Introduction
Clinically, a normal wound healing after a cut,
burn, or injury will repair and close the wound by producing just
sufficient amounts of collagen and tissues and thereafter,
excessive cells, like fibroblasts will be eliminated through
apoptosis (1,2). However, the wound healing process is
truly complex and fragile; for example, interruption or failure of
the normal wound healing will lead to the formation of non-healing
chronic wounds, whereas abnormal wound healing could became
hypertrophic scar (2).
Hypertrophic scar is characterized by excessive deposits of
collagen during skin wound healing and has an adverse influence on
patients psychologically and physically (3). Hypertrophic scar also causes
physical dysfunction and symptoms, such as pain or itch. To date,
the exact pathogenesis or molecular mechanism of hypertrophic scar
formation remains to be defined, although extensive research and
studies were reported during the past decades (3). As known, excessive synthesis of the
extracellular matrix and collagen deposition due to abnormal
fibroblast proliferation and differentiation could be responsible
for hypertrophic scar formation. Previous studies revealed that
there were differences in number and phenotype (4) and lower ability to produce
collagenase between fibroblasts obtained from hypertrophic scar
tissues and normal skin (4).
Moreover, histological assessment of hypertrophic scar tissues
showed characteristics: i) a significant increase in fibroblasts;
ii) thin and disorderly organized wavy collagen III bundles
arranged parallel to the epidermis; and iii) abundant of
myofibroblast nodules and acidic mucopolysaccharides (5,6).
Molecularly, fibroblasts from hypertrophic scar tissues were shown
to overexpress fibronectin (7),
although the defined molecular changes of these fibroblasts from
hypertrophic scar tissues remain to be determined.
Successful treatment of a hypertrophic scar has both
psychological and physical significance. To date, treatment of a
hypertrophic scar remains a challenge to clinicians due to lack of
effective treatment options. For example, current therapeutic
options available to treat hypertrophic scars include intralesion
injection of corticosteroids or 5-fluorouracil (5-FU), silicone
sheeting or gel, and radiotherapy, cryotherapy, excisional surgery,
pressure therapy, fractional CO2 laser, or pulsed dye
laser and their effectiveness on control of a hypertrophic scar is
still debatable (8,9). Originally, extracorporeal shock wave
therapy (ESWT) (10) was used as
an adjunct medical procedure to treat urinary lithotripsy (11) and more recently, its application
has been extended to treat musculoskeletal disorders, fracture
non-union, and soft tissue wounds (12–14); however, the precise ESWT mechanism
on tissues remains unclear, but may be correlated with translating
acoustic energy into mechanical stimulation or physical energy, and
then exerts favorable biological responses to promote repair of
compromised tissue via complex molecular and cellular interactions
and changes (15). The biological
effects of ESWT induced on tissues include increase in blood supply
of tissues, promotion of burn wounds, and diabetic foot ulcer
healing, and improvement of skin graft uptake (10,16). Mechanistically, ESWT could induce
local inflammation reaction and promote angiogenesis by recruitment
of mesenchymal stem cells and endothelial progenitor cells to the
injured site, stimulate cellular proliferation and regeneration,
and decrease bacterial burden of the wound (17). In addition, based on ESWT energy
flux density (EFD) level, ESWT can be divided into two categories,
high EFD ESWT and low EFD ESWT (18,19). Thus, in this study, we performed
ESWT with low- or high-energy flux density versus control to assess
their effects on hypertrophic scar formation and gene expression in
a rabbit model. We expected to provide insightful information and a
support of its clinic application in control of hypertrophic scar
formation.
Materials and methods
Ethical approval
The animal protocol of this study was reviewed and
approved by the Institutional Animal Care and Use Committee (IACUC)
of the Institutional Ethics Committee at The First Hospital of
Jilin University (Jilin, China).
Animals, the rabbit ear hypertrophic scar
model, and treatment
Twenty-five adult laboratory white rabbits with an
initial body weight of 2.3±0.2 kg were obtained from the Laboratory
Animal Center of Jilin University and used in this study. The
rabbit ear hypertrophic scar model was established based on our
previous unpublished study. Briefly, rabbits were anesthetized with
intravenous injection of ketamine (30 mg/kg) and four full-skin
thickness circular wounds (15 mm diameter) with cartilage exposure
were then inflicted on the ventral surface of each ear with a
scalpel and covered with erythromycin eye ointment. The wound
healing occurred spontaneously for three weeks and the control mice
formed hypertrophic scars three weeks after surgery confirmed by
histological examination.
The rabbits were randomly assigned into three groups
with 32 scars for each group on day 21 after surgery, i.e., L-ESWT
(energy flux density of 0.1 mJ/mm2), H-ESWT (energy flux
density of 0.2 mJ/mm2), and sham ESWT group (S-ESWT). We
then treated the rabbits with a shock wave therapy on each
hypertrophic scar of rabbit ear with 500 impulses at a frequency of
8 Hz once a week for 4 weeks using Swiss DolorClast®
Classic (EMS Electro Medical Systems, Nyon, Switzerland), while
S-ESWT group of rabbits received identical treatment without any
shock wave impulses.
Tissue harvest and processing
On day 1, 4, 7, 10, 14, 21, 28, and 35 during or
after ESWT treatment, tissue samples were harvested for
histological analysis and gene expression. The animals were
administered with intravenous injection of ketamine (30 mg/kg) and
half of the scar tissues were excised and fixed in 10% buffered
formalin and embedded into paraffin for histological analysis and
immunohistochemistry and another half of tissue samples were
snap-frozen and stored in liquid nitrogen for RNA isolation and
RT-PCR.
Hematoxylin and eosin and Masson's
trichrome staining
Paraffin blocks of resected tissues from these
rabbits were cut into 4-µM thickness of tissue sections and
stained with hematoxylin and eosin staining kit (cat. no. C0105;
Beyotime Institute of Biotechnology, Jiangsu, China) and Masson's
trichrome staining (Masson's Trichrome Stain kit, cat. no. TR-1303;
ZSGB-Bio Co., Ltd., Beijing, China), respectively, according to the
manufacturer's instructions. The stained tissue sections were then
reviewed and photographed under an Olympus BX51 microscope
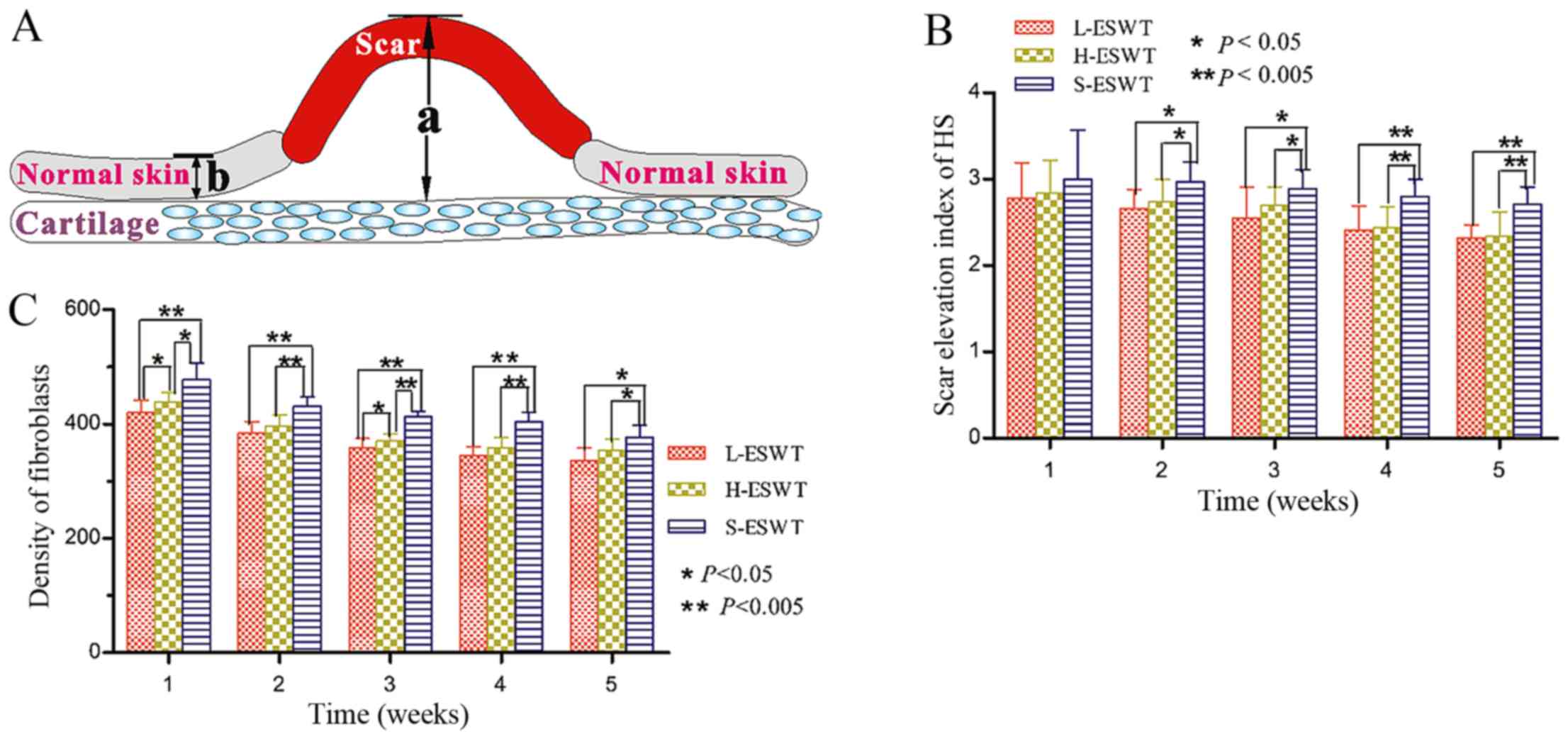
(Olympus, Tokyo, Japan) to evaluate the scar elevation index (SEI),
fibroblasts density, and collagen fiber arrangement by two
investigators blindly.
Assessment of the SEI and fibroblasts
density
For each scar, three photographs were prepared and
evaluated by an investigator in a blinded manner to assess SEI, an
average value to represent the degree of scar hyperplasia.
Specifically, we measured scar thickness versus normal tissues and
the ratio of 1 indicated that there was no difference in
hypertrophy during the wound healing between scar and normal skin,
while SEI 2 denoted 100% increase in wound thickness (20).
To assess the fibroblasts density in rabbit tissues
for ESWT effectiveness, we calculated the numbers of fibroblasts in
hematoxylin and eosin (H&E)-stained tissue sections.
Specifically, ten representative fields of the most fibroblast
density areas were photographed under magnification, ×400 and
individual fibroblasts were counted. Fibroblast density per square
millimeter was then calculated. The data were summarized as mean ±
SD of all tissue sections from each treatment group.
RT-PCR
Hypertrophic scar tissues were homogenized and total
RNA was isolated using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. The resulted RNA samples were quantified by using
Epoch™ Multi-Volume Microplate Spectrophotometer system (BioTek
Instruments, Inc., Winooski, VT, USA) and reversely transcribed
into cDNA using M-MLV Reverse Transcriptase (Promega, Madison, WI,
USA) according to the manufacturer's instructions. After that, PCR
was carried out in a 25 µl reaction mixture containing 12.5
µl GoTaq® Green Master Mix (Promega), 1 µl
of cDNA, 1 µl of each primer, 9.5 µl nuclease-free
water with the following conditions, 94°C for 30 sec and 28–30
cycles of 58°C [proliferating cell nuclear antigen (PCNA)], 56°C
[α-smooth muscle actin (α-SMA)], or 65°C (GAPDH) for 30 sec, 72°C
for 40 sec. PCR products were then separated in 1.5% agarose gel
containing ethidium bromide and images were taken by using the
Tanon 2500 gel imaging system (Tanon Science and Technology Co.,
Ltd., Shanghai, China) for quantification with Tanon gel image
system 1D software (version 4.1.2). Primers were designed and
synthesized by BGI (Shenzhen, China) and primer sequences were:
PCNA forward, 5′-GGTTCTTCCAACTCTGCCACTA-3′ and reverse,
5′-GGTTTTCTCTTTGCCTTCCCTA-3′ to amplify a 215 base pair (bp) of PCR
product; α-SMA forward, 5′-TCGACATCAGGAAGGACCTCT-3′ and reverse,
5′-CATCTGCTGAAAGGTGGACAG-3′ to generate a 206 bp band; and GAPDH
forward, 5′-GCGCCTGGTCACCAGGGCTGCTT-3′ and reverse,
5′-TGCCGAAGTGGTCGTGGATGACCT-3′ to obtain a 464 bp product.
Immunohistochemistry
Immunohistochemistry was used to detect the
expression of cell proliferation marker PCNA and smooth muscle
marker α-SMA in hypertrophic scar tissues. Specifically, tissue
sections were deparaffinized in xylene and rehydrated in a series
of graded ethanol and then subjected to the antigen retrieval by
cooking in 0.01 M citrate buffer (pH 6.0) for 3 min. After washed
with phosphate-buffered saline (PBS) briefly three times, the
endogenous peroxidase activity in tissues was blocked by treating
the tissue sections with 0.3% H2O2 in 70%
methanol at room temperature for 20 min and tissues sections were
incubated in 10% normal goat serum at room temperature for 20 min
and further incubated with a mouse anti-rabbit monoclonal PCNA
antibody (cat. no. BM0104; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) or a mouse monoclonal anti-rabbit α-SMA
antibody (cat no. BM0002; Wuhan Boster Biological Technology, Ltd.)
both at 1:100 dilution at 4°C overnight. The next day, the tissue
sections were washed in PBS three times and then incubated with
UltraSensitive™ SP (mouse) IHC kit (cat. no. KIT-9702; Fujian Maxin
Biological, Fujian, China) at the room temperature for 2 h and then
subsequently with a streptavidin-peroxidase solution at the room
temperature for 2 h. The color reaction was performed using the
3,3′-diaminobenzidine (DAB) detection kit (cat. no. KIT-0017;
Fujian Maxin Biological) according to the manufacturer's
instructions and the tissue sections were briefly counterstained
with hematoxylin. The immunostained tissue sections were then
reviewed and photographed under an Olympus BX51 microscope
(Olympus, Tokyo, Japan) by two investigators in a blinded manner.
Six representative areas of the most PCNA or α-SMA-positive cells
were photographed at ×400 magnification and counted for PCNA or
α-SMA-positive cells. The results were expressed as mean ± SD of
PCNA or α-SMA-positive cells/total number of cells ×100%.
Statistical analysis
All data were expressed as mean ± standard deviation
(SD) and were analyzed using SPSS statistical software, version
19.0 (SPSS, Inc., Chicago, IL, USA). Histology data were compared
and analyzed using the paired t-test and gene expression data were
analyzed using one-way analysis of variance (ANOVA). A P-value
≤0.05 was considered statistically significant.
Results
ESWT reduction of the SEI and fibroblast
density in the rabbit ear hypertrophic scar model
In this study, we first established the rabbit ear
hypertrophic scar model and then treated them with ESWT and found
that only from day 14, there was a significant difference in SEI
between L-ESWT or H-ESWT group and S-ESWT group (2.66±0.22 or
2.74±0.26 vs. 2.97±0.23; P=0.010 and P=0.026 between L-ESWT or
H-ESWT and S-ESWT, respectively). However, there was noticeable
difference between L-ESWT and H-ESWT (Fig. 1 and Table I). We also found that consecutive
ESWT for 35 days significantly inhibited scar hyperplasia in L-ESWT
and H-ESWT groups (P<0.05), while there was no significant
difference between L-ESWT and H-ESWT (Fig. 1 and Table I).
 | Table IEffect of ESWT administration on
suppression of SEI in the rabbit model of hypertrophic scar
formation (mean ± SD). |
Table I
Effect of ESWT administration on
suppression of SEI in the rabbit model of hypertrophic scar
formation (mean ± SD).
| Therapy | 1 week | 2 weeks | 3 weeks | 4 weeks | 5 weeks |
|---|
| L-ESWT | 2.78±0.41 | 2.66±0.22a | 2.55±0.36a | 2.41±0.28a | 2.32±0.15a |
| H-ESWT | 2.84±0.38 | 2.74±0.26a | 2.70±0.20a | 2.44±0.24a | 2.34±0.28a |
| S-ESWT | 3.00±0.57 | 2.97±0.23 | 2.89±0.22 | 2.80±0.20 | 2.71±0.20 |
Furthermore, one week after ESWT, there was a
significant difference in fibroblast density occurring between
L-ESWT or H-ESWT group and S-ESWT group
(420.26±21.44/mm2, or 439.53±16.37/mm2 vs.
477.80±29.56/mm2; P=0.003 or P=0.009 between L-ESWT or
H-ESWT and S-ESWT, respectively). Moreover, there was also
statistical significance in fibrobast density between L-ESWT and
H-ESWT groups (P=0.015). Consecutive administration of ESWT for 35
days significantly reduced the number of fibroblasts between both
ESWT and S-ESWT groups; however, there was no significant
difference between L-ESWT and H-ESWT found in the longer treatment
(Fig. 1).
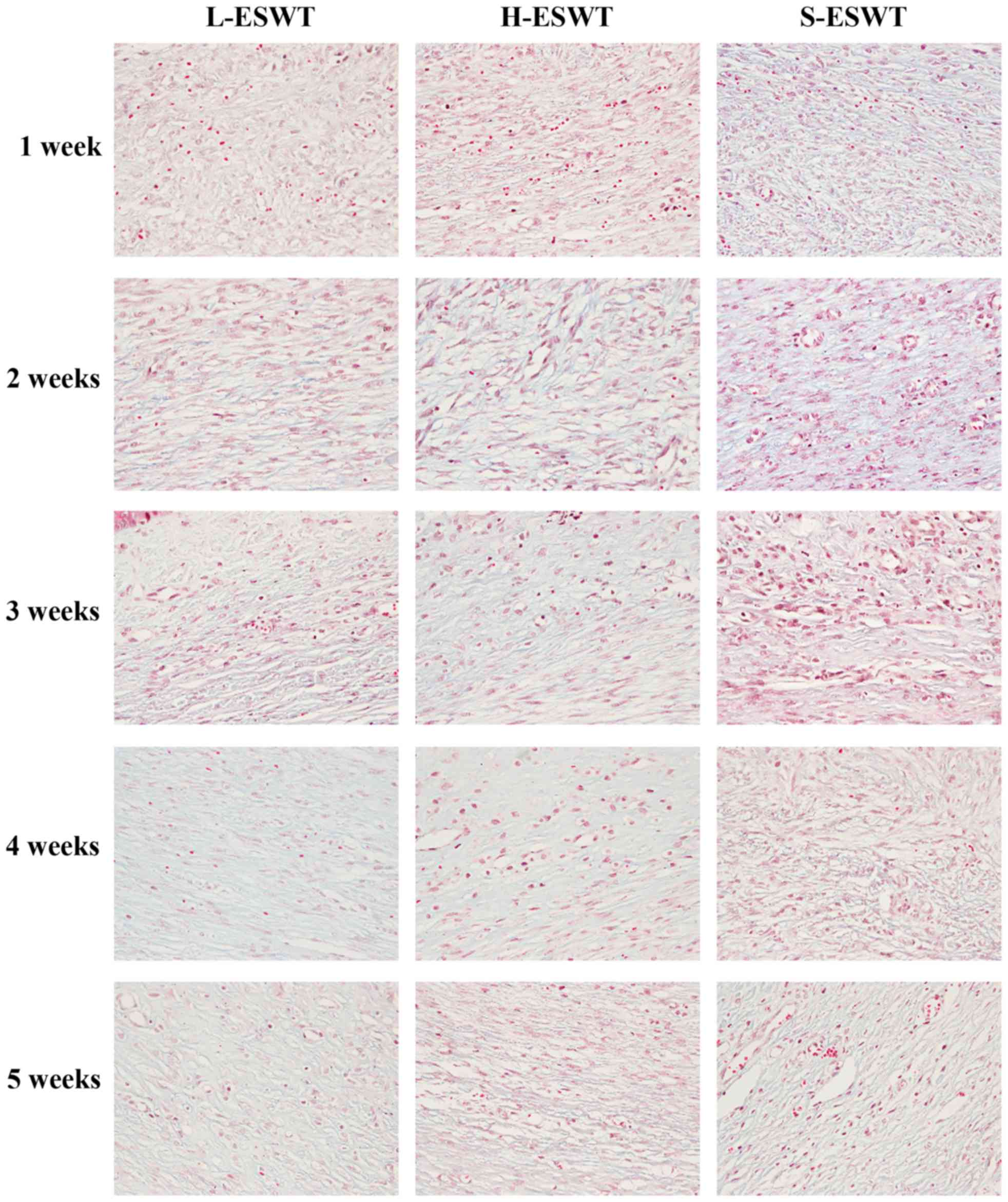
ESWT improvement of collagen arrangement
in the rabbit ear hypertrophic scar model
Masson's trichrome staining showed that number of
the collagen bundles was reduced and the collagen bundles were much
thinner and looser and regularly organized in L-ESWT and
H-ESWT-treated hyper-trophic scar tissues, whereas the collagen
fibers were thicker, denser, more abundant and disorganized in
S-ESWT (Fig. 2).
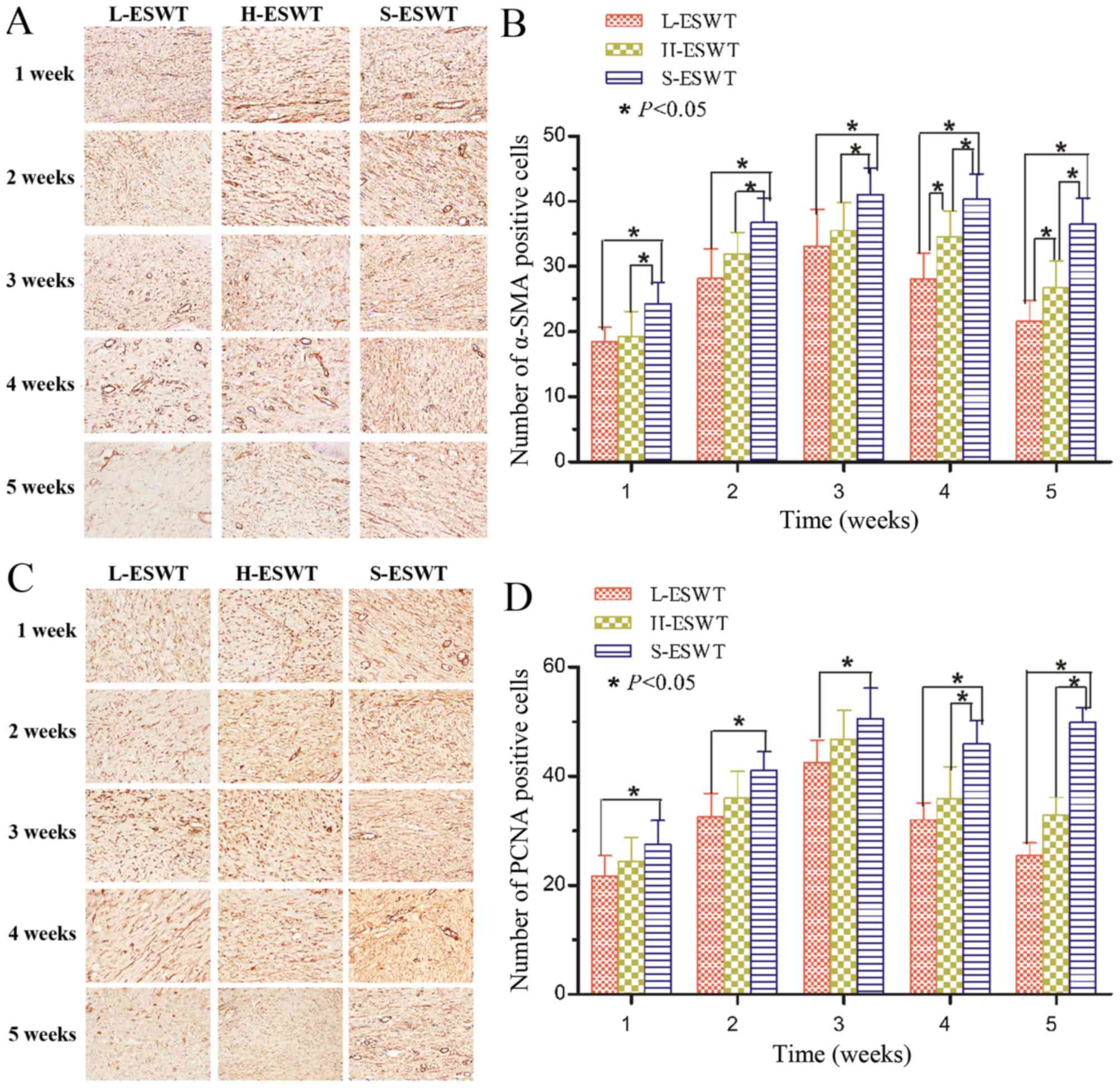
ESWT suppression of PCNA and α-SMA
expression
PCNA and α-SMA proteins were assessed using
immunohistochemistry and the data showed that fibroblasts and
endothelial cells of vessels expressed high levels of PCNA
proteins, whereas L-ESWT or H-ESWT significantly reduced
PCNA-positive cells compared to that of S-ESWT, and L-ESWT showed
much earlier in reduction of PCNA expression than that of H-ESWT
(P<0.05 in 7 and 28 days between L-ESWT or H-ESWT and S-ESWT,
respectively. However, there was no significant difference between
L-ESWT and H-ESWT (Fig. 3 and
Table II).
 | Table IIEffect of ESWT administration on
suppression of PCNA-positive cells in the rabbit model of
hypertrophic scar formation (mean ± SD). |
Table II
Effect of ESWT administration on
suppression of PCNA-positive cells in the rabbit model of
hypertrophic scar formation (mean ± SD).
| Therapy | 1 week | 2 weeks | 3 weeks | 4 weeks | 5 weeks |
|---|
| L-ESWT | 21.74±3.68b | 32.61±4.26b | 42.49±4.18b | 32.01±3.10b | 25.44±2.33b |
| H-ESWT | 24.42±4.28 | 36.04±4.88b | 46.81±5.31 | 35.91±5.83b | 32.89±3.22b |
| S-ESWT | 27.47±4.41 | 41.06±3.45 | 50.62±5.62 | 45.98±4.25 | 49.89±2.67 |
Moreover, levels of α-SMA-positive myofibroblasts
also showed a trend for first increase and late decrease after
L-ESWT or H-ESWT and such a trend was much earlier in L-ESWT group.
The number of α-SMA-positive cells in L-ESWT or H-ESWT groups were
much lower than that of S-ESWT (P<0.05); however, there was no
statistical significance found between L-ESWT and H-ESWT until four
weeks later (P<0.05; Fig. 3
and Table III).
 | Table IIIEffect of ESWT administration on
suppression of α-SMA-positive cells in the rabbit model of
hypertrophic scar formation (mean ± SD). |
Table III
Effect of ESWT administration on
suppression of α-SMA-positive cells in the rabbit model of
hypertrophic scar formation (mean ± SD).
| Therapy | 1 week | 2 weeks | 3 weeks | 4 weeks | 5 weeks |
|---|
| L-ESWT | 18.44±2.26b | 28.21±4.52b | 33.09±5.65b | 28.02±4.01a,b | 21.62±3.11a,b |
| H-ESWT | 19.22±3.88b | 31.94±3.26b | 35.51±4.32b | 34.51±3.99b | 26.73±4.12b |
| S-ESWT | 24.27±3.26 | 36.76±3.67 | 41.02±4.09 | 40.38±3.75 | 36.48±4.01 |
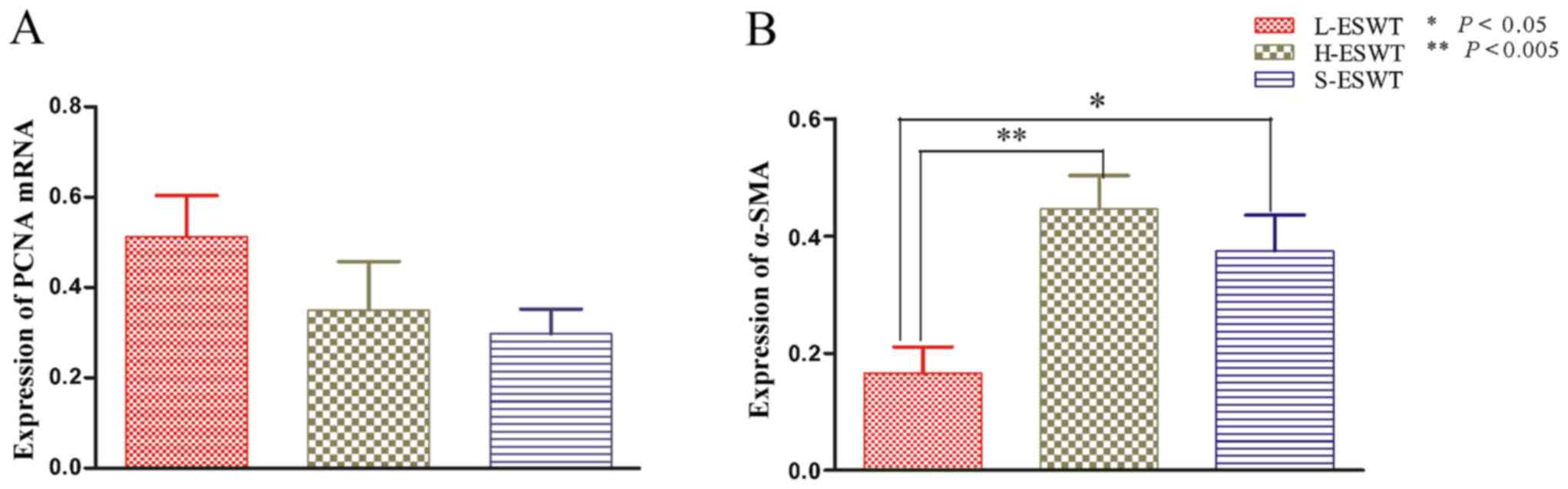
L-ESWT downregulation of α-SMA mRNA
level
The effects of L-ESWT and H-ESWT on PCNA and α-SMA
mRNA expre ssion was also assessed by using RT-PCR. The data showed
that there was no significant difference in PCNA mRNA level among
the three experimental groups, whereas level of α-SMA mRNA was
significantly lower in L-ESWT compared to that in H-ESWT and S-ESWT
groups (P=0.002 and P=0.039, respectively), although there was no
statistical significance observed between H-ESWT and S-ESWT
(Fig. 4).
Discussion
Successful treatment of hypertrophic scar has both
psycho logical and physical significance. Hypertrophic scar causes
cosmetic and social stress on affected persons, especially girls in
addition to the unwanted appearance and physical dysfunction or
symptoms, such as pain or itch. However, to date, treatment of
hypertrophic scar remains a challenge to clinicians due to lack of
effective treatment options. Although various therapeutic
modalities have been developed showing some efficacy on
hypertrophic scar during the past decades, there is still a paucity
of standard of care for hypertrophic scar patients. Better
understanding of the underlying molecular mechanisms responsible
for unwanted wound healing and hypertrophic scar formation could
help us to design or identify novel therapeutic methods. Therefore,
identification and assessment of more effective but less invasive
therapeutic options could help such patients clinically. Thus, in
this study, we performed ESWT with low- or high-energy flux density
versus control to assess their effects on hypertrophic scar
formation and gene expression. We found that both ESWT treatment
groups significantly reduced the scar elevation index and
fibroblast density compared to controls. ESWT treatment also showed
that collagen fibers were more slender and broader and oriented in
parallel to skin surface compared to control tissues. Molecularly,
ESWT treatment suppressed proliferation of PCNA-positive
fibroblasts and α-SMA-positive myofibroblasts compared to the
controls. Nevertheless, both L-ESWT and H-ESWT showed effective
suppression of hypertrophic scar formation by inhibition of scar
elevation index and fibro blast density as well as PCNA and α-SMA
expression in hypertrophic scar tissues. Simultaneously, we also
evaluated the effects of ESWT on regulation of PCNA and α-SMA mRNA
and found that L-ESWT was able to significantly downregulate level
of α-SMA mRNA in the hypertrophic scar tissues, whereas it was
unable to alter level of PCNA mRNA. Future clinical trials will
confirm our current finding.
As a novel adjunct management option for soft tissue
wound healing and musculoskeletal disorders, ESWT has received
extensive attention due to its efficacious, safe, non-invasive, and
cost-effective nature by utilizing abrupt and high amplitude pulses
of mechanical energy, which is similar to sound wave, to treat
human diseases (22–25). Previous studies showed that ESWT
could stimulate angiogenesis and neurogenesis (26) and mechanistically, ESWT may induce
cells to undergo microtrauma and therefore promotes inflammation
and catabolic processes and wound healing (27). However, to date, there has been
only a few of studies evaluated ESWT to manage hypertrophic scars.
For example, Fioramonti et al (28) documented that ESWT could improve
texture and color of post-burn scars in 16 patients. Saggini et
al (29) observed significant
early improvement of pain, mobility, and modified Vancouver Scar
Scale (VSS) in retracting scars of the hands after treatment with
unfocused shock wave treatment. Our current data further supported
the usefulness of ESWT in management of hypertrophic scar and found
that ESWT could histologically improve SEI, fibroblasts density,
and collagen fiber arrangement in hypertrophic scar tissues.
Molecularly, abnormal proliferation and
differentiation of fibroblasts play a key role in hypertrophic scar
formation and remodeling (30).
Thus, in the present study, we assessed PCNA expression, which is a
DNA polymerase delta auxiliary protein that synthesized and
expressed in proliferating cells to be essential for cell
replication and cell cycle progression (31,32). Furthermore, fibroblast
differentiation into myofibroblasts is closely associated with
α-SMA expression during wound healing process and myofibroblasts
have the ability to increase collagen synthesis and contractile
activity, but decrease synthesis of collagenase, and become
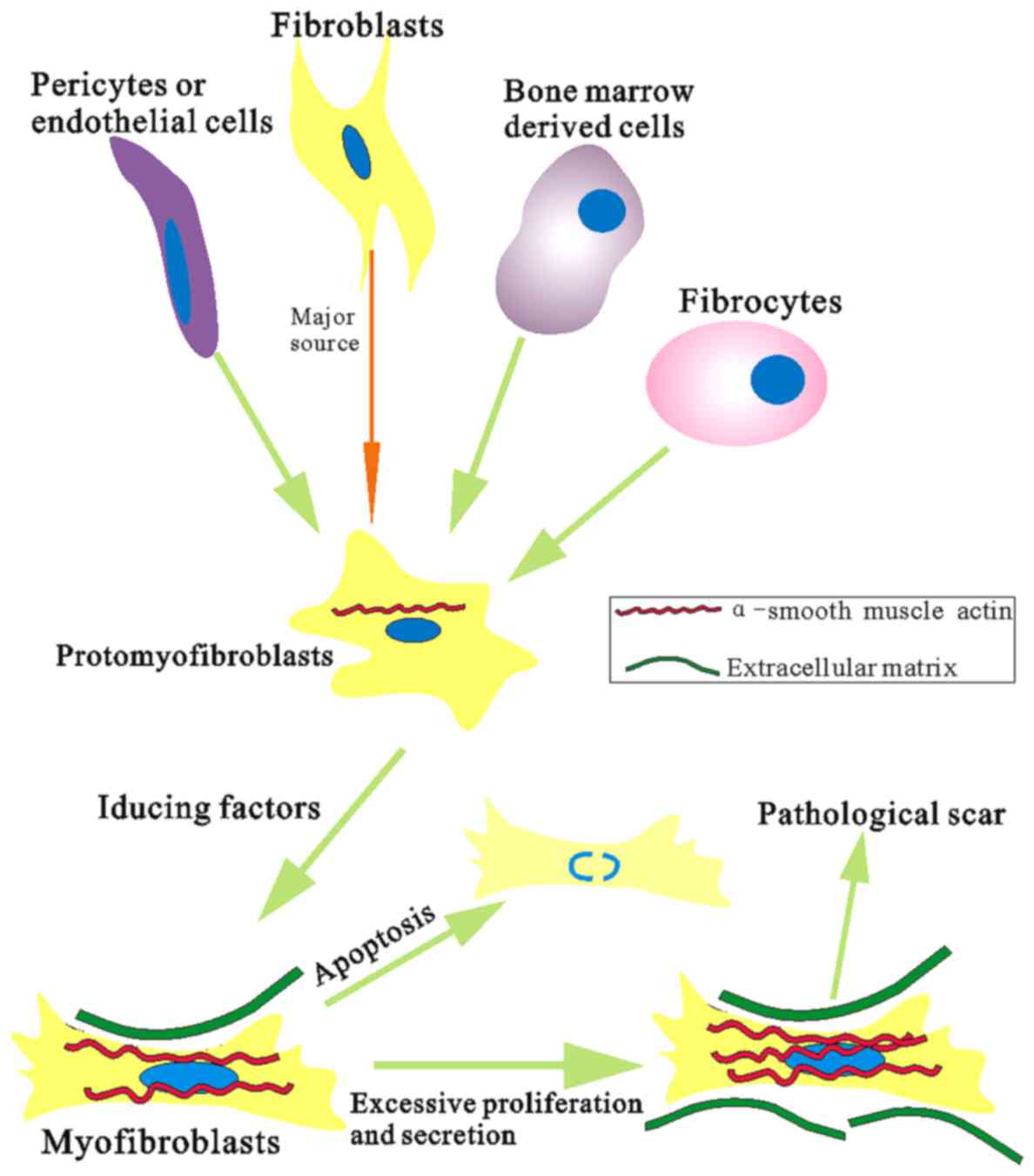
insensitive to apoptotic inductors (33,34) (Fig.
5). Differentiation of myofibroblasts is thought to play a key
role in fibrotic disease and involved in many fibrosis of organs
(35). Our current data showed
that ESWT was able to inhibit expression of α-SMA mRNA and reduce
the number of PCNA and α-SMA-positive cells. Further confirming the
usefulness of ESWT in management of hypertrophic scar. Regarding
the discrepancy between expression of PCNA protein and mRNA in the
present study, it is necessary to further investigate with
carefully controls each time-point using qRT-PCR for confirmation.
Nevertheless, expression of gene protein and mRNA may not always be
parallel since they are regulated differently (21).
In conclusion, both low- and high-energy ESWT was
able to suppress hypertrophic scar formation improving the scar
elevation index and fibroblast density compared to controls in
hypertrophic scar tissues of the rabbit model in an early stage.
This may be associated with the effect of ESWT on inhibition of
α-SMA expression of hypertrophic scar tissues. Future studies are
essential to investigate other potential mechanisms in order to
further develop and improve efficacy of L-ESWT-based therapy of
hypertrophic scar clinically.
Acknowledgments
Not applicable.
Notes
[1]
Funding
No funding was received.
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
ZJC conceived and designed the study, and wrote the
paper. ZBR, HL and SK conducted the experiment and collected the
data. WWW analyzed the data. YJA made scientific revisions. All
authors read and approved the final manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Stadelmann WK, Digenis AG and Tobin GR:
Physiology and healing dynamics of chronic cutaneous wounds. Am J
Surg. 176(Suppl 2A): 26S–38S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Midwood KS, Williams LV and Schwarzbauer
JE: Tissue repair and the dynamics of the extracellular matrix. Int
J Biochem Cell Biol. 36:1031–1037. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gauglitz GG, Korting HC, Pavicic T,
Ruzicka T and Jeschke MG: Hypertrophic scarring and keloids:
pathomechanisms and current and emerging treatment strategies. Mol
Med. 17:113–125. 2011. View Article : Google Scholar :
|
|
4
|
Nedelec B, Shankowsky H, Scott PG, Ghahary
A and Tredget EE: Myofibroblasts and apoptosis in human
hypertrophic scars: the effect of interferon-alpha2b. Surgery.
130:798–808. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slemp AE and Kirschner RE: Keloids and
scars: a review of keloids and scars, their pathogenesis, risk
factors, and management. Curr Opin Pediatr. 18:396–402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang L, Scott PG, Dodd C, Medina A, Jiao
H, Shankowsky HA, Ghahary A and Tredget EE: Identification of
fibrocytes in postburn hypertrophic scar. Wound Repair Regen.
13:398–404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ali SS, Hajrah NH, Ayuob NN, Moshref SS
and Abuzinadah OA: Morphological and morphometric study of cultured
fibroblast from treated and untreated abnormal scar. Saudi Med J.
31:874–881. 2010.PubMed/NCBI
|
|
8
|
Williams CC and De Groote S: Clinical
inquiry: what treatment is best for hypertrophic scars and keloids.
J Fam Pract. 60:757–758. 2011.PubMed/NCBI
|
|
9
|
Shridharani SM, Magarakis M, Manson PN,
Singh NK, Basdag B and Rosson GD: The emerging role of
antineoplastic agents in the treatment of keloids and hypertrophic
scars: a review. Ann Plast Surg. 64:355–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dymarek R, Halski T, Ptaszkowski K,
Slupska L, Rosinczuk J and Taradaj J: Extracorporeal shock wave
therapy as an adjunct wound treatment: a systematic review of the
literature. Ostomy Wound Manage. 60:26–39. 2014.PubMed/NCBI
|
|
11
|
Chaussy C, Brendel W and Schmiedt E:
Extracorporeally induced destruction of kidney stones by shock
waves. Lancet. 2:1265–1268. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rassweiler J, Rassweiler MC, Frede T and
Alken P: Extracorporeal shock wave lithotripsy: an opinion on its
future. Indian J Urol. 30:73–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valchanou VD and Michailov P: High energy
shock waves in the treatment of delayed and nonunion of fractures.
Int Orthop. 15:181–184. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maffulli G, Hemmings S and Maffulli N:
Assessment of the effectiveness of extracorporeal shock wave
therapy (ESWT) for soft tissue injuries (ASSERT): an online
database protocol. Transl Med UniSa. 10:46–51. 2014.PubMed/NCBI
|
|
15
|
Mittermayr R, Hartinger J, Antonic V,
Meinl A, Pfeifer S, Stojadinovic A, Schaden W and Redl H:
Extracorporeal shock wave therapy (ESWT) minimizes ischemic tissue
necrosis irrespective of application time and promotes tissue
revascularization by stimulating angiogenesis. Ann Surg.
253:1024–1032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao JC, Xian CJ and Yu JA: Advancement in
the research of effect of extracorporeal shock wave therapy on
wound angiogenesis. Chin J Inj Repair Wound Healing. 9:80–84.
2014.
|
|
17
|
Zhao J, Xue Y, Yu J, Shi K, Xian C and
Zhou X: Advances in the research of mechanism of enhancement of
wound healing with extracorporeal shock wave therapy. Zhonghua Shao
Shang Za Zhi. 31:315–317. 2015.In Chinese. PubMed/NCBI
|
|
18
|
Speed C: A systematic review of shockwave
therapies in soft tissue conditions: focusing on the evidence. Br J
Sports Med. 48:1538–1542. 2014. View Article : Google Scholar
|
|
19
|
Goertz O, Lauer H, Hirsch T, Ring A,
Lehnhardt M, Langer S, Steinau HU and Hauser J: Extracorporeal
shock waves improve angiogenesis after full thickness burn. Burns.
38:1010–1018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kloeters O, Tandara A and Mustoe TA:
Hypertrophic scar model in the rabbit ear: a reproducible model for
studying scar tissue behavior with new observations on silicone gel
sheeting for scar reduction. Wound Repair Regen. 15(Suppl 1):
S40–S45. 2007. View Article : Google Scholar
|
|
21
|
Zhou M, Li LH, Peng H, Li R, Feng CC, Xu
WD, Leng RX, Pan HF and Ye DQ: Decreased ITGAM and FcγRIIIA mRNA
expression levels in peripheral blood mononuclear cells from
patients with systemic lupus erythematosus. Clin Exp Med.
14:269–274. 2014. View Article : Google Scholar
|
|
22
|
Wang CJ, Ko JY, Chan YS, Weng LH and Hsu
SL: Extra-corporeal shockwave for chronic patellar tendinopathy. Am
J Sports Med. 35:972–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin MC, Ye J, Yao M, Cui XJ, Xia Y, Shen
QX, Tong ZY, Wu XQ, Ma JM and Mo W: Is extracorporeal shock wave
therapy clinical efficacy for relief of chronic, recalcitrant
plantar fasciitis? A systematic review and meta-analysis of
randomized placebo or active-treatment controlled trials. Arch Phys
Med Rehabil. 95:1585–1593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiele S, Thiele R and Gerdesmeyer L:
Lateral epicondylitis: this is still a main indication for
extracorporeal shockwave therapy. Int J Surg. 24(Pt B): 165–170.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang CJ, Cheng JH, Kuo YR, Schaden W and
Mittermayr R: Extracorporeal shockwave therapy in diabetic foot
ulcers. Int J Surg. 24(Pt B): 207–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang CJ, Ko JY, Kuo YR and Yang YJ:
Molecular changes in diabetic foot ulcers. Diabetes Res Clin Pract.
94:105–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Waugh CM, Morrissey D, Jones E, Riley GP,
Langberg H and Screen HR: In vivo biological response to
extracorporeal shockwave therapy in human tendinopathy. Eur Cell
Mater. 29:268–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fioramonti P, Cigna E, Onesti MG, Fino P,
Fallico N and Scuderi N: Extracorporeal shock wave therapy for the
management of burn scars. Dermatol Surg. 38:778–782. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saggini R, Saggini A, Spagnoli AM, Dodaj
I, Cigna E, Maruccia M, Soda G, Bellomo RG and Scuderi N:
Extracorporeal shock wave therapy: an emerging treatment modality
for retracting scars of the hands. Ultrasound Med Biol. 42:185–195.
2016. View Article : Google Scholar
|
|
30
|
Yagmur C, Akaishi S, Ogawa R and Guneren
E: Mechanical receptor-related mechanisms in scar management: a
review and hypothesis. Plast Reconstr Surg. 126:426–434. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bravo R and Macdonald-Bravo H: Existence
of two populations of cyclin/proliferating cell nuclear antigen
during the cell cycle: association with DNA replication sites. J
Cell Biol. 105:1549–1554. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moldovan GL, Pfander B and Jentsch S:
PCNA, the maestro of the replication fork. Cell. 129:665–679. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nedelec B, Ghahary A, Scott PG and Tredget
EE: Control of wound contraction. Basic and clinical features. Hand
Clin. 16:289–302. 2000.PubMed/NCBI
|
|
34
|
Moulin V, Larochelle S, Langlois C,
Thibault I, Lopez-Vallé CA and Roy M: Normal skin wound and
hypertrophic scar myofibroblasts have differential responses to
apoptotic inductors. J Cell Physiol. 198:350–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lim MJ, Ahn J, Yi JY, Kim MH, Son AR, Lee
SL, Lim DS, Kim SS, Kang MA, Han Y, et al: Induction of galectin-1
by TGF-β1 accelerates fibrosis through enhancing nuclear retention
of Smad2. Exp Cell Res. 326:125–135. 2014. View Article : Google Scholar : PubMed/NCBI
|