPA lysis buffer. These tissue samples were
homogenized at 4°C, centrifuged at 12,000 g for 30 min and the
supernatant was retained. Cultured cells were grown in 6-well
plates and lysed using RIPA lysis buffer. Following degeneration,
30 μg samples were separated by 10% SDS-PAGE. The gel was
run at 70 V (at constant voltage) until separation began.
Subsequently the voltage was increased to 110 V. The gel was
transferred to polyvinylidene difluoride membranes at 4°C and 250
mA for 1.5 h. Membranes were blocked with 5% non-fat milk (5 g
non-fat dry milk powder in 100 ml Tris-buffered saline with Tween
20) at room temperature for 1 h, then incubated with specific DRA
antibody (1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
at 4°C overnight. Finally, membranes were incubated with Peroxidase
AffiniPure Rabbit Anti-Goat IgG (1:5,000, #305-035-003; Jackson
ImmunoResearch Laboratories, Inc.), followed by detection with
enhanced chemiluminescence reagents (Pierce; Thermo Fisher
Scientific, Inc.). GAPDH served used as a loading control.
ELISA
Expression levels of TNF-α were determined in the
serum of UC patients and DSS-induced colitis mice, as well as in
the DMEM medium of Caco2BBE cell lines using a Mouse/Human TNF-α
ELISA kit (EMC102a.96 and EHC103a.96; NeoBioscience Technology,
Shenzhen, China) according to the manufacturer’s instructions.
Statistical analysis
Data were presented as the mean ± standard
deviation. One-way analysis of variance or Student’s t tests were
performed to assess the significant differences between treatment
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
UC patients demonstrated decreased
expression levels of DRA and higher expression levels of TNF-α in
peripheral blood serum
The expression level of DRA protein was determined
in sigmoid colonic samples obtained from active UC patients and
healthy control subjects. In addition, to observe the change of
proinflammatory cytokines, the expression level of TNF-α was
detected in the serum of UC patients and healthy control subjects
at the same time. In total, specimens from 11 UC patients were
obtained. In the 11 patients (5 males and 6 females; mean age, 42±3
years), the presence of UC was confirmed by routine
histopathological evaluation. Nine control samples (5 males and 4
females; mean age, 36±4 years) were obtained from patients who did
not suffer from any inflammatory conditions during the physical
examination.
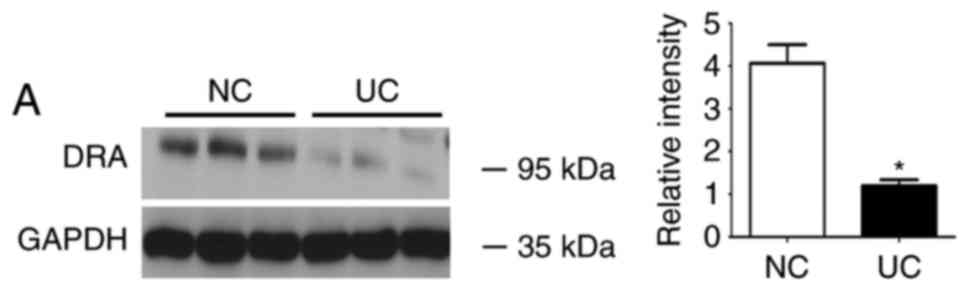
During western blot analyses, DRA was significantly
reduced in UC patients when compared with the control subjects,
whereas the level of TNF-α serum was significantly increased in UC
patients compared with the control subjects (Fig. 1).
Mice suffering from DSS-induced colitis
exhibit reduced levels of DRA expression as well as a higher
expression level of TNF-α in peripheral blood serum
Subsequently, whether the expression level of DRA
correlates with gut inflammation in a mouse model of UC was
investigated (21). Mice were
treated with DSS for 7 days before being exposed to regular water
for another 2 days. In contrast to the untreated mice, DSS-treated
mice exhibited significant body weight loss and bloody stools that
were similar to the symptoms of UC on day 4 of DSS treatment
(Fig. 2A). Additionally, the
water content of stools in DSS-treated mice was significantly more
than that in untreated mice following 3 days of DSS treatment
(Fig. 2B). Macroscopic analysis
of colons in the two groups of mice revealed a marked shortening of
colon length in the DSS-treated mice on day 7 of DSS treatment
(Fig. 2C). DAI results
demonstrated an increased DAI score on day 3 of DSS treatment and
continuous elevation during the process of DSS treatment (Fig. 2D). Consistent with these findings,
histological analysis of H&E-stained colonic tissue samples
demonstrated that intestinal crypts in the colon of DSS-treated
mice were deformed (similar to the clinical symptom of human UC),
exhibited epithelial sloughing and were even destroyed (Fig. 2E).
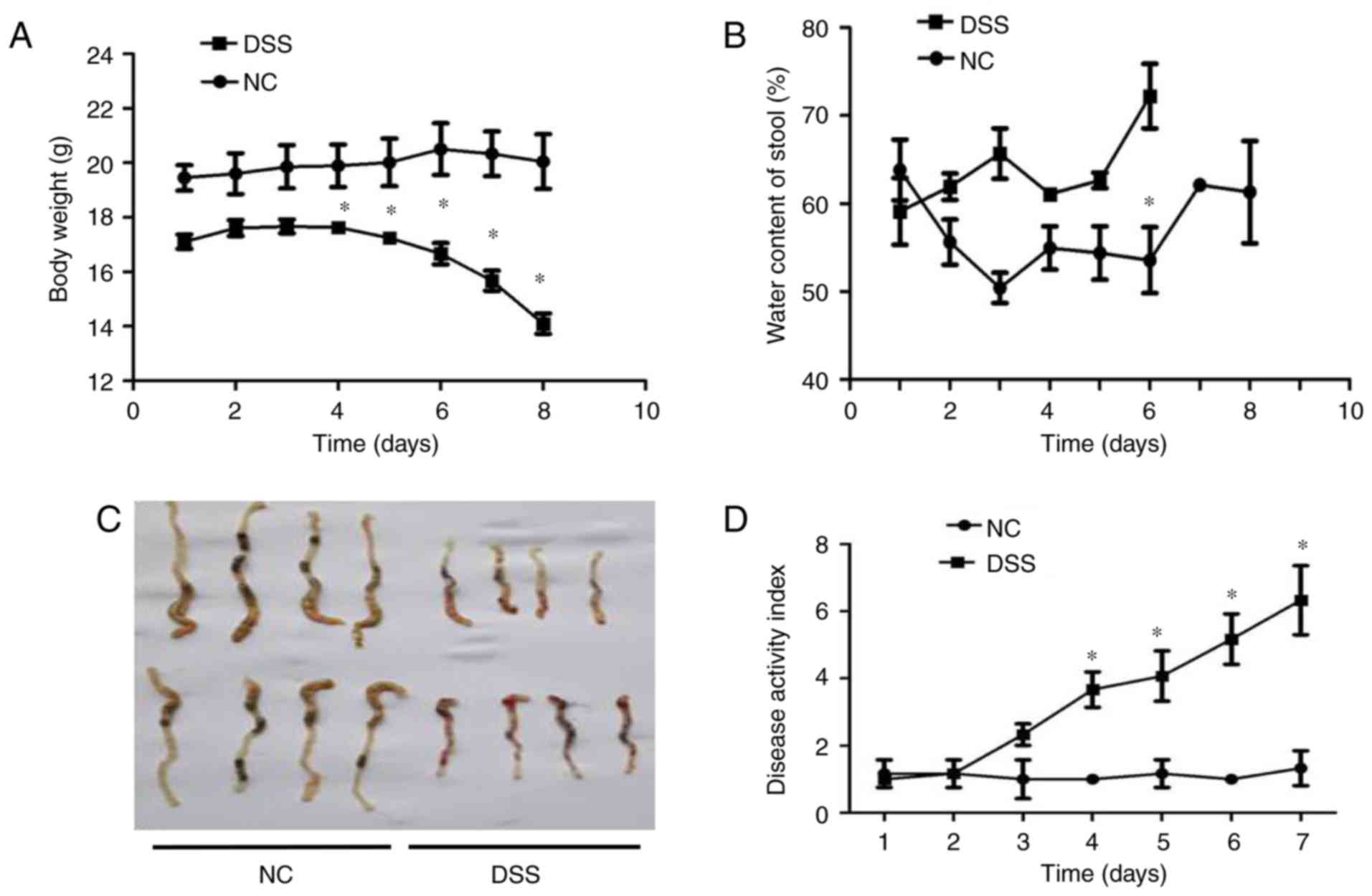 | Figure 2In vivo assessment of colitis
severity and histological characteristics in DSS-induced colitis
mice. (A) Body weight curves for different groups. Compared with
the control group, the DSS group mice exhibited obviously decreased
weight over time, particularly from day 4 of oral DSS treatment
(13.98±2.2 vs. 19.82±1.5 g on day 8). (B) Stool water content
curves for the different groups. Compared with the control group,
the DSS group mice demonstrated significantly increased stool water
content (60.2±4.2 vs. 72.1±3.2% on day 6), and eventually
experienced serious diarrhea and bleeding from day 6 of oral DSS
treatment, so the stools could not be collected. (C) Length of
colon curves in the different groups. Compared with the control
group, the DSS group mice demonstrated markedly shortened lengths
of colon (7.65±0.2 vs. 4.34±0.2 cm). (D) DAI score curves for the
different groups. Compared with the control group, the DSS group
mice exhibited marked increases in their DAI score. (E) Hematoxylin
and eosin staining of the different groups (magnification, x200).
Compared with the control group, the crypts in the colon samples of
the DSS group were deformed and even destroyed in line with
clinical signs of human ulcerative colitis. *P<0.05
vs. NC. DSS, dextran sodium sulfate; DAI, Disease Activity
Index. |
Furthermore, the mRNA and protein expression levels
of DRA in the distal colon of mice, as well as the TNF-α expression
level in the blood serum of mice were analyzed after 7 days of DSS
treatment. Compared with untreated mice, DSS-treated mice displayed
a significantly higher expression level of cytokine TNF-α in blood
serum while a significantly reduced expression level of DRA was
observed (Fig. 3A and B). This
finding was further confirmed by immunostaining of colonic tissue
samples with a DRA antibody. According to IHC and
immunofluorescence staining, DRA was predominantly located in the
colonic surface and its expression level was significantly
decreased in the DSS-treated mice (Fig. 3C and D). In addition, the colonic
crypts in the DSS group were distorted and the colonic surface was
destroyed on day 7 of DSS treatment (Fig. 3C).
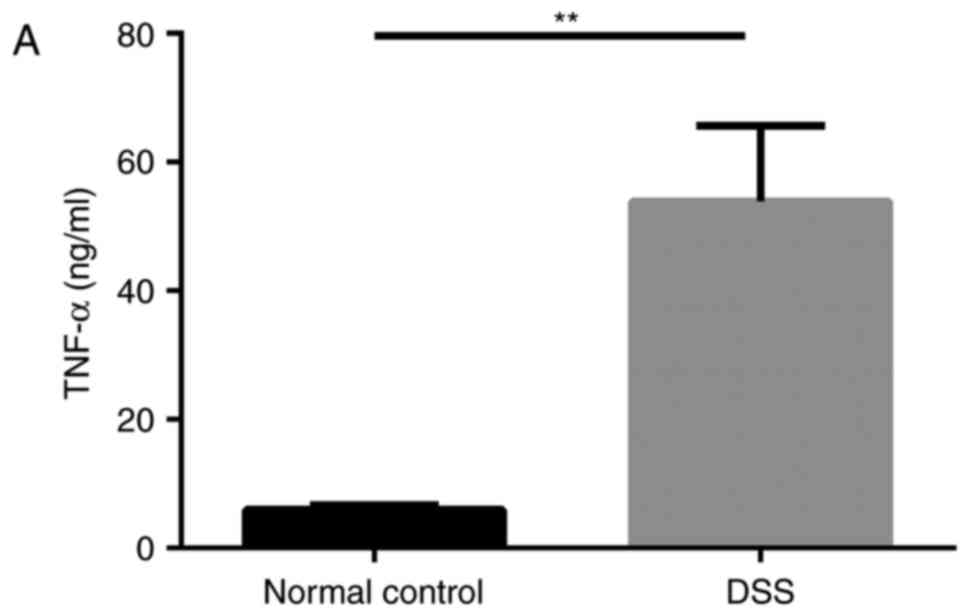 | Figure 3DRA expression levels were decreased
in DSS-induced colitis mice, which was associated with changes in
TNF-α expression in vivo. (A) Compared with the normal
control mice, TNF-α expression level in the serum markedly
increased. (5.98±0.63 vs. 53.89±8.98 ng/ml). (B) In DSS-induced
colitis mice, DRA mRNA and protein expression levels were
significantly decreased compared with the control mice.
*P<0.05 as indicated. (C and D) DRA expression levels
are predominantly observed in the colon crypt surface, and rarely
observed in the cytoplasm. Similarly, in DSS-induced colitis mice,
DRA expression levels were significantly decreased when compared
with control mice, according to immunohistochemistry and
immunofluorescence staining. **P<0.01. DRA,
downregulated-in-adenoma; DSS, dextran sodium sulfate; TNF-α, tumor
necrosis factor-α; NC, normal control; DAPI,
4′,6-diamidino-2-phenylindole. |
TNF-α affects the expression level of DRA
in Caco2BBE cells following stimulation with or silencing of
TNF-α
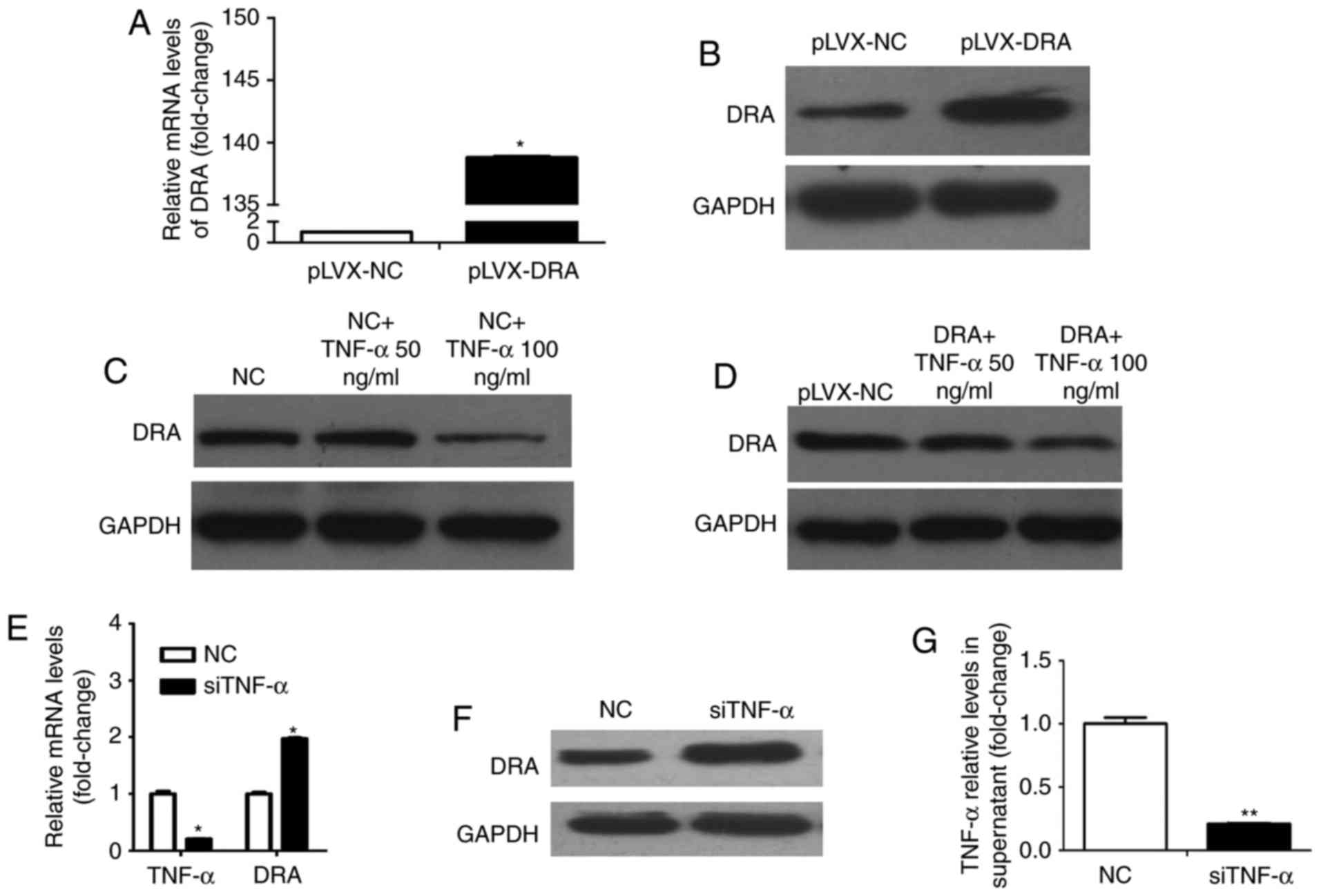
Whether TNF-α affects DRA expression levels was
investigated during the present study. Colonic epithelial cells,
Caco2BBE and lentiviral-mediated DRA overexpressed Caco2BBE cells
were used. As presented in Fig. 4A
and B, the expression levels of DRA mRNA and protein were
significantly increased following infection with recombinant
lentivirus overexpressing DRA. Caco2BBE cells were treated with
TNF-α at different dosages (50 and 100 ng/ml) for 24 h. TNF-α
treatment reduced the expression level of DRA protein and this
effect was apparent at a dosage of 100 ng/ml (Fig. 4C). In addition, DRA overexpressing
cells were treated with TNF-α according to the above-mentioned
method. It was found that while the expression level of DRA in DRA
overexpressing cells was markedly higher than that in control
cells, the expression level of DRA was decreased in response to
TNF-α treatment, particularly at a dosage of 100 ng/ml (Fig. 4D).
Conversely, whether silencing TNF-α would affect the
expression level of DRA was evaluated. Notably, the TNF-α mRNA
level in Caco2BBE cells and the expression level of TNF-α in the
supernatant of Caco2BBE cells following transfection with TNF-α
siRNA were significantly decreased when compared with control cells
and cell supernatant (Fig. 4E and
F). Furthermore, the expression levels of DRA mRNA and protein
were significantly increased when compared with the control cells
(Fig. 4E and G).
Therefore, it was hypothesized that TNF-α may
down-regulate DRA, which may affect intestinal fluid absorption,
enterocyte acid/base balance and barrier function (4–5),
and ultimately exacerbate colon inflammation.
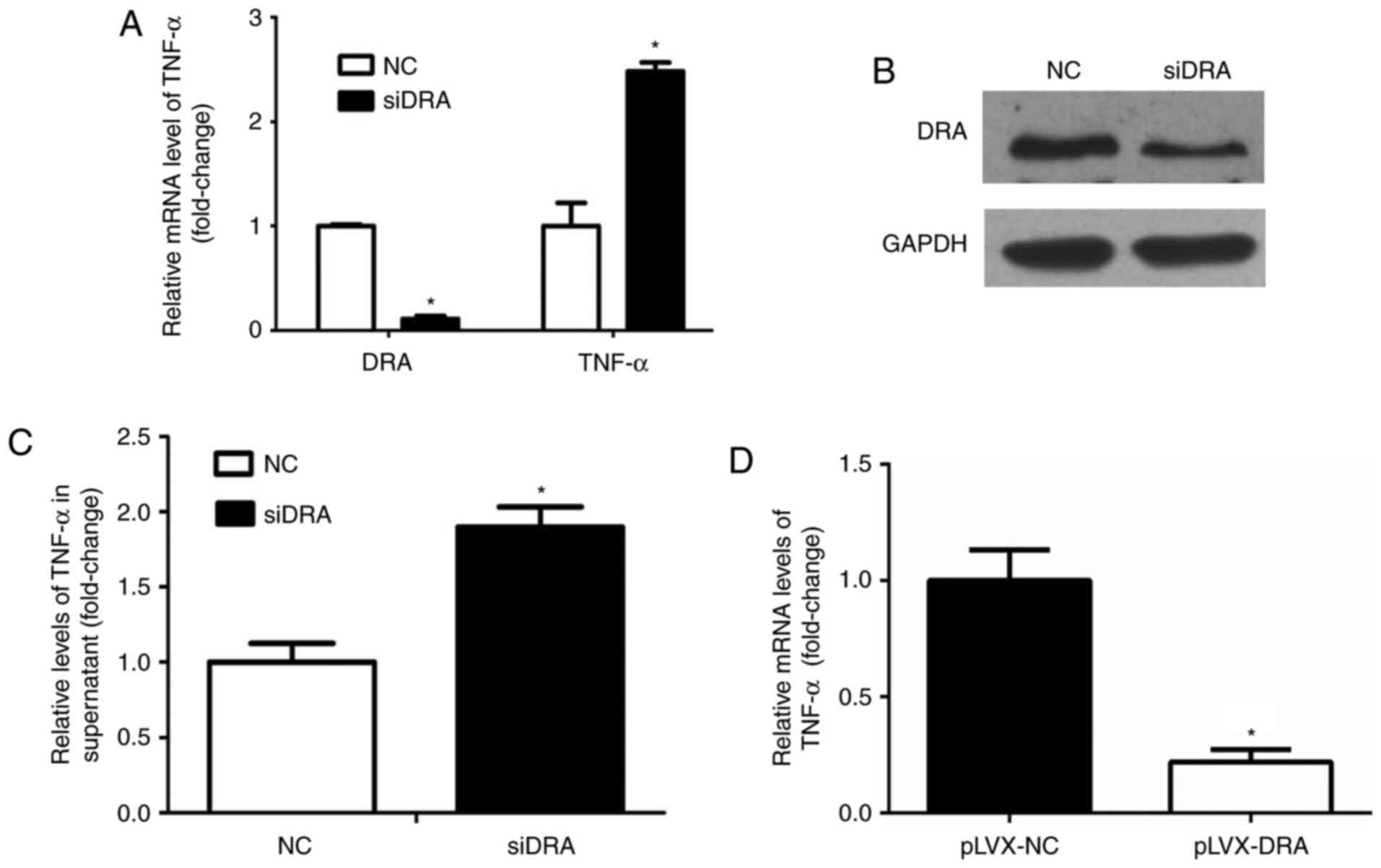
Silencing or overexpressing DRA may
affect TNF-α expression and secretion in Caco2BBE cells
Whether interference with DRA expression levels may
influence TNF-α expression levels was analyzed. As presented in
Fig. 5A and B, the expression
levels of DRA mRNA and protein were significantly decreased
following transfection of DRA siRNA in Caco2BBE cells.
Additionally, TNF-α mRNA and protein expression levels of TNF-α in
the cell culture medium in the DRA-silenced Caco2BBE cells were
significantly increased compared with the control cells (Fig. 5A and C).
Similarly, the expression level of TNF-α mRNA was
determined in DRA overexpression Caco2BBE cells. As expected, the
expression level of TNF-α mRNA was significantly decreased in DRA
overexpression Caco2BBE cells when compared with control cells
(Fig. 5D).
These results indicate that DRA may affect TNF-α
expression levels, and the interaction between TNF-α and DRA may
lead to inflammation progression.
Discussion
IBD-associated mucosal inflammation and the
consequent impaired secretion and absorption of electrolytes often
results in electrolytic and acid-base imbalance in IBD patients
(22,23). DRA, which is an anion exchanger
involved in anion secretion, as well as NaCl absorption, appears to
be the major anion exchanger involved in the pathophysiology of
IBD-associated and enteropathogenic Escherichia
coli-mediated diarrhea disorders (11,24–26). These findings implicated that DRA
expression levels may serve as a potential target during
inflammatory diarrhea.
While a decrease in DRA expression level is
associated with intestinal inflammation, the direct effect of
mediators that trigger intestinal inflammatory mediators on DRA has
not been fully investigated. A previous study provided evidence for
the involvement of hepatocyte nuclear factor 4 and GATA
transcription factors in DRA expression (27). Cytokines, such as interleukin-1β
(24) and interferon (IFN)-γ
(28) also reduce DRA expression
levels in Caco-2 cells. In this regard, TNF-α is also a central
mediator of gut inflammation in IBD (29–31). Whether TNF-α directly modulates
DRA expression remains unknown. It is hypothesized that TNF-α
inhibits DRA expression levels in intestinal epithelial cells, and
that the interaction between TNF-α and DRA promotes the progression
of gut inflammation.
In the present study, the expression level of the
DRA protein was markedly reduced whereas the expression level of
TNF-α in blood serum was significantly increased in UC patients
compared to healthy control subjects. Subsequent studies are
required to establish whether this is the case in UC animal models.
The DSS-induced colitis is a commonly utilized mouse model,
mimicking the clinical pathology of UC (32), and this is characterized by a
significant colonic inflammation, reduction of colon length, weight
loss and bloody diarrhea, which have been demonstrated in the
present study. Furthermore, it was observed that DRA mRNA and
protein expression levels were identified to be markedly decreased
in DSS-induced colitis compared with the normal control subjects.
DRA is predominantly expressed on the apical membrane of intestinal
epithelial cells in IHC and immunofluorescence staining, and thus
exerts the function of NaCl absorption combined with
Na+/H+ exchanger3. Additionally, the present
findings suggested that the expression level of TNF-α was
apparently elevated in the blood serum of colitis mice. Thus, it is
hypothesized that TNF-α may regulate DRA expression levels in
colitis. Colonic epithelial cells (Caco2BBE cells) were used to
perform further investigations.
Caco2BBE cells express a well-developed brush-border
and the cytoskeleton of the microvilli is polarized and structured,
as in human enterocytes in vivo. In addition, Caco2BBE cells
are more homogenous with respect to brush-border expression than
the parental cell line, and the expression level of proteins
associated with the brush- border was similar to that identified in
human enterocytes (33). To avoid
the heterogeneity of different passages of cells, the experiments
were performed in early passages of Caco2BBE cells (between 15 and
25 passages). It was demonstrated that subsequent to stimulating
with TNF-α, the expression level of DRA markedly decreased.
Furthermore, this effect was dose-dependent; when stimulated with
100 ng/ml TNF-α, the decrease in DRA expression levels was more
pronounced than when stimulated with 50 ng/ml TNF-α. Then, whether
overexpression of DRA may block this effect was investigated.
Caco2BBE cells with lentiviral-mediated DRA overexpression were
used and treated with different dosages of TNF-α. The expression
level of DRA decreased following stimulation with 100 ng/ml TNF-α
in DRA overexpression Caco2BBE cells. These results indicate that
TNF-α is a negative regulator of DRA expression in Caco2BBE cells.
Conversely, in order to investigate whether suppression of TNF-α
has an effect on the expression level of DRA, Caco2BBE cells
transfected with siTNF-α were used to perform further experiments.
The expression level of TNF-α was identified to be reduced in the
cell culture medium after silencing TNF-α in the Caco2BBE cells.
Simultaneously, a marked increase in the expression levels of DRA
mRNA and protein was detected, indicating that DRA was a target
site of TNF-α. Based on the results, it was confirmed that TNF-α
downregulates DRA expression. Furthermore, DRA promoter activity
was inhibited by IFN-γ via the Janus kinase/signal transducers and
activators of transcription signaling pathway (28). The overall decrease in DRA
expression may be involved in the process of IBD.
In the development of gut inflammation, many
factors, including cytokines, diet, the microbiome and immunity are
involved in the pathogenetic mechanisms of IBD, and their
interactions lead to abnormalities in individuals (34–36). As TNF-α may regulate ion
transporter DRA, it is proposed that DRA may also affect TNF-α.
Notably, the present results indicated the knockdown of DRA
increased the mRNA expression level of TNF-α and the protein
expression level of TNF-α in the cell culture medium. One
hypothesis is that DRA may function as an important mediator that
drives the negative cycle of TNF-α-induced gut inflammation.
However, a limitation of the present study is that
the result did not indicate the direct regulation of TNF-α on DRA.
Further investigation is required to identify the underlying
molecular mechanisms. Overexpression of DRA was demonstrated to be
beneficial in the inflammatory environment; however, the signaling
pathways have not been well defined.
In conclusion, the current in vitro and in
vivo data demonstrate that DRA is involved in intestinal
inflammation and TNF-α may reduce DRA expression levels; however,
DRA also affects TNF-α expression and secretion. In the clinical
setting, the TNF-α monoclonal antibody has been widely used to
treat IBD patients with favorable results (37,38). The present study increases
knowledge of the underlying mechanisms of TNF-α leading to the
progression of intestinal inflammation and reveals a novel
perspective of the interaction between TNF-α and ion
transporters.
Acknowledgments
The authors would like to thank Professor Ursula
Seidler (Hannover Medical School, Hannover, Germany) for providing
the human colon epithelial cell Caco2BBE cell line. The present
study was supported by a grant from the National Natural Science
Foundation of China (grant no. 81400593).
References
|
1
|
Chi KR: Epidemiology: Rising in the East.
Nature. 540:S100–S102. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nq SC, Tang W, Ching JY, Wong M, Chow CM,
Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, et al: Incidence and
phenotype of inflammatory bowel disease based on results from the
Asia-pacific Crohn’s and colitis epidemiology study.
Gastroenterology. 145:158–165.e2. 2013. View Article : Google Scholar
|
|
3
|
Wang YF, Ouyang Q and Hu RW: Progression
of inflammatory bowel disease in China. J Dig Dis. 11:76–82. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dixon LJ, Kabi A, Nickerson KP and
McDonald C: Combinatorial effects of diet and genetics on
inflammatory bowel disease pathogenesis. Inflamm Bowel Dis.
21:912–922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park JH, Peyrin-Biroulet L, Eisenhut M and
Shin JI: IBD immunopahogenesis: A comprehensive review of
inflammatory molecules. Autoimmun Rev. 16:416–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ng SC, Bernstein CN, Vatn MH, Lakatos PL,
Loftus EV Jr, Tysk C, O’Morain C, Moum B and Colombel JF;
Epidemiology and Natural History Task Force of the International
Organization of Inflammatory Bowel Disease (IOIBD): Geographical
variability and environmental risk factors in inflammatory bowel
disease. Gut. 62:630–649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia W, Yu Q, Riederer B, Singh AK,
Engelhardt R, Yeruva S, Song P, Tian DA, Soleiman M and Seidler U:
The distinct roles of anion transporters Slc26a3 (DRA) and Slc26a6
(PAT-1) in fluid and electrolyte absorption in the murine small
intestine. Pflugers Arch. 466:1541–1556. 2014. View Article : Google Scholar :
|
|
8
|
Wedenoja S, Pekansaari E, Höglund P,
Mäkelä S, Holmberg C and Kere J: Update on SLC26A3 mutations in
congenital chloride diarrhoea. Hum Mutat. 32:715–722. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wedenoja S, Höglund P and Holmberg C:
Review article: The clinical management of congenital chloride
diarrhoea. Aliment Pharmacol Ther. 31:477–485. 2010. View Article : Google Scholar
|
|
10
|
Xiao F, Yu Q, Li J, Johansson ME, Singh
AK, Xia W, Riederer B, Engelhardt R, Montrose M, Soleimani M, et
al: Slc26a3 deficiency is associated with loss of colonic HCO3 (−)
secretion, absence of a firm mucus layer and barrier impairment in
mice. Acta Physiol (Oxf). 211:161–175. 2014. View Article : Google Scholar
|
|
11
|
Farkas K, Yeruva S, Rakonczay Z Jr,
Ludolph L, Molnar T, Nagy F, Szepes Z, Schnur A, Wittmann T,
Hubricht J, et al: New therapeutic targets in ulcerative colitis:
The importance of ion transporters in the human colon. Inflamm
Bowel Dis. 17:884–898. 2011. View Article : Google Scholar
|
|
12
|
Peake ST, Bernardo D, Mann ER, Al-Hassi
HO, Knight SC and Hart AL: Mechanisms of action of anti-tumor
necrosis factor-α agents in Crohn’s disease. Inflamm Bowel Dis.
19:1546–1555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Günther C, Neumann H, Neurath MF and
Becker C: Apoptosis, necrosis and necroptosis: Cell death
regulation in the intestinal epithelium. Gut. 62:1062–1071. 2013.
View Article : Google Scholar
|
|
14
|
Fries W, Muja C, Crisafulli C, Cuzzocrea S
and Mazzon E: Dynamics of enterocyte tight junctions: Effect of
experimental colitis and two different anti-TNF strategies. Am J
Physiol Gastrointest Liver Physiol. 294:G938–G947. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao F, Juric M, Li J, Riederer B, Yeruva
S, Singh AK, Zheng L, Glage S, Kollias G, Dudeja P, et al: Loss of
downregulated in adenoma (DRA) impairs mucosal HCO3(−) secretion in
murine ileocolonic inflammation. Inflamm Bowel Dis. 18:101–111.
2012. View Article : Google Scholar
|
|
16
|
Juric M, Xiao F, Amashen S, May O, Wahl K,
Bantel H, Manns MP, Seidler U and Bachmann O: Increased epithelial
permeability is the primary cause for bicarbonate loss in inflamed
murine colon. Inflamm Bowel Dis. 19:904–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sutherland LR, Martin F, Greer S, Robinson
M, Greenberger N, Saibil F, Martin T, Sparr J, Prokipchuk E and
Borgen L: 5-Aminosalicylic acid enema in the treatment of distal
ulcerative colitis, proctosigmoiditis, and proctitis.
Gastroenterology. 92:1894–1898. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yeruva S, Chodisetti G, Luo M, Chen M,
Cinar A, Ludolph L, Lünnemann M, Goldstein J, Singh AK, Riederer B,
et al: Evidengce for a causal link between adaptor protein PDZK1
downregulation and Na+/H+ exchanger NHE3
dysfunction in human and murine colitis. Pflugers Arch.
467:1795–1807. 2015. View Article : Google Scholar
|
|
19
|
Yu Q, Liu ZY, Chen Q and Lin JS: Mcl-1 as
a potential therapeutic target for human hepatocellular carcinoma.
J Huazhong Univ Sci Technolog Med Sci. 36:494–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liva KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Kolios G: Animal models of inflammatory
bowel disease: How useful are they really? Curr Opin Gastroenterol.
32:251–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Priyamvada S, Gomes R, Gill RK, Saksena S,
Alfefai WA and Dudeja PK: Mechanisms underlying dysregulation of
electrolyte absorption in inflammatory bowel disease associated
diarrhea. Inflamm Bowel Dis. 21:2926–2935. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barkas F, Liberopoulos E, Kei A and Elisaf
M: Electrolyte and acid-base disorders in inflammatory bowel
disease. Ann Gastroenterol. 26:23–28. 2013.PubMed/NCBI
|
|
24
|
Yang H, Jiang W, Furth EE, Wen X, Katz JP,
Sellon RK, Silberg DG, Antalis TM, Schweinfest CW and Wu GD:
Intestinal inflammation reduces expression of DRA, a transporter
responsible for congential chloride diarrhea. Am J Physiol.
275:G1445–G1453. 1998.PubMed/NCBI
|
|
25
|
Gill RK, Borthakur A, Hodges K, Turner JR,
Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G and Dudeja
PK: Mechanisms underlying inhibition of intestinal apical Cl/OH
exchange following infection with enteropathogenic E. coli. J Clin
Invest. 117:428–437. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar A, Anbazhagan AN, Coffing H,
Chatterjee I, Priyamvada S, Gujral T, Saksena S, Gill RK, Alrefai
WA, Borthakur A and Dudeja PK: Lactobacillus acidphilus counteracts
inhibition of NHE3 and DRA expression and alleviates diarrheal
phenotype in mice infected with Citrobacter rodentium. Am J Physiol
Gastrointest Liver Physiol. 311:G817–G826. 2016. View Article : Google Scholar
|
|
27
|
Alrefai WA, Wen X, Jiang W, Katz JP,
Steinbrecher KA, Cohen MB, Williams IR, Dudeja PK and Wu GD:
Molecular cloning and promoter analysis of downregulated in adenoma
(DRA). Am J Physiol Gastrointest Liver Physiol. 293:G923–G934.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saksena S, Singla A, Goyal S, Katyal S,
Bansal N, Gill RK, Alrefai WA, Ramaswamy K and Dudeja PK:
Mechanisms of transcriptional modulation of the human anion
exchanger SLC26A3 gene expression by IFN-{gamma}. Am J Physiol
Gastrointest Liver Physiol. 298:G159–G166. 2010. View Article : Google Scholar
|
|
29
|
Olesen CM, Coskun M, Peyrin-Biroulet L and
Nielsen OH: Mechanisms behind efficacy of tumor necrosis factor
inhibitors in inflammatory bowel disease. Pharmacol Ther.
159:110–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soufli I, Toumi R, Rafa H and
Touil-Boukoffa C: Overview of cytokines and nitric oxide
involvement in immune-pathogenesis of inflammatory bowel diseases.
World J Gastrointest Pharmacol Ther. 7:353–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Strober W and Fuss IJ: Proinflammatory
cytokine in the pathogenesis of inflammatory bowel disease.
Gastroenterology. 140:1756–1767. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
te Velde AA, de Kort F, Sterrenburg E,
Pronk I, ten Kate FJ, Hommes DW and van Deventer SJ: Comparative
analysis of colonic gene expression of three experimental colitis
models mimicking inflammatory bowel disease. Inflamm Bowel Dis.
13:325–330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sambuy Y, De Angelis I, Ranaldi G, Scarino
ML, Stammati A and Zucco F: The Caco-2 cell line as a model of the
intestinal barrier: influence of cell and culture-related factors
on Caco-2 cell functional characteristics. Cell Biol Toxicol.
21:1–26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H, Chao K, Ng SC, Bai AH, Yu Q, Yu J,
Li M, Cui Y, Chen M, Hu JF and Zhang S: Pro-inflammatory miR-223
mediates the cross-talk between the IL23 pathway and the intestinal
barrier in inflammatory bowel disease. Genome Biol. 17:582016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schaubeck M and Haller D: Reciprocal
interaction of diet and microbiome in inflammatory bowel disease.
Curr Opin Gastroenterol. 31:464–470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sandborn WJ, van Assche G, Reinisch W,
Colombel JF, D’Haens G, Wolf DC, Kron M, Tighe MB, Lazar A and
Thakkar RB: Adalimumab induces and maintains clinical remission in
patients with moderate-to-severe ulcerative colitis.
Gastroenterology. 142:257–265.e1-3. 2012. View Article : Google Scholar
|
|
38
|
Hanauer SB, Feagan BG, Lichtenstein GR,
Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson
A, Bao W, et al: Maintenance infliximab for Crohn’s disease: The
ACCENT 1 randomized trial. Lancet. 359:1541–1549. 2002. View Article : Google Scholar : PubMed/NCBI
|