Introduction
Mesenchymal stem cells (MSCs) are multipotent stem
cells derived from the bone marrow (BM), which can differentiate
into osteoblasts, chondrocytes and adipocytes (1–3).
Therefore, MSCs are considered as the most important seed cells for
tissue engineering. Maintenance of the self-renewing and
multipotent properties of MSCs is regulated by molecular signals
from the specific microenvironment where they reside, which is also
known as the stem-cell niche (4–6).
Our previous study reported that MSCs exhibit high
levels of interaction with cluster of differentiation
(CD)31+ endothelial progenitor cells (EPCs) in the BM
microenvironment, thus forming cell clusters in the sinus of the BM
cavity (7). Therefore, it was
hypothesized that EPCs are a significant component of stem-cell
niches, which may promote the self-renewing and multipotent
properties of MSCs. EPCs (8–10)
reside in niches located in the BM and assist in homeostasis by
maintaining vascular function. In addition, combination of EPCs
with MSCs has been reported to enhance neovascularization and bone
regeneration (11–14). These findings provided a novel
basis for clinical bone injury treatment and vascular bone
regeneration; however, what mediates the adhesion between MSCs and
EPCs, and the underlying molecular mechanism, remain unclear.
Intercellular adhesion molecule-1 (ICAM-1, also
known as CD54) is a member of the immunoglobulin supergene family,
and functions in cell-cell and cell-matrix adhesive interactions.
Compared with other adhesion molecules, ICAM-1 is expressed in
hematopoietic and nonhematopoietic cells, and can mediate adhesive
interactions (15–18). Interleukin (IL)-1β is an important
inflammatory mediator that regulates the expression of ICAM-1
(19–21), and serves as a hallmark for bone
damage (22–25) and vascular injury (26,27). Therefore, investigating the
effects of IL-1β on ICAM-1, and the effects of ICAM-1 on cohesion
between MSCs and EPCs may contribute to further understanding of
stem-cell niche adhesion. Nevertheless, adhesion is mediated by
numerous intracellular signaling pathways. Previous studies have
suggested that the p38 mitogen-activated protein kinase (MAPK)
pathway has a critical role in the expression of ICAM-1, and IL-1β
has been reported to activate the p38 MAPK pathway (28–31). Therefore, the present study
hypothesized that the p38 MAPK signaling pathway may be important
in the crosstalk between MSCs and EPCs in the stem-cell niche.
Materials and methods
Animal preparation and cell culture
A total of 24 male C57BL/6J (wild-type) mice (age,
6–8 weeks; weight, 28–35 g) were used to obtain cells for use in
the present study. All mice were purchased from Xinjiang Medical
University (Ürümqi, China; certificate no. SYXK [Xin] 2010-0001).
The mice were maintained in the Animal Facility of the Shihezi
University (Shihezi, China) under controlled conditions
(temperature, 20°C; humidity, 55±5%; 12-h light/dark cycles), with
free access to food and water and were used as a cell source. All
mice used in the present study were matched for age and gender.
Both isolated cell types underwent similar techniques with regards
to culture and harvest; however, the media and materials used for
culture were different between the cell types. Third generation
cells were used for all experiments. All experimental protocols
used in the present study were reviewed and approved by the Animal
Care and Use Committee of Shihezi University.
Isolation and culture of MSCs and EPCs
from murine BM
MSCs and EPCs were isolated from murine BM obtained
from the tibia and femur according to our previous study (7). Briefly, mice (C57BL/6J; age, 6–8
weeks) were euthanized by cervical dislocation and BM cells were
collected. The cells were cultured in low glucose Dulbecco's
modified Eagle's medium (LG-DMEM) supplemented with 10%
lot-selected fetal bovine serum (FBS) (both from Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 2 mM glutamine, 100
U/ml penicillin and 100 µg/ml streptomycin (all from
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C in a
humidified incubator containing 5% CO2 for 48 h of
adhesion. Subsequently, non-adherent cells were collected and
cultured in EBM-2 medium (Lonza Group Ltd., Basel, Switzerland)
supplemented with 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified incubator containing 5%
CO2 for 48 h. In particular, the EPCs were initially
plated in a 60 mm fibronectin (BD Biosciences, Franklin Lakes, NJ,
USA) coated cell culture dish to promote cell adhesion and growth.
Adherent cells were maintained and the non-adherent cells were
removed with medium replacement every 48 h to form uniform cells.
The adherent cells were then retrieved by trypsin digestion
supplemented with 0.25% EDTA (HyClone; GE Healthcare, Logan, UT,
USA) and were incubated at 37°C for 30 min with Sca-1
[-phycoerythrin (PE); cat. no. 108107; 0.2 mg/ml; 1:50], CD29
[-fluorescein isothiocyanate (FITC); cat. no. 102205; 0.2 mg/ml;
1:50], CD45 [-peridinin chlorophyll protein complex (PerCP); cat.
no. 202220; 0.2 mg/ml; 1:50] and CD11b [-allophycocyanin (APC);
cat. no. 201809; 0.2 mg/ml; 1:50] mouse monoclonal antibodies
(BioLegend, Inc., San Diego, CA, USA). The cells underwent flow
cytometry and were analyzed using FACSDIVA software version 6.1.3
(both from BD Biosciences). Sorted Sca-1+
CD29+ CD11b− CD45− cells (MSCs)
were obtained for further culture in the LG-DMEM supplemented with
10% lot-selected FBS (both from Gibco; Thermo Fisher Scientific,
Inc.), 2 mM glutamine, 100 U/ml penicillin and 100 µg/ml
streptomycin (all from Sigma-Aldrich; Merck KGaA) at 37°C in a
humidified incubator containing 5% CO2 for enrichment.
According to the aforementioned technique, sorted CD133+
(-FITC; eBioscience, San Diego, CA, USA) CD31+ (-PE/Cy7;
BioLegend) CD144+ (-PE, BD Biosciences) and
CD11b− (-APC; BioLegend) cells (EPCs) were obtained for
further culture in EBM-2 medium (Lonza Group Ltd) for enrichment.
All of the EPC cells were plated in a 60 mm cell culture dish
coated with fibronectin to promote cell adhesion and growth.
Coculture of MSCs and EPCs
MSCs and EPCs were cocultured in cell number ratios
of 1:1 in 6-well plates or 60 mm cell culture dishes. Cells were
cultured in LG-DMEM without FBS at 37°C in a humidified incubator
containing 5% CO2 for 24 h. Inoculation density was in
accordance with experimental requirements and cells were then
prepared for subsequent experiments.
Determination of IL-1β expression by
ELISA
All of the groups: MSCs, EPCs, MSCs + EPCs,
EPC-CM-MSCs and MSC-CM-EPCs, were seeded at 2×105
cells/well in 6-well plates alongside 2 ml serum- and factor-free
medium for 24 h. We used MSC-CM to stimulate EPC cells and
collected the supernatant to detect the IL-1β expression by ELISA
kit. For the same reason, we named the EPC-CM to stimulate the MSC
cells group as EPC-CM-MSC. We aim to collect the IL-1β expression
in each group for comparison and analysis. Subsequently,
supernatants were collected and centrifuged (4°C; 5,000 × g; 10
min) in order to measure IL-1β expression using an IL-1β ELISA kit
(cat. no. SEA563Mu; Uscn Life Sciences, Inc., Wuhan, China)
according to the manufacturer's protocol.
Detection of ICAM-1 by cell
immunofluorescence
Cells were plated onto coverslips (2×104
cells/sample), which were cultured in 6 mm dishes with 2 ml
corresponding medium (LG-DMEM supplemented with 10% lot-selected
FBS, both from Gibco; Thermo Fisher Scientific, Inc.) for 24 h.
Subsequently, the medium was replaced with serum- and factor-free
medium supplemented with IL-1β (25 µg/ml) or p38 MAPK
inhibitor SB203580 (20 µmol/ml), and cells were incubated
for a further 24 h. After culturing for 24 h, the coverslips were
rinsed in PBS three times and fixed with 4% paraformaldehyde for 20
min. Subsequently, the cells were blocked in 5% bovine serum
albumin (BSA) for 30 min at room temperature and incubated
overnight with anti-mouse ICAM-1 monoclonal antibodies (ab171123,
1:100) or isotype control antibody (ab81032, 1:100) (both from
Abcam, Cambridge, MA, USA) at 4°C. After rinsing in PBS three
times, the cells were incubated with FITC-conjugated goat anti
mouse immunoglobulin (Ig) G (cat. no. ZB2305; 1:100; OriGene
Technologies, Inc., Beijing, China) in the dark for a further 1 h.
After washing, the nuclei were stained with propidium iodide (PI,
10 µg/ml; cat. no. P4170; Sigma-Aldrich; Merck KGaA)
solution and washed a further three times with PBS. Subsequently,
70% glycerol was used to mount the coverslips onto slides and
expression was detected using a confocal laser scanning microscope
(LSM 510 META; Zeiss GmbH, Jena, Germany). The experiments were
performed and repeated >3 times.
Western blotting to detect ICAM-1 and p38
MAPK expression
Following pretreatment, the cells (2×106
cells/sample) were washed three times with ice-cold PBS and protein
samples were extracted using radioimmunoprecipitation assay buffer
(500 µl) with phenylmethylsulfonyl fluoride (5 µl)
(both from Thermo Fisher Scientific, Inc.). Protein concentration
was determined using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Inc.). Subsequently, protein samples were
treated with 5X loading buffer (100 µl; Thermo Fisher
Scientific, Inc.) and heated for 8 min at 100°C. Protein samples
(40 µg) were then separated by 10% SDS-PAGE and were
transferred onto a polyvinylidene difluoride membrane. The membrane
was blocked with 5% skim milk or 5% BSA, and incubated overnight
with anti-ICAM-1 (ab171123, 1:500; Abcam), anti-p38 MAPK (cat. no.
#8690; 1:1,000) and anti-p-p38 MAPK (cat. no. #4511; 1:1,000; both
from Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C.
After washing three times with Tris-buffered saline containing 0.1%
Tween-20, the membrane was incubated with goat anti-mouse
(1:10,000; cat. no. ZB2305) or goat anti-rabbit (1:10,000; cat. no.
ZB2301) IgG horseradish peroxidase-conjugated antibodies (OriGene
Technologies, Inc.) at room temperature for 2 h and was detected by
enhanced chemiluminescence (34094; Thermo Fisher Scientific, Inc.)
for 12 h at 4°C. GAPDH (1:1,000; 12 h, 4°C; cat. no. #51332; Cell
Signaling Technology, Inc.) was used to normalize the relative
protein expression levels. The results and integrated optical
density (IOD) values were analyzed by Gel-Pro Analyzer 4.0 (Media
Cybernetics, Inc., Rockville, MD, USA). Experiments were performed
independently and were repeated three times.
Labeling of MSCs with DAPI and EPCs with
MitoTracker red
MSCs were washed three times with PBS and incubated
with DAPI (2 µl/ml; Sigma-Aldrich; Merck KGaA) for 30 min at
37°C. Subsequently, cells were washed three times with PBS and were
detected using a fluorescence microscope. The same methods were
adopted to label EPCs with MitoTracker red (200 nM/ml; Thermo
Fisher Scientific, Inc.).
MSCs and EPCs adhesion assay
Matrigel (BD Biosciences) was incubated at 4°C
overnight to melt into liquid. Subsequently, a 24-well plate was
coated with Matrigel (200 µl/well) on ice and was incubated
at 37°C for 30 min in a humidified incubator containing 5%
CO2 to allow the Matrigel to solidify. MSCs-DAPI
(5×104 cells/well) and EPCs-MitoTrack red
(5×104 cells/well) were seeded into the pretreated
24-well plate with EPCs culture medium (1 ml; EBM-2 medium; cat.
no. CC-3156; Lonza Group Ltd.) supplemented with anti-ICAM-1
neutralizing antibody (ab171123, 1:50; Abcam), IL-1β (030447, 25
µg/ml; PeproTech, Inc., Rocky Hill, NJ, USA) or SB203580
(cat. no. S8307, lot. no. 104M4605v; 20 µmol/ml;
Sigma-Aldrich). After culturing for 6–8 h at 37°C in a humidified
incubator containing 5% CO2, the plates were randomly
imaged under a fluorescence microscope.
Statistics analysis
Data are expressed as the means ± standard
deviation. SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL,
USA) was used to analyze results. Statistical significance of group
data was assessed using one-way analysis of variance (ANOVA)
followed by SNK post hoc multiple comparisons test. P<0.05 was
considered to indicate a statistically significant difference.
Results
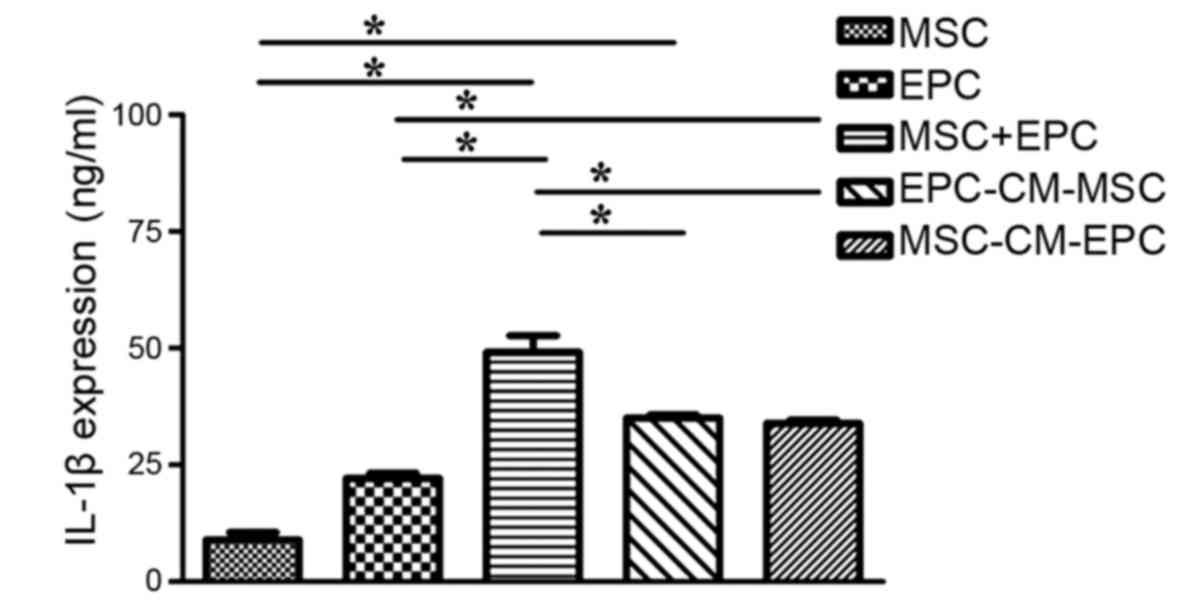
IL-1β expression in culture medium
To determine the expression levels of IL-1β in MSCs,
EPCs, MSCs + EPCs, EPC-CM-MSCs and MSC-CM-EPCs (Fig. 1), the groups were seeded into
6-well plates (2×105 cells/well) and treated with serum-
and factor-free medium for 24 h. The results indicated that both
MSCs and EPCs can secrete IL-1β, and in the MSCs + EPCs (49.13±6.21
ng/ml) group, IL-1β expression was higher than in the MSCs group
(8.96±2.70 ng/ml, P<0.05) and the EPCs group (22.14±1.83 ng/ml,
P<0.05). In addition, in the EPC-CM-MSCs group (35.02±1.19
ng/ml) and the MSC-CM-EPCs group (33.85±1.37 ng/ml) IL-1β
expression was higher compared with in the MSCs group (8.96±2.70
ng/ml, P<0.05) and the EPCs group (22.14±1.83 ng/ml, P<0.05),
but not compared with in the MSCs + EPCs group (49.13±6.21 ng/ml,
P>0.05).
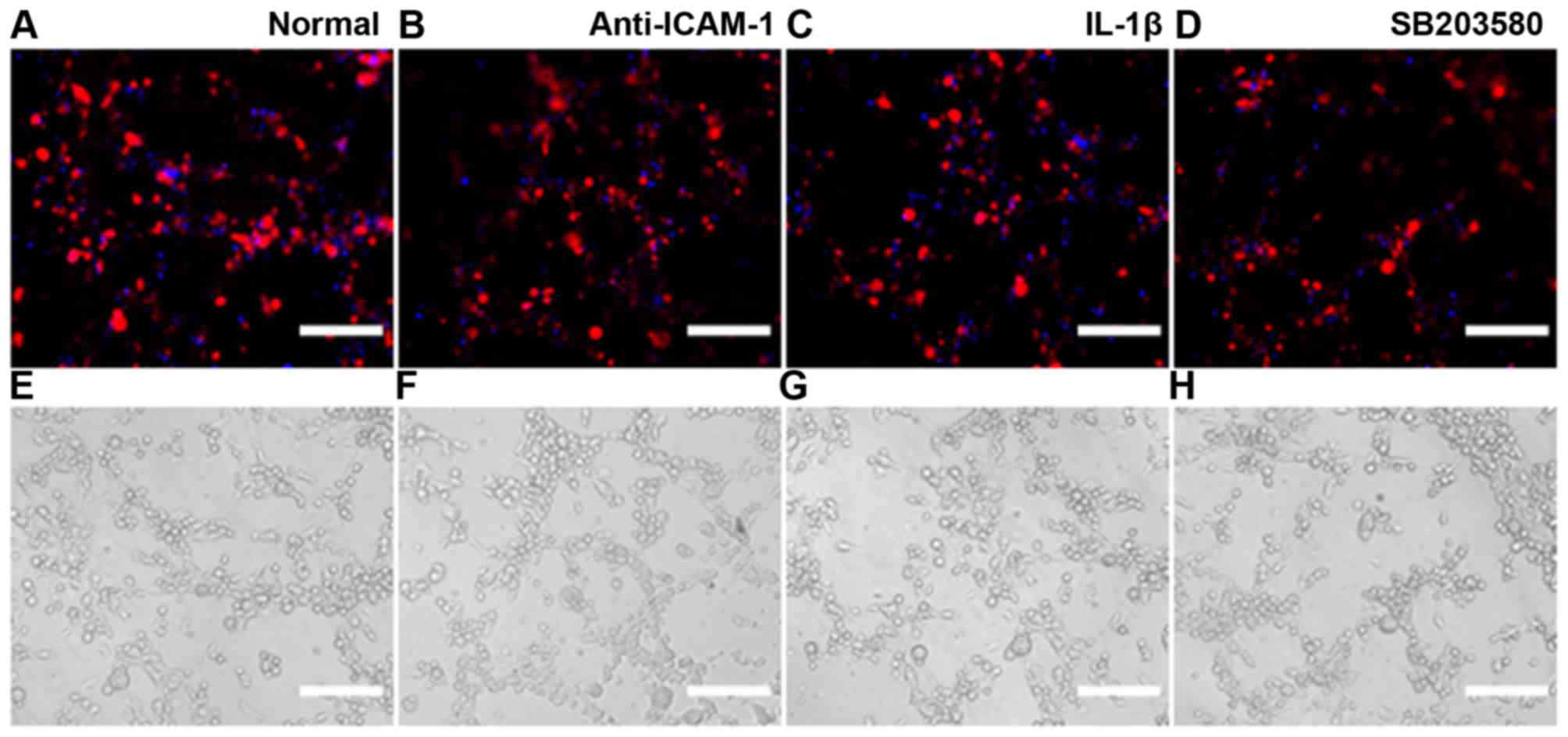
Immunofluorescence detection of ICAM-1
expression
Cells were seeded on coverslips (2×104
cells/sample) and were cultured in 6 mm dishes with 2 ml
corresponding medium for 24 h. Subsequently, medium was replaced
with serum- and factor-free medium supplemented with IL-1β (25
µg/ml) or p38 MAPK inhibitor SB203580 (20 µmol/ml)
for 24 h. ICAM-1 expression was then detected by immunofluorescence
(Fig. 2). The results indicated
that MSCs and EPCs are able to express low levels of ICAM-1;
however, in the MSCs + EPCs group the expression of ICAM-1 was
increased. All of the IL-1β-treated groups (Fig. 2B) exhibited increased ICAM-1
expression compared with in the normal (Fig. 2A) and SB203580 groups (Fig. 2C). ICAM-1 expression was lowest in
the SB203580 groups compared with in the untreated and
IL-1β-treated groups.
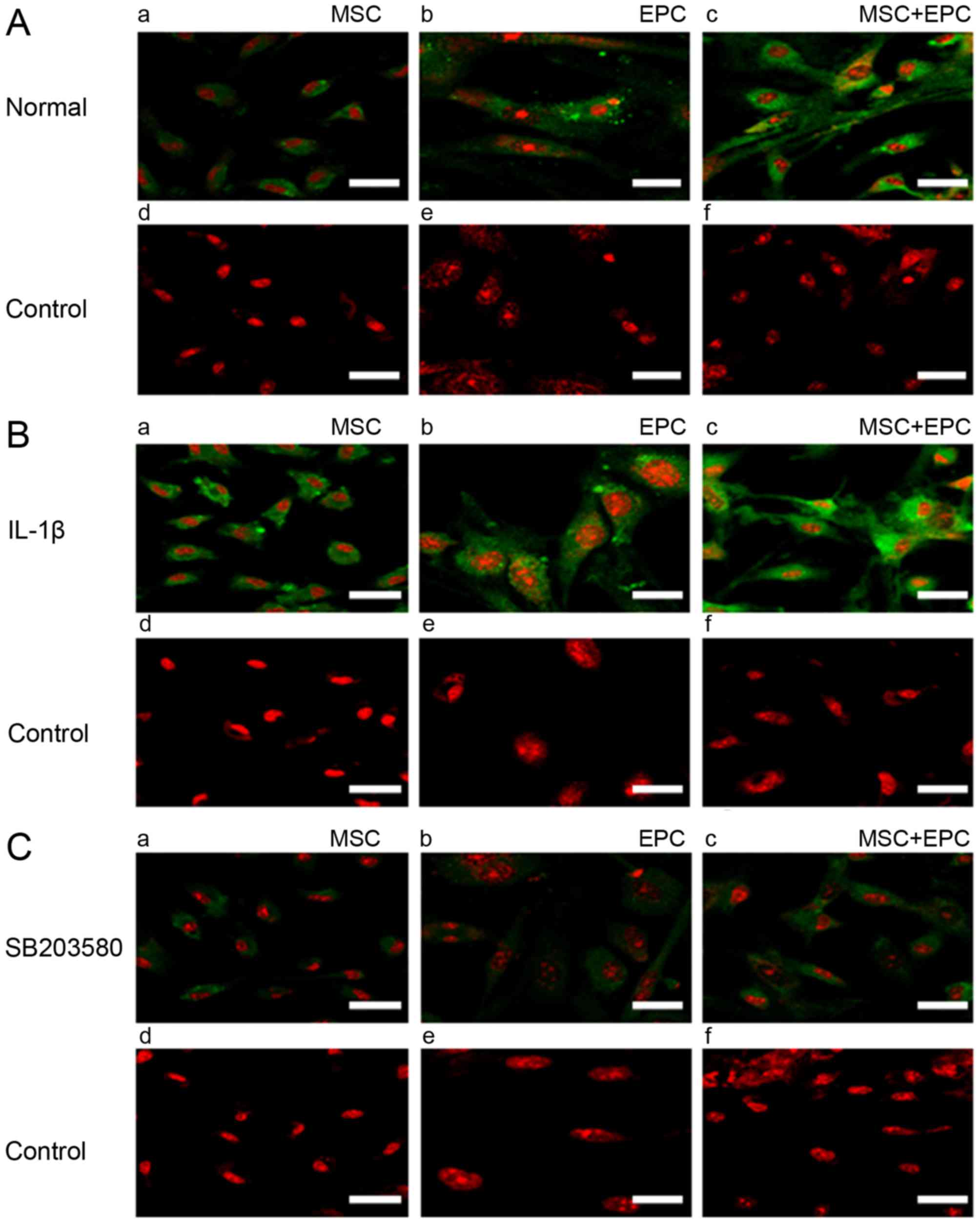 | Figure 2Immunofluorescence detection of
ICAM-1 expression. (A) (a–c) Expression of ICAM-1 (FITC) in MSCs,
EPCs and MSCs + EPCs normally cultured in vitro, as
determined by cell immunofluorescence under a laser confocal
microscope. (d–f) Mouse IgG control (FITC) staining. (B) (a–c)
Expression of ICAM-1 (FITC) in MSCs, EPCs and MSCs + EPCs treated
with IL-1β (25 µg/ml) in vitro. (d–f) Mouse IgG
control (FITC) staining. (C) (a–c) Expression of ICAM-1 (FITC) in
MSCs, EPCs and MSCs + EPCs treated with the p38 mitogen-activated
protein kinase inhibitor SB203580 (20 µmol/ml) in
vitro. (d–f) Mouse IgG control (FITC) staining. Scale bar, 5
µm. EPCs, endothelial progenitor cells; FITC, fluorescein
isothiocyanate; ICAM-1, intercellular adhesion molecule-1; IgG,
immunoglobulin G; IL-1β, interleukin-1β; MSCs, mesenchymal stem
cells. |
Western blotting to detect the expression
levels of ICAM-1 and p38 MAPK
The protein expression levels of ICAM-1 and p38 MAPK
were detected in MSCs, EPCs and MSCs + EPCs using western blotting
(Fig. 3A). The relative
ICAM-1/GAPDH IOD (Fig. 3B) and
p-p38 MAPK/total-p38 MAPK IOD values (Fig. 3C) were then determined for
statistical analysis. Normally cultured MSCs + EPCs (0.21±0.01,
P<0.05) exhibited increased ICAM-1 expression compared with in
the corresponding MSCs group (0.11±0.02, P<0.05) and EPCs group
(0.11±0.02, P<0.05); however, there was no significant
difference between the MSCs and EPCs groups. In the
IL-1β-stimulated MSCs + EPCs group (0.38±0.02, P<0.05) the
expression levels of ICAM-1 were increased compared with in the
corresponding MSCs group (0.23±0.02, P<0.05) and EPCs group
(0.25±0.02, P<0.05). The p38 MAPK inhibitor SB203580-treated
MSCs + EPCs group (0.10±0.02, P<0.05) exhibited increased ICAM-1
expression compared with in the corresponding MSCs group
(0.05±0.01, P<0.05) and EPCs group (0.05±0.01, P<0.05).
ICAM-1 expression was increased in the IL-1β-stimulated groups
(P<0.05), but was decreased in the SB203580-stimulated groups
(P<0.05) compared with in the normal groups. Normally cultured
MSCs + EPCs (0.52±0.02, P<0.05) exhibited increased p-p38 MAPK
expression compared with in the corresponding MSCs group
(0.35±0.02, P<0.05) and EPCs group (0.36±0.01, P<0.05);
however there was no significant difference between the MSCs and
EPCs groups. In the IL-1β-stimulated MSCs + EPCs group (0.74±0.06,
P<0.05), the expression levels of p-p38 MAPK were increased
compared with in the corresponding MSCs group (0.54±0.04,
P<0.05) and EPCs group (0.47±0.03, P<0.05). The p38 MAPK
inhibitor SB203580-stimulated MSCs + EPCs group (0.37±0.02,
P<0.05) exhibited increased p-p38 MAPK expression compared with
in the corresponding MSCs group (0.26±0.03, P<0.05) and EPCs
group (0.17±0.01, P<0.05). The expression levels of p-p38 MAPK
were increased following IL-1β treatment (P<0.05); however, they
were decreased following treatment with the p38 MAPK inhibitor
SB203580 (P<0.05), respectively. The expression levels of total
p38 MAPK remained constant in all groups following various
treatments.
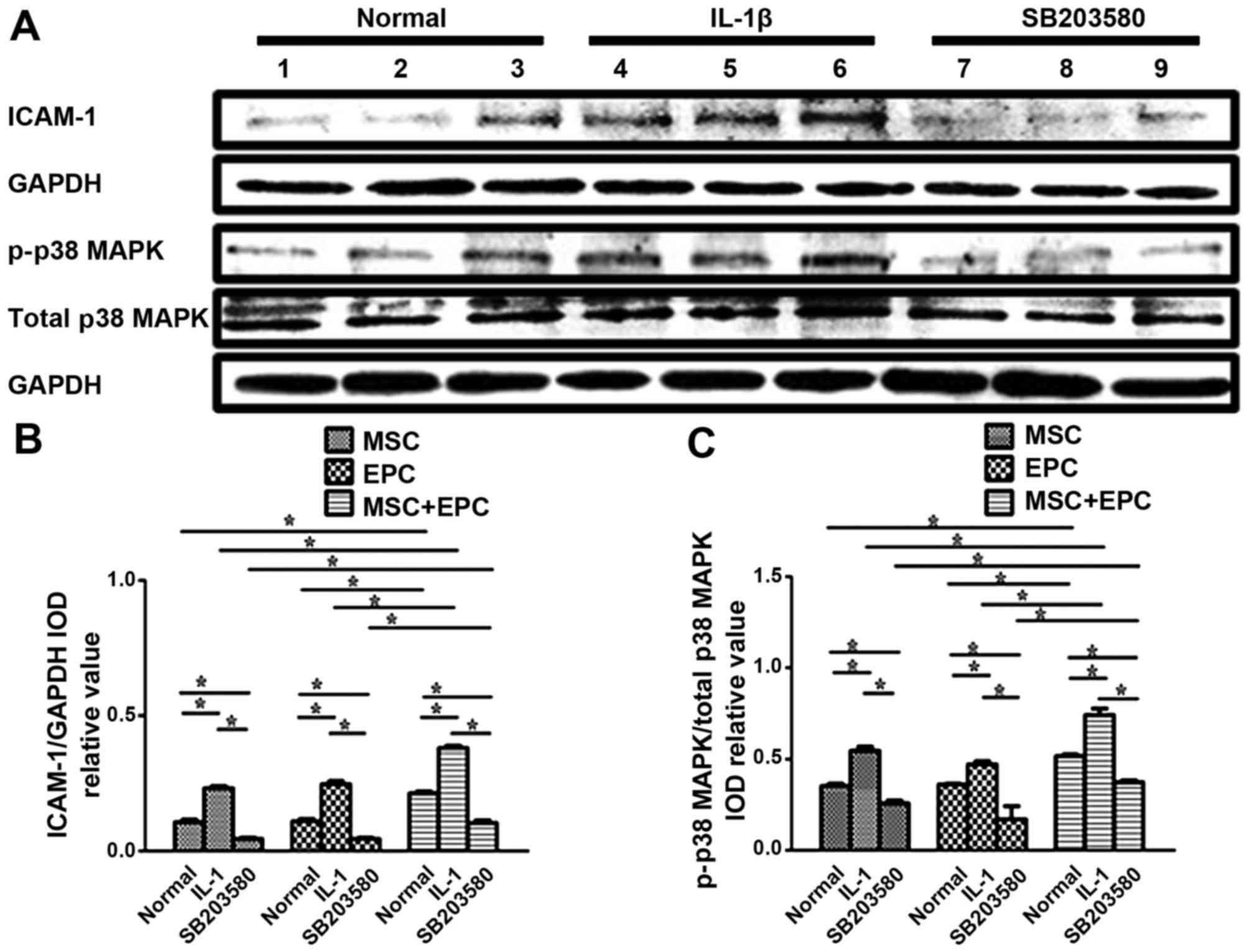 | Figure 3(A) Western blot analysis of ICAM-1,
GAPDH, p-p38 MAPK and total p38 MAPK in MSCs, EPCs and MSCs + EPCs,
with or without IL-1β (25 µg/ml) or p38 MAPK inhibitor
SB203580 (20 µmol/ml) treatment. Lanes 1–3, normally
cultured MSCs, EPCs and MSCs + EPCs groups, respectively; lanes
4–6, IL-1β (25 µg/ml)-stimulated MSCs, EPCs and MSCs + EPCs
groups, respectively; lanes 7–9, p38 MAPK inhibitor SB203580 (20
µmol/ml)-stimulated MSCs, EPCs and MSCs + EPCs groups,
respectively. (B) ICAM-1/GAPDH and (C) p-p38 MAPK/total-p38 MAPK
IOD relative values were determined. Data are presented as the
means ± standard deviation of three independent experiments.
*P<0.05. EPCs, endothelial progenitor cells; ICAM-1,
intercellular adhesion molecule-1; IL-1β, interleukin-1β; IOD,
integrated optical density; MAPK, mitogen-activated protein kinase;
MSCs, mesenchymal stem cells. |
MSCs and EPCs adhesion assay
MSCs were labeled with DAPI and EPCs were labeled
with MitoTracker red. MSCs-DAPI and EPCs-Mitotrack-red were
cocultured in Matrigel-coated 24-well plates (5×104
cells/well, 1:1) with EPCs culture medium supplemented with
anti-ICAM-1 neutralizing antibody (), IL-1β (25 µg/ml) or
p38 MAPK inhibitor SB203580 (20 µmol/ml). Subsequently, the
cells were cultured for 6–8 h at 37°C in a humidified incubator
containing 5% CO2. The results indicated that treatment
with anti-ICAM-1 neutralizing antibody or p38 MAPK inhibitor
SB203580 resulted in a decrease in adhesion between MSCs and EPCs;
however, supplementation with IL-1β markedly increased adhesion
(Fig. 4).
Discussion
The microenvironment surrounding stem cells, which
is also known as the stem-cell niche, is composed of adjacent
cells, extracellular matrix and adhesive molecules, and regulates
stem cell self-renewal and differentiation. The stem-cell niche
also aids regulation of cell fate, and is an interactive structural
unit that functions via the cross-talk of key signaling and
molecular factors (4–6). The functions of the stem-cell niche
are reliant on adhesion molecules, which anchor stem cells in the
niche and regulate communication with surrounding cells or various
molecules.
As previously reported, EPCs likely interact with
MSCs in the BM stem-cell niche (7); interactions between EPCs and MSCs
may enhance neovascularization and bone regeneration (11–14). Therefore, focusing on the adhesion
of MSCs and EPCs may provide information regarding clinical bone
injury and vascular bone regeneration. However, the underlying
mechanism that mediates the adhesion of MSCs and EPCs remains
unclear. ICAM-1 is an important adhesion molecule and a member of
the immunoglobulin supergene family, which participates in
cell-cell and cell-matrix adhesive interactions, including those
between tumor cells, endothelial cells, T cells, leukocytes and
vascular cells (32–34). Furthermore, adhesion may be
decreased by suppressing ICAM-1 expression. As an important
adhesion molecule, previous studies have suggested that ICAM-1 may
regulate IL-1β (19–21), tumor necrosis factor-α (35) and lipopolysaccharide (36). The present study focused on the
stimulatory effects of IL-1β on ICAM-1, since IL-1β is an important
inflammatory mediator, which not only affects nearly every cell
type and interacts with numerous cytokines and small mediator
molecules, but also participates in various physiological functions
and diseases, including clinical bone injury (22–25) and vascular injury (26,27).
The present study demonstrated that both MSCs and
EPCs could express low levels of ICAM-1; however, coculturing MSCs
with EPCs increased ICAM-1 expression, particularly following
treatment with IL-1β; however, treatment with the p38 MAPK
inhibitor SB203580 inhibited ICAM-1 expression. These results
indicated that IL-1β may activate the p38 MAPK pathway.
Furthermore, cocultured MSCs and EPCs were seeded in
Matrigel-coated plates; the results demonstrated that
supplementation with anti-ICAM-1 neutralizing antibody or SB203580
effectively inhibited adhesion between MSCs and EPCs, whereas
supplementation with IL-1β had the opposite effect. These results
highlighted the importance of ICAM-1 for the adhesion between MSCs
and EPCs, and this may be regulated by IL-1β through the p38 MAPK
signaling pathway. However, at present, it remains unknown as to
whether MSCs regulate EPCs function, or whether EPCs regulate MSCs
function; potential joint action may serve an important role in the
stem-cell niche. Previously, our laboratory indicated that EPCs
promoted osteogenic differentiation of MSCs in a paracrine manner
(37). Burlacu et al
reported that MSCs may promote angiogenesis of EPCs in a paracrine
manner (13). Furthermore, Ern
et al demonstrated that EPCs and MSCs can secrete osteogenic
or angiogenic factors to promote proliferation and differentiation
in a paracrine manner (38).
Therefore, the present study hypothesized that paracrine signaling
is critical for the regulation of the effects of MSCs and EPCs
coculture. The present study indicated that MSCs and EPCs could
secrete IL-1β, whereas coculturing MSCs with EPCs enhanced the
secretion of IL-1β. In addition, EPC-CM-MSCs and MSC-CM-EPCs groups
exhibited higher levels of IL-1β compared with MSC-CM and EPC-CM;
however they were not higher than in the MSCs + EPCs group. These
results provided convincing evidence to explain the increased
expression of ICAM-1 in cocultured MSCs and EPCs.
In conclusion, the present study demonstrated that
IL-1β may induce ICAM-1 expression, thus enhancing the cohesion
between MSCs and EPCs via the p38 MAPK signaling pathway. These
findings provide effective evidence to support and explain previous
findings regarding the adhesion of MSCs and EPCs. In addition, the
present study provided a theoretical basis for further stem-cell
niche transplantation to increase understanding regarding the
function of MSCs and for subsequent experimental research. However,
the factors that regulate secretion of IL-1β remain unclear; our
future studies aim to focus on this, and to conduct further in
vitro experiments regarding clinical application (clinical bone
injury treatment, vascular bone regeneration, tissue repair and
immune disorder therapy).
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81760570
and 31271458), the Science and Technology program of Xinjiang
Production and Construction Corps (grant no. 2014AB047), the
Scientific Research Foundation for the Returned Overseas Chinese
Scholars, Ministry of Human Resources and Social Security of the
People's Republic of China (grant no. RSLX201201) and the Shihezi
University Youth Science and Technology Research and Development
program, basis and application research project (grant no.
20142RKXYQ20).
Notes
[1] Competing
interests
The authors declare there is no competing
interest.
References
|
1
|
Friedenstein AJ, Chailakhjan RK and
Lalykina KS: The development of fibroblast colonies in monolayer
cultures of guinea-pig bone marrow and spleen cells. Cell Tissue
Kinet. 3:393–403. 1970.PubMed/NCBI
|
|
2
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moore KA and Elmendorf SC: Propagule vs.
niche limitation: Untangling the mechanisms behind plant species'
distributions. Ecol Lett. 9:797–804. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spradling A, Drummond-Barbosa D and Kai T:
Stem cells find their niche. Nature. 414:98–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moore KA and Lemischka IR: Stem cells and
their niches. Science. 311:1880–1885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Xian L, Lin Z, Yang C, Zhang M,
Feng W, Peng X, Chen X and Wu X: Endothelial progenitor cells as a
possible component of stem cell niche to promote self-renewal of
mesenchymal stem cells. Mol Cell Biochem. 397:235–243. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalka C, Masuda H, Takahashi T, Kalka-Moll
WM, Silver M, Kearney M, Li T, Isner JM and Asahara T:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA.
97:3422–3427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minamino T, Miyauchi H, Yoshida T, Ishida
Y, Yoshida H and Komuro I: Endothelial cell senescence in human
atherosclerosis: Role of telomere in endothelial dysfunction.
Circulation. 105:1541–1544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grellier M, Bordenave L and Amédée J:
Cell-to-cell communication between osteogenic and endothelial
lineages: Implications for tissue engineering. Trends Biotechnol.
27:562–571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu WL, Xiang Z, Huang FG, Gu ZP, Yu XX,
Cen SQ, Zhong G, Duan X and Liu M: Coculture of peripheral
blood-derived mesenchymal stem cells and endothelial progenitor
cells on strontium-doped calcium polyphosphate scaffolds to
generate vascularized engineered bone. Tissue Eng Part A.
21:948–959. 2015. View Article : Google Scholar
|
|
13
|
Burlacu A, Grigorescu G, Rosca AM, Preda
MB and Simionescu M: Factors secreted by mesenchymal stem cells and
endothelial progenitor cells have complementary effects on
angiogenesis in vitro. Stem Cells Dev. 22:643–653. 2013. View Article : Google Scholar :
|
|
14
|
Zigdon-Giladi H, Bick T, Lewinson D and
Machtei EE: Co-transplantation of endothelial progenitor cells and
mesenchymal stem cells promote neovascularization and bone
regeneration. Clin Implant Dent Relat Res. 17:353–359. 2015.
View Article : Google Scholar
|
|
15
|
Zuckerman LA, Pullen L and Miller J:
Functional consequences of costimulation by ICAM-1 on IL-2 gene
expression and T cell activation. J Immunol. 160:3259–3268.
1998.PubMed/NCBI
|
|
16
|
Agarwal SK and Brenner MB: Role of
adhesion molecules in synovial inflammation. Curr Opin Rheumatol.
18:268–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU,
Froio RM, Yang L, Jones T, Liu Y, Nusrat A, et al: Coordinated
redistribution of leukocyte LFA-1 and endothelial cell ICAM-1
accompany neutrophil transmigration. J Exp Med. 200:1571–1580.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sumagin R and Sarelius IH: Intercellular
adhesion molecule-1 enrichment near tricellular endothelial
junctions is preferentially associated with leukocyte
transmigration and signals for reorganization of these junctions to
accommodate leukocyte passage. J Immunol. 184:5242–5252. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang MC, Hung HP, Lin LD, Shyu YC, Wang
TM, Lin HJ, Chan CP, Huang CC and Jeng JH: Effect of interleukin-1β
on ICAM-1 expression of dental pulp cells: Role of PI3K/Akt,
MEK/ERK, and cyclooxygenase. Clin Oral Investig. 19:117–126. 2015.
View Article : Google Scholar
|
|
20
|
Shikama Y, Aki N, Hata A, Nishimura M,
Oyadomari S and Funaki M: Palmitate-stimulated monocytes induce
adhesion molecule expression in endothelial cells via IL-1
signaling pathway. J Cell Physiol. 230:732–742. 2015. View Article : Google Scholar
|
|
21
|
Yamagami H, Yamagami S, Inoki T, Amano S
and Miyata K: The effects of proinflammatory cytokines on
cytokine-chemokine gene expression profiles in the human corneal
endothelium. Invest Ophthalmol Vis Sci. 44:514–520. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Horton JE, Raisz LG, Simmons HA, Oppenheim
JJ and Mergenhagen SE: Bone resorbing activity in supernatant fluid
from cultured human peripheral blood leukocytes. Science.
177:793–795. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dewhirst FE, Stashenko PP, Mole JE and
Tsurumachi T: Purification and partial sequence of human
osteoclast-activating factor: Identity with interleukin 1 beta. J
Immunol. 135:2562–2568. 1985.PubMed/NCBI
|
|
24
|
Stashenko P, Dewhirst FE, Peros WJ, Kent
RL and Ago JM: Synergistic interactions between interleukin 1,
tumor necrosis factor, and lymphotoxin in bone resorption. J
Immunol. 138:1464–1468. 1987.PubMed/NCBI
|
|
25
|
Xiong Y, Donovan KA, Kline MP, Gornet MK,
Moon-Tasson LL, Lacy MQ, Dispenzieri A, Gertz MA, Greipp PR and
Lust JA: Identification of two groups of smoldering multiple
myeloma patients who are either high or low producers of
interleukin-1. J Interferon Cytokine Res. 26:83–95. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang R, Jiang F, Chen CS, Wang T, Feng J,
Tao T and Qin X: Serum levels of IL-1β, IL-6, TGF-β, and MMP-9 in
patients undergoing carotid artery stenting and regulation of MMP-9
in a new in vitro model of THP-1 cells activated by stenting.
Mediators Inflamm. 2015:9560822015. View Article : Google Scholar
|
|
27
|
Alfaidi M, Wilson H, Daigneault M, Burnett
A, Ridger V, Chamberlain J and Francis S: Neutrophil elastase
promotes interleukin-1β secretion from human coronary endothelium.
J Biol Chem. 290:24067–24078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wuyts WA, Vanaudenaerde BM, Dupont LJ,
Demedts MG and Verleden GM: Involvement of p38 MAPK, JNK, p42/p44
ERK and NF-kappaB in IL-1β-induced chemokine release in human
airway smooth muscle cells. Respir Med. 97:811–817. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsu WY, Chao YW, Tsai YL, Lien CC, Chang
CF, Deng MC, Ho LT, Kwok CF and Juan CC: Resistin induces
monocyte-endothelial cell adhesion by increasing ICAM-1 and VCAM-1
expression in endothelial cells via p38 MAPK-dependent pathway. J
Cell Physiol. 226:2181–2188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang B, Wang X, Zhang N, Yang H, Bai R,
Liu M, Bian Y, Xiao C and Yang Z: Angiotensin-(1-7) attenuates
angiotensin II-induced ICAM-1, VCAM-1, and MCP-1 expression via the
MAS Receptor through suppression of P38 and NF-κB pathways in
HUVECs. Cell Physiol Biochem. 35:2472–2482. 2015. View Article : Google Scholar
|
|
31
|
Lee SJ, Drabik K, Van Wagoner NJ, Lee S,
Choi C, Dong Y and Benveniste EN: ICAM-1-induced expression of
proinflammatory cytokines in astrocytes: Involvement of
extracellular signal-regulated kinase and P38 mitogen-activated
protein kinase pathways. J Immunol. 165:4658–4666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park JS, Kim KM, Kim MH, Chang HJ, Baek
MK, Kim SM and Jung YD: Resveratrol inhibits tumor cell adhesion to
endothelial cells by blocking ICAM-1 expression. Anticancer Res.
29:355–362. 2009.PubMed/NCBI
|
|
33
|
Deane JA, Abeynaike LD, Norman MU, Wee JL,
Kitching AR, Kubes P and Hickey MJ: Endogenous regulatory T cells
adhere in inflamed dermal vessels via ICAM-1: Association with
regulation of effector leukocyte adhesion. J Immunol.
188:2179–2188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lacal PM, Petrillo MG, Ruffini F, Muzi A,
Bianchini R, Ronchetti S, Migliorati G, Riccardi C, Graziani G and
Nocentini G: Glucocorticoid-induced tumor necrosis factor receptor
family-related ligand triggering upregulates vascular cell adhesion
molecule-1 and intercellular adhesion molecule-1 and promotes
leukocyte adhesion. J Pharmacol Exp Ther. 347:164–172. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim KH, Lee EN, Park JK, Lee JR, Kim JH,
Choi HJ, Kim BS, Lee HW, Lee KS and Yoon S: Curcumin attenuates
TNF-α-induced expression of intercellular adhesion molecule-1,
vascular cell adhesion molecule-1 and proinflammatory cytokines in
human endometriotic stromal cells. Phytother Res. 26:1037–1047.
2012. View Article : Google Scholar
|
|
36
|
Park GS and Kim JH: LPS Up-regulates
ICAM-1 expression in breast cancer cells by stimulating a
MyD88-BLT2-ERK-linked cascade, which promotes adhesion to
monocytes. Mol Cells. 38:821–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang M, Zhang H, Feng W, Wang Y, Yin S,
Chen X and Wu X: Endothelial progenitor cells promote osteogenic
differentiation of marrow stromal cells in a paracrine manner.
Zhonghua Yi Xue Za Zhi. 95:1253–1257. 2015.In Chinese. PubMed/NCBI
|
|
38
|
Ern C, Krump-Konvalinkova V, Docheva D,
Schindler S, Rossmann O, Böcker W, Mutschler W and Schieker M:
Interactions of human endothelial and multipotent mesenchymal stem
cells in cocultures. Open Biomed Eng J. 4:190–198. 2010. View Article : Google Scholar : PubMed/NCBI
|