1. Introduction
Viral neonatal and paediatric infections are
characterised by a great heterogeneity of clinical manifestations
and are considered as major causes of neonatal and paediatric
morbidity and mortality (1).
Almost 50 years ago, Paediatric Virology was not considered an
isolated discipline and was included in the Paediatric Infectious
Diseases section of the scientific field of Paediatrics (2,3).
However, during the past two decades, new advances in the field of
Clinical Virology and Molecular Medicine have expanded the level of
knowledge on the prevention, diagnosis and treatment of viral
infections occurring in infancy and childhood (4,5).
These developments and changes highlight the demand for
undergraduate and postgraduate medical education in Paediatric
Virology, which combines Paediatrics with Virology, Epidemiology,
Molecular Medicine, Evidence-based Medicine, Clinical Governance,
Quality Improvement, and Pharmacology and Immunology (5).
The 3rd Workshop on Paediatric Virology was entitled
‘Paediatric Virology: Interaction between basic science and
clinical practice’. It was held on October 7th, 2017 in Athens,
Greece, as an official session of the 22nd World Congress on
Advances in Oncology and the 20th International Symposium on
Molecular Medicine. Its aim was to bring together virologists and
paediatric health professionals and encourage them to collaborate
as an international network to promote paediatric health. Moreover,
during the workshop, Nobelist laureate Professor Harald zur Hausen,
Emeritus Professor of Virology at the University of Freiburg in
Germany, who received the 2008 Nobel Prize in Physiology or
Medicine for his discovery of human papilloma viruses (HPVs)
causing cervical cancer and Professor Anne Greenough, Professor of
Neonatology and Clinical Respiratory Physiology at King’s College
London, UK and Vice President of Science and Research at the Royal
College of Paediatrics and Child Health (RCPCH), were honoured by
the Paediatric Virology Study Group (PVSG) for their indisputable
academic, research and publishing contribution to Paediatric
Virology.
The present review provides an overview on the
wealth of new material from different areas of neonatal and
paediatric viral infections presented and discussed during the
workshop. Interestingly, 7 out of the 10 top key messages (Table I) of our meeting, as well as both
statements of Nobelist laureate Professor Harald zur Hausen, on the
occasion of this workshop (Table
II), included recommendations on specific prevention strategies
against viral infections. Along with the significant role of human
breast milk and respiratory syncytial virus (RSV) prophylaxis,
these issues included the necessity of the vaccination policy in
relation to the migration crisis, prevention of hepatitis in
newborns, recent advances on influenza vaccines, male vaccination
against HPVs and the the preventative role of probiotics in the
management of viral infections in children.
 | Table IThe 10 top key messages of the ‘3rd
Workshop on Paediatric Virology’. |
Table I
The 10 top key messages of the ‘3rd
Workshop on Paediatric Virology’.
| Breast feeding | ‘Breast feeding has
beneficial effects on the prevention of infections during the first
years of life and should be promoted by primary paediatric health
care providers.’ |
| RSV
prophylaxis | ‘The increased
chronic respiratory morbidity in prematurely born infants due to
RSV should be taken into consideration when developing of relevant
preventative strategies.’ |
| HPV
vaccination | ‘HPV vaccine uptake
should be promoted in countries with low HPV vaccination rates to
reduce the cervical and anogenital cancer burden, as well as for
the prevention of genital warts. Inclusion of males in childhood
HPV immunisation programmes should be considered.’ |
| Migration
crisis | ‘Children’s
vaccination should be a priority during the current migration
problem; we strongly support childhood vaccination for all children
in Europe.’ |
| HBV
vaccination | ‘Prevention of
mother-to-child transmission of HBV is possible: the vaccine
against HBV should be administered at birth within 24 h regardless
of maternal HBV status.’ |
| Influenza
vaccination | ‘Recent pilot
studies in Europe have shown that LAIV can directly prevent
childhood infections and indirectly protect the elderly population;
continuous enhanced surveillance of circulating influenza viruses
and further study results on LAIV effectiveness over consecutive
influenza seasons are needed in order to develop optimal
vaccination policies.’ |
| Imaging | ‘Imaging of fetal
and neonatal viral infections is unable to provide the specific
diagnosis; however it can contribute in defining the extent of
involvement and reveal the consequences that affect the
outcome.’ |
| Probiotics | ‘To date, several
studies have intimated a role of probiotics in modifying the
frequency and severity of respiratory and gastrointestinal viral
infections in infants and children; however, further studies are
required in order to understand their potential therapeutic and
preventative role.’ |
| Archaeology | ‘Collaboration
between archaeologists and experienced paediatric infectious
diseases specialists is necessary for the better understanding and
interpretation of various recent archaeological findings related to
viral-induced neonatal mortality.’ |
| Medical
education | ‘Medical education
in Paediatric Virology among practicing paediatricians should be
promoted; we strongly support the creation of an educational
e-platform on Paediatric Virology.’ |
 | Table IIStatements of Nobelist laureate
Professor Harald zur Hausen, Emeritus Professor of Virology at the
University of Freiburg, on the occasion of the ‘3rd Workshop on
Paediatric Virology’. |
Table II
Statements of Nobelist laureate
Professor Harald zur Hausen, Emeritus Professor of Virology at the
University of Freiburg, on the occasion of the ‘3rd Workshop on
Paediatric Virology’.
| Breast feeding | ‘Prolonged breast
feeding is clearly of great benefit for babies. Human milk contains
some species-specific sugars, which block the uptake of potentially
dangerous viruses (e.g., noro- and rotaviruses), which are the
cause for high infant mortality for newborn children, specifically
in developing parts of this world. During a period of ~6 months of
breast feeding the immune system matures and subsequently such
infections have a less deleterious impact. The uptake, however, of
5-N-glycolyl-neuraminic acid (not produced in humans) by bovine
milk and meat leads to the incorporation of this sialic acid into
cellular glycoproteins and modifies those receptors, rendering them
susceptible for other virus infections (e.g., human polyomavirus
type 9 and others).’ |
| HPV
vaccination | ‘Vaccination of
boys against HPV should be included in the national vaccination
programmes at the same age as girls (8–14 years). Males are the
main transmitters of high risk HPV, having more HPV-positive
oropharyngeal and anal cancers than females, and in particular
homosexual males would not profit from a herd effect, when 80% or
even more of females had been vaccinated. Since, in addition, in
most countries the vaccination rate ranges between 20–40% or less,
vaccination of boys should be mandatory.’ |
2. Plenary key topics
The importance of diet during the first
years of life
A number of different infections occur during the
early period of life (6). Several
of these infections are viral, e.g., herpes-group viruses,
polyomaviruses and Torque teno viruses, and these result in
long-time persistence and latency within human specific cell types
(6). The fate of the latent
genomes seems to vary substantially. They may remain dormant for
the whole lifetime without any recognisable side effect. During
prolonged latency, their genomes may become defective and can
eventually be degraded. In particular, herpes-group viruses may
become re-activated, leading locally to viral production and cell
lysis. The interaction of the latent genomes with mutagenic
chemical, physical or other biological factors may dysregulate the
control of latency or persistence and may thus result in local
exacerbations, mainly as the result of immunoreactivity (6).
Prolonged human breast feeding (>6 months) has
been documented to have a beneficial effect on the avoidance of
viral infections, e.g., norovirus- and rotavirus-related
infections, which can potentially be lethal for newborn babies
(7). It has also been shown that
it reduces the risk of developing certain malignant tumours arising
in early childhood, e.g., acute lymphoblastic leukemia (ALL), or in
young adolescence, e.g., Hodgkin’s disease (HD). Apparently,
specific sugars in human milk modify the receptors blocking the
uptake of noroviruses and rotaviruses during the breast feeding
phase. The maturation of the immune system during this period,
subsequently, facilitates an effective immune response during the
weaning period. The uptake of specific sialic acids, not produced
in humans, but present in cow milk and meat products, beginning
during the weaning time, results in their incorporation into the
cell surface glycoproteins and gangliosides. The resulting
modification of cell surface receptors alters their susceptibility
to specific viral infections, e.g., human polyomavirus type 9 and
others. Current analyses suggest that these receptors now permit
the binding of infectious agents present in dairy cattle serum and
milk products, leading to their longtime persistence and latency
and their potential pathogenicity under conditions of reactivation.
Thus, the protective effects of breast feeding, which allows for
the maturation of the immune response during the first years of
life, emerges as critical period, which determines the uptake and
latency of specific infections (7).
Long term effects of viral infections on
prematurely born infants
Chronic respiratory morbidity is common in
prematurely born infants and has a multifactorial aetiology
(8). Lower respiratory tract
infections (LRTIs) caused by RSV increase this morbidity. Although
prematurely born infants are functionally (9,10)
and genetically (11) predisposed
to RSV LRTIs, their lung function in infancy is worse after the
LRTI (12,13). RSV LRTIs have more long-term
effects, as evidenced by very prematurely born infants, who have
bronchopulmonary dysplasia (BPD) and then subsequently suffer from
an RSV LRTI hospitalisation; in the first 2 years after birth these
infants need increased healthcare utilisation and have poorer
pulmonary functions at school age (14). There are limited data to suggest
that rhinovirus (RV) may adversely affect on respiratory-related
outcomes in prematurely born infants. In a previous study, in 8
prematurely born infants with BPD, the development of RV LRTIs was
associated with a sustained worsening of their clinical status,
requiring the addition of novel therapies for prolonged periods of
time (15). Furthermore, in
another study, prematurely born infants, who had developed RV LRTI,
during infancy, had higher health-care utilisation costs, including
greater numbers of hospital, outpatient and respiratory-related
general practitioner (GP) attendances, than infants without a viral
LRTI (16).
Ongoing research at King’s College London (UK) has
recently re-examined prematurely born cohorts at school age (5–8
years) for whom data on all respiratory viral infections in
infancy, as well as whether or not hospital admission was required
were recorded (17). The RV group
had a poorer lung function than those who had not suffered an LRTI
during infancy. Cluster analysis of the data demonstrated that
infants with an extremely low birthweight requiring prolonged
ventilation were at an increased risk of suffering from an RV LRTI
in infancy and had higher healthcare utilisation and impaired lung
function at school age (18).
These data suggest that both RSV and RV LRTI increase chronic
respiratory morbidity in prematurely born infants and, as a
consequence, this should be taken into consideration regarding the
development of relevant preventative strategies.
3. Update on current views and advances on
Paediatric Virology
Migration crisis and vaccination
policy
Over the past 2 years, Greece, one of the principal
gateway countries of the European Union (EU), has experienced an
increased inflow of refugees and migrants arriving by sea to the
Greek islands, such as the Dodecanese Islands, Lesbos, Samos and
Agathonisi (19–21). By the end of 2015, 856,723
individuals in total had arrived in the country, while between
January 1st and November 13th, 2016, the corresponding number
reported by the International Organization for Migration (IOM) was
170,553 individuals (21,22). The majority of individuals
arriving in Greece came from Syria (approximately 60%), followed by
Afghanistan, Iraq, Pakistan and Algeria (23). Following the implementation of the
EU-Turkey agreement and the closure of the Northern borders of
Greece in March 2016, >60,000 refugees and migrants remain
stranded in the country, out of which 37% are children (19).
It is worth noting that, to date, several studies
have highlighted lower immunisation coverage among refugees and
migrants compared to European-born individuals (24). This has been attributed to the low
vaccination coverage in their countries of origin due to the
collapse or inadequacy of their healthcare systems. Several
problems have also limited the access of refugees and migrants to
vaccination in Europe, such as the lack of information regarding
their immunisation status, financial problems of the hosting
countries and the lack of collaboration among public health
authorities of European countries. According to the World Health
Organization (WHO), a high vaccination coverage is required for the
protection of refugees, migrants and asylum seekers, as well as for
the population of the hosting countries, which should ensure
appropriate vaccinations, extending their routine immunisation
schedule to individuals on the move irrespective of their legal
status (25).
In Greece, an imperative need emerged for
implementing supplementary immunisation activities, particularly to
refugee children living in the ‘Points of care’, all over the
country (19). Vaccinations were
conducted in the form of a mass campaign by non-governmental
organisations (NGOs) that have significant experience and expertise
in conducting such campaigns, such as Médecins Sans Frontières
(MSF), the Red Cross and Doctors of the World, under the
coordination of the General Secretariat for Public Health of the
Hellenic Ministry of Health. In this framework, the recommendations
on refugees and migrants vaccination of the National Immunisation
Committee were followed and >30,000 vaccines were given
(26). According to data
collected by the Ministry of Health Working Group for the
coordination of refugees/migrants vaccination from May 2016 to 20
January, 2017, the children were vaccinated for 10 infectious
vaccine-preventable diseases, out of which 5 were viral infections
(26). The estimated coverage
(with 1st swoop) among children of appropriate age for each vaccine
was: Measles, mumps and rubella (MMR), 83%; diphtheria, tetanus,
pertussis and poliomyelitis, pertussis, 82%; pneumococcal disease,
76%; haemophilus influenzae type B, 75%; and hepatitis B, 79%.
Taking into account the difficulties, including
vaccine supply, the above-mentioned results are really encouraging
(19). In fact, it is important
that a great number of immunised refugee children are regularly
attending lessons in Greek schools. Public Health Authorities and
NGOs have indeed worked hard together, in order to protect
children, living through one of the biggest crisis of our times,
from infectious diseases. The challenge, today, is to continue the
immunisation of refugees and migrants in Greece, as part of routine
primary healthcare and to develop a plan for providing immunisation
to all newly-arrived refugees and migrants. For this reason, an
effective strategy should be to tailor immunisation services on the
specific needs of the target populations (27).
Mother-to-child transmission of hepatitis
viruses
Chronic infections with hepatitis B virus (HBV) and
hepatitis C virus (HCV) are responsible for 90% of all hepatitis
mortalities globally, with an estimated 257 million individuals
infected with HBV and another 71 million with HCV (28). As 90% of infants infected with HBV
at birth will become chronic carriers, mother-to-child transmission
(MTCT) is responsible for 35–50% of the chronic HBV infections
(29), which can develop into
severe liver disease including hepatocellular carcinoma (HCC) and
cirrhosis. Compared to horizontal transmission, MTCT can increase
the risk of the development of HCC (30). MTCT of HBV occurs mostly during
birth and more rarely in utero or post-natally. The risk is
increased in HBeAg-positive mothers compared to HBeAg-negative
mothers. There are also geographical differences in the risk of
MTCT of HBV, being higher in Asia than in Africa (31). MTCT of HCV occurs at a rate of 5%
and is the major cause of paediatric HCV infection (32).
The prevention of MTCT of HBV and HCV is one of the
5 synergistic measures adopted by the 194 member states of the WHO
in their commitment towards the elimination of viral hepatitis as a
public health threat by 2030 (33). The prevention of MTCT of HBV can
be achieved in 99% of cases, by delivering the birth dose of the
HBV vaccine within 24 h of birth. The risk of MTCT of HBV is
highest in infants born to younger mothers, who are HBeAg-positive
and with high viral loads. Vaccination with <3 doses is also a
risk factor for MTCT of HBV. Testing pregnant women for HBsAg,
treating those positive before delivery and administering the birth
dose and hepatitis B immunoglobulin (HBIg) to infants at birth can
successfully prevent MTCT of HBV (34). The rate of MTCT of HBV can be
reduced by caesarian section delivery (35), with breast feeding not posing a
risk factor for MTCT of HBV (36). By contrast, neither the mode of
delivery nor the type of feeding has been shown to be a risk factor
for MTCT in the case of HCV (37). HIV co-infection doubles the risk
of MTCT of HCV (38).
Although a high HCV viral load is a risk factor for
MTCT (39), it is currently not a
preventable risk factor as direct acting antivirals (DAAs) have not
been approved during pregnancy. The universal screening for HCV has
been shown to be more effective than risk factor-based screening in
preventing MTCT. The approval of DAAs in pregnant women and
children will open new horizons in the prevention of MTCT of HCV.
Interrupting MTCT of HBV and HCV is an intervention that can
contribute to the elimination of viral hepatitis as a public health
threat by 2030 and to averting the high morbidity and mortality of
the severe liver disease associated with these viral
infections.
HPV infections: Aspects of paediatric
relevance
HPVs are small, non-enveloped, double-stranded,
circular DNA viruses, belonging to the family
Papillomaviridae (40–42). HPVs infect epithelial tissue and
their life cycle is closely intertwined with the differentiation of
the epithelium (41,42). HPV requires the host DNA
replication machinery in order to replicate its genome. This
maintains the keratinocytes in a proliferative state via the action
of specific HPV proteins, of which the best characterised are E6
and E7 and thus, creates an environment amenable to carcinogenesis
(43).
HPVs are known to be the aetiologic agents in
cervical cancer and in a significant proportion of anogenital
cancers (90% of anal cancers, 60–90% of vaginal and vulvar cancers
and 30–40% of penile cancers) (44). More recently, it has become
apparent that HPVs are also drivers in a subset of head and neck
squamous cell carcinoma cases, in particular tonsillar and base of
tongue carcinomas (45). In
addition, HPVs are associated with a number of benign neoplasms,
including common cutaneous warts, genital warts, oral papillomas
and recurrent respiratory papillomatosis (RRP) (44). More than 150 types of HPVs have
been discovered to date. These are primarily classified into either
low- or high-risk according to their malignant potential, and into
mucosal and cutaneous types. HPV-16 is the foremost high-risk type
isolated from cervical, anogenital, and head and neck
carcinomas.
Paediatric manifestations of HPV infections include
skin warts, oral papillomas, RRP and anogenital warts (46,47). Additionally, asymptomatic
infections of the oral or tonsillar mucosa, as well as of the
genital mucosa in children have been described (46,47). Studies on HPV seropositivity in
young children indicate a relatively low prevalence of HPV
antibodies (approximately 0–10%) (48). The seroprevalence significantly
increases in adolescence with the commencement of sexual activity.
Mucosal HPV infection is often described as a sexually transmitted
infection; however, the presence of such infections in infants and
young children suggests that the virus may also be vertically and
horizontally transmitted. There is strong evidence that maternal
HPV cervical infection at the time of delivery can be transmitted
to the newborn and there is a strong association between
development of juvenile RRP and the presence of maternal genital
warts during pregnancy (49). The
detection of HPV DNA in spermatozoa and seminal plasma, as well as
in amniotic fluid and placental tissue, suggests that
periconceptual and prenatal transmission may also be possible
(46,47). HPV can be transmitted horizontally
(i.e., by kissing or touch) or to different sites by
auto-inoculation (46,47). In the case of anogenital warts in
particular, sexual abuse remains an important consideration. There
are a lack of data on the persistence of HPV infections in children
and their relation to the subsequent development of HPV-associated
diseases, such as oropharyngeal carcinoma.
The routine vaccination of girls, aged 11–12 years,
has been introduced in many countries to reduce the incidence of
anogenital cancers. The Nobelist laureate Professor Harald zur
Hausen has actively advocated the vaccination of boys for the
eradication of HPV infection and has emphasised that male HPV
vaccination should be included into the current vaccination
programmes. His publication with Professor Karin Michels from
Harvard Medical School entitled ‘HPV vaccine for all’ and published
in 2009 in Lancet has been one of the very first publications
advocating the vaccination of boys (50). To date, the United States, Canada,
Australia, and Austria have currently implemented routine
vaccination of girls as well as boys (51–53). The recent WHO guidelines
recommended the vaccination of girls aged 9–14 years as the primary
target population, and the vaccination of additional target
populations (females 15 years and older, and males) only if
feasible, affordable and cost-effective (54). Gender-neutral vaccination may
achieve greater herd protection effects and direct protection of
males; however gender-neutral programmes have only been implemented
recently and their impact on HPV-related diseases at a population
level remains to be determined (54). Nevertheless, there are strong data
that quadrivalent HPV vaccination is effective in reducing anal
intraepithelial neoplasia (50% reduction in the intention-to-treat
population and 78% in the per-protocol population) (55). Similar prospective data for penile
cancers and oropharyngeal cancers is lacking; however, there are
data suggesting that HPV infections at these sites are reduced
following vaccination (56,57).
Non-vaccinated children will continue to be affected
by paediatric HPV-associated diseases and vaccinated children by
HPV types not covered by vaccination. Increasing vaccine coverage
may reduce HPV prevalence not only for anogenital infections, but
also oral infections. However, vaccination programmes continue to
face challenges, in part due to parental fears regarding vaccine
safety (58). Large
population-based studies have confirmed vaccine safety (59). Educational efforts regarding the
importance of HPV vaccination and the vaccine’s efficacy and safety
profile are necessary to achieve higher vaccination coverage.
Intranasal live-attenuated influenza
vaccination in children
Live-attenuated influenza vaccines (LAIV) have been
used in certain European countries, such as UK and Finland, and in
the USA and Canada for decades (60,61). In addition to their efficient mode
of introduction as a nasal spray, in randomised clinical trials,
they have been shown to provide better protective efficacy than
inactivated influenza vaccines (IIV) in children, which are
considered to be the main drivers of influenza transmission.
However, recent observational studies have shown conflicting
evidence regarding the effectiveness of LAIV. During the 2015/16
influenza season, a reduced vaccine effectiveness (VE) was observed
in the United States, and for this reason the Centers for Disease
Prevention and Control (CDC) recommended the use of the IIV and the
recombinant influenza vaccine (RIV), instead of the LAIV for the
2016/17 season (62). However, in
2015/16, in the UK, Finland and Canada, LAIV VE was shown to be
protective against the drifted influenza A(H3N2) and B viruses and
with suboptimal effectiveness only against A(H1N1)pdm09 compared to
IIV (63–65). Studies have attributed the reduced
effectiveness against A(H1N1)pdm09 to the thermal instability of
this virus component and the handling conditions of the vaccines
(66).
There are certain performance limitations, one of
which is that previous influenza vaccination may modify the immune
response after a repeated vaccination (60). Further studies are warranted to
determine the reasons for reduced VE. A recent systematic
literature review did not find any evidence of the decreased
efficacy of LAIV when administered during two consecutive seasons
(67). On the other hand, the
findings of a study that evaluated the impact of repeated
vaccination among health care workers were suggestive of decreased
serological response among the highly vaccinated group (68). Another consortium, Seasonal
Influenza Vaccination Effectiveness II (SIVE II), is currently
evaluating the safety and effectiveness of the LAIV programme in
children (69). The vaccination
of healthy children has the potential to provide both direct
protection to children, but also indirect protection to vulnerable
groups in the general population. Further studies with large sample
sizes and a greater number of people in the vaccine-naïve group and
in groups with repeated vaccinations are required. Population-based
national health registries are also valuable to generate routine
data for measuring the vaccination impact in a timely manner. As
the LAIV use has been shown to be beneficial over the past years,
continuous enhanced surveillance and further study results are
required in order to better understand the USA findings and the
possible implications for vaccination policies in Europe and
worldwide.
Probiotics and viral infections in
childhood
Probiotics (e.g., Lactobacilli,
Bifidobacteria and Saccharomyces) are live
microorganisms, which can confer beneficial health effects on the
host, when consumed in adequate amounts (70,71). They are non-pathogenic in the
normal host, resist processing and are able to survive in the human
digestive tract. Prebiotics (e.g., Fructo- and
Galacto-oligosaccharides) are non-viable food components, which can
confer a significant health benefit on the host by modulating
intestinal microflora. Synbiotics are a combination of pro- and
prebiotics. The gut microflora is a complex ecosystem supporting
the structure and function of the intestinal mucosa. Probiotics
have the ability to modify the gut microflora either by lowering
colonic pH through production of short-chain fatty acids (SCFAs),
or by producing antimicrobial compounds and antitoxins, and or by
competing with other bacteria for nutrients. They also enhance gut
barrier function and play a role in immunomodulation (72,73).
Probiotics have been used for the management of
children with acute viral diarrhoea as an adjunct to rehydration
therapy (74–76). To date, 5 paediatric meta-analyses
have shown that probiotics reduce the duration of diarrhoea,
ranging between −16.8 h and −30.0 h, as well as the risk of
diarrhoea lasting for >4 days (74). The subgroup with
rotavirus-associated diarrhoea seemed to be more responsive to
probiotics, while Lactobacillus rhamnosus GG (LGG) appeared
to be the most effective probiotic (75). The beneficial effects of
probiotics seem to be more evident when treatment is initiated
early (<48 h) and are strain- and dose-dependent. Probiotics are
not useful for treating bacterial diarrhoea (76).
Probiotics have also been proposed to be helpful in
preventing upper respiratory tract infections (URTIs); however the
interventions and existing evidence are inconsistent (77,78). A recent systematic review
(77) of 14 randomised controlled
trials (RCTs) suggested that probiotic prophylaxis reduced the
number of children, who had one or more URTIs [odds ratio (OR)
0.58, 95% confidence interval (CI) 0.36 to 0.92] and the number of
URTIs per person-year (rate ratio 0.88, 95% CI 0.81 to 0.96). Both
outcomes had inconsistent results in the individual studies,
reflected in estimates of heterogeneity. A better understanding of
the effects of different probiotic strains and a deeper insight
into their mechanisms of action are required for the validation of
specific strains carrying a potential to modify the frequency and
severity of URTIs and gastrointestinal viral infections in infants
and children (78–80).
Fetal and neonatal brain imaging in viral
infections
Fetuses and neonates are susceptible to a wide
variety of viral infections most commonly involving the central
nervous system (CNS) in greater frequency than adults (81). Infections of the CNS are a very
common worldwide health problem in childhood with significant
morbidity and mortality. In children, viruses are the most common
cause of CNS infections, followed by bacteria, and less frequently
by fungi and other causes. Advances in the prenatal and perinatal
care together with technological advent of imaging modalities have
enabled timely detection and detailed exploration of symptoms and
signs in the neonatal population starting from the fetal life.
Although imaging is practically unable to set the diagnosis of
viral infection in the fetuses and neonates, moreover to reveal the
pathogens, it has, however, the potential to accurately suggest
this scenario, map the extent of involvement and direct the
investigation and the consultation accordingly. Additionally, it
may reveal complications from viral infections that may cause
confusion and usually require special treatment (81).
Some imaging findings are highly suggestive of CNS
viral infections in fetuses and neonates (82). Familiarity with the clinical
course, the route of transmission and the imaging appearances
usually proves helpful in reaching the correct diagnosis and in
prompting timely treatment. In general, sequelae of an intrauterine
infection reflect a combination of the pathogens and the stage of
fetal development at which the exposure occurred (83). Congenital infections, occurring
during the second and third trimester, may persist in the neonate
affecting its general and neurologic status (83). However, as a rule of thumb, the
later the diagnosis of congenital infections is made, the more
difficult it is to identify the agent. Additionally, the imaging
findings may become non-specific and less conspicuous as incomplete
white matter myelination may interfere (83).
If maternal viral infection is suspected, combining
prenatal ultrasound and fetal magnetic resonance imaging (MRI) may
document the extent of tissue damage and therefore contribute to
treatment and counselling (84).
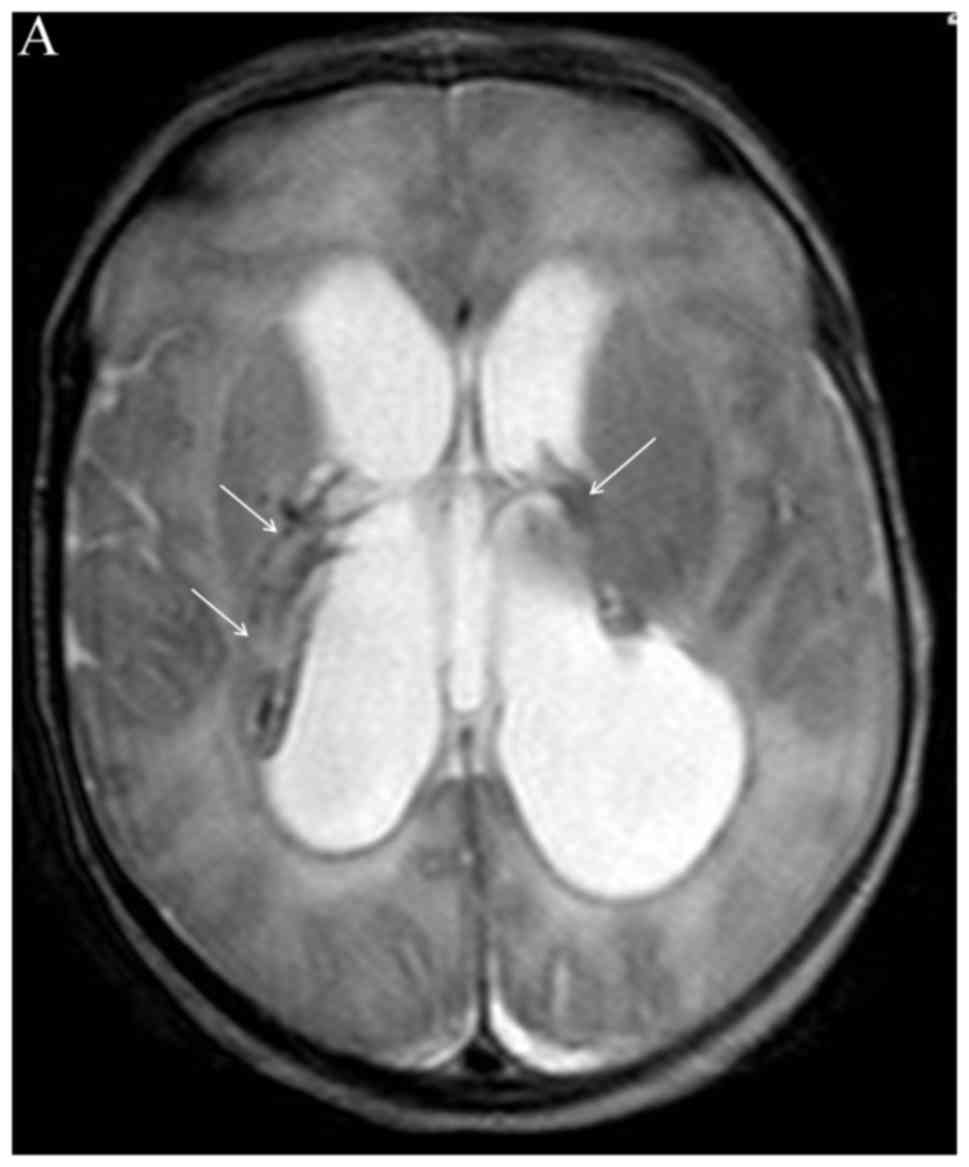
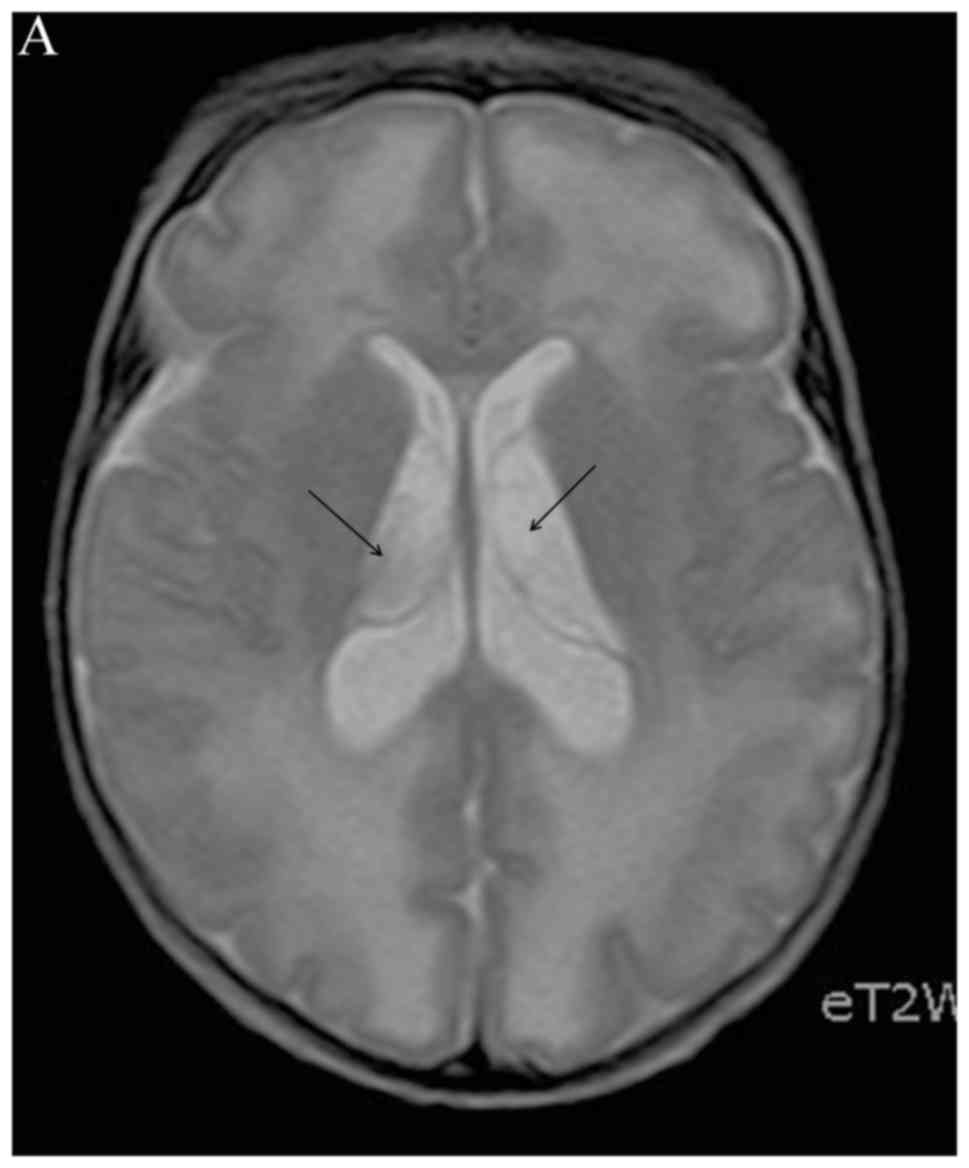
Neonatal head ultrasound, sometimes computed tomography (CT), but
mainly MRI (Figs. 1 and 2) may reveal sequelae from congenital
viral infections (i.e., microcephaly, dystrophic periventricular
calcifications, brain atrophy), which may even suggest the
causative virus, such as cytomegalovirus (CMV) (85). In previously healthy neonates with
viral infection, the imaging investigation of CNS begins with head
ultrasound and if further imaging investigation is required, MRI is
the modality of choice, even in an emergency setting (86). Non-complicated meningitis is
easier to be recognised clinically; however, since complications of
meningitis, such as abscesses, infarcts, venous thrombosis, or
extra-axial empyemas are difficult to diagnose clinically, imaging
plays a crucial role (87).
Viral infections of CNS and paediatric
intensive care
Viral infections can invade the CNS of children,
spreading into the peripheral nervous system (PNS) and more rarely
into the CNS (88). The CNS is
protected by effective immune responses and multi-layer barriers;
however, some viruses enter the CNS with efficiency via the
bloodstream or by directly infecting nerves that innervate
peripheral tissues, resulting in immune-mediated pathology
(89). Viral infections of the
CNS more often cause meningitis, characterised by a high fever,
headaches, vomiting, photophobia and neck stiffness and
encephalitis, which appear with clinical evidence of neurological
dysfunction, such as altered mental status, seizures, behavioral
changes and focal neurologic signs (90). Other clinical manifestations are
myelitis, characterised by weakness, bladder dysfunction, flaccid
paralysis and reduced or absent reflexes, radiculitis, which is
characterised by weakness, shooting pain, dysesthesia and
diminished reflexes and complex syndromes, such as
meningoencephalitis and encephalomyelitis.
Viruses affect different sites of the CNS;
enteroviruses, herpes simplex virus 2 (HSV-2) and mumps virus (MuV)
are responsible for >90% of cases of aseptic meningitis, whereas
herpes simplex virus 1 (HSV-1) is the most common cause of
encephalitis in developed countries. Other types of herpes viruses,
such as Epstein-Barr virus (EBV), CMV, varicella-zoster virus (VZV)
and human herpes viruses 6 and 7 (HHV-6 and -7) can invade the
brain and influence the neurological status of children.
Additionally, there are viruses (Table III), such as West Nile virus
(WNV), Japanese encephalitis virus, Tick-borne encephalitis virus
and Western and Eastern equine encephalitis virus, with certain
geographic distribution and their differential diagnosis should be
taken into account in cases of children, who have travelled in
susceptible areas (91,92). According to the recent data from
the Paediatric Intensive Care Unit (PICU) of the ‘Aglaia Kyriakou’
Children’s Hospital (88) in
Athens, Greece, among 29 children with CNS infection admitted to
the PICU during the past 5 years, 17 patients (59%) had a viral
infection; however, a certain aetiological factor was detected in
only 6 of these patients (4 cases with influenza, 1 case with
enterovirus and 1 case with HHV-7), whereas 2 patients suffered
from autoimmune encephalitis.
 | Table IIIViral causes of central nervous
system (CNS) infections in neonates and children. |
Table III
Viral causes of central nervous
system (CNS) infections in neonates and children.
| Worldwide
distribution | Limited to certain
geographical areas |
|---|
| Herpes viruses | WNV |
| HSV-1, -2 | Japanese
encephalitis virus |
| VZV | St. Louis
encephalitis virus |
| EBV | Tick-borne
encephalitis virus |
| CMV | Western equine
encephalitis virus |
| HHV-6, -7 | Eastern equine
encephalitis virus |
| Non-polio
enteroviruses | |
| MuV | |
| Measles virus | |
| JC
polyomaviruses | |
| BK virus | |
All patients with suspected CNS infection should
undergo lumbar puncture (LP) unless this is contraindicated due to
raised intracranial pressure. Investigations of cerebrospinal fluid
(CSF) include the total and differential white cell count, red cell
count, microscopy, culture, protein, glucose, lactate and a
polymerase chain reaction (PCR) test for HSV-1 and HSV-2, VZV,
enteroviruses and other possible pathogens depending on the
clinical features (92,93). Neuroimaging, a CT scan or
preferably MRI, should be performed as soon as possible. An MRI
performed within 48 h of admission is abnormal in approximately 90%
of patients, with some lesions being specific of certain
aetiological factors (93). An
electroencephalogram (EEG) is considered if seizures are
suspected.
All patients with suspected viral encephalitis start
treatment with acyclovir and if the PCR test is positive for HSVs
or VZV, should continue treatment for at least 14–21 days (93). Corticosteroids can also be used,
although their role remains controversial as they have strong
immunomodulatory effects, which may facilitate viral replication.
Enterovirus infection requires only supportive care and in more
severe cases intravenous immunoglobulin (IVIG) alone or in
combination with high doses of corticosteroids (90). Other drugs, such as oseltamivir or
ganciclovir, are administered according to the underlying viral
cause. Viral CNS infections are a life-threatening emergency.
Supportive care is crucial and includes airway management,
cardiorespiratory support, the maintenance of fluids and
electrolyte balance and the management of seizures, according to
specific guidelines (94).
Approximately 30% of patients require PICU admission. The overall
mortality rates range from 0 to 7%, depending on the pathogen, age
and clinical and laboratory findings at the time of presentation.
Approximately 35% of survivors will suffer from some form of
permanent disability (seizures, learning problems, motor deficits,
developmental delay) with much a higher incidence, two thirds of
patients, in HSV encephalitis cases.
RSV and RV infection and their role in
asthma
Asthma has been considered for >30 years as the
most common chronic disease in childhood (95,96); the role of allergy in its
development is indeed very significant (97). Other risk factors that have also
been associated with the pathogenesis of asthma include
prematurity, parental history of asthma, reduced lung size and
function, chronic lung disease, neuromuscular or cardiovascular
disease, the male sex, low socioeconomic status, Down syndrome,
intrauterine tobacco smoke exposure and passive smoking, daycare
attendance, early exposure to pets, as well as viral respiratory
tract infections in infancy (96,98–100). Among these factors, infections,
such as acute viral bronchiolitis, caused by RSV and RV in
particular, have been recognised as one of the most significant
predictive markers for the development of asthma in later childhood
to young adulthood (99–102).
RSV is a common cause of wheezing that seems to
dominate in the first year of an infant’s life, during the winter
period, causing mainly acute bronchiolitis, while RV is being
detected as a causative agent for acute bronchiolitis and lower
tract viral infections in older infants and children (103). It remains to be clarified
though, whether it is the specific viral respiratory infection that
plays the causative role in asthmatogenesis or whether
viral-induced wheezing is just a marker of predisposed individuals
for the subsequent development of asthma (104). It has been well demonstrated
that there is an interaction of host factors with viral respiratory
infections that promote recurrent virus-induced wheezing and the
subsequent development of asthma (101). The synergistic interaction
between allergies and viruses can be explained via various
mechanisms, such as interferon deficiency, Th2-biased immunity,
defective anti-inflammatory response, airway hyper responsiveness
and reduced barrier function (97,103). In a recent randomised trial of
429 high-risk infants, treatment with palivizumab of otherwise
healthy preterm infants in order to avoid a serious RSV infection
was associated with decreased recurrent wheezing rate when compared
with the placebo group (105).
RSV causes cytopathic damage to the airway epithelium and by that
can affect the function of the epithelium and airway smooth muscle
(100).
To date, there is increasing evidence to indicate
that RV, the most frequent cause of the common cold in children,
can replicate in the lower respiratory tract and can cause
exacerbations of asthma (97,103). Certain features of infection
with RV, including the stimulation of interleukin (IL)-4, IL-13 and
eotaxin production; the immigration of inflammatory cells
(eosinophils, macrophages and neutrophils); and subsequent
increases in factors considered important for airway remodeling
(such as NO and vascular endothelial growth factor), point to the
likelihood that RV infection is asthmatogenic (106). Given the burden of asthma, it is
of utmost importance that future scientific attempts reveal in
detail the causality between viral infections and asthmatogenesis.
Thus, better asthma-predictive indices can be developed and more
effective strategies for asthma prevention can be scheduled,
including research for effective vaccines.
Neonatal mortality and viral infections
in ancient Greece
During the 1937 and 1938 American excavations of the
Agora, in Athens, a well was excavated that was found to contain a
minimum of 459 infant and fetal skeletons (107–109). Although excavated 70 years ago,
the details of its contents were never published. As estimated from
long bone lengths, the age of the infants ranged from 26 weeks
in utero to 6 months post-term, with a peak at 38–40 weeks
(110). The cause of death for
the infants in the Agora’s well has been the topic of much
speculation over the past 70 years. Suggestions have included
war-related famine or epidemic disease, human sacrifice and
large-scale infanticide. One of the goals of this project was to
evaluate the evidence for pathologies in the infants in order to
evaluate the causes of death. Infant death rates from natural
causes in the ancient world were very high, and up to 50% of
neonates may have died early in the postnatal period (111). In addition, approximately 15% of
these skeletons are from infants born pre-term, as young as 26
weeks in utero. Many of these were probably either stillborn
or died from complications associated with prematurity, including
respiratory distress and problems with thermoregulation.
The cranial bones provide evidence for pathologies
and possible infectious causes of death. Most of the lesions are
diffuse areas of new bone formed on the endocranial surface. The
cruciate eminence of the occipital bone was the most common
location for endocranial reactive bone in the infants, and this
corresponds to the findings in other ancient and modern skeletal
collections. Lesions have also been identified on the parietal and
frontal bones, often in areas of venous drainage. The greater wing
and body of the sphenoid bone also exhibited endocranial
proliferative bone lesions. It has been found that that lesions
extending beyond the cruciate eminence of the occipital, and
hair-on-end lesions are more likely to be pathological, rather than
indicative of than normal bone growth (112). More than 30% of the occipital
squamae in this collection exhibited clear pathological bone
deposits.
Infections and birth-related trauma are thought to
have caused most of these lesions. Infections of the meninges are
thought to activate the periosteal membrane, resulting in new bone
deposition, but the exact mechanism is not clear (112). Infectious pathogens were
commonly introduced through cutting the umbilical cord, and through
cultural and dietary practices. It is likely that infants in
ancient Athens suffered from the same array of viral infections to
which modern infants are prone, including HSV, influenza virus,
corona virus, enterovirus, EBV and arbovirus (113). The perinatal period in ancient
Athens was perilous, with considerable risk from viral and
bacterial infections. The skeletons from this well provide a brief
glimpse into the short lives and early deaths of the youngest
Athenians.
Continuing medical education in
Paediatric Virology
To provide the optimal care to paediatric patients,
a paediatrician should commit to lifelong learning (114), following the quote by the
Athenian Solon (640–558 BC), one of the Seven Wise Men of Ancient
Greece, ‘I grow old learning something new every day’ (γηράσκω δ’
αἰεὶ πολλὰ διδασκόμενος) (115).
To date, the importance of continuing medical education (CME), also
known as continuing medical development (CMD), has been recognised
as a method of improving the quality of paediatric care by several
paediatric organisations and institutions, such as the European
Academy of Paediatrics (116)
and the RCPCH (117). It is a
professional responsibility for all practicing paediatricians to
maintain, develop and increase their knowledge, clinical skills and
professional performance with an aim to enhance their clinical
effectiveness and competence (118–122). Paediatric Virology is indeed a
new educational challenge with rapidly increasing and changing
educational needs and preferences (123). This is fostered by several
developments in the fields of Clinical Virology and Molecular
Medicine, emerging viral diseases as well as technical innovations,
which have expanded our diagnostic and therapeutic approaches
towards neonatal and paediatric viral infections.
The feedback received from the participants of the
1st and the 2nd Workshop on Paediatric Virology held in 2015 and
2016, respectively, in Athens, highlighted the need for CME
(114). CME in Paediatric
Virology must not only emphasise the acquisition of knowledge, but
also instruct paediatricians in the process of decision making.
Self-directed learning activity should be encouraged; however, CME
in Paediatric Virology should also instill collegiality,
interaction and collaboration between disciplines. Without a doubt,
Paediatric Virologists should have a leading role in CME in
Paediatric Virology focusing on academic excellence and
state-of-the-art medical education of practicing paediatricians.
Further discussion is required on how different educational
activities, such as on-line learning, interactive case-based
workshops and creation of educational e-platforms, can increase
their potential worth for the promotion of Peadiatrics and Public
Health.
Acknowledgments
The authors would like to thank the plenary
lecturers, the chair persons and the speakers of the ‘3rd Workshop
on Paediatric Virology’ for their significant contribution in
providing the most up-to-date information. The authors would also
like to thank all the participants, who attended the workshop and
provided feedback so that further research on improving the
programme can be achieved for the future. We would like to thank
Nobelist laureate Professor Harald zur Hausen, Emeritus Professor
of Virology at the University of Freiburg, for his detailed
corrections and comments on our manuscript. We would also like to
thank Dr Neda Voutsa-Perdiki, former Director of Exfoliative
Cytology at the University of Florida, USA and academic student of
Dr George N. Papanicοlaou, for her touching presence in our
workshop. Last but not least, this workshop would not have been
possible without the dedicated hard work and strong commitment from
the Congress Secretariat, the members of the PVSG and the whole
organising team of the ‘22nd World Congress on Advances in
Oncology’ and the ‘20th International Symposium on Molecular
Medicine’ for developing the scientific programme, coordinating and
guaranteeing the success of this scientific event.
Notes
[1] Competing
interests
Demetrios Spandidos is the Editor-in-Chief for the
journal, but had no personal involvement in the reviewing process,
or any influence in terms of adjudicating on the final decision,
for this article.
References
|
1
|
Greenough A, Osborne J and Sutherland S:
Congenital, Perinatal, and Neonatal Infections. Churchill
Livingstone; Edinburgh: 1992
|
|
2
|
Shulman ST: The history of pediatric
infectious diseases. Pediatr Res. 55:163–176. 2004. View Article : Google Scholar
|
|
3
|
Starr M: Paediatric infectious diseases:
The last 50 years. J Paediatr Child Health. 51:12–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mammas IN, Greenough A, Theodoridou M,
Kramvis A, Christaki I, Koutsaftiki C, Koutsaki M, Portaliou DM,
Kostagianni G, Panagopoulou P, Sourvinos G and Spandidos DA:
Current views and advances on Paediatric Virology: An update for
paediatric trainees. Exp Ther Med. 11:6–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mammas IN, Greenough A, Theodoridou M and
Spandidos DA: Paediatric Virology: A new paediatric subspecialty? A
proposal at the Workshop on Paediatric Virology, Athens, October
10,2015. Exp Ther Med. 11:3–5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
zur Hausen H: Paediatric Virology and
Oncology: Virus persistence and the important first years of life.
Int J Mol Med. 40(Suppl 41)2017.
|
|
7
|
zur Hausen H, Bund T and de Villiers EM:
Infectious agents in bovine red meat and milk and their potential
role in cancer and other chronic diseases. Curr Top Microbiol
Immunol. 407:83–116. 2017.PubMed/NCBI
|
|
8
|
Greenough A, MacBean V, Lunt A, Drysdale
SB, Rafferty GF and Peacock JL: The long term impact of viral
infections on prematurely born infants. Int J Mol Med. 40(Suppl
41)2017.
|
|
9
|
Broughton S, Bhat R, Roberts A, Zuckerman
M, Rafferty G and Greenough A: Diminished lung function, RSV
infection, and respiratory morbidity in prematurely born infants.
Arch Dis Child. 91:26–30. 2006. View Article : Google Scholar
|
|
10
|
Drysdale SB, Wilson T, Alcazar M,
Broughton S, Zuckerman M, Smith M, Rafferty GF, Johnston SL and
Greenough A: Lung function prior to viral lower respiratory tract
infections in prematurely born infants. Thorax. 66:468–473. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drysdale SB, Prendergast M, Alcazar M,
Wilson T, Smith M, Zuckerman M, Broughton S, Rafferty GF, Johnston
SL, Hodemaekers HM, et al: Genetic predisposition of RSV
infection-related respiratory morbidity in preterm infants. Eur J
Pediatr. 173:905–912. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drysdale SB, Lo J, Prendergast M, Alcazar
M, Wilson T, Zuckerman M, Smith M, Broughton S, Rafferty GF,
Peacock JL, et al: Lung function of preterm infants before and
after viral infections. Eur J Pediatr. 173:1497–1504. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Broughton S, Roberts A, Fox G, Pollina E,
Zuckerman M, Chaudhry S and Greenough A: Prospective study of
healthcare utilisation and respiratory morbidity due to RSV
infection in prematurely born infants. Thorax. 60:1039–1044. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greenough A, Alexander J, Boit P, Boorman
J, Burgess S, Burke A, Chetcuti PA, Cliff I, Lenney W, Lytle T, et
al: School age outcome of hospitalisation with respiratory
syncytial virus infection of prematurely born infants. Thorax.
64:490–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chidekel AS, Rosen CL and Bazzy AR:
Rhinovirus infection associated with serious lower respiratory
illness in patients with bronchopulmonary dysplasia. Pediatr Infect
Dis J. 16:43–47. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Drysdale SB, Alcazar-Paris M, Wilson T,
Smith M, Zuckerman M, Broughton S, Rafferty GF, Peacock JL,
Johnston SL and Greenough A: Rhinovirus infection and healthcare
utilisation in prematurely born infants. Eur Respir J.
42:1029–1036. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
MacBean V, Lunt A, Drysdale S, Rafferty GF
and Greenough A: Predicting healthcare outcomes in prematurely born
infants using cluster analysis. Arch Dis Child. 102:A190–A191.
2017.
|
|
18
|
MacBean V, Yarzi MN, Drysdale S, Rafferty
GF and Greenough A: Impact of respiratory viral infections on
school age outcomes in prematurely born children. Am J Respir Crit
Care Med. 195:A21692017.
|
|
19
|
Theodoridou M: Immigration crisis and
vaccination policy. Int J Mol Med. 40:S412017.
|
|
20
|
United Nations High Commissioner for
Refugees (UNHCR): The sea route to Europe: The Mediterranean
passage in the age of refugees. 2015, http://www.unhcr.org/5592bd059.pdf.
|
|
21
|
Medecins Sans Frontieres (MSF): EU
Migration Crisis Update - February 2016. 2016, http://www.msf.org/en/article/eu-migration-crisis-update-february-2016.
|
|
22
|
International Organization for Migration
(IOM): IOM: 170.553 migrants/refugees have arrived in Greece in
2016. 2016, https://greece.iom.int/en/news/iom-170553-migrantsrefugees-have-arrived-greece-2016.
|
|
23
|
REACH: Situation overview: European
migration crisis, Western Balkans. December. 2015, https://www.impact-repository.org/document/reach/a43ee2d2/reach_eu_situation_overview_european_migration_trends_december_2015_5.pdf.
|
|
24
|
Mipatrini D, Stefanelli P, Severoni S and
Rezza G: Vaccinations in migrants and refugees: A challenge for
European health systems. A systematic review of current scientific
evidence. Pathog Glob Health. 111:59–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
World Health Organization (WHO). Refugee
and migrant health. 2017, http://www.who.int/migrants/en/.
|
|
26
|
Hellenic Ministry of Health. Report.
Vaccination on Points of care of refugees/migrants. February. 2017,
http://www.moh.gov.gr/articles/ministry/grafeio-typoy/press-releases/4489-ekthesh-emboliastikhs-kalypshs-paidiwn-metanastwn-kai-prosfygwn.
|
|
27
|
Giambi C, Del Manso M, Dente MG, Napoli C,
Montaño-Remacha C, Riccardo F and Declich S; Network For The
Control Of Cross-Border Health Threats In The Mediterranean Basin
And Black Sea For The ProVacMed Project: Immunization strategies
targeting newly arrived migrants in non-EU countries of the
Mediterranean Basin and Black Sea. Int J Environ Res Public Health.
14:142017. View Article : Google Scholar
|
|
28
|
World Health Organization (WHO): World
Health Organization Global Hepatitis Report 2017. 2017, http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/.
|
|
29
|
Kramvis A: Mother to child transmission of
hepatitis B and C viruses. Int J Mol Med. 40:S392017.
|
|
30
|
Shimakawa Y, Lemoine M, Bottomley C, Njai
HF, Ndow G, Jatta A, Tamba S, Bojang L, Taal M, Nyan O, et al:
Birth order and risk of hepatocellular carcinoma in chronic
carriers of hepatitis B virus: A case-control study in The Gambia.
Liver Int. 35:2318–2326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kramvis A: The clinical implications of
hepatitis B virus genotypes and HBeAg in pediatrics. Rev Med Virol.
26:285–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tovo PA, Calitri C, Scolfaro C, Gabiano C
and Garazzino S: Vertically acquired hepatitis C virus infection:
Correlates of transmission and disease progression. World J
Gastroenterol. 22:1382–1392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
World Health Organization (WHO). Global
Health Sector Strategy on viral hepatitis 2016–2021. 2016,
http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/.
|
|
34
|
Schillie S, Walker T, Veselsky S, Crowley
S, Dusek C, Lazaroff J, Morris SA, Onye K, Ko S, Fenlon N, et al:
Outcomes of infants born to women infected with hepatitis B.
Pediatrics. 135:e1141–e1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brown RS Jr, McMahon BJ, Lok AS, Wong JB,
Ahmed AT, Mouchli MA, Wang Z, Prokop LJ, Murad MH and Mohammed K:
Antiviral therapy in chronic hepatitis B viral infection during
pregnancy: A systematic review and meta-analysis. Hepatology.
63:319–333. 2016. View Article : Google Scholar
|
|
36
|
Pan CQ, Zou HB, Chen Y, Zhang X, Zhang H,
Li J and Duan Z: Cesarean section reduces perinatal transmission of
hepatitis B virus infection from hepatitis B surface
antigen-positive women to their infants. Clin Gastroenterol
Hepatol. 11:1349–1355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen X, Chen J, Wen J, Xu C, Zhang S, Zhou
YH and Hu Y: Breastfeeding is not a risk factor for mother-to-child
transmission of hepatitis B virus. PLoS One. 8:e553032013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Benova L, Mohamoud YA, Calvert C and
Abu-Raddad LJ: Vertical transmission of hepatitis C virus:
Systematic review and meta-analysis. Clin Infect Dis. 59:765–773.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Elrazek A, Amer M, El-Hawary B, Salah A,
Bhagavathula AS, Alboraie M and Saab S: Prediction of HCV vertical
transmission: What factors should be optimized using data mining
computational analysis. Liver Int. 37:529–533. 2017. View Article : Google Scholar
|
|
40
|
Rusan M: Human papillomavirus infections
in children: Should we be vaccinating earlier? Int J Mol Med.
40:S402017.
|
|
41
|
Doorbar J, Quint W, Banks L, Bravo IG,
Stoler M, Broker TR and Stanley MA: The biology and life-cycle of
human papillomaviruses. Vaccine. 30(Suppl 5): F55–F70. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kajitani N, Satsuka A, Kawate A and Sakai
H: Productive lifecycle of human papillomaviruses that depends upon
squamous epithelial differentiation. Front Microbiol. 3:1522012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lehoux M, D’Abramo CM and Archambault J:
Molecular mechanisms of human papillomavirus-induced
carcinogenesis. Public Health Genomics. 12:268–280. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Trottier H and Burchell AN: Epidemiology
of mucosal human papillomavirus infection and associated diseases.
Public Health Genomics. 12:291–307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Forman D, de Martel C, Lacey CJ,
Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J,
Bray F, Plummer M, et al: global burden of human papillomavirus and
related diseases. Vaccine. 30(Suppl 5): F12–F23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Syrjänen S: Current concepts on human
papillomavirus infections in children. APMIS. 118:494–509. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mammas IN, Sourvinos G and Spandidos DA:
Human papilloma virus (HPV) infection in children and adolescents.
Eur J Pediatr. 168:267–273. 2009. View Article : Google Scholar
|
|
48
|
Tiggelaar SM, Lin MJ, Viscidi RP, Ji J and
Smith JS: Age-specific human papillomavirus antibody and
deoxyribonucleic acid prevalence: A global review. J Adolesc
Health. 50:110–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Silverberg MJ, Thorsen P, Lindeberg H,
Grant LA and Shah KV: Condyloma in pregnancy is strongly predictive
of juvenile-onset recurrent respiratory papillomatosis. Obstet
Gynecol. 101:645–652. 2003.PubMed/NCBI
|
|
50
|
Michels KB and zur Hausen H: HPV vaccine
for all. Lancet. 374:268–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ontario: Getting the HPV vaccine.
Government of Ontario; 2017, http://www.health.gov.on.ca/en/ms/hpv/.
|
|
52
|
Centers for Disease Control and Prevention
(CDC): HPV Vaccines: Vaccinating Your Preteen or Teen. 2016,
https://www.cdc.gov/hpv/parents/vaccine.html.
|
|
53
|
Paul KT: ‘Saving lives’: Adapting and
adopting Human Papilloma Virus (HPV) vaccination in Austria. Soc
Sci Med. 153:193–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
World Health Organization (WHO): Human
papillomavirus vaccines: WHO position paper, May
2017-Recommendations. Vaccine. 2017.In press.
|
|
55
|
Palefsky JM, Giuliano AR, Goldstone S,
Moreira ED Jr, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F,
Stoler MH, et al: HPV vaccine against anal HPV infection and anal
intraepithelial neoplasia. N Engl J Med. 365:1576–1585. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Anic GM and Giuliano AR: Genital HPV
infection and related lesions in men. Prev Med. 53(Suppl 1):
S36–S41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Herrero R, Quint W, Hildesheim A, Gonzalez
P, Struijk L, Katki HA, Porras C, Schiffman M, Rodriguez AC,
Solomon D, et al CVT Vaccine Group: Reduced prevalence of oral
human papillomavirus (HPV) 4 years after bivalent HPV vaccination
in a randomized clinical trial in Costa Rica. PLoS One.
8:e683292013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Drolet M, Bénard É, Boily MC, Ali H,
Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JM, Cummings
T, et al: Population-level impact and herd effects following human
papillomavirus vaccination programmes: A systematic review and
meta-analysis. Lancet Infect Dis. 15:565–580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vichnin M, Bonanni P, Klein NP, Garland
SM, Block SL, Kjaer SK, Sings HL, Perez G, Haupt RM, Saah AJ, et
al: An overview of quadrivalent human papillomavirus vaccine
safety: 2006 to 2015. Pediatr Infect Dis J. 34:983–991. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Melidou A: Intranasal live-attenuated
influenza vaccination in children: Evidence, limitations and
proposals. Int J Mol Med. 40:S392017.
|
|
61
|
Rodgers L, Pabst LJ and Chaves SS:
Increasing uptake of live attenuated influenza vaccine among
children in the United States, 2008-2014. Vaccine. 33:22–24. 2015.
View Article : Google Scholar
|
|
62
|
Centers for Disease Prevention and Control
(CDC). Seasonal Influenza: Flu Basics. 2016, https://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm.
|
|
63
|
Pebody R, Warburton F, Ellis J, Andrews N,
Potts A, Cottrell S, Johnston J, Reynolds A, Gunson R, Thompson C,
et al: Effectiveness of seasonal influenza vaccine for adults and
children in preventing laboratory-confirmed influenza in primary
care in the United Kingdom: 2015/16 end-of-season results. Euro
Surveill. 21:212016.
|
|
64
|
Nohynek H, Baum U, Syrjänen R, Ikonen N,
Sundman J and Jokinen J: Effectiveness of the live attenuated and
the inactivated influenza vaccine in two-year-olds - a nationwide
cohort study Finlandm influenza season 2015/16. Euro Surveill.
21:212016. View Article : Google Scholar
|
|
65
|
Kwong JC, Pereira JA, Quach S, Pellizzari
R, Dusome E, Russell ML, Hamid JS, Feinberg Y, Winter AL, Gubbay
JB, et al Public Health Agency of Canada/Canadian Institutes of
Health Research Influenza Research Network (PCIRN) Program Delivery
and Evaluation Group: Randomized evaluation of live attenuated vs.
inactivated influenza vaccines in schools (RELATIVES) cluster
randomized trial: Pilot results from a household surveillance study
to assess direct and indirect protection from influenza
vaccination. Vaccine. 33:4910–4915. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Caspard H, Coelingh KL, Mallory RM and
Ambrose CS: Association of vaccine handling conditions with
effectiveness of live attenuated influenza vaccine against
H1N1pdm09 viruses in the United States. Vaccine. 34:5066–5072.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Caspard H, Heikkinen T, Belshe RB and
Ambrose CS: A systematic review of the efficacy of live attenuated
influenza vaccine upon revaccination of children. Hum Vaccin
Immunother. 12:1721–1727. 2016.PubMed/NCBI
|
|
68
|
Leung VKY, Carolan LA, Worth LJ, Harper
SA, Peck H, Tilmanis D, Laurie KL, Slavin MA and Sullivan SG:
Influenza vaccination responses: Evaluating impact of repeat
vaccination among health care workers. Vaccine. 35:2558–2568. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Simpson CR, Lone NI, Kavanagh K, Robertson
C, McMenamin J, von Wissmann B, et al: Evaluating the
effectiveness, impact and safety of live attenuated and seasonal
inactivated influenza vaccination: protocol for the Seasonal
Influenza Vaccination Effectiveness II (SIVE II) study. BMJ Open.
7:e0142002017.70. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Korovessi P: Probiotics and viral
infections in childhood. Int J Mol Med. 40:S392017.
|
|
71
|
Food and Agriculture Organization of the
United Nations, World Health Organization: Guidelines for the
Evaluation of Probiotics in Food. In: Report of a Joint FAO/WHO
Working Group on Drafting Guidelines for the Evaluation of
Probiotics in Food; London, Ontario, Canada. April 30 and May 1,
2002;
|
|
72
|
Ahrne S and Hagslatt ML: Effect of
lactobacilli on paracellular permeability in the gut. Nutrients.
3:104–117. 2011. View Article : Google Scholar
|
|
73
|
Morais MB and Jacob CM: The role of
probiotics and prebiotics in pediatric practice. J Pediatr (Rio J).
82(Suppl 5): S189–S197. 2006. View Article : Google Scholar
|
|
74
|
Guarino A, Lo Vecchio A and Canani RB:
Probiotics as prevention and treatment for diarrhea. Curr Opin
Gastroenterol. 25:18–23. 2009. View Article : Google Scholar
|
|
75
|
Szajewska H, Guarino A, Hojsak I, Indrio
F, Kolacek S, Shamir R, Vandenplas Y and Weizman Z; European
Society for Pediatric Gastroenterology, Hepatology and Nutrition:
Use of probiotics for management of acute gastroenteritis: A
position paper by the ESPGHAN Working Group for Probiotics and
Prebiotics. J Pediatr Gastroenterol Nutr. 58:531–539. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Guarino A, Ashkenazi S, Gendrel D, Lo
Vecchio A, Shamir R and Szajewska H; European Society for Pediatric
Gastroenterology, Hepatology, and Nutrition; European Society for
Pediatric Infectious Diseases: European Society for Pediatric
Gastroenterology, Hepatology, and Nutrition/European Society for
Pediatric Infectious Diseases evidence-based guidelines for the
management of acute gastroenteritis in children in Europe; Update
2014. J Pediatr Gastroenterol Nutr. 59:132–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hao Q, Lu Z, Dong BR, Huang CQ and Wu T:
Probiotics for preventing acute upper respiratory tract infections.
Cohrane Database Syst Rev. 9:CD0068952015. View Article : Google Scholar
|
|
78
|
Esposito S, Rigante D and Principi N: Do
children’s upper respiratory tract infections benefit from
probiotics? BMC Infect Dis. 14:1942014. View Article : Google Scholar
|
|
79
|
Allan GM and Arroll B: Prevention and
treatment of the common cold: Making sense of the evidence. CMAJ.
186:190–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Amaral MA, Guedes GHBF, Epifanio M, Wagner
MB, Jones MH and Mattiello R: Network meta-analysis of probiotics
to prevent respiratory infections in children and adolescents.
Pediatr Pulmonol. 52:833–843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Papaioannou G: Foetal and neonatal brain
imaging in viral infections. Int J Mol Med. 40:S392017.
|
|
82
|
Lo CP and Chen CY: Neuroimaging of viral
infections in infants and young children. Neuroimaging Clin N Am.
18:119–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shaw DWW and Cohen WA: Viral infections of
the CNS in children: Imaging features. AJR Am J Roentgenol.
160:125–133. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Verstraelen H, Vanzieleghem B, Defoort P,
Vanhaesebrouck P and Temmerman M: Prenatal ultrasound and magnetic
resonance imaging in fetal varicella syndrome: Correlation with
pathology findings. Prenat Diagn. 23:705–709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sanchez TR, Datlow MD and Nidecker AE:
Diffuse periventricular calcification and brain atrophy: A case of
neonatal central nervous system cytomegalovirus infection.
Neuroradiol J. 29:314–316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gupta K, Banerjee A and Saggar K,
Ahluwalia A and Saggar K: A prospective study of magnetic resonance
imaging patterns of central nervous system infections in pediatric
age group and young adults and their clinico-biochemical
correlation. J Pediatr Neurosci. 11:46–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Acosta JH, Rantes CI, Arbelaez A, Restrepo
F and Castillo M: Noncongenital central nervous system infections
in children: Radiology review. Top Magn Reson Imaging. 23:153–164.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Papatheodoropoulou AE: Viral infections of
central nervous system in children. Int J Mol Med. 40:S402017.
|
|
89
|
Koyuncu OO, Hogue IB and Enquist LW: Virus
infections in the nervous system. Cell Host Microbe. 13:379–393.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Teoh HL, Mohammad SS, Britton PN, Kandula
T, Lorentzos MS, Booy R, Jones CA, Rawlinson W, Ramachandran V,
Rodriguez ML, et al: Clinical characteristics and functional motor
outcomes of Enterovirus 71 neurological disease in children. JAMA
Neurol. 73:300–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Erasmus M: Viral infections of Central
Nervous System. CME. 29:190–193. 2011.
|
|
92
|
Venkatesan A, Tunkel AR, Bloch KC, Lauring
AS, Sejvar J, Bitnun A, Stahl JP, Mailles A, Drebot M, Rupprecht
CE, et al International Encephalitis Consortium: Case definitions,
diagnostic algorithms, and priorities in encephalitis: Consensus
statement of the international encephalitis consortium. Clin Infect
Dis. 57:1114–1128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kneen R, Michael BD, Menson E, Mehta B,
Easton A, Hemingway C, Klapper PE, Vincent A, Lim M, Carrol E, et
al National Encephalitis Guidelines Development and Stakeholder
Groups: Management of suspected viral encephalitis in children -
Association of British Neurologists and British Paediatric Allergy,
Immunology and Infection Group national guidelines. J Infect.
64:449–477. 2012. View Article : Google Scholar
|
|
94
|
Pillai SC, Hacohen Y, Tantsis E, Prelog K,
Merheb V, Kesson A, Barnes E, Gill D, Webster R, Menezes M, et al:
Infectious and autoantibody-associated encephalitis: Clinical
features and long-term outcome. Pediatrics. 135:e974–e984. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Koutsaftiki C: RSV and rhinovirus
infection in early childhood and their role in asthma. Int J Mol
Med. 40:S402017.
|
|
96
|
Gidaris D, Urquhart D and Anthracopoulos
MB: ‘They said it was bronchiolitis; is it going to turn into
asthma doctor?’. Respirology. 19:1158–1164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kloepfer KM and Gern JE: Virus/allergen
interactions and exacerbations of asthma. Immunol Allergy Clin
North Am. 30:553–563. vii2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chong Neto HJ, Rosario N, Solé D and
Mallol J: Associated factors for recurrent wheezing in infancy.
Allergy. 65:406–407. 2010. View Article : Google Scholar
|
|
99
|
Jackson DJ, Gangnon RE, Evans MD, Roberg
KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf
E, et al: Wheezing rhinovirus illnesses in early life predict
asthma development in high-risk children. Am J Respir Crit Care
Med. 178:667–672. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kakumanu S: Virus-induced wheezing and
asthma: An overview. UpToDate. 2017, https://www.uptodate.com/contents/virus-induced-wheezing-and-asthma-an-overview.
|
|
101
|
Gern JE: Viral respiratory infection and
the link to asthma. Pediatr Infect Dis J. 27(10 Suppl): S97–S103.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Rantala AK, Jaakkola MS, Mäkikyrö EM, Hugg
TT and Jaakkola JJ: Early respiratory infections and the
development of asthma in the first 27 years of life. Am J
Epidemiol. 182:615–623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gern JE: The ABCs of rhinoviruses,
wheezing, and asthma. J Virol. 84:7418–7426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bartlett NW, McLean GR, Chang YS and
Johnston SL: Genetics and epidemiology: Asthma and infection. Curr
Opin Allergy Clin Immunol. 9:395–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Blanken MO, Rovers MM, Molenaar JM,
Winkler-Seinstra PL, Meijer A, Kimpen JL and Bont L; Dutch RSV
Neonatal Network: Respiratory syncytial virus and recurrent wheeze
in healthy preterm infants. N Engl J Med. 368:1791–1799. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Stone CA Jr and Miller EK: Understanding
the association of human rhinovirus with asthma. Clin Vaccine
Immunol. 23:6–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liston MA: The causes and contexts of
perinatal mortality in ancient Greece: A case study from the ‘bone
well’ of the Athenian Agora. Int J Mol Med. 40:S402017.
|
|
108
|
Liston MA, Rotroff SI and Snyder LM: The
Agora bone well. Hesperia Supplements Am Sch Class Stud Athens. In
press.
|
|
109
|
Liston MA and Rotroff SI: Babies in the
well: Archaeological evidence for newborn disposal in Hellenistic
Greece. The Oxford Handbook of Childhood and Education in the
Classical World. Parkin T and Evans-Grubbs J: Oxford University
Press; Oxford: 2013, https://doi.org/10.1093/oxfordhb/9780199781546.013.003.
|
|
110
|
Scheuer L and Black S: Developmental
Juvenile Osteology. Elsevier Academic Press; London: 2000
|
|
111
|
Becker MJ: Etruscan infants: children’s
cemeteries at Tarquinia, Italy, as indicators of an age of
transition. (Re)thinking the Little Ancestor: New Perspectives on
the Archaeology of Infancy and Childhood. Lally M and Moore A:
Archaeopress; Oxford: 2011
|
|
112
|
Lewis ME: Endocranial lesions in non-adult
skeletons: Understanding their aetiology. Int J Osteoarchaeol.
14:82–97. 2004. View
Article : Google Scholar
|
|
113
|
Santos RP and Tristram D: A practical
guide to the diagnosis, treatment, and prevention of neonatal
infections. Pediatr Clin North Am. 62:491–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Mammas IN and Spandidos DA: Paediatric
Virology: Focusing on medical education and training research. Int
J Mol Med. 40:S252017.
|
|
115
|
Plutarch: Vioi Paralliloi; Solon
- Poplikolas. 1. Kaktos Editions; 1992, In Greek.
|
|
116
|
European Academy of Paediatrics:
Continuous Medical Education (CME). 2017, http://eapaediatrics.eu/working-group/continuous-medical-education-cme/.
|
|
117
|
Royal College of Paediatrics and Child
Health: Continuing Professional Development (CPD). 2017, https://www.rcpch.ac.uk/training-examinations-professional-development/continuing-professional-development-cpd/continuing-pr.
|
|
118
|
Confederation of European Specialists in
Paediatrics Working Group on Continuing Medical Education: Charter
on continuing medical education in paediatrics in the European
Union. Eur J Pediatr. 157:192–193. 1998. View Article : Google Scholar
|
|
119
|
Djuricich AM, Turco MG and Sachdeva AK:
Changing continuing medical education. JAMA. 314:1072–1073. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Holl RA and Alden ER: Continuing medical
education in Europe. Pediatr Rev. 23:335–336. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Costa A, Van Hemelryck F, Aparicio A,
Gatzemeier W, Leer JW, Maillet B and Hossfeld DK: Continuing
medical education in Europe: Towards a harmonised system. Eur J
Cancer. 46:2340–2343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
James E: Clinical education in
paediatrics. J Child Health Care. 2:178–181. 1998. View Article : Google Scholar
|
|
123
|
Mammas IN, Theodoridou M, Kramvis A,
Thiagarajan P, Gardner S, Papaioannou G, Melidou A, Koutsaki M,
Kostagianni G, Achtsidis V, et al: Paediatric Virology: A rapidly
increasing educational challenge. Exp Ther Med. 13:364–377. 2017.
View Article : Google Scholar : PubMed/NCBI
|