Introduction
Cisplatin (cis-dichlorodiammine platinum, cis-DDP)
is widely used in the treatment of various solid tumors, for
example, testicular cancer, in which remission rates of up to 90%
have been obtained (1). However,
the development of drug resistance and undesirable side-effects
have limited the clinical application of DDP. These side-effects
include nephrotoxicity, neurotoxicity and ototoxicity (2). DDP-induced nephrotoxicity primarily
occurs in the renal proximal tubule, with manifestations of
epithelial cell necrosis, glomerular sclerosis, renal dysfunction
and renal failure (3–6). Therefore, the development of
therapeutic strategies and drugs that relieve the side-effects of
DDP may enable expansion of its clinical applications.
Previous studies have confirmed that DDP-induced
kidney injury is primarily caused by apoptosis (7) due to oxidative stress injury and the
inflammatory infiltration of epithelial cells in the renal proximal
tubule (8). In a previous study
conducted by the present research team, it was shown that DDP
induces apoptosis by causing oxidative stress in HK-2 proximal
tubule epithelial cells (9).
Mitogen-activated protein kinase (MAPK) signaling pathways have
been demonstrated to serve a key role in this pathological process.
MAPKs are composed of three key kinases, namely extracellular
signal-regulated kinase (ERK), c-Jun-N-terminal kinase (JNK) and
p38 (10,11). Following induction by DDP, renal
tissues produce large amounts of reactive oxygen species (ROS) and
inflammatory factors, which activate and initiate apoptosis through
MAPK signaling (12,13). The nuclear factor-κB (NF-κB)
signaling pathway inhibits apoptosis. The activation of NF-κB and
its translocation to the nucleus regulates the expression of
Fas-associated death domain-like interleukin-1β-converting
enzyme-inhibitory protein (FLIP) (14) and X-linked inhibitor-of-apoptosis
protein (XIAP) (15,16). The expression of FLIP then
inhibits the activation of caspase-8 (17), whereas the expression of XIAP
inhibits the function of second mitochondria-derived activator of
caspase (also known as Diablo) (18). Therefore, abrogation of
DPP-induced renal cell apoptosis may be achieved by blocking MAPK
signaling or activating NF-κB signaling.
Schizandrin B (SchB) is extracted from the fruit of
Magnoliaceae Schisandra chinensis (Turcz.) Baill (19). SchB has been shown to alleviate
damage in a number of different types of tissues and cells,
including hepatocytes (20,21), nerve cells (22), renal tissues (23) and cardiomyocytes (24,25). Additionally, SchB has been
demonstrated to have a potent anticancer effect (26). However, the cytoprotective
mechanism of SchB has not been fully elucidated. Therefore, the
effect of SchB on DDP-exposed proximal tubular epithelial HK-2
cells was evaluated in the present study with the aim of
elucidating the protective mechanism of SchB.
Materials and methods
Antibodies and reagents
Cis-DDP and SchB were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). The primary antibodies targeting
cleaved-caspase-3 (cat. no. 9661), ERK (cat. no. 4695), phospho
(p)-ERK (cat. no. 4370), IκB kinase (IKK)β (cat. no. 8943), IKKα
(cat. no. 2682), p-IKKα/β (cat. no 2697), inhibitor of NF-κBα
(IκBα; cat. no. 4812), p-IκBα (cat. no. 2859), NF-κB p65 (cat. no.
8242), p-NF-κB p65 (cat. no. 3033), survivin (cat. no. 2808) and
GAPDH (cat. no: 5174), all used at 1:1,000 dilution, were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). IRDye
800CW goat anti-mouse secondary antibodies (P/N 926-80010; 1:5,000
dilution) and Alexa IRDye 700RD goat anti-rabbit secondary
antibodies (P/N 925-68070; 1:5,000 dilution) were purchased from
LI-COR Biosciences (Lincoln, NE, USA), and
4′,6-diamidino-2-phenylindole (DAPI), pyrollidine dithiocarbamate
(PDTC) and U0126 were purchased from Beyotime Institute of
Biotechnology (Shanghai, China).
Cell culture
HK-2 cells were purchased from American Type Culture
Collection (Manassas, VA, USA). The cells were cultured in
keratinocyte serum-free medium containing 0.05 mg/ml bovine
pituitary extract and 5 ng/ml human recombinant epidermal growth
factor (all HyClone; GE Healthcare Life Sciences, Logan, UT, USA).
The culture was incubated under an atmosphere of 5% CO2
at 37°C and passaged the next day.
Cell viability assay
HK-2 cells (1×106 cells/well) were seeded
in 96-well plates. Following stimulation with cis-DDP and/or SchB
as indicated, the medium was exchanged, and 10 µl Cell
Counting kit (CCK)-8 test solution (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) was added to each well for 1 h at 37°C The
absorbance of the culture solution was measured at 450 nm using a
microplate reader.
Apoptosis rate assay
HK-2 cells (1×107 cells/well) were seeded
into 6-well plates, and SchB or cis-DDP was added 24 h later.
Following the appropriate incubation period, the cells were stained
with Annexin V-fluorescein isothiocyanate and propidium iodide
(Beyotime Institute of Biotechnology) for 30 min. A flow cytometer
(BD FACSVerse; BD Biosciences, Franklin Lakes, NJ, USA) was used to
determine the apoptotic rate using CellQuest Pro 3.3 software (BD
Biosciences).
DAPI staining
HK-2 cells (1×107 cells/well) were seeded
into 6-well plates, and SchB or cis-DDP was added 24 h later.
Following the appropriate incubation period, the cells were fixed
in 4% paraformaldehyde for 30 min at room temperature and then
stained with DAPI (Beyotime Institute of Biotechnology) for 1 h at
room temperature. An Olympus IX71 inverted microscope (Olympus
Corporation, Tokyo, Japan) was used to evaluate the nuclear
morphology.
Double-wavelength in-cell western
blotting assay
HK-2 cells (1×106 cells/well) were seeded
in a 96-well plate. Following treatment with cis-DPP and/or SchB as
indicated, the cells were fixed with 4% paraformaldehyde for 20 min
at room temperature and then washed three times with 0.1%
Triton-100%/phosphate-buffered saline (PBS). The cells were then
incubated with rabbit and mouse antibodies against
cleaved-caspase-3 and GAPDH, respectively, overnight at 4°C,
followed by incubation with goat anti-rabbit antibody labelled with
Alexa Fluor® 700 or goat anti-mouse antibody labelled
with IRDye 800 for 2 h at room temperature. The results were
observed using an Odyssey imaging system (LI-COR Biosciences).
Western blotting
Following the aforementioned treatments, the HK-2
cells were washed with pre-chilled PBS and then lysed with cell
lysis buffer (Beyotime Institute of Biotechnology) on ice for 40
min. Next, the lysate was collected and mixed with 2X loading
buffer for sodium dodecyl sulfate-polyacrylamide (12% separation
gel and 4% concentrated gel) gel electrophoresis. The proteins were
then transferred to a nitrocellulose membrane, and the membrane was
incubated in blocking solution (5% skimmed milk) at room
temperature for 2 h. The membrane was incubated overnight at 4°C
with the primary antibody, and then incubated with a fluorescent
secondary antibody for 1 h at room temperature and imaged using the
Odyssey imaging system (LI-COR Biosciences).
Cell transfection and interference
Small interfering RNAs (siRNAs) against survivin
(Survivin RNAi-1: Sense, ACA GAC AGA CAG UCU GUG GU and antisense,
UUU GUC UGU CUG UCA GAC ACC; Survivin RNAi-2: Sense, CAG ACA GAC
AGU CUG UGG UUU and antisense, UUGUCUGUCUGUCAGACACCA) and a
pGL3-human survivin promoter luciferase (pG3L-hSurvivin-Luc)
plasmid were synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China). The survivin siRNAs or pGL3-hSur-vivin-Luc and
Lipofectamine® 2000 (Gibco; Thermo Fisher Scientific,
Inc., MA, USA) were mixed in opti-MEM (Gibco; Thermo Fisher
Scientific, Inc.) and incubated with HK-2 cells for 72 h. Survivin
expression was detected by western blotting. The luciferase
activity of pG3L-hSurvivin-Luc was measured using a GloMax 20/20
Luminometer (Promega Corporation, Madison, WI, USA).
Signaling pathway screening
HK-2 cells were pre-incubated with SchB for 2 h
and/or stimulated with cis-DDP for various time periods at 37°C, as
indicated. The cell lysates were collected and analyzed using
PathScan Antibody Array kits (Cell Signaling Technology, Inc.)
according to the instructions provided by the manufacturer.
Fluorescence emission points on the chip were observed at 680 nm
using an Odyssey imaging system. The grey-scale value was
calculated and statistically analyzed.
Statistical analysis
All data are presented as the mean ± standard
deviation. One-way analysis of variance followed by Dunnett’s test
were used for inter-group comparisons. Statistical analysis of the
data was performed using SPSS 13.0 software (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of SchB on the cis-DDP-induced
loss of viability of HK-2 cells
In a previous study, in vitro experiments
indicated that SchB is able to alleviate the toxicity of DDP to
HK-2 cells (27). In the present
study, the effect of SchB on the cis-DDP-induced apoptosis of HK-2
cells was further evaluated. To test the effect of SchB on the
viability of HK-2 cells, the cells were incubated with SchB at
various concentrations (0, 2.5, 5, 10, 20, 40 and 80 µM) for
24 h. Then, cell viability was determined using a CCK-8 assay. The
experimental results are shown in Fig. 1A. When the concentration of SchB
was 20 or 40 µM, the cell viability was significantly
increased compared with that of the untreated cells. However, a
SchB concentration of 80 µM was toxic to the cells, and
significantly reduced their viability. To evaluate the
time-dependent response to SchB, HK-2 cells were incubated with 40
µM SchB for various time periods (0, 6, 12, 18, 24, 30, 36
and 42 h). As shown in Fig. 1B,
SchB significantly increased the viability of the HK-2 cells
compared with that of the untreated cells when the incubation time
was ≥18 h. To evaluate the effect of cis-DPP on cell viability,
HK-2 cells were incubated with cis-DDP at different concentrations
(0, 2.5, 5, 10, 20 and 30 µM) for 24 h, and cell viability
was determined. As shown in Fig.
1C, concentrations of cis-DDP ≥5 µM were significantly
cytotoxic to the HK-2 cells, and cis-DDP concentrations ≥10
µM strongly inhibited the viability of the HK-2 cells.
Therefore, 10 µM cis-DDP was considered the toxic dose for
subsequent experiments. To evaluate the effect of SchB on that of
cis-DPP, HK-2 cells were pre-incubated with different
concentrations of SchB (0, 2.5, 5, 10, 20, 40 and 80 µM) for
2 h and then stimulated with 10 µM cis-DDP for 24 h. The
viability of the cells was subsequently determined. The results
indicated that SchB concentrations ≥10 µM significantly
alleviated the reduction in HK-2 cell viability induced by cis-DDP.
A SchB concentration of 80 µM exhibited no additional impact
compared with that of a 40 µM concentration on the decreased
viability of the HK-2 cells induced by cis-DDP. Therefore, 10, 20
and 40 µM SchB were used as the low, medium and high dose
groups, respectively, in the subsequent experiments.
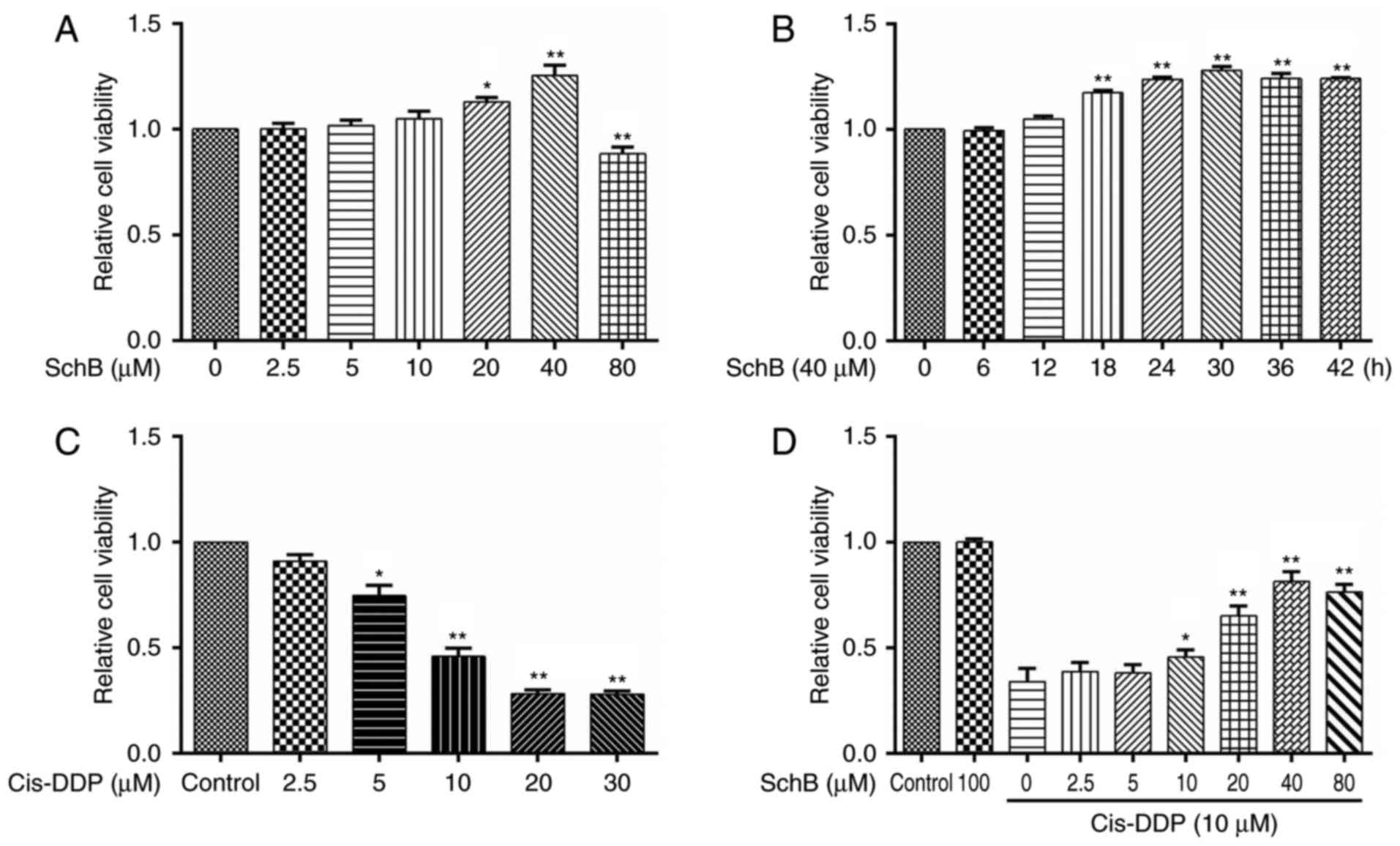 | Figure 1Effects of SchB on the
cis-DDP-induced loss of viability of HK-2 cells. (A) HK-2 cells
were incubated with SchB at different concentrations (0, 2.5, 5,
10, 20, 40 and 80 µM) for 24 h. Cell viability was then
determined using a Cell Counting kit-8 assay. (B) HK-2 cells were
incubated with 40 µM SchB for 0, 6, 12, 18, 24, 30, 36 and
42 h, and the cell viability was then determined. (C) HK-2 cells
were incubated with cis-DDP at different concentrations (0, 2.5, 5,
10, 20 and 30 µM) for 24 h, and cell viability was then
determined. (D) Cells were pre-incubated with different
concentrations of SchB (0, 2.5, 5, 10, 20, 40 and 80 µM) for
2 h and then stimulated with 10 µM cis-DDP for 24 h. The
viability of the cells was determined. Data are presented as the
mean ± standard deviation (n=3). (A-C) *P<0.05 and
**P<0.01 vs. the control group (0 µM SchB);
(D) *P<0.05 and **P<0.01 vs. the group
treated with cis-DDP alone. SchB, schizandrin B; cis-DDP,
cis-dichlorodiammine platinum. |
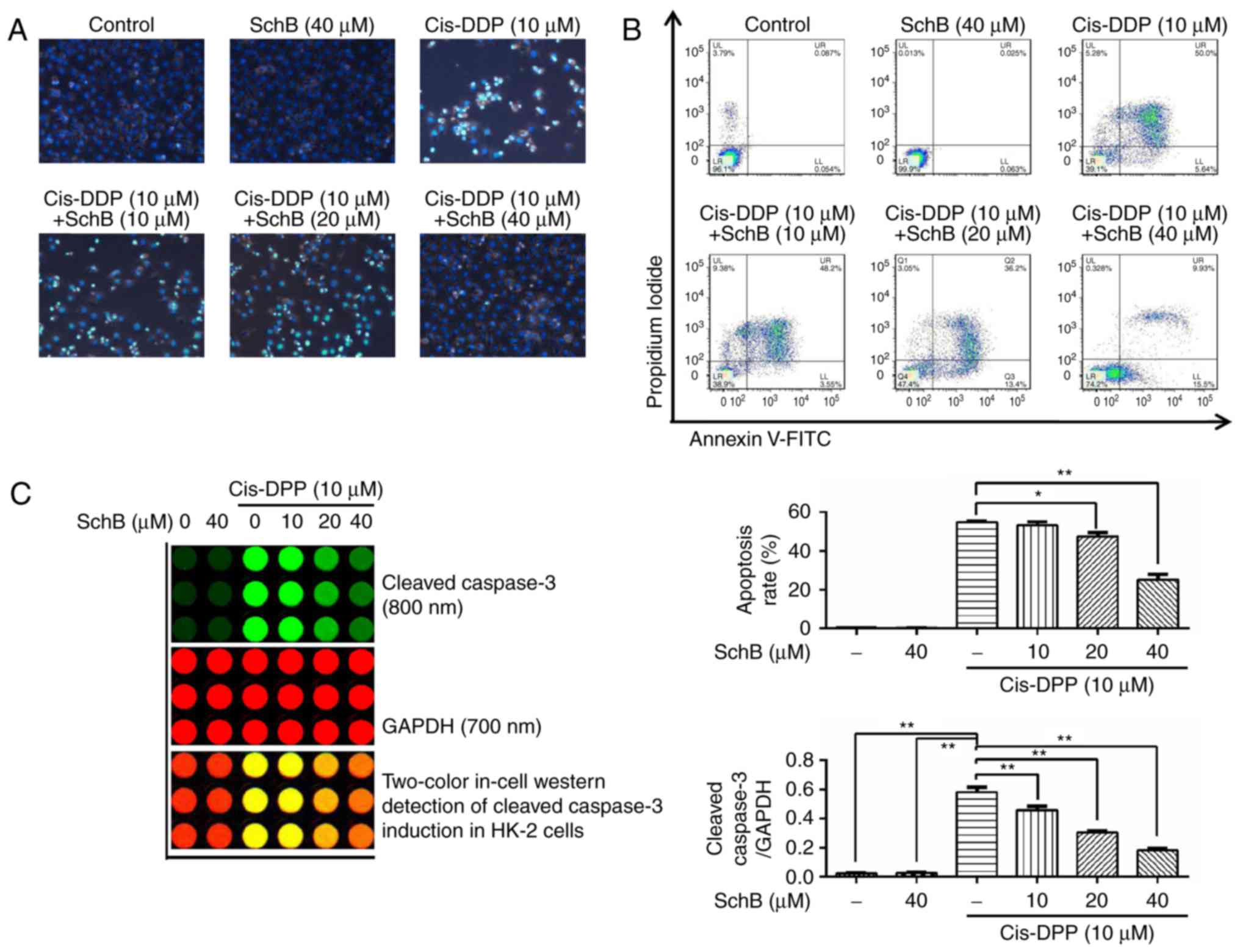
Effects of SchB on the cis-DDP-induced
apoptosis of HK-2 cells
The effects of SchB on the cis-DDP-induced apoptosis
of HK-2 cells were evaluated. Following the pre-incubation of HK-2
cells with 10, 20 and 40 µM SchB for 2 h, the cells were
stimulated with 10 µM cis-DDP for 24 h and stained with DAPI
to observe the nuclear morphology. The results (Fig. 2A) demonstrated that cis-DDP
induced nuclear DNA shrinkage and fragmentation in the HK-2 cells,
whereas SchB significantly attenuated the cis-DDP-induced nuclear
condensation and fragmentation. Following co-staining of the HK-2
cells with Annexin V-fluorescein isothiocyanate and propidium
iodide, the apoptotic and survival rates of the HK-2 cells were
determined by flow cytometry. The results (Fig. 2B) demonstrate that SchB
significantly reduced the cis-DDP-induced apoptosis of HK-2 cells.
Caspase-3 is an executive apoptotic protein (28). Following the pre-incubation of
HK-2 cells with 10, 20 and 40 µM SchB for 2 h, the cells
were stimulated with 10 µM cis-DDP for 24 h. Then, the
activation of caspase-3 was determined by in-cell western blot
analysis. The results (Fig. 2C)
indicate that the activation of cleaved caspase-3 was increased
during cis-DDP stimulation. However, when the cells were
pre-incubated with SchB, the activation of caspase-3 by cis-DDP was
significantly attenuated compared with that in the cells that did
not undergo SchB pretreatment. These results indicate that SchB
effectively blocked the cis-DDP-induced apoptosis of HK-2
cells.
Effects of SchB and cis-DDP on the
apoptosis/survival signaling pathway of HK-2 cells
The signaling targets in HK-2 cells on which SchB
may exert cytoprotective effects were screened. HK-2 cells were
used to create a SchB (40 µM) incubation group, a cis-DDP
(10 µM) incubation group and a SchB (40 µM) + cis-DDP
(10 µM) co-incubation group, which were incubated for 1, 2,
4, 8 and 16 h. The activation of apoptosis/survival signal
transduction pathways was determined using protein chips. As shown
in Fig. 3A and B, at 1 h after
cis-DDP stimulation, p53, p38 and JNK were phosphorylated to
different extents. After 16 h, cis-DDP induced the activation of
cleaved caspases-3 and -7, leading to poly(ADP-ribose) polymerase
(PARP) degradation. These results suggest that cis-DDP may have
induced the apoptosis of HK-2 cells via the activation of p53, p38
and JNK signaling. Incubation with SchB alone did not activate p53,
p38 and JNK and exhibited no effect on caspases-3 and -7 or PARP.
However, with SchB incubation, ERK was strongly phosphorylated in
the HK-2 cells, with decreased expression of total IκBα and
increased expression of phosphorylated (p)-lκBα. Notably, following
incubation with SchB for 16 h, survivin protein was strongly
expressed. Survivin is an inhibitor of apoptosis (29) and may be the key protein involved
in the protective effect of SchB against DDP nephrotoxicity. When
the HK-2 cells were co-incubated with SchB (40 µM) and
cis-DDP (10 µM), and when combined with the effects of
p-p38, p-JNK, p-lκBα, p-ERK and survivin, the cleaved-caspases-3
and -7 and cleaved-PARP apoptosis proteins were significantly
inhibited. On the basis of these results (Fig. 3B), it appears that ERK and NF-κB
signaling, and survivin serve important roles in the alleviation of
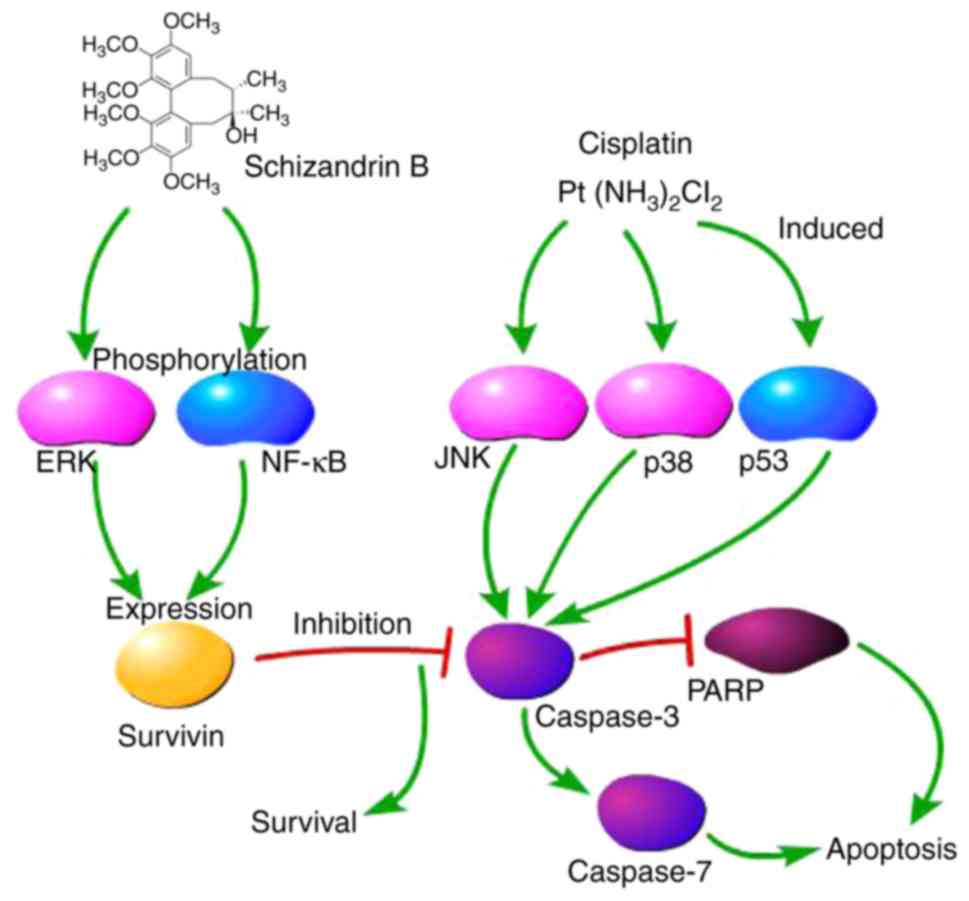
cis-DDP-induced apoptosis by SchB in HK-2 cells. The predicted
signaling pathways in the cis-DDP-induced apoptosis and
SchB-induced survival of HK-2 cells are shown in Fig. 3C.
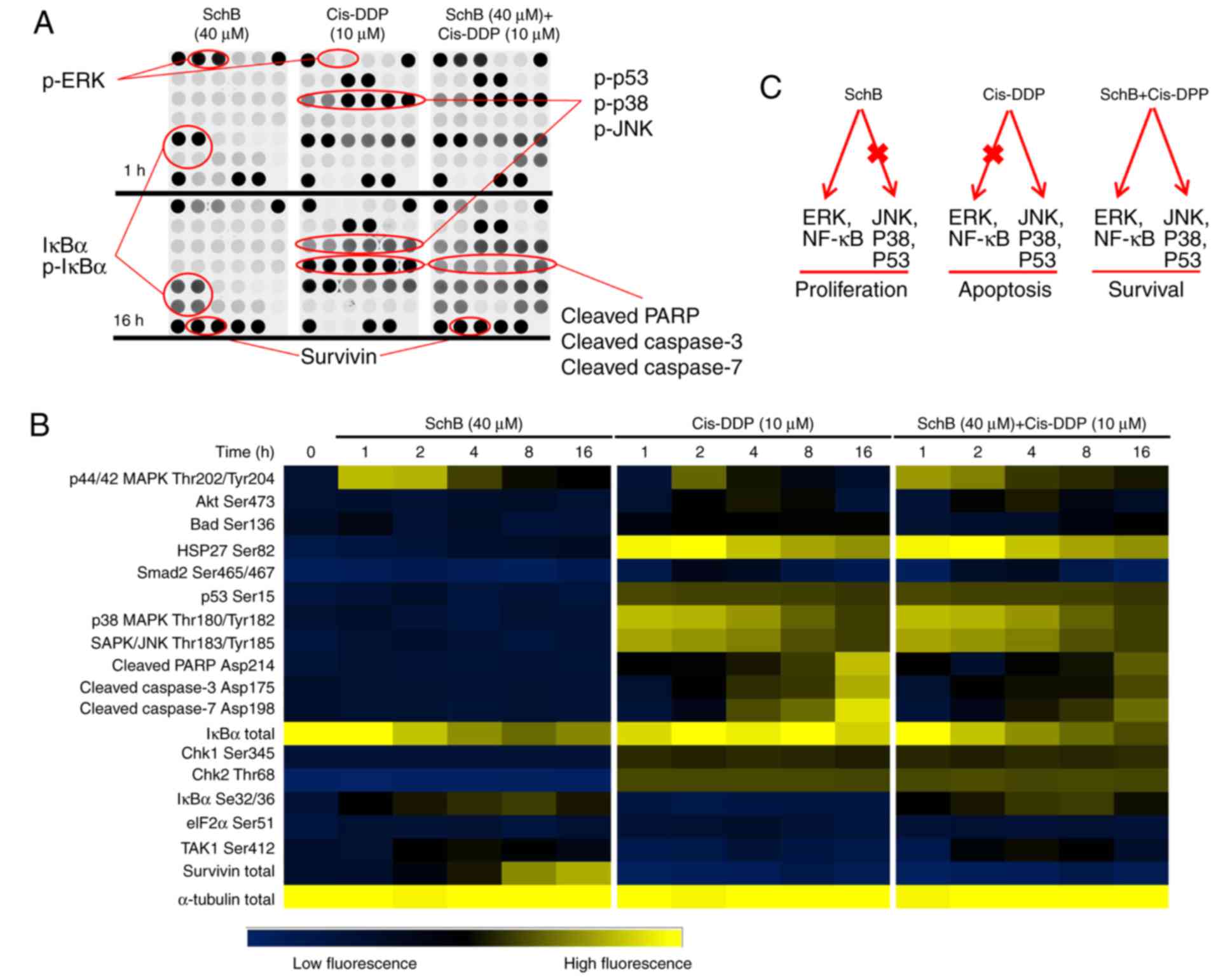 | Figure 3Effects of SchB and cis-DDP on the
apoptosis/survival signaling pathway of HK-2 cells. HK-2 cells were
used to establish a SchB (40 µM) incubation group, a cis-DDP
(10 µM) incubation group and a SchB (40 µM) + cis-DDP
(10 µM) co-incubation group and the cells were incubated for
1, 2, 4, 8 and 16 h. (A) The activation of associated signal
transduction pathways was determined using protein chips. (B)
Heat-maps of the protein chip results. (C) The predicted signaling
pathways in the cis-DDP-induced apoptosis and SchB-induced survival
of HK-2 cells. SchB, schizandrin B; cis-DDP, cis-dichlorodiammine
platinum; ERK, extracellular signal-regulated kinase; JNK,
c-Jun-N-terminal kinase; NF-κB, nuclear factor κB; IκBα, inhibitor
of NF-κBα; PARP, poly(ADP-ribose) polymerase; p,
phosphorylated. |
Effects of the activation of ERK/NF-κB
signaling by SchB on the cis-DDP-induced apoptosis of HK-2
cells
To confirm the activation of ERK and NF-κB by SchB,
HK-2 cells were incubated with 40 µM SchB for 0, 0.5, 1, 2,
4 and 8 h, and the cell lysates were collected. Western blotting
was performed to determine the phosphorylation of ERK, IKKα/β, IκBα
and p65. As shown in Fig. 4A,
SchB activated ERK and NF-κB signaling at the five time points
tested, with maximum activation for ERK, IKKα/β, IκBα and p65 at
0.5, 2, 2 and 8 h, respectively. The HK-2 cells were incubated with
10, 20 and 40 µM SchB for 0.5, 2, 2 and 8 h, and the
phosphorylation levels of ERK, IKKα/β, IκBα and p65, respectively,
were determined. As shown in Fig.
4B, SchB activated ERK and NF-κB signaling at the three
concentrations tested. To confirm the roles of activated ERK and
NF-κB signaling in the DDP-induced apoptosis of HK-2 cells, the
HK-2 cells were incubated with ERK inhibitor U0126 (10 µM)
or NF-κB inhibitor PDTC (20 µM) for 2 h, followed by
incubation with SchB for 4 h and stimulation with cis-DDP for 24 h.
Changes in the expression of cleaved caspase-3 protein were
determined (Fig. 5). As shown in
Fig. 5A, SchB effectively
attenuated the cis-DDP-induced expression of cleaved caspase-3,
indicating that it reduced apoptosis, whereas U0126 and PDTC
inhibited the anti-apoptotic effect of SchB to different extents.
These results suggest that the activation of ERK and NF-κB
signaling serves a critical role in the inhibitory effect of SchB
against cis-DDP-induced apoptosis in HK-2 cells.
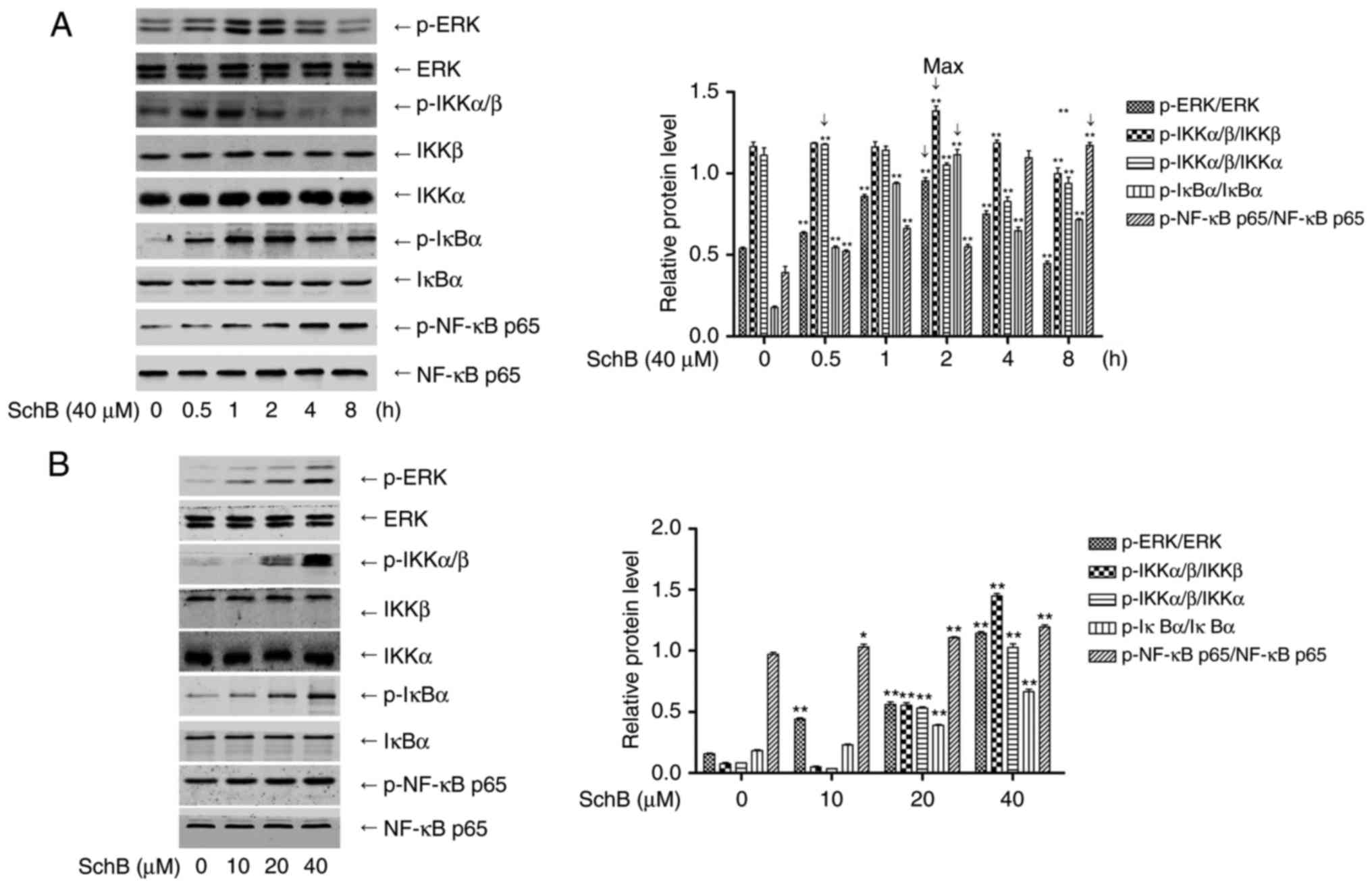 | Figure 4Signaling via ERK/NF-κB activation in
the cis-DDP-induced apoptosis of HK-2 cells. (A) HK-2 cells were
incubated with 40 µM SchB for 0, 0.5, 1, 2, 4 and 8 h, and
the cell lysates were collected. Western blotting was performed to
determine the phosphorylation of ERK, IKKα/β, IκBα and p65. (B)
HK-2 cells were incubated with 10, 20 and 40 µM SchB for
0.5, 2, 2 and 8 h, and the phosphorylation of ERK, IKKα/β, IκBα and
p65 was determined, respectively. Data are presented as the mean ±
standard deviation (n=3). *P<0.05 and
**P<0.01 vs. the control group [(A), 0 h; (B), 0
µM]. SchB, schizandrin B; cis-DDP, cis-dichlorodiammine
platinum; ERK, extracellular signal-regulated kinase; NF-κB,
nuclear factor κB; IκBα, inhibitor of NF-κBα; IKK, IκB kinase; p,
phosphorylated. |
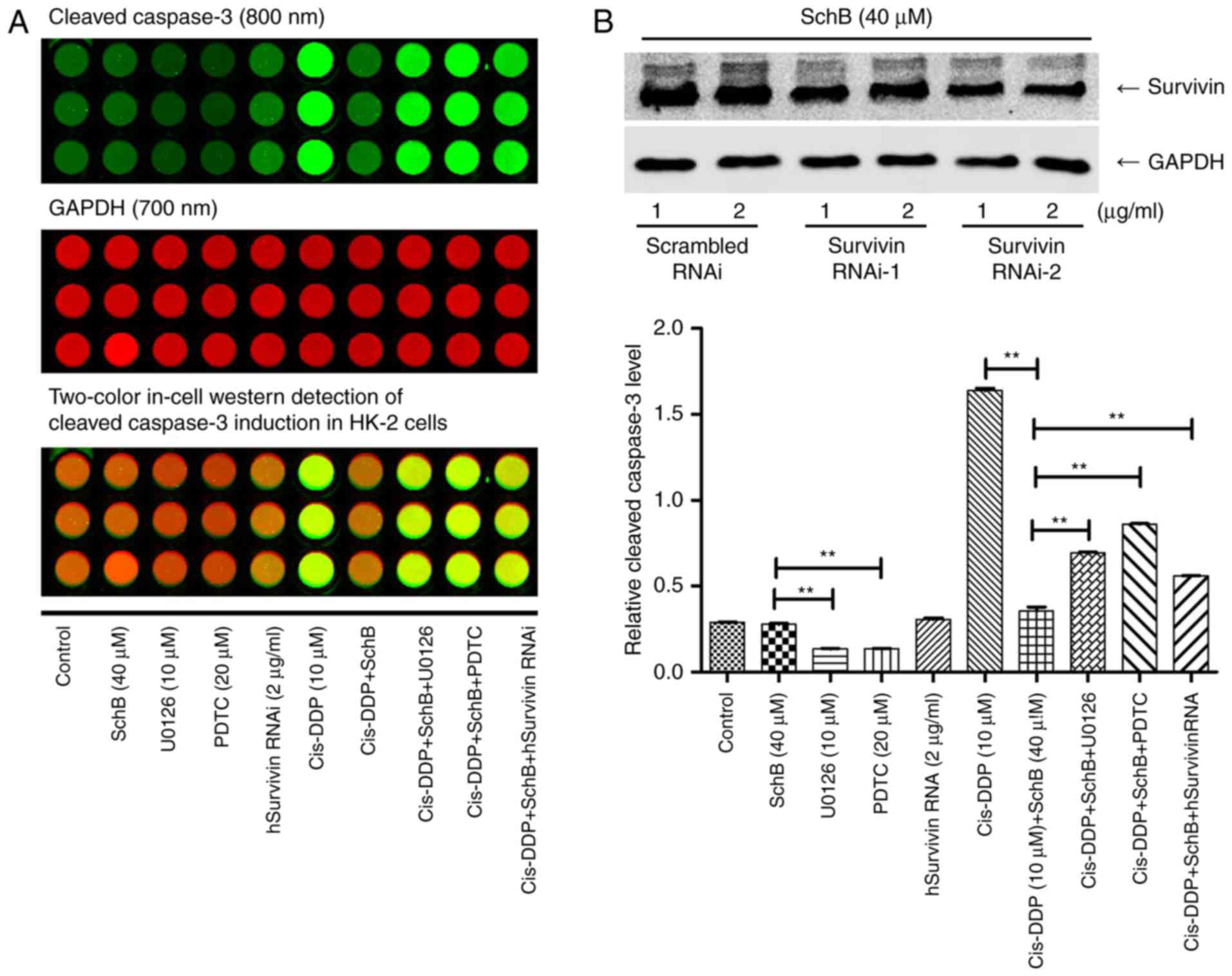
Effect of the regulation of survivin
expression by NF-κB on the cis-DDP-induced apoptosis of HK-2
cells
Survivin is an anti-apoptotic protein with a
molecular weight of 16 kDa. Survivin binds to and inhibits
caspase-3 activity, thereby inhibiting apoptosis and promoting cell
division (29,30). The aforementioned antibody chip
results indicate that SchB might act as a survivin inducer in an
anti-apoptotic role. Thus, the effect of survivin activation on
cis-DDP nephrotoxicity was evaluated. HK-2 cells were incubated
with 0, 10, 20 and 40 µM SchB for 16 h, and the expression
of survivin protein was determined. As shown in Fig. 6A, SchB significantly activated
survivin expression at the three concentrations tested. The
expression of survivin was also determined following the incubation
of HK-2 cells with 40 µM SchB for 0, 1, 4, 8 and 16 h. As
shown in Fig. 6B, SchB
significantly increased survivin expression at the three time
points tested. HK-2 cells were incubated with ERK inhibitor U0126
or NF-κB inhibitor PDTC for 2 h, followed by incubation with SchB
for 16 h, and then the expression of survivin was determined. As
shown in Fig. 6C, PDTC and U0126
inhibited the SchB-induced expression of survivin.
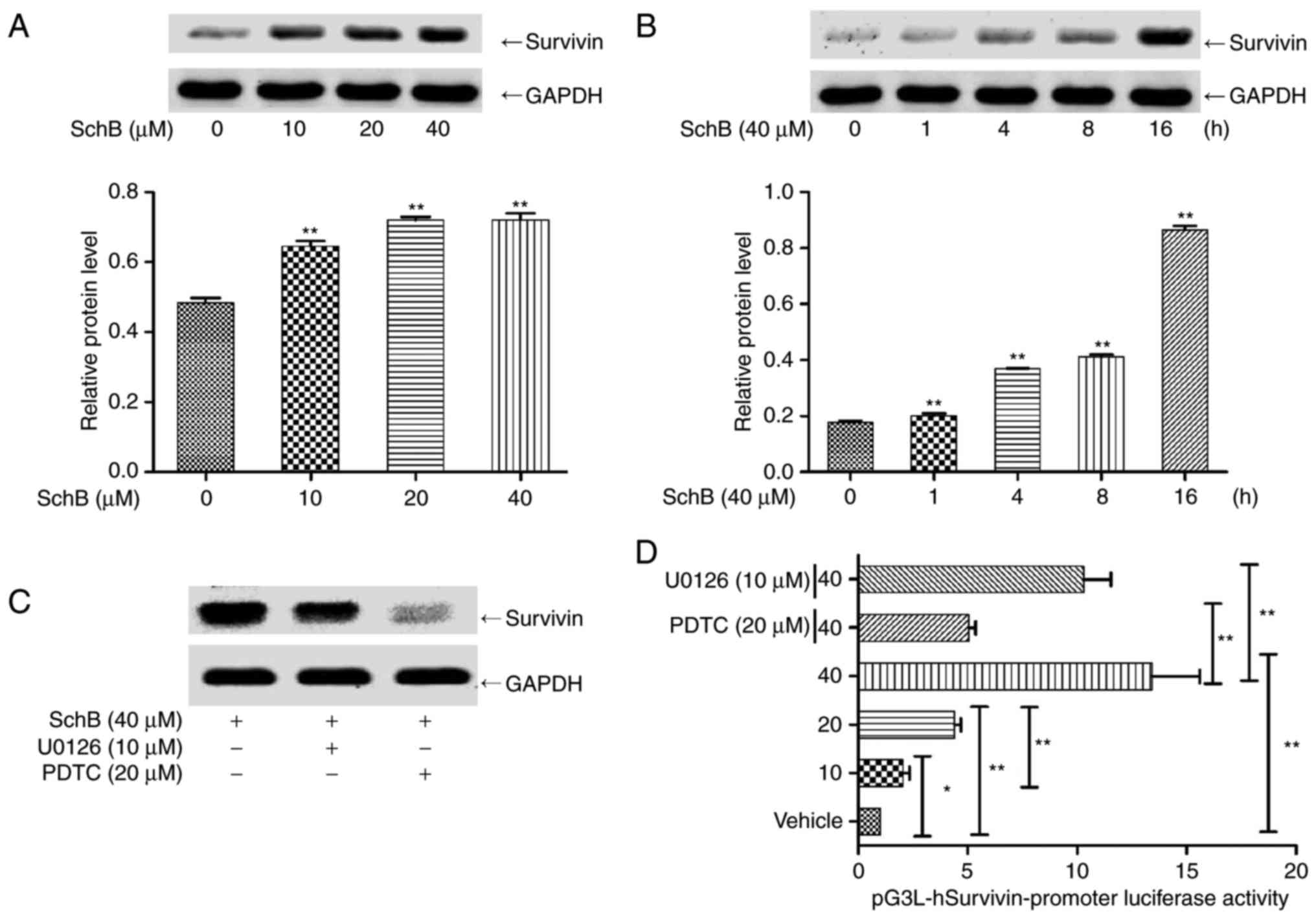 | Figure 6ERK/NF-κB mediates SchB-induced
survivin expression in HK-2 cells. (A) HK-2 cells were incubated
with 0, 10, 20 and 40 µM SchB for 16 h, and the expression
of survivin protein was determined. (B) HK-2 cells were incubated
with 40 µM SchB for 0, 1, 4, 8 and 16 h, and the expression
of survivin protein was determined. (C) HK-2 cells were incubated
with ERK inhibitor U0126 and NF-κB inhibitor PDTC for 2 h, followed
by incubation with SchB for 16 h, and then the expression of
survivin was determined. (D) The luciferase reporter gene
pGL3-hSurvivin promoter luciferase was transfected into HK-2 cells.
These cells were then incubated with 10, 20 and 40 µM SchB
for 16 h or pre-incubated with U0126 and PDTC and subsequently
incubated with 40 µM SchB. The fluorescence intensity of the
HK-2 cells was then detected using a fluorescence microplate
reader. Data are presented as the mean ± standard deviation (n=3).
*P<0.05 and **P<0.01. vs. the control
group [(A), 0 µM; (B), 0 h] or as indicated. SchB,
schizandrin B; cis-DDP, cis-dichlorodiammine platinum; PDTC,
pyrollidine dithiocarbamate; ERK, extracellular signal-regulated
kinase; NF-κB, nuclear factor κB. |
Subsequently, a survivin promoter luciferase
reporter construct (pGL3-hSurvivin-Luc) was constructed and
transfected into HK-2 cells. The transfected cells were incubated
with 10, 20 or 40 µM SchB for 16 h, or pre-incubated with 10
µM U0126 or 20 µM PDTC and subsequently incubated
with 40 µM SchB for 16 h. As shown in Fig. 6D, SchB induced pGL3-hSurvivin-Luc
fluorescence in a dose-dependent manner. Pre-incubation with PDTC
or U0126 significantly inhibited the SchB-induced fluorescence
intensity of the luciferase reporter gene compared with that in the
unpretreated cells. These results indicate that the activation of
NF-κB and ERK regulated the expression of survivin. To confirm the
effect of survivin activation on the cis-DDP-induced apoptosis of
HK-2 cells, survivin siRNA was synthesized for the RNA interference
(RNAi) of survivin. Survivin siRNA was transfected into HK-2 cells,
which were incubated with 40 µM SchB for 16 h. The
expression of survivin was then determined. As shown in Fig. 5B, hSurvivin RNAi successfully
inhibited the expression of survivin. The interference
efficiencies, presented as the fractional reductions in survivin
expression, for Survivin RNAi-1 were 0.82 and 0.85 at 1 and 2
µg/ml. The interference efficiencies of Survivin RNAi-2 were
0.69 and 0.58 at 1 and 2 µg/ml. Therefore, 2 µg/ml
Survivin RNAi-2 was selected for the subsequent experiments. The
survivin siRNA was transfected into HK-2 cells, which were then
incubated with 40 µM SchB for 4 h and stimulated with
cis-DDP for 24 h. The activation of cleaved caspase-3 protein was
then determined. As shown in Fig.
5A, SchB effectively inhibited the activation of cleaved
caspase 3, indicating that it abrogated the cis-DDP-induced
apoptosis of HK-2 cells. Thus, blocking the expression of survivin
is indicated to inhibit the anti-apoptotic effect of SchB, which
indicates that survivin serves an anti-apoptotic role as an
activation target of SchB. In summary, it appears that SchB
alleviated the cis-DDP-induced apoptosis of HK-2 cells by
activating NF-κB/ERK and thereby regulating the expression of
survivin.
Discussion
Since the FDA approved platinum compounds for the
treatment of various solid tumors in the 1970s, DDP has been an
important front-line anticancer drug, but its severe side have
greatly limited its clinical application. The development of a
suitable treatment strategy to alleviate the side-effects of DDP
should greatly increase its clinical applicability. SchB is a
naturally existing small molecule that has been shown to have a
variety of cytoprotective activities (31). To the best of our knowledge, the
present study is the first to provide evidence that SchB is able to
alleviate DDP-induced renal injury by activating the ERK/NF-κB
signaling pathway in vitro.
DDP-induced apoptosis is a well-known process for
killing proliferative cancer cells. However, the excessive
re-absorption of DDP by the renal proximal tubule may reduce the
proliferation rate of the epithelial cells in the tubule (32,33). In addition, an excessive
concentration of DDP may induce renal tubular and peripheral tissue
cells to produce inflammatory factors, such as tumor necrosis
factor-α, or large amounts of ROS and reactive nitrogen clusters;
to induce mitochondrial apoptotic pathways and death receptor
apoptotic pathways; to activate caspases-3, -6, -9 and -12; and to
eventually induce epithelial cell apoptosis in the renal proximal
tubule (34,35). In the present study, 10 µM
DDP significantly decreased cell viability, activated caspase-3,
induced nuclear condensation and fragmentation, and increased the
apoptosis rate of HK-2 cells. However, when the HK-2 cells were
pre-incubated with SchB, the DDP-induced apoptosis was
significantly inhibited. These experimental results confirm the
potential therapeutic effect of SchB on cis-DDP-induced renal
injury.
The nephrotoxicity of cis-DDP is associated with
multiple apoptosis-related signaling pathways, the most important
of which are MAPKs (36); for
example, p53 serves a key role in DDP-induced apoptosis (37). The experiments conducted in the
present study verified the possible involvement of SchB in the
protection of HK-2 cells. Protein chip screening was used to
determine the possible target pathways via which SchB acts as an
anti-apoptotic agent. Under cis-DDP stimulation, p53, p38 and JNK
were phosphorylated to varying extents, with subsequent enhanced
activation of caspases-3 and -7 and PARP degradation. Thus, p53,
JNK and p38 may mediate the DDP-induced apoptosis of HK-2 cells.
When pre-incubated with SchB, the cis-DDP-induced activation of
caspases-3 and -7 and PARP degradation in the HK-2 cells was
inhibited, but the cis-DDP-induced activation of p53, JNK and p38
was not inhibited. For the HK-2 cells incubated with SchB alone,
ERK was phosphorylated, the expression of total IκBα decreased
while that of p-IκBα increased, and survivin was highly activated.
The CCK-8 assay demonstrated that incubation with SchB alone
promoted an increase in HK-2 cell viability. Therefore, it may be
speculated that with cis-DDP incubation, the activation of JNK, p38
and p53 leads to apoptosis. Furthermore, with SchB incubation, the
activation of NF-κB and ERK leads to cell proliferation, and
co-incubation with cis-DDP and SchB promotes HK-2 cell survival
compared with that for cells incubated with cis-DDP alone.
Therefore, it appears that MAPKs serve a dual role in the
nephrotoxicity of cisplatin. The activation of JNK and p38 induces
the apoptosis of HK-2 cells, whereas the activation of ERK protects
HK-2 cells.
Survivin is a small molecular anti-apoptotic protein
that binds to caspase-3 and inhibits its activation (30,38). In the present study, it was
demonstrated that the incubation of HK-2 cells with SchB alone
induced the expression of survivin and activated survivin promoter
luciferase. When siRNA was used to interfere with the expression of
survivin, the cis-DDP-induced induction of cleaved caspase-3 in the
HK-2 cells was markedly inhibited, indicating that the activation
of survivin by SchB may be the mechanism underlying its
anti-apoptotic effects. Western blot assays confirmed that SchB
activated ERK and that the ERK inhibitor U0126 downregulated the
expression of survivin and blocked the anti-apoptotic effect of
SchB. The anti-apoptotic effect of NF-κB has been demonstrated in a
number of studies, as summarized in a previous review (39). In the present study, SchB
activated NF-κB, and the NF-κB inhibitor PDTC downregulated
survivin expression and the activity of the survivin promoter,
which is indicative of the ability of SchB to inhibit
cis-DDP-induced apoptosis. PDTC inhibited the expression of
survivin more strongly than did U0126. Therefore, it appears that
NF-κB signaling has a dominant role in the regulation of survivin
expression. These results indicate that SchB regulates the
expression of survivin via ERK/NF-κB to alleviate the
cis-DDP-induced apoptosis of HK-2 cells.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that SchB is able to
alleviate the cis-DDP-induced apoptosis of HK-2 cells in
vitro. SchB likely activates the ERK/NF-κB signaling pathway,
which in turn, activates survivin (Fig. 7). These results suggested that
SchB may serve as a survivin inducer in the treatment of DDP
nephrotoxicity.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Einhorn LH: Curing metastatic testicular
cancer. Proc Natl Acad Sci USA. 99:4592–4595. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karasawa T and Steyger PS: An integrated
view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol
Lett. 237:219–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brock PR, Knight KR, Freyer DR, Campbell
KC, Steyger PS, Blakley BW, Rassekh SR, Chang KW, Fligor BJ, Rajput
K, et al: Platinum-induced ototoxicity in children: A consensus
review on mechanisms, predisposition, and protection, including a
new International Society of Pediatric Oncology Boston ototoxicity
scale. J Clin Oncol. 30:2408–2417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McWhinney SR, Goldberg RM and McLeod HL:
Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 8:10–16.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: A cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao X, Panichpisal K, Kurtzman N and
Nugent K: Cisplatin nephrotoxicity: A review. Am J Med Sci.
334:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nozaki Y, Kinoshita K, Hino S, Yano T,
Niki K, Hirooka Y, Kishimoto K, Funauchi M and Matsumura I:
Signaling Rho-kinase mediates inflammation and apoptosis in T cells
and renal tubules in cisplatin nephrotoxicity. Am J Physiol Renal
Physiol. 308:F899–F909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hagar H, Medany AE, Salam R, Medany GE and
Nayal OA: Betaine supplementation mitigates cisplatin-induced
nephrotoxicity by abrogation of oxidative/nitrosative stress and
suppression of inflammation and apoptosis in rats. Exp Toxicol
Pathol. 67:133–141. 2015. View Article : Google Scholar
|
|
9
|
Chen Q, Peng H, Dong L, Chen L, Ma X, Peng
Y, Dai S and Liu Q: Activation of the NRF2-ARE signalling pathway
by the Lentinula edodes polysaccharose LNT alleviates ROS-mediated
cisplatin nephrotoxicity. Int Immunopharmacol. 36:1–8. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma X, Dang C, Kang H, Dai Z, Lin S, Guan
H, Liu X, Wang X and Hui W: Saikosaponin-D reduces
cisplatin-induced nephrotoxicity by repressing ROS-mediated
activation of MAPK and NF-κB signalling pathways. Int
Immunopharmacol. 28:399–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Francescato HD, Costa RS, Silva CG and
Coimbra TM: Treatment with a p38 MAPK inhibitor attenuates
cisplatin nephrotoxicity starting after the beginning of renal
damage. Life Sci. 84:590–597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mishima K, Baba A, Matsuo M, Itoh Y and
Oishi R: Protective effect of cyclic AMP against cisplatin-induced
nephrotoxicity. Free Radic Biol Med. 40:1564–1577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramesh G and Reeves WB: p38 MAP kinase
inhibition ameliorates cisplatin nephrotoxicity in mice. Am J
Physiol Renal Physiol. 289:F166–F174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shao Y, Le K, Cheng H and Aplin AE: NF-κB
regulation of c-FLIP promotes TNFα-mediated RAF inhibitor
resistance in melanoma. J Invest Dermatol. 135:1839–1848. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsubaki M, Ogawa N, Takeda T, Sakamoto K,
Shimaoka H, Fujita A, Itoh T, Imano M, Satou T and Nishida S:
Dimethyl fumarate induces apoptosis of hematopoietic tumor cells
via inhibition of NF-κB nuclear translocation and down-regulation
of Bcl-xL and XIAP. Biomed Pharmacother. 68:999–1005. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang T, Lan J, Huang Q, Chen X, Sun X, Liu
X, Yang P, Jin T, Wang S and Mou X: Embelin sensitizes acute
myeloid leukemia cells to TRAIL through XIAP inhibition and NF-κB
inactivation. Cell Biochem Biophys. 71:291–297. 2015. View Article : Google Scholar
|
|
17
|
Hughes MA, Powley IR, Jukes-Jones R, Horn
S, Feoktistova M, Fairall L, Schwabe JW, Leverkus M, Cain K and
MacFarlane M: Co-operative and hierarchical binding of c-flip and
caspase-8: A unified model defines how c-flip isoforms
differentially control cell fate. Mol Cell. 61:834–849. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elsawy MA, Martin L, Tikhonova IG and
Walker B: Solid phase synthesis of Smac/DIABLO-derived peptides
using a ‘Safety-Catch’ resin: Identification of potent XIAP BIR3
antagonists. Bioorg Med Chem. 21:5004–5011. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwan HY, Niu X, Dai W, Tong T, Chao X, Su
T, Chan CL, Lee KC, Fu X, Yi H, et al: Lipidomic-based
investigation into the regulatory effect of schisandrin B on
palmitic acid level in non-alcoholic steatotic livers. Sci Rep.
5:91142015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pao TT, Hsu KF, Liu KT, Chang LG, Chuang
CH and Sung CY: Protective action of schizandrin B on hepatic
injury in mice. Chin Med J. 3:173–179. 1977.PubMed/NCBI
|
|
21
|
Yang T, Liu S, Zheng TH, Tao YY and Liu
CH: Comparative pharmacokinetics and tissue distribution profiles
of lignan components in normal and hepatic fibrosis rats after oral
administration of Fuzheng Huayu recipe. J Ethnopharmacol.
166:305–312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang EP, Li H, Yu CR, Yu CY, Jing S, Sun
HX, Wang CM, Fan XT, Chen JG and Wang S: Schisandrin B protects
PC12 cells against oxidative stress of neurodegenerative diseases.
Neuroreport. 26:360–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stacchiotti A, Li Volti G, Lavazza A,
Schena I, Aleo MF, Rodella LF and Rezzani R: Different role of
schisandrin B on mercury-induced renal damage in vivo and in vitro.
Toxicology. 286:48–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thandavarayan RA, Giridharan VV, Arumugam
S, Suzuki K, Ko KM, Krishnamurthy P, Watanabe K and Konishi T:
Schisandrin B prevents doxorubicin induced cardiac dysfunction by
modulation of DNA damage, oxidative stress and inflammation through
inhibition of MAPK/p53 signaling. PLoS One. 10:e01192142015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiu PY, Leung HY, Poon MK, Mak DH and Ko
KM: (−) Schisandrin B is more potent than its enantiomer in
enhancing cellular glutathione and heat shock protein production as
well as protecting against oxidant injury in H9c2 cardiomyocytes.
Mol Cell Biochem. 289:185–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv XJ, Zhao LJ, Hao YQ, Su ZZ, Li JY, Du
YW and Zhang J: Schisandrin B inhibits the proliferation of human
lung adenocarcinoma A549 cells by inducing cycle arrest and
apoptosis. Int J Clini Exp Med. 8:6926–6936. 2015.
|
|
27
|
Bunel V, Antoine MH, Nortier J, Duez P and
Stévigny C: Protective effects of schizandrin and schizandrin B
towards cisplatin nephrotoxicity in vitro. J Appl Toxicol.
34:1311–1319. 2014. View
Article : Google Scholar
|
|
28
|
Fernandes-Alnemri T, Litwack G and Alnemri
ES: CPP32, a novel human apoptotic protein with homology to
Caenorhabditis elegans cell death protein Ced-3 and mammalian
interleukin-1 beta-converting enzyme. J Biol Chem. 269:30761–30764.
1994.PubMed/NCBI
|
|
29
|
Reed JC and Reed SI: Survivin’
cell-separation anxiety:. Nat Cell Biol. 1:E199–E200. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li F, Ackermann EJ, Bennett CF, Rothermel
AL, Plescia J, Tognin S, Villa A, Marchisio PC and Altieri DC:
Pleiotropic cell-division defects and apoptosis induced by
interference with survivin function. Nat Cell Biol. 1:461–466.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong Q, Hou H, Wu J and Chen Y: The
Nrf2-ARE pathway is associated with Schisandrin B attenuating
benzo(a) pyrene-Induced HTR cells damages in vitro. Environ
Toxicol. 31:1439–1449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Filipski KK, Mathijssen RH, Mikkelsen TS,
Schinkel AH and Sparreboom A: Contribution of organic cation
transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin
Pharmacol Ther. 86:396–402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pabla N, Murphy RF, Liu K and Dong Z: The
copper transporter Ctr1 contributes to cisplatin uptake by renal
tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal
Physiol. 296:F505–F511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Juo P, Kuo CJ, Yuan J and Blenis J:
Essential requirement for caspase-8/FLICE in the initiation of the
Fas-induced apoptotic cascade. Curr Biol. 8:1001–1008. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu H and Baliga R: Endoplasmic reticulum
stress-associated caspase 12 mediates cisplatin-induced LLC-PK1
cell apoptosis. J Am Soc Nephrol. 16:1985–1992. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Omar HA, Mohamed WR, Arab HH and Arafa
el-SA: Tangeretin alleviates cisplatin-induced acute hepatic injury
in rats: Targeting MAPKs and apoptosis. PLoS One. 11:e01516492016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsumoto M, Nakajima W, Seike M, Gemma A
and Tanaka N: Cisplatin-induced apoptosis in non-small-cell lung
cancer cells is dependent on Bax- and Bak-induction pathway and
synergistically activated by BH3-mimetic ABT-263 in p53 wild-type
and mutant cells. Biochem Biophys Res Commun. 473:490–496. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
O’Connor DS, Grossman D, Plescia J, Li F,
Zhang H, Villa A, Tognin S, Marchisio PC and Altieri DC: Regulation
of apoptosis at cell division by p34cdc2 phosphorylation of
survivin. Proc Natl Acad Sci USA. 97:13103–13107. 2000. View Article : Google Scholar
|
|
39
|
Perkins ND: Post-translational
modifications regulating the activity and function of the nuclear
factor kappa B pathway. Oncogene. 25:6717–6730. 2006. View Article : Google Scholar : PubMed/NCBI
|