Introduction
Voltage-gated sodium channel, a transmembrane
protein, plays a major role in the generation and propagation of
action potential in sensory neurons, which is a critical
determinant of electrical excitability for nociception (1,2).
The three dimensional structure of voltage-gated sodium channel is
still unknown, but is supposed to compose a large, pore-forming
α-subunit and two small β-auxiliary subunits (3). The α-subunit is the core or major
components of voltage-gated sodium channel, which can function
independently (3–15). So far, nine distinct voltage-gated
sodium channel α-subunits, namely Nav1.1–Nav1.9, have been cloned
from various mammalian tissues (4). Of the nine distinct α-subunits, all
were observed in adult dorsal root ganglia (DRG) neurons except for
Nav1.4 which was expressed abundantly in skeletal muscle (6,7).
Voltage-gated sodium channel Nav1.5, a tetrodotoxin-resistant
(TTX-R) sodium channel, was first cloned from the heart and has
been considered as the cardiac sodium channel (8,9).
However, as a matter of fact, it could also be detected in various
mammalian tissues other than the heart, such as the brain and DRG
neurons (9–119).
Previous studies confirmed the expression of Nav1.5
mRNA, including Nav1.5a and Nav1.5c isoforms, in adult mouse and
rat DRG neurons (14,15). Furthermore, Nav1.5 current was
supposed to underlie the 'third TTX-R sodium current' in rat small
DRG neurons (13). So far, nine
Nav1.5 splice variants have been discovered (Nav1.5a–f and E28B-D)
and four of them (Nav1.5a, Nav1.5c, Nav1.5d and Nav1.5e) have been
proved to generate functional sodium channels (9). Whether other Nav1.5 splice variants,
especially the neonatal Nav1.5 isoform (Nav1.5e) which shows
different functional properties compared with adult Nav1.5
(20), express in the DRG neurons
is still unknown. In this study, we systematically investigated the
expression of neonatal and adult Nav1.5 in the DRG and axon of
peripheral sensory neurons of rats with spared nerve injury (SNI)
by RT-PCR, DNA sequencing, restriction enzyme digestion methods.
The expression level of Nav1.5, as well as the expression ratio of
neonatal Nav1.5 versus adult Nav1.5, in the DRG neurons and axon of
peripheral sensory neurons was explored in this study. The
expression and distribution of total Nav1.5 protein in these
structures was also detected by immunohistochemistry and
immunofluorescence method. In addition, the expression of protein
kinase C (PKC), the activation of which was supposed to modulate
the biophysical properties of TTX-R INa, in the DRG neurons and
axon of peripheral sensory neurons of SNI rats was also detected in
this study.
Materials and methods
Animal models
Healthy male Sprague-Dawley rats at the age of 8–110
weeks and weighing 200–1250 g were provided by the Animal
Experimentation Center of Capital Medical University. They were
housed two per group and maintained on a 12-h light/dark cycle with
food and water available ad libitum under constant
temperature (23±2°C). The investigation was approved by the Ethics
Committee of Animal Experimentation of Capital Medical University
and conformed to the principles outlined in the NIH Guide for the
Care and Use of Laboratory Animals (NIH Publications no.
80–123).
The SNI rat models were established according to the
previous study (21). In
conclusion, the SD rats were anesthetized with pentobarbital sodium
(0.06 g/kg) and the surgery was conducted at room temperature
(18–122°C). After exposing the sciatic nerve and its three terminal
branches (the sural, common peroneal and tibial nerves) by incising
the skin and separating the muscle, axotomy and ligation of the
tibial and common peroneal nerves was performed, leaving the sural
nerve intact. Then the peroneal and the tibial nerves were
tight-ligated with 4–10 surgical silk and sectioned distal to the
ligation, removing 5 mm of the distal nerve stump.
RNA isolation and RT-PCR
On day 14 after surgery, the DRG (L4–16) and axon of
the peripheral sensory neurons was removed. Total RNA was extracted
by using RNA out kit (Takara, Shiga, Japan) according to the
manufacturer's instructions. cDNAs were generated from 1 µg
of total RNA using the reverse transcription polymerase chain
reaction (RT-PCR) kit (Life Technologies, Carlsbad, CA, USA). The
PCR primer used for amplification of adult and neonatal Nav1.5 was
designated to target both exon 6 and 6A: forward,
5′-TTCTGCCTGCATGCATTCACCTT-3′ and reverse primer,
5′-GCAGAAGACAGTGAGGACCA-3′, with a product length of 240 bp. The
PCR reaction conditions consisted of 95°C for 5 min followed by 36
cycles of 95°C for 30 sec, 66°C for 30 sec, 72°C for 30 sec and
final elongation at 72°C for 5 min. Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used for reference gene: forward,
5′-GACCACCCAGCCCAGCAAGG-3′ and reverse primer,
5′-TCCCCAGGCCCCTCCTGTTG-3′, with a product length of 144 bp. The
mRNA expression was analyzed with the gel analysis system, and the
relative expression level of Nav1.5 was calculated by dividing the
gray value of its band by the gray value of the GAPDH band.
DNA sequencing
PCR products were separated on 2% agarose gel by
electrophoresis. The fragments of the expected size were extracted
and purified by using gel extraction kit (Qiagen, Valencia, CA,
USA) and then sequenced directly by using 3730xl DNA sequencer
(Applied Biosystem, Foster City, CA, USA).
Restriction enzyme digestion
Restriction enzyme SacIII was used to digest
the total PCR products in order to distinguish the neonatal Nav1.5
splice variant from the total Nav1.5 cDNAs. The reaction system was
30 µl in total, containing 8 µl PCR products, 0.5
µl SacIII enzyme, 3 µl loading buffer and 18.5
µl super-purified water. After 1 h incubation at 38°C or
overnight at room temperature, the PCR products were seperated on
2% agarose gel by electrophoresis to detect the digestion
results.
Immunohistochemistry assay
Fresh specimens of the DRG (L4–16) and axon of the
peripheral sensory neurons were fixed with 1–14% paraformaldehyde
to prepare the paraffin-embedded specimens in 4-µm thick
sections. The streptavidin-peroxidase (SP) immunohistochemical
method was applied. Immunohistochemistry was performed according to
manufacturer's instructions of Histostain-SP kit (Invitrogen,
Carlsbad, CA, USA). Sections were incubated with primary Nav1.5
antibody (rabbit, 1:100; Alomone, Jerusalem, Israel), and then
incubated with the second antibody (biotinylated goat anti-rabbit
IgG, 1:500; Invitrogen). Phosphate-buffered saline (PBS) replaced
the primary antibody to serve as the negative control and the slide
with known positive Nav1.5 expression in human atrial muscle served
as the positive control.
Western blot analysis
The antibodies used for western blot analysis were
as follows: rabbit anti-Nav1.5 polyclonal antibody (1:200;
Alomone); rabbit polyclonal anti-PKC-γ antibody (1:1,000; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA); rabbit anti-α
tubulin monoclonal antibody (1:10,000; Millipore, Billerica, MA,
USA); horseradish peroxidase-linked anti-rabbit and anti-mouse IgG
secondary antibody (1:2,000; Cell Signaling Technology, Danvers,
MA, USA). Approximately 100 mg of tissue was rinsed with precooled
PBS (4°C), mixed with 500 µl of RIPA strong lysate buffer,
homogenized with a homogenizer, and left for lysis at 4°C
overnight. After centrifugation at 12,000 rpm for 30 min at 4°C,
the supernatants were collected and assayed for protein content.
The protein concentration was determined using the BCA method, 100
µg of protein was loaded into each lane for a 6–110% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
running at a constant voltage of 80 V for 2 h. The proteins were
then transferred onto a polyvinylidene fluoride (PVDF) membrane at
a constant current of 400 mA for 2 h. The membrane was blocked with
10% non-fat milk for 2 h and incubated with primary antibody
overnight at 4°C. Then the membrane was rinsed with TBST 3 times
and incubated with horseradish peroxidase-labeled secondary
antibody with shaking at room temperature for 1 h, and finally
exposed and developed using the ECL system. Immunoreactive protein
bands were detected with an enhanced chemiluminescence reagent
(ECL-Plus) and densitometrically quantitated according to the
manufacturer's instructions (Amersham Pharmacia Biotech,
Piscataway, NJ, USA). The protein expression was analyzed with the
gel analysis system, and the relative expression levels of targeted
protein were calculated by dividing the gray value of its band by
the gray value of the α-tubulin band. The experiments were repeated
3 times.
Results
Both neonatal and adult Nav1.5 mRNA are
expressed in the DRG neurons and axon of peripheral sensory neurons
of adult rat
In order to detect the expression of neonatal Nav1.5
(Nav1.5e) isoform in the DRG neurons and axon of peripheral sensory
neurons of rats, we used a special PCR primer targeting both exon 6
(adult Nav1.5) and exon 6A (neonatal Nav1.5) of SCN5A.
Electrophoresis was made after PCR and the results demonstrated
that a single band with the expected size (240 bp) appeared on the
2% agarose gel (Fig. 1). Direct
DNA sequencing of the PCR products was performed after gel
extraction. The results showed that a single sequence presented in
exon 5 or 7 coding region, but a dual sequence appeared in exon 6
or 6A coding region (Fig. 1). DNA
sequence analysis revealed that both exon 6 and 6A of SCN5A gene
were inclusively expressed in the PCR products, indicating the
expression of both adult and neonatal Nav1.5 channels (Nav1.5e) in
the DRG neurons and axon of peripheral sensory neurons of adult
rats.
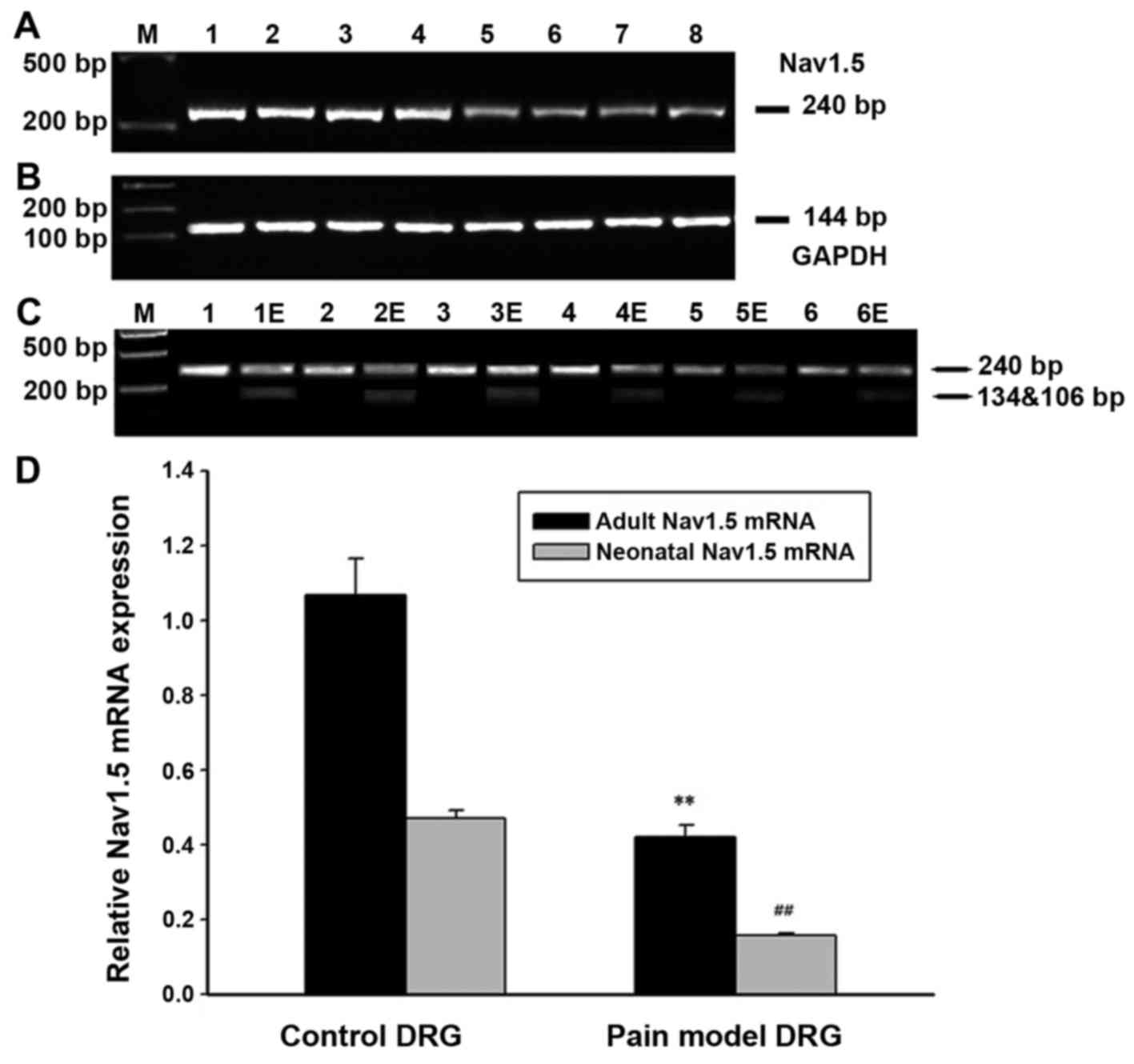
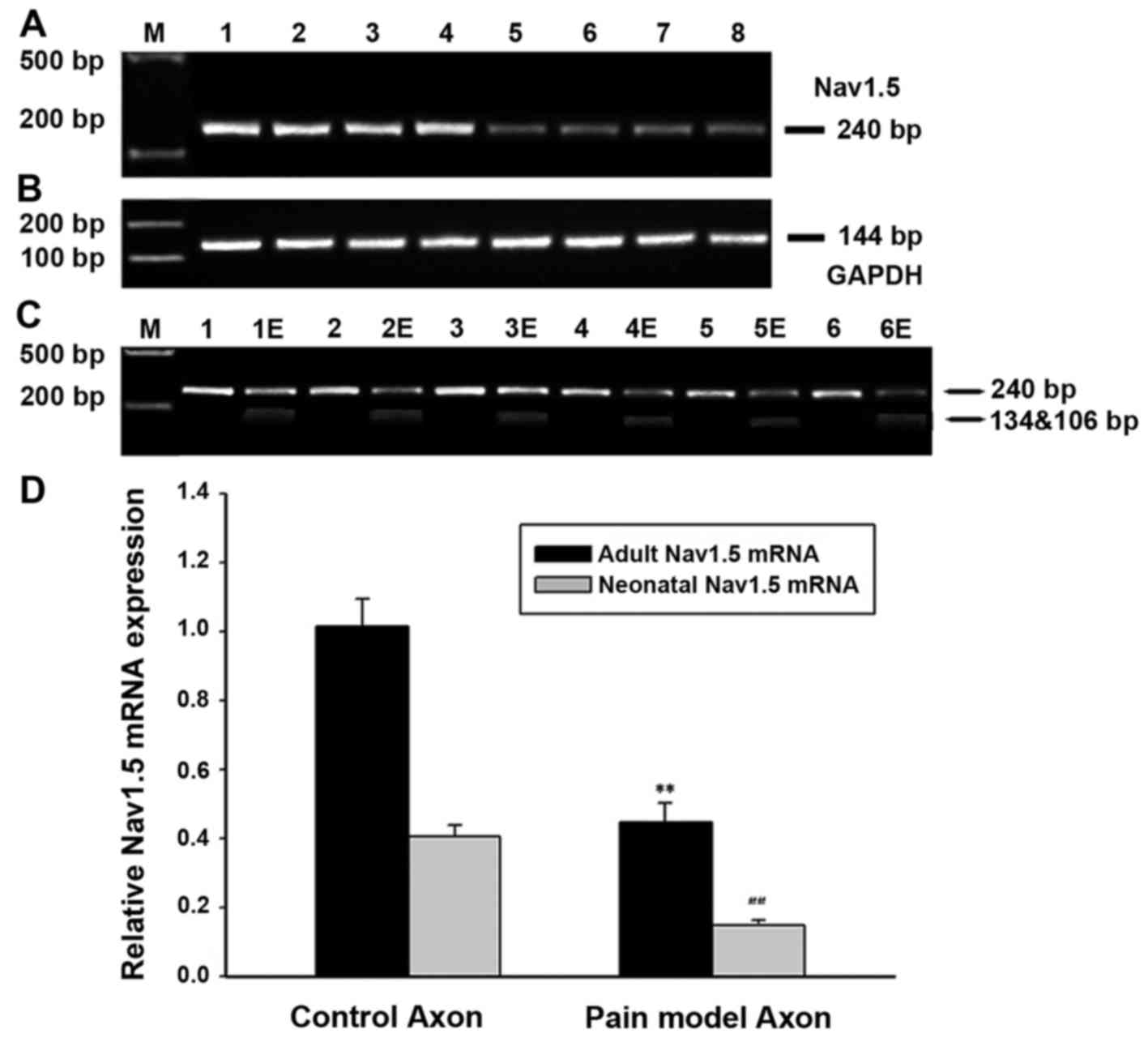
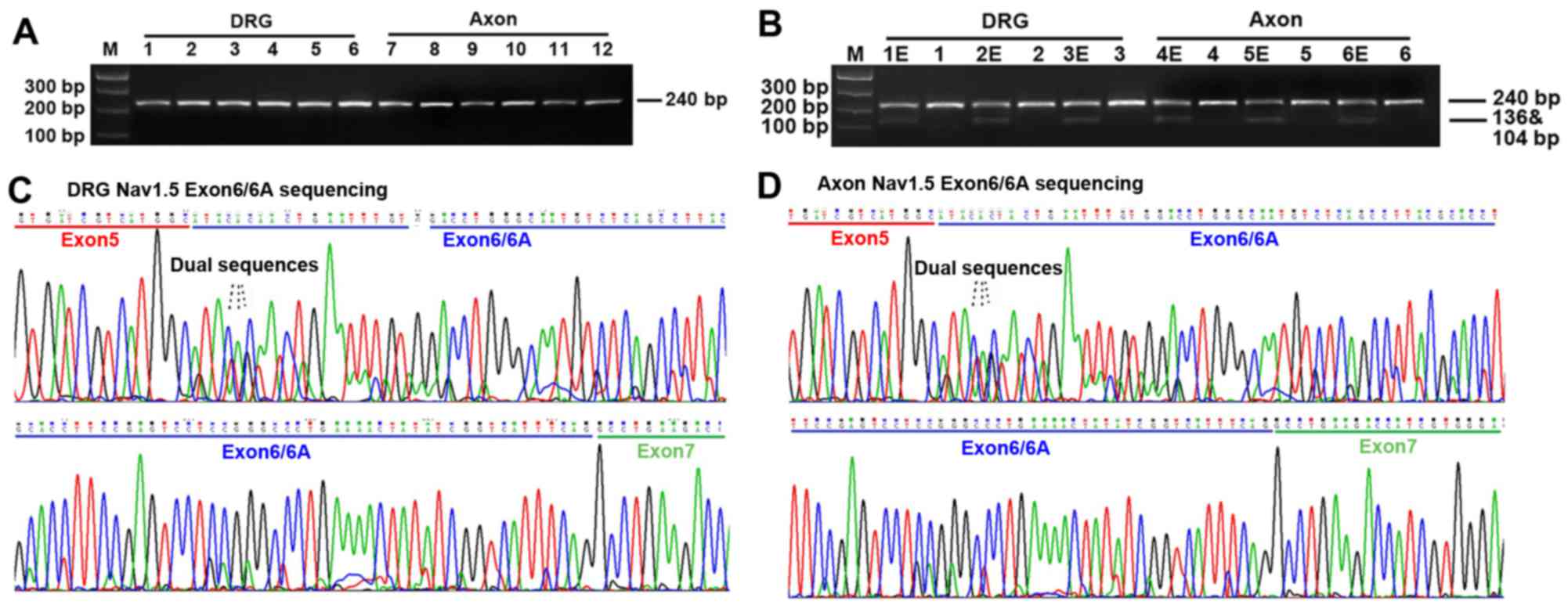 | Figure 1RT-PCR and direct DNA sequencing
results. (A) RT-PCR results. Electrophoresis showed a single and
clear band with the expected size (240 bp) on the agarose gel. M,
Marker; 1–16, normal dorsal root ganglia (DRG) neurons; 7–112,
normal axon of peripheral sensory neurons. (B) Digestion results by
restriction enzyme SacIII. 1E-6E, PCR products with
restriction enzyme SacIII; 1–16, PCR products without
restriction enzyme SacIII. As indicated by the
electrophoresis results, PCR products with restriction enzyme
SacIII can generate two more bands on the agarose gel, with
the expected size of 136 and 104 bp, respectively, indicating that
the fragments including exon 6A were digested into another two
fragments while that containing exon 6 was preserved. (C and D) DNA
sequencing results. Direct DNA sequencing demonstrated that single
sequence presented in the exon 5 or 7 coding region, but a dual
sequence appeared in exon 6 coding region, indicating that both
exon 6 and 6A were expressed in the DRG neurons and axon of
peripheral sensory neurons. |
In order to explore the expression quantification of
the adult and neonatal Nav1.5 splice variants, we used restriction
enzyme SacIII to digest the PCR products for a specific
restriction enzyme site of SacIII was found in exon 6A
rather than exon 6 of SCN5A gene. As indicated in Fig. 1, PCR fragments including exon 6A
were digested into two bands while that containing exon 6 was
preserved. Therefore, the relative amounts of digested and
undigested PCR products represented the quantification of neonatal
and adult Nav1.5 cDNA, respectively. The expression ratio of adult
(wild Nav1.5) versus neonatal Nav1.5 (Nav1.5e) in the DRG neurons
was ~2.5:1 judged from the signal quantification of pre- and
post-digestion by autoradiography (Fig. 1) (n=6). Similar results were found
in the axon of peripheral sensory neurons of adult rats (Fig. 1) (n=6).
The expression of Nav1.5 mRNA is
decreased in the DRG neurons and axon of peripheral sensory neurons
of rat with spared nerve injury
RT-PCR methods was used to detect the expression of
neonatal and adult Nav1.5mRNA in the DRG neurons and axon of
peripheral sensory neurons of rats with spared nerve injury. The
results demonstrated the expression of total Nav1.5 mRNA (adult
with neonatal Nav1.5) decreased by ~55% in SNI rat models compared
with that in controls, indicating a downregulation of Nav1.5mRNA
expression in the neuropathic pain models. Further investigation
was made in order to clarify whether the neonatal and adult Nav1.5
decreased simultaneously. As indicated in Figs. 2 and 3, restriction enzyme digestion method
was used to figure out the quantification of neonatal and adult
Nav1.5 mRNA expressed in the DRG neurons and axon of peripheral
sensory neurons of rats with spared nerve injury. The results
confirmed that both adult and neonatal Nav1.5 mRNA decreased in the
DRG neurons of SNI rats, with the adult and neonatal Nav1.5 mRNA
decreased by ~53 and 56% (n=12 for SNI rats; n=6 for controls),
respectively. Similar expression pattern of Nav1.5 mRNA was found
in the axon of peripheral sensory neurons of SNI rats, with the
adult and neonatal Nav1.5 mRNA decreased by ~55 and 57% (n=12 for
SNI rats; n=6 for controls), respectively.
Expression of total Nav1.5 protein in the
DRG neurons and axon of peripheral sensory neurons of rats
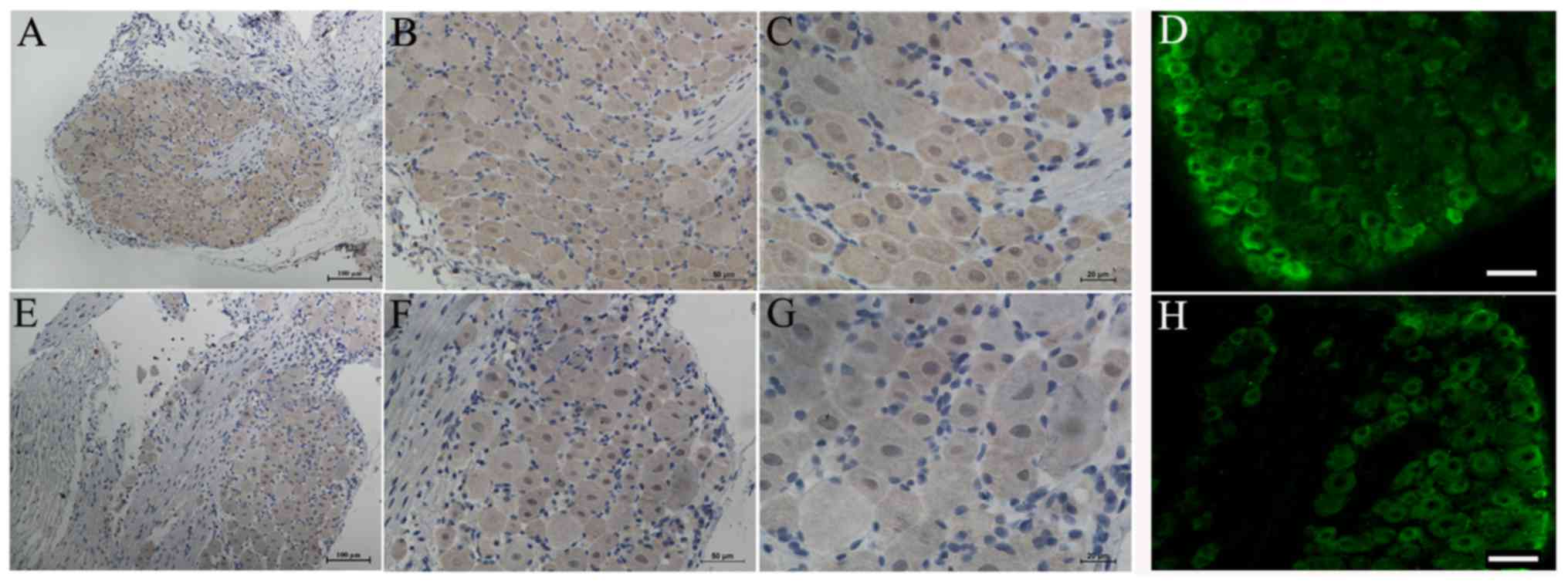
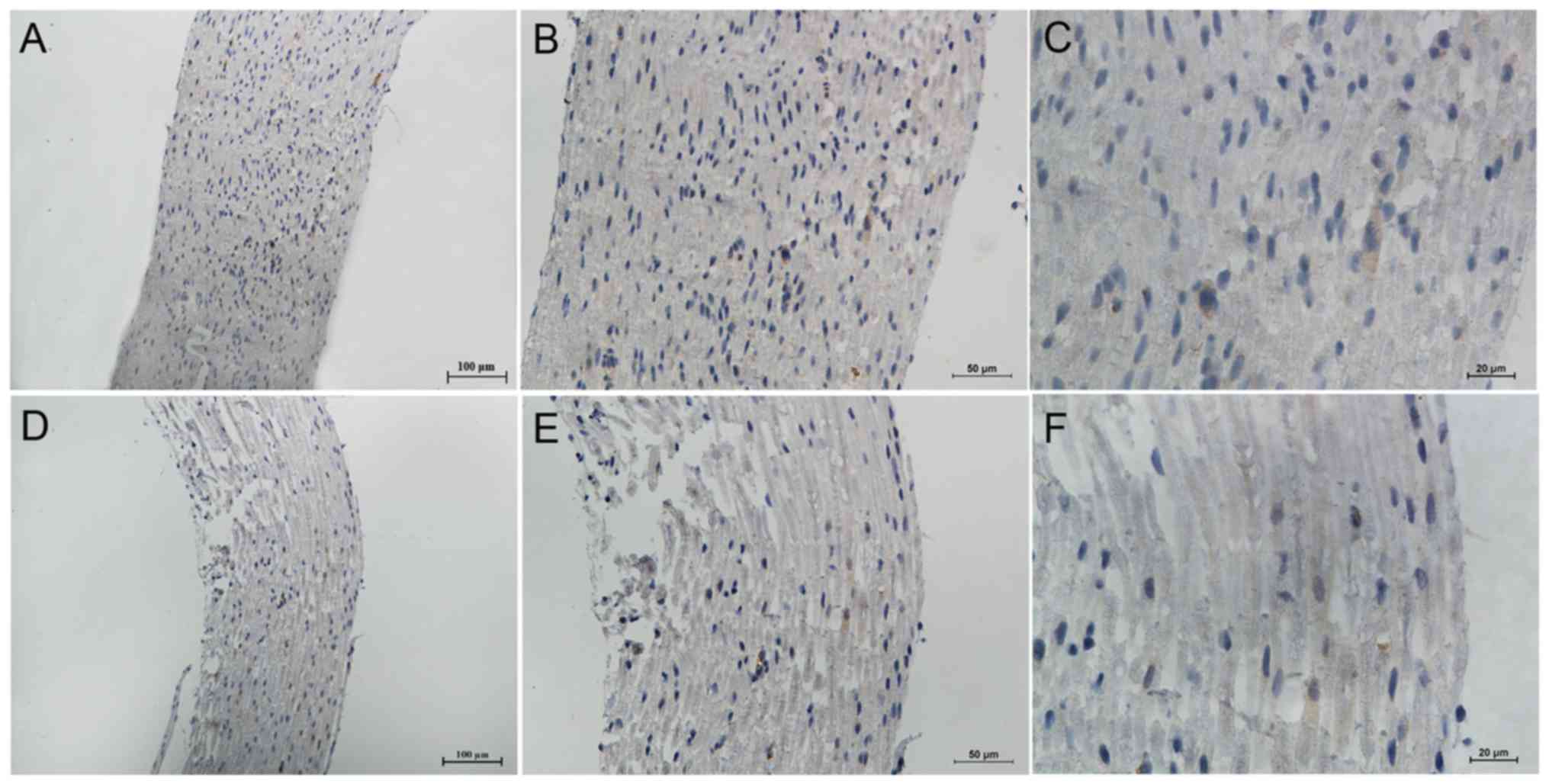
In order to investigate whether Nav1.5 protein was
expressed in the DRG neurons and axon of peripheral sensory neurons
of rat, we used immunohistochemical and immunofluorescence methods
to detect the expression and distribution of total Nav1.5 protein
in these structures. The results demonstrated that Nav1.5
immunoreactivity could be detected in the membrane and plasma of
DRG neurons and it showed stronger immunoreactivity in the small
diameter sensory neurons compared with large ones (Fig. 4). Positive Nav1.5 staining results
were also observed in the axon of peripheral sensory neurons,
including the myelin sheath (Schwann cells) (Fig. 5). In general, the Nav1.5
immunoreactivity in the axon and myelin sheath was weaker than that
in the DRG neurons. Similar to the RT-PCR results, the
immunohistochemistry results of Nav1.5 also indicated a decreased
expression of total Nav1.5 protein in the DRG neurons and axon of
peripheral sensory neurons of SNI rats (Figs. 4 and 5).
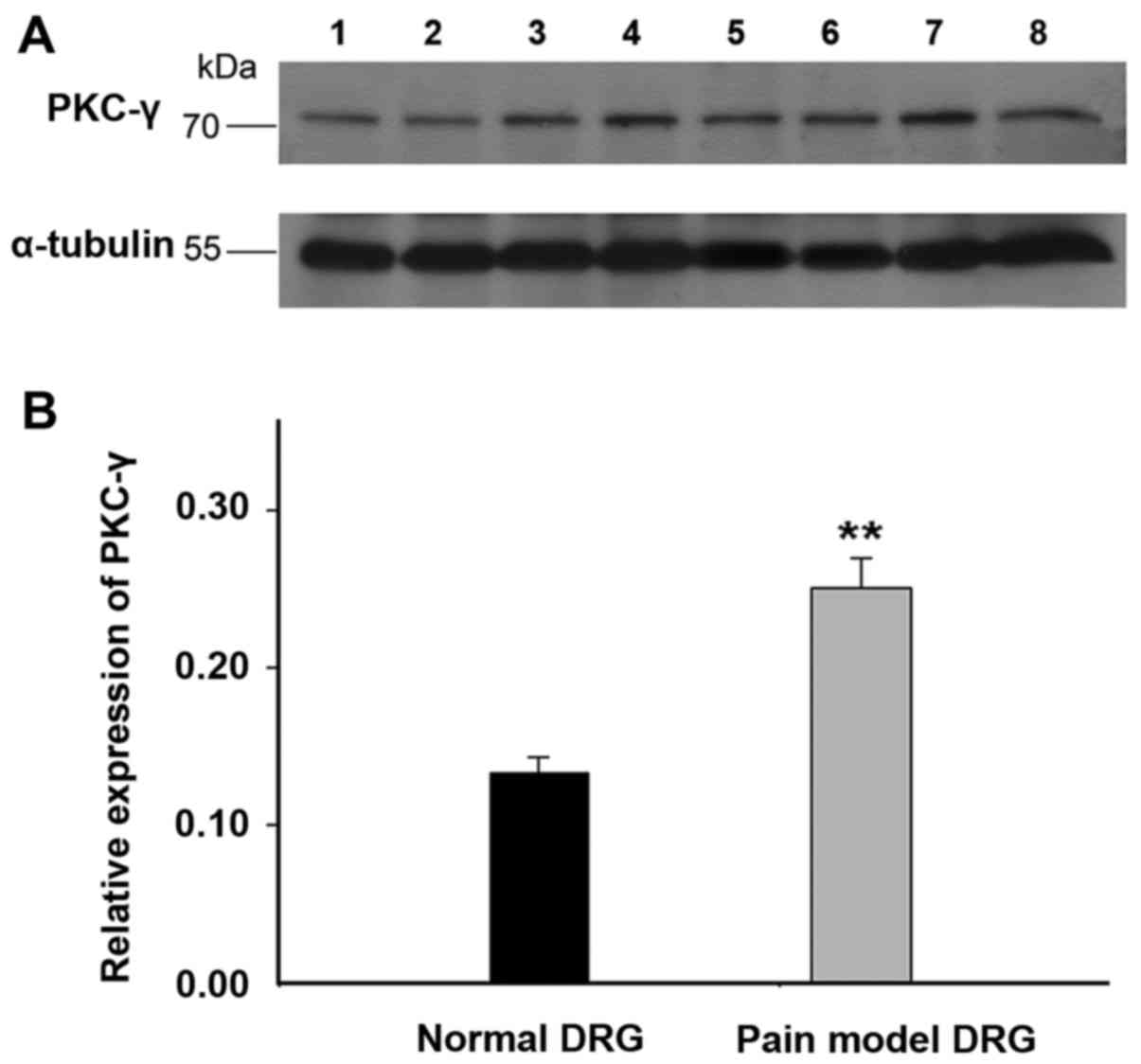
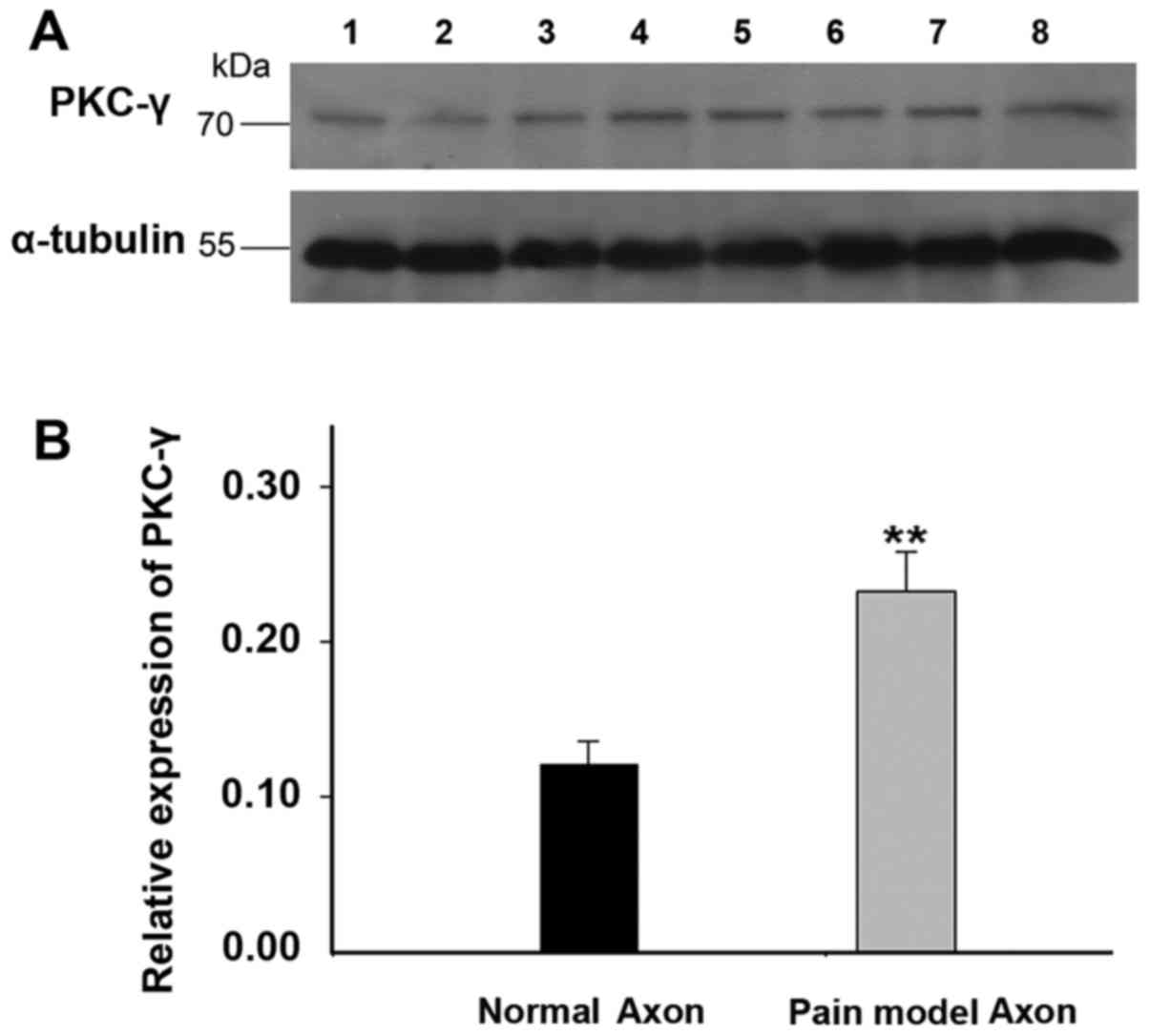
Upregulation of PKC-γ in the DRG neurons
and axon of peripheral sensory neurons of rats with spared nerve
injury
The activation of PKC was supposed to modulate the
biophysical properties of Nav1.5 channels, therefore, the
expression of PKC-γ, which expressed abundantly in nervous system,
was detected in the DRG neurons and axon of peripheral sensory
neurons of rats with spared nerve injury by western blot method.
The results demonstrated that the expression of PKC-γ increased by
~1-fold in the DRG neurons of rats with spared nerve injury
(Fig. 6, n=12 for SNI rats; n=6
for controls). Similar expression pattern of PKC-γ was found in the
axon of peripheral sensory neurons of SNI rats (Fig. 7, n=12 for SNI rats; n=6 for
controls). The results confirmed the upregulation of PKC-γ in both
the DRG neurons and axons of peripheral sensory neurons of pain
model rats.
Discussion
Both adult and neonatal Nav1.5 are
expressed in the DRG neurons and axons of peripheral sensory
neurons
Previous studies have confirmed the expression and
distribution of Nav1.5 in the adult and neonatal rat and mouse DRG
neurons in both mRNA and protein levels (14). Moreover, the expression ratio of
various Nav1.5 alternative splicing variants, including Nav1.5a and
Nav1.5c, in the rat and mouse DRG neurons has also been
investigated (15). These studies
demonstrated that Nav1.5a was the major Nav1.5 isoform expressed in
the DRG neurons and it showed different functional properties
compared with full-length Nav.1.5 (wild Nav1.5) isoform (14,15). Subsequently, Nav1.5 channel was
also proved to produce the third TTX-R current in rat small DRG
neurons by electrophysiological studies (13). However, since nine Nav1.5 splice
variants have been discovered (Nav1.5a-f, E28B-D), whether other
Nav1.5 isoforms are expressed in the DRG neurons is still unknown.
To our knowledge, there was no report on the expression of neonatal
Nav1.5 isoform in the DRG neurons. Therefore, in this study, we
systematically investigated in the expression of neonatal Nav1.5 in
the DRG neurons and the axons of peripheral sensory neurons of SNI
rats. The results confirmed, for the first time, the expression of
both neonatal and adult Nav1.5 isoforms in the DRG neurons and axon
of peripheral sensory neurons.
The neonatal Nav1.5, also called Nav1.5e or nNav1.5,
is characterized by an exon 6A encoding for the S3/S4 region in
domain I (residues 205–1234 in hNav1.5) (22,23). Instead, the adult Nav1.5 is
encoded by exon 6 of SCN5A. Both exon 6A and exon 6 have 92
nucleotides but differ at 31 nucleotide positions, resulting in 7
amino acid differences (10,11,16,22,23). Notably, this alternative splicing
from exon 6 to 6A replaces a conserved negative aspartate residue
in the adult Nav1.5 with a positive lysine (20). Thus, this amino acid change
reversed the local charge, which may alter the electrophysiological
properties of Nav1.5. Subsequently, this hypothesis was confirmed
by Onkal et al (20). As
reported in their study, compared with the 'adult' Nav1.5 isoform,
the 'neonatal' Nav1.5 channel exhibited i) a depolarized threshold
of activation and voltage at which the current peaked; ii) much
slower kinetics of activation and inactivation; iii) 50% greater
transient charge (Nat) influx; iv) a stronger voltage dependence of
time to peak; and v) a slower recovery from inactivation (20). Therefore, the alternative splicing
of exon 6 and 6A generated two electrophysiologically different
Nav1.5 channels. In this study, the co-expression of neonatal and
adult Nav1.5 in the DRG neurons and axons of peripheral sensory
neurons was identified. However, the expression ratio of the two
distinct Nav1.5 isoforms, which may affect the total Nav1.5
functional properties, in these structures was still uncertain.
Therefore, we analyzed the difference between the sequence exon 6
and 6A. Occasionally, a specific restriction enzyme site of
SacIII was found in the exon 6A rather than exon 6.
Therefore, the restriction enzyme SacIII was used to digest
the PCR products in order to distinguish these two variants. The
results demonstrated the expression ratio of adult (wild Nav1.5)
versus neonatal Nav1.5 (Nav1.5e) in the DRG neurons and axon of
peripheral sensory neurons was ~2.5:1. Therefore, the results
indicated that the Nav1.5 sodium current detected before was a
compound product by both adult and neonatal Nav1.5 isoforms. Our
findings provided a basic step for further investigation in the
significance of the co-expression of adult and neonatal Nav1.5 in
the DRG neurons.
Previous studies have detected the expression of
Nav1.5 protein in the DRG neurons by western blot method (15). In this study, we used
immunochemistry and immunofluorescence method to detect the
expression of Nav1.5 protein in the DRG neurons and axon of
peripheral sensory neurons. The results demonstrated that Nav1.5
immunoreactivity could be detected both in the DRG neurons and axon
of peripheral sensory neurons, and it showed stronger
immunoreactivity in the small diameter DRG neurons compared with
large ones. These results, combined with the previous
electrophysiological studies, confirmed the expression of Nav1.5 in
the protein level. Although Nav1.5 was proved to express
functionally in the DRG neurons, its specific role in the
generation and propagation of action potential still remains
unknown. Previous studies demonstrated that the functional
properties of Nav1.5 in the DRG neurons was proved to be different
from other TTX-R sodium channels, such as Nav1.8 and Nav1.9
channels, in the following aspects: half-time for activation, the
time constant for inactivation, and the midpoints of activation and
inactivation (13). Future
studies should make deep investigation in clarifying the specific
function of Nav1.5 in the DGR neurons and axons of peripheral
sensory neurons.
Downregulation of adult and neonatal
Nav1.5 in the DRG neurons and axons of peripheral sensory neurons
of SNI model rats
Voltage-gated sodium channels in the DRG neurons and
axons of peripheral sensory neurons are essential for the
generation and propagation of action potential for nociception
(1,7,24,25). Since at least eight sodium
channels have been detected to be expressed in the DRG neurons
axons of peripheral sensory neurons (6,7,25).
It is important to clarify the specific contributions of various
sodium channels to the generation and propagation of nociceptive
information. Previous studies demonstrated that the expression of
Nav1.5a and Nav1.5/Nav1.5c mRNA decreased by 48.1 and 46.4%,
respectively, in mouse lumbar L4 and L5 DRG neurons seven days
after peripheral axotomy (15).
In this study, we investigated the expression of neonatal and adult
Nav1.5 mRNA and protein in the DRG neurons, as well as the axon of
peripheral sensory neurons of rats with spared nerve injury.
Similar to the expression of Nav1.5a and Nav1.5/Nav1.5c in the DRG
neurons of peripheral nerve transection (axotomy) rat model, RT-PCR
results demonstrated the expression of neonatal and adult Nav1.5
mRNA decreased approximately by half in the DRG neurons and axon of
peripheral sensory neurons of rats with spared nerve injury.
Immunochemistry and immunofluorescence results also indicated a
downregulation of total Nav1.5 protein in these structures. In
general, others and our findings confirmed the downregulation of
Nav.5 channel in both mRNA and protein level in the neuropathic
pain model.
The role of PKC-γ in the modulation of
sodium current and sodium channel gene expression in the DRG
neurons
PKC-γ, one of the conventional PKC (cPKC) isozymes,
is expressed abundantly in the nervous systems. Previous studies
demonstrated that activation of PKC led to a reduction in the
sodium current in both brain and heart (26–129). This effect was due to the
phosphorylation of site Ser1503 in the Nav1.5 channel by
activated PKC. Other studies further demonstrated that Nav1.5
channels stably expressed in human embryonic kidney cells were
preferentially trafficked away from the plasma membrane by PKC
activation, with a major contribution by Ca2+ sensitive
or conventional PKC isoforms (30). Removal of the conserved PKC site
Ser1503 eliminated the PKC-mediated effect to alter
Nav1.5 channel trafficking (30).
These results demonstrated PKC may play a role to alter both the
localization and functional properties of Nav1.5 channels. In this
study, we investigated the expression of PKC-γ and other PKC
isoforms, including PKC-α, PKC-δ and PKC-ε in the DRG neurons and
axons of peripheral sensory neurons of rats with spared nerve
injury. Of these isoforms, PKC-γ showed a reproducible upregulation
in the DRG neurons and axons of peripheral sensory neurons of pain
model rats. The expression of phosphorylated PKC-γ, the activated
form of PKC-γ, in the DRG neurons and axons of peripheral sensory
neurons of SNI rats was also detected in this study (unpublished
data) and the result was similar to that of total PKC-γ. In
general, both the total and phosphorylated PKC-γ increased in the
DRG neurons and axons of SNI rat model. Although the upregulation
of PKC-γ may modulate the sodium current and intracellular sodium
channel trafficking, previous studies and our data provided no
direct evidence to support the modulation of Nav1.5 mRNA and
protein by PKC-γ. Combined with previous studies, we hypothesized
that the activation of PKC may suppress the sodium current and
activity, which in turn decreased the intracellular Na+
and inhibit the activation of PKA, which may modulate the
expression of Nav1.5 mRNA and protein. However, we admit that the
mechanisms must be more complex than that we hypothesized. Future
studies should make further investigation to reveal the detailed
mechanisms underlying this phenomenon.
In conclusion, this study first confirmed the
expression of both adult and neonatal Nav1.5 isoforms in the DRG
neurons and axon of peripheral sensory neurons. In DRG neurons and
axons of SNI pain model rats, the expression of both adult and
neonatal Nav1.5 decreased by approximately a half in mRNA and
protein level, however, the expression of PKC-γ increased by
~1-fold. The upregulation of PKC-γ may downregulate the expression
of adult and neonatal Nav1.5 in SNI rat models and then further
involve in the generation of neuropathic pain. Further study need
to clarify the specific relation between the upregulation of PKC-γ
and the downregulation of Nav1.5 in pain models.
Acknowledgments
The authors would like to thank the staff of the
Department of Neurosurgery of the First Hospital of China Medical
University (Shenyang, China) for their technical assistance.
Notes
[1]
Funding
The present study was supported by the Liaoning
Provincial Natural Science Foundation of China (grant no.
2014021097, awarded to JW) and the National Natural Science Foun
dation of China (grant no. 31100770, awarded to JW).
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
GML and ZQDX designed the expriment. JW performed
the experiment, and was a major contributor in writing the
manuscript. YFB established the animal model of pain. SWO and YJW
interpreted the data and proofread the manuscript. All authors read
and approved the final manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu M and Wood JN: The roles of sodium
channels in nociception: Implications for mechanisms of neuropathic
pain. Pain Med. 12(Suppl 3): S93–S99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cummins TR, Sheets PL and Waxman SG: The
roles of sodium channels in nociception: Implications for
mechanisms of pain. Pain. 131:243–1257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Catterall WA: From ionic currents to
molecular mechanisms: The structure and function of voltage-gated
sodium channels. Neuron. 26:13–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu FH and Catterall WA: Overview of the
voltage-gated sodium channel family. Genome Biol. 4:2072003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Catterall WA: Voltage-gated sodium
channels at 60: Structure, function and pathophysiology. J Physiol.
590:2577–12589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laedermann CJ, Pertin M, Suter MR and
Decosterd I: Voltage-gated sodium channel expression in mouse DRG
after SNI leads to re-evaluation of projections of injured fibers.
Mol Pain. 10:192014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Devor M: Sodium channels and mechanisms of
neuropathic pain. J Pain. 7(Suppl 1): S3–S12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rook MB, Evers MM, Vos MA and Bierhuizen
MF: Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc
Res. 93:12–123. 2012. View Article : Google Scholar
|
|
9
|
Schroeter A, Walzik S, Blechschmidt S,
Haufe V, Benndorf K and Zimmer T: Structure and function of splice
variants of the cardiac voltage-gated sodium channel Na(v)1.5. J
Mol Cell Cardiol. 49:16–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Ou SW, Wang YJ, Kameyama M,
Kameyama A and Zong ZH: Analysis of four novel variants of
Nav1.5/SCN5A cloned from the brain. Neurosci Res. 64:339–1347.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Ou SW, Wang YJ, Zong ZH, Lin L,
Kameyama M and Kameyama A: New variants of Nav1.5/SCN5A encode
Na+ channels in the brain. J Neurogenet. 22:57–175.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xing D, Wang J, Ou S, Wang Y, Qiu B, Ding
D, Guo F and Gao Q: Expression of neonatal Nav1.5 in human brain
astrocytoma and its effect on proliferation, invasion and apoptosis
of astrocytoma cells. Oncol Rep. 31:2692–12700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Renganathan M, Dib-Hajj S and Waxman SG:
Na(v)1.5 underlies the 'third TTX-R sodium current' in rat small
DRG neurons. Brain Res Mol Brain Res. 106:70–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kerr NC, Holmes FE and Wynick D: Novel
isoforms of the sodium channels Nav1.8 and Nav1.5 are produced by a
conserved mechanism in mouse and rat. J Biol Chem.
279:24826–124833. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kerr NC, Gao Z, Holmes FE, Hobson SA,
Hancox JC, Wynick D and James AF: The sodium channel Nav1.5a is the
predominant isoform expressed in adult mouse dorsal root ganglia
and exhibits distinct inactivation properties from the full-length
Nav1.5 channel. Mol Cell Neurosci. 35:283–1291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren CT, Li DM, Ou SW, Wang YJ, Lin Y, Zong
ZH, Kameyama M and Kameyama A: Cloning and expression of the two
new variants of Nav1.5/SCN5A in rat brain. Mol Cell Biochem.
365:139–1148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hartmann HA, Colom LV, Sutherland ML and
Noebels JL: Selective localization of cardiac SCN5A sodium channels
in limbic regions of rat brain. Nat Neurosci. 2:593–1595. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu L, Nishiyama K, Hollyfield JG and Wang
Q: Localization of Nav1.5 sodium channel protein in the mouse
brain. Neuroreport. 13:2547–12551. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frenz CT, Hansen A, Dupuis ND, Shultz N,
Levinson SR, Finger TE and Dionne VE: NaV1.5 sodium channel window
currents contribute to spontaneous firing in olfactory sensory
neurons. J Neurophysiol. 112:1091–11104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Onkal R, Mattis JH, Fraser SP, Diss JK,
Shao D, Okuse K and Djamgoz MB: Alternative splicing of Nav1.5: An
electrophysiological comparison of 'neonatal' and 'adult' isoforms
and critical involvement of a lysine residue. J Cell Physiol.
216:716–1726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Decosterd I and Woolf CJ: Spared nerve
injury: An animal model of persistent peripheral neuropathic pain.
Pain. 87:149–1158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ou SW, Kameyama A, Hao LY, Horiuchi M,
Minobe E, Wang WY, Makita N and Kameyama M: Tetrodotoxin-resistant
Na+ channels in human neuroblastoma cells are encoded by
new variants of Nav1.5/SCN5A. Eur J Neurosci. 22:793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fraser SP, Diss JK, Chioni AM, Mycielska
ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, et
al: Voltage-gated sodium channel expression and potentiation of
human breast cancer metastasis. Clin Cancer Res. 11:5381–15389.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang W, Gu J, Li YQ and Tao YX: Are
voltage-gated sodium channels on the dorsal root ganglion involved
in the development of neuropathic pain? Mol Pain. 7:162011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moldovan M, Alvarez S, Romer Rosberg M and
Krarup C: Axonal voltage-gated ion channels as pharmacological
targets for pain. Eur J Pharmacol. 708:105–1112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Numann R, Catterall WA and Scheuer T:
Functional modulation of brain sodium channels by protein kinase C
phosphorylation. Science. 254:115–1118. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schreibmayer W: Isoform diversity and
modulation of sodium channels by protein kinases. Cell Physiol
Biochem. 9:187–1200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grant AO and Wendt DJ: Block and
modulation of cardiac Na+ channels by antiarrhythmic
drugs, neurotransmitters and hormones. Trends Pharmacol Sci.
13:352–1358. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tateyama M, Kurokawa J, Terrenoire C,
Rivolta I and Kass RS: Stimulation of protein kinase C inhibits
bursting in disease-linked mutant human cardiac sodium channels.
Circulation. 107:3216–13222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hallaq H, Wang DW, Kunic JD, George AL Jr,
Wells KS and Murray KT: Activation of protein kinase C alters the
intracellular distribution and mobility of cardiac Na+
channels. Am J Physiol Heart Circ Physiol. 302:H782–H789. 2012.
View Article : Google Scholar
|