Introduction
With the increasing level of neonatal treatment, an
increasing proportion of premature children born at an early
gestational age and with a low birth weight survive, although the
subsequent incidence of preterm birth-associated complications
continues to increase. Bronchopulmonary dysplasia (BPD) is a
chronic respiratory disease, which is one of the most common
sequelae of premature birth, particularly in those <30 weeks
gestational age (1,2). According to the Neonatal Research
Network of the National Institute of Child Health and Human
Development, the incidence of the new classification of
severity-based BPD has been reported to be as high as 68% among
preterm infants born between gestational weeks 22 and 28
(traditional definition, 42%; physiological definition, 40%)
(3). BPD not only causes
relatively high infant mortality soon after birth, but can also
induces multiple respiratory and nervous system complications
during adolescence (1,4,5).
Alveolar simplification and disorganized vasculature
are the major pathological characteristics of 'new' BPD (6,7).
It is widely accepted that neonatal hyperoxia promotes a BPD-like
pathology characterized by alveolar simplification and angiogenic
impairment (8,9). Simplified alveolar epithelium in the
lungs was also observed in previous studies on hyperoxia-treated
neonatal rats by our group (10,11). A compensatory increase in
transdifferentiation of alveolar epithelial cells (AECs) was
identified in the lungs of the hyperoxic group rather than their
suppression (10). In addition,
decreased proliferation of type II AECs (AEC II) was observed in
vitro, which may, at least partially, contribute to the
development of simplified alveolar epithelium in hyperoxia
(10). However, the factors
affecting such changes of AECs have remained elusive.
Zonula occludens 1 (ZO-1)-associated nucleic
acid-binding protein (ZONAB) is a Y-box transcription factor that
is recruited to tight junctions (TJs) by binding to the Src
homology 3 domain of ZO-1 (12,13) and has selectivity for sequences of
specific promoters containing an inverted CCAAT box (12). ZONAB shuttles between the TJs and
nuclei, while the sequestration with ZO-1 at the TJs prevents
accumulation of ZONAB in the nucleus, where ZONAB engages in
transcriptional activities (13–15). As a transcription factor, the
first discovered role of ZONAB was the promotion of cell
proliferation. Cyclin-dependent kinase (CDK)4 is a core factor that
regulates G1/S transition in the cell cycle, with transposition
between the nucleus and cytoplasm (16). Balda et al (13) reported that ZONAB modulates the
accumulation of CDK4 in the nucleus to affect the regulation of the
cell cycle. When the concentration of ZONAB in the nucleus is
reduced, CDK4 is reduced and cell proliferation is subsequently
inhibited. Proliferating-cell nuclear antigen (PCNA) is a basic
regulator of eukaryotic DNA replication and repair (17). Sourisseau et al (18) confirmed that PCNA is the target
gene of ZONAB and that PCNA expression is upregulated by increased
ZONAB expression, which promotes DNA replication and cell
proliferation. Cyclin D1, the core factor of cell cycle regulation,
is also a target gene of ZONAB (18–20). Cyclin D1 expression is upregulated
by overexpression of ZONAB, which activates CDK4 and CDK6 in order
to promote G1/S transition (18).
At the same time, ZONAB also inhibits epithelial cell
differentiation. During normal kidney development, epithelial cells
undergo a transition from proliferation to differentiation,
resulting in polarization of the epithelial cell layers. However,
this polarization disappears during tumor formation, resulting in
excessive cell proliferation and regression of tissue repair.
Expression of the megalin and cubilin genes is a marker of
epithelial differentiation (21).
Lima et al (22)
identified the megalin and cubilin genes as target genes of ZONAB,
and ZONAB was able to decrease megalin and cubilin expression and
inhibit epithelial cell differentiation. ZONAB expression in human
renal clear cell carcinoma tissue is abnormally increased,
resulting in further proliferation of differentiated cells, loss of
normal polarized morphology and promotion of tumorigenesis.
Therefore, the present study focused on ZONAB
expression in AECs in a hyperoxia-induced BPD model and its
possible function in the regulation of the transdifferentiation and
proliferation of AECs. The primary objectives of the present study
were to determine whether the transcription factor ZONAB was
differentially expressed in the lung of newborn rats exposed to
hyperoxia and to identify an alternative pathway that promotes
BPD-like alveolar simplification.
Materials and methods
Animal model
All animal procedures were approved by the Medical
Ethics Committee of Shengjing Hospital affiliated to China Medical
University (Shenyang, China). BPD animal models were established
according to methods previously described by our group (10). Adult Wistar rats (age, 3–4 months
old; 20 females of weight 250–300 g and 5 males of weight 350–400
g) were purchased from the Animal Lab of the Experimental Research
Center of Shengjing Hospital (China Medical University, Shenyang,
China) and mated at a male to female ratio of 1:4. The 211 newborn
Wistar rats were bred in house and randomly divided into two
groups: A BPD model group, which inhaled 85% oxygen and a control
group, which inhaled air. The rats were housed in the following
conditions: 12-h light/dark cycle; temperature, 25–26°C; humidity,
60–70%; boxes were opened for 30 min each day to add water and
feed, and replace bedding. Food and water were available ad
libitum. The oxygen concentration in the glass chamber was
monitored with an oxygen measurement instrument (model no. 572;
Servomex, Inc., Norwood, MA, USA). Maternal rats in the two groups
were switched every 24 h to avoid oxygen toxicity. Neonatal rats
were kept under constant hyperoxia (8,10)
and were not kept for longer than 21 days.
Lung sample collection
According to a method previously described by our
group (10), eight rats were
randomly selected from the two groups on days 1, 3, 7, 14 and 21,
and under intra-peritoneal anesthesia (chloral hydrate 300 mg/kg
body weight), the chest cavity was immediately opened and the left
atrial appendage was cut. Subsequently, the right ventricle was
punctured with a sterile infusion needle and irrigated with saline
solution at a pressure of 18 cm H2O to wash out any
blood in the lungs. The left lung tissue was fixed in 4%
paraformaldehyde, embedded in paraffin and routinely sliced into
4-µm tissue sections, which were stained with hematoxylin (5
min) and eosin (1–3 min) at room temperature. For the preparation
of frozen sections, the tissues fixed in 4% paraformaldehyde were
dehydrated in 30% sucrose for 12 h and frozen at −80°C.
Subsequently, the tissues were sliced into 7-µm sections for
immunofluorescence double staining. The right lung tissue was
placed in Eppendorf tubes and frozen at −80°C for western blot and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analyses.
AEC II isolation and culture
In accordance with the method described in a
previous study by our group (10), 200 newborn rats within 24 h after
birth were anesthetized and lavaged to isolate cardiopulmonary
tissues, which were subsequently placed in pre-chilled D-Hanks
solution. The isolated lung tissue was repeatedly washed to remove
residual blood, cut into small pieces (1 mm3) and
repeatedly rinsed in D-Hanks solution. The pieces of tissue were
immersed in 0.25% Trypsin-EDTA solution (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in each well and incubated for
30 min at 37°C in a water bath. The digestion was terminated by the
addition of an equal volume of 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.). The mixture was filtered through a
200-mesh filter and then centrifuged (4°C, 235 × g, 5 min). The
supernatant was removed and the pellet was incubated with 0.1%
collagenase I (Gibco; Thermo Fisher Scientific, Inc.) for 20 min at
37°C in a water bath. The mixture was centrifuged again (4°C, 235 ×
g, 5 min) and the pellet was resuspended in medium containing 10%
FBS, then inoculated in a Petri dish and incubated in a humidified
atmosphere of 5% CO2 at 37°C. The medium was replaced
three times at 50-min intervals to remove adherent fibroblasts.
Non-adherent cells were transferred to rat immunoglobulin G
(IgG)-coated dishes for 1 h to remove macrophages. The cells were
adjusted to a density of 2–3×106 cells/ml and inoculated
in a 50-ml flask or six-well plate to continue the culture. After
24 h, relatively pure AECs II were obtained by removing blood cells
and dead cells. Of the cells, >95% were viable as demonstrated
by trypan blue staining, with 90% purity by immunofluorescence
staining against surfactant protein C (SP-C). After 24 h of
culture, the cells were randomly allocated to a model group or a
control group. Cells in the model group were incubated in an
atmosphere of 85% O2/5% CO2 in a standard
incubator (CB150; Binder GmbH, Tuttlingen, Germany) and the control
group was maintained under an atmosphere of 21% O2/5%
CO2 in a Series 11 3111 Water Jacketed CO2
Incubator (Thermo Fisher Scientific, Inc.) for 48 h of continuous
culture.
The extraction of AEC II from the rats in the BPD
model group and control group was performed on days 7, 14 and 21
after hyperoxia or air exposure, in accordance with specific
methods for extraction, as described above. The cells were purified
by differential adhesion and IgG adhesion, centrifuged, and the
supernatant was discarded. The cells were stored at −80°C for
western blot and RT-qPCR analyses.
Trypan blue staining
Dead cells can be stained by Trypan blue, but live
cells prevent the dye from entering; therefore, trypan blue
staining may be used to distinguish between dead and live cells. A
single-cell suspension (106 cells/ml) was prepared and
mixed with 0.4% trypan blue solution. Cells were loaded into a
hemocytometer and observed under inverted phase contrast
microscope. The cell viability was determined as the total number
of live cells divided by the sum of the total number of live and
dead cells, multiplied by 100.
Immunofluorescence staining
The primary and secondary antibodies for double
staining of ZONAB/SP-C in lung tissue were polyclonal rabbit
anti-ZONAB (1:50 dilution; cat. no. 402800; Invitrogen; Thermo
Fisher Scientific, Inc.), DyLight 488 AffiniPure donkey anti-rabbit
IgG (H&L; 1:100 dilution; cat. no. E032221-02; EarthOx Life
Sciences, San Francisco, CA, USA), polyclonal goat anti-SP-C (1:100
dilution; cat. no. sc-7705; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and donkey anti-goat IgG (H&L) Affinity Pure,
DyLight594 Conjugate (1:100 dilution; cat. no. DkxGt-003-D;
ImmunoReagents, Raleigh, NC, USA). The cultured cells were
incubated with polyclonal rabbit anti-ZONAB (1:50 dilution; cat.
no. 402800; Invitrogen; Thermo Fisher Scientific, Inc.) and DyLight
549 AffiniPure goat anti-rabbit IgG (H&L; 1:100 dilution; cat.
no. E032320; EarthOx Life Sciences) for ZONAB staining. The primary
and secondary antibodies for immunofluorescence double staining of
cultured cells for aquaporin (AQP)5/SP-C were polyclonal rabbit
anti-SP-C (1:50 dilution; cat. no. sc-13979; Santa Cruz
Biotechnology, Inc.), DyLight 488 AffiniPure donkey anti-rabbit IgG
(H&L; 1:100 dilution; cat. no. E032221; EarthOx Life Sciences),
poly-clonal goat anti-AQP5 (1:100 dilution; cat. no. sc-9891; Santa
Cruz Biotechnology, Inc.) and donkey anti-goat IgG (H&L)
Affinity Pure, DyLight594 Conjugate (1:100 dilution; cat. no.
DkxGt-003-D; ImmunoReagents). Immunofluorescence staining of lung
tissue was performed with frozen sections (7 µm) fixed in
methanol for 30 min at room temperature and blocked with 10% FBS
for 30 min at room temperature, which were then washed and
incubated with primary antibody at room temperature overnight,
washed and then incubated with immunofluorescence secondary
antibody at room temperature for 3–4 h, washed, and incubated for 5
min with DAPI (1:1,000 dilution; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Immunofluorescence was then observed under a
confocal laser-scanning microscope (C1; Nikon Corp., Tokyo, Japan).
Immunofluorescence staining of cultured AECs was performed on
tissue culture-treated polycarbonate filter inserts (Transwell;
Corning-Costar, Corning, NY, USA), which were fixed with 4%
paraformaldehyde for 30 min at room temperature and then fixed in
methanol for another 30 min at room temperature, followed by
incubation with primary antibody overnight at 4°C. The remaining
steps were identical to those for immunofluorescent staining of
tissue.
Western blot analysis
Total protein was extracted from lung tissues or
cells using lysis buffer for western blot analysis (cat. no. P0013;
Beyotime Institute of Biotechnology, Shanghai, China). EpiQuik
Nuclear Extraction kit (EpiGentek Group, Inc., New York, NY, USA)
was used to extract total nuclear protein from cells. Protein
concentration was determined by a Bradford assay, and equal amounts
of protein (50 µg) from extracts were mixed with sample
loading buffer. After electrophoresis on 12% precast SDS-PAGE gels
(70 V for 90 min), proteins were transferred to polyvinylidene
difluoride membranes (cat. no. LC2005; Thermo Fisher Scientific,
Inc.) (70 V for 90 min). The membranes were blocked with 5% (w/v)
nonfat milk in a buffer of 100 mM Tris base (pH 7.5) containing
0.9% (w/v) NaCl and 0.1% (v/v) Tween 20 (TBST) for 1 h at room
temperature and then incubated overnight at 4°C with the following
primary antibodies: Rabbit anti-ZONAB (1:800 dilution; Invitrogen;
Thermo Fisher Scientific, Inc.), goat anti-AQP5 (1:500 dilution;
Santa Cruz Biotechnology), rabbit anti-SP-C (1:500 dilution; Santa
Cruz Biotechnology), mouse anti-PCNA (1:1,000 dilution; Abcam,
Cambridge, MA, USA), rabbit anti-cyclin D1 (1:1,000 dilution;
Abcam), rabbit anti-LAMIN B1 (cat. no. ab16048; 1:1,000 dilution;
Abcam) or rabbit anti-β-actin (1:1,000 dilution; Sigma-Aldrich;
Merck KGaA). Following extensive washing in TBS-T three times, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibody (Cell Signaling Technologies, Inc., Beverly, MA,
USA) at room temperature for 2 h. The membranes were then washed
and treated with western blotting luminol reagent (cat. no.
sc-2048; Santa Cruz Biotechnology, Inc.) and the signal was
visualized using an Alpha Ease RFC Imaging System (Alpha Innotech
Corp., San Leandro, CA, USA). Integrated density values of the
protein bands were analyzed using a Fluor Chen 2.0 system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and normalized to those of
β-actin or LAMIN B.
RT-qPCR
Total RNA was extracted from lung tissues and cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
A total of 1 µg RNA was reverse-transcribed into
complementary DNA using the SuperScript III kit (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. IQ SYBR Green Supermix (Bio-Rad Laboratories, Inc.)
and a 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) were used for quantitative real-time PCR.
Gene-specific primers were designed and synthesized by Takara
Biotechnology (Dalian, China). The primer sequences are listed in
Table I. The threshold cycle was
recorded to reflect the mRNA expression levels and dissociation
curve analysis was performed to ensure the specificity of the PCR
products. The relative expression of target gene/internal reference
mRNA was expressed according to the 2−ΔΔCq method
(23).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene name | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| ZONAB |
CCCTACAACTATAGGCGCCGTTC |
GCACTGCTCTGTTCGGTAGCTG |
| AQP5 |
CTCCGAGCTGTCTTCTACGTG |
CAGGCGTTGTGTTGTTGTTC |
| SP-C |
GGTAGCAAAGAGGTACTGATGG |
CACCACGACGACAAGGACTA |
| PCNA |
TAAGGGCTGAAGATAATGCTGAT |
CCTGTTCTGGGATTCCAAGTT |
| β-actin |
ACCGTGAAAAGATGACCCAGAT |
CAGTGGTACGACCAGAGGCATA |
Transfection of ZONAB plasmids
AEC II was isolated from neonatal rat lungs and
cultured in six-well cell plates at a density of 2–3×106
cells/ml. The cells were incubated at 37°C in an atmosphere of 5%
CO2 for 24 h. The plasmid DNA clones for rat ZONAB and
the empty vector pCMV6 were purchased from Origene Technologies,
Inc. (Rockville, MD, USA) and were amplified using an
endotoxin-free plasmid Maxi-prep kit (Qiagen, Valencia, CA, USA).
The cells were transfected at a ratio of 1 µg plasmid DNA to
2 µl Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in 100 µl Opti-MEM Reduced Serum Media
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. After incubation at room temperature for
20 min, the transfection complex was added to the cell culture.
Cells were cultured for 6 h, and the medium with the transfection
solution was then removed and replaced with complete Dulbecco's
modified Eagle's medium: Nutrient mixture F-12 (DMEM/F12) (Gibco;
Thermo Fisher Scientific, Inc.). The cells were harvested after 48
h and subjected to the abovementioned assays. The AEC viability
assay was performed using trypan blue staining.
Transfection of small interfering RNA
(siRNA) of ZONAB
Cultured AEC II were seeded into six-well plates at
a density resulting in 70% confluence. The siRNA sequence targeting
rat ZONAB (siZONAB; 5′-CCGAAACGACACCAAAGAAdTdT-3′) was designed and
synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
siZONAB or control non-targeting siRNA complexed with 2 µl
Lipofectamine 2000 in 100 µl of Opti-MEM Reduced Serum Media
was added concurrently and incubated at room temperature for 20
min. The transfection complex was then gently added to the cells.
The medium was replaced with complete DMEM/F12 medium after 6 h.
Cells were analyzed after 48 h. The cell viability was determined
by trypan blue staining.
Statistical analysis
All analyses were performed using SPSS 18.0 software
(SPSS, Inc., Chicago, IL, USA). Values are expressed as the mean ±
standard deviation and the mean values were obtained from at least
three independent experiments. Statistical significance between two
groups was determined using Student's t-test. One-way analysis of
variance followed by Dunnett's test was used for multiple group
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Animal condition
The mortality rate as a result of hyperoxia was
~6.7%, and the mortality rate observed in the present study as a
result of hyperoxia was expected according to our previous studies
(data not shown). The signs of hyperoxia-induced toxicity were poor
response, reduced activity, dull body hair, low body weight, short
body height, high mortality, as well as cyanosis and shortness of
breath following oxygen exposure, which were in agreement with
previous studies (10,11,17). These symptoms were considered to
be humane endpoints in the present study. In the present study, 1%
of the animals exhibited a body weight loss >20% and were
immediately sacrificed. Although infrequent, this phenomenon has
been observed in previous studies (10,11,17). Animals meeting the humane
endpoints were subjected to cardiac puncture following
intraperitoneal anesthesia with chloral hydrate (300 mg/kg body
weight). Animal death was confirmed by lack of heartbeat.
ZONAB expression is deregulated in the
lung tissues of the BPD model group
ZONAB expression in lung tissues of the model group
and the control group was first detected and the total amount of
ZONAB protein expressed in the lung tissues was determined by
western blot analysis. The results demonstrated that, as compared
with the control group, ZONAB expression increased from days 1–21
of hyperoxia (Fig. 1A). In order
to eliminate interference with other cellular components in lung
tissues and to specifically observe ZONAB expression in AEC II, the
lung tissue sections were assessed using immunofluorescence double
staining. As presented in Fig.
1B, the AEC II-specific marker SP-C was labeled with red
fluorescence, ZONAB was labeled with green fluorescence, and
double-stained cells (yellow) represent AEC II expressing ZONAB.
The results indicated that ZONAB expression in the AEC II of the
model group was significantly reduced compared with that in the
control group (Fig. 1B).
Conversely, excessive expression of ZONAB was identified in the
bronchial epithelial cells of the model group (Fig. 1C). At the same time, AEC II was
isolated and purified from the lungs of the control and model
groups at different time-points (days 7, 14 and 21), and the target
protein and mRNA were extracted from the obtained cell samples for
further quantification (Fig. 1D).
The results indicated that ZONAB expression in the control and
model groups gradually decreased with prolonged exposure time,
while the average ZONAB expression in the model group was lower
than that in the control group (P<0.05).
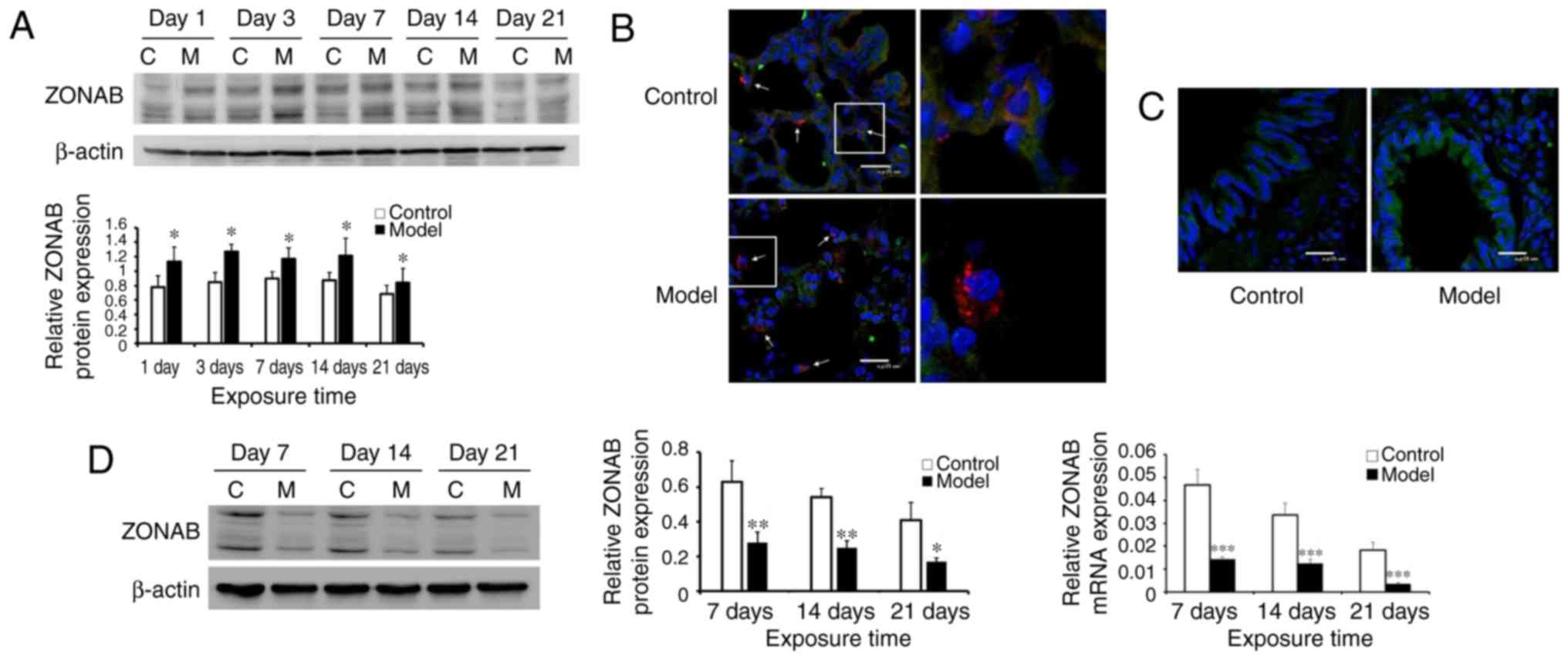 | Figure 1Abnormal ZONAB expression in lung
tissues of the bronchopulmonary dysplasia model group. (A) Western
blot analysis was used to measure ZONAB expression in lung tissues
of the control and model groups. (B) At day 21, lungs of animals
from the two groups were double-stained (magnification, ×800; scale
bar, 25 µm). SP-C, the AEC II marker, is labeled with red
fluorescence and ZONAB is labeled with green fluorescence.
Double-stained cells are displayed in yellow, representing AEC II
expression of ZONAB. ZONAB expression in the AEC II was
significantly decreased in the model group (shown by arrows).
Images in the boxes were magnified and displayed in the right-hand
panel to indicate ZONAB and SP-C localization (magnification, ×800;
scale bar, 25 µm). (C) Immunofluorescence staining of lung
tissues on day 21 demonstrated excessive expression of ZONAB in
bronchial epithelial cells in the model group (magnification, ×800;
scale bar, 25 µm). (D) Western blot analysis was used to
measure the protein expression, while reverse-transcription
quantitative polymerase chain reaction analysis was employed to
analyze the mRNA expression of ZONAB in AEC II isolated from lung
tissues of rats in the control and model groups.
*P<0.05, **P<0.01,
***P<0.001 vs. control group on the same day. C,
control group; M, model group; ZONAB, zonula occludens 1-associated
nucleic acid binding protein; SP-C, surfactant protein C; d, days;
AEC II, type II alveolar epithelial cells. |
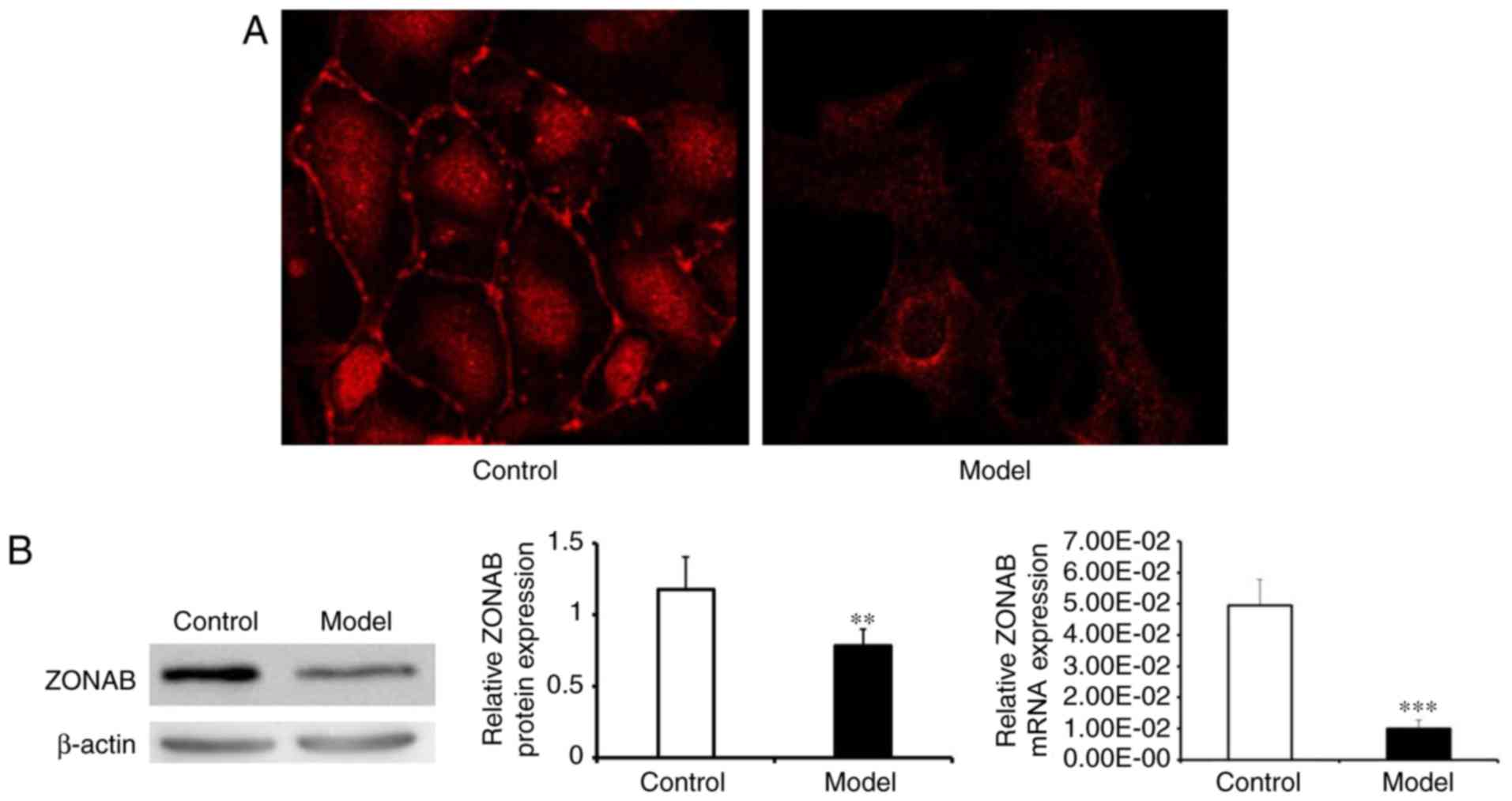
ZONAB expression in primary cultured AEC
II
In order to better understand the effect of ZONAB on
the transdifferentiation and proliferation of AECs, an in
vitro AEC II model was established. The expression levels and
cellular localization of ZONAB were detected by immunofluorescence
staining, which demonstrated that compared with that in the model
group, the expression of ZONAB in the control group was higher, and
that the protein was mainly located at the cellular junctions or in
the nuclei, whereas in the model group, it was aggregated in the
cytoplasm with little remaining at the cell junctions or in the
nuclei (Fig. 2A). Western blot
and RT-qPCR analyses were used to quantify the expression of ZONAB
protein and mRNA, both of which suggested that ZONAB expression in
the model group was lower than that in the control group (Fig. 2B).
ZONAB overexpression inhibits
transdifferentiation and promotes proliferation of AEC II
ZONAB expression was decreased in AEC II of the BPD
model group and a previous study by our group had confirmed
increased transdifferentiation and decreased proliferation of AEC
II in the BPD model group (10).
In the present study, it was further verified whether ZONAB is
involved in the regulation of transdifferentiation and
proliferation of AEC II. ZONAB expression in primary AEC II was
detected by RT-qPCR and western blot analyses after pCMV6-ZONAB
transfection. The results indicated that ZONAB protein and mRNA
levels in the pCMV6-ZONAB-transfected group were significantly
higher than those in the blank control group (P<0.05). ZONAB
protein expression in the nuclei was also significantly increased
compared with the level of the LAMIN B control (Fig. 3A) (24). The expression of the AEC I marker
AQP5 and the AEC II marker SP-C was examined, which indicated that
AQP5 expression in the pCMV6-ZONAB-transfected group was
significantly lower than that in the control group, and SP-C
expression was significantly increased, suggesting decreased
transdifferentiation (Fig. 3B).
The expression levels of PCNA and cyclin D1 in the
pCMV6-ZONAB-transfected group were significantly higher than those
in the control group (Fig. 3C).
Cell immunofluorescence double staining demonstrated that AQP5
expression was decreased, while SP-C expression was increased in
the pCMV6-ZONAB-transfected group. The double-stained cells in the
pCMV6-ZONAB-transfected group were reduced compared with those in
the control group (Fig. 3D),
which indicated decreased transdifferentiation and increased
proliferation. The cell viability assay performed by trypan blue
staining indicated increased cell viability in the
pCMV6-ZONAB-transfected group (Fig.
3E).
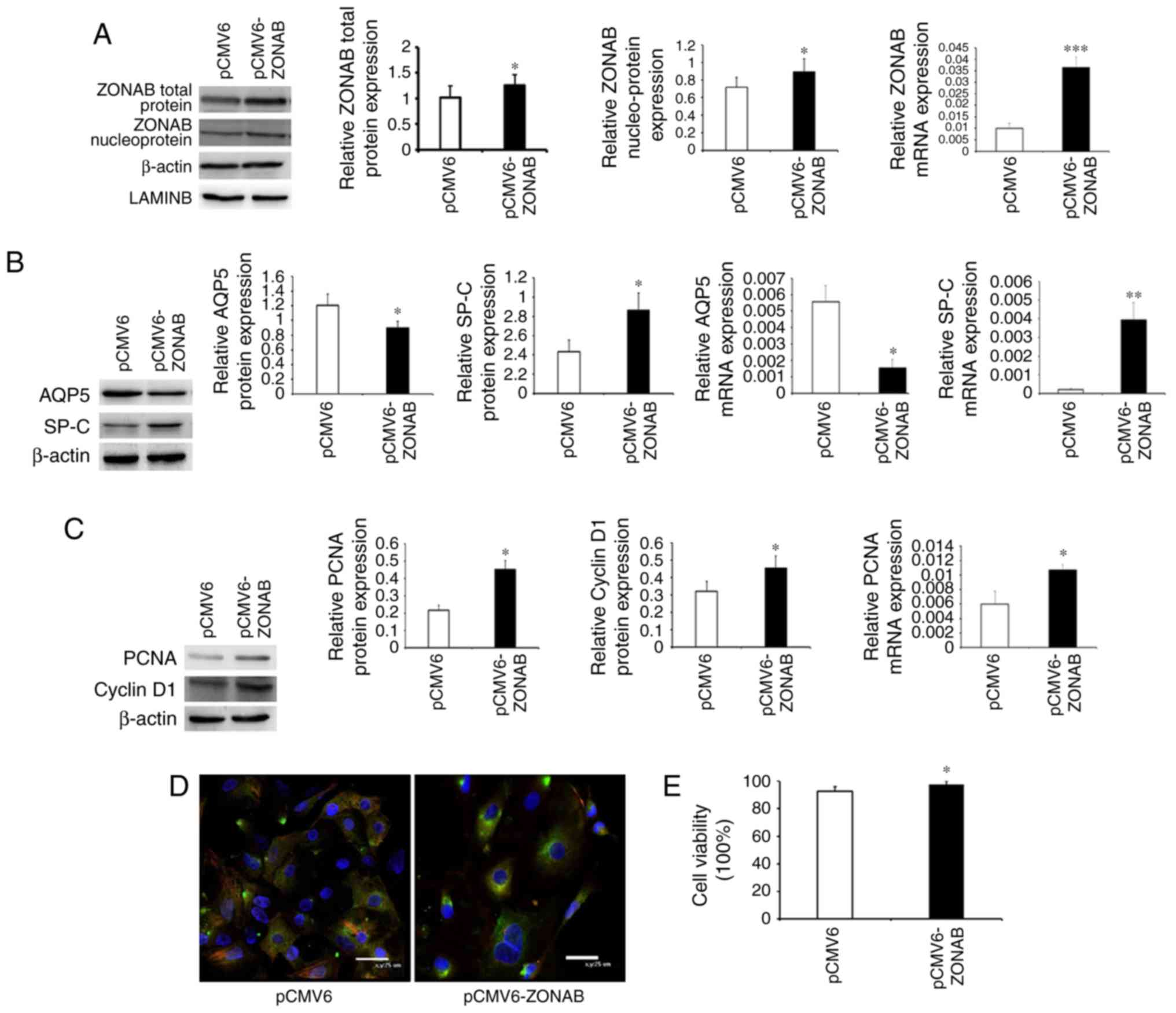 | Figure 3Overexpression of ZONAB inhibits
transdifferentiation and promotes proliferation of AEC II. (A)
Expression of ZONAB protein and mRNA in the pCMV6-ZONAB-transfected
and blank control groups. (B) Protein and mRNA expression of AQP5
and SP-C in the two groups of cells. (C) Protein and mRNA
expression of PCNA and cyclin D1 in the two groups. (D)
Immunofluorescent double staining results for the two groups of
cells (magnification, ×800; scale bar, 25 µm). Red
fluorescent-labeled AQP5 represents AEC I, green
fluorescent-labeled SP-C represents AEC II, and the double-stained
cells are displayed in yellow, representing cells transformed from
AEC II to AEC I. The nuclei were stained with DAPI (blue). (E) Cell
viability in the two groups. *P<0.05,
**P<0.01, ***P<0.001 vs. pCMV6 group.
ZONAB, zonula occludens 1-associated nucleic acid binding protein;
SP-C, surfactant protein C; AEC II, type II alveolar epithelial
cells; PCNA, proliferating cell nuclear antigen; AQP, aquaporin;
pCMV, cytomegalovirus plasmid. |
Knockdown of ZONAB expression promotes
transdifferentiation and inhibits proliferation of AEC II
In order to further investigate the effects of ZONAB
on the transdifferentiation and proliferation of AEC II, anti-ZONAB
siRNA (siZONAB) was transfected to inhibit ZONAB expression.
Indicators of AEC II transdifferentiation and proliferation were
detected. The results indicated that ZONAB expression in
siZONAB-transfected cells was significantly lower than that in the
blank control group (transfected with nonspecific siRNA), and ZONAB
expression in the nuclei was also significantly decreased
(P<0.05; Fig. 4A). At the same
time, the expression of the AEC I marker AQP5 was significantly
increased and the expression of the AEC II marker SP-C was
significantly decreased in the siZONAB-transfected group,
suggesting increased transdifferentiation of the cells (Fig. 4B). PCNA and cyclin D1 expression
levels were significantly decreased in the siZONAB-transfected
group, indicating decreased cell proliferation (Fig. 4C). The double immunofluorescence
staining results demonstrated that AQP5 expression was increased,
SP-C expression was decreased and double-stained cells increased in
the siZONAB-transfected group (Fig.
4D), suggesting increased transdifferentiation and decreased
proliferation of AEC II in the siZONAB-transfected group. Cell
viability was decreased in the siZONAB-transfected group compared
with that in the control group (Fig.
4E).
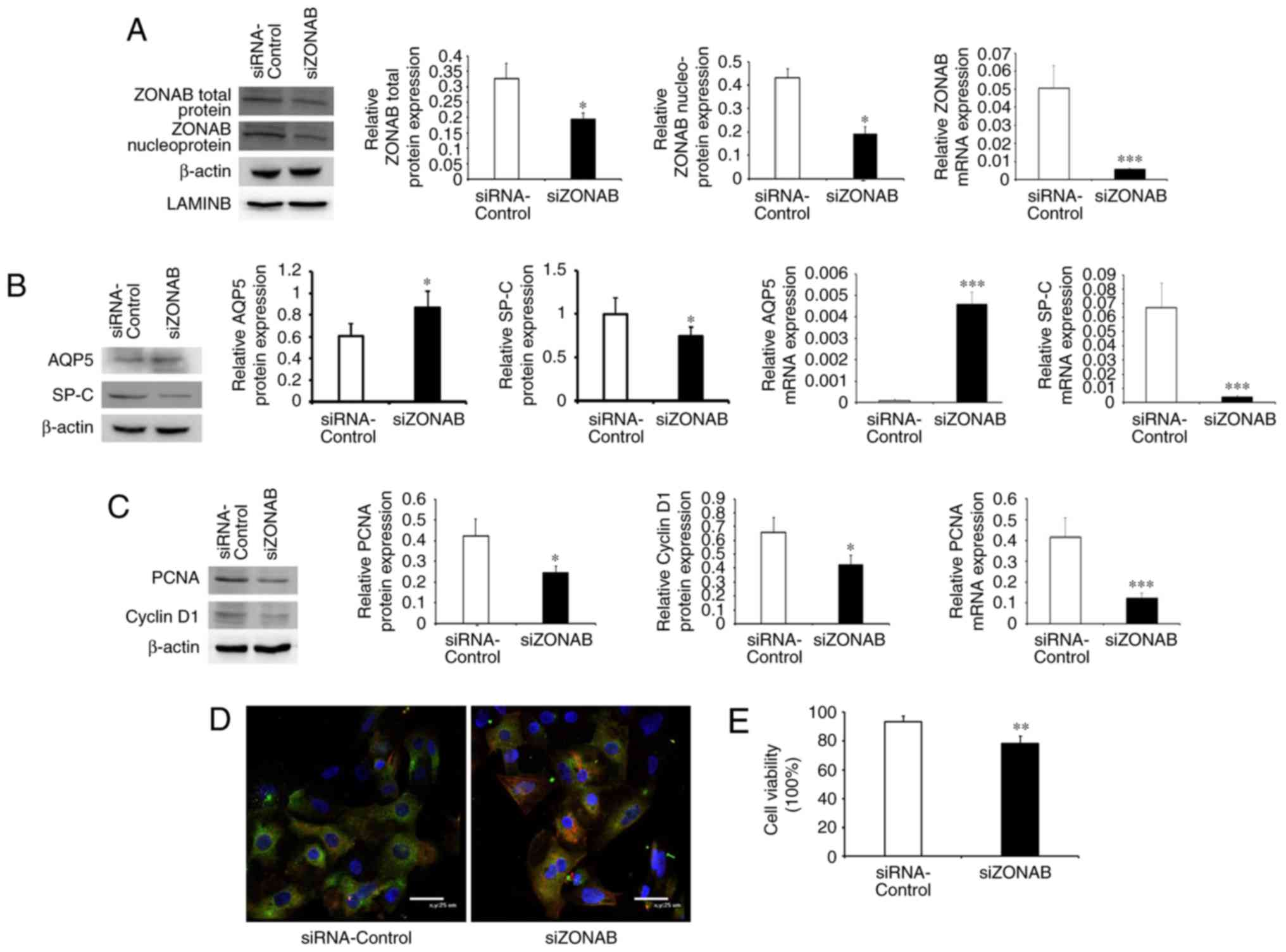 | Figure 4Knockdown of ZONAB expression
promotes transdifferentiation and inhibits proliferation of AEC II.
(A) Protein and mRNA expression of ZONAB in AEC II of the
siZONAB-transfected and blank control groups. (B) Protein and mRNA
expression levels of AQP5 and SP-C in the two groups of cells. (C)
Protein and mRNA expression of PCNA and cyclin D1 in the two
groups. (D) Immunofluorescent double staining results for the two
groups of cells (magnification, ×800; scale bar, 25 µm). The
red fluorescent-labeled AQP5 represents AEC I, the green
fluorescent-labeled SP-C represents AEC II, the double-stained
cells (yellow) represent cells transformed from AEC II to AEC I,
and nuclei were stained with DAPI (blue). (E) Cell viability in the
two groups. *P<0.05, **P<0.01,
***P<0.001 vs. control group. ZONAB, zonula occludens
1-associated nucleic acid binding protein; SP-C, surfactant protein
C; AEC II, type II alveolar epithelial cells; PCNA, proliferating
cell nuclear antigen; AQP, aquaporin; siRNA, small interfering
RNA. |
ZONAB overexpression partially alleviates
hyperoxia-induced abnormal transdifferentiation and proliferation
of AEC II
To investigate whether ZONAB is involved in
mediating the effects of hyperoxia on AEC II transdifferentiation
and proliferation, the pCMV6-ZONAB plasmid or pCMV6 blank plasmid
was transfected into primary AEC II, which were then exposed to
hyperoxia or normoxia treatment to observe cell
transdifferentiation and proliferation. ZONAB mRNA expression in
the pCMV6-ZONAB-transfected and blank control groups was decreased
after hyperoxia treatment. However, after hyperoxia treatment, the
level of ZONAB expression in the pCMV6-ZONAB-transfected group was
higher than that in the control group, but did not reach the
concentration of that in the normoxic control group. AQP5 mRNA
expression increased after hyperoxia treatment in each of the two
groups, while the increase in the pCMV6-ZONAB-transfected group was
significantly lower than that in the control group, suggesting that
high ZONAB expression partially mitigated the excessive
transdifferentiation caused by hyperoxia. SP-C and PCNA mRNA
expression levels decreased in each of the two groups after
hyperoxia treatment. Although the decrease in the
pCMV6-ZONAB-transfected group was less significant than that in the
pCMV6-transfected group under hyperoxia, it was still lower than
that in the control group under normoxia conditions, suggesting
that high ZONAB expression partially alleviated the
hyperoxia-induced inhibition of proliferation (Fig. 5A). The changes in the protein
expression of the above indicators were similar to the trends in
the mRNA expression, and the changes in the protein expression of
cyclin D1 were similar to those of PCNA (Fig. 5B). Hyperoxia caused significant
cell death, and transfection of pCMV6-ZONAB partly alleviated the
cell death (Fig. 5C).
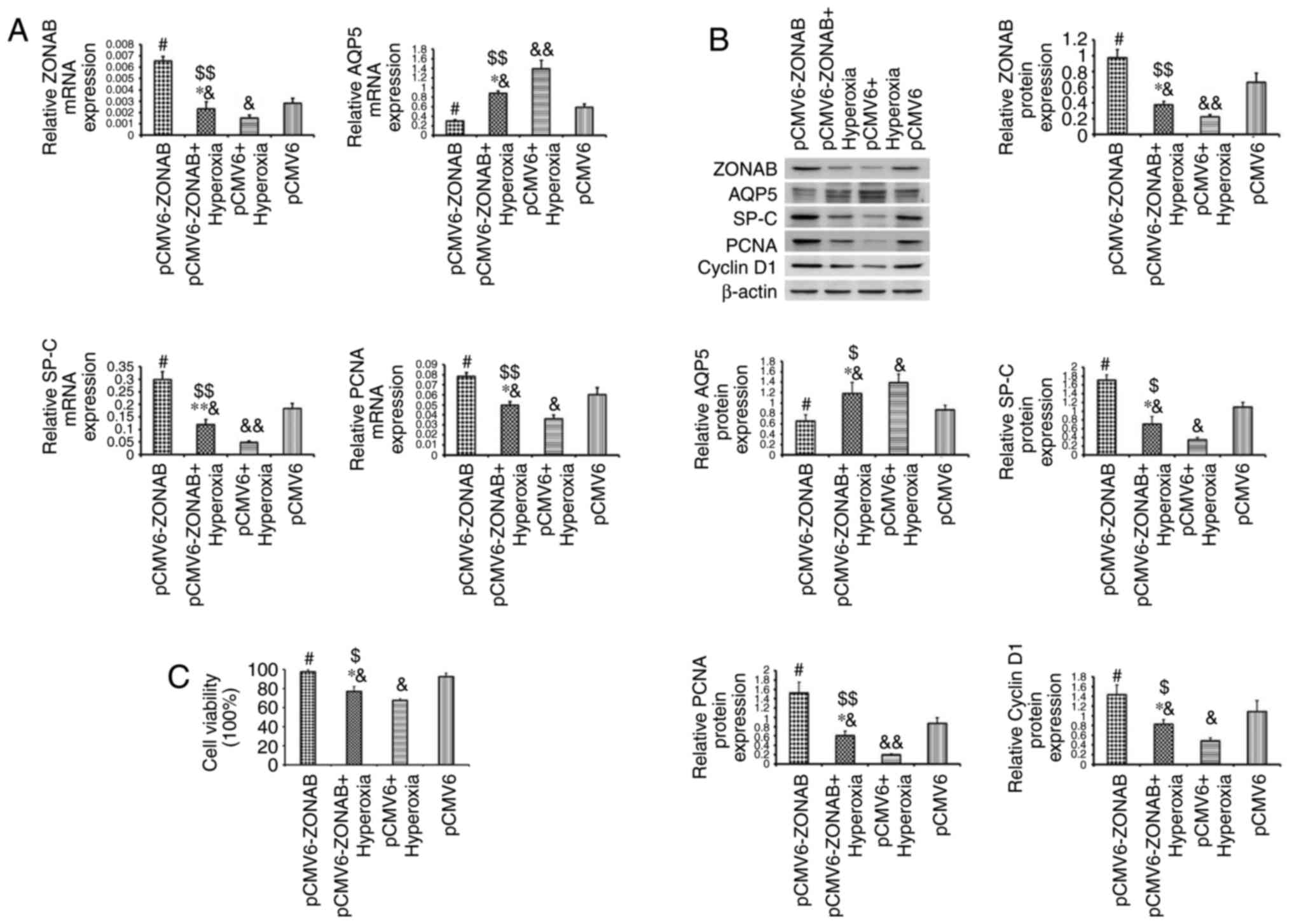 | Figure 5ZONAB overexpression partially
alleviates hyperoxia-induced excessive transdifferentiation and
decreased proliferation of AEC II. (A) mRNA expression levels of
ZONAB, AQP5, SP-C and PCNA in primary AEC II of the
pCMV6-ZONAB-transfected and blank control groups, with hyperoxia or
normoxia exposure. (B) Protein expression of ZONAB, AQP5, SP-C,
PCNA and cyclin D1 in each group. (C) Cell viability in each group.
#P<0.05 for comparison with the pCMV6 group,
$P<0.05, $$P<0.01 for comparison with
the pCMV6-ZONAB group, *P<0.05,
**P<0.01 for comparison with the pCMV6+hyperoxia
group, &P<0.05, &&P<0.01
for comparison with the pCMV6 group. ZONAB, zonula occludens
1-associated nucleic acid binding protein; SP-C, surfactant protein
C; AEC II, type II alveolar epithelial cells; PCNA, proliferating
cell nuclear antigen; AQP, aquaporin; pCMV, cytomegalovirus
plasmid. |
Discussion
Previous studies on the pathogenesis of BPD mainly
focused on AEC II injury, which is thought to be the pathological
basis of BPD. Sureshbabu et al (25) reported that apoptosis of a large
number of AEC II occurred in the BPD model. Roper et al
(26) and O'Reilly et al
(27) identified DNA damage in
in vivo and in vitro models of BPD. Chen et al
(28) demonstrated that AEC II
produce a large number of reactive oxygen species under hyperoxia
conditions, resulting in apoptosis and necrosis. However, the
results of other studies differed from this. For instance, Crapo
et al (29) revealed that
the number of AEC II in BPD lung tissues was increased rather than
decreased and Yee et al (8) reported that the number of AEC I
increased in a hyperoxia-induced BPD model, suggesting that AEC II
injury is not the only mechanism of BPD injury in the lung
epithelium. A previous study by our group demonstrated that
transdifferentiation of AECs in the BPD model significantly
increased at the tissue and cellular levels of AECs, accompanied by
proliferative dysregulation (10). The transdifferentiation of AEC II
to AEC I is an important mechanism in lung injury repair (30–32), but the elevated
transdifferentiation level of AECs in the BPD model does not
compensate for injury of the lung epithelium, in which AEC II as
well as AEC I are structurally and functionally abnormal, resulting
in destruction of the blood-gas barrier and opening of the
pulmonary epithelial TJs (10).
These changes are likely to be associated with excessive
dysregulation of transdifferentiation and proliferation of AECs,
which provides a novel pathway for further studies on the
pathogenesis of BPD.
To further study the regulatory mechanism of the
trans-differentiation and proliferation of AECs, the present study
focused on the transcription factor ZONAB. A previous study by our
group indicated decreased expression of ZO-1 in AECs in a model of
hyperoxia-induced lung injury (33), which resulted in the opening of
the TJs between AECs and destruction of the epithelial barrier. In
addition to participating in the regulation of TJs, ZO-1 interacts
with ZONAB to regulate cell differentiation and proliferation
(13,22,34). The role of ZONAB in the regulation
of cell differentiation and proliferation has been studied in
numerous diseases, including human hepatocellular carcinoma
(35), uveal melanoma (36), proliferative vitreoretinopathy
(37) and renal clear cell
carcinoma (22). However,
relatively few studies have assessed ZONAB expression in lung
tissue and no study has investigated the effects of ZONAB on the
onset and development of BPD. Therefore, the present study examined
ZONAB expression in the lung tissues of rats with BPD and further
confirmed that ZONAB participates in the regulation of the
transdifferentiation and proliferation of AECs in vitro in
order to explore the pathogenesis of BPD.
Evaluation of the western blot results of tissue
samples indicated that ZONAB expression was greater in the in the
lung tissues of the BPD model group as compared with that the
control group. However, immunofluorescence double staining clearly
demonstrated that ZONAB expression was decreased in AEC II of the
model group. By isolating and purifying AEC II from neonatal rats
and quantifying the protein and gene expression levels of ZONAB
after subjecting the cells to hyperoxia in vitro, it was
revealed that the ZONAB expression was reduced in the BPD model
group, as compared with that the control group, indicating that
ZONAB expression was unequally distributed in dozens of cells in
the lung tissues of the hyperoxia-induced in vivo model of
BPD. ZONAB expression may be increased in other cell types,
including bronchial epithelial cells, as demonstrated by the
immunofluorescence staining results. Other sources, such as
fibroblasts, may have also contributed to the increased ZONAB
expression in the in vivo model and are probably involved in
the epithelial-mesenchymal transition (10,38), although this speculation must be
confirmed in future studies. In addition, the effect of hyperoxia
on endogenous and exogenous ZONAB, as well as the mechanism of how
hyperoxia causes a decrease in ZONAB in AEC II are questions
requiring further exploration.
The regulatory effects of ZONAB on
transdifferentiation and proliferation of AECs was further verified
by manipulating ZONAB expression through gene transfection of
primary AEC II. The results indicated that when ZONAB was
overexpressed, the protein and mRNA expression levels of the AEC I
marker AQP5 were downregulated, while those of the AEC II marker
SP-C were upregulated, and the expression levels of PCNA and cyclin
D1 were increased. The results of the immunofluorescence double
staining also demonstrated that the transdifferentiation of AECs
was decreased in the plasmid-transfected group, although a single
channel and line scan for the intensity may be able to better
demonstrate this, which should be included in a future study. The
present results confirmed that overexpression of ZONAB inhibited
the transdifferentiation and promoted the proliferation of AECs. In
addition, the siZONAB transfection experiment confirmed that
knockdown of ZONAB expression promoted the transdifferentiation and
inhibited the proliferation of AECs. In the AECs, ZONAB inhibited
transdifferentiation and promoted proliferation, which is
consistent with the function of ZONAB in renal tubular epithelium
(22), retina (34,36,37), cornea (39), intestine (40), liver (35), mammary gland (18) and brain (41,42), which is likely to be similar in
lung tissue.
Given that ZONAB has a role in the regulation of AEC
transdifferentiation and proliferation, and that excessive
dysregulation of transdifferentiation and proliferation was
observed in the BPD model (10),
the present study investigated whether this dysregulation was
mediated by ZONAB in the hyperoxia-induced BPD model. Since
hyperoxia caused a decrease in ZONAB expression in AEC II, plasmids
overexpressing ZONAB were transfected into AEC II, and the cells
were then cultured under hyperoxic or normoxic conditions to
further compare the cell differentiation and proliferation levels
between the groups. The results indicated that ZONAB overexpression
counteracted the effects of hyperoxia and partially attenuated the
hyperoxia-induced dysregulation of AEC transdifferentiation and
proliferation, but did not completely offset the effect of
hyperoxia. Although the effects of ZONAB on transdifferentiation
and proliferation of AECs are not hyperoxia-specific, it does take
part in the hyperoxia-induced alveolar epithelial injury. These
results indicated that the transdifferentiation and proliferation
of AECs were partially dependent on ZONAB during the course of
hyperoxia injury, although other factors may also be involved.
Numerous factors participate in the regulation of AEC
transdifferentiation and proliferation, and clinical studies on BPD
patients and animal models reported abnormal expression of certain
cytokines, including insulin-like growth factor I (43–45), transforming growth factor-β1
(46–48), keratinocyte growth factor
(48,49) and bone morphogenetic protein
(46,50), which act to accelerate or inhibit
AEC transdifferentiation (45,51–53). Therefore, it is important to
identify the factors involved in the mediation of AEC
transdifferentiation and proliferation in the process of
hyperoxia-induced BPD, and to investigate how these factors
interact with one another and regulate cell transdifferentiation
and proliferation.
In conclusion, the in vivo and in
vitro experiments of the present study demonstrated that ZONAB
expression was reduced in AEC II of the hyperoxia-induced BPD
model. Transfection of ZONAB plasmids or siRNA confirmed that ZONAB
inhibited the transdifferentiation and promoted the proliferation
of AECs. Transfection of plasmids overexpressing ZONAB in AEC II
ameliorated the hyperoxia-induced cell transdifferentiation and
proliferation abnormalities in vitro, but did not completely
offset the impact of hyperoxia, indicating that ZONAB is involved
in mediating hyperoxia-induced BPD development of lung epithelial
disorders. These results may provide a novel pathway to explore as
a mechanism of pulmonary epithelial damage in BPD, while the
specific regulatory mechanisms of ZONAB on AEC transdifferentiation
and proliferation remain to be elucidated.
Acknowledgments
This study was funded by the Natural Science
Foundation of China (grant nos. 81601331 and 81471489).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Islam JY, Keller RL, Aschner JL, Hartert
TV and Moore PE: Understanding the short- and long-term respiratory
outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir
Crit Care Med. 192:134–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H, St Julien KR, Stevenson DK,
Hoffmann TJ, Witte JS, Lazzeroni LC, Krasnow MA, Quaintance CC,
Oehlert JW, Jelliffe-Pawlowski LL, et al: A genome-wide association
study (GWAS) for bronchopulmonary dysplasia. Pediatrics.
132:290–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stoll BJ, Hansen NI, Bell EF, Shankaran S,
Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et
al: Neonatal outcomes of extremely preterm infants from the NICHD
Neonatal Research Network. Pediatrics. 126:443–456. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhandari A and McGrath-Morrow S: Long-term
pulmonary outcomes of patients with bronchopulmonary dysplasia.
Semin Perinatol. 37:132–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson PJ and Doyle LW:
Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin
Perinatol. 30:227–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jobe AH and Bancalari E: Bronchopulmonary
dysplasia. Am J Respir Crit Care Med. 163:1723–1729. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alvira CM: Aberrant pulmonary vascular
growth and remodeling in bronchopulmonary dysplasia. Front Med
(Lausanne). 3:212016.
|
|
8
|
Yee M, Buczynski BW and O'Reilly MA:
Neonatal hyperoxia stimulates the expansion of alveolar epithelial
type II cells. Am J Respir Cell Mol Biol. 50:757–766. 2014.
View Article : Google Scholar :
|
|
9
|
Thebaud B and Abman SH: Bronchopulmonary
dysplasia: Where have all the vessels gone? Roles of angiogenic
growth factors in chronic lung disease. Am J Respir Crit Care Med.
175:978–985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou A, Fu J, Yang H, Zhu Y, Pan Y, Xu S
and Xue X: Hyperoxia stimulates the transdifferentiation of type II
alveolar epithelial cells in newborn rats. Am J Physiol Lung Cell
Mol Physiol. 308:L861–L872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang H, Fu J, Xue X, Yao L, Qiao L, Hou A,
Jin L and Xing Y: Epithelial-mesenchymal transitions in
bronchopulmonary dysplasia of newborn rats. Pediatr Pulmonol.
49:1112–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balda MS and Matter K: The tight junction
protein ZO-1 and an interacting transcription factor regulate
ErbB-2 expression. EMBO J. 19:2024–2033. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balda MS, Garrett MD and Matter K: The
ZO-1-associated Y-box factor ZONAB regulates epithelial cell
proliferation and cell density. J Cell Biol. 160:423–432. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matter K and Balda MS: Epithelial tight
junctions, gene expression and nucleo-junctional interplay. J Cell
Sci. 120:1505–1511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balda MS and Matter K: Tight junctions and
the regulation of gene expression. Biochim Biophys Acta.
1788:761–767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sherr CJ, Beach D and Shapiro GI:
Targeting CDK4 and CDK6: From discovery to therapy. Cancer Discov.
6:353–367. 2016. View Article : Google Scholar :
|
|
17
|
Xing Y, Fu J, Yang H, Yao L, Qiao L, Du Y
and Xue X: MicroRNA expression profiles and target prediction in
neonatal Wistar rat lungs during the development of
bronchopulmonary dysplasia. Int J Mol Med. 36:1253–1263. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sourisseau T, Georgiadis A, Tsapara A, Ali
RR, Pestell R, Matter K and Balda MS: Regulation of PCNA and cyclin
D1 expression and epithelial morphogenesis by the ZO-1-regulated
transcription factor ZONAB/DbpA. Mol Cell Biol. 26:2387–2398. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Darzynkiewicz Z, Zhao H, Zhang S, Lee MY,
Lee EY and Zhang Z: Initiation and termination of DNA replication
during S phase in relation to cyclins D1, E and A, p21WAF1, Cdt1
and the p12 subunit of DNA polymerase delta revealed in individual
cells by cytometry. Oncotarget. 6:11735–11750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frankel P, Aronheim A, Kavanagh E, Balda
MS, Matter K, Bunney TD and Marshall CJ: RalA interacts with ZONAB
in a cell density-dependent manner and regulates its
transcriptional activity. EMBO J. 24:54–62. 2005. View Article : Google Scholar :
|
|
21
|
Nielsen R, Christensen EI and Birn H:
Megalin and cubilin in proximal tubule protein reabsorption: From
experimental models to human disease. Kidney Int. 89:58–67. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lima WR, Parreira KS, Devuyst O, Caplanusi
A, N'kuli F, Marien B, Van Der Smissen P, Alves PM, Verroust P,
Christensen EI, et al: ZONAB promotes proliferation and represses
differentiation of proximal tubule epithelial cells. J Am Soc
Nephrol. 21:478–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Mukherjee N, Cardenas E, Bedolla R and
Ghosh R: SETD6 regulates NF-kB signaling in urothelial cell
survival: Implications for bladder cancer. Oncotarget.
8:15114–15125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sureshbabu A, Syed M, Das P, Janér C,
Pryhuber G, Rahman A, Andersson S, Homer RJ and Bhandari V:
Inhibition of regulatory-associated protein of mechanistic target
of rapamycin prevents hyperoxia-induced lung injury by enhancing
autophagy and reducing apoptosis in neonatal mice. Am J Respir Cell
Mol Biol. 55:722–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roper JM, Mazzatti DJ, Watkins RH,
Maniscalco WM, Keng PC and O'Reilly MA: In vivo exposure to
hyperoxia induces DNA damage in a population of alveolar type II
epithelial cells. Am J Physiol Lung Cell Mol Physiol.
286:L1045–L1054. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Reilly MA, Staversky RJ, Finkelstein JN
and Keng PC: Activation of the G2 cell cycle checkpoint enhances
survival of epithelial cells exposed to hyperoxia. Am J Physiol
Lung Cell Mol Physiol. 284:L368–L375. 2003. View Article : Google Scholar
|
|
28
|
Chen Y, Chang L, Li W, Rong Z, Liu W, Shan
R and Pan R: Thioredoxin protects fetal type II epithelial cells
from hyperoxia-induced injury. Pediatr Pulmonol. 45:1192–1200.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Crapo JD, Barry BE, Foscue HA and
Shelburne J: Structural and biochemical changes in rat lungs
occurring during exposures to lethal and adaptive doses of oxygen.
Am Rev Respir Dis. 122:123–143. 1980.PubMed/NCBI
|
|
30
|
Barkauskas CE, Cronce MJ, Rackley CR,
Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW and Hogan BL:
Type 2 alveolar cells are stem cells in adult lung. J Clin Invest.
123:3025–3036. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu HY, Shao GB, Li WB and Wang H: Effects
of hyperoxia on transdifferentiation of primary cultured typeII
alveolar epithelial cells from premature rats. In Vitro Cell Dev
Biol Anim. 47:64–72. 2011. View Article : Google Scholar
|
|
32
|
Liebler JM, Marconett CN, Juul N, Wang H,
Liu Y, Flodby P, Laird-Offringa IA, Minoo P and Zhou B:
Combinations of differentiation markers distinguish subpopulations
of alveolar epithelial cells in adult lung. Am J Physiol Lung Cell
Mol Physiol. 310:L114–L120. 2016. View Article : Google Scholar :
|
|
33
|
You K, Xu X, Fu J, Xu S, Yue X, Yu Z and
Xue X: Hyperoxia disrupts pulmonary epithelial barrier in newborn
rats via the deterioration of occludin and ZO-1. Respir Res.
13:362012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Georgiadis A, Tschernutter M, Bainbridge
JW, Balaggan KS, Mowat F, West EL, Munro PM, Thrasher AJ, Matter K,
Balda MS and Ali RR: The tight junction associated signalling
proteins ZO-1 and ZONAB regulate retinal pigment epithelium
homeostasis in mice. PLoS One. 5:e157302010. View Article : Google Scholar
|
|
35
|
Arakawa Y, Kajino K, Kano S, Tobita H,
Hayashi J, Yasen M, Moriyama M, Arakawa Y and Hino O: Transcription
of dbpA, a Y box binding protein, is positively regulated by E2F1:
Implications in hepatocarcinogenesis. Biochem Biophys Res Commun.
322:297–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jayagopal A, Yang JL, Haselton FR and
Chang MS: Tight junction-associated signaling pathways modulate
cell proliferation in uveal melanoma. Invest Ophthalmol Vis Sci.
52:588–593. 2011. View Article : Google Scholar :
|
|
37
|
Tamiya S and Kaplan HJ: Role of
epithelial-mesenchymal transition in proliferative
vitreoretinopathy. Exp Eye Res. 142:26–31. 2016. View Article : Google Scholar
|
|
38
|
Zhu Y, Fu J, Yang H, Pan Y, Yao L and Xue
X: Hyperoxia-induced methylation decreases RUNX3 in a newborn rat
model of bronchopulmonary dysplasia. Respir Res. 16:752015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Russ PK, Pino CJ, Williams CS, Bader DM,
Haselton FR and Chang MS: Bves modulates tight junction associated
signaling. PLoS One. 6:e145632011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nie M, Balda MS and Matter K: Stress- and
Rho-activated ZO-1-associated nucleic acid binding protein binding
to p21 mRNA mediates stabilization, translation, and cell survival.
Proc Natl Acad Sci USA. 109:10897–10902. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Penes MC, Li X and Nagy JI: Expression of
zonula occludens-1 (ZO-1) and the transcription factor
ZO-1-associated nucleic acid-binding protein (ZONAB)-MsY3 in glial
cells and colocalization at oligodendrocyte and astrocyte gap
junctions in mouse brain. Eur J Neurosci. 22:404–418. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu LB, Liu XB, Ma J, Liu YH, Li ZQ, Ma T,
Zhao XH, Xi Z and Xue YX: Bradykinin increased the permeability of
BTB via NOS/NO/ZONAB-mediating down-regulation of claudin-5 and
occludin. Biochem Biophys Res Commun. 464:118–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Löfqvist C, Hellgren G, Niklasson A,
Engström E, Ley D and Hansen-Pupp I; WINROP Consortium: Low
postnatal serum IGF-I levels are associated with bronchopulmonary
dysplasia (BPD). Acta Paediatr. 101:1211–1216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hellström A, Ley D, Hansen-Pupp I,
Hallberg B, Ramenghi LA, Löfqvist C, Smith LE and Hård AL: Role of
insulinlike growth factor 1 in fetal development and in the early
postnatal life of premature infants. Am J Perinatol. 33:1067–1071.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ghosh MC, Gorantla V, Makena PS, Luellen
C, Sinclair SE, Schwingshackl A and Waters CM: Insulin-like growth
factor-I stimulates differentiation of ATII cells to ATI-like cells
through activation of Wnt5a. Am J Physiol Lung Cell Mol Physiol.
305:L222–L228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alejandre-Alcázar MA, Kwapiszewska G,
Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B,
Köbrich S, Hesse M, et al: Hyperoxia modulates TGF-beta/BMP
signaling in a mouse model of bronchopulmonary dysplasia. Am J
Physiol Lung Cell Mol Physiol. 292:L537–L549. 2007. View Article : Google Scholar
|
|
47
|
Luan Y, Zhang L, Chao S, Liu X, Li K, Wang
Y and Zhang Z: Mesenchymal stem cells in combination with
erythropoietin repair hyperoxia-induced alveoli dysplasia injury in
neonatal mice via inhibition of TGF-β1 signaling. Oncotarget.
7:47082–47094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Been JV, Debeer A, van Iwaarden JF,
Kloosterboer N, Passos VL, Naulaers G and Zimmermann LJ: Early
alterations of growth factor patterns in bronchoalveolar lavage
fluid from preterm infants developing bronchopulmonary dysplasia.
Pediatr Res. 67:83–89. 2010. View Article : Google Scholar
|
|
49
|
Franco-Montoya ML, Bourbon JR, Durrmeyer
X, Lorotte S, Jarreau PH and Delacourt C: Pulmonary effects of
keratinocyte growth factor in newborn rats exposed to hyperoxia. Am
J Physiol Lung Cell Mol Physiol. 297:L965–L976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hines EA and Sun X: Tissue crosstalk in
lung development. J Cell Biochem. 115:1469–1477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bhaskaran M, Kolliputi N, Wang Y, Gou D,
Chintagari NR and Liu L: Trans-differentiation of alveolar
epithelial type II cells to type I cells involves autocrine
signaling by transforming growth factor beta 1 through the Smad
pathway. J Biol Chem. 282:3968–3976. 2007. View Article : Google Scholar
|
|
52
|
Zhao L, Yee M and O'Reilly MA:
Transdifferentiation of alveolar epithelial type II to type I cells
is controlled by opposing TGF-β and BMP signaling. Am J Physiol
Lung Cell Mol Physiol. 305:L409–L418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qiao R, Yan W, Clavijo C, Mehrian-Shai R,
Zhong Q, Kim KJ, Ann D, Crandall ED and Borok Z: Effects of KGF on
alveolar epithelial cell transdifferentiation are mediated by JNK
signaling. Am J Respir Cell Mol Biol. 38:239–246. 2008. View Article : Google Scholar
|