Introduction
Inflammation is a complex immunological response
that protects the body from infection by bacteria, viruses and
fungi. Inflammatory responses are self-limited through the balance
between the inhibition of proinflammatory proteins and the
upregulation of anti-inflammatory proteins (1,2).
Macrophages are the essential first line of defense against common
pathogens. Among the various stimuli of macrophages,
lipopolysaccharide (LPS) is an inflammatory stimulator that
functions through toll-like receptor 4 (TLR4) on the macrophage
membrane surface (3,4). Macrophages activated by LPS produce
several proinflammatory mediators, such as nitric oxide (NO),
prostaglandin E2 (PGE2), interleukin (IL)-6
and -1β, and tumor necrosis factor (TNF)-α (5,6).
The enhanced production of inflammatory mediators is necessary for
the host defense against external stimuli; however, this is
reportedly involved in several inflammatory diseases, including
rheumatoid arthritis, systemic lupus erythematosus and inflammatory
bowel disease (7,8). Therefore, agents that inhibit
excessive production of inflammatory mediators in activated
macrophages may be candidates for the treatment of inflammatory
diseases.
Guettarda speciosa Linn. (G.
speciosa), a member of the Rubiaceae family, is widely
distributed from East Africa to South Asia. In East Africa, the
stem of G. speciosa is used in a preparation for the
treatment of maternal postpartum infection (9). In Tahiti, G. speciosa has
been used in anti-diarrheic, febrifugal and anti-cholinergic
treatments (10). In addition,
the decoction of G. speciosa leaves has been used to treat
cough, cold, sore throats, fever, dysentery and headache (10). The anti-inflammatory effects of
the extracts from G. speciosa on intestinal mucosa
inflammation in mice have been previously demonstrated (10,11). Several studies have reported a
positive correlation between the effects of the therapeutic use of
drugs isolated from plants and of the traditional use of the plant
extracts from which they are derived (12,13). The phytochemical composition of
G. speciosa comprises phenolic components, including
squalene, cladosporide A, nonacosane and campesterol, and steroidal
components, such as stigmasterol (14). Certain major active components,
including squalene, campesterol and stigmasterol, have demonstrated
anti-inflammatory, anti-cancer, anti-bacterial and anti-oxidant
effects (15–17).
Although G. speciosa has been used as a folk
medicine and several of its pharmacological activities have been
evaluated, the anti-inflammatory mechanisms of this plant have
remained to be determined. In the present study, the
anti-inflammatory effects of the methanol extract of G.
speciosa (MGS) in LPS-activated RAW 264.7 macrophages and the
underlying mechanisms of action were investigated.
Materials and methods
MGS preparation
A methanol extract of G. speciosa (Rubiaceae)
(voucher no: PBID 11237) collected from Indonesia was purchased
from the International Biological Material Research Center (Korea
Research Institute of Bioscience and Biotechnology, Daejeon, Korea)
(18). The extract was dissolved
in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and added to the culture media to achieve the final
concentration as described in each assay. In all experiments, the
DMSO concentrations did not exceed 0.1%.
Cell culture and reagents
RAW 264.7 macrophages were purchased from the
American Type Culture Collection (Manassas, VA, USA). RAW 264.7
cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS, Invitrogen; Thermo
Fisher Scientific, Inc.), and 1% penicillin/streptomycin (Thermo
Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
containing 5% CO2. Mouse polyclonal anti-p38 (cat no.
sc-7972), mouse monoclonal anti-c-Jun N-terminal kinase (JNK; cat
no. sc-7345), rabbit polyclonal anti-inhibitor of nuclear factor
(NF)-κB [IκBα; (C-32); cat no. sc-371], rabbit polyclonal phospho
(p)-anti-IκBα (Ser32/36; cat no. sc-101713), mouse polyclonal
anti-spleen tyrosine kinase (Syk; cat no. sc-1240), mouse
monoclonal anti-c-proto-oncogene tyrosine-protein kinase Src (c-Src
cat no. sc-19), rabbit polyclonal anti-p-c-Src (Tyr424; cat no.
sc-81521), rabbit polyclonal anti-Akt1/2/3 (cat no. sc-8312),
rabbit polyclonal anti-p-Akt1/2/3 (Ser473; cat no. sc-7985), goat
polyclonal anti-cyclooxygenase 2 (COX-2; cat no. sc-1745), rabbit
polyclonal anti-inducible NO synthase (iNOS; cat no. 651) and mouse
monoclonal anti-α-tubulin (cat no. sc-5286) antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Rabbit polyclonal transforming growth factor-β-activated kinase 1
(TAK1; cat no. 4505), rabbit monoclonal anti-p-TAK1 (Thr184/187;
cat no. 4508), rabbit polyclonal anti-mitogen-activated protein
kinase (MAPK) kinase 4 (MKK4; cat no. 9152), rabbit polyclonal
anti-MKK7 (cat no. 9264), rabbit polyclonal anti-p-MKK4 (Thr261;
cat no. 9151), rabbit polyclonal anti-p-MKK7 (Ser217/Thr275; cat
no. 4171), rabbit polyclonal anti-p-p38 (Thr180/Tyr182; cat no.
9211), rabbit polyclonal anti-extracellular signal-regulated kinase
(ERK; cat no. 9102), rabbit monoclonal anti-p-ERK (Thr202/Tyr204;
cat no. 9106), rabbit polyclonal anti-p-JNK (Thr183/Tyr185; cat no.
9252) and rabbit polyclonal anti-p-Syk (Tyr525/526; cat no. 2711)
antibodies were purchased from Cell Signaling Technology (Danvers,
MA, USA). All primary antibodies were diluted at 1:1,000 in 5%
non-fat dried milk. Polyclonal anti-rabbit IgG-HRP (1:5,000; cat
no. LF-SA8002) and polyclonal anti-mouse IgG Fc-HRP (1:5,000; cat
no. LF-SA8001) were from AbFrontier (Young In Frontier Co., Ltd.,
Seoul, Korea). Ready-SET-Go! ELISA kits were used for the detection
of IL-6 (cat no. 88-7084) and TNF-α (cat no. 88-7324) were from
eBioscience (Thermo Fisher Scientific, Inc).
Cell viability assay
RAW 264.7 macrophages were seeded in 96-well plates
(4.5×104 cells/well) and treated with various
concentrations of MGS (50, 100, 200, 300 and 600 µg/ml) and
LPS (1 µg/ml) at 37°C for 24 h. The cytotoxic effects were
evaluated using the EZ-Cytox cell viability assay kit (cat no.
EZ-3000, Daeil Lab, Seoul, Korea). EZ-Cytox solution, which
contained a water-soluble tetrazolium salt, was added to the cell
culture (1/10 culture medium), followed by incubation for 1 h at
37°C. The relative absorbance were measured at 450 nm (absorbance
for viable cells) and 650 nm (reference absorbance) with a Synergy
H1 microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Nitrite assay
RAW 264.7 macrophages were seeded in 96-well plates
at a density of 4.5×104 cells/well and incubated at 37°C
overnight. Subsequently, the cells were incubated with various
concentrations of MGS (50, 100, 200 and 300 µg/ml) and LPS
(1 µg/ml) at 37°C for 24 h. Culture media (100 µl)
was transferred to a new 96 well-plate and 100 µl Griess
reagent [1% sulfanilamide, 2.5% phosphoric acid
(H3PO4), and 0.1% N-(1-naphthyl)
ethylenediamine in distilled water] was added. Sodium nitrite was
used to generate a standard curve. After incubation at 37°C for 10
min, the absorbance was measured at 540 nm using a Synergy H1
microplate reader.
ELISA
RAW 264.7 macrophages were treated with various
concentrations of MGS (50, 100, 200 and 300 µg/ml) and LPS
(1 µg/ml) at 37°C for 24 h. After stimulation, the
concentrations of TNF-α and IL-6 in the supernatants were measured
using a sandwich ELISA with monoclonal antibodies specific for each
mediator in accordance with the manufacturer's protocol. Prior to
the application of samples, the plate was pre-coated with the
capture antibody in the supplied buffer. Following incubation
overnight at 4°C, the plate was washed with 1X PBS with 0.05% Tween
20 (PBST) and blocked with 1X assay diluents at room temperature
for 1 h. The solutions were added to each well and incubated for 2
h at room temperature. The plate was washed with 1X PBST and
treated with a biotinylated detection antibody solution at room
temperature for 1 h, which was followed by treatment with a
horseradish peroxidase-streptavidin solution at room temperature
for 30 min. After one further wash, 3,3′,5,5′-tetramethylbenzidine
was added and the plate was incubated at room temperature for 10
min in the dark. Subsequently, 1N H3PO4 was
added and the absorbance of individual wells was measured at 450 nm
using a Synergy H1 microplate reader.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RAW 264.7 macrophages were treated with various
concentrations of MGS (50, 100, 200 and 300 µg/ml) and LPS
(1 µg/ml) at 37°C for 3 h. Total RNA was isolated from the
cells using Accuzol total RNA extraction solution (Bioneer Corp.,
Daejeon, Korea) and was reverse transcribed into complementary DNA
(cDNA) by using a TOPscript cDNA synthesis kit in accordance with
the manufacturer's protocol (Enzynomics, Daejeon, Korea). The
amplification of cDNA was performed using a RT-qPCR premix and iTaq
Universal SYBR Green Master Mix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) in accordance with the manufacturer's protocol.
The PCR was run for 40 cycles of denaturation at 95°C (10 sec) and
annealing/extension at 55°C (30 sec) in a CFX Connect real-time
thermal cycler (Bio-Rad Laboratories, Inc.). The gene expressions
were quantified using the 2−ΔΔCq method, and normalized
to the reference genes β-actin and GAPDH (19). PCR primer sequences used in this
study were as follows: Mouse iNOS (forward,
5′-TGGCCACCAAGCTGAACT-3′ and reverse,
5′-TCATGATAACGTTTCTGGCTCTT-3′); COX-2 (forward,
5′-GATGCTCTTCCGAGCTGTG-3′ and reverse,
5′-GGATTGGAACAGCAAGGATTT-3′), TNF-α (forward,
5-CTGTAGCCCACGTCGTAGC-3′ and reverse, 5′-TTGAGATCCATGCCGTTG-3′),
IL-6 (forward, 5′-TCTAATTCATATCTTCAACCAAGAGG-3′ and reverse,
5′-TGGTCCTTAGCCACTCCTTC-3′), IL-1β (forward,
5′-TTGACGGACCCCAAAAGAT-3′ and reverse, 5′-GATGTGCTGCTGCGAGATT-3′),
β-actin (forward, 5′-CGTCATACTCCTGCTTGCTG-3′ and reverse,
5′-CCAGATCATTGCTCCTCCTGA-3′) and GAPDH (forward,
5′-GCTCTCTGCTCCTCCTGTTC-3′ and reverse,
5′-ACGACCAAATCCGTTGACTC-3′).
Western blot analysis
RAW 264.7 macrophages were pre-treated with MGS (50,
100, 200 and 300 µg/ml) for 2 h at 37°C and stimulated with
LPS (1 µg/ml) at 37°C for the indicated times to
specifically detect the target proteins: 3 min (for IκBα, Src, Syk
and Akt), 15 min (for MAPKs) or 24 h (for iNOS and COX-2). Cells
were washed twice with cold PBS (pH 7.4) and lysed in lysis buffer
[150 mM NaCl, 20 mM Tris-HCl (pH 8.0), 0.5% IGEPAL®
CA-630, 0.5% Triton X-100, 1 mM EDTA, 1% glycerol, 2 mM
phenylmethylsulfonyl fluoride, 10 mM NaF and 1 mM
Na3VO4]. The lysates were centrifuged at
15,814 × g for 30 min at 4°C and the supernatants were transferred
to a new tube. The protein concentration was determined by a
Bradford protein assay (Bio-Rad Laboratories, Inc.) in accordance
with the manufacturer's protocol and immunoblotting was performed
as described previously (20). In
brief, equal amounts (30 µg per lane) of protein were mixed
with 5X SDS sample buffer [12 mM Tris-HCl (pH 6.8), 0.4% SDS, 5%
glycerol, 1% β-mercaptoethanol and 0.02% bromophenol blue] and
boiled at 100°C for 5 min. Samples were separated by 10% SDS-PAGE
and transferred onto nitrocellulose membranes. Membranes were
blocked with 5% nonfat-dried skimmed milk for 1 h at room
temperature in 1X Tris-buffered saline with 0.05% Tween 20 (TBST)
and each membrane was incubated at 4°C overnight with a specific
primary antibody. Membranes were washed four times with TBST and
then incubated with the appropriate secondary antibody for 2 h at
room temperature. Protein bands were visualized by application of
enhanced chemiluminescence immunoblotting detection reagent
(Pierce; Thermo Fisher Scientific, Inc.) and protein levels were
quantified using LabWorks software version 4.6 (UVP Inc., Upland,
CA, USA).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. The differences between experimental conditions were
assessed using one-way analysis of variance and Dunnett's
multiple-comparisons test, which were computed using Prism 3.0
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant difference.
Data from nine replicates, comprised of three independent
experiments with three replicates, were analyzed for each test
condition.
Results
Effect of MGS on viability of LPS-treated
or -untreated RAW 264.7 cells
G. speciosa has been used in folk medicine
from East Africa to Asia for the treatment of inflammatory
diseases. Recently, several components of G. speciosa
extract have been reported to exhibit anti-inflammatory,
anti-oxidative, anti-microbial or anti-epileptic effects (10,16). However, to the best of our
knowledge, systemic studies to investigate the anti-inflammatory
effects and precise mechanisms of G. speciosa have not been
performed. Therefore, the anti-inflammatory effects of MGS in
macrophages were explored by performing cell viability assays to
determine the non-cytotoxic concentrations of MGS in RAW 264.7
macrophages. As illustrated in Fig.
1A, MGS exerted only a minor or no effect on cell viability
compared with the untreated control group at concentrations of ≤300
µg/ml in the absence or presence of LPS. However,
cytotoxicity was observed at MGS concentrations of >300
µg/ml. Therefore, MGS concentrations of up to 300
µg/ml were used in the subsequent experiments.
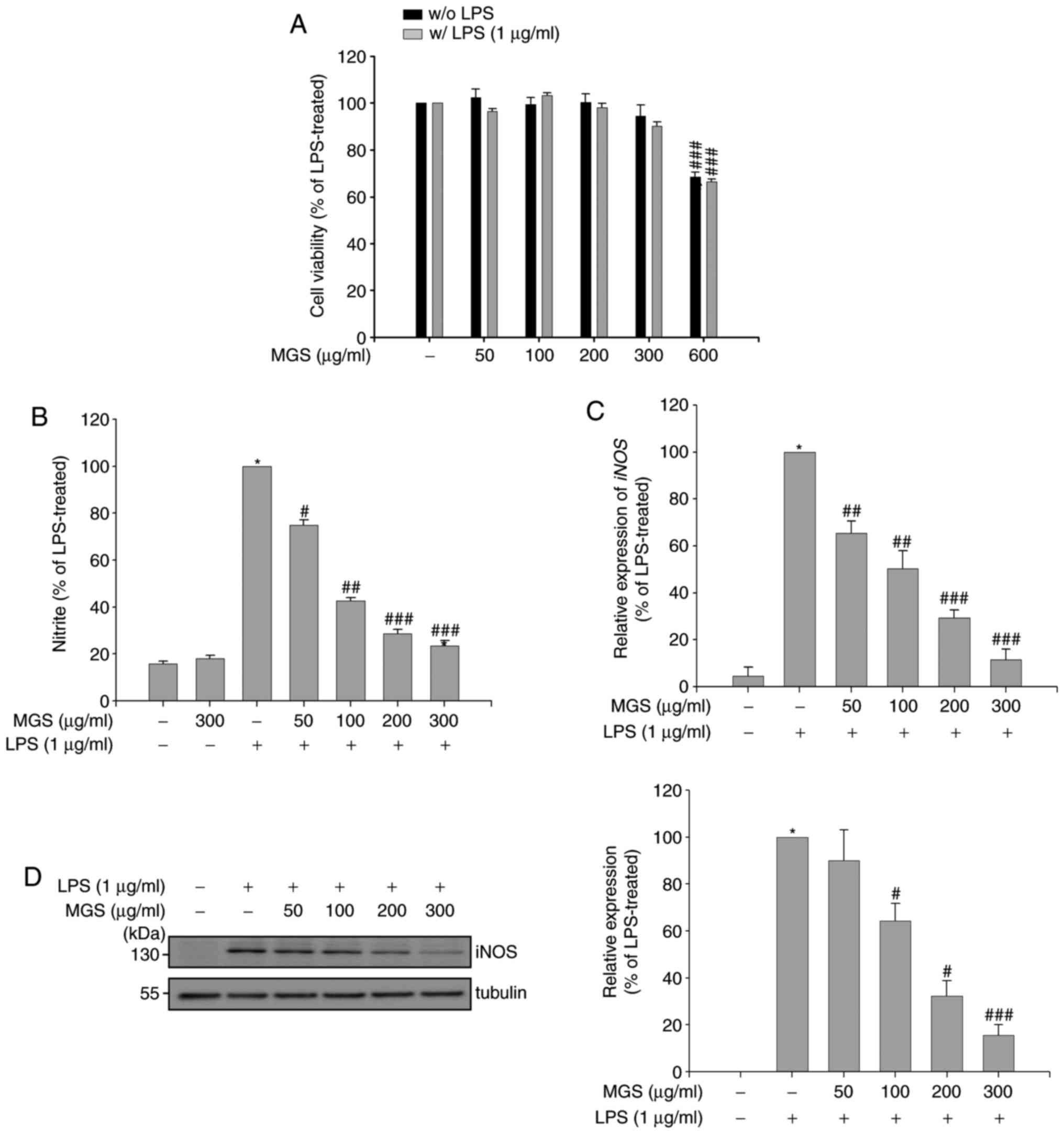 | Figure 1Effects of MGS on cell viability and
LPS-mediated NO production. (A) RAW 264.7 macrophages were
pretreated with MGS (50, 100, 200, 300, and 600 µg/ml) for 2
h, followed by incubation with or without LPS and the cell
viability was measured using the EZ-Cytox assay kit. (B) After
stimulation for 24 h, NO levels in the supernatants were measured
using the Griess reagent. After stimulation for 3 h, the iNOS (C)
mRNA and (D) protein expression levels in each group were compared
to those in the LPS-treated group using reverse-transcription
quantitative polymerase chain reaction analysis or western blot
analysis with normalization to α-tubulin, respectively. Values are
expressed as the mean ± standard error of the mean.
*P<0.01 vs. LPS-untreated or LPS-treated control
group; #P<0.05, ##P<0.01 and
###P<0.001 relative to the LPS-treated group. NO,
nitric oxide; iNOS, inducible NO synthase; LPS, lipopolysaccharide;
MGS, methanol extract of G. speciosa. |
Inhibitory effects of MGS on LPS-induced
NO production
Under physiological conditions, NO has several
functions, including the elimination of bacteria, control of blood
pressure and mediation of neurotransmission (21). However, when an inflammatory
reaction occurs, NO levels are increased by inducing iNOS in cells
and the generated NO exerts dual roles in immunity and inflammatory
responses (22). The
anti-inflammatory effects of MGS with regard to its capability to
suppress NO production in LPS-treated RAW 264.7 cells was
evaluated. The production of NO decreased by MGS in LPS-stimulated
RAW 264.7 cells in a dose-dependent manner (Fig. 1B). As iNOS is a key enzyme in the
production of NO in LPS-stimulated macrophages, RT-qPCR and
immunoblotting were employed to assess whether MGS regulated the
expression of iNOS at the mRNA and protein levels, respectively. As
demonstrated in Fig. 1C, the mRNA
expression levels of iNOS were decreased by MGS. In addition, MGS
reduced the LPS-induced increases of iNOS protein expression
(Fig. 1D). These results
demonstrated that MGS reduced NO production in LPS-activated
macrophages through the inhibition of iNOS expression.
Effect of MGS on LPS-induced production
of proinflammatory mediators
The biosynthesis of PGE2, which is
tightly regulated by COX-2, is significantly increased in inflamed
tissue, contributing to the development of the cardinal signs of
acute inflammation (23).
Therefore, the inhibitory effects of MGS on the mRNA and protein
expression of COX-2 were investigated. In contrast to the reduction
of iNOS mRNA expression, MGS did not affect the mRNA expression of
COX-2 (Fig. 2A). Similarly, no
significant inhibition of LPS-induced COX-2 protein expression by
MGS was observed (Fig. 2B). These
results suggested that MGS suppressed the expression of iNOS, but
not COX-2, in macrophages.
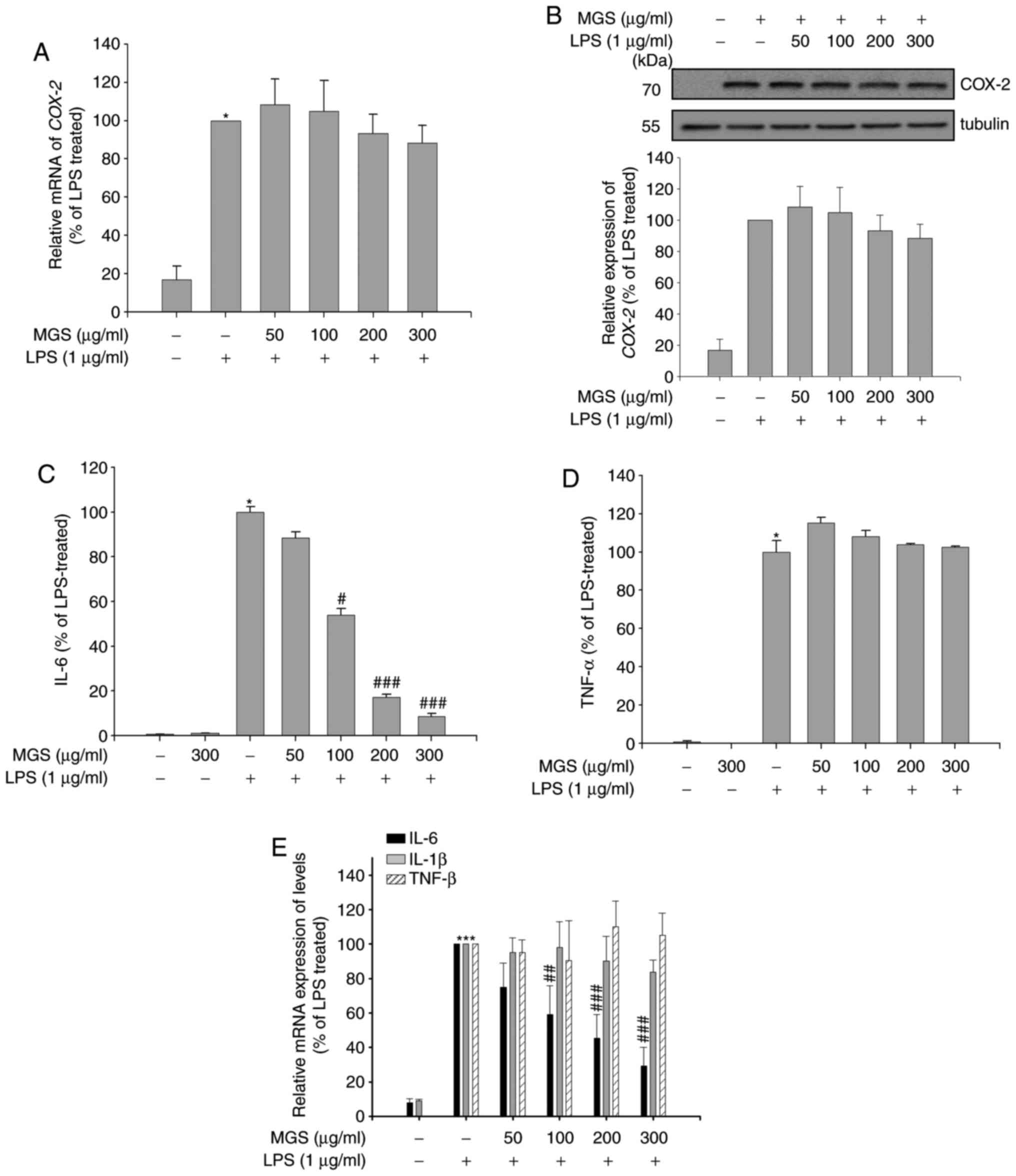 | Figure 2Effect of MGS on the LPS-induced
production of proinflammatory mediators. RAW 264.7 macrophages were
pretreated with MGS (50, 100, 200, and 300 µg/ml) for 2 h
and stimulated with LPS for the indicated times. After stimulation
for 3 h, COX2 (A) mRNA and (B) protein expression levels were
measured in each group using RT-qPCR analysis or western blot
analysis with normalization to α-tubulin, respectively. After
stimulation for 24 h, protein expression levels of (C) IL-6 and (D)
TNF-α were measured by ELISA and (E) relative mRNA expression
levels of IL-1β, IL-6 and TNF-α were measured by RT-qPCR. Values
are expressed as the mean ± standard error or the mean.
*P<0.01 vs. LPS-untreated group;
#P<0.05, ##P<0.01 and
###P<0.001 relative to the LPS-treated group. COX,
cyclooxygenase; IL, interleukin; LPS, lipopolysaccharide; MGS,
methanol extract of G. speciosa; TNF, tumor necrosis factor;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. |
Proinflammatory cytokines are predominantly produced
by activated macrophages and are involved in the upregulation of
inflammatory reactions (24).
There is evidence that certain proinflammatory cytokines, including
IL-1β, IL-6 and TNF-α, are involved in the mechanisms of
pathological pain (25).
Therefore, the inhibitory effect of MGS on cytokine production in
LPS-stimulated macrophages was tested to further investigate the
anti-inflammatory actions of MGS. As presented in Fig. 2C and D, LPS-stimulated RAW 264.7
macrophages produced large amounts of the cytokines IL-6 and TNF-α.
MGS treatment reduced the LPS-stimulated production of IL-6, but
did not change TNF-α production. To determine whether the effect of
MGS on the production of proinflammatory cytokines was regulated at
the transcriptional level, the mRNA expression of proinflammatory
cytokines was assessed in LPS-stimulated RAW 264.7 cells after
treatment with MGS. RT-qPCR analysis revealed that MGS inhibited
the expression of IL-6 mRNA in LPS-stimulated RAW 264.7 cells, but
did not inhibit TNF-α and IL-1β mRNA levels (Fig. 2E). These results indicated that
MGS selectively regulated the production of the proinflammatory
cytokine IL-6 at the mRNA and protein level.
Selective inhibitory effect of MGS on
MAPK phosphorylation and NF-κB activation in RAW 264.7
macrophages
Owing to the relevance of the MAPK and NF-κB
signaling pathways in LPS-induced cytokine gene expression in
inflammation (26,27), the modulatory effect of MGS on
these pathways in macrophages was investigated. The transcription
factor NF-κB performs critical roles in inflammation, immunity,
cell proliferation, differentiation and survival (28). NF-κB activation depends on
phosphorylation of IκBα at Ser32/36; in unstimulated cells, the
proteasomal degradation of phosphorylated IκBα retains NF-κB as
heterodimers in the cytosol (29). Therefore, the levels of total and
p-IκBα were measured to assess the involvement of the NF-κB pathway
in the anti-inflammatory mechanism of MGS in LPS-induced
macrophages. As demonstrated in Fig.
3A, LPS treatment increased the phosphorylation of IκBα and
reduced the levels of total, unphosphorylated IκBα; however, in the
MGS-treated groups, the LPS-induced phosphorylation of IκBα and
reduction of its expression were decreased.
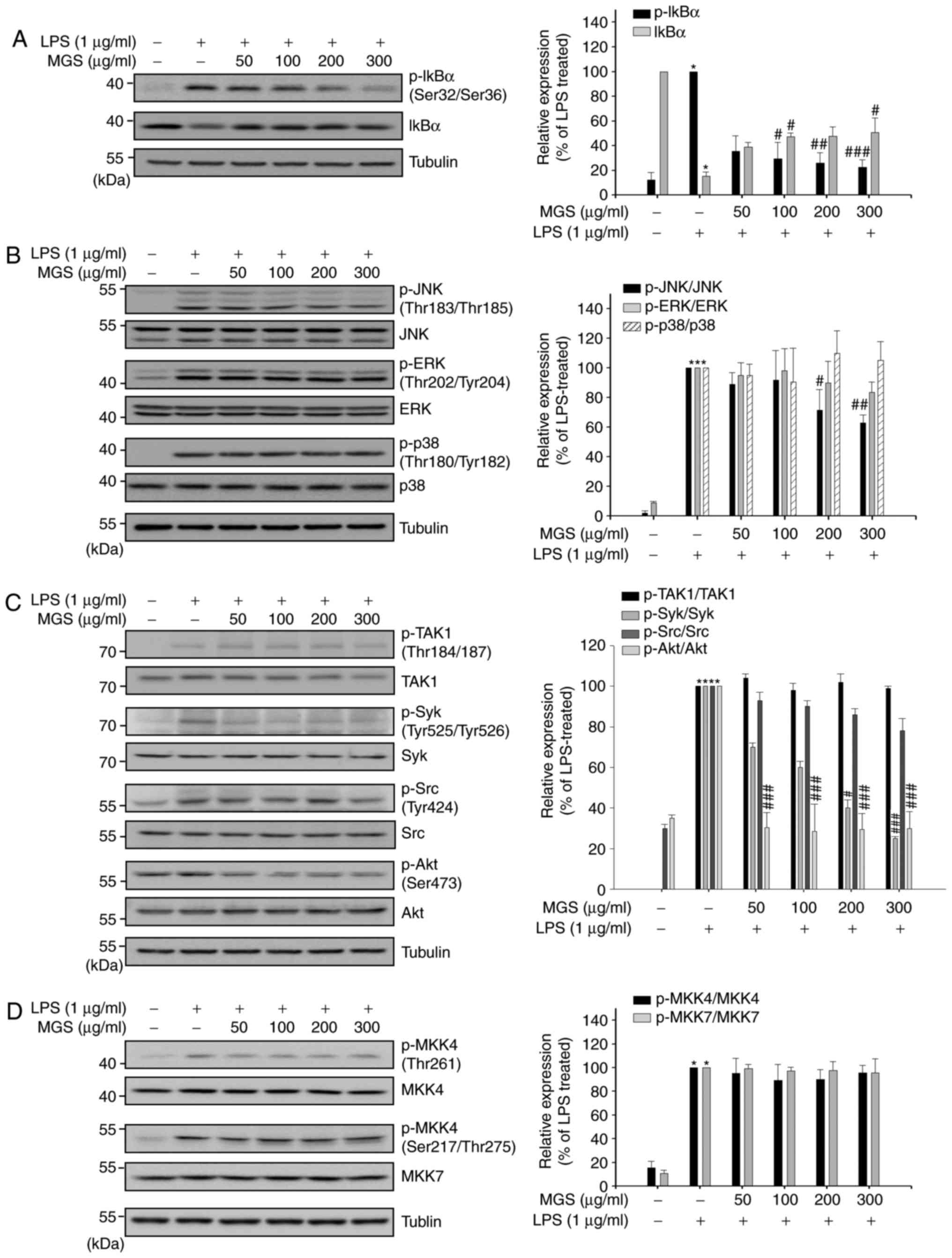 | Figure 3Selective inhibition of MGS on MAPK
phosphorylation and NF-κB activation in RAW 264.7 macrophages. RAW
264.7 macrophages were pretreated with MGS (50, 100, 200 and 300
µg/ml) for 2 h and stimulated with LPS for indicated times.
(A) After stimulation for 3 min, protein expression levels of
p-IκBα and IκBα were measured by western blot analysis. (B) After
stimulation for 15 min, protein expression levels of JNK, p-JNK,
ERK, p-ERK, p38 and p-p38 were measured by western blot analysis.
(C) After stimulation for 3 min, protein expression levels of TAK1,
p-TAK1, Syk, p-Syk, Src, p-Src, Akt and p-Akt and (D) after
stimulation for 15 min, protein expression levels of MKK4, p-MKK4
and-7 were measured by western blot analysis. Values are expressed
as the mean ± standard error of the mean. *P<0.01 vs.
LPS-untreated group; #P<0.05, ##P<0.01
and ###P<0.001 relative to the LPS-treated group.
ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; LPS, lipopolysaccharide; MGS, methanol extract of G.
speciosa ; MKK, mitogen-activated protein kinase kinase; p,
phosphorylated; Src, sarcoma; Syk, spleen tyrosine kinase; IκB,
inhibitor of nuclear factor κB. |
Studies have demonstrated that MAPKs respond to
extra- and intracellular stimuli and regulate immune responses,
including proinflammatory cytokine production, as well as cell
proliferation, differentiation and survival (30,31). In the present study, the
suppression of the phosphorylation status in the activation loops
of MAPKs was examined by immunoblotting analysis to identify the
role of MGS in MAPK signaling pathways. As illustrated in Fig. 3B, the phosphorylation of all MAPKs
was induced in LPS-stimulated RAW 264.7 macrophages. However, only
the LPS-induced phosphorylation of JNK was moderately inhibited
after MGS treatment; that of ERK1/2 and p38 was not changed. As the
phosphorylation status of MAPKs is directly associated with their
kinase activity (32), the
results suggested that MGS reduced the production of
proinflammatory mediators through the inhibition of NF-κB and JNK
signaling.
In addition, Syk/Src/Akt signaling pathways have a
key role in NF-κB activation, whereas JNK activation is induced by
MKK4/7 (33,34). To clarify the target of MGS in the
regulation of NF-κB and JNK signaling pathways, the effects of MGS
on the phosphorylation of Syk/Src/Akt and MKK4/7 were determined.
The LPS-increased phosphorylation of Syk and Akt was decreased by
MGS treatment (Fig. 3C). However,
the LPS-induced phosphorylation of MKK4/7 did not appear to be
affected by MGS treatment (Fig.
3D). In conclusion, these results suggested that, in RAW 264.7
macrophages, MGS regulated NF-κB activity through the inhibition of
the Syk/Akt axis and that its inhibitory effect on JNK activation
did not proceed via the regulation of MKK4/7.
Discussion
The production of NO by iNOS after certain stimuli
is one of the most important steps in the inflammatory process
(35). Numerous studies have
attempted to identify novel anti-inflammatory agents that inhibit
iNOS expression and elicit their mechanisms of action in
inflammation (36,37). For instance, COX-2, which
ultimately induces inflammation and fever, catalyzes the production
of PGE2 from the lipid arachidonic acid (38). The results of the present study
demonstrated that MGS inhibited the expression of iNOS, but did not
suppress COX-2 expression. Previous studies have reported that the
selective regulation of proinflammatory signaling pathways by
anti-inflammatory extracts led to the differential inhibition of
proinflammatory mediators (39,40). Based on the results of the present
study and those of previous reports, the selective inhibitory
effects of MGS on the production of proinflammatory mediators are
likely to be a result of MGS-mediated selective inhibition of
certain associated upstream signaling pathways.
MGS reduced the production of IL-6, but not IL-1β
and TNF-α, in LPS-induced RAW 264.7 cells. Although the promoter
region of each proinflammatory cytokine is known to contain binding
sites for NF-κB and downstream transcription factors of MAPKs,
previous studies by our group demonstrated that extracts of natural
plants exert different inhibitory effects on the production of
proinflammatory cytokines as a result of differential regulation of
NF-κB and MAPKs (41). Of note,
the inhibition of TNF-α production was only detected when the
extract inhibited NF-κB and all MAPKs, including JNK, ERK and p38
(42). In one instance, TNF-α
production was not attenuated when the extract only inhibited one
MAPK in addition to NF-κB (43).
Therefore, it may be speculated that the MGS-mediated selective
inhibition of cytokine production is a consequence of the selective
regulation of inflammatory signal transduction, but further studies
are needed to confirm the above.
In the present study, MGS suppressed the LPS-induced
phosphorylation of IκBα and JNK. The MGS-mediated dephosphorylation
of TAK1 was measured, as TAK1 is a well-known upstream kinase in
the activation of NF-κB and MAPKs, including JNK and p38 (44). However, no significant inhibition
of TAK1 phosphorylation was detected by MGS treatment (Fig. 3C). Previous studies to determine
the regulatory effects of MGS on the upstream signaling molecules
of IκBα and JNK revealed that MGS regulated the phosphorylation of
Syk/Akt, upstream signaling proteins of NF-κB activation, whereas
MKK4 and MMK7, the specific upstream signaling proteins of JNK,
were not affected by MGS treatment. Further studies, including
those on the measurement of MKK4/7 kinase activity after MGS
treatment, are needed to clarify the underlying mechanism(s) of the
regulatory effect of MGS on JNK activation.
Several studies have reported that the
anti-inflammatory properties of the individual active components
define the anti-inflammatory functions of an extract and its signal
regulatory ability (36,45). Phytochemical analysis of MGS
performed by gas chromatography tandem mass spectrometry
demonstrated that MGS contained several anti-inflammatory
components, including squalene, campesterol and stigmasterol, that
are known to selectively regulate inflammatory responses in
different ways (14). Squalene
was reported to inhibit inflammatory responses in macrophages via
the inhibition of JNK and NF-κB pathways (15). Campesterol and stigmasterol
inhibit the production of inflammatory mediators via the
suppression of the NF-κB signaling pathway in chondrocytes
(16,46,47). It may be proposed that the
selective inhibition of NF-κB and JNK pathways by MGS is a result
of the regulatory effects of its anti-inflammatory components on
these signaling pathways, but further studies are needed to
identify specific roles of the components (Fig. 4).
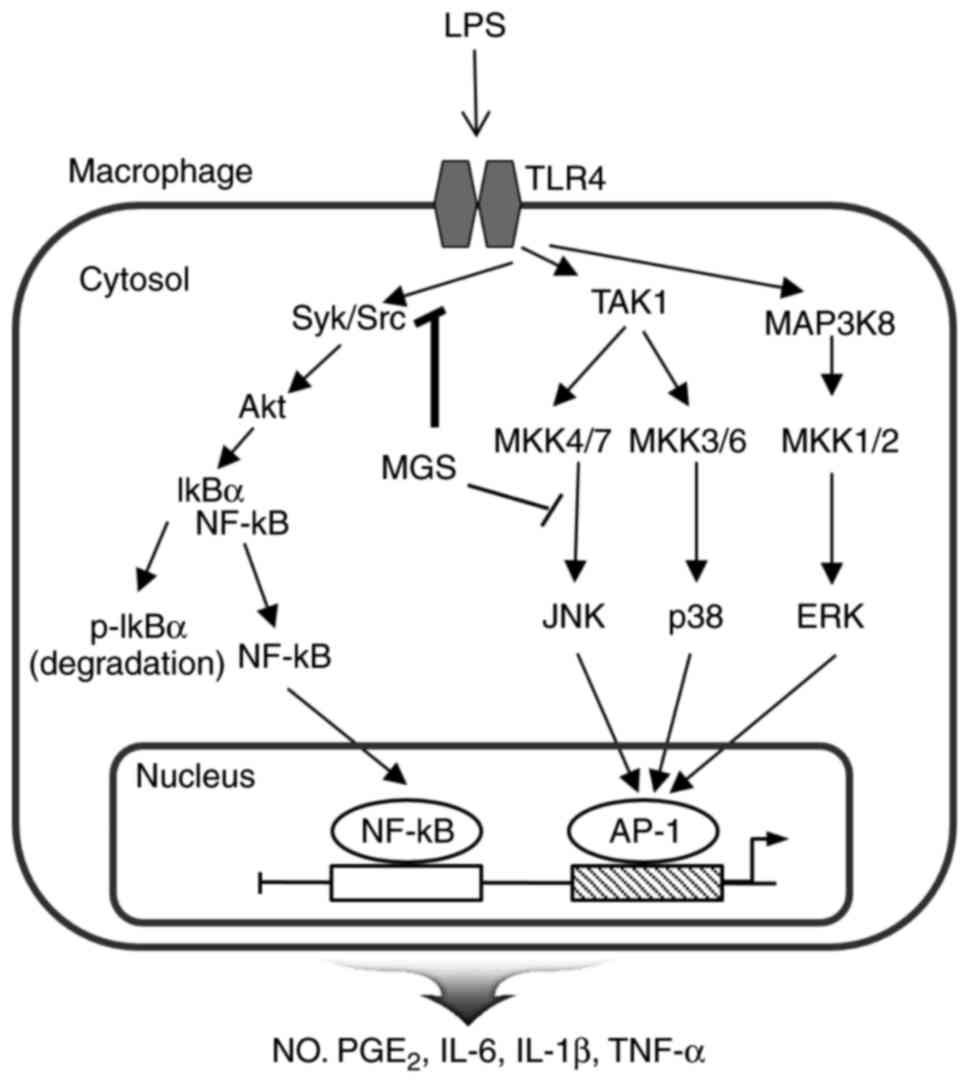 | Figure 4Schematic representation of the
proposed anti-inflammatory mechanism of MGS in LPS-activated RAW
264.7 macrophages. MGS may inhibit the inflammatory mediators via
regulation of the NF-κB and JNK signaling pathways. The molecules
and inflammatory mediators regulated by MGS are stated in bold
letters. JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide;
MGS, methanol extract of G. speciosa; NF-κB, nuclear factor
κB; MAPK, mitogen-activated protein kinase; MKK, MAPK kinase; IκB,
inhibitor of NF-κB; TLR, Toll-like receptor; AP-1, activator
protein 1; ERK, extracellular signal-regulated kinase; p,
phosphorylated; Src, sarcoma; Syk, spleen tyrosine kinase; TAK1,
transforming growth factor-β-activated kinase 1; NO, nitric oxide;
LPS, lipopolysaccharide; TNF, tumor necrosis factor; PGE2,
prostaglandin E2. |
In conclusion, MGS inhibited NO and IL-6 production
through the suppression of certain molecular signaling molecules
associated with the inflammatory response, including NF-κB and JNK
activation. Therefore, owing to the observed anti-inflammatory
effect of MGS, this extract may be considered for the development
of an effective anti-inflammatory agent for the treatment of severe
inflammation.
Acknowledgments
The present study was supported by the National
Research Foundation of Korea (NRF) with funding from the Ministry
of Science, ICT & Future Planning (grant nos.
NRF-2015R1A2A2A11001446 and NRF-2015R1A5A1008958).
References
|
1
|
Abdalla SI, Sanderson IR and Fitzgerald
RC: Effect of inflammation on cyclooxygenase (COX)-2 expression in
benign and malignant oesophageal cells. Carcinogenesis.
26:1627–1633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Franchi J, Marteau C, Crola da Silva C,
Mitterrand M, André P and Kieda C: Cell model of inflammation.
Biosci Rep. 28:23–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Billack B: Macrophage activation: Role of
toll-like receptors, nitric oxide, and nuclear factor kappa B. Am J
Pharm Educ. 70:1022006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar
|
|
5
|
Lee KJ, Kim YK, Krupa M, Nguyen AN, Do BH,
Chung B, Vu TT, Kim SC and Choe H: Crotamine stimulates phagocytic
activity by inducing nitric oxide and TNF-α via p38 and NF κ-B
signaling in RAW 264.7 macrophages. BMB Rep. 49:185–190. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng F and Lowell CA: Lipopolysaccharide
(LPS)-induced macrophage activation and signal transduction in the
absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med.
185:1661–1670. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evans SS, Repasky EA and Fisher DT: Fever
and the thermal regulation of immunity: The immune system feels the
heat. Nat Rev Immunol. 15:335–349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Juhn SK, Jung MK, Hoffman MD, Drew BR,
Preciado DA, Sausen NJ, Jung TT, Kim BH, Park SY, Lin J, et al: The
role of inflammatory mediators in the pathogenesis of Otitis media
and Sequelae. Clin Exp Otorhinolaryngol. 1:117–138. 2008.
View Article : Google Scholar
|
|
9
|
Cai WH, Matsunami K, Otsuka H, Shinzato T
and Takeda Y: A glycerol α-D-glucuronide and a megastigmane
glycoside from the leaves of Guettarda speciosa L. J Nat Med.
65:364–369. 2011. View Article : Google Scholar
|
|
10
|
Gandhimathi R, Saravana KA, Senthil Kumar
KK, Kusuma PK and Uma MJ: Pharmacological studies of
anti-diarrhoeal activity of Guettarda speciosa (L.) in experimental
animals. J Pharm Sci Res. 2:61–67. 2009.
|
|
11
|
Saravana KA and Gandhimathi R: Effect of
Guettarda speciosa extracts on antioxidant enzymes levels in rat
brain after induction of seizures by MES and PTZ. J Nat Prod.
3:80–85. 2010.
|
|
12
|
Atanasov AG, Waltenberger B,
Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L,
Schwaiger S, Heiss EH, et al: Discovery and resupply of
pharmacologically active plant-derived natural products: A review.
Biotechnol Adv. 33:1582–1614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oliveira AB, Dolabela MF, Braga FC, Jácome
RL, Varotti FP and Póvoa MM: Plant-derived antimalarial agents: New
leads and efficient phythomedicines. Part I Alkaloids. An Acad Bras
Cienc. 81:715–740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Revathi D and Rajeswari M: Chemical
profiling of Guettarda speciosa Linn. by GC-MS. Int J Adv Res
Technol. 5:2015.
|
|
15
|
Cárdeno A, Aparicio-Soto M, Montserrat-de
la Paz S, Bermudez B, Muriana FJG and Alarcón-de-la-Lastra C:
Squalene targets pro- and anti-inflammatory mediators and pathways
to modulate over-activation of neutrophils, monocytes and
macrophages. J Funct Foods. 14:779–790. 2015. View Article : Google Scholar
|
|
16
|
Gabay O, Sanchez C, Salvat C, Chevy F,
Breton M, Nourissat G, Wolf C, Jacques C and Berenbaum F:
Stigmasterol: A phytosterol with potential anti-osteoarthritic
properties. Osteoarthritis Cartilage. 18:106–116. 2010. View Article : Google Scholar
|
|
17
|
Sabeva NS, McPhaul CM, Li X, Cory TJ,
Feola DJ and Graf GA: Phytosterols differentially influence ABC
transporter expression, cholesterol efflux and inflammatory
cytokine secretion in macrophage foam cells. J Nutr Biochem.
22:777–783. 2011. View Article : Google Scholar :
|
|
18
|
Chemat F, Vian MA and Cravotto G: Green
extraction of natural products: Concept and principles. Int J Mol
Sci. 13:8615–8627. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Le HTT, Cho YC and Cho S: Inhibition of
protein tyrosine phosphatase non-receptor type 2 by PTP inhibitor
XIX: Its role as a multiphosphatase inhibitor. BMB Rep. 50:329–334.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Förstermann U and Sessa WC: Nitric oxide
synthases: Regulation and function. Eur Heart J. 33:829–837. 2012.
View Article : Google Scholar :
|
|
22
|
Tripathi P, Tripathi P, Kashyap L and
Singh V: The role of nitric oxide in inflammatory reactions. FEMS
Immunol Med Microbiol. 51:443–452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liclican EL, Nguyen V, Sullivan AB and
Gronert K: Selective activation of the Prostaglandin E(2) circuit
in chronic injury-induced pathologic angiogenesis. Invest
Ophthalmol Vis Sci. 51:6311–6320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang JM and An J: Cytokines,
inflammation, and pain. Int Anesthesiol Clin. 45:27–37. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JH, Min DS, Lee CW, Song KH, Kim YS
and Kim HP: Ginsenosides from Korean Red Ginseng ameliorate lung
inflammatory responses: Inhibition of the MAPKs/NF-kB/c-Fos
pathways. J Ginseng Res. 2017. View Article : Google Scholar
|
|
27
|
Zhang P, Martin M, Michalek SM and Katz J:
Role of mitogen-activated protein kinases and NF-κB in the
regulation of proinflammatory and anti-inflammatory cytokines by
porphyromonas gingivalis Hemagglutinin B. Infect Immun.
73:3990–3999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caamaño J and Hunter CA: NF-κB family of
transcription factors: Central regulators of innate and adaptive
immune functions. Clin Microbiol Rev. 15:414–429. 2002. View Article : Google Scholar
|
|
29
|
Oeckinghaus A and Ghosh S: The NF-κB
family of transcription factors and its regulation. Cold Spring
Harb Perspect Biol. 1:a0000342009. View Article : Google Scholar
|
|
30
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muralidharan S and Mandrekar P: Cellular
stress response and innate immune signaling: Integrating pathways
in host defense and inflammation. J Leukoc Biol. 94:1167–1184.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Whitmarsh AJ: Regulation of gene
transcription by mitogen-activated protein kinase signaling
pathways. Biochim Biophys Acta. 1773:1285–1298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lopez-Bergami P and Ronai Z: Requirements
for PKC-augmented JNK activation by MKK4/7. Int J Biochem Cell
Biol. 40:1055–1064. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lowell CA: Src-family and Syk kinases in
aActivating and inhibitory pathways in innate immune
cells-signaling crosstalk. Cold Spring Harb Perspect Biol.
3:a0023522011. View Article : Google Scholar
|
|
35
|
Miljković D and Spasojević I: Multiple
sclerosis: Molecular mechanisms and therapeutic opportunities.
Antioxid Redox Signal. 19:2286–2334. 2013. View Article : Google Scholar
|
|
36
|
Aggarwal BB, Prasad S, Reuter S, Kannappan
R, Yadev VR, Park B, Kim JH, Gupta SC, Phromnoi K, Sundaram C, et
al: Identification of novel anti-inflammatory agents from ayurvedic
medicine for prevention of chronic diseases: 'Reverse pharmacology'
and 'Bedside to bench' approach. Curr Drug Targets. 12:1595–1653.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gilroy DW: New insights into the
anti-inflammatory actions of aspirin-induction of nitric oxide
through the generation of epi-lipoxins. Mem Inst Oswaldo Cruz.
100(Suppl 1): S49–S54. 2005. View Article : Google Scholar
|
|
38
|
Ricciotti E and FitzGerald GA:
Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol.
31:986–1000. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miyahara K, Murayama H, Wakabayashi H,
Kurihara T, Hashimoto K, Satoh K, Motohashi N and Sakagami H:
Inhibition of LPS-stimulated NO production in mouse macrophage-like
cells by benzocycloheptoxazines. Anticancer Res. 28:2657–2662.
2008.PubMed/NCBI
|
|
40
|
Suga A, Narita T, Zhou L, Sakagami H,
Satoh K and Wakabayashi H: Inhibition of NO production in
LPS-stimulated mouse macrophage-like cells by
benzo[b]cyclohept[e][1,4] oxazine and 2-aminotropone derivatives.
In Vivo. 23:691–697. 2009.PubMed/NCBI
|
|
41
|
Wullaert A, Bonnet MC and Pasparakis M:
NF-κB in the regulation of epithelial homeostasis and inflammation.
Cell Res. 21:146–158. 2011. View Article : Google Scholar
|
|
42
|
Seong YA, Hwang D and Kim GD: The
anti-inflammatory effect of Gnaphalium affine through inhibition of
NF-κB and MAPK in lipopolysaccharide-stimulated RAW264.7 cells and
analysis of its phytochemical components. Cell Biochem Biophys.
74:407–417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cho YC and Cho S: c-Jun N-terminal
kinase-mediated anti-inflammatory effects of Garcinia subelliptica
in macrophages. Mol Med Rep. 13:2293–2300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji
J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O and Akira S:
Essential function for the kinase TAK1 in innate and adaptive
immune responses. Nat Immunol. 6:1087–1095. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Choi RJ, Ngoc TM, Bae K, Cho HJ, Kim DD,
Chun J, Khan S and Kim YS: Anti-inflammatory properties of
anthraquinones and their relationship with the regulation of
P-glycoprotein function and expression. Eur J Pharm Sci.
48:272–281. 2013. View Article : Google Scholar
|
|
46
|
Moreno-Anzurez NE, Marquina S, Alvarez L,
Zamilpa A, Castillo-España P, Perea-Arango I, Torres PN,
Herrera-Ruiz M, Díaz García ER, García JT and Arellano-García J: A
cytotoxic and anti-inflammatory campesterol derivative from
genetically transformed hairy roots of Lopezia racemosa Cav.
(Onagraceae). Molecules. 22:E1182017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pandith H, Zhang X, Thongpraditchote S,
Wongkrajang Y, Gritsanapan W and Baek SJ: Effect of Siam weed
extract and its bioactive component scutellarein tetramethyl ether
on anti-inflammatory activity through NF-κB pathway. J
Ethnopharmacol. 147:434–441. 2013. View Article : Google Scholar : PubMed/NCBI
|


















