Introduction
Cerebral stroke is a common neurological event
(1). Cerebral
ischemia/reperfusion may cause oxygen and nutrient deprivation
(2) and induce neuronal injury
(3). Minimizing neuronal injury
has been considered a hot topic among investigators and
clinicians.
Thymosin β4 (Tβ4) is a pleiotropic polypeptide
(4). It sequesters G-actin and is
necessary for cell motility and organogenesis. Previous studies
have indicated that Tβ4 promotes tissue repair (5,6).
The safety, tolerability and efficacy of Tβ4 are being evaluated in
clinical applications (7,8).
Tβ4 expression in developing brain tissue correlates
with neuronal migration and neurite extension. A series of studies
have suggested that Tβ4 may also have neuroprotective effects. Choi
et al (9) reported that
Tβ4 suppressed staurosporine-induced neuronal apoptosis in
vitro, Popoli et al (10) indicated that Tβ4 attenuated
glutamate-induced toxicity, and Morris et al (11) and Xiong et al (12) demonstrated that Tβ4 improves the
outcome for rats subjected to acute stroke or traumatic brain
injury. Tβ4 treatment also induced oligodendrocyte differentiation
and myelin gene expression (13),
but the direct effect of Tβ4 on neurons has remained to be fully
elucidated.
As mentioned above, cerebral ischemia/reperfusion
causes severe brain injury and results in neuronal death through
programmed cell death mechanisms (14), including necrosis, apoptosis and
autophagy; the latter two are more commonly observed in in
vitro experiments (15).
Autophagy is an intracellular bulk degradation
process that is essential to maintain cellular metabolism and
homeostasis (16). Vast evidence
indicates that excessive autophagic activity triggers autophagic
cell death in numerous diseases (17), including neurodegenerative
diseases (18) and cerebral
ischemia (19,20).
However, the role of Tβ4 in autophagy and apoptosis
still requires clarification. In the present study, PC12 cells were
used in a model of oxygen-glucose deprivation and reoxygenation
(OGD/R) (21) in order to
investigate the effect of Tβ4 on neural cells subjected to cerebral
ischemia/reperfusion injury.
Materials and methods
Materials
Tβ4 was purchased from ProSpec (Ness-Ziona, Israel)
and dissolved in distilled water. The PC12 pheochromocytoma cell
line was obtained from the cell bank of the Chinese Academy of
Sciences (Shanghai, China). The reagents for cell culture,
including Dulbecco's Modified Eagle's Medium (DMEM) and fetal
bovine serum (FBS), were obtained from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). MTT was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The assay kits for
the determination of superoxide dismutase (SOD) activity (cat. no.
S0107) and malondialdehyde (MDA) content (cat. no. S0131) were
purchased from Beyotime Institute of Biotechnology (Haimen, China).
The assay kit for the determination of glutathioneperoxidase
(GSH-Px) activity (cat. no. A005) was purchased from Jiancheng
(Nanjing, China). TRIzol for RNA isolation and the Power
SYBR® Master Mix were from Invitrogen (Thermo Fisher
Scientific, Inc.). Primary mouse monoclonal antibodies against
B-cell lymphoma 2 (Bcl-2; cat. no. sc-7382), Beclin-1 (cat. no.
sc-48341), microtubule-associated protein 1 light chain 3 I/II
(LC3I/II; cat. no. sc-398822) and β-actin (cat. no. sc-130300) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Primary antibodies for active caspase-3 (cat. no. ab2302) and P62
(cat. no. ab56416) were purchased from Abcam (Cambridge, MA, USA).
Secondary antibodies (cat. no. 31430) and a lactase dehydrogenase
(LDH) cytotoxicity assay kit (cat. no. 88953) were from Pierce
(Thermo Fisher Scientific, Inc.). An Annexin V-fluorescein
isothiocyanate (FITC) cell apoptosis assay kit (cat. no. C1063) was
purchased from Beyotime Institute of Biotechnology.
Cell culture and the OGD/R cell
model
The PC12 cells were cultured in DMEM with 10% FBS at
37°C in a humid atmosphere containing 5% CO2. Cells were
divided into a control group, an OGD/R group and an OGD/R+Tβ4 group
(Tβ4 intervention group; 0.1, 1 or 10 mg/l Tβ4 was added). In the
OGD/R group, DMEM was replaced with serum-free, glucose-free
Earle's buffer supplemented with 10 mmol/l
Na2S2O4 (Sigma-Aldrich; Merck
KGaA), followed by incubation at 37°C for 4 h in air containing 5%
CO2, and then the
Na2S2O4 was removed. Subsequent
culture in DMEM supplemented with serum and glucose in air
containing 5% CO2 for 2 h was performed. In the control
group, the cells were incubated in DMEM in a normoxic atmosphere
for the same duration. In the Tβ4 intervention group, the cells
were pre-treated with 0.1–10 mg/l Tβ4 for 2 h and cultured in
serum-free, glucose-free Earle's buffer supplemented with 10 mmol/l
Na2S2O4 for 4 h. The
Na2S2O4 was then removed and the
cells were cultured in DMEM supplemented with serum and glucose for
a further 2 h.
MTT assay
The PC12 cells from all groups were cultured at 37°C
with 5% CO2 for 24 h. MTT solution was added to each
well, followed by incubation for 4 h at 37°C. The culture medium
was then removed and dimethylsulfoxide was added to each well,
followed by incubation with agitation for 10 min. The viability was
determined by measuring the optical density (OD) at 562 nm. The
cell viability in each group was calculated as a percentage of the
control group.
Measurement of LDH release
In brief, after OGD/R, the supernatant of the four
groups were assessed using the LDH Cytotoxicity Assay kit. The LDH
release, which is associated with cell damage, was measured at 490
nm using a microplate reader (Thermo Fisher Scientific, Inc.).
Lipid peroxidation assay
In brief, after OGD/R, the cells were washed,
homogenized and centrifuged at 12,000 × g for 10 min at 4°C. The
MDA content was measured using an MDA assay kit, according to the
manufacturer's protocol. The results are expressed in
μmol/l.
Analysis of SOD and GSH-Px activity
In brief, after OGD/R, the cells were resuspended
and homogenized. The supernatant was collected for further
experiments. The activity levels of the intracellular antioxidant
enzymes SOD and GSH-Px were measured using commercial assay kits,
according to the manufacturer's protocols. SOD and GSH-Px activity
was expressed in U/ml.
Flow cytometric analysis
The cells were analyzed by flow cytometry using a BD
FACS Aria flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
The cells were stained with Annexin V-FITC and propidium iodide
using the Annexin V-FITC apoptosis detection kit according to the
manufacturer's instructions.
Western blot analysis
Samples were homogenized in lysis buffer (50 mM
Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% sodium
deoxycholate). Protein concentrations were determined using a BCA
kit (Haoji Biotec, Inc., Hangzhou, China). The protein samples (60
μg per lane) were separated using 5–10% SDS-PAGE. Following
electrophoresis, separated proteins were transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were then blocked in 5% non-fat milk and probed
overnight at 4°C with the following primary antibodies: Anti-LC3
(1:1,000 dilution), anti-Beclin 1 (1:500 dilution), anti-Bcl-2
(1:500 dilution), anti-cleaved (active) caspase-3 (1:1,000
dilution), anti-P62 (1:500 dilution) and anti-β-actin (1:5,000
dilution). The membranes were then washed 3 times with
Tris-buffered saline containing Tween-20 and subsequently incubated
with the respective secondary antibodies (1:5,000 dilution) for 1 h
at room temperature. The blots were visualized using enhanced
chemiluminescence SuperSignal® West Dura Extended
Duration Substrate (cat. no. 34075; Pierce; Thermo Fisher
Scientific, Inc.) and images were captured on X-ray film (Kodak,
Rochester, NY, USA), which was scanned and quantified. The density
of bands corresponding to proteins of interest was normalized to
the density of the control β-actin band. Five independent
experiments were performed using duplicate samples.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from cells using TRIzol reagent,
and 5 μg RNA was reverse transcribed into cDNA using a
1st-Strand cDNA Synthesis kit (Haoji Biotec, Inc.) to a final
reaction volume of 20 μl (including RT buffer mix, Primer
mix and RT Enzyme mix). The RT reaction was as follows: 30°C for 10
min and cooling on ice and 42°C for 30 min, according to the
manufacturer's protocol. The reaction was terminated by heating at
70°C for 15 min. Primers for Beclin-1 forward,
5′-GGCAGTGGCGGCTCCTATTC-3′ and reverse,
5′-CTGTGAGGACACCCAAGCAAGAC-3′; autophagy-related protein-5 (Atg5)
forward, 5′-TCAGCTCTGCCTTGGAACATCA-3′ and reverse,
5′-AAGTGAGCCTCAACTGCATCCTT-3′ and control 18S RNA (18S) forward,
5′-GAATTCCCAGTAAGTGCGGGTCATA-3′ and reverse,
5′-CGAGGGCCTCACTAAACCATC-3′ were designed with Primer Premier 6.0
(Premier Biosoft International, Palo Alto, CA, USA) and Beacon
Designer 7.8 (Premier Biosoft International) and synthesized by
Sangon Biotech (Shanghai, China). The relative mRNA expression
levels were then evaluated by qPCR on an ABI 7300 PCR application
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with a Power
SYBR® Master mix (Invitrogen; Thermo Fisher Scientific,
Inc.). PCR was performed under the following conditions: 95°C for 1
min, followed by 40 cycles of 95°C for 10 sec and 64°C for 25 sec.
Rat 18S RNA was used as the reference gene. The 2−ΔΔCq
method (22) was used to quantify
the mRNA of target genes.
Statistical analysis
Values are expressed as the mean ± standard
deviation and were analyzed using Prism 6 software (GraphPad
Software, Inc., La Jolla, CA, USA). Statistical analyses were
performed using one-way analysis of variance followed by Dunnett's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
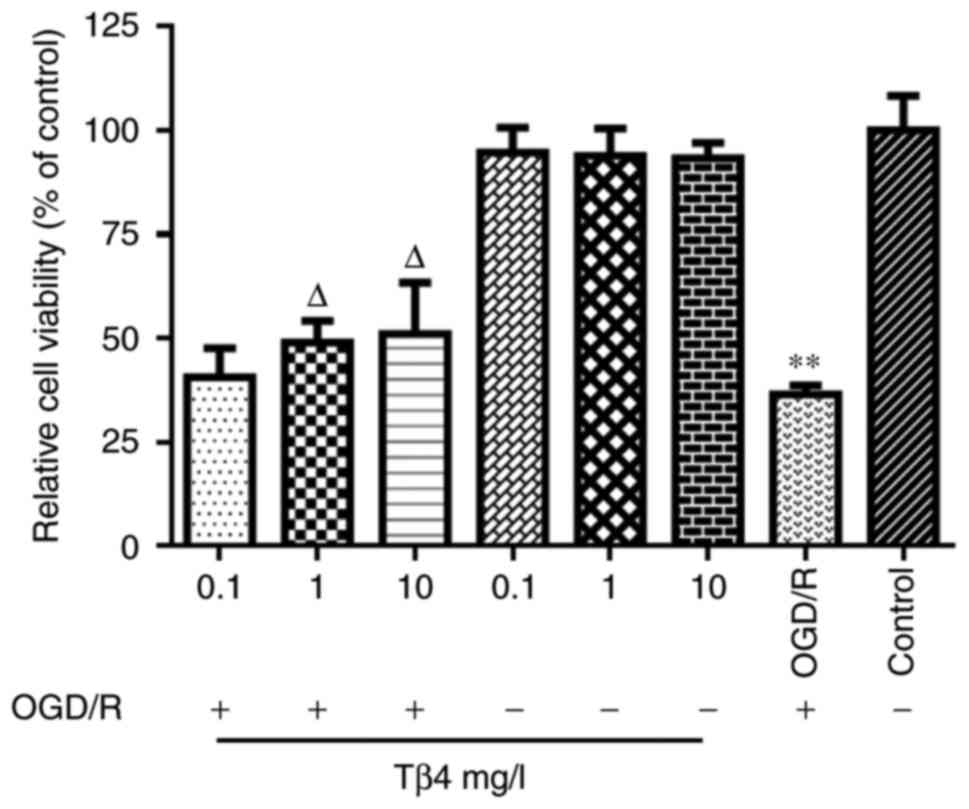
Tβ4 reduces OGD-induced cell damage
As indicated by the MTT assay, PC12-cell viability
in the OGD/R group was reduced by nearly 60%. In the OGD/R+Tβ4
groups, the cells were pre-treated with 0.1, 1 or 10 mg/l Tβ4 for 2
h and then cultured under OGD/R conditions for 6 h. The results
indicated that pre-treatment with Tβ4 did not affect the cell
viability in the groups that were not exposed to OGD/R, but Tβ4 at
the 1 and 10 mg/l concentrations reduced the OGD/R-induced cell
death of PC12 cells in a dose-dependent manner (Fig. 1).
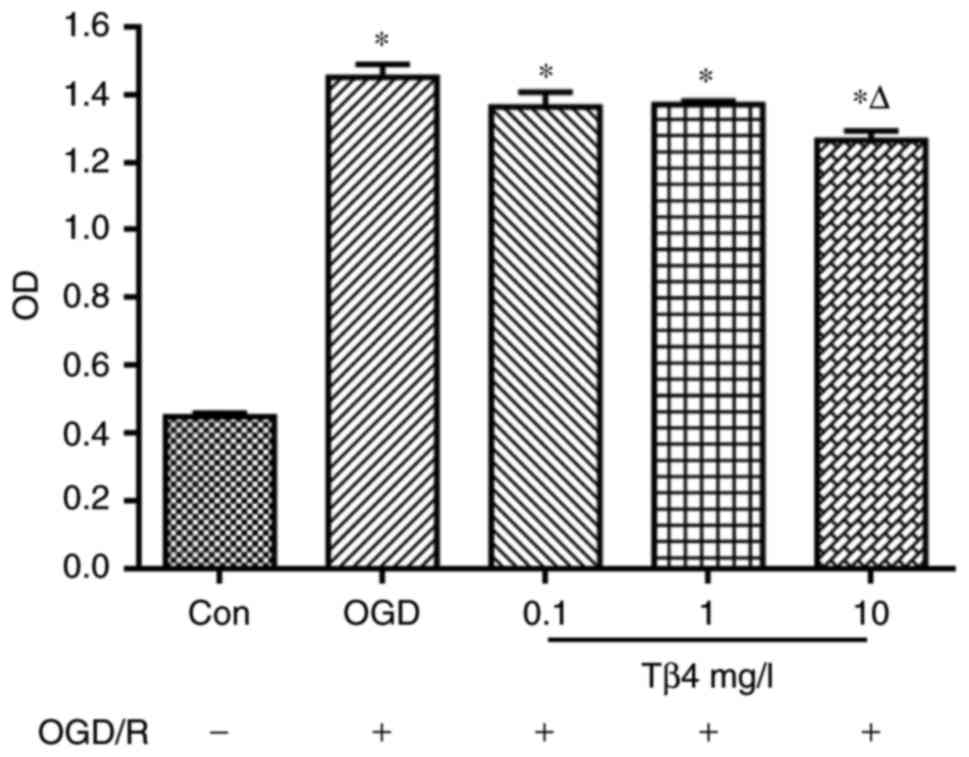
Tβ4 reduces OGD-induced LDH leakage
In the OGD/R+Tβ4 groups, 0.1, 1 or 10 mg/l Tβ4 was
added to the culture for 2 h and then cultured under OGD/R
conditions for 6 h. The results indicated that 10 mg/l Tβ4
effectively suppressed OGD/R-induced LDH release from PC12 cells
(Fig. 2).
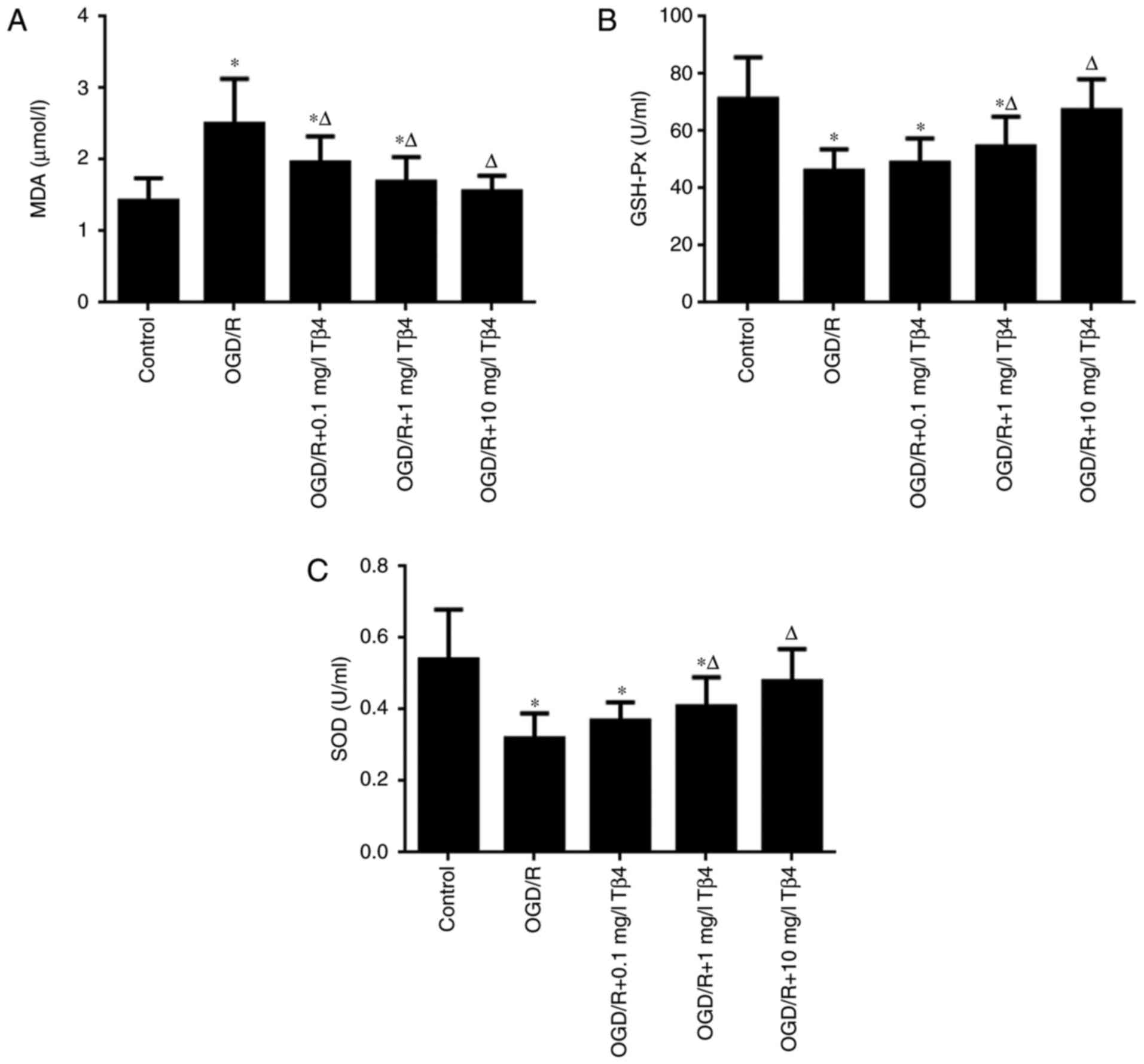
Tβ4 reduces OGD-induced changes in MDA
levels, as well as SOD and GSH-Px activities
As presented in Fig.
3A, the MDA content was significantly increased under OGD/R
conditions compared with that in the control group (P<0.05).
However, pre-treatment with Tβ4 at the concentrations of 0.1, 1 or
10 mg/l Tβ4 markedly decreased the MDA content by 21.60, 32.4 and
37.60%, respectively, of that in the OGD/R group. The activities of
the antioxidant enzymes SOD and GSH-Px under OGD/R conditions were
then investigated. As presented in Fig. 3B and C, OGD/R significantly
decreased the activities of SOD and GSH-Px compared with those in
the control group (P<0.05). However, SOD and GSH-Px activities
were significantly increased by pre-treatment with 1 and 10 mg/l
Tβ4 in a concentration-dependent manner compared with those in the
OGD/R group (P<0.05). These results suggested that Tβ4
attenuated the oxidative damage to cells under OGD/R conditions and
maintained the activity of antioxidant enzymes.
Tβ4 attenuates OGD/R-induced
apoptosis
The flow cytometry results indicated that different
concentrations of Tβ4 (0.1, 1 or 10 mg/l) significantly increased
the cell survival percentage (Q1) after exposure to OGD/R
(26.85±0.61, 25.22±0.53 and 24.75±0.50%, respectively, vs.
5.35±0.13% in the OGD/R group) and reduced the percentage of cells
in late apoptosis/necrosis (Q2; 6.35±0.34, 5.50±0.23 and
12.11±1.35%, respectively, vs. 21.83±0.57% in the OGD/R group). In
addition, the high concentration of Tβ4 reduced the number of early
apoptotic cells (Q3) (58.65±0.94 vs. 72.03±1.32% in the OGD/R
group). These results demonstrated that Tβ4 has an obvious
protective effect on PC12 cells against OGD/R damage (Fig. 4).
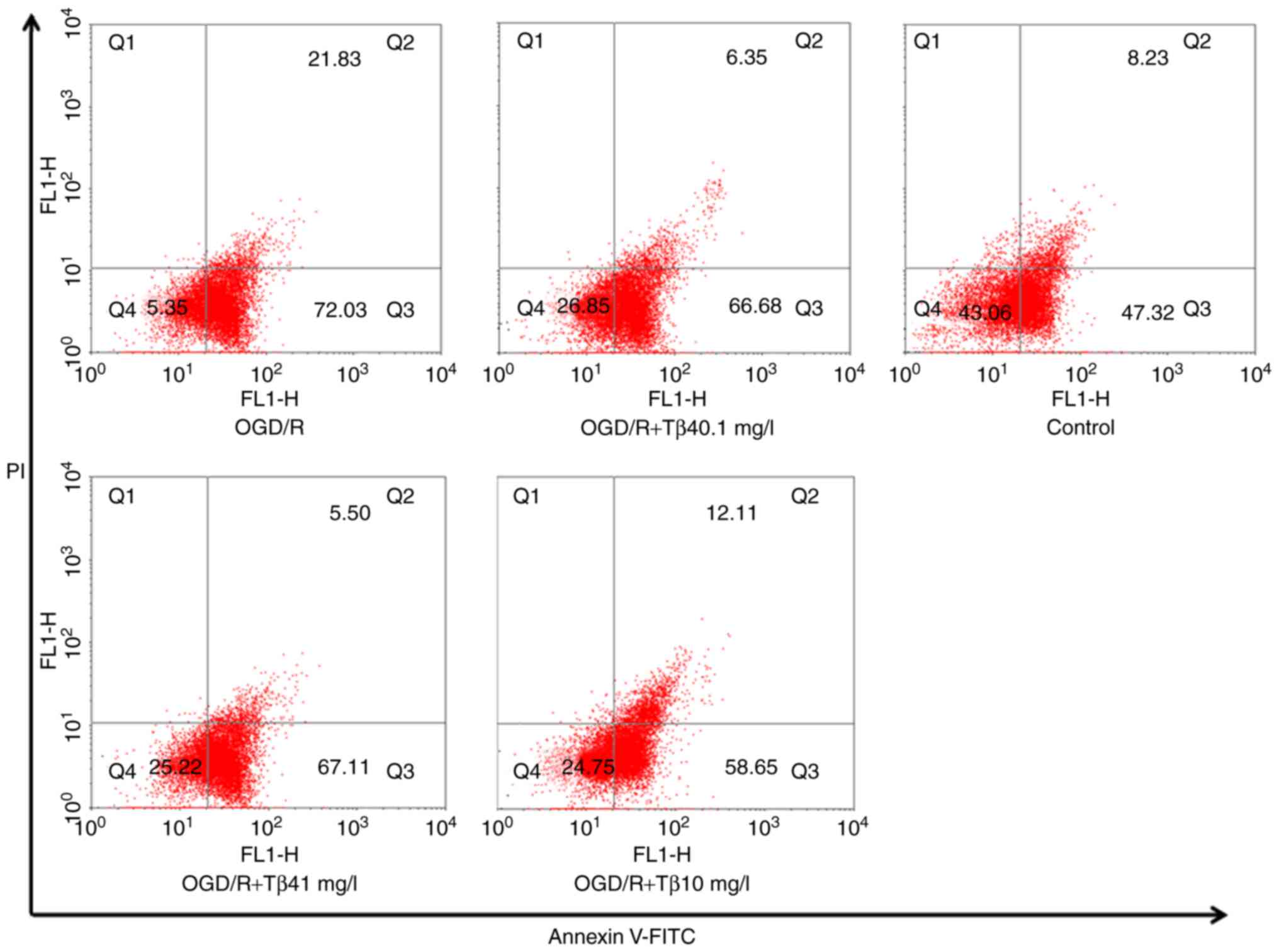 | Figure 4Influence of Tβ4 on OGD/R-induced cell
apoptosis determined by flow cytometry. Quantitative analysis of
Annexin V-FITC/PI staining in PC12 cells under OGD/R conditions and
pre-treatment with different doses of Tβ4 by flow cytometry. The
results indicated that pre-treatment with different concentrations
of Tβ4 (0.1, 1 or 10 mg/l) significantly increased the percentage
of surviving cells (Q1) after exposure to OGD/R (26.85±0.61,
25.22±0.53 and 24.75±0.50%, respectively, vs. 5.35±0.13% in the
OGD/R group; n=3) and reduced the percentage of cells in late
apoptosis/necrosis (Q2; 6.35±0.34, 5.50±0.23 and 12.11±1.35%,
respectively, vs. 21.83±0.57% in the OGD/R group). In addition, the
high concentration of Tβ4 reduced the number of early apoptotic
cells (Q3; 58.65±0.94 vs. 72.03±1.32% in the OGD/R group). Tβ4,
thymosin β4; OGD/R, oxygen-glucose deprivation and reoxygenation;
FITC, fluorescein isothiocyanate; PI, propidium iodide; Q,
quadrant. |
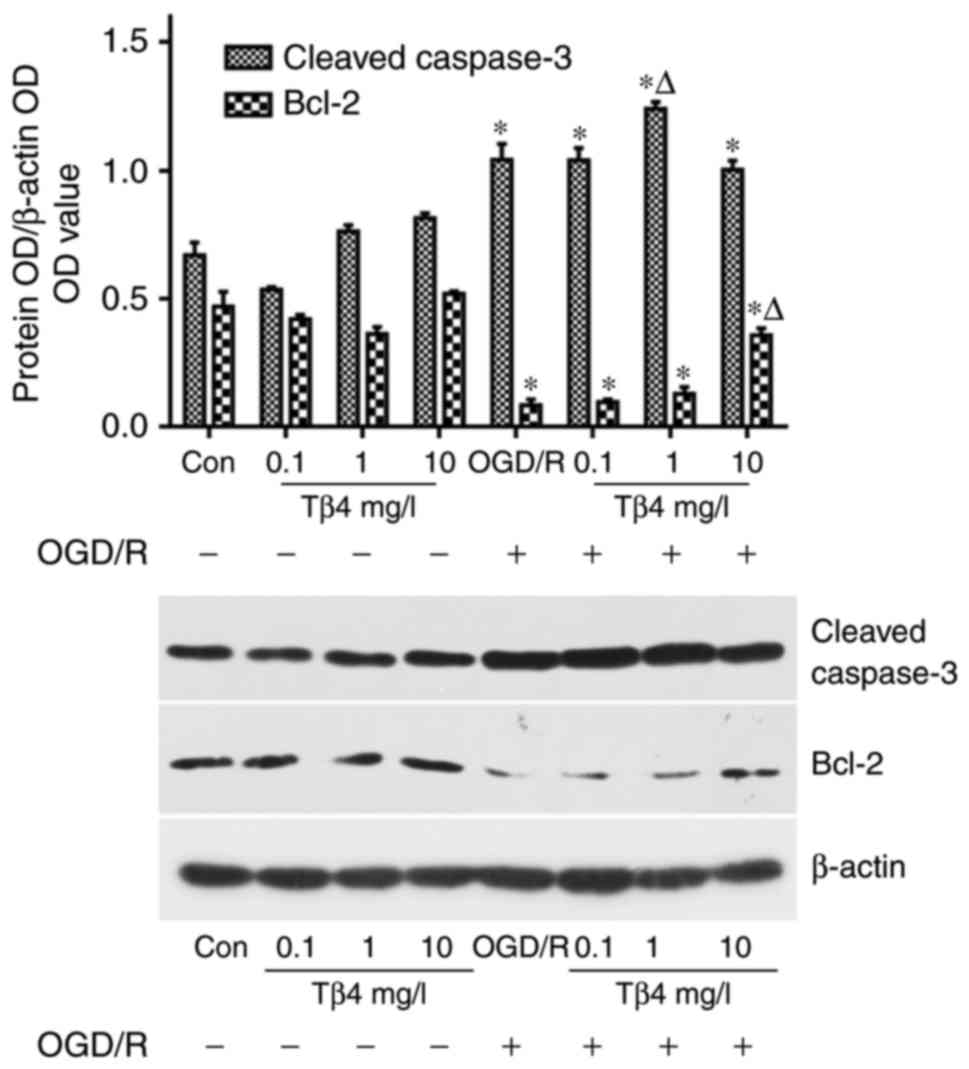
Tβ4 reduces OGD/R-induced apoptotic
signalling
Western blot analysis indicated that the levels of
Bcl-2 were decreased in PC12 cells following OGD/R; however, these
levels were significantly increased in the presence of Tβ4 compared
with those in the OGD/R group (Fig.
5).
Tβ4 reduces OGD/R-induced
autophagy-associated gene expression
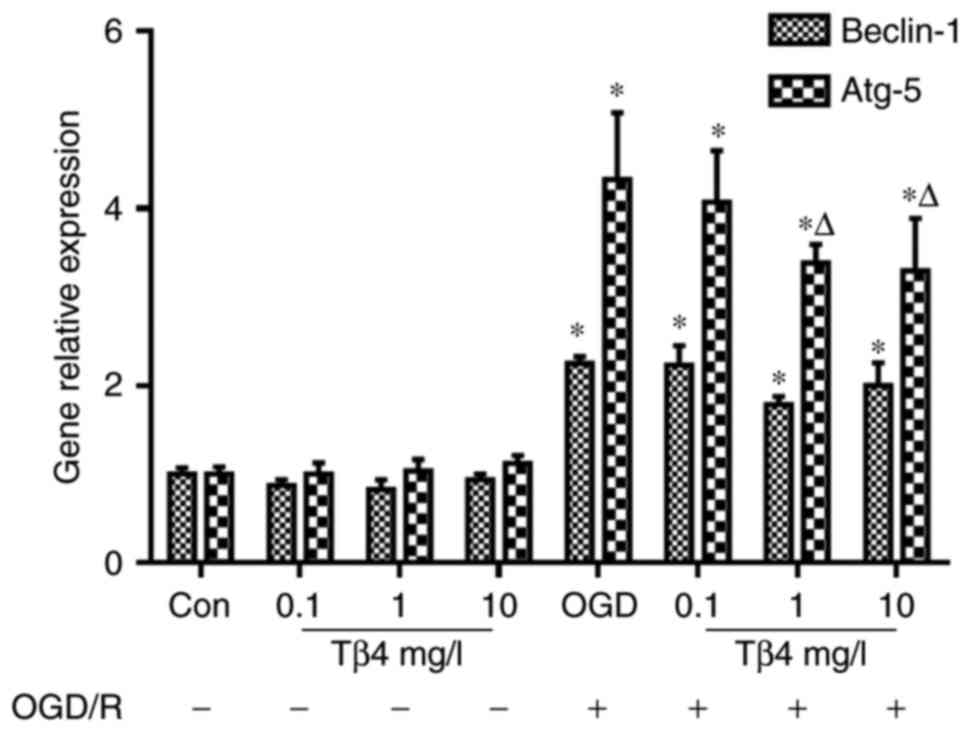
RT-qPCR analysis demonstrated that following OGD/R,
the expression levels of Beclin-1 and Atg-5 were increased compared
with those in the control group, and only the Atg-5 levels were
significantly decreased in the presence of Tβ4 (1 and 10 mg/l)
compared with those in the OGD/R group (Fig. 6).
Tβ4 regulates OGD/R-induced
autophagy-associated protein expression
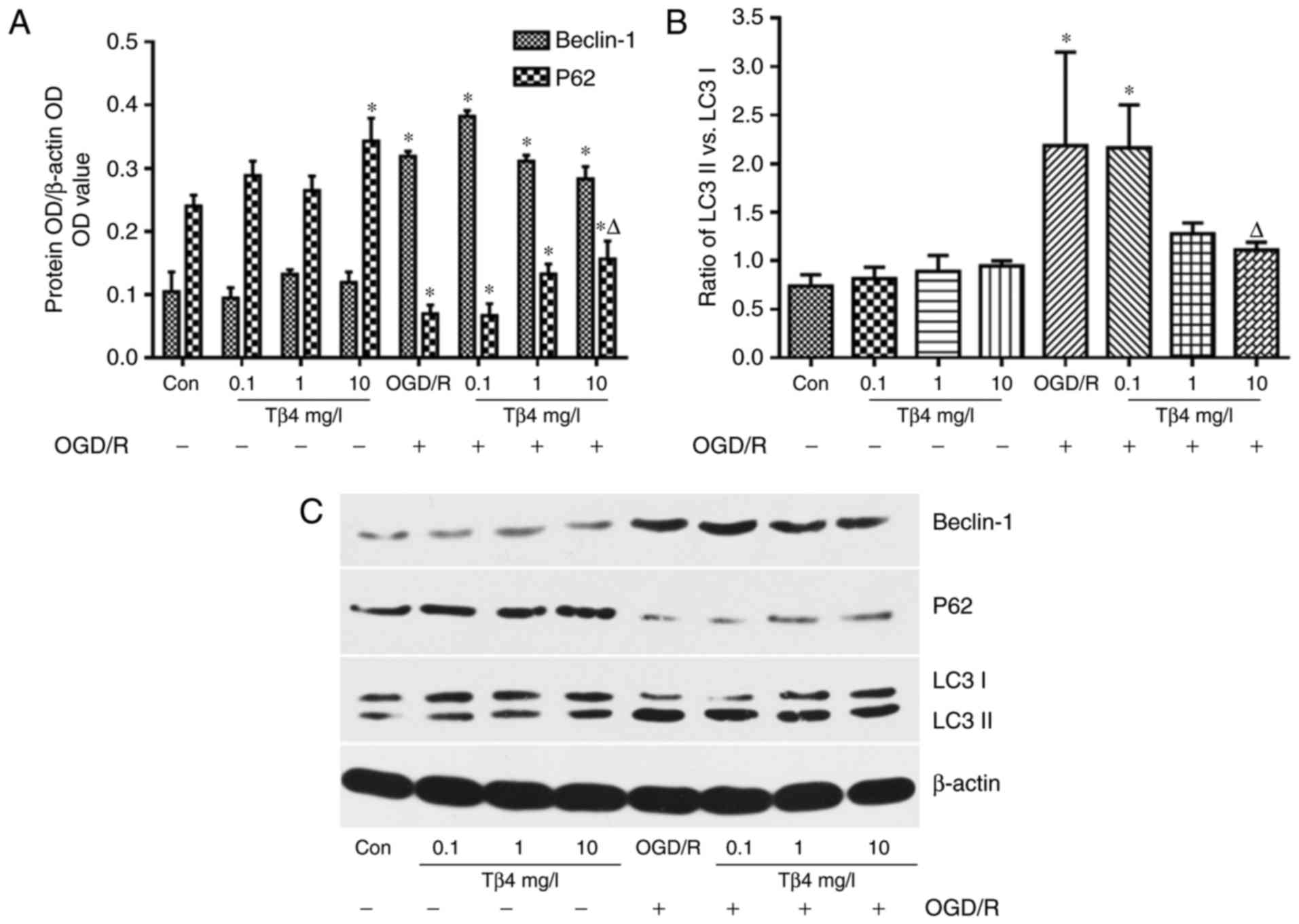
Western blot analysis demonstrated that the levels
of Beclin-1 and the ratio of LC3 II vs. LC3 I were increased in
PC12 cells subjected to OGD/R, and the ratio of LC3 II vs. LC3 I
was significantly inhibited in the presence of Tβ4 (10 mg/l). The
levels of P62 were decreased in the OGD/R group; however, these
levels were significantly increased in the presence of Tβ4 (10
mg/l) compared with those in the OGD/R group (Fig. 7).
Discussion
Cerebral ischemia induces neuronal cell death
through mechanisms including apoptosis and autophagy. In the
present study, the MTT assay and flow cytometric analysis indicated
that pre-treatment with Tβ4 reduced OGD/R-induced PC12 cell death.
Tβ4 also effectively suppressed OGD/R-induced LDH release,
decreased the MDA content and significantly increased SOD and
GSH-Px activities, which suggests that Tβ4 attenuated the oxidative
damage to PC12 cells following OGD/R. Western blot analysis
revealed that Tβ4 reduced the OGD/R-induced decreases in Bcl-2
expression, which suggests that Tβ4 has an obvious anti-apoptotic
effect on PC12 cells subjected to OGD/R.
Distinct from apoptosis, autophagy is a type of
programmed cell death that is mediated by self-digestion (23) and degradation of organelles
(24). Therefore, the role of
autophagy in various diseases has become a hot research topic
(25).
Excessive autophagic activity may lead to cell death
in acute neurological disorders, including cerebral ischemia
(26). Xie et al (27) reported that selective deletion of
Atg-7 prevented hypoxia-ischemia-induced autophagy and protected
against neuronal death after cerebral ischemia. Koike et al
(19) also demonstrated that
inhibition of autophagy prevents neuronal death after
hypoxic-ischemic injury, suggesting that autophagy is involved in
cerebral ischemia.
Ischemia/reperfusion is known to stimulate autophagy
through a Beclin-1-dependent mechanism (28). Furthermore, P62, a long-lived
protein, which is assembled on selective autophagic cargos and
preferentially degraded via autophagy, was assessed in the present
study. P62 has been reported to be a possible marker of autophagic
flux in vivo (29) and it
also regulates autophagy (30).
In the present study, it was demonstrated that OGD/R
increased the mRNA and protein expression of Beclin-1 and increased
Atg-5 mRNA expression, indicating that OGD/R enhanced autophagy in
PC12 cells. By contrast, P62 expression was significantly reduced
after OGD/R. Of note, pre-treatment with 1 or 10 mg/l Tβ4 led to a
downregulation of the mRNA expression of Atg-5, as well as the
ratio of LC3 II vs. LC3 I, and an upregulation of the protein
expression of P62.
Autophagy is closely associated with apoptosis
(31). As mentioned above,
autophagy during OGD/R is mediated by a Beclin-1-dependent pathway.
Beclin-1, an autophagy- associated protein that contains a Bcl-2
homology-3 (BH3) domain (32),
may be inhibited via activation of apoptosis-associated proteins
that possess BH3-binding domains, including Bcl-2 (33). Therefore, Bcl-2 may reduce the
pro-autophagic activity of Beclin-1, while upregulation of Bcl-2
may decrease autophagic cell death and reduce cellular autophagy by
binding to Beclin-1.
Based on the finding that Tβ4 increased the
expression of Bcl-2, it was hypothesized that the anti-autophagic
effect of Tβ4 may be linked to the increased Bcl-2 expression,
which may have promoted the interaction of Bcl-2 with Beclin-1. Tβ4
also increased the expression of the autophagy regulatory protein
P62, thereby inhibiting autophagy, but the specific mechanisms
require to be elucidated in further studies.
In conclusion, the present study demonstrated that
OGD/R in PC12 cells induced excessive autophagic flux, likely
leading to autophagic cell death. Tβ4 was demonstrated to reduce
oxidative stress-induced cell damage and inhibit cell apoptosis and
autophagy to partly prevent OGD/R-induced injury. The ability of
Tβ4 to protect against OGD/R in PC12 cells may provide new
opportunities for clinical therapeutic strategies in the
future.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Matsumaru Y, Ishikawa E, Yamamoto T and
Matsumura A: Recent trends in neuro-endovascular treatment for
acute ischemic stroke, cerebral aneurysms, carotid stenosis, and
brain arteriovenous malformations. Neurol Med Chir (Tokyo).
57:253–260. 2017. View Article : Google Scholar
|
|
2
|
Rocha-Ferreira E, Kelen D, Faulkner S,
Broad KD, Chandrasekaran M, Kerenyi Á, Kato T, Bainbridge A, Golay
X, Sullivan M, et al: Systemic pro-inflammatory cytokine status
following therapeutic hypothermia in a piglet hypoxia-ischemia
model. J Neuroinflammation. 14:442017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li N, Yuan Q, Cao XL, Zhang Y, Min ZL, Xu
SQ, Yu ZJ, Cheng J, Zhang C and Hu XM: Opposite effects of HDAC5
and p300 on MRTF-A-related neuronal apoptosis during
ischemia/reperfusion injury in rats. Cell Death Dis. 8:e26242017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldstein AL, Hannappel E and Kleinman HK:
Thymosin beta4: Actin-sequestering protein moonlights to repair
injured tissues. Trends Mol Med. 11:421–429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim S and Kwon J: Thymosin beta 4 improves
dermal burn wound healing via downregulation of receptor of
advanced glycation end products in db/db mice. Biochim Biophys
Acta. 1840:3452–3459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marks ED and Kumar A: Thymosin β4: Roles
in development, repair, and engineering of the cardiovascular
system. Vitam Horm. 102:227–249. 2016. View Article : Google Scholar
|
|
7
|
Zhu J, Song J, Yu L, Zheng H, Zhou B, Weng
S and Fu G: Safety and efficacy of autologous thymosin β4
pre-treated endothelial progenitor cell transplantation in patients
with acute ST segment elevation myocardial infarction: A pilot
study. Cytotherapy. 18:1037–1042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guarnera G, DE Rosa A and Camerini R:
Thymosin beta-4 and venous ulcers: Clinical remarks on a European
prospective, randomized study on safety, tolerability, and
enhancement on healing. Ann N Y Acad Sci. 1112:407–412. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi SY, Noh MR, Kim DK, Sun W and Kim H:
Neuroprotective function of thymosin-beta and its derivative
peptides on the programmed cell death of chick and rat neurons.
Biochem Biophys Res Commun. 362:587–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Popoli P, Pepponi R, Martire A, Armida M,
Pèzzola A, Galluzzo M, Domenici MR, Potenza RL, Tebano MT,
Mollinari C, et al: Neuroprotective effects of thymosin beta4 in
experimental models of excitotoxicity. Ann N Y Acad Sci.
1112:219–224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morris DC, Cui Y, Cheung WL, Lu M, Zhang
L, Zhang ZG and Chopp M: A dose-response study of thymosin β4 for
the treatment of acute stroke. J Neurol Sci. 345:61–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong Y, Mahmood A, Meng Y, Zhang Y, Zhang
ZG, Morris DC and Chopp M: Treatment of traumatic brain injury with
thymosin β4 in rats. J Neurosurg. 114:102–115. 2011. View Article : Google Scholar
|
|
13
|
Santra M, Chopp M, Zhang ZG, Lu M, Santra
S, Nalani A, Santra S and Morris DC: Thymosin β4 mediates
oligodendrocyte differentiation by upregulating p38 MAPK. Glia.
60:1826–1838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Northington FJ, Chavez-Valdez R and Martin
LJ: Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol.
69:743–758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang J and Klionsky DJ: Autophagy and
human disease. Cell Cycle. 6:1837–1849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levine B, Mizushima N and Virgin HW:
Autophagy in immunity and inflammation. Nature. 469:323–335. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oh BM, Lee SJ, Cho HJ, Park YS, Kim JT,
Yoon SR, Lee SC, Lim JS, Kim BY, Choe YK and Lee HG: Cystatin SN
inhibits auranofin-induced cell death by autophagic induction and
ROS regulation via glutathione reductase activity in colorectal
cancer. Cell Death Dis. 8:e30532017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nixon RA: Autophagy in neurodegenerative
disease: Friend, foe or turncoat? Trends Neurosci. 29:528–535.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koike M, Shibata M, Tadakoshi M, Gotoh K,
Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, et
al: Inhibition of autophagy prevents hippocampal pyramidal neuron
death after hypoxic-ischemic injury. Am J Pathol. 172:454–469.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ginet V, Puyal J, Clarke PG and Truttmann
AC: Enhancement of autophagic flux after neonatal cerebral
hypoxia-ischemia and its region-specific relationship to apoptotic
mechanisms. Am J Pathol. 175:1962–1974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tabakman R, Jiang H, Levine RA, Kohen R
and Lazarovici P: Apoptotic characteristics of cell death and the
neuroprotective effect of homocarnosine on pheochromocytoma PC12
cells exposed to ischemia. J Neurosci Res. 75:499–507. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai R, Zhang S, Duan W, Wei R, Chen H, Cai
W, Yang L and Wang Q: Enhanced autophagy contributes to protective
effects of GM1 ganglioside against Aβ1-42-induced neurotoxicity and
cognitive deficits. Neurochem Res. 42:2417–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rabinowitz JD and White E: Autophagy and
metabolism. Science. 330:1344–1348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rubinsztein DC, DiFiglia M, Heintz N,
Nixon RA, Qin ZH, Ravikumar B, Stefanis L and Tolkovsky A:
Autophagy and its possible roles in nervous system diseases, damage
and repair. Autophagy. 1:11–22. 2005. View Article : Google Scholar
|
|
26
|
Rami A, Langhagen A and Steiger S: Focal
cerebral ischemia induces upregulation of Beclin 1 and
autophagy-like cell death. Neurobiol Dis. 29:132–141. 2008.
View Article : Google Scholar
|
|
27
|
Xie C, Ginet V, Sun Y, Koike M, Zhou K, Li
T, Li H, Li Q, Wang X, Uchiyama Y, et al: Neuroprotection by
selective neuronal deletion of Atg7 in neonatal brain injury.
Autophagy. 12:410–423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsui Y, Takagi H, Qu X, Abdellatif M,
Sakoda H, Asano T, Levine B and Sadoshima J: Distinct roles of
autophagy in the heart during ischemia and reperfusion: Roles of
AMP-activated protein kinase and Beclin 1 in mediating autophagy.
Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geng J and Klionsky DJ: Direct
quantification of autophagic flux by a single molecule-based probe.
Autophagy. 13:639–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ichimura Y, Waguri S, Sou YS, Kageyama S,
Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, et
al: Phosphorylation of p62 activates the Keap1-Nrf2 pathway during
selective autophagy. Mol Cell. 51:618–631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song S, Tan J, Miao Y, Li M and Zhang Q:
Crosstalk of autophagy and apoptosis: Involvement of the dual role
of autophagy under ER stress. J Cell Physiol. 232:2977–2984. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maiuri MC, Criollo A, Tasdemir E, Vicencio
JM, Tajeddine N, Hickman JA, Geneste O and Kroemer G: BH3-only
proteins and BH3 mimetics induce autophagy by competitively
disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L).
Autophagy. 3:374–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Du Y and Ji X: Bcl-2 down-regulation by
small interfering RNA induces Beclin1-dependent autophagy in human
SGC-7901 cells. Cell Biol Int. 38:1155–1162. 2014. View Article : Google Scholar : PubMed/NCBI
|